Abstract
Cellular therapies are becoming a major focus for the treatment of demyelinating diseases such as multiple sclerosis (MS), therefore it is important to identify the most effective cell types that promote myelin repair. Several components contribute to the relative benefits of specific cell types including the overall efficacy of the cell therapy, the reproducibility of treatment, the mechanisms of action of distinct cell types and the ease of isolation and generation of therapeutic populations. A range of distinct cell populations promote functional recovery in animal models of MS including neural stem cells and mesenchymal stem cells derived from different tissues. Each of these cell populations has advantages and disadvantages and likely works through distinct mechanisms. The relevance of such mechanisms to myelin repair in the adult central nervous system is unclear since the therapeutic cells are generally derived from developing animals. Here we describe the isolation and characterization of a population of neural cells from the adult spinal cord that are characterized by the expression of the cell surface glycoprotein NG2. In functional studies, injection of adult NG2+ cells into mice with ongoing MOG35-55-induced experimental autoimmune encephalomyelitis (EAE) enhanced remyelination in the CNS while the number of CD3+ T cells in areas of spinal cord demyelination was reduced approximately three-fold. In vivo studies indicated that in EAE, NG2+ cells stimulated endogenous repair while in vitro they responded to signals in areas of induced inflammation by differentiating into oligodendrocytes. These results suggested that adult NG2+ cells represent a useful cell population for promoting neural repair in a variety of different conditions including demyelinating diseases such as MS.
Keywords: NG2 glycoprotein, myelin, oligodendrocytes, experimental autoimmune encephalomyelitis, multiple sclerosis, remyelination
INTRODUCTION
Cell therapies for central nervous system (CNS) insults are becoming recognized as an important approach to facilitating functional recovery[1–5]. Such studies raise issues about the most effective population of cells and defining their mode of action. We have previously described the functional benefit of bone marrow-derived mesenchymal stem cells (MSCs) in animal models of experimental autoimmune encephalomyelitis (EAE) and proposed that these cells both suppress the immune response and promote the development of myelinating oligodendrocytes from neural stem cells (NSCs)[6]. Similar functional benefits have been described following treatment of animals with NSCs[7,8]. Bone marrow is not the only source of MSCs and such cells have been isolated from adipose and a number of other tissues[9–12] although in lower numbers. One hypothesis that has gained considerable attention is that MSCs are found in all tissues and that they represent pericytes that are closely associated with tissue vasculature[13]. Consistent with this hypothesis, both MSCs and pericytes express a number of stem-cell properties. For example, MSCs are multipotent and give rise to a variety of different cell types[14–16]. In general, bone marrow-derived MSCs generate cells associated with mesodermal derivatives including cartilage, muscle and connective tissue. Likewise in the CNS, vascular or capillary-associated pericytes have stem cell-like properties[17] and those derived from the developing retina or CNS capillaries give rise to cells with properties of mesodermal or ectodermal derivatives[18–20] suggesting they are capable of generating diverse cell types including neurons and oligodendrocytes, the myelinating cells of the CNS[21,22].
One characteristic shared by pericytes and the precursors of oligodendrocytes (OPCs) is the expression of the cell surface glycoprotein NG2[23–25]. The NG2 protein was originally defined by antibodies directed against surface proteins in a rat cell line with glial and neuronal properties[26], and more recently has been shown to identify a population of cells in both the developing and adult CNS that have the capacity to generate oligodendrocytes[27]. These adult OPCs or polydendrocytes[28], which may constitute up to 5% of the cells in the adult rodent CNS, have unique properties in that they are multi-processed, electrically coupled to axons, and have been proposed to modulate neuronal connectivity in the CNS[29]. The most intense expression of NG2 in the adult CNS is, however, on pericytes associated with the vasculature[30] where they are thought to play a role in regulating tissue homeostasis[31–33] and the blood-brain barrier[34,35].
Given that NG2 is expressed by cells with stem cell-like properties[36] and other stem cell populations promote functional recovery in models of demyelinating diseases, we developed an isolation protocol that allows for the selection of NG2+ cells from the adult mouse spinal cord and characterized their functional properties.
MATERIALS AND METHODS
Animals
All cell cultures were obtained from 6–8-week-old C57BL/6 mice using techniques approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Access to food and water was facilitated for paralyzed mice to prevent dehydration. In some studies, to distinguish between transplanted and host oligodendrocyte-lineage cells, female mice expressing enhanced green fluorescent protein (EGFP) driven by the promoter for proteolipid protein promoter (PLP) on a C57BL/6J background[37] were used as hosts following induction of EAE.
Purification of Mouse Adult Spinal Cord NG2+ Cells Using Percoll Gradient
For each isolation, the spinal cords from two adult (6–8 weeks) C57BL/6J mice were rapidly dissected and placed in cold minimum essential medium (MEM) (Gibco, Grand Island, NY, Cat. no. 12360). The spinal cords were dissociated in trypsin/EDTA (1:1) for 30 min at 37°C in 5% CO2. After incubation, tissue was mixed with 0.6 mL 0.5% DNase in DMEM/F12 with 10% FBS for 10 min homogenization and centrifuged at 800 g for 5 min. The cells were washed three times before layering onto a Percoll gradient (GE Healthcare, Cleveland, OH, Cat. no. 17-0891-02). The gradient was prepared from stock isotonic Percoll (SIP) at 70% in 1× PBS, 30% in 1× MEM without Ca2+ and Mg2+. Tissue was homogenized in 10 mL of 30% SIP and slowly layered on top of the 70% SIP. After centrifugation at 2 000 rpm for 30 min, the lipid layer was carefully removed from the top of the tube and 2.0–3.0 mL of the 70%–30% interface collected into a clean conical tube and washed at 1 200 rpm for 5 min. Cells were plated at 1×106 cells/mL in poly-L-lysine (PLL) (Sigma, St. Louis, MO, Cat. no. P1274-100MG)-coated 75-cm2 flasks at 37°C in 5% CO2 for at least 3 weeks with replenishment of 2 mL DMEM/F12 once a week. After ~3 weeks, colonies of cells developed and the medium was changed every 3 days. After 5 weeks, the cells reached 85% confluence, and the cultures were harvested with trypsin and passaged 1:4 into fresh PLL-coated 75-cm2 flasks. Cells were grown to confluence and passaged again as described. All cells for immunocytochemical analyses were seeded onto PLL-coated coverslips and grown for 3 days unless indicated differently. Cell purity was determined by NG2 antibody staining and the cells were passaged at least once before use. To label cells prior to transplantation, purified adult NG2+ cells were labeled with either 5-chlormethyfluorescein diacetate (Cell Tracker™ red CMFDA, Invitrogen, Grand Island, NY, No. 2925) or CFSE (Renovar, Madison, WI) and 1×106 cells were injected via the tail vein on day 15 post-immunization.
Antibodies
Polyclonal rabbit anti-NG2 chondroitin sulfate proteoglycan was from Millipore, Billerica, MA (Cat. no. AB5320). Monoclonal rabbit anti-PDGFβ-receptor was from AbCam, Cambridge, UK (Cat. no. ab32570). Monoclonal mouse anti-α-smooth muscle actin (α-SMA, Cat. no. ab5694-100) and anti-glial fibrillary acidic protein (GFAP, Cat. no. z0334) antibodies were from AbCam and DAKO, Denmark. Monoclonal mouse anti-CD3 antibody was from BD Pharmingen San Diego, CA (Cat. no. 553058). The Alexa Fluor 488 (Cat no. A11029) and Alexa Fluor 594 (Cat. no. A11012) conjugated secondary antibodies against rabbit IgG and mouse IgG were from Invitrogen.
Immunofluorescence and Microscopy
Cell cultures were fixed in 5% acid methanol (−20°C) for 12 min and then washed 2× for 5 min in room-temperature DMEM with 5% normal goat serum (NGS). Cultures were incubated with antibodies (diluted 1:100 in DMEM) for 30 min at 37°C in a humidified chamber and washed 6× for 5 min in DMEM with 5% NGS, followed by incubation with fluorescent conjugated secondary antibodies diluted 1:200 in DMEM with 5% NGS for 30 min at 37°C, washed briefly and mounted in Vectashield with DAPI (Sigma). For proliferation assays, NG2+ cells were grown until they reached ~75% confluence. Bromodeoxyuridine (5-bromo-2'-deoxyuridine, BrdU) (Roche, Indianapolis, IN, Cat. no. 11170376001) was added 18 h before fixation. Cells were washed with DMEM/F12, and double-labeled for NG2 and BrdU. Each experiment was repeated at least 3 times with data from duplicate coverslips. Proliferation was assessed by quantifying the number of BrdU-positive cells as a proportion of the total number of NG2-positive cells, and the results expressed as the mean ± SEM of three experiments. Statistical significance (P <0.05) was assessed by Student's t-test.
Animals were perfused with 4% paraformaldehyde and selected tissues cryoprotected in 30% sucrose overnight, frozen in OCT mounting medium, and sectioned (20 μm) on a Leica cryostat. Black gold staining for myelin was conducted according to the manufacturer's instructions (Garbelli et al., 2011)[38]. All stained cells and spinal cord sections were imaged with a Leica microscope (DM5500B) and analyzed with Leica Microsystem (CH-9435).
EAE Induction and Animal Groups
Peptide of myelin oligodendrocyte glycoprotein, MOG35-55 (MEVGWYRSPFSRVVH LYRNGK-COOH), was from United Biochemical Research, Inc., Seattle, WA (>70% HPLC, Cat. no. UBR110920A-2). C57/BL/6 mice were immunized subcutaneously with an emulsion of MOG35-55 antigen peptide (200 μg/mouse) in complete Freund's adjuvant and paraffin oil (EM Science, Gibbstown, NJ, Cat. no. PX0045-3) mixed with mannide monooleate (Sigma, M8546-500G) at a 1:9 ratio containing 0.3 mg heat-inactivated Mycobacterium tuberculosis h37ra (Difco Laboratories, Detroit, MI, Cat. no. 231141). Each animal also received 300 ng pertussis toxin (List Biological Laboratories, Inc., Campbell, CA, Cat. no. 181) on days 0 and 1 post-immunization. Mice were randomly divided into a control group (n =10) with PBS (0.2 mL) and a treatment group with 1×106 NG2 cells in the volume injected into the tail vein of EAE mice on day 15 post-immunization at the peak of clinical disease[3]. Animals in both groups were scored daily for clinical symptoms of EAE as follows: 0, healthy; 1, loss of tail tone; 2, ataxia and/or paresis of hindlimbs; 3, paralysis of hindlimbs and/or paresis of forelimbs; 4, tetraparalysis; 5, moribund or dead[8]. Animals were tested daily for 48 days after the onset of clinical symptoms. For histopathological and immunohistochemical analyses, spinal cords from both NG2+ cell-treated and control animals with the best functional scores were selected.
Preparation of EAE slice cultures and EAE-conditioned Media
For slice cultures, brains were isolated from 8-week-old mice with ongoing EAE and 1-mm coronal slices (EAE-S) were cut on a Vibratome (Leica, VT1000S). Slices were placed on PLL-coated dishes (3 slices/dish) with DMEM/F12, 15% horse serum and 2% GN2 (Gibco, Cat. no. 17504-044)[39]. The medium was changed every other day and collected after 48 h of incubation and defined as EAE-conditioned medium (EAE-CM). Media were pooled and stored at −20°C prior to use. For NG2+ cell differentiation, passage 2 NG2+ cells were allowed to expand in either EAE-CM or EAE-S for 3–7 days, and assayed for the proportion of cells expressing the mature oligodendrocyte markers O1, CC1, and MBP as described above.
Statistical Analysis
All experiments were conducted in triplicate and repeated at least twice. To quantify the cellular composition of cultures, four experiments were performed in duplicate within each experiment at each passage. All data are presented as mean ± SEM, and statistical significance was defined as P <0.05 by Student's t-test[40]. Group means and standard deviations were calculated for each parameter. Statistical differences between two groups were evaluated using the two-tailed Student's t-test. Differences were considered statistically significant when the P value was <0.05.
RESULTS
Purification and characterization of Adult Mouse spinal cord-Derived NG2+ cells
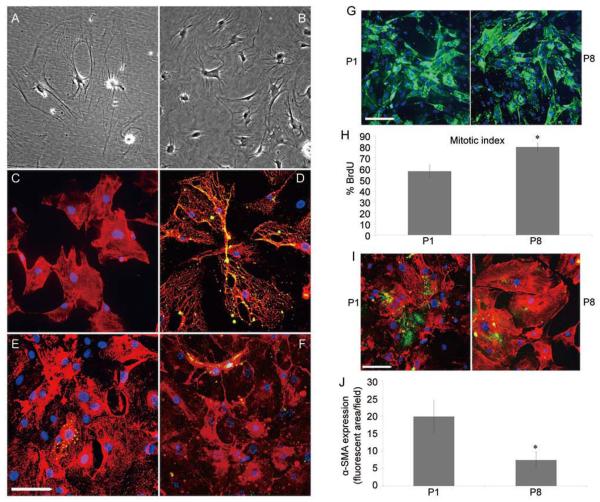
After isolation of adult mouse spinal cord cells from the Percoll gradient and growth in DMEM/F12 with 10% FBS, cell colonies began to emerge after ~3 weeks in vitro. These cells initially had a characteristic morphology with a discoid nucleus, dense cytoplasm and an irregular shape with multiple processes (Fig. 1A)[41]. When the cells reached confluence, they were passaged and most of the passaged cells assumed a rhomboid morphology (Fig. 1B). To characterize these cells in more detail, cultures were labeled with antibodies to cell-type-specific markers including NG2 and platelet-derived growth factor-beta receptor (PDGFR-β) to identify pericytes, the endothelial cell marker, VWF (von Willebrand factor) and antibodies to GFAP (glial fibrillary acidic protein), a characteristic of adult spinal cord astrocytes. By the second passage >98% of the cells were labeled with antibody to NG2 (Fig. 1C) and >80% of the NG2-expressing cells were co-labeled with antibody to PDGFR-β (Fig. 1D). By contrast, <3% of the NG2-expressing cells were positive for VWF (Fig. 1E). To determine whether the cultures contained substantial numbers of astrocytes, they were labeled with antibody to GFAP, an intermediate filament found predominantly in astrocytes in the adult spinal cord. Less than 2% of second passage NG2+ cells expressed detectable levels of GFAP (Fig. 1F) suggesting there were few astrocytes in these cultures. These results suggested that our purification procedure yielded cells that shared some characteristics with pericytes.
Fig. 1.
Characterization of NG2+ cells at low (P1) and high (P8) passage numbers. Enriched NG2+ cells were isolated on a Percoll gradient. A and b Phase-contrast images of an early culture of mouse adult spinal cord NG2+ cells (A) and a confluent monolayer of mouse adult spinal cord passage 2 NG2+ cells (b). Note that the cultures from the early primary to subsequent passages show that most cells have a largely rhomboid morphology more reflective of pericytes than OPCs. C: The NG2+ cells were relatively homogenous with limited contamination from other cell types. D–F: Dual-immunofluorescence staining of NG2+ cells with PDGFR-β (D), VWF (E) and GFAP (F) shows only a small fraction of the population expressed markers of endothelial cells (E) or astrocytes (F). G: cell cultures from P1 and P8 stained with immunofluorescent antibodies against NG2 and the differentiation marker α-SMA. The morphology of the NG2+ cells was similar in P1 and P8. H: comparison of the rates of proliferation in P1 and P8 NG2+ cells using brdU incorporation demonstrated a significant increase in the proliferation of late-passage cells. All points represent the mean ± SEM of 3 experiments, *P <0.05. I: The expression of α-SMA by NG2+ cells decreased with passage number. While P1 cells expressed low but detectable levels of a-SMA, this decreased significantly at P8. J: Quantification of α-SMA expression by fluorescent imaging using Image J. Note that P1 NG2+ cells expressed very low levels of α-SMA, that decreased with further passages. *P <0.05 for P1 versus P8. Mean ± SEM of duplicate preparations from three independent experiments. Scale bars in A–F, G and I, 100 μm.
Purified Adult NG2+ cells remained Undifferentiated Over Many Passages
To determine whether the phenotype and fundamental properties of adult NG2+ cells changed during long-term culture as has been reported for pericytes[42], cells from low (P1) and high (P8) passage numbers were compared with respect to morphology, proliferation rate and phenotype. The NG2+ cells retained similar characteristics through the 8 passages. For example, the morphology was similar for P1 and P8 cells and both cell populations strongly expressed NG2 (Fig. 1G). Somewhat unexpectedly, the P8 passage cells had a slightly higher mitotic index (~80% per 18-h pulse) than the P1 cells (~57% per 18-h pulse) suggesting that these cells retained a robust proliferative capacity (Fig. 1H). Since these cells initially shared some characteristics with pericytes, the expression of α-SMA was compared between P1 and P8 cells (Fig. 1I). In P1 cultures, 19.1 ± 5.5% of the cells expressed α-SMA and this proportion decreased to 10.2 ± 3.5% in P8 cultures (Fig. 1J) suggesting not only no enhancement of differentiation with increased passage number but also some loss of mature pericyte characteristics with extended culture. Less than 2% of the NG2 cells at either passage number expressed detectable levels of oligodendrocyte lineage markers (PDGF-αR, Sox10 or olig-1). Given the lack of evident differences in the cell population up to P8, all subsequent studies were conducted with cells at P8 or lower.
NG2+ cells Improved Functional recovery and Promoted remyelination in EAE
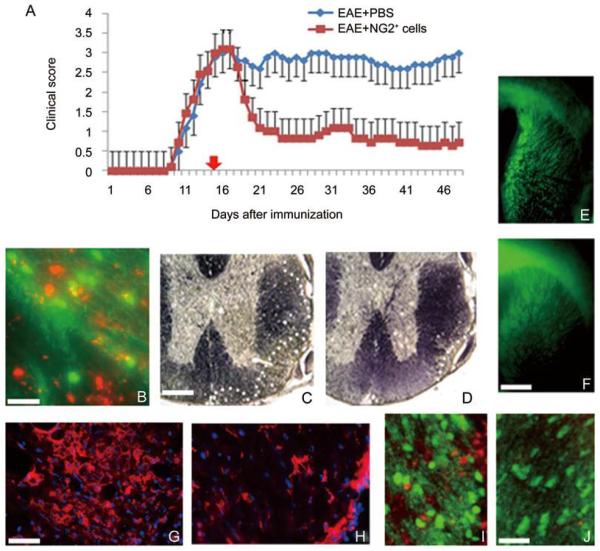
To determine if adult NG2+ cells had the capacity to promote remyelination in the setting of adult CNS demyelination, the functional effects of intravenous infusion of cells into mice with ongoing MOG35-55-induced EAE was assayed. Cells were engrafted 15 days after the induction of EAE, at the peak of the disease, and the animals were evaluated histologically up to 47 days after engraftment. The MOG35-55 paradigm generated functional deficits that developed 8 days after initial immunization, plateaued after 15–16 days and remained relatively constant in control animals treated with PBS (Fig. 2A). By contrast, in animals that received NG2+ cells on day 15, a considerable functional improvement was seen within 5 days and this was maintained for the duration of the study (Fig. 2A). To determine whether the functional improvement was associated with the migration of adult NG2+ cells into the CNS, cells were labeled prior to injection into PLP-EGF host animals. Within 24 h, labeled cells were detected in white-matter tracts of the brain and spinal cord (Fig. 2B), particularly in regions of demyelination. The areas of demyelination in the spinal cord in cell-treated animals were also smaller than in controls (Fig. 2C, D) and the levels of endogenous myelin expression as revealed by expression of EGFP in the PLPEGFP hosts were elevated in those that received adult NG2+ cell transplants (Fig. 2E, F). To assess the extent of immune cell infiltration, sections were labeled with antibodies to CD3, a T lymphocyte marker. In lesion areas, the number of CD3+ T cells was reduced four-fold in animals treated with NG2 cells compared to controls (Fig. 2G, H). For example, lesion areas in controls contained 44 ± 10 CD3+ cells, while lesion areas in animals treated with NG2+ cells contained 10 ± 7 CD3+ cells (P <0.05). Consistent with the increase in endogenous myelination seen in NG2-treated animals, 48 h after cell treatment, lesion areas containing transplanted cells had a >25% increase in the number of PLP-EGFP oligodendrocyte lineage cells compared to regions devoid of transplanted cells (Fig. 2I, J). These data suggest that NG2+ cells have the capacity to suppress inflammation and promote myelin repair in the adult CNS.
Fig. 2.
Purified NG2+ cells ameliorate EAE, migrate into the demyelinated CNS, promote remyelination and reduce inflammatory T cell infiltration. A: Injection of 1×106 NG2+ cells into mice with MOG35-55-induced EAE resulted in significant neurological improvement compared to control animals that received Pbs. b: Labeled NG2+ cells (red) were found in lesion areas of white matter identified by reduced expression of PLP-EGFP 24 h after injection. c and D: the lesion load was reduced in the spinal cord of animals that received NG2+ cells compared to controls as shown by black gold staining. the relative area of demyelination at the periphery of the spinal cord is delineated by white dots. E and F: treatment with NG2+ cells promoted endogenous myelination in PLP-EGFP host animals with EAE. Control animals with EAE (E) showed significantly reduced EGFP expression in the corpus callosum and forebrain compared to animals treated with NG2+ cells (F). G and H: Demyelinated lesion areas in the spinal cord of control animals (G) were characterized by infiltrating CD3+ t cells that were substantially reduced in animals treated with NG2+ cells (H). I and J: The number of endogenous oligodendrocytes (green) increased in regions of demyelination that contained NG2+ cells (red) (I) compared to regions devoid of NG2+ cells (J). Scale bars: B, 50 μm; C–F, 500 μm; G–J, 100 μm.
NG2 cells Generated Oligodendrocytes in response to EAE-Induced cues
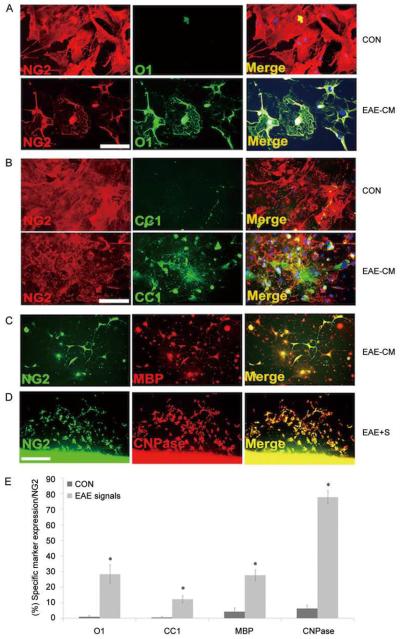
The enhancement of myelination and the increase in PLP-EGFP+ oligodendrocytes in lesion areas of mice treated with NG2+ cells suggested that they stimulate endogenous oligodendrogenesis. The NG2+ cells may also contribute to repair by directly differentiating into oligodendrocytes in lesion areas. To assess whether soluble signals from CNS tissue with EAE lesions promoted the conversion of NG2+ cells into oligodendrocytes, two distinct approaches were taken. First, NG2 cells were plated at low-density (1×105) in the presence of EAE tissue-derived conditioned medium and the number of oligodendrocyte lineage cells that developed was compared with cultures grown in conditioned medium derived from naïve animals. After 24 h, 28 ± 3% of the cells were O1+ oligodendrocytes, 12 ± 2% were CC1+ cells and 27 ± 6% were MBP+ mature oligodendrocytes in EAE-derived conditioned medium compared with <2% O1+ and CC+ cells, and <5% MBP+ cells in naïve conditioned medium (Fig. 3A–C, E). Second, NG2 cells were plated in conditioned medium from living slices of naïve and EAE spinal cord, and the proportion of CNPase+ cells was assayed. When grown in the presence of EAE-derived slices, >77% of the cells expressed CNPase while <5% of cells grown in the presence of naïve slices expressed detectable CNPase (Fig. 3D, E). These findings suggested that NG2+ cells have the capacity to differentiate into mature oligodendrocyte lineage cells in response to signals from demyelinated CNS tissue.
Fig. 3.
Adult NG2+ cells generate mature oligodendrocytes in response to cues from demyelinating tissue. Adult spinal cord NG2+ cells grown in the presence of EAE-conditioned medium for 3–5 days generated increased numbers of differentiated mature oligodendrocytes that were O1+ (A), CC1+ (B), and MBP+ (C) compared with parallel cultures grown in control medium. When 1 × 104 NG2+ cells were co-cultured with 1-mm slices from EAE brain tissue (EAE-S) for 3 days, higher numbers of CNPase+ cells differentiated from the NG2+ cells (D). Quantification of cells differentiated from the NG2+ cells in the presence of EAE-cM or EAE-s is shown in E. *Control versus EAE-cM/s: O1 P <0.05, CC1 P <0.05, MBP P <0.05, CNPase P <0.001. Mean ± SEM of duplicate preparations from three independent experiments. Scale bars in A–C, 150 μm and D, 300 μm.
DISCUSSION
Cellular therapies are becoming increasingly important in developing treatments for neurological disorders. The ability to isolate distinct cell populations and characterize their functional properties will lead to the identification of the mechanisms mediating repair and the cell populations appropriate for the treatment of specific neural insults. Here, we describe a novel approach for the isolation of a population of cells from the adult mouse spinal cord that share some characteristics with pericytes and promote functional recovery in a animal model of MS. The cell population was isolated through a dissociation and Percoll gradient selection process that resulted in a relatively homogenous population of cells that expressed the cell surface glycoprotein NG2 and had a flat non-process-bearing morphology. When infused into mice with ongoing MOG35-55-induced chronic EAE, these cells promoted functional recovery that was associated with a reduction in the extent of demyelinating lesions, stimulation of endogenous oligodendrocyte numbers in lesion areas, and lower levels of T cell infiltration into the CNS. Exposure of these adult NG2 cells to medium conditioned by EAE spinal cords, or slice cultures derived from the spinal cords of animals with EAE, stimulated the generation of mature oligodendrocytes suggesting that fate determination in these cells can be modulated by pathologically-derived signals.
The in vivo correlate of the isolated NG2+ cells is currently unclear although they share several characteristics with pericytes but relatively few with adult OPCs[43]. First, they expressed both NG2 and PDGFR-β[44]. While OPCs can also be identified by the expression of NG2, they are characterized by the expression of the PDGFR-α and their major mitogen is PDGF-AA[45,46]. Second, in early passages, a small proportion of the cells expressed α-SMA, a characteristic shared with pericytes and not OPCs. This expression was lost with later passage (P8), suggesting its expression is not irreversibly constitutive to the cells. Third, the morphology of the cells was not consistent with that of OPCs. In vitro, OPCs are largely bipolar with long processes and are highly motile[47]. As OPCs are cultured, the number of processes increases but they seldom assume a flattened morphology such as that expressed by the NG2+ cells. One aspect of NG2+ cells that is not currently clear is their ability to generate multiple cell types. We demonstrated that these cells gave rise to oligodendrocytes in response to signals from demyelinated CNS tissues and they were also capable of generating astrocytes and neurons (data not shown). All these cellular products are, however, neuroepithelial in nature and it is not clear if the NG2+ cells, like pericytes[36] can generate cells of mesodermal origin given the correct environment.
One activity NG2+ cells share with some stem cell populations is the ability to promote functional recovery in animal models of MS. Previous studies have demonstrated that treatment with MSCs derived from either human or mouse sources promotes functional recovery in EAE[3,6,48]. Similarly, NSCs promote functional recovery in EAE, suggesting that this is a common characteristic of stem cells[8,49,50]. In both cases, modulation of the immune response is associated with the promotion of repair. A clear function of MSCs is to promote a switch in the immune response from a Th1-based pro-inflammatory to a Th2 anti-inflammatory response[3,6]. What the contribution of this switch is to the overall functional improvement in EAE is not yet defined.
The specific mechanisms by which NG2+ cells promote recovery in EAE is unclear but may be closely tied to the process of myelin repair in the intact adult CNS. The reduction in CD3+ T cells seen in the lesions of animals treated with NG2+ cells suggests that they may directly influence the immune system. Alternatively, the reduction in T cells and the enhanced functional and histological improvement may reflect a primary contribution of the NG2+ cells to neural repair and remyelination. Several lines of evidence support the hypothesis that there is a direct effect of NG2+ cells on CNS repair. Transplantation of NG2+ cells promoted endogenous myelination and increased the oligodendrocyte number in lesion areas. Furthermore, in a model of spinal cord injury, transplantation of NG2+ cells resulted in enhanced axonal survival in the regions of injury. One hypothesis for this phenomenon is that contact of growth cones with these NG2+ cells stabilizes the growth cone against macrophage-induced collapse[51]. In addition, it may be that in the setting of demyelination, NG2+ cells home in on areas of insult and differentiate into oligodendrocytes that contribute to myelin repair[52]. Consistent with this hypothesis are the findings that in our in vitro and ex vivo co-culture systems, NG2+ cells differentiated into mature oligodendrocytes in response to demyelinating signals released from the spinal cord of animals with ongoing EAE, and it may be that in normal conditions they are recruited for turnover and replacement functions that are upregulated in response to insults.
The nature of the signals that mediate enhanced host myelination and oligodendrocyte numbers in response to NG2 cell infiltration is currently unknown but may include growth factors such as PDGFAA, IGF-1 and HGF, all of which are implicated in modulating myelin repair. Likewise, whether the NG2+ cells promote oligodendrogenesis from OPCs, or promote the survival of existing oligodendrocytes, will require additional analysis. The signals that promote the responses of NG2+ cells to demyelination cues are also currently unknown and their identification may reveal important targets for future therapeutic approaches to demyelinating diseases. Our studies suggest that the microenvironment in demyelinated lesions such as those in EAE exerts an influence on NG2+ cell differentiation, and OPCs that also express NG2 can be stimulated by extracellular signals to revert to NSCs[53]. While conversion to NSCs or generation of OPCs by NG2+ cells could provide support for repair in the CNS, conversion of those cells into astrocytes that contribute to glial scar formation may actually inhibit repair in some situations. The evidence for astrocytes derived from NG2+ cells in vivo is not particularly strong. The reactive astrocytes in the setting of spinal cord demyelination or injury appear to be mostly derived from astrocyte precursors and not NG2+ cells[54–56], while in mouse models of neuronal degeneration such as hereditary amyotrophic lateral sclerosis, a variant of the fatal motor neuron disease[57,58], there is also no strong evidence that NG2+ cells generate astrocytes although they appear to generate oligodendrocytes[59,60].
In conclusion, using a novel Percoll gradient approach we have isolated a population of cells from the adult mouse spinal cord that promotes functional recovery in EAE, an animal model of demyelinating diseases, and promotes regeneration in spinal cord injury. These cells share some characteristics with pericytes including the expression of the glycoprotein NG2 and the tyrosine kinase receptor PDGFR-β. Treatment of animals with ongoing EAE with NG2+ cells resulted in rapid infiltration of the cells into the CNS and significant functional improvement. This improvement was correlated with enhanced generation of host myelin and oligodendrocytes as well as the potential conversion of NG2 cells to oligodendrocytes. Given that NG2+ cells are isolated in relatively small numbers and are generated from adult CNS tissue, they are unlikely to be a direct source for cell therapy in the immediate future. Further analysis of their origin, and the mechanisms underlying their ability to promote remyelination in demyelinating diseases, as well as their ability to promote axonal regeneration in the CNS will, however, provide valuable insights into the cellular and molecular pathways that mediate recovery from CNS insults. Such data are fundamentally important to the future development of novel therapies for neural repair in demyelinating diseases such as MS.
ACKNOWLEDGEMENTS
We specially thank Drs. Abdelmadjid Belkadi and Andrew Caprariello for technical assistance. This work was supported by NIH grants (NS 030800 and NS 077942) to RHM.
REFERENCES
- [1].Miller RH. The promise of stem cells for neural repair. Brain Res. 2006;1091:258–264. doi: 10.1016/j.brainres.2006.01.073. [DOI] [PubMed] [Google Scholar]
- [2].Miller RH, Bai L, Lennon DP, Caplan AI. The potential of mesenchymal stem cells for neural repair. Discov Med. 2010;9:236–242. [PubMed] [Google Scholar]
- [3].Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sharp J, Keirstead HS. Stem cell-based cell replacement strategies for the central nervous system. Neurosci Lett. 2009;456:107–111. doi: 10.1016/j.neulet.2008.04.106. [DOI] [PubMed] [Google Scholar]
- [5].Auletta JJ, Bartholomew AM, Maziarz RT, Deans RJ, Miller RH, Lazarus HM, et al. The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy. 2012;4:529–547. doi: 10.2217/imt.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ben-Hur T. Cell therapy for multiple sclerosis. Neurotherapeutics. 2011;8:625–642. doi: 10.1007/s13311-011-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- [9].Bassi G, Pacelli L, Carusone R, Zanoncello J, Krampera M. Adipose-derived stromal cells (ASCs) Transfus Apher Sci. 2012;47:193–198. doi: 10.1016/j.transci.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [10].Fouraschen SM, Pan Q, de Ruiter PE, Farid WR, Kazemier G, Kwekkeboom J, et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 2012;21:2410–2419. doi: 10.1089/scd.2011.0560. [DOI] [PubMed] [Google Scholar]
- [11].Hoffman AM, Paxson JA, Mazan MR, Davis AM, Tyagi S, Murthy S, et al. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev. 2011;20:1779–1792. doi: 10.1089/scd.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang Y, Chen P, Zhang CB, Ko GJ, Ruiz M, Fiorina P, et al. Kidney-derived mesenchymal stromal cells modulate dendritic cell function to suppress alloimmune responses and delay allograft rejection. Transplantation. 2010;90:1307–1311. doi: 10.1097/TP.0b013e3181fdd9eb. [DOI] [PubMed] [Google Scholar]
- [13].Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [14].Vaculik C, Schuster C, Bauer W, Iram N, Pfisterer K, Kramer G, et al. Human dermis harbors distinct mesenchymal stromal cell subsets. J Invest Dermatol. 2012;132:563–574. doi: 10.1038/jid.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Caplan AI. New era of cell-based orthopedic therapies. Tissue Eng Part B Rev. 2009;15:195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29:1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- [17].Dore-Duffy P, Mehedi A, Wang X, Bradley M, Trotter R, Gow A. Immortalized CNS pericytes are quiescent smooth muscle actin-negative and pluripotent. Microvasc Res. 2011;82:18–27. doi: 10.1016/j.mvr.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Canfield AE, Sutton AB, Hoyland JA, Schor AM. Association of thrombospondin-1 with osteogenic differentiation of retinal pericytes in vitro. J Cell Sci. 1996;109(Pt 2):343–353. doi: 10.1242/jcs.109.2.343. [DOI] [PubMed] [Google Scholar]
- [19].Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- [20].Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–1593. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- [21].Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- [22].Lundberg C, Martinez-Serrano A, Cattaneo E, McKay RD, Bjorklund A. Survival, integration, and differentiation of neural stem cell lines after transplantation to the adult rat striatum. Exp Neurol. 1997;145:342–360. doi: 10.1006/exnr.1997.6503. [DOI] [PubMed] [Google Scholar]
- [23].Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- [25].Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- [26].Stallcup WB. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981;83:154–165. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- [27].Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- [28].Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- [29].Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].MacFadyen J, Savage K, Wienke D, Isacke CM. Endosialin is expressed on stromal fibroblasts and CNS pericytes in mouse embryos and is downregulated during development. Gene Expr Patterns. 2007;7:363–369. doi: 10.1016/j.modgep.2006.07.006. [DOI] [PubMed] [Google Scholar]
- [31].Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- [32].Edelman DA, Jiang Y, Tyburski J, Wilson RF, Steffes C. Pericytes and their role in microvasculature homeostasis. J Surg Res. 2006;135:305–311. doi: 10.1016/j.jss.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [33].von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- [34].Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [35].Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- [36].Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS micro-vascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- [37].Wight PA, Duchala CS, Shick HE, Gudz TI, Macklin WB. Expression of a myelin proteolipid protein (Plp)-lacZ transgene is reduced in both the CNS and PNS of Plp(jp) mice. Neurochem Res. 2007;32:343–351. doi: 10.1007/s11064-006-9202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garbelli R, Zucca I, Milesi G, Mastropietro A, D'Incerti L, Tassi L, et al. Combined 7-T MRI and histopathologic study of normal and dysplastic samples from patients with TLE. Neurology. 2011;76:1177–1185. doi: 10.1212/WNL.0b013e318212aae1. [DOI] [PubMed] [Google Scholar]
- [39].Sun HS, French RJ, Feng ZP. A method for identifying viable and damaged neurons in adult mouse brain slices. Acta Histochem. 2009;111:531–537. doi: 10.1016/j.acthis.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [40].Jariyapongskul A, Nakano A, Yamaguchi S, Nageswari K, Niimi H. Maturity of pericytes in cerebral neocapillaries induced by growth factors: fluorescence immuno-histochemical analysis using confocal laser microscopy. Clin Hemorheol Microcirc. 2003;29:417–421. [PubMed] [Google Scholar]
- [41].Sims DE. The pericyte--a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- [42].Orlidge A, D'Amore PA. Cell specific effects of glycosaminoglycans on the attachment and proliferation of vascular wall components. Microvasc Res. 1986;31:41–53. doi: 10.1016/0026-2862(86)90005-1. [DOI] [PubMed] [Google Scholar]
- [43].Yang Z, Watanabe M, Nishiyama A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 2005;149:50–56. doi: 10.1016/j.jneumeth.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [44].Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB. Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. J Neuroinflammation. 2011;8:158. doi: 10.1186/1742-2094-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pringle N, Collarini EJ, Mosley MJ, Heldin CH, Westermark B, Richardson WD. PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J. 1989;8:1049–1056. doi: 10.1002/j.1460-2075.1989.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tsai HH, Macklin WB, Miller RH. Distinct modes of migration position oligodendrocyte precursors for localized cell division in the developing spinal cord. J Neurosci Res. 2009;87:3320–3330. doi: 10.1002/jnr.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kaplan JM, Youd ME, Lodie TA. Immunomodulatory activity of mesenchymal stem cells. Curr Stem Cell Res Ther. 2011;6:297–316. doi: 10.2174/157488811797904353. [DOI] [PubMed] [Google Scholar]
- [49].Payne NL, Sun G, Herszfeld D, Tat-Goh PA, Verma PJ, Parkington HC, et al. Comparative study on the therapeutic potential of neurally differentiated stem cells in a mouse model of multiple sclerosis. PLoS One. 2012;7:e35093. doi: 10.1371/journal.pone.0035093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Miller RH, Bai L. Translating stem cell therapies to the clinic. Neurosci Lett. 2012;519:87–92. doi: 10.1016/j.neulet.2012.01.043. [DOI] [PubMed] [Google Scholar]
- [51].Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, et al. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci. 2010;30:255–265. doi: 10.1523/JNEUROSCI.3705-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Whitman LM, Blanc CA, Schaumburg CS, Rowitch DH, Lane TE. Olig1 function is required for remyelination potential of transplanted neural progenitor cells in a model of viral-induced demyelination. Exp Neurol. 2012;235:380–387. doi: 10.1016/j.expneurol.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- [54].Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what's in a name? J Anat. 2005;207:687–693. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dincman TA, Beare JE, Ohri SS, Whittemore SR. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 2012;209:219–226. doi: 10.1016/j.jneumeth.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Staugaitis SM, Trapp BD. NG2-positive glia in the human central nervous system. Neuron Glia Biol. 2009;5:35–44. doi: 10.1017/S1740925X09990342. [DOI] [PubMed] [Google Scholar]
- [57].Carlesi C, Pasquali L, Piazza S, Lo Gerfo A, Caldarazzo Ienco E, Alessi R, et al. Strategies for clinical approach to neurodegeneration in Amyotrophic lateral sclerosis. Arch Ital Biol. 2011;149:151–167. doi: 10.4449/aib.v149i1.1267. [DOI] [PubMed] [Google Scholar]
- [58].Naganska E, Matyja E. Amyotrophic lateral sclerosis - looking for pathogenesis and effective therapy. Folia Neuropathol. 2011;49:1–13. [PubMed] [Google Scholar]
- [59].Lepore AC, Dejea C, Carmen J, Rauck B, Kerr DA, Sofroniew MV, et al. Selective ablation of proliferating astrocytes does not affect disease outcome in either acute or chronic models of motor neuron degeneration. Exp Neurol. 2008;211:423–432. doi: 10.1016/j.expneurol.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurode-generation. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]