Abstract
Repeated, extreme, or traumatic stressors can elicit pathological effects leading to many negative physical and psychological outcomes. Stressors can precipitate the onset of psychiatric diseases, or exacerbate pre-existing disorders including various anxiety and mood disorders. As stressors can negatively impact human psychiatric health, it is essential to identify neurochemicals that may confer protection from the negative sequelae of repeated or extreme stress exposure. Elucidating the neurobiological underpinnings of stress resilience will enhance our ability to promote resilience to, or recovery from, stress-related psychiatric disease. Herein, we will review the evidence for neuropeptide Y as an endogenous mediator of resilience and its potential relevance for the treatment of stress-related psychiatric diseases.
Keywords: neuropeptide Y, stress resilience, stress-related psychiatric disorders, rodent models, emotionality
1. Introduction
Stressors elicit a cascade of neuronal, endocrine, and behavioral responses that promote homoeostatic adaptation to changing or threatening environments. Stressors maintained over prolonged periods of time or perceived as extreme can lead to maladaptive responses within stress-integrative circuitry. Pathological neurochemical and behavioral mechanisms can then manifest in the form of stress-related psychiatric diseases including anxiety disorders, post-traumatic stress disorder (PTSD), and depression. Neuropeptides have been shown to be influential neuromodulators of stress-related emotionality [1]. A growing body of evidence supports a role for neuropeptide Y (NPY) as a protective neurochemical that mediates stress resilience. NPY is a 36-amino acid peptide derived from preproNPY and belonging to a family that also includes pancreatic polypeptide (PP) and peptide YY (PYY) [2]. NPY is highly conserved across mammalian species and is expressed throughout the central nervous system (CNS) [3–7]. In the periphery, NPY is expressed primarily in sympathetic ganglia, the adrenal medulla, and in platelets [3–7]. NPY is the most abundant and widely distributed neuropeptide in the human brain [4], and has been shown to have a significant impact on brain activity. In the CNS, NPY and its receptors (Y1, Y2, Y4, Y5) play important roles in the control of food intake, energy homeostasis, pain, and many behavioral and physiological processes associated with stress and stress resilience [7, 8]. In this review, we will discuss the role of NPY in stress-related behaviors and its relevance to select psychiatric disorders.
2. Neuropeptide Y (NPY)
2.1. NPY and NPY receptor subtypes in the brain
NPY immunopositive cell bodies and fibers are generally found in cortical, limbic, hypothalamic, and brainstem regions [5]. Expression of NPY in the human and rodent brain is similar, with abundant NPY mRNA or immunoreactivity located in the neocortex, amygdala, hippocampus, basal ganglia, hypothalamus, periaqueductal grey, dorsal raphe nucleus, and the A1–3 and A6 noradrenergic cells groups in the brainstem [4, 5, 9–13]. The effects of NPY are mediated by at least four subtypes of G-protein coupled receptors termed Y1, Y2, Y4, and Y5. Y6 receptors are expressed in the mouse brain, but this isoform is absent in the rat and nonfunctional in human and non-human primates [14]. Autoradiographic and immunohistochemical examinations indicate that Y1 and Y2 receptors (Y1R and Y2R) exhibit the greatest expression in the brain, whereas lower levels of Y4 and Y5 receptors (Y4R and Y5R) are also present [15–20]. Significant differences in the distribution of NPY receptors are detectable between the rodent and human brain, warranting caution in the generalization of the role of NPY receptors from preclinical animal models to humans [18]. NPY receptors can couple to various effectors systems by associating with inhibitory Gi/o proteins (see review [21]). NPY receptors inhibit adenylyl cyclase and the accumulation of cAMP, mobilize calcium through phospholipase C and phosphatidylinositol 3-kinase activity, and have effects on multiple ion channels [21]. Within stress responsive brain regions such as the cortex, amygdala, hypothalamus, and locus coeruleus, NPY receptors are localized on or impact the function of neurons expressing GABA, glutamate, corticotropin-releasing factor (CRF), and norepinephrine (NE) [22–27]. It has been hypothesized that NPY serves as a functional “brake” to tone down the excitatory effects of pro-stress neurotransmitters such as CRF and NE [21, 27, 28]. This hypothesis is supported by studies demonstrating that NPY is frequently contained within the same neuroanatomical brain structures as CRF and NE, and the function of NPY is often physiologically and behaviorally opposite to pro-stress neurotransmitters (reviewed in [19, 21, 29]). Although clear interactions between NPY and pro-stress systems in the regulation of stress-related emotionality still need to be established, it is likely that the balance of these neuropeptides and transmitters in stress-related circuits plays a pivotal role in mediating resilience to stress-associated responses discussed in this review.
3. NPY in stress-related psychiatric disorders: insight from human studies
3.1. Stress and Anxiety
Human studies have identified associations between NPY and stress resilience. In healthy human subjects, plasma NPY levels have been shown to rise in response to stress [30–32]. For example, when military soldiers underwent an interrogation model of extreme psychological stress to mimic the captive experience of prisoners of war, higher levels of NPY following interrogation were present in soldiers displaying lower psychological distress or belonging to special operations forces [31, 32]. NPY levels were positively associated with feelings of dominance and self-confidence, and superior performance under interrogation stress [30, 31, 33].
Genetic variants of the preproNPY gene have been associated with differential stress responses and emotionality [34, 35]. Specific NPY haplotypes have been correlated to postmortem levels of NPY mRNA in the brain, plasma NPY concentrations, and brain activity in response to stressful challenges [35]. Individuals possessing a genotype associated with low NPY expression report more negative emotional experiences during a painful stressor, exhibit greater amygdalar reactivity in response to threat-related facial images, and exhibit low stress resilience compared to high NPY genotype carriers [34, 35]. Haplotype-driven NPY expression is also inversely correlated to trait anxiety in healthy individuals [35].
Studies in humans with stress-related psychiatric disorders have also revealed a role for NPY in resilience [27, 36–39], although the evidence stems primarily from populations with PTSD and depression. Rodent studies have provided a wealth of evidence for NPY in resilience to anxiety (see below), but few human studies have been conducted to determine the profile of NPY in generalized anxiety, obsessive compulsive, social anxiety, and panic disorders. One study found an association between a single-nucleotide polymorphism of the NPY gene and increased risk for generalized anxiety disorder in individuals exposed to high stress [40]. Genetic variants of the Y5 receptor gene have been significantly associated with panic disorder [41]. Elevated plasma NPY was detected in a study of individuals with panic disorder, in which the authors suggest that an increase in NPY may be compensatory to buffer enhanced sympathetic activation in this disorder [42]. Other studies have not detected differences in NPY levels between healthy controls and persons with obsessive compulsive, social anxiety, or panic disorders [43, 44], or have failed to identify genetic associations between NPY and anxiety disorders [45].
3.2. Depression
Clinical investigations have revealed that the plasma and CSF of depressed individuals contain decreased concentrations of NPY compared to healthy controls [46–50]. Additional studies have shown lower NPY in clinically depressed patients with a history of suicide attempts compared to healthy persons, and that NPY levels are lowest in individuals with a recent suicide attempt [51]. Likewise, low NPY immunoreactivity has been found in postmortem brain tissue of suicide victims, with the most robust reductions in NPY occurring in the brains of persons with a history of depression [52]. Low levels of NPY mRNA expression are also found in persons with bipolar disorder [53, 54]. Genetic variants of the preproNPY gene have been associated with resilience or vulnerability to depression [47, 55, 56]. For instance, a genetic polymorphism resulting in higher levels of mature NPY appears to be protective against depression despite exposure to environmental risk factors [56], and the presence of this polymorphism is less frequent in depressed patients [47]. In another study, a genotype associated with low NPY expression was found to be overrepresented in persons with major depression compared to healthy controls [34]. Interestingly, antidepressant strategies are associated with parallel elevations in NPY and decreases in corticotropin-releasing hormone (CRH), thereby supporting peptidergic interactions in the mechanisms underlying clinically efficacious treatments for depression. For example, CSF levels of NPY are elevated in depressed patients following electroconvulsive therapy, while levels of corticotropin-releasing hormone decrease concurrently [57, 58]. Increased NPY after treatment with the selective serotonin reuptake inhibitor citalopram is associated with a reduction in depression severity and the levels of CRH [59].
3.3. Post-traumatic stress disorder (PTSD)
Reduced concentrations of cerebrospinal and plasma NPY have been reported in both individuals with PTSD and those who have been exposed to traumatic stress [37–39]. NPY is inversely related to PTSD symptomology, with low NPY correlating specifically to the presence of intrusion symptoms [60]. Higher NPY is predicative of PTSD symptom improvement and shows a positive association with coping following a traumatic event [61]. Aberrant NPY and norepinephrine function have been linked in PTSD. Yohimbine, an antagonist of the presynaptic α2-adrenergic receptor that increases norepinephrine levels, elicits panic attacks and exacerbates the core symptoms of PTSD [62]. Yohimbine has also been shown to stimulate increases in plasma NPY and levels of the norepinephrine metabolite MHPG (3-methyl-4-hydroxy-phenyl-glycol) in healthy subjects. However, yohimbine-stimulated increases in NPY are significantly blunted in persons with PTSD [63, 64]. Additionally, baseline concentrations of plasma NPY correlated negatively to yohimbine-induced increases in MHPG in the same study [63]. This correlation suggests that low basal levels of NPY were associated with an exaggerated increase in MHPG following yohimbine [63]. Both basal and yohimbine-stimulated levels of NPY were negatively correlated to scores on a combat-exposure scale, indicating that greater combat exposure was associated with blunted levels of NPY [63].
4. Potential therapeutic applications of NPY: evidence from animal models
Pathological responses to stress manifest in behaviors that include enhanced anxiety, arousal, and fear. In this section, we review the findings in animal models utilized to examine these three behavioral responses, as well as the effects of NPY in rodent models of PTSD and depression-like behavior. Examples provided in the text are summarized in Table 1.
Table 1.
Behavioral observations following pharmacological interventions or genetic manipulations of the NPY system.
| Rodent Models | Pharmacological Intervention or Genetic Manipulation |
Route of Administration/ Region |
Direction of Behavioral Effect |
Reference |
|---|---|---|---|---|
| Anxiety-like Behavior | NPY | i.c.v. BLA, CeA Hippocampus Lateral Septum LC |
Decrease | [67–70] [73–75] [71, 72] [77] [76] |
| NPY knockout | Increase | [65] | ||
| Y1R agonists | i.c.v. CeA Hippocampus |
Decrease | [69, 86] [75, 88] [87] |
|
| Y1R antagonists | i.c.v. PAG LC Hypothalamus CeA |
Increase No effect |
[89–91] [89] |
|
| Y1R knockout | Increase | [83–85] | ||
| Y2R agonist | i.c.v. LC Lateral septum BLA (high dose) |
No effect Decrease |
[67–69, 86] [76] [94] [96] |
|
| Y2R antagonists | CeA | Decrease | [98] | |
| Y2R knockout | Global, BLA or CeA GABAergic neurons in CeA |
Decrease Increase |
[99–103] [97] |
|
| Y4R knockout | Decrease | [100, 102] | ||
| Y5R agonist | i.c.v. | Decrease | [86] | |
| Y5R antagonist | BLA | Decrease | [105] | |
| Arousal | NPY | i.c.v., BLA CeA |
Decrease No effect |
[67, 92, 110] [110] |
| NPY knockout | Increase | [66] | ||
| Y1R agonists | i.c.v. | Decrease | [67] | |
| Y2R | i.c.v. | No effect | [67] | |
| Y2R knockout | Increase | [66] | ||
| Fear | NPY | i.c.v., Amygdala | Decrease | [110, 116–118] |
| NPY knockout | Increase | [119] | ||
| Y1R agonists | i.c.v. | Decrease | [116] | |
| Y1R antagonists | i.c.v. Amygdala |
Block NPY effects Increase |
[116] [110] |
|
| Y1R knockout | Increase | [117] | ||
| Y2R knockout | No effect | [119] | ||
| Depression-like Behavior | NPY | i.c.v., Hippocampus | Decrease | [122–125] |
| Y1R agonists | i.c.v. | Decrease | [124] | |
| Y1R antagonists | i.c.v. | Block NPY effects | [124] | |
| Y1R knockout | Increase | [81] | ||
| Y2R antagonists | i.c.v. | Decrease | [124] | |
| Y2R knockout | Decrease | [101] | ||
| Y4R knockout | Decrease | [100, 102] | ||
|
Depression Models (OBX or FSL) |
NPY | Decrease (OBX) | [139] | |
| Y1R agonist | i.c.v. | Decrease (OBX) | [139] | |
| Y2R agonist | i.c.v. | Increase (OBX) | [141] | |
| Y2R antagonist | i.c.v. | Decrease (OBX) | [140] | |
| Y5R antagonist | i.c.v. | Decrease (FSL) | [134] | |
|
PTSD models (PSS and SPS) |
NPY | Intranasal, Hippocampus |
Decrease anxiety, arousal, fear, depression-like behaviors |
[147, 149, 151] |
| Y1R antagonist | Hippocampus | Increase anxiety, arousal |
[147] |
4.1. Anxiety
Genetic rodent models and pharmacological studies have provided insight into the anxiolytic properties of NPY in multiple paradigms of anxiety-like behavior [19, 29]. NPY deficiency is associated with an anxiogenic phenotype in rodents [65], and highly anxious rats are more sensitive to the anxiolytic actions of NPY [66]. Intracerebroventricular (i.c.v.) administration of NPY decreases anxiety-like behavior in the elevated plus maze, Vogel’s drinking conflict test [67, 68], and other operant conflict tasks [69, 70]. Site specific-studies have revealed the amygdala, locus coeruleus, lateral septum, and hippocampus as regions that are involved in the anxiolytic properties of NPY [71–77]. For example, infusion of NPY into the basolateral amygdala decreases social anxiety [74], produces anti-conflict effects via the central nucleus of the amygdala [75], and decreases anxiety upon injection into the locus coeruleus [76]. The effects of NPY may be related to interactions with CRF signaling, as NPY attenuates anxiety and avoidance behavior induced by CRF and CRF agonists upon i.c.v. or direct delivery into subregions of the amygdala [78–80]. An interaction with norepinephrine systems has also been implicated, as pretreatment with idazoxan, an α2-adrenergic receptor antagonist, blocks the anxiolytic effects of NPY [68].
The receptor subtypes mediating the anxiolytic properties of NPY are currently under investigation. Studies largely support a role for the activation of Y1R in the attenuation of anxiety-like behavior. For example, the anxiolytic effects of NPY are absent in mice lacking the Y1R [81, 82], and Y1R knockout mice exhibit an anxiogenic phenotype [83, 84]. Selective knockout of Y1R from excitatory forebrain neurons also results in increased anxiety [85]. Centrally administered Y1R agonists are anxiolytic in a number of behavioral paradigms [69, 86], while site-specific examinations implicate the central nucleus of the amygdala and hippocampus as regions of Y1R-mediated anxiolysis [75, 87, 88]. Administration of Y1R antagonists centrally or into the periaqueductal grey produces anxiogenic effects [89, 90], but has no reported effects when delivered into the locus coeruleus, hypothalamus, or central nucleus of the amygdala [89]. The lack of effect in these regions may be due to their low level of expression of Y1R [19]. Central blockade of Y1R is also sufficient to elicit conditioned place aversion, supporting the notion that Y1R are necessary for endogenous anxiolytic actions of NPY [91]. Y1R are found to be preferentially expressed on pyramidal cells in the basolateral amygdala [25], therefore it is likely that Y1R mediate anxiolysis here by influencing glutamatergic input to the central nucleus of the amygdala and subsequent output to the brainstem [92].
The function of Y2R in anxiety is allegedly opposite of the Y1R subtype; however conflicting reports demonstrating both anxiogenic and anxiolytic effects mediated by Y2R make the role of this subtype in anxiety less clear. Y2R are generally considered NPY autoreceptors and evidence for their pre-synaptic localization has been demonstrated in humans and rodents [17, 93]. Central administration of Y2R agonists have failed to alter anxiety-like behavior in a number of studies [67–69, 86]. However, agonism of Y2R in the locus coeruleus and lateral septum produces anxiolytic effects, whereas Y2R are required for NPY-mediated anxiolysis in the hippocampus [76, 94, 95]. Y2R agonism in the basolateral amygdala has bidirectional effects on anxiety in the social interaction test, with low agonist doses generating anxiety and high doses decreasing anxiety [96]. A recent study indicates that knockout of the Y2R in GABAergic neurons located in the central nucleus of the amygdala was anxiogenic specifically in female mice [97]. Contrasting reports indicate that Y2R antagonism in the central nucleus of the amygdala is anxiolytic [98], and that ablation of Y2R in either the basolateral or central nucleus of the amygdala produces an anxiolytic phenotype [99]. Global deletion of Y2R reduces anxiety in the elevated plus maze, light-dark, open-field, and marble burying tests [100–103], and Y2R deficient mice exhibit reduced neuronal activation upon exposure to an anxiogenic environment [104]. Taken together, this evidence suggests that Y2R may function in a regionally specific and neurochemically selective fashion.
The Y4R and Y5R also have putative roles in rodent anxiety-like behavior. Similar to Y2R mutant mice, deletion of the Y4R also reduces anxiety-like behavior in a number of rodent paradigms [100, 102]. Knockout of the Y4R with the Y2R enhances the anxiolytic phenotype observed following deletion of either receptor alone [100]. Finally, pharmacological studies indicate that Y5R ligands may have promising anxiolytic properties. A Y5R antagonist blocked the anxiolytic effects of a Y2R agonist in the basolateral amygdala [105], while i.c.v. delivery of a Y5R agonist produced anxiolytic effects [86]. Y5R can form heterodimers with Y1R [106], and these receptor subtypes are colocalized in the basolateral amygdala, hippocampus, and hypothalamus [20, 84, 107, 108]. Y1 and Y5 receptors act synergistically in the regulation of energy homeostasis [109]. Although the combined effects of Y1 and Y5 receptor agonists have not been tested in the context of anxiety thus far, the notion of co-activating these receptors could be valuable in the development of pharmacotherapeutics for enhanced anxiolytic effects.
4.2. Arousal
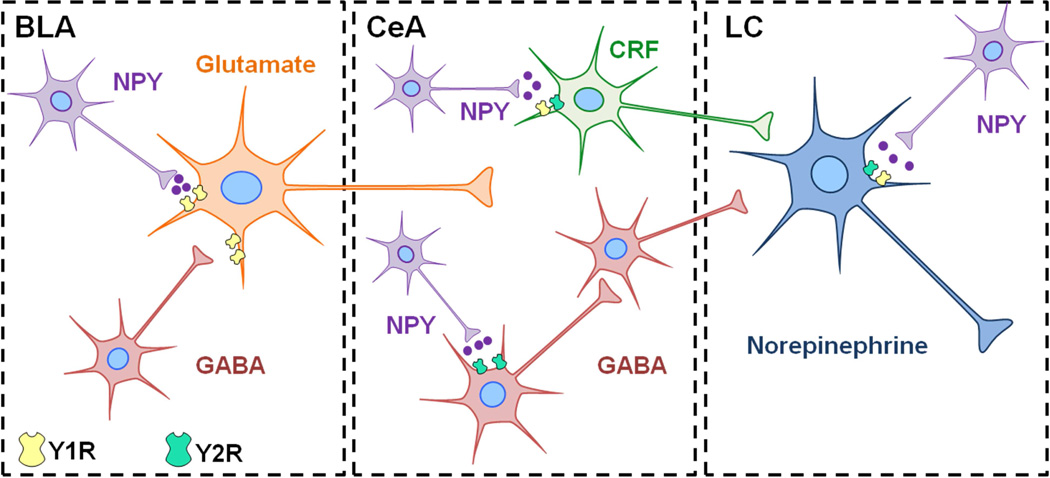
Hypervigilance is a characteristic symptom of stress-related psychiatric disorders that may reflect dysregulation of brain arousal systems. Startle responses can be measured in rodents using loud acoustic tones, and can be enhanced in fear-potentiated startle, a paradigm in which startle is tested in an environment previously paired with footshocks. Central administration of NPY inhibits both basal acoustic startle and fear-potentiated startle in rodents [67, 92, 110]. Another study demonstrated that NPY infusion into the basolateral, but not central nucleus, of the amygdala mimics the effects of NPY on acoustic startle and fear-potentiated responses [110]. Central administration of a Y1R agonist attenuates fear-potentiated startle, whereas a Y2R agonist was reported to have no effect [67]. In genetically modified rodents, knockout of NPY or Y2R enhances acoustic startle [65], whereas deletion of the Y1R yields impaired habituation of startle responses [111]. These studies indicate a role for NPY in the modulation of startle and potential for NPY as a therapeutic for hyperarousal in stress-related psychiatric disorders. However the receptor subtypes and brain regions dictating NPY-induced resilience to this behavioral response remain unclear. The NE system originating in the locus coeruleus (LC) is a brainstem region contributing to arousal responses [112, 113], thus NPY may mediate arousal behavior by directly acting in the LC or by influencing brain regions upstream. Figure 1 demonstrates putative neurochemical interactions and circuitry that may influence the function of the LC-NE system and arousal behavior. NPY inhibits the firing rate of NE neurons in the LC, and potentiates the effect of NE on presynaptic autoinhibition of neuronal firing [26, 114]. This electrophysiological evidence suggests that NPY may act to restrain the activity of noradrenergic neurons, which may have important implications for stress-psychiatric diseases in which the LC-NE system is disrupted. In combination with anatomical evidence demonstrating rich NPY innervation of the LC [115] (shown in Figure 2),these studies suggest that NPY may play an important role in the regulation of noradrenergic stress responses and arousal via NE circuitry.
Figure 1. Putative modulation of arousal behavior by NPY within stress-integrative circuitry.
Excitatory glutamatergic (Glu) projections from the basolateral amygdala (BLA) activate the central nucleus of the amygdala (CeA) in response to stress. Subsequent activation of afferents expressing corticotropin-releasing factor (CRF) leads to enhanced activity of norepinephrine (NE) neurons in the locus coeruleus (LC), which then project to and activate regions of the forebrain to regulate arousal behavior. Putative interactions of NPY with stress responsive regions are shown. Activation of Y1 receptors on Glu neurons in the BLA may decrease activation of the CeA in response to stress [25]. NPY may suppress noradrenergic activation in the LC via Y2R located on NE neurons [26, 114], or suppress Y2R–expressing GABAergic interneurons in the CeA leading to disinhibition of GABA output to the LC (not shown) [176]. Alternatively, we hypothesize that NPY axon terminals may directly interact with CRF neurons in the CeA to suppress the activity of the LC-NE system in response to stress.
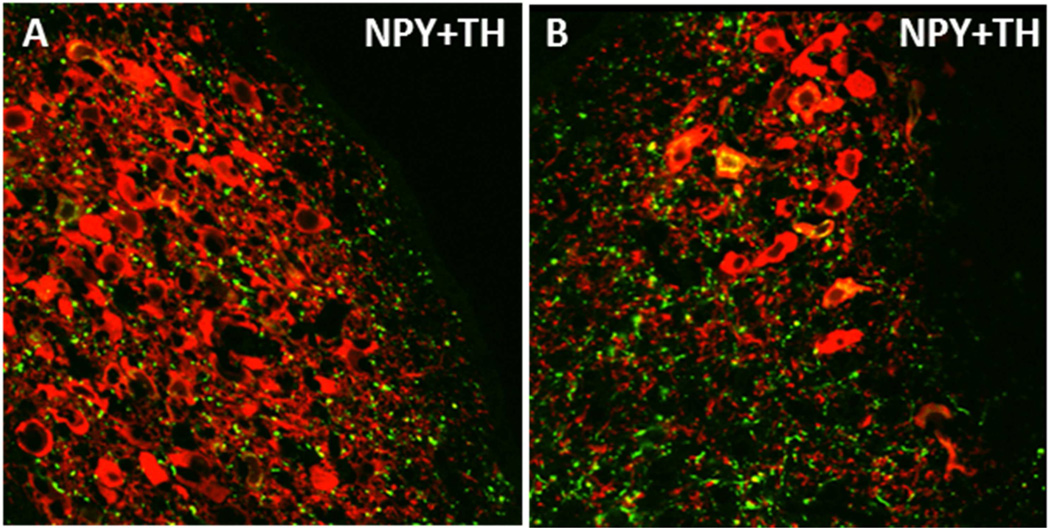
Figure 2. NPY innervation of the rat locus coeruleus.
NPY fibers (green) innervating the nuclear core (A) and the ventral dendritic region (B) of the locus coeruleus are shown. Noreprinephrine neurons in the locus coeruleus are represented by staining of the biosynthetic enzyme tyrosine hydroxylase (TH) (red). Colocalization of NPY and TH can be observed in cell bodies and fibers (yellow). NPY and TH in this high magnification image were visualized by immunofluorescence and confocal laser microscopy. Tissues were obtained from a non-colchicine treated Sprague-Dawley rat, which may contribute to the minimal observation of NPY synthesizing neurons in this image.
4.3. Fear
Recent rodent studies suggest that NPY may be useful in the treatment of psychiatric diseases such as PTSD, which is heavily characterized by behavioral sequelae associated with fear. NPY has been found to influence multiple fear-related behaviors including the acquisition, incubation, expression, and extinction of conditioned fear. For example, i.c.v. administration of NPY or a Y1R agonist inhibits freezing behavior in both the acquisition and consolidation phases of fear conditioning, and these effects are blocked by pretreatment with a Y1R antagonist [116]. Y1R may not be necessary for the cued-expression of fear, as intra-amygdalar administration of NPY robustly decreases the expression of conditioned fear, but these effects are not replicated by Y1R agonists and are not blocked by pretreatment with a Y1R antagonist [117]. In this particular study, Y1R knockout mice showed slight elevations in freezing behavior during fear conditioning, but did not show an enhanced phenotype upon testing for the cued-expression of fear compared to wildtype mice [117]. In addition, NPY was still capable of reducing the cued-expression of fear in these Y1R deficient mice, suggesting that the Y1R may not be involved in this phase [117]. NPY can suppress the long-term incubation of conditioned fear, while delivery of NPY prior to extinction training attenuates freezing and enhances retention of extinguished fear memories [110, 116, 118]. Y1R antagonism blocks NPY-induced reductions in freezing and blockade of amygdalar Y1R leads to deficient extinction retention [110, 116]. Consistent with pharmacological studies, NPY knockout mice display accelerated acquisition of conditioned fear, excessive recall of fear, and impaired fear extinction [119]. Interestingly, deletion of the Y1R has moderately similar effects, whereas knockout of the Y2R has no effect on fear [119]. However, double Y1R and Y2R knockout mice exhibit a remarkably similar phenotype to NPY deficient mice, indicating that both receptor subtypes do play a role in aspects of fear conditioning [119]. In an inescapable footshock paradigm, interactions between the NPY and CRF systems were evident as increased amygdalar CRFR1 and decreased Y1R mRNA were found concurrently in animals displaying enhanced freezing time, and all of these effects were reversed in parallel following re-exposure to the footshock-paired environment [120]. Indirect evidence for NPY interactions with norepinephrine was obtained using auditory fear conditioning, in which centrally administered NPY and a Y1R agonist blunted fear-induced tachycardia [121]. These effects were blocked by a Y1R antagonist [121].
4.4. Rodent models of depression
NPY is implicated in depression-like behavior and produces antidepressant effects. For example, central administration of NPY dose-dependently reduces immobility and increases swimming time in the forced swim test [122–124], a screening paradigm for pharmacological anti-depressant activity. Y1R agonists and Y2R antagonists also produce anti-depressant effects in forced swim [124], whereas Y1R antagonists block the anti-depressant effects of NPY [124]. Intra-hippocampal infusion of NPY has anti-depressant properties in a learned helplessness paradigm, which is blocked by co-administration of a Y1R, but not a Y2R antagonist [125]. Y1R knockout mice display increased immobility in the forced swim test, indicative of a depression-like phenotype [81]. Both Y2R and Y4R knockout mice exhibit reduced depression-like behavior in the tail suspension test, another common screening assay for antidepressant potential [100–102]. Knockout of both Y2R and Y4R results in augmented anti-depressant effects compared to single-knockout of either receptor [100]. Anti-depressant strategies including imipramine and electroconvulsive stimuli increase NPY immunoreactivity or receptor mRNA and binding sites, respectively [126, 127]. The anti-depressant properties of NPY may be mediated through interactions with the serotonin system, as administration of a tryptophan hydroxylase inhibitor blocked the anti-depressant effects of NPY in the forced swim test [122].
The Flinders-sensitive line (FSL) is a transgenic model of depression in which abnormalities in NPY, serotonin, and catecholaminergic systems have been identified [128, 129]. Depression-like behavior has been associated with impaired hippocampal neurogenesis, and enhanced NPY and serotonin activities been shown to increase cell proliferation in the dentate gyrus of the hippocampus [130]. Hippocampal and amygdalar NPY immunoreactivity is lower in FSL rats compared to Flinders-resistant controls [131–133], and aging is associated with exacerbated loss of hippocampal NPY immunoreactivity in the FSL line [130]. In FSL rats, Y5R antagonism produces anti-depressant effects in the forced swim test [134]. Electroconvulsive stimuli and the selective serotonin reuptake inhibitor fluoxetine increase NPY mRNA or immunoreactivity in the hippocampus and hypothalamus, and upregulate amygdalar Y1R binding sites in FSL rats [93, 135]. Exercise and escitalopram are associated with similar alterations in hippocampal NPY and Y1 receptor mRNA [136]. NPY has also been examined in olfactory bulbectomized rats (OBX), which are utilized as a rodent model due to depression-like disruptions in behavior, physiology, and neurochemistry [137, 138]. Anti-depressant effects are observed following chronic treatment with NPY, a Y1R agonist, and a Y2R antagonist in OBX rats [139, 140]. In contrast, chronic administration of a Y2R agonist enhanced depression-like behavior in OBX rats in the forced swim test [141].
Future studies investigating the efficacy of NPY in depression-like behavior induced by chronic psychosocial stress using the resident-intruder model of social defeat would be interesting. Social defeat reproduces behavioral and physiological indices of depression including disruption of CRF and NE systems [142–146], and would likely yield important information regarding the role of NPY in depressive behavior and disorders.
4.5. Rodent models of PTSD
Several rodent models of PTSD indicate that NPY expression in the brain following stress may be associated with susceptibility to PTSD-associated impairments. For example, rats displaying extreme anxiety and arousal following exposure to predator scent stress (PSS) had lower NPY protein levels in the cortex, amygdala, hippocampus, and periaqueductal grey compared to rodents that were less impaired or to unstressed controls [147, 148]. Injection of NPY into the hippocampus 1 hour after PSS reduced the development of anxiety-like behavior, hyperarousal, and cue-elicited freezing. Additionally, NPY administration reduced the prevalence of an extreme behavioral response [148].
Delivery of NPY to the brain by intranasal (IN) infusion has been used to examine its efficacy in the single prolonged stress (SPS) model of PTSD [149–151]. Intranasal NPY can elevate CSF concentrations to a range that reduces anxiety behavior after i.c.v. administration, while also reaching multiple stress responsive brain regions and leaving plasma NPY levels unchanged [149, 150]. Pretreatment with IN NPY slowed the development of immobility during the forced swim portion of SPS, and reduced the induction of gene expression of the NE biosynthetic enzymes, tyrosine hydroxylase and dopamine beta hydroxylase, in the locus coeruleus shortly after SPS [149]. SPS-induced increases in plasma corticosterone and ACTH were also attenuated by IN NPY, suggesting either less activation or more rapid recovery of the hypothalamic-pituitary-adrenal (HPA) axis [149]. Intranasal NPY administered prior to or immediately after SPS led to pronounced and long-lasting effects on the development of behavioral, neuroendocrine, and molecular impairments associated with PTSD. NPY greatly attenuated, and in many cases prevented, increases in anxiety, hyperarousal, and depression-like behavior observed 1–2 weeks after exposure to traumatic stress [149]. NPY prevented SPS-triggered induction of CRF, glucocorticoid receptor (GR), and FKBP5 mRNAs and the reduction in phosphorylated-GR in the mediobasal hypothalamus [150]. NPY also increased the expression and phosphorylation of GR in the hippocampus [150]. These studies suggest that early intervention with intranasal NPY may prevent dysregulation of the HPA axis by restoring proper negative feedback inhibition by GR. Intranasal NPY also attenuated long-term changes in the central noradrenergic system induced by SPS, including the development of increased sensitization of the LC to re-experiencing the forced swim [149]. Taken together, PSS and SPS studies indicate that a single treatment with NPY near the time of the traumatic stress could provide long-lasting resilience to the development of PTSD and co-morbid impairments such as depression. Moreover, recent work also suggests that NPY may be efficacious as a treatment once PTSD-like symptoms have already manifested. Rats given IN NPY one week after SPS, when PTSD-like symptoms have manifested, exhibit anxiety-like behavior similar to unstressed controls up to 2 days later [151]. Rats administered NPY after SPS also had reduced depression-like behavior [151]. Further studies are necessary to determine if intranasal NPY reverses other impairments associated with PTSD, as well as the duration and sustainability of the improvements.
5. Therapeutic Implications
The examples presented herein demonstrate that pharmacological interventions targeting the NPY system display much promise for the treatment of numerous stress-related psychiatric disorders. Future pharmacotherapeutic studies should consider targeting the central NPY system in stress-related emotionality and resilience. The preponderance of data suggests that NPY itself has significant therapeutic potential as a mediator of stress resilience. There are two major challenges associated with the development of NPY as a drug for psychiatric disorders; it is a peptide and it has a broad range of activities that may result in undesirable side-effects. The attractiveness and challenges of peptide therapeutics for CNS disorders has recently been reviewed [152]. Peptides do not accumulate in tissues and are effectively metabolized by endogenous enzymes; therefore they have limited potential for drug-drug interactions. However, peptides have short half-lives and several methods have been introduced to prolong their stability in vivo. Encouragingly, as demonstrated in rodent models [149–151], NPY may confer long-lasting benefits for stress resilience despite its short half-life.
Although this review has concentrated on the beneficial effects of NPY in the CNS, NPY also has multiple actions in the periphery [7, 153, 154]. For example, NPY is a co-transmitter in sympathetic nerves, plays a role in vascular tone, and contributes to cardiovascular remodeling [155–158]. Rodent studies have demonstrated NPY-induced disruption of metabolic homeostasis, as chronic NPY administration in rodents leads to abnormal baroreflex sensitivity, abdominal obesity, and dyslipidemia [159]. NPY release from sympathetic nerves also stimulates fat angiogenesis, macrophage infiltration, and proliferation and differentiation of new adipocytes leading to abdominal obesity and a metabolic syndrome in rodents [160]. NPY also plays a role in bone physiology, gastrointestinal function, and cancer progression [8]. Peripheral administration of NPY may result in undesirable side effects on these physiological processes, increasing the value and necessity for strategies of NPY administration to the brain. Moreover, peptides do not typically cross the blood-brain barrier unless carried by specific transporters. Although no such transporter is known to exist for NPY, studies have shown that NPY can enter the brain to some extent [161].
Intranasal (IN) infusion represents a clinically relevant and non-invasive approach for the delivery of NPY to the brain. IN administration allows peptides to rapidly and directly enter the CNS via intracellular neuronal olfactory and extracellular trigeminal-associated pathways bypassing the blood–brain barrier to affect multiple sites within the brain [162–165]. As demonstrated in rodent models [149–151], NPY delivered to the brain by IN infusion has beneficial effects on stress-related emotionality and pathology, which is likely achieved by influencing NPY responsive systems in all regions regulating stress responses. A potential disadvantage of IN infusion is the lack of selective targeting and potential for CNS-mediated side effects. For example, NPY is also a powerful orexigenic agent and regulates circadian rhythms [8, 166]. Although not used for stress-related implications, studies have administered NPY by IN infusion in humans [167–171]. One small clinical trial aimed to test the effect of IN NPY on mood and anxiety (NCT 00748956)[172], while another is currently underway to investigate the safety of IN NPY using a dose escalation in PTSD (NCT 01533519) [173]. To date no side effects have been reported. The viability of this route of administration makes it much more feasible to consider clinical proof of concept studies for severe stress-related disorders such as PTSD, for which there are no truly effective treatments and the initiating stress is often known. In the event that CNS-mediated side effects prove to be significant obstacles to the chronic use of NPY as a therapeutic, it is possible that the selective activation or inhibition of individual receptor subtypes may be a safer yet still effective alternative. There is already considerable preclinical data demonstrating the therapeutic potential of Y1R agonists and Y2R antagonists for the treatment of stress-related disorders and these targets clearly merit additional study.
6. Future Directions
Elucidating the neuroanatomical interactions of the NPY system with other neurotransmitters and peptides within stress-integrative circuitry would greatly advance our knowledge regarding the role of NPY in stress resilience and emotionality in future studies. In addition, future studies should consider the impact of sex differences on NPY-mediated effects. Human and rodent studies indicate that females may be more vulnerable to stress and stress-related psychiatric diseases than males [174]. Psychiatric symptomology and treatments responses also vary based on sex [175]. Future studies examining the efficacy of NPY on stress and emotionality in females with direct comparisons to males would advance our understanding of sex differences in stress resilience. Neuroanatomical and molecular studies conducted across sexes would reveal potential mechanisms underlying effective coping to stress and intervention strategies for stress-induced psychiatric diseases.
Highlights.
Overview of neuropeptide Y and receptor subtypes in the central nervous system
Alterations of neuropeptide Y in human stress-related psychiatric disorders
Evidence for neuropeptide Y in resilience to stress-related emotionality in rodent behavioral models
Pharmacotherapeutic implications for neuropeptide Y in the treatment of stress-related psychiatric disorders
Acknowledgements
This work was supported by DA09082 (EJV) from the National Institutes of Health and DM102281(ELS) from US Army, Department of Defense Medical Research and Development Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicole M. Enman, Email: nicole.enman@drexelmed.edu.
Esther L. Sabban, Email: esther_sabban@nymc.edu.
Paul McGonigle, Email: paul.mcgonigle@drexelmed.edu.
Elisabeth J. Van Bockstaele, Email: elisabeth.vanbockstaele@drexelmed.edu.
References
- 1.Kormos V, Gaszner B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides. 2013;47(6):401–419. doi: 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Larhammar D, Blomqvist AG, Soderberg C. Evolution of neuropeptide Y and its related peptides. Comp Biochem Physiol C. 1993;106(3):743–752. doi: 10.1016/0742-8413(93)90236-e. [DOI] [PubMed] [Google Scholar]
- 3.Larhammar D, et al. Origins of the many NPY-family receptors in mammals. Peptides. 2001;22(3):295–307. doi: 10.1016/s0196-9781(01)00331-x. [DOI] [PubMed] [Google Scholar]
- 4.Adrian TE, et al. Neuropeptide Y distribution in human brain. Nature. 1983;306(5943):584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- 5.Allen YS, et al. Neuropeptide Y distribution in the rat brain. Science. 1983;221(4613):877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg JM, Hokfelt T. Multiple co-existence of peptides and classical transmitters in peripheral autonomic and sensory neurons--functional and pharmacological implications. Prog Brain Res. 1986;68:241–262. doi: 10.1016/s0079-6123(08)60242-3. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32(5):645–659. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med. 2010;2(11):429–439. doi: 10.1002/emmm.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caberlotto L, Fuxe K, Hurd YL. Characterization of NPY mRNA-expressing cells in the human brain: co-localization with Y2 but not Y1 mRNA in the cerebral cortex, hippocampus, amygdala, and striatum. Journal of Chemical Neuroanatomy. 2000;20(3–4):327–337. doi: 10.1016/s0891-0618(00)00107-1. [DOI] [PubMed] [Google Scholar]
- 10.Wahlestedt C, Ekman R, Widerlov E. Neuropeptide Y (NPY) and the central nervous system: distribution effects and possible relationship to neurological and psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(1–2):31–54. doi: 10.1016/0278-5846(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 11.Yamazoe M, et al. Distribution of neuropeptide Y in the lower brainstem: an immunohistochemical analysis. Brain Res. 1985;335(1):109–120. doi: 10.1016/0006-8993(85)90281-1. [DOI] [PubMed] [Google Scholar]
- 12.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system--II. Immunohistochemical analysis. Neuroscience. 1986;18(3):545–618. doi: 10.1016/0306-4522(86)90057-6. [DOI] [PubMed] [Google Scholar]
- 13.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system--I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986;18(3):527–543. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- 14.Larhammar D, Salaneck E. Molecular evolution of NPY receptor subtypes. Neuropeptides. 2004;38(4):141–151. doi: 10.1016/j.npep.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Dumont Y, et al. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13(1):73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanic D, et al. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol. 2006;499(3):357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- 17.Stanic D, et al. Characterization of NPY Y2 receptor protein expression in the mouse brain. II. Coexistence with NPY, the Y1 receptor, and other neurotransmitter-related molecules. J Comp Neurol. 2011;519(7):1219–1257. doi: 10.1002/cne.22608. [DOI] [PubMed] [Google Scholar]
- 18.Dumont Y, et al. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. The Journal of Comparative Neurology. 1998;402(3):372–384. [PubMed] [Google Scholar]
- 19.Kask A, et al. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neuroscience & Biobehavioral Reviews. 2002;26(3):259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 20.Wolak ML, et al. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464(3):285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- 21.Sah R, Geracioti TD. Neuropeptide Y and posttraumatic stress disorder. Mol Psychiatry. 2013;18(6):646–655. doi: 10.1038/mp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grove KL, et al. Neuropeptide Y Y5 receptor protein in the cortical/limbic system and brainstem of the rat: expression on gamma-aminobutyric acid and corticotropin-releasing hormone neurons. Neuroscience. 2000;100(4):731–740. doi: 10.1016/s0306-4522(00)00308-0. [DOI] [PubMed] [Google Scholar]
- 23.Dimitrov EL, et al. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148(8):3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- 24.Giesbrecht CJ, et al. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J Neurosci. 2010;30(50):16970–16982. doi: 10.1523/JNEUROSCI.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rostkowski AB, et al. Cell-specific expression of neuropeptide Y Y1 receptor immunoreactivity in the rat basolateral amygdala. J Comp Neurol. 2009;517(2):166–176. doi: 10.1002/cne.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illes P, Finta EP, Nieber K. Neuropeptide Y potentiates via Y2-receptors the inhibitory effect of noradrenaline in rat locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(5):546–548. doi: 10.1007/BF00173217. [DOI] [PubMed] [Google Scholar]
- 27.Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7(17):1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- 28.Heilig M, et al. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 29.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38(4):225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Morgan CA, 3rd, et al. Relationship among plasma cortisol, catecholamines, neuropeptide Y, and human performance during exposure to uncontrollable stress. Psychosom Med. 2001;63(3):412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Morgan CA, 3rd, et al. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47(10):902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
- 32.Morgan CA, 3rd, et al. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol Psychiatry. 2002;52(2):136–142. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- 33.Morgan CA, 3rd, et al. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biological Psychiatry. 2002;52(2):136–142. doi: 10.1016/s0006-3223(02)01319-7. [DOI] [PubMed] [Google Scholar]
- 34.Mickey BJ, et al. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011;68(2):158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 37.Sah R, et al. Low Cerebrospinal Fluid Neuropeptide Y Concentrations in Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66(7):705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmusson AM, et al. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biological Psychiatry. 2000;47(6):526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 39.Morgan CA, 3rd, et al. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol Psychiatry. 2003;54(10):1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- 40.Amstadter AB, et al. NPY moderates the relation between hurricane exposure and generalized anxiety disorder in an epidemiologic sample of hurricane-exposed adults. Depress Anxiety. 2010;27(3):270–275. doi: 10.1002/da.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domschke K, et al. Chromosome 4q31-34 panic disorder risk locus: association of neuropeptide Y Y5 receptor variants. Am J Med Genet B Neuropsychiatr Genet. 2008;147b(4):510–516. doi: 10.1002/ajmg.b.30629. [DOI] [PubMed] [Google Scholar]
- 42.Boulenger JP, et al. Elevated plasma levels of neuropeptide Y in patients with panic disorder. Am J Psychiatry. 1996;153(1):114–116. doi: 10.1176/ajp.153.1.114. [DOI] [PubMed] [Google Scholar]
- 43.Stein MB, et al. Plasma neuropeptide Y in anxiety disorders: findings in panic disorder and social phobia. Psychiatry Res. 1996;59(3):183–188. doi: 10.1016/0165-1781(95)02776-9. [DOI] [PubMed] [Google Scholar]
- 44.Altemus M, et al. Normal CSF oxytocin and NPY levels in OCD. Biol Psychiatry. 1999;45(7):931–933. doi: 10.1016/s0006-3223(98)00263-7. [DOI] [PubMed] [Google Scholar]
- 45.Lindberg C, et al. No association between the −399 C > T polymorphism of the neuropeptide Y gene and schizophrenia, unipolar depression or panic disorder in a Danish population. Acta Psychiatr Scand. 2006;113(1):54–58. doi: 10.1111/j.1600-0447.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto H, et al. Plasma neuropeptide Y in patients with major depressive disorder. Neurosci Lett. 1996;216(1):57–60. doi: 10.1016/0304-3940(96)13008-1. [DOI] [PubMed] [Google Scholar]
- 47.Heilig M, et al. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res. 2004;38(2):113–121. doi: 10.1016/s0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 48.Hou C, et al. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res. 2006;1095(1):154–158. doi: 10.1016/j.brainres.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson C, et al. Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides. 1996;17(3):359–362. doi: 10.1016/0196-9781(96)00013-7. [DOI] [PubMed] [Google Scholar]
- 50.Widerlov E, et al. Neuropeptide Y and peptide YY as possible cerebrospinal fluid markers for major depression and schizophrenia, respectively. J Psychiatr Res. 1988;22(1):69–79. doi: 10.1016/0022-3956(88)90030-1. [DOI] [PubMed] [Google Scholar]
- 51.Westrin A, Ekman R, Traskman-Bendz L. Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur Neuropsychopharmacol. 1999;9(3):205–211. doi: 10.1016/s0924-977x(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 52.Widdowson PS, Ordway GA, Halaris AE. Reduced neuropeptide Y concentrations in suicide brain. J Neurochem. 1992;59(1):73–80. doi: 10.1111/j.1471-4159.1992.tb08877.x. [DOI] [PubMed] [Google Scholar]
- 53.Caberlotto L, Hurd YL. Reduced neuropeptide Y mRNA expression in the prefrontal cortex of subjects with bipolar disorder. Neuroreport. 1999;10(8):1747–1750. doi: 10.1097/00001756-199906030-00022. [DOI] [PubMed] [Google Scholar]
- 54.Kuromitsu J, et al. Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res Gene Expr Patterns. 2001;1(1):17–21. doi: 10.1016/s1567-133x(01)00003-5. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, et al. A neuropeptide Y variant (rs16139) associated with major depressive disorder in replicate samples from Chinese Han population. PLoS One. 2013;8(2):57042. doi: 10.1371/journal.pone.0057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sjoholm LK, et al. PreproNPY Pro7 protects against depression despite exposure to environmental risk factors. J Affect Disord. 2009;118(1–3):124–130. doi: 10.1016/j.jad.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Mathé AA, et al. Effects of electroconvulsive treatment on somatostattn, neuropeptide Y, endothelin, and neurokinin a concentrations in cerebrospinal fluid of depressed patients: A pilot study. Depression. 1995;3(5):250–256. [Google Scholar]
- 58.Nikisch G, Mathe AA. CSF monoamine metabolites and neuropeptides in depressed patients before and after electroconvulsive therapy. Eur Psychiatry. 2008;23(5):356–359. doi: 10.1016/j.eurpsy.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Nikisch G, et al. Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int J Neuropsychopharmacol. 2005;8(3):403–410. doi: 10.1017/S1461145705005158. [DOI] [PubMed] [Google Scholar]
- 60.Sah R, et al. Cerebrospinal fluid neuropeptide Y in combat veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:277–283. doi: 10.1016/j.psyneuen.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yehuda R, Brand S, Yang R-K. Plasma Neuropeptide Y Concentrations in Combat Exposed Veterans: Relationship to Trauma Exposure, Recovery from PTSD, and Coping. Biological Psychiatry. 2006;59(7):660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 62.Bremner JD, et al. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54(3):246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 63.Rasmusson AM, et al. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47(6):526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- 64.Rasmusson AM, et al. Plasma neuropeptide Y (NPY) increases in humans in response to the alpha 2 antagonist yohimbine. Neuropsychopharmacology. 1998;19(1):95–98. doi: 10.1016/S0893-133X(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 65.Bannon AW, et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868(1):79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- 66.Sudakov SK, et al. Differences in genetic predisposition to high anxiety in two inbred rat strains: role of substance P, diazepam binding inhibitor fragment and neuropeptide Y. Psychopharmacology (Berl) 2001;154(4):327–335. doi: 10.1007/s002130000651. [DOI] [PubMed] [Google Scholar]
- 67.Broqua P, et al. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6(3):215–222. [PubMed] [Google Scholar]
- 68.Heilig M, et al. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98(4):524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- 69.Britton KT, et al. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacology (Berl) 1997;132(1):6–13. doi: 10.1007/s002130050313. [DOI] [PubMed] [Google Scholar]
- 70.Heilig M, et al. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regul Pept. 1992;41(1):61–69. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- 71.Lin EJ, et al. Adult-onset hippocampal-specific neuropeptide Y overexpression confers mild anxiolytic effect in mice. Eur Neuropsychopharmacol. 2010;20(3):164–175. doi: 10.1016/j.euroneuro.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Thorsell A, et al. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97(23):12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Primeaux SD, et al. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30(9):1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- 74.Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368(2–3):143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 75.Heilig M, et al. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8(4):357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- 76.Kask A, Rago L, Harro J. Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13-36 microinjected into vicinity of locus coeruleus in rats. Brain Res. 1998;788(1–2):345–348. doi: 10.1016/s0006-8993(98)00076-6. [DOI] [PubMed] [Google Scholar]
- 77.Trent NL, Menard JL. Infusions of neuropeptide Y into the lateral septum reduce anxiety-related behaviors in the rat. Pharmacol Biochem Behav. 2011;99(4):580–590. doi: 10.1016/j.pbb.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Ide S, et al. Opposing roles of corticotropin-releasing factor and neuropeptide Y within the dorsolateral bed nucleus of the stria terminalis in the negative affective component of pain in rats. J Neurosci. 2013;33(14):5881–5894. doi: 10.1523/JNEUROSCI.4278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9(1):21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- 80.Britton KT, et al. Neuropeptide Y blocks anxiogenic-like behavioral action of corticotropin-releasing factor in an operant conflict test and elevated plus maze. Peptides. 2000;21(1):37–44. doi: 10.1016/s0196-9781(99)00169-2. [DOI] [PubMed] [Google Scholar]
- 81.Karlsson RM, et al. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2008;195(4):547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- 82.Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59(2):201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- 83.Karl T, Burne THJ, Herzog H. Effect of Y1 receptor deficiency on motor activity, exploration, and anxiety. Behavioural Brain Research. 2006;167(1):87–93. doi: 10.1016/j.bbr.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 84.Longo A, et al. Conditional Inactivation of Neuropeptide Y Y1 Receptors Unravels the Role of Y1 and Y5 Receptors Coexpressing Neurons in Anxiety. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Bertocchi I, et al. Regulatory functions of limbic Y1 receptors in body weight and anxiety uncovered by conditional knockout and maternal care. Proc Natl Acad Sci U S A. 2011;108(48):19395–19400. doi: 10.1073/pnas.1109468108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sorensen G, et al. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J Neurosci Res. 2004;77(5):723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- 87.Olesen MV, et al. Neuropeptide Y Y1 receptor hippocampal overexpression via viral vectors is associated with modest anxiolytic-like and proconvulsant effects in mice. J Neurosci Res. 2012;90(2):498–507. doi: 10.1002/jnr.22770. [DOI] [PubMed] [Google Scholar]
- 88.Lyons AM, Thiele TE. Neuropeptide Y conjugated to saporin alters anxiety-like behavior when injected into the central nucleus of the amygdala or basomedial hypothalamus in BALB/cJ mice. Peptides. 2010;31(12):2193–2199. doi: 10.1016/j.peptides.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kask A, Rago L, Harro J. Anxiogenic-like effect of the NPY Y1 receptor antagonist BIBP3226 administered into the dorsal periaqueductal gray matter in rats. Regul Pept. 1998;75–76:255–262. doi: 10.1016/s0167-0115(98)00076-7. [DOI] [PubMed] [Google Scholar]
- 90.Kask A, Rago L, Harro J. NPY Y1 receptors in the dorsal periaqueductal gray matter regulate anxiety in the social interaction test. Neuroreport. 1998;9(12):2713–2716. doi: 10.1097/00001756-199808240-00005. [DOI] [PubMed] [Google Scholar]
- 91.Kask A, et al. Neuropeptide Y Y1 receptor antagonist BIBP3226 produces conditioned place aversion in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):705–711. doi: 10.1016/s0278-5846(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 92.Gilpin NW, et al. Effects of neuropeptide Y and ethanol on arousal and anxiety-like behavior in alcohol-preferring rats. Alcohol. 2011;45(2):137–145. doi: 10.1016/j.alcohol.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caberlotto L, et al. Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Brain Res Mol Brain Res. 1998;59(1):58–65. doi: 10.1016/s0169-328x(98)00137-5. [DOI] [PubMed] [Google Scholar]
- 94.Trent NL, Menard JL. Lateral septal infusions of the neuropeptide Y Y2 receptor agonist, NPY13–36 differentially affect different defensive behaviors in male, Long Evans rats. Physiology & Behavior. 2013;110–111(0):20–29. doi: 10.1016/j.physbeh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Smialowska M, et al. The effect of intrahippocampal injection of group II and III metobotropic glutamate receptor agonists on anxiety; the role of neuropeptide Y. Neuropsychopharmacology. 2007;32(6):1242–1250. doi: 10.1038/sj.npp.1301258. [DOI] [PubMed] [Google Scholar]
- 96.Sajdyk TJ, et al. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002;71(3):419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 97.McCall NM, et al. Effects of sex and deletion of neuropeptide Y2 receptors from GABAergic neurons on affective and alcohol drinking behaviors in mice. Front Integr Neurosci. 2013;7:100. doi: 10.3389/fnint.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kallupi M, et al. Neuropeptide Y Y2R blockade in the central amygdala reduces anxiety-like behavior but not alcohol drinking in alcohol-dependent rats. Addict Biol. 2013 doi: 10.1111/adb.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tasan RO, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30(18):6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tasan RO, et al. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158(4):1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Painsipp E, Herzog H, Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008;55(1):117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Painsipp E, et al. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 2008;7(5):532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tschenett A, et al. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18(1):143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- 104.Nguyen NK, et al. Effect of neuropeptide Y Y2 receptor deletion on emotional stress-induced neuronal activation in mice. Synapse. 2009;63(3):236–246. doi: 10.1002/syn.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43(7):1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 106.Gehlert DR, et al. Co-expression of neuropeptide Y Y1 and Y5 receptors results in heterodimerization and altered functional properties. Biochem Pharmacol. 2007;74(11):1652–1664. doi: 10.1016/j.bcp.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 107.Oberto A, et al. Expression patterns of promoters for NPY Y(1) and Y(5) receptors in Y(5)RitTA and Y(1)RVenus BAC-transgenic mice. Eur J Neurosci. 2007;26(1):155–170. doi: 10.1111/j.1460-9568.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- 108.Fetissov SO, Kopp J, Hokfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides. 2004;38(4):175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Mashiko S, et al. Synergistic interaction between neuropeptide Y1 and Y5 receptor pathways in regulation of energy homeostasis. Eur J Pharmacol. 2009;615(1–3):113–117. doi: 10.1016/j.ejphar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 110.Gutman AR, et al. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28(48):12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karl T, et al. Acoustic startle response and sensorimotor gating in a genetic mouse model for the Y1 receptor. Neuropeptides. 2010;44(3):233–239. doi: 10.1016/j.npep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 112.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6(3):235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 114.Finta EP, Regenold JT, Illes P. Depression by neuropeptide Y of noradrenergic inhibitory postsynaptic potentials of locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1992;346(4):472–474. doi: 10.1007/BF00171093. [DOI] [PubMed] [Google Scholar]
- 115.Smialowska M. Neuropeptide Y immunoreactivity in the locus coeruleus of the rat brain. Neuroscience. 1988;25(1):123–131. doi: 10.1016/0306-4522(88)90011-5. [DOI] [PubMed] [Google Scholar]
- 116.Lach G, de Lima TC. Role of NPY Y1 receptor on acquisition, consolidation and extinction on contextual fear conditioning: dissociation between anxiety, locomotion and non-emotional memory behavior. Neurobiol Learn Mem. 2013;103:26–33. doi: 10.1016/j.nlm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 117.Fendt M, et al. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology (Berl) 2009;206(2):291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- 118.Pickens CL, et al. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience. 2009;164(4):1398–1406. doi: 10.1016/j.neuroscience.2009.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Verma D, et al. NPY controls fear conditioning and fear extinction by combined action on Y(1) and Y(2) receptors. Br J Pharmacol. 2012;166(4):1461–1473. doi: 10.1111/j.1476-5381.2012.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hendriksen H, et al. Re-exposure and environmental enrichment reveal NPY-Y1 as a possible target for post-traumatic stress disorder. Neuropharmacology. 2012;63(4):733–742. doi: 10.1016/j.neuropharm.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 121.Tovote P, et al. Central NPY receptor-mediated alteration of heart rate dynamics in mice during expression of fear conditioned to an auditory cue. Regul Pept. 2004;120(1–3):205–214. doi: 10.1016/j.regpep.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 122.Redrobe JP, et al. Role of serotonin (5-HT) in the antidepressant-like properties of neuropeptide Y (NPY) in the mouse forced swim test. Peptides. 2005;26(8):1394–1400. doi: 10.1016/j.peptides.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 123.Stogner KA, Holmes PV. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur J Pharmacol. 2000;387(2):R9–R10. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
- 124.Redrobe JP, et al. The neuropeptide Y (NPY) Y1 receptor subtype mediates NPY-induced antidepressant-like activity in the mouse forced swimming test. Neuropsychopharmacology. 2002;26(5):615–624. doi: 10.1016/S0893-133X(01)00403-1. [DOI] [PubMed] [Google Scholar]
- 125.Ishida H, et al. Infusion of neuropeptide Y into CA3 region of hippocampus produces antidepressant-like effect via Y1 receptor. Hippocampus. 2007;17(4):271–280. doi: 10.1002/hipo.20264. [DOI] [PubMed] [Google Scholar]
- 126.Heilig M, et al. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur J Pharmacol. 1988;147(3):465–467. doi: 10.1016/0014-2999(88)90182-3. [DOI] [PubMed] [Google Scholar]
- 127.Madsen TM, et al. Electroconvulsive stimuli enhance both neuropeptide Y receptor Y1 and Y2 messenger RNA expression and levels of binding in the rat hippocampus. Neuroscience. 2000;98(1):33–39. doi: 10.1016/s0306-4522(00)00078-6. [DOI] [PubMed] [Google Scholar]
- 128.Overstreet DH, et al. The Flinders Sensitive Line rat: A selectively bred putative animal model of depression. Neuroscience & Biobehavioral Reviews. 2005;29(4–5):739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 129.Serova L, et al. Altered gene expression for catecholamine biosynthetic enzymes and stress response in rat genetic model of depression. Brain Res Mol Brain Res. 1998;63(1):133–138. doi: 10.1016/s0169-328x(98)00270-8. [DOI] [PubMed] [Google Scholar]
- 130.Husum H, et al. Exacerbated loss of cell survival, neuropeptide Y-immunoreactive (IR) cells, and serotonin-IR fiber lengths in the dorsal hippocampus of the aged flinders sensitive line "depressed" rat: Implications for the pathophysiology of depression? J Neurosci Res. 2006;84(6):1292–1302. doi: 10.1002/jnr.21027. [DOI] [PubMed] [Google Scholar]
- 131.Jimenez Vasquez PA, et al. Neuropeptide Yin brains of the Flinders Sensitive Line rat, a model ofdepression. Effects of electroconvulsive stimuli and d-amphetamine on peptide concentrations and locomotion. Behav Brain Res. 2000;111(1–2):115–123. doi: 10.1016/s0166-4328(00)00142-x. [DOI] [PubMed] [Google Scholar]
- 132.Jimenez-Vasquez PA, Overstreet DH, Mathe AA. Neuropeptide Yin male and female brains of Flinders Sensitive Line a rat model of depression. Effects of electroconvulsive stimuli. J Psychiatr Res. 2000;34(6):405–412. doi: 10.1016/s0022-3956(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 133.Zambello E, et al. Acute stress differentially affects corticotropin-releasing hormone mRNA expression in the central amygdala of the "depressed" flinders sensitive line and the control flinders resistant line rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):651–661. doi: 10.1016/j.pnpbp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 134.Walker MW, et al. The novel neuropeptide Y Y5 receptor antagonist Lu AA33810 [N-[[trans-4-[(4,5-dihydro[1]benzothiepino[5,4-d]thiazol-2-yl)amino]cyclohexyl]me thyl]-methanesulfonamide] exerts anxiolytic- and antidepressant-like effects in rat models of stress sensitivity. J Pharmacol Exp Ther. 2009;328(3):900–911. doi: 10.1124/jpet.108.144634. [DOI] [PubMed] [Google Scholar]
- 135.Caberlotto L, et al. Alterations in neuropeptide Y levels and Y1 binding sites in the Flinders Sensitive Line rats, a genetic animal model of depression. Neurosci Lett. 1999;265(3):191–194. doi: 10.1016/s0304-3940(99)00234-7. [DOI] [PubMed] [Google Scholar]
- 136.Bjornebekk A, Mathe AA, Brene S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus. 2010;20(7):820–828. doi: 10.1002/hipo.20683. [DOI] [PubMed] [Google Scholar]
- 137.Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neuroscience & Biobehavioral Reviews. 2005;29(4–5):627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 138.Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: An update. Pharmacology & Therapeutics. 1997;74(3):299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 139.Goyal SN, et al. Neuropeptide Y modulates the antidepressant activity of imipramine in olfactory bulbectomized rats: involvement of NPY Y1 receptors. Brain Res. 2009;1266:45–53. doi: 10.1016/j.brainres.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 140.Morales-Medina JC, et al. Chronic administration of the Y2 receptor antagonist, JNJ-31020028, induced anti-depressant like-behaviors in olfactory bulbectomized rat. Neuropeptides. 2012;46(6):329–334. doi: 10.1016/j.npep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 141.Morales-Medina JC, et al. Role of neuropeptide Y Y(1) and Y(2) receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology. 2012;62(1):200–208. doi: 10.1016/j.neuropharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 142.Wood SK, et al. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151(4):1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wood SK. Individual differences in the neurobiology of social stress: implications for depression-cardiovascular disease comorbidity. Curr Neuropharmacol. 2014;12(2):205–211. doi: 10.2174/1570159X11666131120224413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chaijale NN, et al. Repeated Social Stress Increases Reward Salience and Impairs Encoding of Prediction by rat Locus Coeruleus Neurons. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chaijale NN, et al. Social stress engages opioid regulation of locus coeruleus norepinephrine neurons and induces a state of cellular and physical opiate dependence. Neuropsychopharmacology. 2013;38(10):1833–1843. doi: 10.1038/npp.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Russo SJ, et al. Neurobiology of resilience. Nat Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cohen H, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cohen H, et al. The Neuropeptide Y (NPY)-ergic System is Associated with Behavioral Resilience to Stress Exposure in an Animal Model of Post-Traumatic Stress Disorder. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serova LI, et al. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 150.Laukova M, et al. Early Intervention with Intransala NPY Prevents Single Prolonged Stress-Triggered Impairments in Hypothalamus and Ventral Hippocampus in Male Rats. Endocrinology. 2014 doi: 10.1210/en.2014-1192. in press. [DOI] [PubMed] [Google Scholar]
- 151.Serova LI, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 152.McGonigle P. Peptide therapeutics for CNS indications. Biochem Pharmacol. 2012;83(5):559–566. doi: 10.1016/j.bcp.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 153.Held K, et al. Neuropeptide Y (NPY) shortens sleep latency but does not suppress ACTH and cortisol in depressed patients and normal controls. Psychoneuroendocrinology. 2006;31(1):100–107. doi: 10.1016/j.psyneuen.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 154.Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: the universal soldier. Cell Mol Life Sci. 2003;60(2):350–377. doi: 10.1007/s000180300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zukowska-Grojec Z, Neuropeptide Y. A novel sympathetic stress hormone and more. Ann N Y Acad Sci. 1995;771:219–233. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]
- 156.Edvinsson L, et al. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol. 1984;83(2):519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schuerch LV, et al. Human neuropeptide Y potentiates alpha1-adrenergic blood pressure responses in vivo. Am J Physiol. 1998;275(3 Pt 2):H760–H766. doi: 10.1152/ajpheart.1998.275.3.H760. [DOI] [PubMed] [Google Scholar]
- 158.Abe K, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr Top Med Chem. 2007;7(17):1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- 159.Xie F, et al. Long-term neuropeptide Y administration in the periphery induces abnormal baroreflex sensitivity and obesity in rats. Cell Physiol Biochem. 2012;29(1–2):111–120. doi: 10.1159/000337592. [DOI] [PubMed] [Google Scholar]
- 160.Kuo LE, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 161.Kastin AJ, Akerstrom V. Nonsaturable entry of neuropeptide Y into brain. Am J Physiol. 1999;276(3 Pt 1):E479–E482. doi: 10.1152/ajpendo.1999.276.3.E479. [DOI] [PubMed] [Google Scholar]
- 162.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 163.Ionescu IA, et al. Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons. Neuropsychopharmacology. 2012;37(6):1323–1337. doi: 10.1038/npp.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thorne RG, et al. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692(1–2):278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 165.Thorne RG, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 166.Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33(5):329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- 167.Lacroix JS, Mosimann BL. Attenuation of allergen-evoked nasal responses by local pretreatment with exogenous neuropeptide Y in atopic patients. J Allergy Clin Immunol. 1996;98(3):611–616. doi: 10.1016/s0091-6749(96)70095-7. [DOI] [PubMed] [Google Scholar]
- 168.Lacroix JS, et al. Intranasal administration of neuropeptide Y in man: systemic absorption and functional effects. Br J Pharmacol. 1996;118(8):2079–2084. doi: 10.1111/j.1476-5381.1996.tb15647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cervin A, et al. Functional effects of neuropeptide Y receptors on blood flow and nitric oxide levels in the human nose. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1724–1728. doi: 10.1164/ajrccm.160.5.9902102. [DOI] [PubMed] [Google Scholar]
- 170.Hallschmid M, et al. NPY attenuates positive cortical DC-potential shift upon food intake in man. Psychoneuroendocrinology. 2003;28(4):529–539. doi: 10.1016/s0306-4530(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 171.Hallschmid M, et al. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol Behav. 2004;83(1):55–64. doi: 10.1016/j.physbeh.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 172.U.S. National Institutes of Health; Dennis Charney, Mount Sinai School of Medicine. Bethesda, MD: National Library of Medicine US; 2012. [Last accessed 13 Aug 2014]. Intranasal administration of neuropeptide Y in health male volunteers (NPY) Available at: http://clinicaltrials.gov/ct2/show/NCT00748956?term=NCT+00748956&rank=1. NLM Identifier: NCT00748956. [Google Scholar]
- 173.U.S. National Institutes of Health; James Murrough, Mount Sinai School of Medicine. Bethesda, MD: National Library of Medicine (US; 2013. [Last accessed 13 Aug 2014]. A dose escalation study of intranasal neuropeptide Y in post-traumatic stress disorder (PTSD) Available at http://clinicaltrials.gov/ct2/show/NCT01533519?term=NCT+01533519&rank=1. NLM Identifier: NCT01533519. [Google Scholar]
- 174.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology. 2014;0 doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014 doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46(4):329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]