Abstract
Little is known about the use of pharmacologic rhythm or rate control in younger atrial fibrillation (AF) patients in clinical practice. Using commercial health data from 2006 through 2010, patients aged <65 years with an initial AF encounter were categorized as receiving pharmacologic rhythm- or rate-control treatment. Factors associated with each treatment were determined. Cox models with inverse propensity-weighted estimators were used to compare times to AF, heart failure, cardiovascular, non-cardiovascular, and any-cause hospitalizations. Of 79,232 patients meeting the study criteria, 12,408 (16%) received a rhythm-control drug and 66,824 (84%) received only rate-controlling drugs. Only 2% and 0.1%, respectively, received electrical cardioversion and AF ablation during the initial AF encounter. Patients who were men (OR 1.10, 95% CI 1.06–1.15), had index encounters in later years (2010 versus 2006: OR 1.34, 95% CI 1.23–1.45), were in the southern United States, and had other cardiac comorbidities were more likely to receive a rhythm-control drug. There was a greater risk of AF (HR 1.40, 95% CI 1.31–1.50), cardiovascular (HR 1.26, 95% CI 1.20–1.33), and all-cause (HR 1.11, 95% CI 1.07–1.16) hospitalizations in the rhythm-control group, but there was no difference between groups in heart failure (HR 1.01, 95% CI 0.88–1.17) or non-cardiovascular (HR 1.04, 95% CI 0.99–1.09) hospitalizations. Among younger AF patients receiving initial pharmacologic treatment, antiarrhythmic drugs were used less frequently than only rate-controlling drugs, and were associated with a higher risk of subsequent hospitalization.
Keywords: Fibrillation, Rhythm Control, Rate Control
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia affecting up to 6.1 million people in the United States, with estimates increasing to 12 million by 2050.1,2 For more than a decade, there has been debate as to whether a rhythm- or rate-control strategy is superior for managing AF. Sinus rhythm is generally thought to be superior to AF due to the risks of stroke and myocardial remodeling associated with AF, but the risks associated with long-term use of antiarrhythmic drugs to restore and maintain sinus rhythm may outweigh the potential benefits.3 In a recently published meta-analysis and in an Agency for Healthcare Research and Quality effective health care evidence report comparing 1) the use of antiarrhythmic drugs with or without electrical cardioversion and 2) the use of rate-control drugs in elderly and nonelderly patients in randomized controlled trials, there was no statistically significant difference between strategies in terms of mortality, cardiac mortality, stroke, worsening heart failure, or bleeding.4,5 Among studies in which the mean patient age was <65 years, there was a significantly lower risk of mortality in those receiving the antiarrhythmic drugs with or without electrical cardioversion (risk ratio [RR] 0.33, 95% confidence interval [CI] 0.17–0.63), indicating that age may be an important consideration in strategy selection.4 Little is known about the use of different medical treatments in younger AF patients in clinical practice. The purpose of this study was to explore the use of pharmacologic rhythm control and rate control immediately following the first AF event in patients aged <65 years in clinical practice, and to compare risk of subsequent hospitalization between the 2 initial pharmacologic treatments.
Materials and Methods
Data Source
This retrospective cohort study used data from the Thomas Reuters MarketScan® Commercial Claims and Encounters Database, which comprises inpatient, outpatient, and prescription claims and health plan enrollment data from large U.S. employers and health plans for employees and their spouses and dependents. Patient data are linked across calendar years. The MarketScan® databases have been used for more than 450 publications of health care utilization and outcomes in a variety of diseases, including atrial fibrillation.6–8 Data were obtained from all patients with an inpatient or outpatient encounter that included a diagnosis of AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9] code 427.31) between January 1, 2006 and December 31, 2010. The database does not include Medicare claims data nor any data on patients > 65 years of age, and therefore, the study cohort consists only of patients aged <65 years. The Duke University Health System Institutional Review Board determined that the study was exempt from review.
Selection Of Study Cohort
For this study, we were interested in identifying adult patients aged <65 years with their first AF encounter for whom subsequent antiarrhythmic drug prescriptions would most likely be for AF treatment. Only patients with individual-level and pharmacy benefit data were included. The first inpatient or outpatient encounter with a primary or secondary diagnosis of AF (ICD-9 code 427.31) was identified. The date of hospital discharge or the end of the outpatient encounter was used as the index AF encounter date. Exclusion criteria comprised the following: age <18 years, death during the index AF encounter, <6 months of continuous enrollment in the health plan before the index AF encounter, diagnosis of ventricular arrhythmias during the 6 months before the index AF encounter (ICD-9 codes 427.1, 427.4x, and 427.5), prescription claim for an antiarrhythmic drug before the index AF encounter, and heart transplantation or left ventricular assist device implantation at any point (ICD-9 codes 37.5x, 33.6, 37.6x, and V42.1). Also excluded were patients who underwent cardiothoracic surgery (ICD-9 codes 35.x–39.x) within 30 days before or after the index AF encounter, unless the patient experienced a subsequent AF encounter >30 days after the cardiothoracic surgery and there was no prescription claim for an antiarrhythmic drug during the 6 months before this subsequent encounter. In this situation, the subsequent AF encounter became the index AF encounter for this analysis.
Categorization Of Patients Into Pharmacologic Rhythm- Or Rate-Control Groups
For this study, we were interested in the use of rhythm- or rate-control drugs immediately following each patient’s initial AF encounter. Patients included in the pharmacologic rhythm-control group had to have a prescription claim for a >30-day supply of 1 of the following oral antiarrhythmic drugs that was filled within 14 days of the index AF encounter: Class Ia drugs (quinidine, procainamide, or disopyramide), Class Ic drugs (flecainide or propafenone), or Class III drugs (amiodarone, sotalol, dofetilide, or dronedarone). Patients in the pharmacologic rate-control group were selected from those who were not assigned to the pharmacologic rhythm-control group. Patients in the rate-control arm had to have a prescription claim for a >30-day supply of 1 of the following oral drugs that was 1) filled within 14 days after the index AF encounter, or 2) continued from before the index AF encounter if ≥1 prescription claim covered the 30-day period after the index AF encounter: digitalis glycosides (digoxin or digitoxin), calcium channel blockers (verapamil or diltiazem, including combination products containing these drugs), and beta-blockers without primary intrinsic sympathomimetic activity (excluding sotalol).
Outcome Measures
The primary outcome measure was time to AF hospitalization defined as the number of days from the index AF encounter to the hospitalization admission for a primary diagnosis of AF. Secondary outcome measures included the following: time to heart failure hospitalization (hospitalization with a primary diagnosis of heart failure [ICD-9 codes 428.xx, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 398.91]); time to cardiovascular hospitalization (hospitalization with primary diagnosis of ischemic heart disease [codes 410–414, 429.2, and V45.81], heart failure, cardiomyopathy [codes 425.0, 425.1, 425.2, 425.3, 425.5, 425.7, 425.8, and 425.9], cardiac arrhythmias [code 427.x], or cerebral hemorrhage/stroke [codes 431.x–435]); time to non-cardiovascular hospitalization; and time to all-cause hospitalization.
Since the analyses used an intention to treat approach, we also determined the number of patients who transitioned from the pharmacologic rhythm-control group to the rate-control group and vice-versa during the 1 year following the index AF encounter. Rhythm-control patients were considered to have switched to rate-control if a gap in the supply of all antiarrhythmic drugs occurred for >30 days and a rate-control drug was present (as determined by a >30-day supply of 1 or more rate-control drugs after the rhythm-control gap). Rate-control patients were considered to have switched to rhythm control if a prescription claim for a >30-day supply of an antiarrhythmic drug was filled. Median times to change (25th and 75th percentiles) were also calculated for both types of transition.
Statistical Analysis
The following characteristics were compared between AF patients in the rhythm-control and rate-control groups: age; sex; geographic region; year of index AF encounter; inpatient versus outpatient index AF encounter; proportion of hospitalized patients discharged to self-care; electrical cardioversion or AF ablation during index AF encounter; proportion of hospitalized patients with AF as the primary diagnosis; cardiovascular and non-cardiovascular hospitalizations in the preceding 6 months; history (within 6 months before or during the index AF encounter) of atrial flutter, ischemic heart disease, diabetes, hypertension, heart failure, cardiomyopathy, chronic rheumatic heart disease, other atrial arrhythmias, bradyarrhythmias, pacemaker use, renal failure, liver disease, thyroid disease, pulmonary disease, cancer, stroke, cerebral hemorrhage, depression, obesity, non-rheumatic valvular heart disease, or bleeding; and use of rate-controlling drugs, QT-prolonging drugs, warfarin, and dabigatran during or within the 6 months before the index AF encounter. ICD-9 and Current Procedural Terminology (CPT) codes for all diagnoses and procedures and all drug names are listed in Appendix 1. Categorical variables are presented as numbers (percentages) and were compared using chi-square tests. Continuous variables are presented as medians (25th and 75th percentiles) and were compared using Wilcoxon rank sum tests. The number (percentage) of drugs initially used for pharmacologic rhythm or rate control by study arm was also determined.
Factors associated with initial use of pharmacologic rhythm control versus pharmacological rate control were determined using a logistic regression model. All variables listed above were entered into the model using a stepwise selection process to identify variables that were significantly associated with an initial pharmacologic rhythm- versus rate-control approach. A p-value <0.05 was considered statistically significant and was required to remain in the model. Age was the only continuous variable, and to account for potential nonlinearity between age and treatment group, logistic regression models with restricted cubic splines were assessed to identify the best fit for age. It was determined that the relationship between age and rhythm control versus rate control was nonlinear and could be approximated by a piecewise linear spline with a change point at 55 years.
Substantial differences were anticipated between inpatient versus outpatient index AF encounters. Although this variable was included in the logistic regression model, we also conducted a post-hoc subgroup analysis to explore the effect of this variable on the other factors associated with use of pharmacological rhythm versus rate control. In this analysis, the logistic regression model was repeated and included only patients who had an outpatient index AF encounter.
To assess differences in the primary outcome and time to AF hospitalization, and to address potential treatment-selection bias, an inverse propensity-weighted estimation method was used. For each patient, a propensity score for the initial pharmacologic rhythm-versus rate-control approach was calculated using the variables presented above. The patient’s data contribution to the Cox regression model for time to AF hospitalization was then inverse-weighted by the probability of receiving the patient’s actual treatment (that is, by propensity score for patients receiving rhythm control and by 1– propensity score for patients receiving rate control). To assess balance before and after inverse propensity score weighting, Cramér’s phi measure of association was calculated for each categorical variable (Appendix 2) and an R2 value was calculated for the continuous age variables (age <55 and age >55). Censoring occurred at the end of the data-collection period (December 2010) or at the end of enrollment for the individual patient, whichever came first. A second Cox model with inverse propensity-weighted estimators and an interaction term for treatment (initial pharmacologic rhythm or rate control) and inpatient/outpatient index AF encounter was then developed to explore potential differences in patients with an inpatient versus an outpatient index AF encounter. The methods described above for the primary outcome measure were also used to assess the secondary outcome measures of times to heart failure, cardiovascular, non-cardiovascular, and all-cause hospitalizations. Hazard ratios (HRs) and 95% CIs are presented for comparisons of the initial pharmacologic rhythm- versus rate-control approaches.
Results
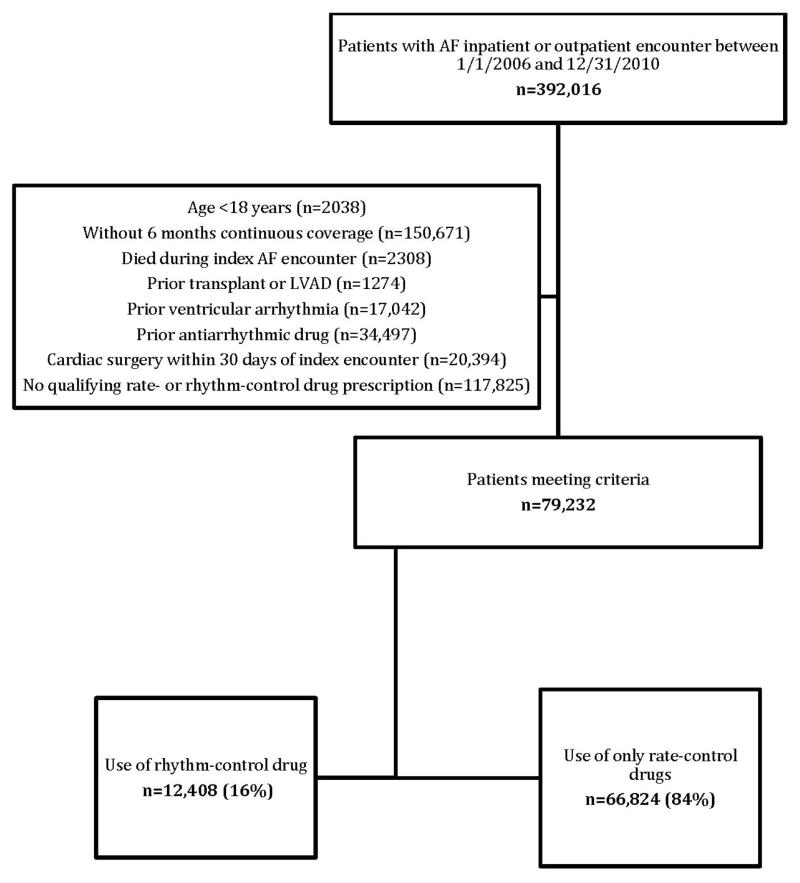
Of the 392,016 unique patients with an inpatient or outpatient encounter with a diagnosis of AF between January 1, 2006 and December 31, 2010, a total of 79,232 patients (20%) were included in the study—12,408 patients (16%) were categorized in the pharmacologic rhythm-control group, and 66,824 patients (84%) were categorized in the pharmacologic rate-control group (Figure 1). Baseline characteristics of the patients in each group are presented in Table 1. A higher proportion of patients in the rhythm-control group, compared with patients in the rate-control group, were hospitalized for their index AF encounter (40% versus 21%, p<0.0001), had a prior cardiovascular hospitalization (28% versus 15%, p<0.0001) or non-cardiovascular hospitalization (21% versus 16%, p<0.0001), underwent electrical cardioversion during the index AF encounter (7% versus 1%, p<0.0001), and had 1 or more prescription claims for warfarin or dabigatran during or within the 6 months before the index AF encounter (41% versus 32%, p<0.0001). Among those with a hospitalization for the index AF encounter, the median length of stay was 4 (interquartile range [IQR] 2–7) days for the rhythm-control group and 3 (IQR 1–6) days for the rate-control group (p<0.0001). AF ablation during the index AF encounter was very uncommon, but occurred in a greater proportion of patients in the rhythm-control group versus the rate-control group (0.4% versus 0.05%, p<0.0001).
Figure 1. Study cohort.
AF indicates atrial fibrillation; LVAD, left ventricular assist device
Table 1.
Baseline Characteristics
| Characteristic | All Patients (n=79,232) | Rhythm Control (n=12,408) | Rate Control (n=66,824) | p-Value |
|---|---|---|---|---|
| Age, yrs, median (IQR) | 57 (51–61) | 57 (51–61) | 57 (51–61) | 0.08 |
| Male sex, % | 64 | 67 | 64 | <0.0001 |
| Geographic region, % | <0.0001 | |||
| North central | 30 | 30 | 30 | |
| Northeast | 14 | 9 | 15 | |
| South | 39 | 45 | 38 | |
| West | 15 | 14%) | 15 | |
| Unknown | 1 | 1 | 1 | |
| Year of IE, % | <0.0001 | |||
| 2006 | 9 | 8 | 10 | |
| 2007 | 17 | 17 | 17 | |
| 2008 | 24 | 23 | 24 | |
| 2009 | 27 | 26 | 27 | |
| 2010 | 23 | 25 | 22 | |
| Index AF encounter hosp., % | 24 | 40 | 21 | <0.0001 |
| Discharged to self-care, n/N (%) | 15,251/18,987 (80) | 3913/4945 (79) | 11,338/14,042 (81) | 0.01 |
| Electrical cardioversion during IE, % | 2 | 7 | 1 | <0.0001 |
| AF ablation during IE, % | 0.1 | 0.4 | 0.05 | <0.0001 |
| AF is the primary diagnosis during IE, % | 82 | 87 | 81 | <0.0001 |
| Hosp. in 6 months before IE, % | ||||
| Cardiovascular | 17 | 28 | 15 | <0.0001 |
| Non-cardiovascular | 17 | 21 | 16 | <0.0001 |
| Medical history, % | ||||
| Atrial flutter | 9 | 17 | 8 | <0.0001 |
| Ischemic heart disease | 20 | 27 | 19 | <0.0001 |
| Diabetes | 20 | 20 | 20 | 0.3 |
| Hypertension | 51 | 56 | 50 | <0.0001 |
| Heart failure | 12 | 17 | 11 | <0.0001 |
| Cardiomyopathy | 1 | 2 | 1 | <0.0001 |
| Chronic rheumatic heart disease | 2 | 4 | 2 | <0.0001 |
| Other atrial arrhythmias | 5 | 6 | 4 | <0.0001 |
| Bradyarrhythmias | 2 | 3 | 2 | <0.0001 |
| Pacemaker | 0.1 | 0.4 | 0.1 | <0.0001 |
| Renal failure | 6 | 7 | 5 | <0.0001 |
| Liver disease | 3 | 3 | 3 | 0.1 |
| Thyroid disease | 10 | 10 | 10 | 0.1 |
| Pulmonary disease | 14 | 17 | 13 | <0.0001 |
| Cancer | 9 | 12 | 9 | <0.0001 |
| Stroke | 5 | 6 | 5 | <0.0001 |
| Cerebral hemorrhage | 0.3 | 0.3 | 0.3 | 0.8 |
| Depression | 7 | 6 | 7 | 0.6 |
| Obesity | 8 | 11 | 7 | <0.0001 |
| Non-rheumatic valvular heart disease | 15 | 22 | 14 | <0.0001 |
| Bleeding | 3 | 3 | 3 | 0.1 |
| Drug use during or within 6 months before IE, % | ||||
| Rate-controlling drugs | ||||
| Beta-blocker alone | 35 | 73 | 67 | <0.0001 |
| Digoxin alone | 2 | 2 | 4 | <0.0001 |
| CCB alone | 6 | 10 | 11 | <0.0001 |
| Combination of drugs | 9 | 7 | 9 | <0.0001 |
| QT-prolonging drugs | ||||
| Definite | 3 | 3 | 3 | 0.96 |
| Possible | 17 | 16 | 17 | <0.0001 |
| Warfarin/dabigatran | 33 | 41 | 32 | <0.0001 |
AF indicates atrial fibrillation; IE, index encounter; CCB, calcium channel blocker; hosp., hospitalization; IQR, interquartile range.
The initial rhythm- and rate-control drugs used following the index AF encounter for each group are shown in Figures 2A and 2B, respectively. The most commonly used antiarrhythmic drug in the rhythm-control group was amiodarone (37%), and the most commonly used rate-control drugs in the rate-control group were beta-blockers (63%). Of patients receiving rate-control drugs, 10,532 (15.8%) received the drug prior to the index AF encounter.
Figure 2. Rhythm-control drugs used in rhythm-control group Rate-control drugs used in rate-control group.
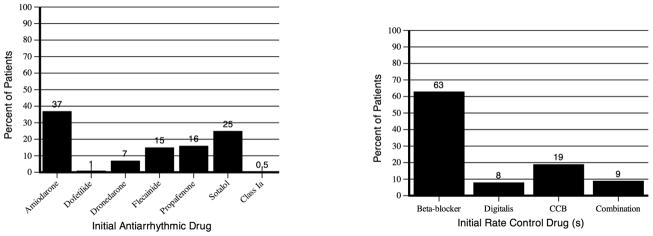
CCB indicates calcium channel blocker
Changes In Drug Treatment Groups
A total of 2294 (18%) patients in the rhythm-control group switched to the use of only a rate-control drug during the 1 year following the index AF encounter. The median time to the change was 129 (IQR 66–219) days. A total of 7824 (12%) patients in the rate-control group switched to the use of an antiarrhythmic drug during the 1 year following the index AF encounter. The median time to the change was 69 (IQR 35–140) days.
Factors Associated With An Initial Pharmacologic Rhythm- Versus Rate-Control Approach
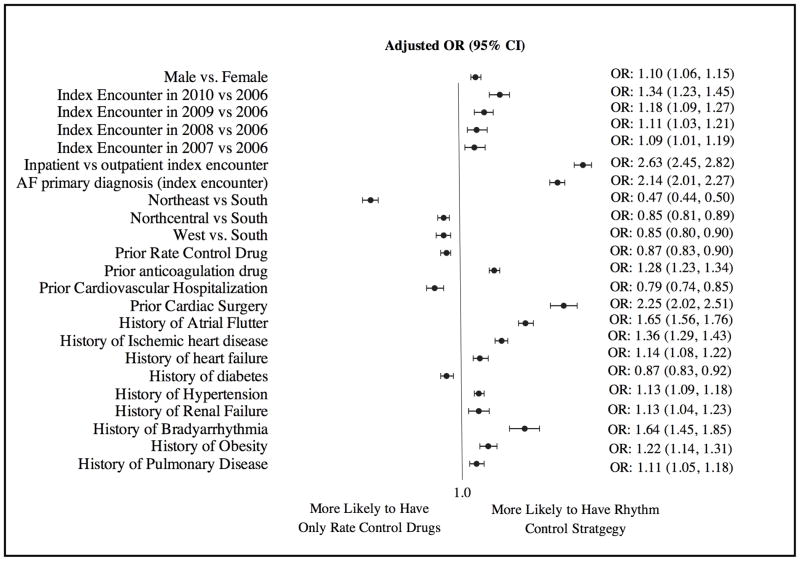
Factors associated with use of a rhythm-control drug versus a rate-control approach in patients with their first AF encounter are shown in Figure 3. The concordance statistic for the final model was 0.70, indicating good fit. Patient age (continuous variables for age <55 years and age 55–65 years) was not retained in the final model because its association with a pharmacologic rhythm- versus rate-control approach was not statistically significant after other variables were included into the model using the stepwise selection process. Men (odds ratio [OR] 1.10, 95% CI 1.06–1.15) and patients with index encounters in later years (2010 versus 2006: OR 1.34, 95% CI 1.23–1.45; 2009 versus 2006: OR 1.18, 95% CI 1.09–1.27; 2008 versus 2006: OR 1.11, 95% CI 1.03–1.21; 2007 versus 2006: OR 1.09, 95% CI 1.01–1.19) were more likely to receive an antiarrhythmic drug than only a rate-control drug. Patients in the southern United States were more likely than patients in the other regions to receive an antiarrhythmic drug than only a rate-control drug. In addition, in general, patients with other comorbidities were more likely to receive an antiarrhythmic drug than a rate-control drug only. However, patients with a prior cardiovascular hospitalization (OR 0.79, 95% CI 0.74–0.85) or diabetes mellitus (OR 0.87, 95% CI 0.83–0.92) were less likely to receive an antiarrhythmic drug following the first AF encounter.
Figure 3. Factors independently associated with receiving pharmacological rhythm control versus rate control following the first AF encounter.
AF indicates atrial fibrillation; CI, confidence interval; OR, odds ratio
In the subgroup analysis in which only patients with outpatient index AF encounters were included (n=60,245), factors associated with use of a pharmacologic rhythm- versus rate-control approach were the same except that increasing age from 55 to 65 years was associated with a lower likelihood of receiving an antiarrhythmic drug (OR per 1-year increase 0.98, 95% CI 0.98–0.99), and history of thyroid disease became a new factor significantly associated with receiving an antiarrhythmic drug (OR 1.10, 95% CI 1.01–1.19) with the first AF encounter.
Hospitalizations
The number of patients with an AF, a heart failure, a cardiovascular, a non-cardiovascular, or an all-cause hospitalization by treatment arm is shown in Table 2. The median (IQR) number of days of follow-up was 408 days (177–749) in the rhythm-control group and 446 days (196–789) in the rate-control group. After adjustment, the rhythm-control group was associated with a greater risk of AF hospitalizations compared with the rate-control group (HR 1.40, 95% CI 1.31–1.50).
Table 2.
Hospitalizations in the Pharmacological Rhythm-Control Versus Rate-Control Groups
| Hospitalizations | Patients with Events | Adjusted HR (95% CI) | |
|---|---|---|---|
| Rhythm Control (n=12,408) | Rate Control (n=66,824) | ||
| Atrial fibrillation | 1279 (10.3%) | 4845 (7.3%) | 1.40 (1.31–1.50) |
| Heart failure | 310 (2.5%) | 1379 (2.1%) | 1.01 (0.88–1.17) |
| Cardiovascular | 2160 (17.4%) | 8807 (13.2%) | 1.26 (1.20–1.33) |
| Non-cardiovascular | 2669 (21.5%) | 13,000 (19.5%) | 1.04 (0.99–1.09) |
| All-cause | 4060 (32.7%) | 18,888 (28.3%) | 1.11 (1.07–1.16) |
CI indicates confidence interval; HR, hazard ratio.
As shown in Table 2, there was also a significantly greater risk of cardiovascular hospitalization (HR 1.26, 95% CI 1.20–1.33) and all-cause hospitalization (HR 1.11, 95% CI 1.07–1.16) in the rhythm-versus rate-control group, but there was no statistically significant difference in heart failure or non-cardiovascular hospitalizations between groups.
In the models in which an interaction between treatment and inpatient/outpatient index AF encounter was included, a statistically signification interaction was found for AF, heart failure, non-cardiovascular, and all-cause hospitalizations. For the primary outcome measure, the risk of AF hospitalization was still significantly greater in the rhythm- versus rate-control group; however, the magnitude of the effect was lower for those with an inpatient index AF encounter (HR 1.19, 95% CI 1.07–1.32) than for those with an outpatient AF encounter (HR 1.46, 95% CI 1.35–1.58). There was a statistically significantly greater risk of heart failure hospitalization with rhythm versus rate control for those with an inpatient index AF encounter after including the interaction term (HR 1.34, 95% CI 1.11–1.62), but there was no observed difference in those with an outpatient index AF encounter (HR 0.90, 95% CI 0.76–1.08). The risk of non-cardiovascular hospitalization (HR 1.60, 95% CI 1.50– 1.70) and all-cause hospitalization (HR 1.48, 95% CI 1.40–1.56) was significantly greater with rhythm versus rate control in those with an inpatient index encounter. The risk of non-cardiovascular hospitalization was decreased (HR 0.85, 95% CI 0.80–0.91), and there was no difference in all-cause hospitalization (HR 0.99, 95% CI 0.94–1.04) with rhythm versus rate control in those with an outpatient index AF encounter.
Discussion
Most of the published randomized controlled studies assessing outcomes of different AF treatment strategies included primarily older patients (mean age >65 years).9–12 Little is known about outcomes associated with AF treatment in younger patients, but at least 1 sub-analysis indicated that outcomes may vary by patient age.12 With few evidence-based recommendations for treatment decisions in younger AF patients, clinicians must rely on data derived from older patients and their clinical judgment. In this study, we explored pharmacologic therapy initiated following the first identified AF event in patients aged <65 years to better understand its use and associated outcomes. Among patients with qualifying prescriptions, use of only rate-controlling drugs was much more common than use of antiarrhythmic drugs (84% versus 16%), and amiodarone was the most frequently used antiarrhythmic drug (37%). Electrical cardioversion and AF ablation procedures were rare during the initial AF event. Men, patients with AF events in later years, and patients with concomitant heart disease were more likely to initially receive a rhythm-control drug than only a rate-control approach. In addition, even after adjustment for baseline characteristics, patients who received an initial rhythm-control drug were more likely to have an AF, a cardiovascular, or an all-cause hospitalization than were patients receiving only rate-controlling drugs. These results provide new insight into the current management of younger AF patients.
There are no other published assessments of the initial use of rhythm- and rate-controlling therapies within clinical practice in patients aged <65 years. However, 1 study using prescription data from 1999–2008 found that of 3094 patients with AF at a mean age of 66 years, 13% were receiving an antiarrhythmic drug.13 The proportions of patients who were men and had ischemic heart disease and heart failure were similar to those in our study; however, our study included a larger proportion of patients with hypertension and diabetes. In a study conducted in Canada, 25% of AF patients between 1999 and 2007 received an initial rhythm-control drug, but all patients were aged >72 years.14 In 2 registry studies conducted completely or partly in the United States, 46% and 64%, respectively, of enrolled patients received an initial pharmacologic rhythm-control treatment, but the mean age of patients was 66 years.15,16 Other AF registries conducted primarily outside of the United States also included patients with a mean age of >66 years and tended to have a higher proportion of patients receiving rhythm-control due to the registry design.17–23,3
Patient age might be a factor in deciding whether to use antiarrhythmic drugs, as 2 registry studies found that the use of a rhythm-control strategy was more common in younger patients.18,19 However, it is not clear how much of that association is due to the increasing likelihood of comorbidities with increasing age. Ionescu-Ittu et al found that in a population of patients aged 72–85 years who were hospitalized with an initial AF event, increasing age was independently associated with lower odds of receiving pharmacologic rhythm-control versus rate-control treatment (OR 0.95, 95% CI 0.95–0.96).14 However, in our study of patients aged <65 years with an initial inpatient or outpatient AF encounter, age was not found to be independently and statistically significantly associated with the pharmacologic treatment group. In addition, unlike Ionescu-Ittu et al who found that hypertension, heart failure, and prior warfarin use were associated with use of only rate-controlling drugs, we found that these were associated with initial use of antiarrhythmic drugs. This may indicate differences in perceived risk of antiarrhythmic drugs in patients with these characteristics in an older, hospitalized population as opposed to a younger population with a mix of inpatients and outpatients.
In this study, we also explored hospitalizations following the initial AF encounter. It is important to acknowledge that a hospitalization for a primary diagnosis of AF does not necessarily mean that the therapy failed due to a recurrence of AF, worsening of AF symptoms, or an adverse event from the prescribed therapy. However, each hospitalization may represent a burden to the patient and to the health care system, regardless of the reason. In this study, we found a greater risk of hospitalization for a primary diagnosis of AF, cardiovascular disease, and any cause for patients categorized in the rhythm- versus rate-control group. There was no difference in risk of heart failure hospitalization or non-cardiovascular hospitalization. These results are similar to those from a meta-analysis of randomized controlled trials comparing pharmacologic rhythm- versus rate-control strategies, despite differences in study populations.4 In the meta-analysis, the pooled estimate for risk of all-cause rehospitalization was 1.49 (95% CI 1.11–2.00), which is greater than our estimate of effect for all-cause hospitalization (HR 1.11, 95% CI 1.07– 1.16). However, the data on hospitalizations varied widely among the included studies. Also, the authors cautioned that some of the hospitalizations may have been required by the study protocols and thus may not necessarily represent clinical practice.4 The Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation (RECORDAF) included patients with a mean age of 66 years and found no difference between the proportions of patients with a cardiovascular hospitalization at 1 year in the rhythm- versus rate-control groups (17%, p=0.9).19
There are several limitations to this study. First, because the MarketScan® database does not include death data unless the death occurred during a hospitalization, we were unable to assess differences in mortality between groups. Second, in our hospitalization analyses, we used inverse propensity-weighted estimators to adjust for potential treatment selection bias. However, this method is most effective when all factors associated with outcomes and treatment selections are included. It is possible that there were some important factors not captured in the MarketScan® database and, thus, were not available for our analysis. Despite this, this method appeared to provide good balance in the available variables (Appendix 2); however, these results should be replicated using other data sources. Third, in the rare instances in which drugs such as amiodarone were used as rate-controlling drugs, we would have misclassified their use as rhythm-control drugs. The prescriber’s intent for use of any of the rhythm or rate controlling drugs is not available in the claims data. Fourth, the study population included only those with commercial health insurance and thus these results may not be applicable to AF patient populations without commercial health insurance. Lastly, there is the possibility of miscoded diagnoses, cash payment for prescription medications, and gaps in coverage that may result in inaccuracies in the selected covariates or missing patients or events.
Conclusion
Among patients aged <65 years with an initial AF event receiving pharmacologic therapy, an antiarrhythmic drug was used much less frequently than treatment with only a rate-control drug. The initial pharmacologic approach remained consistent for 82% of patients started on an antiarrhythmic drug and for 88% of patients started on only a rate-control drug during the 1 year following the initial AF encounter. The risk of hospitalization was greater in those with an initial rhythm-control versus a rate-control approach, but this needs to be confirmed with other data sources and evaluated in context with other clinical outcomes.
Appendix 1. ICD-9 or CPT Codes for Identification of Variables Included in the Models
| Variables | Codes* |
|---|---|
| Atrial flutter | 427.32 |
| Ischemic heart disease | 410–414, 429.2, V45.81 |
| Diabetes | 250.x |
| Hypertension | Without LVH: 401.x, 403.xx, 404.00, 404.02, 404.10, 404.12, 404.90, 404.92, 405.xx, 437.2 With LVH: 402.00, 402.10, 402.90, any prior HTN code along with 429.3 |
| Heart failure | 428.xx, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 398.91 |
| Cardiomyopathy | 425.0, 425.1, 425.2, 425.3, 425.5, 425.7, 425.8, 425.9 |
| Chronic rheumatic heart disease | 393–398 |
| Acute rheumatic fever with heart involvement | 391.x, 392.0 |
| Other atrial arrhythmias | 427.0, 426.89 |
| Bradyarrhythmias | 427.81 |
| Pacemaker | 00.50, 00.52, 00.53, 37.71–37.79, 37.81–37.89 |
| Renal failure | Chronic: 403.01, 403.11, 403.91, 404.03, 404.12, 404.92, 404.13, 404.93, 404.93, 585.x, 586.x, 588.0, V42.0, V45.1, V56.x Acute: 584.0–584.9 |
| Liver disease | 70.32, 70.23, 70.32, 70.33, 70.44, 70.54, 70.6, 70.9, 456.0–456.2, 570.x, 571.x, 572.2–572.8, 573.3, 573.4, 573.8, 573.9, V42.7 |
| Thyroid disease | 240–246 |
| Pulmonary disease | 416.8, 416.9, 490.x–505.x, 506.4, 508.1, 508.8 |
| Cancer | 196.x–199.x, 200.x–202.x, 203.0, 238.6, 140.x–172.x, 174.x–195.x |
| Stroke | 433–435 |
| Cerebral hemorrhage | 431–432 |
| Depression | 296.2, 296.3, 296.5, 300.4, 309.x, 311 |
| Obesity | 278.0 |
| Non-rheumatic valvular heart disease | 424.xx |
| Cardiothoracic surgery | 35.x–39.x >30 days from index AF encounter date |
| Electrical cardioversion | ICD-9 codes 99.61, 99.62, and 99.60 CPT codes 00410, 92960, and 92961 |
| AF ablation | ICD-9-CM 37.33, 37.34 CPT 93651 |
| Bleeding | ICD-9 codes 528.0–528.9, 530.7, 531.0, 531.2, 531.4, 531.6, 532.0, 532.2, 532.4, 532.6, 533.0, 533.2, 533.4, 533.6, 534.0, 534.2, 534.4, 534.6, 362.8, 379.2, 441.0, 441.1, 441.3, 161.7, 599.7, 786.3, 784.7, 431.0–432.9 |
AF indicates atrial fibrillation; CPT, Current Procedural Terminology; HTN, hypertension; ICD-9, International Classification of Diseases, Ninth Revision, Clinical Modification; LVH, left ventricular hypertrophy.
All are ICD-9 codes, except where indicated as CPT.
Drugs Included in the Analysis
| Drug Category | Drugs | |
|---|---|---|
| Rhythm-control drugs | Amiodarone Disopyramide Dofetilide Dronedarone Flecainide |
Procainamide Propafenone Quinidine Sotalol |
| Beta-blockers | Acebutolol Atenolol Betaxolol Bisoprolol Carteolol Carvedilol Labetalol |
Metoprolol Nadolol Nebivolol Penbutolol Propranolol Timolol |
| Digitalis glycosidesq | Digitalis Digitoxin Digoxin |
|
| Calcium channel blockers | Diltiazem Verapamil |
|
| Definite QT-prolonging drugs | Azithromycin Bepridil Chloroquine Chlorpromazine Citalopram Clarithromycin Droperidol Erythromycin |
Halofantrine Haloperidol Mesoridazine Moxifloxacin Pentamidine Pimozide Thioridazine Vandetanib |
| Possible QT-prolonging drugs | Amantadine Amitriptyline Atazanavir Chloral Hydrate Ciprofloxacin Clomipramine Clozapine Desipramine Diphenhydramine Dolasetrone Doxepin Escitalpram Famotidine Felbamate Fingolimod Fluconazole Fluoxetine Foscarnet Fosphenytoin Galantamine Iloperidone Imipramine Indapamide Isradipine Itraconazole Ketoconazole Lapatinib |
Levofloxacin Lithium Moexipril Nicardipine Nilotinib Nortriptyline Octeotide Ofloxacin Ondansetron Oxytocin Paroxetine Protriptyline Quetiapine Risperidone Ritonavir Sertraline Sulfamethoxazole/trimethroprim Sunitinib Tacrolimus Telithromycin Tizanidine Trazodone Trimipramine Vardenafil Venlafaxine Voriconazole Ziprasidone |
| Anticoagulants | Warfarin Dabigatran |
Appendix 2: Balance of Categorical Variables after IPW Adjustment
Open circles represent values before IPW adjustment, and closed circles represent values after IPW adjustment.
AF indicates atrial fibrillation; DCC, direct current cardioversion; poss, possible; def, definite; DX, diagnosis; Dz, disease; CV, cardiovascular; IHD, ischemic heart disease; IPW, inverse propensity weighted; Hx, history; arrhy, arrhythmia; RF, rheumatic fever; RHD, rheumatic heart disease; rheum, rheumatic.
Footnotes
Disclosures:
NM Allen LaPointe, GD Sanders, ED Peterson, and SM Al-Khatib: All disclosures are listed at https://www.dcri.org/about-us/conflict-of-interest
Y. Lokhnygina and J Rimmler: will provide disclosures upon revision.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CW American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Sardar P, Lichstein E, Mukherjee D, Aikat S. Pharmacologic rate versus rhythm-control strategies in atrial fibrillation: an updated comprehensive review and meta-analysis. Pacing Clin Electrophysiol. 2013;36:122–133. doi: 10.1111/j.1540-8159.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Allen Lapointe N, Chatterjee R, Crowley MJ, Dupre ME, Kong DF, Lopes RD, Povsic TJ, Raju SS, Shah BR, Kosinski A, McBroom AJ, Chobot MM, Gray R, Sanders GD. Comparative Effectiveness Review #119. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Treatment of Atrial Fibrillation. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-2007-10066-I). AHRQ Publication No.13-EHC095-EF. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 6.Naccarelli GV, Johnston SS, Dalal M, Lin J, Patel PP. Rates and implications for hospitalization of patients ≥65 years of age with atrial fibrillation/flutter. Am J Cardiol. 2012;109:543–549. doi: 10.1016/j.amjcard.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds MR, Gunnarsson CL, Hunter TD, Ladapo JA, March JL, Zhang M, Hao SC. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- 8.Truven Health Analytics website. [Accessed July 11, 2013];Databases and Online Tools. http://www.truvenhealth.com/your_healthcare_focus/pharmaceutical_and_medical_device/data_databases_and_online_tools.aspx.
- 9.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U STAF Investigators. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 12.Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV, Bosker HA, Kamp O, Tijssen JG, Veeger NJ, Van Gelder IC RAte Control versus Electrical cardioversion for persistent atrial fibrillation study group. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–1111. doi: 10.1016/j.ahj.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Kashyap A, Li C. Trends in utilization of management strategies for newly diagnosed atrial fibrillation patients in the United States: 1999 to 2008. J Pharm Pract. 2012;25:151–159. doi: 10.1177/0897190011424803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, Essebag V, Eisenberg MJ, Wynant W, Richard H, Pilote L. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172:997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 15.Reiffel JA, Kowey PR, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, Reiter MJ, Waldo AL AFFECTS Scientific Advisory Committee and Investigators. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry) Am J Cardiol. 2010;105:1122–1129. doi: 10.1016/j.amjcard.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Zimetbaum P, Ho KK, Olshansky B, Hadjis T, Lemery R, Friedman PA, Cannom DS, Chen XH, Josephson ME FRACTAL Investigators. Variation in the utilization of antiarrhythmic drugs in patients with new-onset atrial fibrillation. Am J Cardiol. 2003;91:81–83. doi: 10.1016/s0002-9149(02)03004-7. [DOI] [PubMed] [Google Scholar]
- 17.Alam M, Bandeali SJ, Shahzad SA, Lakkis N. Real-life global survey evaluating patients with atrial fibrillation (REALISE-AF): results of an international observational registry. Expert Rev Cardiovasc Ther. 2012;10:283–291. doi: 10.1586/erc.12.8. [DOI] [PubMed] [Google Scholar]
- 18.Andrade JG, Connolly SJ, Dorian P, Green M, Humphries KH, Klein GJ, Sheldon R, Talajic M, Kerr CR. Antiarrhythmic use from 1991 to 2007: insights from the Canadian Registry of Atrial Fibrillation (CARAF I and II) Heart Rhythm. 2010;7:1171–1177. doi: 10.1016/j.hrthm.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey JY, Merioua I, Pedrazzini L, Prystowsky EN, Schwartz PJ, Torp-Pedersen C, Weintraub W. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation) J Am Coll Cardiol. 2011;58:493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O’Neill J, Silva-Cardoso J, Zharinov O, Gamra H, Alam S, Ponikowski P, Lewalter T, Rosenqvist M, Steg PG. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophsiol. 2012;5:632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 21.Meiltz A, Zimmermann M, Urban P, Bloch A Association of Cardiologists of the Canton of Geneva. Atrial fibrillation management by practice cardiologists: a prospective survey on the adherence to guidelines in the real world. Europace. 2008;10:674–680. doi: 10.1093/europace/eun086. [DOI] [PubMed] [Google Scholar]
- 22.Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, Goette A, Lewalter T, Ravens U, Meinertz T, Breithardt G, Steinbeck G. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steg PG, Alam S, Chiang CE, Gamra H, Goethals M, Inoue H, Krapf L, Lewalter T, Merioua I, Murin J, Naditch-Brûlé L, Ponikowski P, Rosenqvist M, Silva-Cardoso J, Zharinov O, Brette S, Neill JO RealiseAF investigators. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98:195–201. doi: 10.1136/heartjnl-2011-300550. [DOI] [PubMed] [Google Scholar]