Abstract
Lymphangioleiomyomatosis (LAM) is a female-predominant lung disease that can lead to respiratory failure. LAM cells typically have inactivating TSC2 mutations, leading to mTORC1 hyperactivation. The gender specificity of LAM suggests that female hormones contribute to disease progression. Clinical findings indicate that estradiol exacerbates LAM behaviors and symptoms. Although hormonal therapy with progesterone has been employed, the benefit in LAM improvement has not been achieved. We have previously found that estradiol promotes the survival and lung metastasis of cells lacking tuberin in a preclinical model of LAM. In this study, we hypothesize that progesterone alone or in combination with estradiol promote metastatic behaviors of TSC2-deficient cells. In cell culture models of TSC2-deficient LAM patient-derived and rat uterine leiomyoma-derived cells, we found that progesterone treatment or progesterone plus estradiol resulted in increased phosphorylation of Akt and ERK1/2, induced the proliferation, and enhanced the migration and invasiveness. In addition, treatment of progesterone plus estradiol synergistically decreased the levels of reactive oxygen species, and enhanced cell survival under oxidative stress. In a murine model of LAM, treatment of progesterone plus estradiol promoted the growth of xenograft tumors; however, progesterone treatment did not affect the development of xenograft tumors of Tsc2-deficient cells. Importantly, treatment of progesterone plus estradiol resulted in alteration of lung morphology, and significantly increased the number of lung micrometastases of Tsc2-deficient cells compared with estradiol treatment alone. Collectively, these data indicate that progesterone increases the metastatic potential of TSC2-deficient LAM patient-derived cells in vitro and lung metastasis in vivo. Thus, targeting progesterone-mediated signaling events may have therapeutic benefit for LAM and possibly other hormonally dependent cancers.
Keywords: tuberin, Akt, ERK1/2, lung metastasis, alveolar integrity, migration, invasiveness, oxidative stress, progesterone, estradiol, tuberin
Introduction
LAM is a progressive lung disease that affects almost exclusively women. The pathogenesis of the sporadic form of LAM is unusual: LAM cells are histological benign smooth muscle cells that metastasize to the lungs [1], where they cause emphysema-like cystic lung degeneration in young, otherwise healthy, non-smoking women [2, 3]. The majority of LAM patients also have renal angiomyolipomas, which are benign tumors containing smooth muscle cells that are indistinguishable from LAM cells. LAM and angiomyolipoma cells have mutations in TSC1 or TSC2, leading to activation of the mammalian target of rapamycin complex 1 (mTORC1). mTORC1 regulates cell growth, protein translation, and metabolism. Treatment with Rapamycin, an mTORC1 inhibitor, can stabilize lung function in LAM but lung function decline continues when the drug is discontinued [4]. The only proven treatment for end-stage disease is lung transplantation, after which LAM can recur in the transplanted lungs [5].
The female predominance of LAM, coupled with the genetic data indicating that estradiol may promote LAM pathogenesis. Both LAM cells and renal angiomyolipoma cells express estradiol receptor alpha and progesterone receptor [6–13]. LAM occurs exclusively in women during their reproductive age, and the symptoms of LAM become complicated during pregnancy [3, 14, 15], and by administration of exogenous estradiol [16–18]. These clinical findings provided rationales for hormonal therapy, particularly using progesterone, for the treatment of women with LAM; however, the benefit has not been successfully demonstrated [19–21].
In preclinical models of LAM, estradiol promoted the survival and metastasis of TSC2-deficient cells [22–24]. Faslodex, a pure estradiol receptor antagonist, blocked estradiol-induced lung metastasis of Tsc2-deficient cells in a metastatic model [25]. In addition, it has been shown that estradiol promoted whereas tamoxifen suppressed the development of liver hemangiomas in Tsc1 heterozygous mice [26]. Furthermore, in a recently developed uterine-specific Tsc2 knockout mouse model, estradiol treatment increased myometrial proliferation, which was suppressed by ovariectomy and aromatase inhibition. Interestingly, progesterone treatment did not affect the proliferation of myometrial [24]. Despite these findings, the impact of progesterone on the proliferation, survival, and metastasis of cells lacking TSC2 has not been extensively investigated.
We report here that progesterone treatment or progesterone plus estradiol activated Akt and ERK1/2 signaling pathways in LAM patient-derived cells. Importantly, progesterone alone or in combination with estradiol strongly enhanced the migration and invasiveness of TSC2-deficient cells. In addition, treatment of progesterone plus estradiol synergistically decreased the cellular levels of reactive oxygen species (ROS), and enhanced cell survival under oxidative stress. Furthermore, treatment of progesterone plus estradiol promoted the growth of xenograft tumors; however, progesterone treatment did not affect the development of xenograft tumors of Tsc2-deficient cells. Importantly, treatment of progesterone plus estradiol promoted the lung metastasis of Tsc2-deficient cells compared with estradiol treatment alone. Collectively, these data demonstrate that progesterone, in addition to estradiol, increases the metastatic potential of TSC2-deficient LAM patient-derived cells in vitro and lung metastasis in vivo. Thus, targeting progesterone-mediated signaling and/or cellular events may have therapeutic benefit for LAM and possibly other hormonally dependent neoplasm.
Results
Progesterone activates ERK1/2 and Akt and enhances the proliferation of TSC2-deficient cells
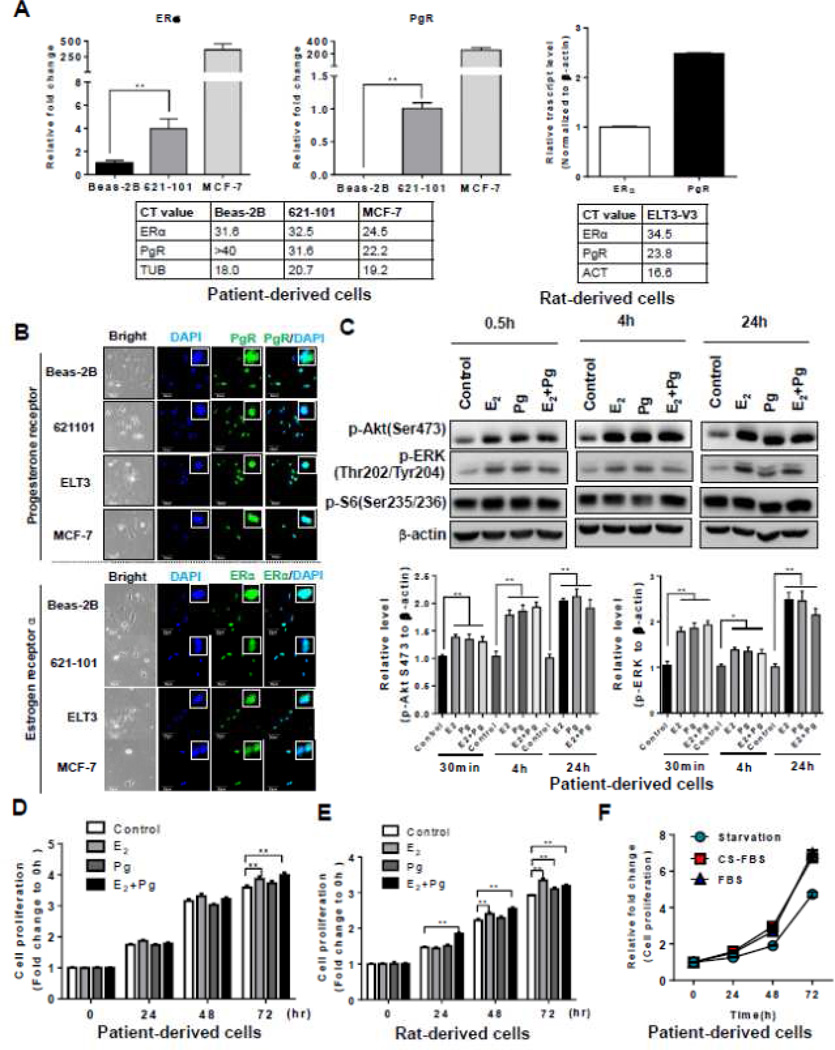
LAM patient-associated angiomyolipoma-derived cells and rat uterine leiomyoma-derived cells express estrogen receptor alpha (ERα) and progesterone receptor (PgR), and respond to estradiol stimulation [27, 28]. The patient-derived cells were developed from a sporadic LAM-associated renal angiomyolipoma. These cells carry bi-allelic mutations of the TSC2 gene that are identical to the mutations found in the patient’s pulmonary LAM cells [28]. The rat cells were developed from an Eker rat uterine leiomyoma, which is composed of smooth muscle cells lacking functional TSC2 [27, 29]. To validate the expression of ERα and PgR, we measured their transcript levels using quantitative RT-PCR. The relative transcript level of ERα was 4-fold higher in 621-101 cells (CT = 32.5) relative to normal human lung bronchial epithelial cells (BEAS-2B) (CT = 31.6) (Figure 1A). Interestingly, the transcript level of ERα was much lower in 621-101 cells relative to that in breast cancer MCF-7 cells (CT = 24.5) (Figure 1A). Moreover, the transcript level of PgR was detectable in 621-101 cells (CT = 31.6), although the value was lower than that of MCF-7 cells (CT = 22.2) (Figure 1A). Furthermore, the expression of ERα (CT = 34.5) and PgR (CT = 23.8) was confirmed in rat uterine leiomyoma-derived ELT3 cells (Figure 1A), consistent with previous findings [27, 28]. To further determine the accumulation of ERα and PgR in TSC2-deficient LAM patient-derived and rat-derived cells, we performed immunofluorescent staining of ERα and PgR in 621-101, ELT3-V3, BEAS-2B and MCF-7 cells. Nuclear staining of ERα and PgR was evident in both 621-101 and ELT3-V3 cells (Figure 1B). Intense nuclear staining of ERα and PgR was also found in MCF-7 cells as expected [30, 31]. Interestingly, nuclear staining of ERα and PgR was also observed in BEAS-2B, consistent with previous findings [32].
Figure 1. Progesterone activates Akt and ERK1/2 and enhances the proliferation of TSC2-deficient cells in vitro.
(A) The relative transcript levels of ERα and PgR in patient-derived cells, rat-derived cells, MCF-7 and BEAS-2B cells. The actual CT values of the ERα and PgR transcript were shown in the tables. (B) Representative immunofluorescent staining images of ERα and PgR in patient-derived cells (621-101), rat-derived cells (ELT3-V3), BEAS-2B and MCF-7 cells. (C) Patient-derived cells were treated with 10 nM E2, 10 nM progesterone (Pg), 10 nM E2 + 10 nM Pg, or vehicle control for 0.5, 4, and 24 hr. Immunoblot analysis of phospho-Akt (Ser473), phospho-ERK1/2 (Thr202/Tyr204) and phospho-S6 (S235/236). A densitometry of phospho-ERK1/2 and ERK1/2 was performed. (D) LAM patient-derived cells were treated with 10 nM E2, 10 nM progesterone (Pg), 10 nM E2 + 10 nM Pg, or vehicle control for 24, 48, and 72 hr. Cell proliferation was measured using crystal violet staining assay. (E) Tsc2-deficient rat uterus-derived ELT3 cells were treated with 10 nM E2, 10 nM progesterone (Pg), 10 nM E2 + 10 nM Pg, or vehicle control for 24, 48, and 72 hr. Cell proliferation was measured using crystal violet staining assay. (F) Patient-derived cells were in complete media (containing steroids) or in phenol red-free media supplemented with charcoal-dextran stripped FBS (depleting steroids) for 72 hr, and measured cell growth at 24, 48 and 72 hr post cell seeding. Cell proliferation was measured using crystal violet staining assay. * p < 0.05, ** p < 0.01, Student t test.
To determine the impact of progesterone stimulation on TSC2 deficient cells, we examined the activation of Akt and ERK1/2, which are known signaling molecules influenced by E2 in TSC2-deficient cells [23, 25, 33–37]. We found that E2 treatment for 0.5, 4 and 24 hr induced a biphasic activation of ERK1/2 (T202/Y204) by 70%, 20% and 140%, respectively, compared with vehicle control (p < 0.01, Figure 1C), as previously reported [23, 25, 33–37]. Interestingly, progesterone treatment for 0.5, 4 and 24 hr also induced a biphasic activation of ERK1/2 (T202/Y204) by 80%, 20% and 140%, respectively, compared with vehicle control, in TSC2-deficient LAM patient-derived cells (p < 0.01, Figure 1C). Moreover, progesterone treatment induced phosphorylation of Akt (Ser 473) at 0.5, 4 and 24 hr by 40%, 85% and 100% (Figure 1C). Importantly, the combination of progesterone and E2 did not further increase the levels of phospho-Akt (Ser 473) or phospho-ERK1/2 (T202/Y204) compared with progesterone or E2 treatment alone in LAM patient-derived cells (Figure 1C).
To determine the impact of progesterone stimulation on cell growth, we treated TSC2-deficient LAM patient-derived and rat-derived cells with progesterone, E2, or progesterone plus E2, for 24, 48, and 72 hr. Progesterone treatment did not affect the proliferation of LAM patient-derived cells (Figure 1D). Interestingly, progesterone plus E2 enhanced the proliferation of LAM patient-derived cells (p < 0.01, Figure 1D). In rat-derived cells, progesterone alone had a modest effect in cell proliferation, but progesterone plus E2 significantly increased cell proliferation (p < 0.01, Figure 1E). We also observed that E2 promoted the growth of both LAM patient-derived and rat-derived cells, as previously reported [23, 33, 35, 36]. These data indicate that progesterone exerts differential actions on phosphorylation of signaling pathway components and the growth in TSC2-deficient cells in nutrient-poor conditions.
To determine whether patient-derived cells require steroids to grow in nutrient-rich conditions, we cultured these cells in complete media (containing steroids) or in phenol red-free media supplemented with charcoal-dextran stripped FBS (depleting steroids) for 72 hr, and measured cell growth at 24, 48 and 72 hr post cell seeding. We found that the growth rates of patient-derived cells were similar between steroids-containing and steroid-depleting conditions (Figure 1F), suggesting these patient-derived cells do not require steroids to grow in nutrient-rich conditions.
Progesterone enhances the migration of TSC2-deficient cells
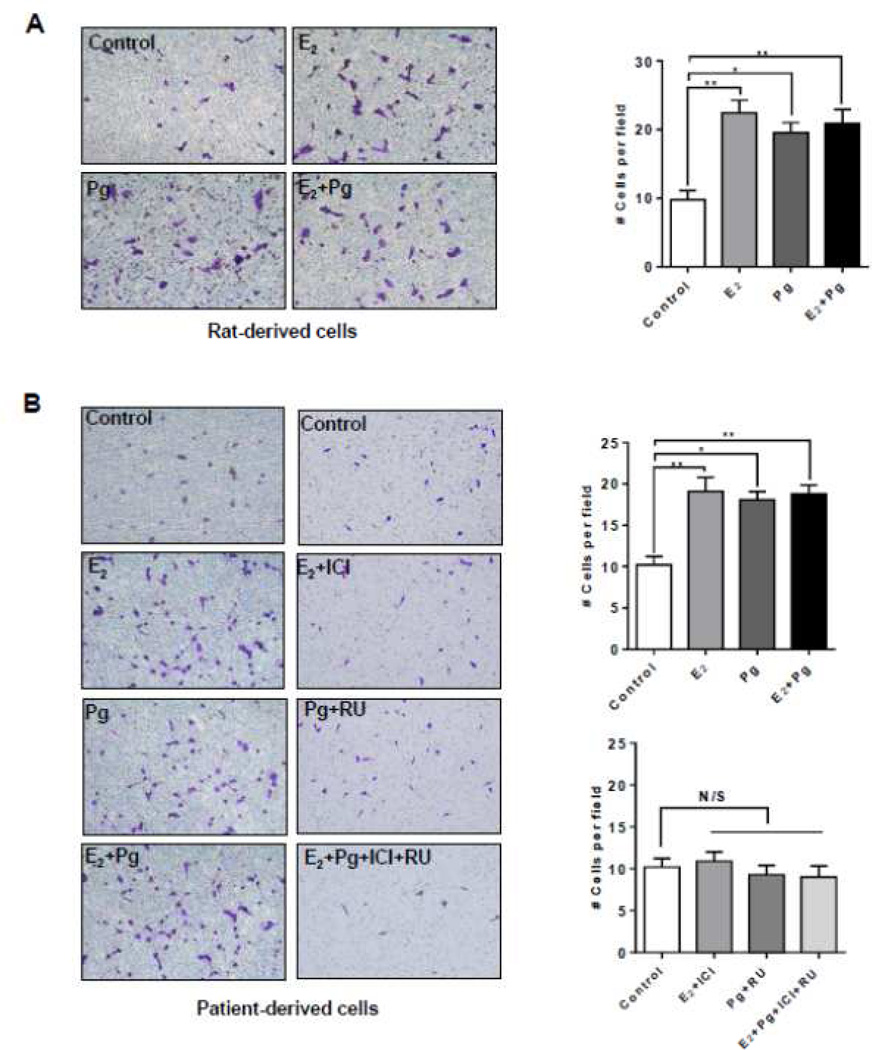
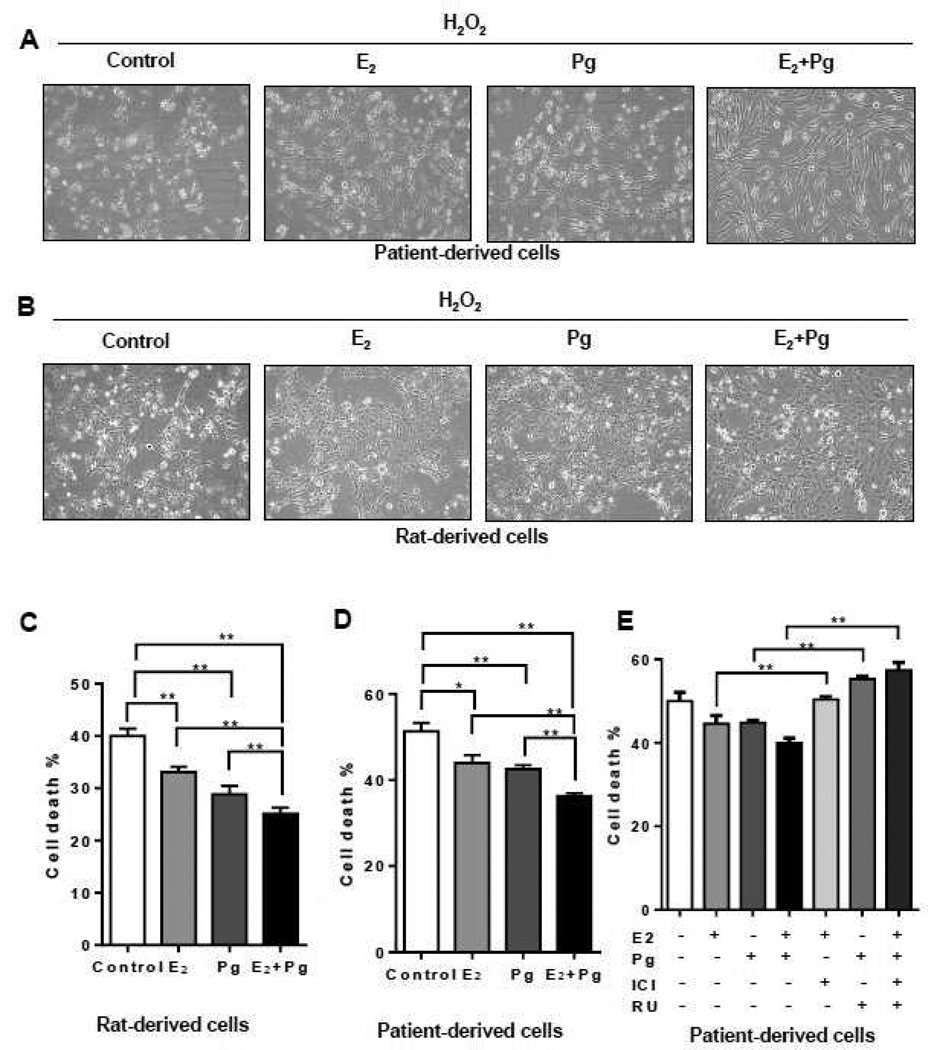
We asked whether progesterone treatment affects the migration of TSC2-deficient. In rat-derived cells, progesterone increased the migration of cells by ~90% (p < 0.01, Figure 2A). E2 stimulation also increased the number of migrating cells by ~110%. Interestingly, combination of progesterone and E2 did not further enhance cell migration (Figure 2A). In LAM patient-derived cells, progesterone increased the migration of by ~80% (p < 0.05, Figure 2B). E2 stimulation also increased the number of migrating cells by ~90%, confirming the finding by Gu et al [35]. Interestingly, combination of progesterone and E2 did not further enhance cell migration compared to either steroid alone (Figure 2B).
Figure 2. Progesterone increases the migration of TSC2-deficient cells.
(A) Rat-derived cells and (B) Patient-derived cells were treated with 10 nM E2, 10 nM progesterone (Pg), 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr. 20,000 cells were seeded in the upper chamber in the presence of steroids or vehicle. Cell migration was measured using the Boyden chamber transwell assay. Number of cells migrated through the chamber after 24 hr incubation was detected by Crystal Violet staining and quantitated. (B) Patient-derived cells were pre-treated with ICI 182,780 (ICI, 10 µM) or RU-486 (RU, 20 µM) for 12 hr, and then stimulated with 10 nM E2, 10 nM Pg, 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr. Cell migration was measured using the Boyden chamber transwell assay. Number of cells migrated through the chamber was detected by Crystal Violet staining and quantitated. Data are mean ± SEM, n = 3 in triplicate. * p < 0.05, ** p < 0.01, Student t test.
To examine the effect of steroid hormone receptor antagonists in cell culture models, we tested the estrogen receptor antagonist ICI 182,780 (Fulvestrant) and PgR antagonist RU-486 (Mifepristone), singly or in combination, in TSC2-deficient patient-derived cells. First, ICI 182,780 or RU-486 single treatment suppressed E2 or Pg-enhanced cell migration. The combination of ICI 182,780 and RU-486 also inhibited cell migration compared to either inhibitor alone (Figure 2B). These results point toward a critical role of progesterone/estradiol and PgR/ERα in the migration of TSC2-deficient cells.
Progesterone enhances the invasiveness of TSC2-deficient cells
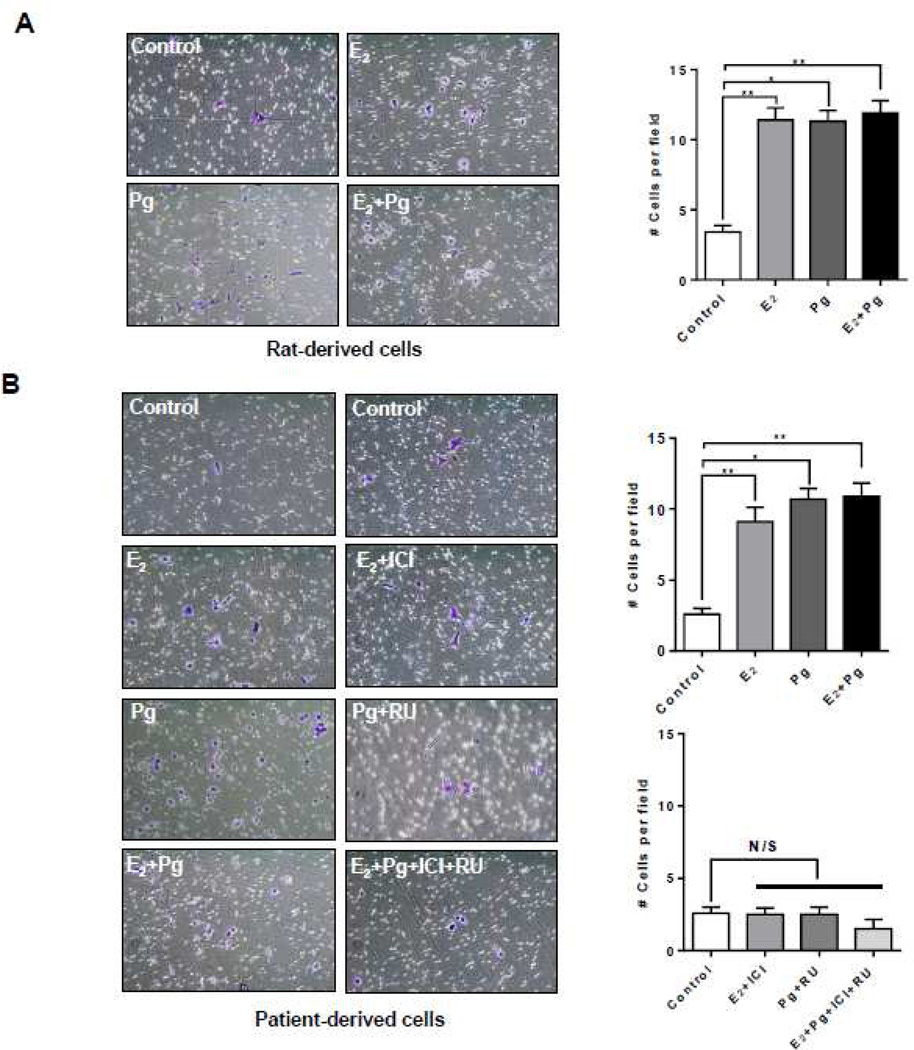
We have previously reported that E2 increased the invasion of rat-derived cells [25]. To examine whether progesterone treatment stimulates the invasiveness of TSC2-deficient, we treated rat-derived and LAM patient-derived cells with progesterone, E2, or progesterone plus E2, for 24 hr, and then performed a Matrigel invasion assay. In rat-derived cells, progesterone increased the invasion of cells by 4-fold (p < 0.05, Figure 3A). E2 stimulation also increased the number of invading cells by 4-fold, confirming the finding by Gu et al. [35]. Interestingly, combination of progesterone and E2 did not further enhance cell invasion (Figure 3A). In patient-derived cells, progesterone increased the number of invading LAM patient-derived cells by ~3.5-fold (p < 0.01, Figure 3B). E2 stimulation increased cell invasion by 4-fold, consistent with previous studies [25, 35]. Interestingly, the combination of progesterone and E2 did not further increase cell invasion (Figure 3B). These results indicate that progesterone enhances the invasion of TSC2-deficient cells.
Figure 3. Progesterone enhances the invasiveness of TSC2-deficient cells.
(A) Rat-derived cells and (B) Patient-derived cells were treated with 10 nM E2, 10 nM progesterone (Pg), 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr. 2 × 106 cells were seeded in a Matrigel-coated upper chamber in the presence of steroids or vehicle. Cell invasion was assessed using Matrigel invasion assay. (B) Patient-derived cells were pre-treated with ICI 182,780 (ICI, 10 µM) or RU-486 (RU, 20 µM) for 12 hr, and then stimulated with 10 nM E2, 10 nM Pg, 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr. Cell invasion was assessed using Matrigel invasion assay. Number of cells invaded through the Matrigel was detected by Crystal Violet staining and quantitated. Data are mean ± SEM, n = 3 in triplicate. * p < 0.05, ** p < 0.01, Student t test.
Next, we examined the consequence of ICI 182,780 and RU-486, singly or in combination, in the invasiveness of TSC2-deficient patient-derived cells. ICI 182,780 or RU-486 single treatment suppressed E2 or Pg-enhanced cell invasion. The combination of ICI 182,780 and RU-486 also blocked cell invasion compared to vehicle control (Figure 3B). These results indicate a critical role of progesterone/estradiol and PgR/ERα in the invasion of TSC2-deficient cells.
Progesterone and estradiol synergistically decrease ROS in TSC2-deficient cells
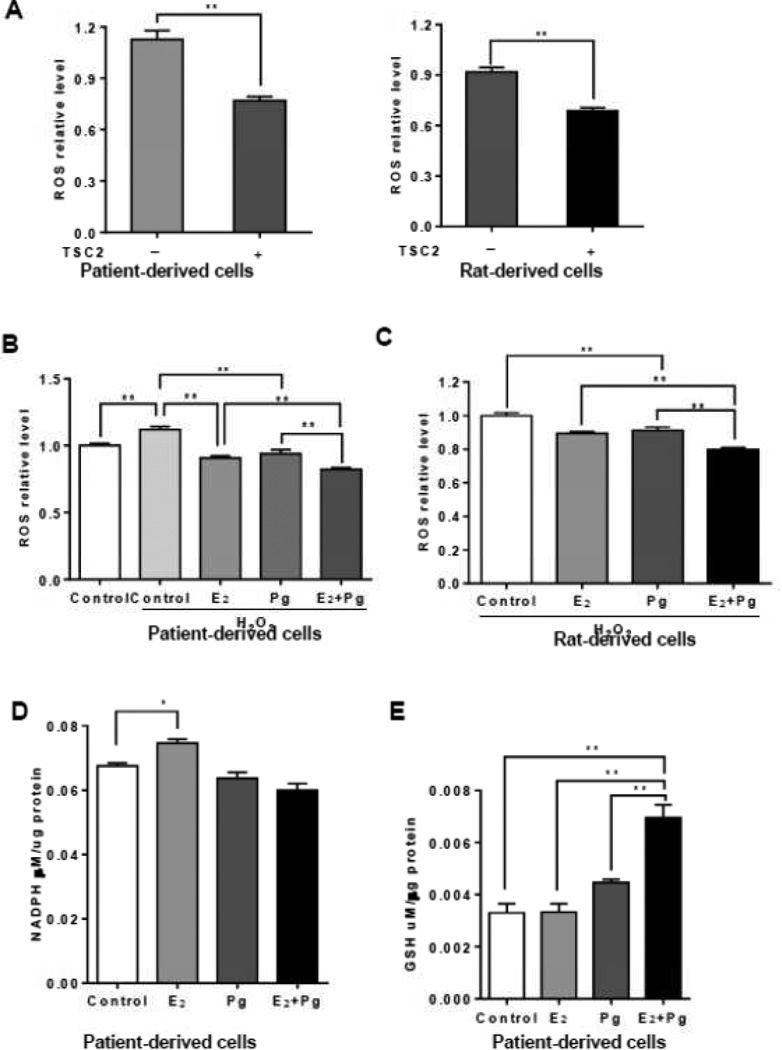
We recently found that E2 enhances the survival of TSC2-deficient cells [23], in part through the pentose phosphate pathway (PPP) [36]. We found that TSC2-deficient LAM patient-derived cells produced higher levels of ROS by 32% relative to TSC2-addback cells (p < 0.01, Figure 4A). Similarly, rat-derived cells produced higher levels of ROS levels by 28% relative to TSC2-addback cells (p < 0.01, Figure 4A), consistent with previous studies [33, 36].
Figure 4. Progesterone and estradiol synergistically decrease ROS and increase GSH in TSC2-deficient cells.
(A) Cellular levels of ROS were measured using DCFH-DA in patient-derived or rat-derived cells grown in serum-free conditions. (B) Rat-derived cells and (C) LAM patient-derived cells were treated with 10 nM E2, 10 nM Pg, 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr. Cells were incubated with hydrogen peroxide (0.5 µM) for 30 min prior to the measurement. Cellular levels of ROS were quantified using DCFH-DA. Data was normalized to total cell number. Results are representative of eight independent samples per group from three experiments. (D) Cellular levels of NADPH were measured in LAM patient-derived cells treated with 10 nM E2, 10 nM Pg, 10 nM E2 +10 nM Pg, or vehicle control for 24 hr. Data was normalized to total protein level. (E) Cellular levels of GSH were measured in patient-derived cells treated with 10 nM E2, 10 nM Pg, 10 nM E2 +10 nM Pg, or vehicle control for 24 hr. Data was normalized to total protein levels. Results are representative of three sets of independent samples per group from three experiments. * p < 0.05, ** p < 0.01, Student t test.
We next measured the cellular levels of ROS in the presence or absence of hydrogen peroxide (H2O2). As expected, H2O2 treatment led to a 20% increase of ROS (p < 0.01, Figure 4B), and E2 treatment decreased ROS levels by ~30% (p < 0.01, Figure 4B). Interestingly, progesterone treatment caused ~30% reduction of ROS levels relative to vehicle control (p < 0.01, Figure 4B). Importantly, progesterone plus E2 decreased ROS levels, more so than either progesterone or E2 in LAM patient-derived cells (p < 0.01, Figure 4B). Similar results on ROS production were also seen in Tsc2-deficient rat-derived cells (Figure 4C). These data indicate that progesterone and E2 reduce the cellular levels of ROS. We then assessed the impact of progesterone and/or E2 on the production of NADPH, a co-enzyme involved in protecting against the toxicity of ROS. E2 increased the cellular levels of NADPH (p < 0.05, Figure 4D), as expected. However, progesterone or progesterone plus E2 did not affect the levels of NADPH in LAM patient-derived cells (Figure 4D). These data indicate that progesterone and E2 affect the oxidative stress response in cells lacking TSC2.
To define the mechanism responsible for the steroid-induced alteration of oxidative stress, we measured the intracellular levels of glutathione (GSH), an important antioxidant molecule protecting cells from damage caused by ROS. We found that E2 plus Pg treatment resulted in 2.5-fold increase in intracellular GSH levels compared with the treatment of E2 or Pg alone in patient-derived cells (Figure 4E), suggesting an important action of E2 and Pg in oxidative defense in LAM patient-derived cells.
Progesterone and estradiol synergistically attenuate the death of TSC2-deficient cells
To assess the effect of progesterone and/or E2 in the survival of TSC2-deficient cells under oxidative stress, cells were treated with H2O2 or vehicle control for 0.5 hr in the presence or absence of Pg, E2, or Pg plus E2 for additional 24 hr. Cell morphology showed that H2O2-triggered cell death was modestly rescued by Pg or E2 treatment (Figure 5A), and strongly rescued by Pg plus E2 stimulation in LAM patient-derived cells (Figure 5A) and rat-derived cells (Figure 5B). Using the PI exclusion assay, E2 or Pg single treatment reduced cell death by 20% and 30% relative to vehicle control. The combination of progesterone and E2 significantly reduced H2O2-induced cell death by 40% relative to control in rat-derived cells (p < 0.01, Figure 5C). In LAM patient-derived cells, E2 or Pg single treatment reduced cell death by 10% and 10% relative to vehicle control. The combination of progesterone and E2 significantly reduced H2O2-induced cell death by 30% relative to control LAM patient-derived cells (p < 0.01, Figure 5D). These data suggest that Pg and E2 synergistically enhance the survival of TSC2-deficient cells particularly under conditions of oxidative stress.
Figure 5. Progesterone and estradiol synergistically attenuate the death of TSC2-deficient cells under oxidative stress.
(A) Patient-derived TSC2-deficient cells or (B) Rat-derived ELT3 cells were treated with 10 nM E2, 10 nM progesterone (Pg), 10 nM E2 +10 nM Pg, or vehicle control for 24 hr, and then incubated with 0.5 µM H2O2 for 30 min. Cell morphology was recorded using phase-contrast microscopy. (C) Rat-derived cells and (D) patient-derived cells were treated with 10 nM E2, 10 nM Pg, 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr, and then incubated with 0.5 µM H2O2 for 30 min. Cell death was measured using the propidium iodide (PI) exclusion assay. Proportion of dead cells was normalized to the total number of variable cells. Results are representative of eight independent samples per group from three experiments. (E) Patient-derived TSC2-deficient cells were pre-treated with ICI 182,780 (ICI, 10 µM) or RU-486 (RU, 20 µM) for 12 hr, stimulated with with 10 nM E2, 10 nM Pg, 10 nM E2 + 10 nM Pg, or vehicle control for 24 hr, and then incubated with 0.5 µM H2O2 for 0.5 hr. Cell death was measured using the propidium iodide (PI) exclusion assay. Proportion of dead cells was normalized to the total number of variable cells. Results are representative of eight independent samples per group from three experiments.* p < 0.05, ** p < 0.01, Student t test.
Next, we examined the consequence of ICI 182,780 and RU-486, singly or in combination, in the survival of TSC2-deficient patient-derived cells. ICI 182,780 or RU-486 single treatment moderately increased E2-reduced cell death by 5% and Pg-reduced cell death by 10%. The combination of ICI 182,780 and RU-486 more strongly increased cell death by 17% compared to either inhibitor alone (Figure 5F). These results indicate a critical role of progesterone/estradiol and PgR/ERα in the survival of TSC2-deficient cells under oxidative stress.
Progesterone and estradiol synergistically promote lung metastasis of Tsc2-deficient cells in a preclinical model of LAM
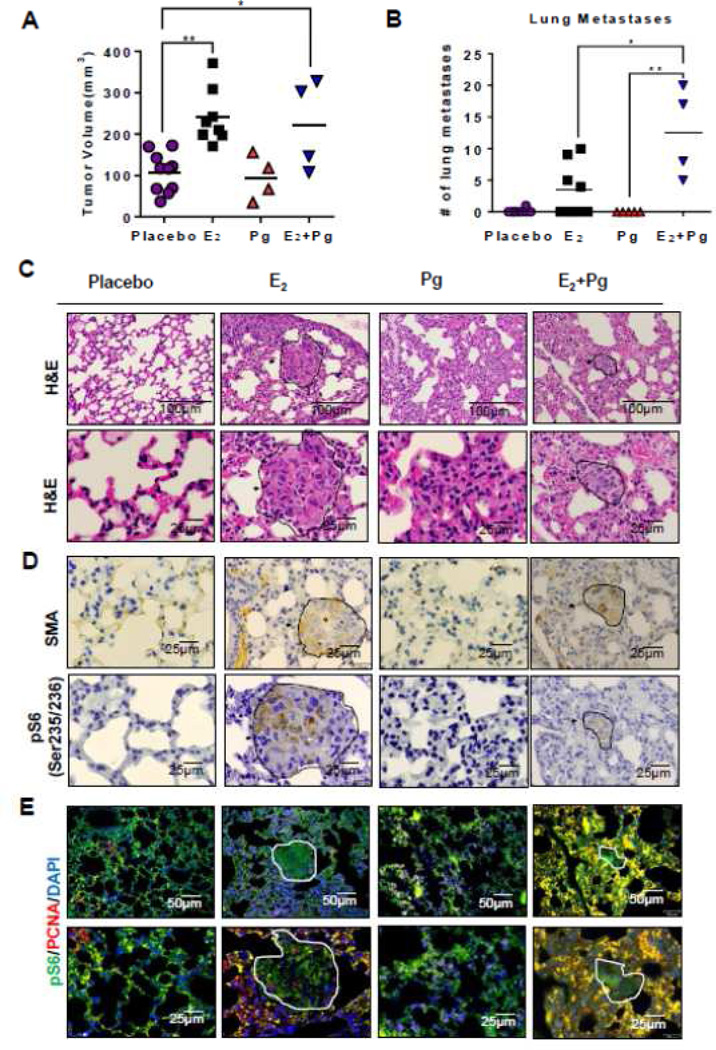
We previously found that E2 promotes the lung metastasis of Tsc2-deficient ELT3 cells [23]. To determine whether progesterone singly or in combination with E2 affects the metastasis of ELT3 cells, we supplemented female ovariectomized scid mice with slow-releasing pellets of progesterone, E2, or the combination of progesterone and E2, and then subcutaneously inoculated ELT3 cells. At week seven post cell inoculation, E2 treatment increased the volume of xenograft tumors by 2.4-fold (p < 0.01, Figure 6A), consistent with our previous work [23]. Progesterone treatment did not affect the growth of xenograft tumors, and the combination of progesterone and E2 increased the growth of xenograft tumors to the same extent as that of E2 treatment (Figure 6A).
Figure 6. Progesterone and estradiol synergistically promote lung metastasis of Tsc2-deficient cells in a preclinical model of LAM.
ELT3 cells were subcutaneously injected into female ovariectomized mice implanted with slow-releasing pellets of E2, progesterone, E2+progesterone, or placebo. (A) The tumor volume was calculated at eight-week post cell inoculation. (B) The number of lung metastases in female mice was scored: placebo (P) (n = 10), E2 (n = 8), progesterone (Pg) (n = 4), and E2 plus progesterone (E2+Pg) (n = 4). *p<0.05, ** p < 0.01, Student t test. (C) Lung sections from female mice were stained with H&E. Arrowheads point to metastatic lesions in mouse lungs. (D) Immunohistochemical staining of smooth muscle actin (SMA) and phospho-S6 (Ser235/235) in mouse lung sections. (E) Immunofluorescent double staining of phospho-S6 and PCNA in mouse lung sections.
Lung metastases were scored by an observer blinded to the experimental conditions. Five of the ten E2-treated mice (50%) developed lung metastases, with an average of four micrometastases/mouse (range 3–10) (Figure 6B). In contrast, only one of the ten placebo-treated mice (10%) developed a single metastasis, and none of mice treated with progesterone developed lung metastases. Importantly, lung metastases were identified in all four of the progesterone plus E2 treated mice (100%) (Figure 6B), with an average of 12 micrometastases/mouse (range 5–20) (Figure 6B). Together, these data suggest that the combination of progesterone and E2 more potently promote lung metastasis of Tsc2-deficient cells compared with E2 treatment alone.
To qualify the in vivo findings that E2 and Pg synergistically promote the lung metastasis of Tsc2-deficient cells, we first examined the lung morphology and identified the accumulation of abnormal cell clusters in the E2 or E2 plus Pg-treated mouse lungs compared with the vehicle treatment (Figure 6C). Interestingly, Pg treatment had no effect on lung metastasis of Tsc2-deficient ELT3 cells (Figure 6C). Next, we performed immunostaining of lung lesions for smooth muscle actin (SMA), phospho-S6 (S235/236) and PCNA. We found that E2- and E2 plus Pg-promoted lung metastatic lesions cells are positive for smooth muscle actin (Figure 6D), confirming the leiomyoma origin [23, 27, 29]; positive for phospho-S6 (Figure 6D), indicating the mTORC1 hyperactivation [38]; and positive for PCNA, suggesting the proliferating tumor cells in the lung (Figure 6E).
Progesterone induces alveolar wall thickening in a preclinical model of LAM
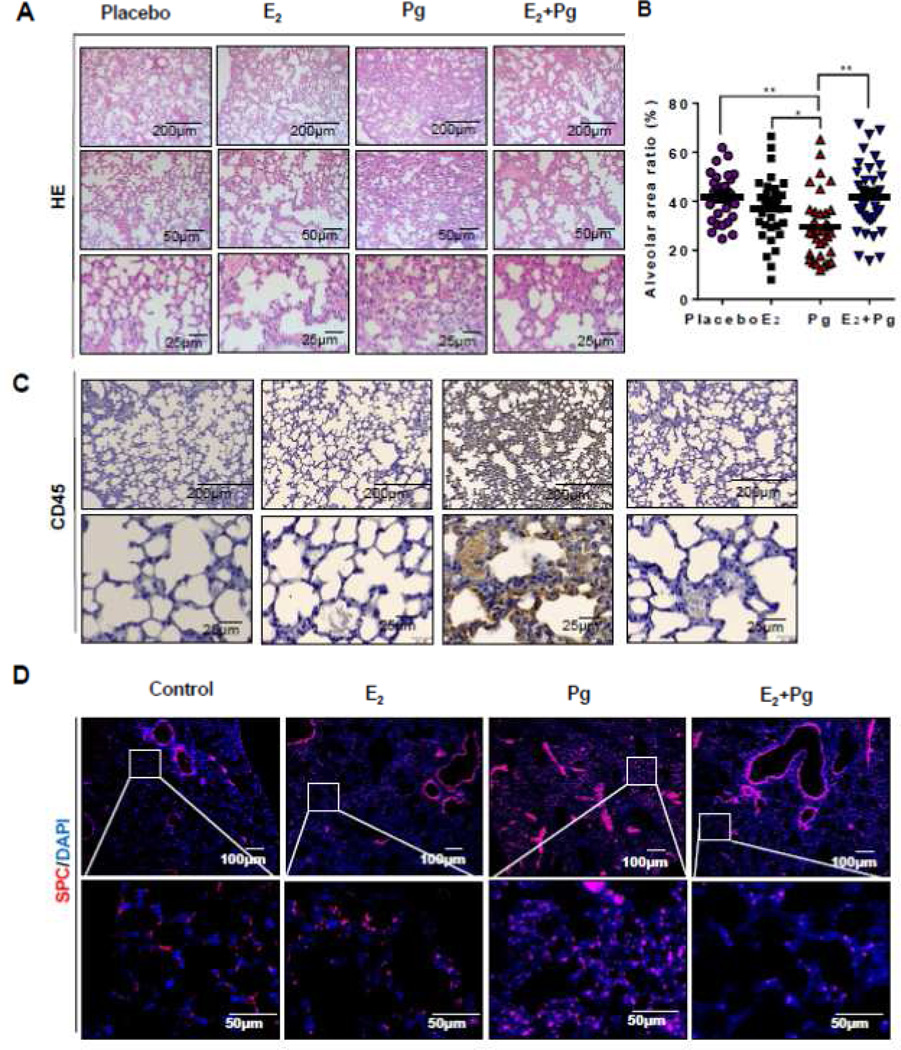
Despite the fact that progesterone did not affect lung metastasis of Tsc2-deficient cells (Figure 6), the evaluation of lung morphology from hematoxylin and eosin stained section showed striking interstitial alterations in Pg-treated mouse lungs compared with all other treatment groups (Figure 7A). To define the lung interstitial alterations, we first quantified the area of the alveolar space and found that Pg treatment markedly reduces the alveolar area relative to all other treatment groups (Figure 7B), indicating the development of alveolar thickening. Next, we tested the hypothesis of immune cell infiltration by immunostaining for lymphocyte common antigen (LCA, or CD45). We observed abundant accumulation of LCA-positive cells in the alveolar wall of lungs from a Pg-treated mouse (Figure 7C), suggesting that Pg-induced lung alteration is associated with lymphocyte infiltration. We also hypothesized that progesterone treatment causes thickening of alveolar epithelium. We performed immunofluorescent staining of lung sections with surfactant protein C (SPC), a marker for alveolar epithelial type II cells. Surprisingly, we found that Pg-treatment increased the accumulation of SPC-positive cells in alveolar space compared with all other treatments (Figure 7D), suggesting a specific role of progesterone in regulating alveolar wall integrity.
Figure 7. Progesterone induces alveolar wall thickening in a preclinical model of LAM.
Rat-derived ELT3 cells were subcutaneously injected into female ovariectomized mice implanted with slow-releasing pellets of E2, Pg, E2 + Pg, or placebo. (A) Lung sections from female mice 29 were stained with H&E. (B) Quantification of alveolar air space in mouse lungs from all treatment groups. The area of alveolar space was scored from 30 random fields per lung section. The ratio of alveolar space to the corresponding lung area was calculated and illustrated in a scatterplot. (C) Immunohistochemical staining of CD45 in mouse lung sections. (D) Immunofluorescent staining of SPC in mouse lung sections. *p<0.05, ** p < 0.01, Student t test.
Discussion
LAM is a female predominant lung disease characterized by the abnormal proliferation of smooth muscle cells and progressive cystic destruction that can lead to respiratory failure and fatality [3, 15]. The gender specificity of LAM suggests that circulating female hormones including progesterone and estradiol may contribute to disease development. However, the mechanisms responsible for the action of progesterone and estradiol in cells with TSC2 deficiency have not been extensively studied. In current study we found that progesterone or progesterone plus estradiol activated Akt and ERK1/2, stimulated the proliferation, and promoted the migration and invasiveness of TSC2-deficient LAM patient-derived and rat-uterine leiomyoma-derived ELT3 cells in vitro. Moreover, progesterone plus estradiol synergistically reduced the cellular levels of ROS, increased the levels of glutathione, and enhanced cell survival under oxidative stress. In vivo, combination of progesterone and estradiol promoted the growth of xenograft tumors of Tsc2-deficient cells. Importantly, treatment of progesterone plus estradiol increased the number of lung micrometastases of Tsc2-deficient cells compared with estradiol treatment. Interestingly, progesterone induced immune cell infiltration and alveolar wall thickening.
In the past 35 years, anti-estrogen therapies have been used in the treatment of LAM; however, the benefit of progesterone therapy has not been promising in most of the clinical studies [20, 21, 39–46]. Our in vitro and in vivo results differ from previous studies. We hypothesize that E2 drives and Pg enhances the metastatic potential of Tsc2-deficient cells via the cognate receptors ERα and PgR. Importantly, PgR is one of the well-defined targets of E2 regulation. In the absence of E2, the Pg:PgR stoichiometry may be imbalance. Consequently, the actions of Pg may be limited. In the presence of E2, the expression of PgR is elevated, allowing more binding of Pg with PgR and subsequent higher PgR-mediated transactivation of targeted genes, leading to a synergistic effect of E2 and Pg. Recently, Gao et al. have reported that the expression of progesterone receptor is significantly higher than that of estrogen receptor in pulmonary LAM, suggesting a potential role of PgR in LAM pathogenesis [47]. In a preclinical model of LAM, Prizant et al. reported that E2 or E2 plus Pg increase the growth of uterine tumors and lung metastasis of uterine tumor cells, although Pg alone did not affect these alterations [24], consistent with our preclinical findings. Despite the lack of effect of Pg in lung metastasis of tumor cells, we observed striking alveolar alterations of immune cell infiltration and alveolar thickening in the lungs of mice treated with Pg. To our knowledge, this is the first report describing a direct effect of progesterone in promoting immune responses and alveolar integrity, suggesting that progesterone may play an important role in LAM pathogenesis and progression.
The impact of estradiol on the growth and metastatic behaviors of TSC2-deficient cells have been reported. Estradiol treatment activated p44/42 MAPK and the proliferation of rat uterine leiomyoma-derived cells [23, 33]. Gu et al. found that estradiol stimulation activates ERK1/2, increases migration and invasion, and promotes epithelial-to-mesenchymal transition of LAM patient-derived cells [35]. Sun et al. reported that estradiol treatment promoted glucose metabolism via enhanced pentose phosphate pathway addiction, increased the glucose uptake in vitro via Akt reactivation, and increased the survival of LAM patient-derived cells in a G6PD-dependent manner [36]. In addition, Li et al. demonstrated that estradiol treatment increased phospholipid-arachidonate breakdown, the expression of COX-2, and the production of prostaglandin metabolites in LAM patient-derived cells [34]. In this study, the effects of E2 and/or Pg on cell proliferation and survival are subtle, but we repeatedly observe similar trends. One explanation for this subtle hormonal effect on cell proliferation might be caused by decreased ERα and PgR expression in cultures, with more striking effects likely in vivo. In agreement with this, we found that E2 treatment led to a 2-fold increase of the growth of subcutaneous tumors of ELT3 cells at 8-weeks post cell injection in a xenograft tumor model [23].
Importantly, the migration and invasion of TSC2-deficient cells were more dramatically affected by E2 and/or Pg treatment. Recent studies showed that E2 treatment enhanced the migration of human-derived cells [35] and rat-derived ELT3 cells. More importantly, Faslodex treatment blocked the E2-promoted lung metastasis of ELT3 cells without affecting the growth of xenograft tumors [25]. Collectively, these studies imply a critical action of E2 in regulating metastatic behaviors of TSC2-deficient cells more than their proliferation.
We previously showed that estradiol promotes the survival and lung metastasis of Tsc2-deficient ELT3 cells in a MEK1/2-dependent manner [23]. Although the impact of estradiol on the growth and metastatic behaviors of TSC2-deficient cells have been reported, the influence of progesterone in these cellular outcomes has not been extensively investigated in the context of TSC2 deficiency and LAM. Recently, Prizant et al. developed a novel uterine-specific Tsc2 knockout mouse model in which estradiol or estradiol plus progesterone promotes the growth of myometrial tumors and lung metastasis, although progesterone alone had no effect on these phenotypes [24]. In current study, we found that progesterone was sufficient to activate ERK1/2 and Akt signaling pathways, although not sufficient to promote tumor growth or lung metastasis of Tsc2-deficient cells, indicating a potentially key pathogenic mechanism of progesterone action underlying the female hormone-driven progression of LAM.
LAM can lead to respiratory failure and death [3, 15]. Despite many advances in understanding mTOR-dependent and independent pathways and the clinical care of women with LAM, there remains a critical need for improved therapeutic options. The recent MILES trial (Multicenter International LAM Efficacy of Sirolimus Trial) demonstrated that the mTORC1 inhibitor Sirolimus stabilizes lung function in women with LAM; however, lung function decline resumed upon drug discontinuation [4], indicating a curable therapeutic strategy is urgently needed. Our data highlight a mechanism for LAM pathogenesis, for the first time, that progesterone activates MEK1/2 and PI3K/Akt signaling pathways and enhances the migration and invasiveness of TSC2-deficient LAM patient-derived cells. It will be important to further investigate the molecular mechanisms responsible for the action of progesterone in LAM progression and test the efficacy of inhibition of progesterone and progesterone receptor in vitro and in vivo. We anticipate that targeting progesterone signaling may be beneficial in the treatment and/or in the prevention of women with LAM.
Materials and Methods
Cell line and culture
Cell culture media and supplements were from GIBCO (Frederick, MD). Eker rat uterine leiomyoma-derived (ELT3) were developed by Howe et al. [29, 48]. ELT3 cells were transduced with a retroviral plasmid pMSCVneo-hTSC2 or its corresponding empty vector pMSCVneo, and then selected with neomycin for two weeks. Stable clones were characterized for TSC2 expression [49]. The patient-derived cells were developed from a sporadic LAM-associated renal angiomyolipoma. These cells carry bi-allelic mutations of the TSC2 gene that are identical to the mutations found in the patient’s pulmonary LAM cells [28]. An E6/E7 immortalized patient-derived cell line was developed [6]. Its corresponding TSC2-rescued control cell line has been described previously [50]. In brief, patient-derived cells were transfected with pcDNA3.1zeo-hTSC2 or its corresponding empty vector control pcDNA3.1zeo. Stable clones expressing TSC2 were selected using zeocin for two weeks as described previously [50]. Cells were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 0.2 µM hydrocortisone, 0.1 nM triiodothyronine, 0.01 µU/ml vasopressin, 1.6 µM FeSO4, cholesterol, human insulin-transferrin-sodium selenite (ITS), 100 ng/ml epidermal growth factor (EGF), 100 µg/ml zeomycin, and 1% penicillin-streptomycin-amphotericin B (PSA). One week prior to hormonal treatment, cells were cultured in phenol red-free media supplemented with charcoal-dextran stripped FBS (steroids depleted). All hormonal treatments were performed in phenol red-free and serum-free media.
Antibodies and Chemicals
The following chemicals were used: 17-beta-estradiol, progesterone, ICI 182,780 (Fulvestrant), RU-486 (Mifepristone), and propidium iodide (Sigma-Aldrich). Antibodies included phospho-ERK1/2 (T202/Y204), phospho-Akt (Ser473), phospho-S6 (S235/236), ERK1/2 and PCNA (Cell Signaling Technologies); tuberin (TSC2) (Santa Cruz); CD45 (BD BIOSCIENCES); SPC (Santa Cruz); smooth muscle actin (Biogenex); and β-actin (Sigma-Aldrich).
Immunofluorescent staining
Cells were seeded on glass coverslips adn cultured in complete media. Cells were fixed with 4% Paraformaldehyde for 10 min, blocked in 1% BSA/PBS/Tween 20 (0.05%) for 30 min, and incubated with primary antibody for 1 hr and secondary antibodies for 1 hr. Images were captured with an FluoView FV-10i Olympus Laser Point Scanning Confocal Microscope Olympus.
Cell proliferation assay
Cell were seeded at a density of 3 × 104/mL in 96-well plate for 24 hr, and then treated with control, estradiol (10 nM), progesterone (10 nM). Cell proliferation was measured using crystal violet. Briefly, cells were fixed with formalin for 5 minutes, stained with 0.05% crystal violet (CV) for 30 minutes, washed three times with tap water, and air dried. 100 µL methanol was added to each well to solubilize the dye thoroughly. Absorbance at 540 nm was read on a plate reader (BioTek Synergy HT).
Transwell migration assay
Cells were pre-incubated with control, estradiol (10 nM), progesterone (10 nM), and estradiol plus progesterone for 24 hr, and then seeded into 24-well Corning transwell. Twenty-four hours later, migrating cells were stained with Crystal violet and quantitated.
Matrigel invasion assay
Cells were pre-incubated with control, estradiol (10 nM), progesterone (10 nM), and estradiol plus progesterone for 24 hr, and then seeded into 6-well BD BioCoat™ Matrigel™ Invasion Chamber (BD Biosciences). Twenty-four hours later, invading cells were stained with Crystal violet and quantitated.
Cellular NADPH quantification
Cells were seeded in 12-well plates, and then stimulated with control, estradiol (10 nM), progesterone (10 nM), and estradiol plus progesterone. 1 × 105 cells were harvested and lysates were extracted with 200 µL of extraction buffer following the manufacture’s instruction. Levels of NADPH were measured using NADP/NADPH Assay kit (Abcam) and normalized to total cellular proteins.
Quantification of reactive oxygen species (ROS)
Cells were seeded in 96-well plates and incubated with 50 µM DCFH-DA in 1x-DPBS for 45 min at 37°C. Fluorescence was read at 485 nm/525 nm. Levels of cellular ROS were normalized to the total number of cells.
Quantification of intracellular glutathione (GSH)
Cells were seeded in 6-well plates and then treated with control, estradiol (10 nM), progesterone (10 nM), and estradiol plus progesterone for 24 hr. 5 × 105 cells were harvested and whole cell lysates were extracted with 300 µL extraction buffer by freeze-thaw cycles. Intracellular levels of glutathione (GSH) were quantified (Cayman Glutathione Assay) and normalized to protein amounts.
Measurement of cell survival
Cells were plated in the 96-well plates, treated with control, estradiol (10 nM), progesterone (10 nM), and estradiol plus progesterone for 24 hr, and then exposure to 0.5 µM H2O2 for 30 min. Cells were incubated with 5 µM propidium iodide in 100 µL 1x-DPBS for 30 min at 37°C. Fluorescence was read at 530 nm/620 nm. Data was represented as percentage of dead cells relative to the total number of cells.
Immunoblotting
Cells were lysed in M-PER buffer (Pierce) supplemented with protease inhibitors and phosphatase inhibitor cocktails. Cleared cell lysates were obtained by centrifugation at 14,000 × rpm for 10 min at 4°C and then subjected to immunoblotting.
Animal studies
All animal work was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee-Fox Chase Cancer Center, Philadelphia. For xenograft tumor establishment, one week prior to cell inoculation, mice were implanted with estradiol pellets (90-day release, 1.7 mg/pellet), progesterone pellets (90-day release, 25 mg/pellet), estradiol plus progesterone (1.7 mg + 25 mg/pellet), or placebo pellets (Innovative America). Two million Tsc2-deficient ELT3 cells were inoculated bilaterally into the rear flanks of the mice as described in our previous study[23]. Subcutaneous tumors were developed four weeks post cell inoculation. The volume of xenograft tumor (width × length × depth) was measured using a digital caliper. Mice were sacrificed eight-week post cell injection. To identify metastatic lesions, lung sections were stained with smooth muscle actin (SMA), phospho-S6 (Ser235/236), CD45, SPC, and dual staining of PCNA and phospho-S6. The clusters of double positive cells were considered as lung metastases, resembling the proliferative phenotype of human LAM [38]. Furthermore, lung metastases were scored from 5-micron H&E-stained sections of each lobe by observers blinded to the experimental conditions as described [23]. Sections were deparaffinized and stained with Gill’s Hematoxylin and eosin (H&E). The area of alveolar space was scored from 30 random fields per lung section. The ratio of alveolar space to the corresponding lung area was calculated and illustrated in a scatterplot.
Quantitative RT-PCR
Total RNA was extracted using the RNeasy mini kit (Qiagen). cDNA was synthesized from 2 µg of total RNA using a high-capacity cDNA reverse transcription kit (Life Technologies) with random primers, according to the manufacturer's protocol. Gene expression was quantified using SYBR green real-time PCR Master Mixes kit (Life Technologies) in the Applied Biosystems Real-Time PCR System and normalized to tubulin. The primers were used:
ERS1 (ERα): Forward: GCTTACTGACCAACCTGGCAGA.
Reverse: GGATCTCTAGCCAGGCACATTC.
Tubulin forward: GAGGAGATGACTCCTTCAACACC.
Reverse: TGATGAGCTGCTCAGGGTGGAA.
PgR Forward: GTCGCCTTAGAAAGTGCTGTCAG.
Reverse: GCTTGGCTTTCATTTGGAACGCC.
Rat derived cells gene expression was quantified using One-Step qRT-PCR kits (Invitrogen) in the Applied Biosystems Step One Plus Real-Time PCR System and normalized to β-actin. PgR primer mix (Applied Biosystem Rn01448227_m1), EsR1 primer mix (Applied Biosystem Rn01640372_m1) and Rat actin primer mix (Applied Biosystem 4352931).
Statistical analyses
All data are shown as mean ± S.E.M. Measurements at single time points were analyzed by ANOVA and, if they demonstrated significance, were further analyzed by a two-tailed t-test. All statistical tests were conducted using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). P < 0.05 was used to define statistical significance.
Acknowledgments
We are grateful to Dr. C. Walker (Texas A&M Health Science Center) for providing ELT3 cells, Ms. Chunrong Wang for animal experiments, and Mr. Anthony Larro (Fox Chase Cancer Center) for assistance with animal studies, and Dr. E.P. Henske for her critical reading of this manuscript. C.L. is a postdoctoral research fellow funded by The LAM Foundation Fellowship. This study is supported by the National Heart Lung and Blood Institute to (HL98216) to J.J.Y.
Footnotes
Conflict of interest. The authors declare that there are no conflicts of interest.
References
- 1.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97(11):6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 3.Henske EP, McCormack FX. Lymphangioleiomyomatosis - a wolf in sheep's clothing. J Clin Invest. 2012;122(11):3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP, 3rd, Goldberg HJ, Young LR, Kinder BW, Downey GP, Sullivan EJ, Colby TV, McKay RT, Cohen MM, Korbee L, Taveira-DaSilva AM, Lee HS, Krischer JP, Trapnell BC, National Institutes of Health Rare Lung Diseases C, Group MT. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, McCormack FX, Henske EP. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167(7):976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Astrinidis A, Howard S, Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L694–L700. doi: 10.1152/ajplung.00204.2003. [DOI] [PubMed] [Google Scholar]
- 7.Brentani MM, Carvalho CR, Saldiva PH, Pacheco MM, Oshima CT. Steroid receptors in pulmonary lymphangiomyomatosis. Chest. 1984;85(1):96–99. doi: 10.1378/chest.85.1.96. [DOI] [PubMed] [Google Scholar]
- 8.Colley MH, Geppert E, Franklin WA. Immunohistochemical detection of steroid receptors in a case of pulmonary lymphangioleiomyomatosis. Am J Surg Pathol. 1989;13(9):803–807. doi: 10.1097/00000478-198909000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Logginidou H, Ao X, Russo I, Henske EP. Frequent estrogen and progesterone receptor immunoreactivity in renal angiomyolipomas from women with pulmonary lymphangioleiomyomatosis. Chest. 2000;117(1):25–30. doi: 10.1378/chest.117.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Ohori NP, Yousem SA, Sonmez-Alpan E, Colby TV. Estrogen and progesterone receptors in lymphangioleiomyomatosis, epithelioid hemangioendothelioma, and sclerosing hemangioma of the lung. Am J Clin Pathol. 1991;96(4):529–535. doi: 10.1093/ajcp/96.4.529. [DOI] [PubMed] [Google Scholar]
- 11.Tazelaar HD, Kerr D, Yousem SA, Saldana MJ, Langston C, Colby TV. Diffuse pulmonary lymphangiomatosis. Hum Pathol. 1993;24(12):1313–1322. doi: 10.1016/0046-8177(93)90265-i. [DOI] [PubMed] [Google Scholar]
- 12.Usuki J, Horiba K, Chu SC, Moss J, Ferrans VJ. Immunohistochemical analysis of proteins of the Bcl-2 family in pulmonary lymphangioleiomyomatosis: association of Bcl-2 expression with hormone receptor status. Arch Pathol Lab Med. 1998;122(10):895–902. [PubMed] [Google Scholar]
- 13.Berger U, Khaghani A, Pomerance A, Yacoub MH, Coombes RC. Pulmonary lymphangioleiomyomatosis and steroid receptors. An immunocytochemical study. Am J Clin Pathol. 1990;93(5):609–614. doi: 10.1093/ajcp/93.5.609. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli A, Catalini G, Fianchini A. Pregnancy exacerbating unsuspected mediastinal lymphangioleiomyomatosis and chylothorax. Int J Gynaecol Obstet. 1996;52(3):289–290. doi: 10.1016/0020-7292(95)02619-3. [DOI] [PubMed] [Google Scholar]
- 15.Taveira-DaSilva AM, Pacheco-Rodriguez G, Moss J. The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis. Lymphat Res Biol. 2010;8(1):9–19. doi: 10.1089/lrb.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberstein EM, Fleming LE, Gomez-Marin O, Glassberg MK. Pulmonary lymphangioleiomyomatosis (LAM): examining oral contraceptive pills and the onset of disease. J Womens Health (Larchmt) 2003;12(1):81–85. doi: 10.1089/154099903321154176. [DOI] [PubMed] [Google Scholar]
- 17.Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002;57(12):1085–1086. doi: 10.1136/thorax.57.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen A, Iseman MD, Waldron JA, King TE. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous estrogens. Chest. 1987;91(5):782–785. doi: 10.1378/chest.91.5.782. [DOI] [PubMed] [Google Scholar]
- 19.Matsui K, Takeda K, Yu ZX, Valencia J, Travis WD, Moss J, Ferrans VJ. Downregulation of estrogen and progesterone receptors in the abnormal smooth muscle cells in pulmonary lymphangioleiomyomatosis following therapy. An immunohistochemical study. Am J Respir Crit Care Med. 2000;161(3 Pt 1):1002–1009. doi: 10.1164/ajrccm.161.3.9904009. [DOI] [PubMed] [Google Scholar]
- 20.Seyama K, Kira S, Takahashi H, Ohnishi M, Kodama Y, Dambara T, Kobayashi J, Kitamura S, Fukuchi Y. Longitudinal follow-up study of 11 patients with pulmonary lymphangioleiomyomatosis: diverse clinical courses of LAM allow some patients to be treated without anti-hormone therapy. Respirology. 2001;6(4):331–340. doi: 10.1046/j.1440-1843.2001.00343.x. [DOI] [PubMed] [Google Scholar]
- 21.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126(6):1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Lunsford EP, Tong J, Ashitate Y, Gibbs SL, Yu J, Choi HS, Henske EP, Frangioni JV. Real-time monitoring of tumorigenesis, dissemination, & drug response in a preclinical model of lymphangioleiomyomatosis/tuberous sclerosis complex. PLoS One. 2012;7(6):e38589. doi: 10.1371/journal.pone.0038589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, Wang C, Hernandez-Cuebas L, Seeholzer LF, Nicolas E, Hensley H, Jordan VC, Walker CL, Henske EP. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci U S A. 2009;106(8):2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prizant H, Sen A, Light A, Cho SN, DeMayo FJ, Lydon JP, Hammes SR. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Mol Endocrinol. 2013;27(9):1403–1414. doi: 10.1210/me.2013-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Zhou X, Sun Y, Zhang E, Mancini JD, Parkhitko A, Morrison TA, Silverman EK, Henske EP, Yu JJ. Faslodex inhibits estradiol-induced extracellular matrix dynamics and lung metastasis in a model of lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2013;49(1):135–142. doi: 10.1165/rcmb.2012-0476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Hashemite N, Walker V, Kwiatkowski DJ. Estrogen enhances whereas tamoxifen retards development of Tsc mouse liver hemangioma: a tumor related to renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Cancer Res. 2005;65(6):2474–2481. doi: 10.1158/0008-5472.CAN-04-3840. [DOI] [PubMed] [Google Scholar]
- 27.Howe SR, Everitt JI, Gottardis MM, Walker C. Estrogen/antiestrogen responsiveness in an in vivo/in vitro model for myometrial tumorigenesis. Ann N Y Acad Sci. 1995;761:373–375. doi: 10.1111/j.1749-6632.1995.tb31396.x. [DOI] [PubMed] [Google Scholar]
- 28.Yu JJ, Astrinidis A, Howard S, Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L694–L700. doi: 10.1152/ajplung.00204.2003. [DOI] [PubMed] [Google Scholar]
- 29.Howe SR, Gottardis MM, Everitt JI, Walker C. Estrogen stimulation and tamoxifen inhibition of leiomyoma cell growth in vitro and in vivo. Endocrinology. 1995;136(11):4996–5003. doi: 10.1210/endo.136.11.7588234. [DOI] [PubMed] [Google Scholar]
- 30.Olea-Serrano N, Devleeschouwer N, Leclercq G, Heuson JC. Assay for estrogen and progesterone receptors of breast cancer cell lines in monolayer culture. Eur J Cancer Clin Oncol. 1985;21(8):965–973. doi: 10.1016/0277-5379(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 31.Kawahara K, Shimazu A. Expression and intracellular localization of progesterone receptors in cultured human gingival fibroblasts. J Periodontal Res. 2003;38(3):242–246. doi: 10.1034/j.1600-0765.2003.00654.x. [DOI] [PubMed] [Google Scholar]
- 32.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37(2):153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 33.Finlay GA, Hunter DS, Walker CL, Paulson KE, Fanburg BL. Regulation of PDGF production and ERK activation by estrogen is associated with TSC2 gene expression. Am J Physiol Cell Physiol. 2003;285(2):C409–C418. doi: 10.1152/ajpcell.00482.2002. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Lee PS, Sun Y, Gu X, Zhang E, Guo Y, Wu CL, Auricchio N, Priolo C, Li J, Csibi A, Parkhitko A, Morrison T, Planaguma A, Kazani S, Israel E, Xu KF, Henske EP, Blenis J, Levy BD, Kwiatkowski D, Yu JJ. Estradiol and mTORC2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient LAM cells. J Exp Med. 2014;211(1):15–28. doi: 10.1084/jem.20131080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu X, Yu JJ, Ilter D, Blenis N, Henske EP, Blenis J. Integration of mTOR and estrogen-ERK2 signaling in lymphangioleiomyomatosis pathogenesis. Proc Natl Acad Sci U S A. 2013;110(37):14960–14965. doi: 10.1073/pnas.1309110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Yang, G X, Zhang Erik, Park Mi-Ae, Pereira Ana M, Wang Shuyan, Morrison Tasha, Li Chenggang, Blenis John, Gerbaudo Victor H, Henske Elizabeth Petri, Yu Jane J. Estradiol promotes pentose phosphate pathway addiction and cell survival via reactivation of Akt in mTORC1 hyperactive cells. Cell Death & Disease. 2014 doi: 10.1038/cddis.2014.204. In print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16(1):231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA, Jr, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277(34):30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 39.McCarty KS, Jr, Mossler JA, McLelland R, Sieker HO. Pulmonary lymphangiomyomatosis responsive to progesterone. N Engl J Med. 1980;303(25):1461–1465. doi: 10.1056/NEJM198012183032506. [DOI] [PubMed] [Google Scholar]
- 40.Denoo X, Hermans G, Degives R, Foidart JM. Successful treatment of pulmonary lymphangioleiomyomatosis with progestins: a case report. Chest. 1999;115(1):276–279. doi: 10.1378/chest.115.1.276. [DOI] [PubMed] [Google Scholar]
- 41.Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160(2):628–633. doi: 10.1164/ajrccm.160.2.9901027. [DOI] [PubMed] [Google Scholar]
- 42.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, Cordier JF. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P) Medicine (Baltimore) 1999;78(5):321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Schiavina M, Contini P, Fabiani A, Cinelli F, Di Scioscio V, Zompatori M, Campidelli C, Pileri SA. Efficacy of hormonal manipulation in lymphangioleiomyomatosis. A 20-year-experience in 36 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24(1):39–50. doi: 10.1007/s11083-007-9058-0. [DOI] [PubMed] [Google Scholar]
- 44.Harari S, Cassandro R, Chiodini I, Taveira-DaSilva AM, Moss J. Effect of a gonadotrophin-releasing hormone analogue on lung function in lymphangioleiomyomatosis. Chest. 2008;133(2):448–454. doi: 10.1378/chest.07-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldi BG, Medeiros Junior P, Pimenta SP, Lopes RI, Kairalla RA, Carvalho CR. Evolution of pulmonary function after treatment with goserelin in patients with lymphangioleiomyomatosis. J Bras Pneumol. 2011;37(3):375–379. doi: 10.1590/s1806-37132011000300015. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SR. Lymphangioleiomyomatosis. Eur Respir J. 2006;27(5):1056–1065. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- 47.Gao L, Yue MM, Davis J, Hyjek E, Schuger L. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Arch. 2014;464(4):495–503. doi: 10.1007/s00428-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 48.Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C. Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am. J. Pathol. 1995;146(6):1568–1579. [PMC free article] [PubMed] [Google Scholar]
- 49.Astrinidis A, Cash TP, Hunter DS, Walker CL, Chernoff J, Henske EP. Tuberin, the tuberous sclerosis complex 2 tumor suppressor gene product, regulates Rho activation, cell adhesion and migration. Oncogene. 2002;21(55):8470–8476. doi: 10.1038/sj.onc.1205962. [DOI] [PubMed] [Google Scholar]
- 50.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30(6):701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]