Abstract
Neuroimaging studies have found that adult male psychopaths show reduced engagement of limbic and paralimbic circuitry while making moral judgments. The goal of this study was to investigate whether these findings extend to adolescent males with psychopathic traits. Functional MRI was used to record hemodynamic activity in 111 incarcerated male adolescents while they viewed unpleasant pictures that did or did not depict moral transgressions and rated each on “moral violation severity”. Adolescents were assessed for psychopathic traits using the Psychopathy Checklist-Youth Version (PCL-YV), Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (KSADS-PL) Conduct Disorder supplement, and Inventory of Callous and Unemotional Traits–Youth version (ICU-Y). While viewing pictures depicting moral transgressions, CD scores were negatively correlated with hemodynamic responses in the anterior temporal cortex. Adolescents scoring low on the ICU-Y showed a positive correlation between right amygdala responses and severity of violation ratings; those with high ICU-Y scores showed a negative correlation. While viewing unpleasant pictures with and without moral transgressions, PCL-YV scores were negatively correlated with hemodynamic responses in the left amygdala. Overall, the results are consistent with those previously found in adult male psychopaths, but vary depending on the type of psychopathy assessment.
Keywords: moral, adolescent, psychopathy, fMRI, amygdala
1. Introduction
Antisocial behaviors are influenced by several factors that peak during adolescence including peer pressure, impulsivity, and poor emotion regulation. Antisocial behaviors do not usually persist beyond adolescence (Moffit, 1993). However, some adolescents continue to engage in frequent antisocial behavior throughout adulthood. Life-course persistent antisocial behavior is a central characteristic of individuals who meet diagnostic criteria for psychopathy. Psychopathy is a disorder characterized by interpersonal, affective and behavioral traits such as grandiosity, callousness, and irresponsibility (Hare, 2003). Psychopaths are known for diminished “moral emotions” such as guilt and empathy, which contribute to rule-breaking and harming others.
The diminished moral emotions that accompany psychopathy may have an early-emerging neurobiological basis. Blair (2007) proposed a neurodevelopmental account of psychopathy in which early-onset dysfunction within ventromedial prefrontal cortex (vmPFC) and amygdala contributes to impaired moral socialization. The proposal is based on the importance of stimulus-reinforcement associations in moral socialization (learning that certain behaviors are harmful to others and should be avoided) and the role of amygdala and vmPFC in these processes - the former in valence representation (“good” /“bad”), the latter in outcome expectancy. In other words, dysfunction within these regions makes psychopaths less sensitive to aversive consequences of moral transgressions and less likely to avoid committing them.
Neuroimaging studies of adult psychopathy have reported findings consistent with this hypothesis. Harenski et al. (2010) used fMRI to compare brain responses in incarcerated psychopaths and nonpsychopaths while they viewed pictures that did or did not depict moral transgressions (e.g. a hand breaking into a house vs. an injured hand) and rated their degree of “moral violation severity”. While psychopaths and nonpsychopaths gave similar severity ratings to moral and non-moral pictures, nonpsychopaths showed greater hemodynamic responses compared to psychopaths when viewing moral relative to non-moral pictures in the vmPFC and a lateral subregion of the anterior temporal cortex posterior to the temporal pole (ATC). Nonpsychopaths also showed a positive correlation between amygdala response and severity ratings that was absent in psychopaths, suggesting that moral judgments were associated with emotional responses in nonpsychopaths but not psychopaths. Other studies have reported negative associations between psychopathy and hemodynamic responses in the amygdala, mPFC, and additional regions including the posterior cingulate (PC), during the evaluation of moral dilemmas (Glenn et al., 2009; Pujol et al., 2012). The amygdala, mPFC, PC, and ATC have been implicated in non-clinical studies of moral judgment (Greene & Haidt, 2002; Moll et al., 2005; Raine & Yang, 2006).
The goal of this study was to investigate whether aberrant brain responses associated with moral judgment in adult psychopathy extend to adolescents with psychopathic traits. Neuroimaging studies have found that adolescents with psychopathic traits show reduced hemodynamic responses in limbic regions, particularly the amygdala, when processing emotional stimuli such as unpleasant pictures and fearful facial expressions (Marsh et al., 2008; Sterzer et al., 2005). Given the integral role of emotion in moral judgment, it is reasonable to expect that reduced limbic responses in adolescents with psychopathic traits might occur during the evaluation of moral transgressions. We used fMRI to scan a large sample of incarcerated adolescents with varying levels of psychopathic traits while they completed the moral judgment task used in our study of adult psychopaths (Harenski et al., 2010). Based on our prior findings in adult psychopaths, we predicted that psychopathic traits would be negatively correlated with hemodynamic responses in the vmPFC and ATC during the evaluation of pictures depicting moral transgressions, and that hemodynamic responses in the amygdala would be positively correlated with moral violation severity ratings in adolescents scoring low vs. high on psychopathic traits. We also predicted that psychopathic traits would be negatively correlated with amygdala responses when viewing unpleasant pictures regardless of moral content, consistent with the prior literature. The moral picture task has been shown in studies of non-clinical populations to engage brain regions implicated in psychopathy, including mPFC, amygdala, and ATC (Harenski et al., 2006; 2008; 2010); thus provides a robust test of psychopathy-related brain dysfunction during moral judgment.
To be consistent with the measurement of psychopathy in our adult study (Harenski et al., 2010), which used the Psychopathy Checklist-Revised (PCL-R; Hare, 2003), we used the Psychopathy Checklist-Youth Version (PCL-YV; Forth et al., 2003) as our primary measure of psychopathic traits. While the PCL-R is the “gold standard” for psychopathy assessment in adult populations, the PCL-YV is one of several instruments used to assess psychopathic traits in youth. Other instruments assess Conduct Disorder (CD), the closest approximation of youth psychopathy in the Diagnostic and Statistical Manual for Mental Disorders (4th ed.; American Psychiatric Association, 1994). CD diagnostic criteria have been criticized for emphasizing disruptive behaviors over the affective and interpersonal traits. Recently developed instruments focus on callous-unemotional (CU) traits, which are associated with worse conduct problems in youth (Edens et al., 2008; Wootton et al., 1997), conduct problems that are less related to environmental factors (Viding et al. 2005; Viding & Jones, 2008) and exhibit a more stable pattern over time (Frick et al. 2003, 2005). The importance of evaluating CU traits in youth with conduct problems was demonstrated in a neuroimaging study which reported suppressor effects of CD symptoms and CU traits during an affective task (Sebastian et al., 2012). CD symptoms were positively correlated and CU traits negatively correlated with hemodynamic responses in the amygdala. These results suggest that youth with CD but without CU traits show exaggerated responses to emotional stimuli whereas those with CD and CU traits show the opposite pattern.
Given the emphasis on different psychopathic traits across instruments, divergent brain responses associated with the instruments, and research showing poor convergent validity among the instruments (Fink et al., 2012), to ensure comprehensive measurement of psychopathic traits we included measures of CD symptoms and CU traits in addition to the PCL-YV. This allowed us to examine whether our prior findings in adult psychopaths using the PCL-R would extend to adolescent psychopathic traits measured with the PCL-YV, and whether brain responses associated with moral judgment would show unique associations with specific psychopathic traits (e.g., CD vs. CU).
2. Method
2. 1. Participants
The study included 119 adolescent males recruited from a maximum-security youth detention facility who met study inclusion criteria. Participants were excluded for any of the following: reading level below 4th grade (n = 7), IQ below 75 (n = 12), current DSM-IV Axis I diagnosis other than Conduct Disorder (n = 22) or lifetime history of a psychotic disorder (n = 1). Of participants excluded for DSM-IV diagnosis, most had a diagnosis of current substance abuse or dependence and admitted to using alcohol or drugs in the past 30 days (n = 10). Other diagnoses included post-traumatic stress disorder (n = 3), attention deficit hyperactivity disorder (n = 6), major depression (n = 1) or other anxiety disorder (n = 2). Participants were incarcerated for crimes that included murder, assault, rape, arson, weapons possession, burglary, fraud, drug possession/distribution, and criminal mischief. Apart from the participants excluded for current substance abuse or dependence, none of the participants used cigarettes, alcohol, or illegal drugs, which were prohibited at the facility.
Eight participants were excluded from analysis due to poor task performance (e.g., drowsiness during scanning observed with real-time eye tracking, n = 4) or the participants' fMRI data showed signal dropout in the vmPFC (n = 4). Signal dropout extending at least halfway into the vmPFC region of interest (i.e., z </= -17) was used as the threshold for exclusion. The resulting final sample included 111 incarcerated adolescents. Demographic characteristics are provided in Table 1. The study was conducted in accordance with institutional ethical standards. All participants provided written informed consent (>=18 years of age) or written informed assent and parent/guardian written informed consent (<18 years of age).
Table 1.
Descriptive statistics for analyzed sample.
| Correlation with Psychopathic Traits (r) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | M(SD) | Range | PCL-YV Total | PCL-YV Factor 1 | PCL-YV Factor2 | CD | ICU | |
| Age | 111 | 17.2(1.07) | 14-19 | 0.02 | -0.01 | -0.02 | -0.11 | -0.04 |
| IQ Substance | 99 | 94.8 (10.89) | 77-129 | 0.03 | 0.08 | -0.05 | 0.14 | -0.06 |
| Dependence diagnoses | 108 | 2.1 (1.48) | 0-8 | 0.24* | 0.16 | 0.25* | 0.37** | 0.16 |
| PCL-YV Total | 111 | 23.7 (6.38) | 2-35 | --- | 0.82** | 0.84** | 0.38** | 0.37** |
| PCI-YV Factor 1 | 111 | 6.8 (3.22) | 1-15 | --- | 0.54** | 0.22* | 0.26* | |
| PCL-YV Factor 2 | 111 | 14.8(3.41) | 1-20 | --- | 0.53** | 0.39** | ||
| CD | 103 | 39.2 (4.54) | 23-18 | --- | 0.44** | |||
| ICUY | 101 | 28.4 (8.89) | 11-56 | --- | ||||
p < .05;
p < .0001
2.2. Assessment measures
2.2.1. The Psychopathy Checklist-Youth Version (PCL-YV)
The PCL-YV (Forth et al., 2003) includes a review of institutional records and a semi-structured interview regarding school, family, work, and criminal histories, and interpersonal/emotional skills. Individuals are scored on 20 items that measure traits and behaviors characteristic of psychopathy. Scores range from 0 to 40. We also examined the two factor model of the PCL-YV (Forth et al., 2003) with Factor 1 composed of interpersonal and affective traits and Factor 2 composed of lifestyle and antisocial traits.
For adults evaluated with the PCL-R, the typical diagnostic cutoff for psychopathy is 30. Twenty five of the 111 participants scored 30 or higher on the PCL-YV. However, we did not use this to identify “adolescent psychopaths”. When assessing adolescents it is more appropriate to refer to them as “high in psychopathic traits”, or “at-risk” for developing psychopathic personality, than to label them as psychopaths due to developmental issues (Seagrave & Grisso, 2002; Vincent, 2006). We focused on dimensional analyses using continuous PCL-YV scores. However, to enable comparisons of the results to previous studies in adults, a supplemental analysis comparing the 25 highest PCL-YV scorers (30 and higher) and 25 lowest PCL-YV scorers (19 and lower) can be found in Tables S1-S2.
PCL-YV interviews were conducted by trained researchers and videotaped for reliability assessment (12% of randomly selected interviews were scored by a second independent rater). Reliability results (intraclass correlation coefficient = 0.90) were consistent with those reported in the PCL-YV manual (Forth et al., 2003).
2.2.2. Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (KSADS-PL)
The KSADS-PL involves a semi-structured interview used to evaluate psychiatric symptoms in youth. Current and past episodes of psychopathology are assessed based on DSM-IV criteria. Questions are designed to be open-ended and used as guidelines. As reported in Kaufman et al. (1997), the inter-rater reliability for diagnostic items is high (range: 93% - 100%), as is test-retest reliability for major depressive and bipolar disorders, generalized anxiety disorder, conduct disorder, and oppositional defiant disorder (r =.77-1.00). Test-retest reliability for attention-deficit-hyperactivity disorder and post-traumatic stress disorder is good (r = .63-.67). Trained research staff conducted the abbreviated research version of the KSADS-PL, which requires an interview with only the participant. Research staff were trained by a clinical psychologist for one to two months prior to the study.
In addition to evaluating the presence or absence of DSM-IV disorders, the KSADS-PL was used to examine CD symptom severity. Each of 18 symptoms (e.g., physical cruelty to persons, truancy) are scored from 1-3 (1 = not present 2 = subthreshold, 3 = threshold), for a maximum total of 54. The CD supplement was not used for six participants who did not meet CD screening criteria, and two participants did not complete the KSADS-PL. These participants were not included in CD analyses. The KSADS-PL was also used to evaluate past alcohol and drug use severity, by counting the total number of substances for which a participant met lifetime dependence criteria.
2.2.3. Inventory of Callous and Unemotional Traits - Youth Version
CU traits were assessed using the Inventory of Callous and Unemotional Traits - Youth Version (ICU-Y; Frick, 2003). The ICU-Y is a 24-item self-report measure developed from the Callous-Unemotional scale of the Antisocial Process Screening Device (APSD; Frick & Hare, 2001). Each item (e.g., “I do not care who I hurt to get what I want”) is scored from 0 (not at all true) to 3 (definitely true). The scale has shown acceptable internal consistency (coefficient alpha = .77; Essau et al., 2006). The validity of the self-report ICU-Y is comparable to other-report (e.g., teacher) versions (Roose et al., 2010. Ten participants did not complete the ICU-Y.
2.2.4. Wechsler Intelligence Scale (WAIS; WISC)
IQ was estimated from the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (Wechsler, 1997; Ryan et al., 1999) for participants 16 and older and from the Wechsler Intelligence Scale for Children-Fourth Edition (Wechsler, 2003; Sattler & Dumont, 2004) for participants younger than 16.
2.3. Stimuli and Task
Three picture sets (25 moral, 25 non-moral, 25 neutral) were selected primarily from the International Affective Picture System (Lang et al., 1995) and supplemented with pictures from media sources1. Moral pictures depicted unpleasant social scenes indicating a moral transgression (e.g., a drunk driver). Non-moral pictures depicted unpleasant social scenes without moral content (e.g., an angry driver). Neutral pictures depicted social scenes without moral content (e.g., a normal driver). Moral and non-moral pictures were selected based on ratings from a pilot study in which participants rated 100 unpleasant pictures on severity of moral violation. Pictures which were rated highest and lowest on moral violation severity were assigned to the moral and non-moral condition, respectively. Ratings of emotional arousal and social complexity were also obtained to ensure pictures were matched on these variables. The selected moral pictures have consistently been rated significantly higher on moral violation severity than non-moral pictures in adult females (Harenski et al., 2006), adult males (Harenski et al., 2008), and adolescent males and females (Harenski et al., 2012).
Participants were instructed to rate the severity of moral violation in each picture from 1-5, with 5 representing high violation severity and 1 representing no violation. They were encouraged to make ratings based on their own moral values, not what others or society would think was a moral violation. All participants completed five practice trials to ensure they understood how to perform the task. Pictures were displayed for six seconds, followed by a four-second rating scale in which a moving red bar progressed from 1 (none) to 5 (high). The participant pressed a button to stop the bar when it reached their desired rating. This format was chosen for simplicity (pressing one rather than several buttons). Participants with more than 5 (out of 75) missed ratings were excluded from analysis (n = 4). Following each rating, a four-second rest period occurred before the next trial.
Moral, non-moral, and neutral trials were randomized along with 25 “jitter” fixation trials (10 seconds each) randomly interspersed between picture trials. The 100 total trials (25 moral, 25 non-moral, 25 neutral, and 25 fixation) were presented across two runs. Trial order was fixed across participants. Responses were recorded using a 4-button box.
2.4. MRI Data Acquisition and Analysis
All participants were scanned at a maximum-security youth detention facility using The Mind Research Network's mobile MRI scanner. The mobile 1.5T Avanto MRI system is equipped with advanced SQ gradients (max slew rate 200T/m/s; 346T/m/s vector summation, rise time 200us) and a 12-element head coil. The EPI gradient-echo pulse sequence (TR/TE: 2000/39 ms, flip angle: 90°, FOV: 24×24 cm, 64×64 matrix, voxel size: 3.4×3.4×4mm (1mm gap), 30 slices) covered the entire brain (150mm) in 2 seconds. Head motion was restricted by a custom apparatus that interfaced with the head coil.
To correct residual head motion, “bad” images (confounded by motion or radio-frequency spikes) were estimated and removed using ART-Repair (Mazaika et al., 2009). The offending images were determined by calculating the mean intensity for a given time series. Any individual images whose intensity was greater than four standard deviations were replaced in the time series by a rolling mean image. The mean number of images removed across participants was 15.3 (of 712). Regression vectors were included in the General Linear Model (GLM) to remove the offending images from the analyses. Realignment parameters (3 translation; 3 rotations) were included as covariates of no interest in the GLM.
Imaging data were analyzed using Statistical Parametric Mapping software (SPM5). Functional images were spatially normalized to the MNI template and spatially smoothed (8 mm FWHM). The events of interest, picture viewing (moral, non-moral, neutral) were modeled with a six second hemodynamic response function. The rating period, collapsed across all picture presentations, was also modeled as an event with a four second hemodynamic response function. The moral > non-moral picture comparison evaluated hemodynamic responses associated with moral salience while controlling for social and emotional content. The moral > neutral and non-moral > neutral picture comparisons evaluated hemodynamic responses to general affective stimuli while controlling for social content. Participants' “severity of moral violation” ratings of each picture were included as covariates of no interest to model variance associated with individual differences in ratings.
PCL-YV scores were entered in a regression with individual moral > non-moral, moral > neutral, and non-moral > neutral images. A separate regression with these contrast images included PCL-YV Factor 1 and 2 scores. A third separate regression with these contrast images included ICU-Y and CD scores. The latter two regressions examined correlations between hemodynamic response and affective and interpersonal traits (Factor 1/ICU-Y) while controlling for disruptive and antisocial behavior (Factor 2/CD), and vice versa. Age and IQ were not correlated with PCL-YV, Factor 1, Factor 2, CD, or ICU-Y scores (Table 1). Number of substance dependence diagnoses was positively correlated with PCL-YV total, Factor 2, and CD scores. Thus, all three regressions included substance dependence as a covariate.
We also analyzed hemodynamic responses associated with “severity of moral violation” ratings of pictures depicting moral transgressions using parametric modulation analysis in SPM5. Participants' ratings of each picture were analyzed as covariates of interest for hemodynamic responses during picture viewing. Functional images were computed for each participant which revealed brain regions whose hemodynamic responses during picture viewing were associated with higher (positive correlation) or lower (negative correlation) severity ratings. Median split was used to create lower and higher scoring groups for PCL-YV, Factor 1, Factor 2, CD, and ICU-Y scores (Table 2). This enabled us to test the hypothesis that participants scoring lower vs. higher on psychopathic traits would show a positive correlation between amygdala response and severity ratings. One-sample t-tests were first conducted on the parametrically modulated images within each group, followed by between-group ANOVA to identify differential correlations across groups. Analyses with PCL-YV, Factor 2, and CD scores included substance dependence as a covariate.
Table 2.
Descriptive statistics for median split groups.
| M (SD) | Range | |
|---|---|---|
| PCL-YV High | 27.5 (2.35) | 23-35 |
| PCL-YV Low | 17.9 (4.21) | 2-23 |
| Factor 1 High | 9.6 (1.80) | 7-12 |
| Factor 1 Low | 4.2 (1.57) | 0-6 |
| Factor 2 High | 17.4 (1.35) | 15-20 |
| Factor 2 Low | 12.3 (3.00) | 1-15 |
| CD High | 42.2 (1.94) | 4048 |
| CD Low | 35.5 (3.38) | 23-40 |
| ICU-Y High | 35.6 (5.13) | 29-56 |
| ICU-Y Low | 21.2 (5.42) | 11-28 |
Analyses were performed on a voxel-by-voxel basis over the entire brain using the general linear model in SPM5. Monte Carlo simulation (Ward, 2000) determined that a 20 voxel extent at a height threshold of p <.001 yielded a family-wise error corrected threshold of p <.05. In addition to whole brain analysis, hypotheses were tested in a priori regions of interest (right anterior temporal cortex, ventromedial prefrontal cortex, amygdala). Peak coordinates for these regions were drawn from our adult study (regions showing differences in psychopaths vs. nonpsychopaths; Harenski et al., 2010). This study found psychopathy-related differences only in right amygdala, in contrast to other functional imaging studies showing psychopathy-related results in right and left amygdala (e.g., Kiehl et al., 2001; Birbaumer et al., 2005; Glenn et al., 2009). Given the theoretical significance of the amygdala in psychopathy we felt it was important to examine the left and right amygdala in the current study. Thus, the left amygdala ROI was obtained from a prior published study of healthy controls using the same task (Harenski et al., 2008). Small volume correction within SPM5 was used to correct for 10-mm sphere search volumes for each region.
3. Results
3.1. Behavioral Data
All participants rated moral pictures (M = 3.8, SD = 0.58) higher on violation severity than non-moral (M = 2.7, SD = 0.75; p < .0001) and neutral (M = 1.6, SD = 0.35; p < .0001) pictures. Non-moral pictures were rated higher on violation severity than neutral pictures (p < .0001). Moral and non-moral picture ratings were negatively correlated with PCL-YV scores (r (108) = -0.23; p < .02; -0.23, p < .02; respectively), Factor 1 scores (r (111) = -0.21; p < .03; -0.23, p < .02), and Factor 2 scores ((r (108) = -0.19; p < .05; -0.16, p < .09). Neutral picture ratings were negatively correlated with PCL-YV total and Factor 1 scores (r (108) = -0.17, p < .08; r (111) = -0.19, p < .05), but not Factor 2 scores (r (108) = -0.11, ns). Moral, non-moral and neutral picture ratings were not significantly correlated with CD scores (r (99) = -0.12; ns; -0.06, ns; -0.05, ns). Moral (r (98) = -0.18; p < .08) but not non-moral or neutral (r (98) = -0.05; ns; -0.04; ns) picture ratings were marginally negatively correlated with ICU-Y scores2.
3.2. Imaging data
Main effect results of viewing moral > non-moral pictures across all incarcerated participants, in addition to those from a group of community adolescents from a prior study (Harenski et al., 2012), are presented in Table S3.
We used regression to examine correlations between psychopathic traits and hemodynamic responses to moral > non-moral pictures. The regression with CD scores (controlling for ICU-Y scores) revealed a negative correlation with hemodynamic responses in the right anterior temporal cortex (x = 60, y = -9, z = -15, t = 3.26, p = .046 (FWE); k = 63; Figure 1). No other significant negative correlations between psychopathic traits and regions of interest were found.
Figure 1.
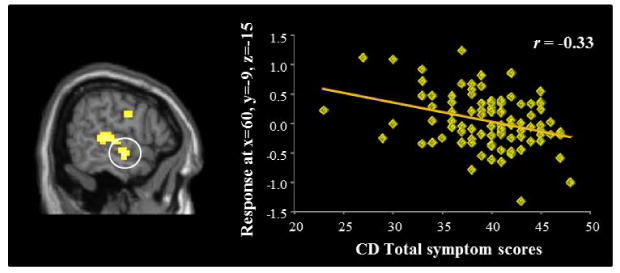
Negative correlation between CD symptoms and ATC response during moral > non-moral picture viewing.
We next used parametric modulation to examine the correlation between hemodynamic responses and severity of moral violation ratings in adolescents scoring low vs. high on the PCL-YV, Factor 1, Factor 2, CD and ICU-Y. Low ICU-Y adolescents showed a positive correlation between severity ratings and hemodynamic responses in the right amygdala (Figure 2). High ICU-Y adolescents showed a negative correlation between severity ratings and hemodynamic responses in the right amygdala. The between-group difference was significant (x = 27, y = -3, z = -15, t = 3.27, p = .05 (FWE); k = 28). No other significant correlations between psychopathic traits and severity ratings in regions of interest were found. Results for the full incarcerated sample and from a group of community adolescents from a prior study (Harenski et al., 2012) are presented in Table S4.
Figure 2.
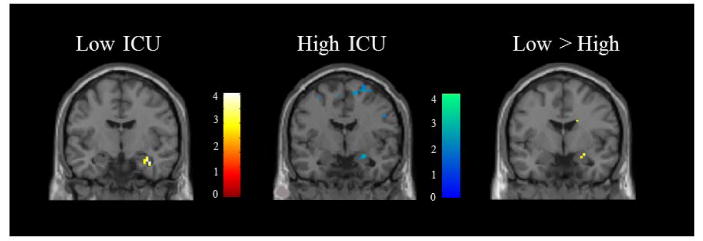
Positive correlation between right amygdala activity and severity of moral violation ratings in low CU adolescents (N = 50): negative correlation between right amygdala activity and severity of moral violation ratings in high CU adolescents (N = 51); group difference in right amygdala correlation with severity of moral violation ratings between low vs. high CU adolescents.
We also explored the association between psychopathic traits and general affective stimuli, by examining hemodynamic responses to moral > neutral and non-moral > neutral pictures. Consistent with prior fMRI studies of emotion and psychopathy, PCL-YV scores were negatively correlated with hemodynamic responses in the left amygdala during moral > neutral (x = -18, y = -3, z = -21, t = 3.04, p = .07 (FWE); k = 68) and non-moral > neutral (x = -15, y = -3, z = -21, t = 3.92, p = .007 (FWE); k = 103) picture processing. ICU-Y scores (controlling for CD scores) were marginally negatively correlated with hemodynamic responses in the left amygdala responses during moral > neutral (x = -15, y = -6, z = -30, t = 2.94, p = .10 (FWE); k = 24) and non-moral > neutral (x = -18, y = 0, z = -18, t = 3.03, p = .07 (FWE); k = 17) picture processing. CD scores (controlling for ICU-Y scores) were negatively correlated with hemodynamic responses in the right ATC (x = 57, y = -12, z = -12; t = 3.57, p = .02 (FWE), k = 88) during moral > neutral picture processing. No other significant correlations between psychopathic traits and regions of interest were present. Results of the whole brain analysis are presented in Table S5.
All results reported above were substantively the same when substance dependence was not included as a covariate, with two exceptions: the negative correlation between CD scores and ATC response during moral > non-moral picture was no longer significant (p = .07) and a significant negative correlation between PCL-YV total scores and left amygdala response was present during moral > neutral picture processing (x = -21, y = 0, z = -24; t = 3.38, p = .03 FWE, k = 72). Results were also substantively the same when ICU-Y was examined without controlling for CD, and vice versa. Finally, the results were substantively the same when accounting for prescription medication use, which was more prevalent among adolescents scoring higher in psychopathic traits (see Supplementary data).
When PCL-YV Factor 1 scores were examined without controlling for PCL-YV Factor 2 scores, negative correlations with left amygdala response during moral > neutral (x = -21, y = 0, z = -24, t = 3.33, p = .04 (FWE); k = 55) and non-moral > neutral (x = -15, y = -6, z = -24, t = 3.50, p = .02 (FWE); k = 77) picture processing were present. Similarly, when Factor 2 scores were examined without controlling for Factor 1 scores, negative correlations with left amygdala response during moral > neutral (x = -15, y = -3, z = -21, t = 3.07, p = .07 (FWE); k = 42) and non-moral > neutral (x = -30, y = -3, z = -30, t = 3.55, p = .02 (FWE); k = 101) picture processing were present.
4. Discussion
We previously found that incarcerated adult male psychopaths showed reduced engagement of the anterior temporal cortex/ATC and amygdala during moral judgments (Harenski et al., 2010). In the present study using an independent incarcerated adolescent sample, responses within these regions were significantly associated with psychopathic traits. Higher CD scores were associated with reduced hemodynamic responses in the ATC during judgments of morally-salient images. Correlations between hemodynamic responses in the amygdala and severity of moral violation ratings were positive in low-CU adolescents and negative in high-CU adolescents. Amygdala responses to unpleasant pictures with and without moral content were negatively correlated with PCL-YV scores.
CD symptoms were negatively correlated with right ATC responses during moral picture processing. Reduced ATC responses to moral pictures have been previously shown in adult psychopaths (Harenski et al., 2010) as well as studies showing reduced ATC gray matter volume in adult psychopaths (Ermer et al., 2012), adolescents with CD (Huebner et al., 2008; Kruesi et al., 2004) and high PCL-YV scores (Ermer et al., 2013). Because the analysis with CD symptoms covaried CU scores, this result appears to be more strongly related to the behavioral and antisocial features of psychopathy. The role of the ATC in moral judgment is not well understood. Heekeren et al. (2005) proposed that the ATC enhances processing depth via the generation of semantic and emotional context during moral judgment (Heekeren et al., 2005). High-CD adolescents may have been less able or inclined to engage in deeper contextual processing of morally-salient pictures, perhaps focusing more narrowly on specific aspects of pictures (e.g., a gun) to identify moral transgressions. Future studies employing methods such as eye tracking could shed light on differences in moral picture processing among high-CD and low-CD adolescents.
Adolescents scoring low on CU traits showed a positive correlation between right amygdala responses and severity of moral violation ratings, while those scoring high on CU traits showed a negative correlation. The former result was in line with our predictions, while the latter was not (no significant association was predicted for the high-CU group). These results suggest that amygdala responses during moral picture processing convey different information to low vs. high-CU adolescents. In the low-CU group, the positive amygdala-rating correlation may represent higher severity ratings influenced by affective cues from morally-salient pictures. In the high-CU group, the negative amygdala-rating correlation may represent lower severity ratings of moral pictures influenced by enhanced attention to other cues from morally salient pictures. For example, during informal discussions following scanning participants sometimes made comments such as “I didn't rate that picture high because I thought maybe the person with the gun was defending himself”. If different participants orient to different features of a moral picture (e.g., victim vs. perpetrator), this could elicit a similar amygdala response but different rating outcome. Future research replicating this effect and examining potential alternative explanations is warranted.
PCL-YV scores were negatively correlated with left amygdala responses during moral and non-moral picture processing. This is consistent with prior studies showing reduced amygdala responses to emotional stimuli in youth scoring high on psychopathic traits (Marsh et al., 2008; Sterzer et al., 2005) and suggests that adolescents scoring high on the PCL-YV had reduced affective responses to unpleasant pictures regardless of moral content. ICU-Y scores showed weaker negative correlations with amygdala responses to moral and non-moral pictures, and CD scores did not show significant correlations with amygdala responses. Overall, the results are consistent with the expectation that psychopathic traits, especially those that comprise affective and interpersonal features of psychopathy, would be associated with reduced amygdala activation in response to emotional pictures.
No single measure of psychopathic traits in the present study produced results that showed greater consistency with our findings in adults using the PCL-R (Harenski et al., 2010). What these results mean for the assessment of psychopathic traits in adolescents is not a simple question to answer, but at the very least emphasizes the importance of comprehensive assessment battery in neuroimaging studies rather than using different assessments across studies. The latter can make it challenging to interpret seemingly discrepant results across studies. For example, in contrast to the present findings of psychopathic trait-related reductions in brain response to moral transgressions, Marsh et al. (2011) found no psychopathic trait-related differences in adolescent brain responses to statements describing illegal (and presumably immoral) behaviors in a moral judgment implicit association task in a non-incarcerated community sample. This could be due to sample differences (incarcerated vs. community sample) or task differences, but could also be related to the use of different psychopathy assessments (PCL-YV, CD, and ICU-Y vs. PCL-YV and Antisocial Process Screening Device) across studies.
The adolescents in this study comprised a highly antisocial group. All were incarcerated in a maximum-security detention facility. In contrast, most published neuroimaging studies of adolescents with psychopathic traits have been conducted in community samples, usually with lower levels of psychopathic traits. This may explain why we did not find a double dissociation between amygdala response and CD symptoms vs. CU traits, with high-CD individuals showing greater amygdala responses to affective stimuli and high-CU individuals showing reduced amygdala responses, similar to the findings reported in Sebastian et al. (2012). Our group had a restricted range on the CD symptom measure, which may have obscured associations with amygdala response. More generally, while our population included adolescents with high levels of psychopathic traits, the results may not generalize to studies that examined subclinical psychopathic traits or non-incarcerated populations.
In our adult study, psychopaths and nonpsychopaths did not differ significantly on violation severity ratings of moral or non-moral pictures (Harenski et al., 2010). Here we observed significant negative correlations between severity ratings of moral pictures and PCL-YV, Factor 1, and Factor 2 scores. Negative correlations were also present with non-moral and neutral picture ratings. CU traits were marginally negatively correlated with moral picture ratings only. This could indicate that adolescents with higher levels of psychopathic traits were less sensitive to moral transgressions. However, because negative correlations with non-moral and neutral pictures were also present, these results should be interpreted with caution. The violation severity rating is associated with response time due to the progressive scale (i.e., more severe ratings necessarily accompanied by longer response times). Thus the effects may be related to an overall tendency of the higher-scoring participants to give lower ratings or respond faster, not to actual perceived moral salience.
All participants rated non-moral pictures higher on moral violation severity than neutral pictures. This is consistent with prior studies of incarcerated adults (Harenski et al., 2010) and non-incarcerated healthy adults and adolescents (Harenski et al., 2012). The elevated ratings of non-moral pictures are likely because participants occasionally over-interpret the content. For example, if a non-moral picture depicted someone in distress a participant might infer that another person caused it (approximately 60% of both the moral and non-moral pictures depicted someone in distress). This could raise the question of whether certain null findings in the moral versus non-moral picture comparison (e.g., lack of correlations with PCL-YV scores) are due to the non-moral pictures being more similar to moral pictures in perceived moral salience than the neutral pictures. We believe this is unlikely, given that moral pictures were rated significantly higher on moral violation severity than the non-moral pictures, yet no correlations between hemodynamic responses and PCL-YV scores were observed in the moral versus non-moral picture comparison at thresholds as low as p < .005, uncorrected. In addition, correlations between hemodynamic responses and PCL-YV scores were not present in the moral versus neutral picture comparison, with the exception of the left amygdala. Regarding the latter, the amygdala-PCL-YV correlation in the non-moral moral versus neutral picture comparison was stronger than the one observed in the moral versus neutral picture comparison (p = .007 vs. .07, respectively), which is consistent with an affective salience rather than moral salience interpretation.
Limitations of this study should be considered. Most participants were in late adolescence, thus it will be important to examine younger adolescents and children with psychopathic traits to determine the onset of dysfunction within the moral neural network. The current study focused on males; however, research has identified divergent neural correlates of moral judgment in males and females (Harenski et al., 2008). Whether the results extend to female adolescents with psychopathic traits is a question for future research. The present results also may not necessarily generalize to different moral judgment tasks (e.g., moral dilemmas).
In summary, we observed negative associations between psychopathic traits and hemodynamic responses in the ATC and amygdala during moral judgments of emotional pictures. These associations varied across the type of psychopathic traits examined. While the PCL-YV was our primary measure of psychopathic traits, we included additional measures of psychopathic and antisocial traits (ICU, CD), so that our results could be compared with other published imaging studies of psychopathic traits in youth and also to address the low correlations between different measures that has been previously reported (Fink et al., 2012). The results of analyses examining these additional measures (ICU, CD) should thus be considered preliminary pending replication. Overall the results strengthen the evidence of aberrant brain responses related to moral judgment in psychopathy and underscore the conceptualization of psychopathy as a neurodevelopmental disorder.
Highlights.
We examine brain responses during moral judgment in youth with psychopathic traits.
Conduct disorder symptoms negatively predicted anterior temporal responses.
Callous-unemotional traits negatively predicted amygdala-moral rating associations.
Brain dysfunction patterns during moral judgment vary by psychopathic trait type.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (R01MH071896). The authors thank Katherine Tremba, Alisa Clark, Amy Byrd, Olga Antonenko, and Rachel Kahn for assistance with data collection.
Footnotes
IAPS pictures included #s 2751, 3010, 3530, 4233, 4621, 6250, 6312, 6313, 6350, 6360, 6821, 9520, 9800, 9810 (moral); 1300, 2120, 2205, 2661, 3080, 3150, 3170, 8230, 8480, 9220, 9432, 9921 (non-moral); and 2058, 2200, 2206, 2210, 2215, 2410, 2480, 2495, 2840, 7130, 7503, 7550 (neutral).
When the incarcerated adolescent participants in the current study are compared to the community adolescents in Harenski et al. (2012), they show similar ratings of moral pictures (M = 3.79, M = 3.57, p = .17), and higher ratings of non-moral pictures (M = 2.73, M = 2.01, p < .008). We believe the most likely explanation for this finding is longer response time due to initial uncertainty in determining that an unpleasant picture does not contain a moral transgression. Importantly, the incarcerated adolescents, like community adolescents, rate moral pictures significantly higher compared to non-moral pictures, indicating they recognize the moral salience of moral pictures.
Conflict of Interest Statement: The authors report no financial relationships with commercial interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Keith A. Harenski, Email: kharenski@mrn.org.
Kent A. Kiehl, Email: kkiehl@mrn.org.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: A functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cerebral Cortex. 2012;22:209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- Edens JF, Skopp NA, Cahill MA. Psychopathic features moderate the relationship between harsh and inconsistent parental discipline and adolescent antisocial behavior. Journal of Clinical Child & Adolescent Psychology. 2008;37:472–476. doi: 10.1080/15374410801955938. [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. Journal of the Academy of Child and Adolescent Psychiatry. 2013;52:94–103. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S, Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13:454–469. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Fink B, Tant A, Tremba K, Kiehl KA. Assessment of psychopathic traits in an incarcerated adolescent sample: a methodological comparison. Journal of Abnormal Child Psychology. 2012;40:971–986. doi: 10.1007/s10802-012-9614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth AE, Kosson DS, Hare RD. Hare Psychopathy Checklist: Youth Version (PCL:YV) Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Frick PJ, Hare RD. The antisocial process screening device (APSD) Toronto: Multi-Health Systems; 2001. [Google Scholar]
- Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. Journal of Abnormal Child Psychology. 2003;31:457–70. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Stickle TR, Dandreaux DM, Farrell JM, Kimonis ER. Callous–unemotional traits in predicting the severity and stability of conduct problems and delinquency. Journal of Abnormal Child Psychology. 2005;33:471–487. doi: 10.1007/s10648-005-5728-9. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–523. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Antonenko O, Shane M, Kiehl K. Gender differences in neural mechanisms underlying moral sensitivity. Social, Cognitive, and Affective Neuroscience. 2008;3:313–321. doi: 10.1093/scan/nsn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Neural development of mentalizing in moral judgment from adolescence to adulthood. Developmental Cognitive Neuroscience. 2012;2:162–73. doi: 10.1016/j.dcn.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision making. Neuroimage. 2005;24:887–897. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Huebner T, Vloet TD, Marx IVO, Konrad K, Fink GR, Herpertz SC, Herpertz-Dahlmann Morphometric brain abnormalities in boys with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kruesi MJP, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS) Bethesda, MD: National Institute of Mental Health Center for the Study of Emotion and Attention; 1995. [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJR. Reduced amygdala response to fearful expressions in adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IN, Schechter JC, Yu HH, Pine DS, Blair RJR. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research. 2011;194:279–286. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Golver GH, Reiss AL. Methods and software for fMRI analysis for clinical subjects. Human Brain Mapping. 2009 [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nature Reviews: Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Pujol J, et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Social Cognitive and Affective Neuroscience. 2012;7:917–923. doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social, Cognitive, Affective, and Behavioral Neuroscience. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose A, Bijttebier P, Decoene S, Claes L, Frick P. Assessing the affective features of psychopathy in adolescence: a further validation of the Inventory of Callous and Unemotional Traits. Assessment. 2010;17:44–57. doi: 10.1177/1073191109344153. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Lopez SJ, Werth TR. Development and preliminary validation of a Satz-Mogel short form of the WAIS-III in a sample of persons with substance abuse disorders. International Journal of Neuroscience. 1999;98:131–40. doi: 10.3109/00207459908994796. [DOI] [PubMed] [Google Scholar]
- Sattler JM, Dumont R. Assessment of Children: WISC-IV and WPPS-IIII Supplement. San Diego, CA: Jerome M. Sattler Publishing Company; 2004. [Google Scholar]
- Seagrave D, Grisso T. Adolescent development and the measurement of juvenile psychopathy. Law and Human Behavior. 2002;26:219–239. doi: 10.1023/a:1014696110850. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJP, Cecil CAM, Lockwood PL, De Brito SA, Fontaine NMG, Viding E. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry. 2012;69:814–22. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Viding E, Jones AP, Frick PJ, Moffitt TE, Plomin R. Heritability of antisocial behaviour at 9: do callous-unemotional traits matter? Developmental Science. 2008;11:17–22. doi: 10.1111/j.1467-7687.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. Journal of Child Psychology and Psychiatry. 2005;46:592–597. doi: 10.1111/j.1469-7610.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- Vincent GM. Psychopathy and violence risk assessment in youth. Child Psychiatric Clinics of North America. 2006;15:407–428. doi: 10.1016/j.chc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for fMRI data. Milwaukee, WI: Author; 2000. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]


