Abstract
The cellular requirements of Dicer, an essential miRNA processing enzyme, and the consequences of altered levels of it on tumorigenesis are incompletely understood. We review the effects of Dicer loss in cells of different origin and whether loss of p53 permits cell survival and growth in the absence of Dicer.
Keywords: Dicer, p53, tumorigenesis, lymphoma
Review
microRNA (miRNA) are small non-coding RNA that post-transcriptionally regulate gene expression. miRNA are linked to biological processes associated with cancer initiation and progression.1 In tumorigenesis, the requirements for miRNA and the enzymes that process them remain unresolved. While tumor development may be facilitated by the gain or loss of specific miRNA, an overall reduction in miRNA has been reported in human malignancies.1 Reduced activity or expression of Dicer, an enzyme crucial for miRNA biogenesis, causes downregulation of miRNA in some cancers.1 These observations led to the hypothesis that reduced Dicer levels facilitate tumorigenesis.
In support of this concept, Dicer was reported to function as a haploinsufficient tumor suppressor in mouse models of soft-tissue sarcoma, lung adenocarcinoma, and retinoblastoma.2-4 Contrastly, Dicer hypomorphic mice, expressing 20% of normal Dicer levels, did not have an increased cancer incidence.5 Moreover, we determined Dicer did not function as a haploinsufficient tumor suppressor in B-cell lymphoma, as loss of one Dicer allele did not affect the rate of B-cell lymphomagenesis, regardless of the p53 tumor suppressor status. Additionally, Dicer heterozygosity did not alter Dicer protein levels or mature miRNA production in B-cell lymphomas, indicating one Dicer allele was sufficient for normal Dicer function in B-cells.6, 7 Therefore, reduced levels of Dicer have differential effects in different tissues in transformation.
There is mounting evidence Dicer and thus, miRNA are required for tumorigenesis. Although it was initially reported that Dicer deletion in the lung led to tumor development, it was later determined the lung cancer cells in these mice had retained one Dicer allele.2 Additionally, attempts at homozygous Dicer deletion in other mouse models, including data we generated with a Myc oncogene-driven B-cell lymphoma model, have revealed selection against complete Dicer ablation in cancers.2, 3, 6, 7 We showed Myc-induced lymphomas are delayed in Cre-expressing B cells in Dicer conditional knockout mice due to B-cell apoptosis, and none of the lymphomas that developed had deleted both Dicer alleles. Biallelic deletion of Dicer in established lymphomas also resulted in apoptosis. Screening of 1,303 lymphoma clones that survived Dicer deletion over two studies revealed all had retained at least one Dicer allele.6, 7 In addition, homozygous deletion of Dicer in sarcoma cells was possible, but these cells had impaired proliferation.4 Thus, a certain level of Dicer expression appears to be necessary for tumor cell development, survival, and growth.
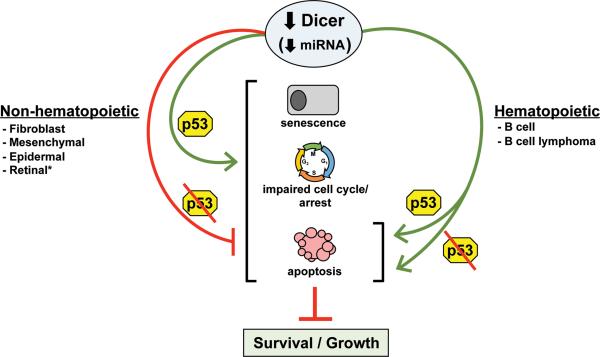
Inactivation of the p53 pathway is a prerequisite to cancer, as this allows cells to evade apoptotic and growth arrest signals. Loss of miRNA biogenesis can lead to cellular stress and activation of p53 (Fig. 1). Dicer ablation in untransformed murine fibroblasts or epidermis led to p53 activation and premature senescence or apoptosis, respectively.8, 9 We detected an elevated frequency of p53 inactivation in B-cell lymphomas upon Dicer deletion, indicating an increase in p53 activation during transformation.7 Deleting p53 or Arf (tumor suppressor and regulator of p53) delayed the premature senescence in fibroblasts,9 and loss of p53 inhibited the apoptosis in epidermal cells8 (Fig. 1). Additionally, a small fraction of p53-null, mutant K-Ras expressing sarcoma cells and SV40-immortalized (p53 and Rb inactivated) mesenchymal stem cells survived Dicer ablation4 (Fig. 1). These data suggested p53 inactivation may extend or be required for cell survival when Dicer is deleted. However, a p53 deficiency did not rescue B-cell apoptosis caused by Dicer deletion (Fig. 1), and did not prevent the resultant delay in lymphomagenesis. p53 deletion was also insufficient to allow established B-cell lymphomas to survive Dicer deletion in vitro or in vivo.6 Arf deletion also did not confer a survival advantage to B-cell lymphomas that had deleted Dicer. Furthermore, B-cell lymphomas frequently overexpress the Bcl-2 anti-apoptotic protein, which may have partially protected developing B cells, but not lymphomas from Dicer deletion-induced apoptosis.6, 10 Additionally, synthetic lethality occurred when p53 was inactivated in Dicer and Rb deficient retinal cells.1 We observed a p53 deficiency did restore development of the characteristic B-cell lymphoma in our mouse model in comparison to the early precursor B-cell lymphoma that arose in 40% of the lymphomas from mice born with two p53 alleles.6, 7 Together, the data reveal tissue-specific requirements for Dicer and that p53 pathway inactivation is unlikely to rescue or may only delay the effects of Dicer deletion. Moreover, in contrast to non-hematopoietic cells, miRNA appear to have an irreplaceable function in B-cell survival, regardless of cellular transformation or p53 status.
Figure 1. p53 inactivation protects some cells from the negative consequences of Dicer deletion.
The p53 tumor suppressor responds to impaired miRNA processing by inducing senescence, cell cycle arrest, or apoptosis. However, non-hematopoietic cells that harbor inactivated p53 can survive and grow (possibly more slowly) in the absence of Dicer. However, hematopoietic cells are extremely sensitive to Dicer loss and rapidly undergo apoptosis, regardless of p53 status. *If the Rb pathway is co-inactivated with p53, there is synthetic lethality.
Hematopoietic cells preferentially undergo apoptosis, whereas non-hematopoietic cells favor senesce when stressful situations, such as impaired miRNA processing, are encountered (Fig. 1). We demonstrated that inactivation of the p53 pathway was neither protective against nor cooperated with Dicer loss during B-cell lymphomagenesis.6, 7 Although it remains to be determined whether specific genetic alterations would permit B-cell lymphomas to survive without Dicer, our data establish Dicer as a possible therapeutic target for the treatment of B-cell lymphomas, and likely other hematopoietic malignancies. A concern for moving forward on such a strategy may be that Dicer appears to function as a haploinsufficient tumor suppressor for lung, muscle, and retina, but this has only been shown in the context of p53 inactivation and after prolonged Dicer deficiency.2-4 B-cell lymphomas were profoundly sensitive to loss of Dicer and rapidly underwent apoptosis,6, 7 suggesting short-term Dicer inactivation results in B-cell lymphoma death. Moreover, standard DNA damage-inducing chemotherapeutics can result in cancer development and thus, transient Dicer inactivation is unlikely to be more tumor causing.
Acknowledgments
Funding
Our studies on Dicer were supported by F31CA165728 (CMA) and R01CA148950 (CME).
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- 1.Jansson MD, Lund AH. MicroRNA and cancer. Molecular oncology. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, Kirsch DG, Golub TR, Jacks T. Dicer1 functions as a haploinsufficient tumor suppressor. Genes & development. 2009;23:2700–4. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, Marine JC. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell death and differentiation. 2010;17:633–41. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi A, Gurtan AM, Kumar MS, Bhutkar A, Chin C, Lu V, Lees JA, Jacks T, Sharp PA. Proliferation and tumorigenesis of a murine sarcoma cell line in the absence of DICER1. Cancer cell. 2012;21:848–55. doi: 10.1016/j.ccr.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita S, Hara A, Kojima I, Horii T, Kimura M, Kitamura T, Ochiya T, Nakanishi K, Matoba R, Matsubara K, et al. Dicer is required for maintaining adult pancreas. PloS one. 2009;4:e4212. doi: 10.1371/journal.pone.0004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams CM, Eischen CM. Inactivation of p53 Is Insufficient to Allow B Cells and B-Cell Lymphomas to Survive Without Dicer. Cancer research. 2014;74:3923–34. doi: 10.1158/0008-5472.CAN-13-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arrate MP, Vincent T, Odvody J, Kar R, Jones SN, Eischen CM. MicroRNA biogenesis is required for Myc-induced B-cell lymphoma development and survival. Cancer research. 2010;70:6083–92. doi: 10.1158/0008-5472.CAN-09-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyle S, Hoover K, Colpan C, Zhu Z, Matijasevic Z, Jones SN. Dicer Cooperates with p53 to Suppress DNA Damage and Skin Carcinogenesis in Mice. PloS one. 2014;9:e100920. doi: 10.1371/journal.pone.0100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mudhasani R, Zhu Z, Hutvagner G, Eischen CM, Lyle S, Hall LL, Lawrence JB, Imbalzano AN, Jones SN. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. The Journal of cell biology. 2008;181:1055–63. doi: 10.1083/jcb.200802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–74. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]