Abstract
Objective
To evaluate gastrointestinal and cardiovascular adverse event risks associated with optical colonoscopy (OC) among Medicare outpatients who received computed tomography colonography (CTC) as their initial method of colorectal evaluation.
Methods
Medicare claims were compared between 6,114 outpatients ≥ 66 years who received initial CTC and 149,202 outpatients who received initial OC between January 2007 and December 2008. OC patients were matched on county of residence and year of evaluation. Outcomes included lower gastrointestinal bleeding, gastrointestinal perforation, other gastrointestinal events and cardiovascular events resulting in an emergency department visit or hospitalization within 30 days.
Results
Among 1,000 outpatients undergoing initial CTC, 12.4 experienced lower gastrointestinal bleeding, 0.7 perforation, 18.0 other gastrointestinal events and 45.5 cardiovascular events within 30 days. After multivariate adjustment, risks of lower gastrointestinal bleeding, other gastrointestinal events and cardiovascular events were higher with initial OC than CTC, with or without subsequent OC (OR 1.91 95CI [1.47,2.49], OR 1.35 95CI [1.07,1.69] and OR 1.38 95CI [1.18,1.62], respectively); however, perforation risk did not differ (p=0.10). This pattern is similar in older and symptomatic populations.
Conclusion
Rates of gastrointestinal bleeding, other gastrointestinal events and cardiovascular events are lower following initial CTC than OC, but rates of perforation do not differ.
Introduction
CT Colonography (CTC) is an alternative to optical colonoscopy (OC) for colorectal evaluation, including cancer screening and diagnostic evaluation. Although CTC demonstrates a per patient sensitivity of 0.78 in the detection of adenomas at least 6 mm, and an overall sensitivity of 89% in the detection of polyps at least 6 mm in diameter 1,2 it may be safer than OC given that CTC does not involve passage of an endoscope or require sedation. Prior case series suggest low rates of gastrointestinal perforation and chest pain among patients who receive CTC; particularly in the screening population.3,4,5 However, there is a paucity of population based studies directly evaluating the risk of adverse events following CTC among the elderly where the risks are thought to be higher.6-12 This lack of data about the risks and benefits of CTC in the older population, in addition to concerns over the cost effectiveness of this modality, was cited in the Centers for Medicare and Medicaid Services (CMS) decision to deny coverage for screening CTC in March 2009 13,14.
Given this background, our objective was to evaluate the risks of gastrointestinal and cardiovascular events among symptomatic and asymptomatic outpatient Medicare beneficiaries who received CTC as their first method of colorectal evaluation during the two years preceding this CMS coverage determination; January 2007 through December 2008. Adverse events known to be associated with OC were selected as OC is the traditional method of colorectal evaluation. We focused on patients undergoing initial CTC to capture adverse events attributable to the CTC and to any subsequent testing within 30 days (including OC with or without biopsy9,10,15) driven by the findings of the CTC. To provide clinical context CTC patients were compared to a cohort of patients who received initial OC and also stratified by asymptomatic (i.e. screening) and symptomatic (i.e. diagnostic) indications.3,5
Materials & Methods
Design Overview
This Health Insurance Portability and Accountability Act compliant study using de-identified Medicare claims data was exempt from institutional review board approval. We performed a retrospective cohort study of all Medicare beneficiaries ≥ 66 years of age in the United States with a claim for CTC between January 2007 and December 2008 and a randomly selected group of patients with a claim for OC during the same time period, matched 9:1 by county of residence and year of colorectal evaluation. Approximately 98% of adults in the United States ages 65 and older are enrolled in Medicare, making Medicare data a robust source of health care utilization.16 Adverse events resulting in an emergency department visit or inpatient admission 30 days following either procedure were determined from Medicare Provider Analysis and Review, Outpatient and Carrier files using appropriate Current Procedural Terminology / Healthcare Common Procedure Coding System (CPT / HCPCS) and International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) codes (eTable 1). We used a 30 day time interval for the assessment of complications to ensure a comparable follow-up interval in both cohorts based on prior data about complications from OC.9 Claims were also used to identify comorbidities associated with increased likelihood of adverse events in the year preceding either CTC or OC and comparable adverse events in the preceding 90 days. The analysis focused on patients who had either initial CTC, defined as no OC on the same day or within the prior 12 months, or initial OC, defined as no OC within the prior 12 months. Although 14% of the initial CTC patients in our study underwent OC within the 12 months after CTC, similar to published rates of 13-15% in the literature17,18, only 3% (187 / 6,114) of initial CTC patients received OC within 30 days. Due to this small sample size a separate analysis of adverse events among patients who received OC within 30 days of CTC was not performed. However, additional analyses stratified patients by asymptomatic (i.e. screening) and symptomatic (i.e. diagnostic) indications using ICD-9 codes from the CTC or OC claim, based on a previously utilized algorithm; 19,20 a full list of codes is provided in e-table 1.
Setting and Participants
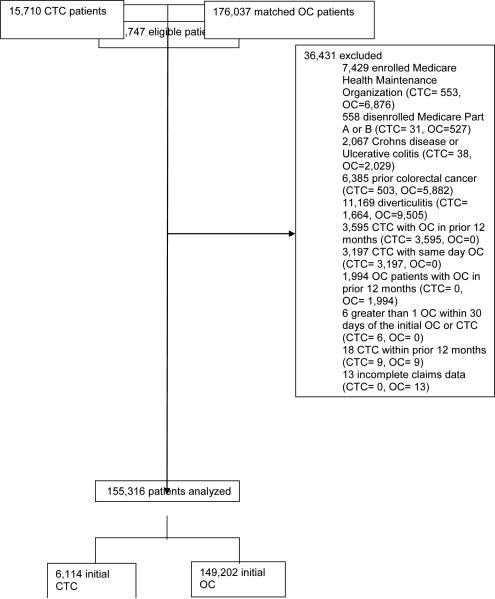
We excluded patients (a) enrolled in a Medicare Health Maintenance Organization within the year preceding or 30 days following CTC (n = 7,429); or (b) disenrolled from Medicare Part A or B coverage during the same time interval (n = 558). Similar to prior methodology9, we excluded patients with significant prior colonic disease that increased the risk of perforation including Crohns disease or Ulcerative colitis (n = 2,067), prior colorectal cancer (n = 6,385), or diverticulitis in the preceding year (n=11,169). Because the objective of our study was to evaluate patients who received CTC as the first method of colorectal evaluation we excluded patients who received CTC with OC either in the prior 12 months (n = 3,595) or on the same day (n = 3,197). Similarly, OC patients who received OC within the prior 12 months were excluded (n = 1,994). We also excluded patients with more than one OC within 30 days of the initial OC or CTC (n = 6). Eighteen patients were excluded for CTC claims in the year preceding OC or CTC and thirteen patients were excluded for incomplete billing data. No patients were excluded on the basis of an incomplete procedure. The final study population was comprised of 153,316 patients (Figure 1). These 153,316 patients included 3,609 CTC and 115,691 OC patients from a prior publication 20Differences in study populations are due to inclusion and / or exclusion criteria stemming from the disparate foci of these publications.
Figure 1.
Flow chart of study population
Outcomes and Follow Up
Four groups of adverse events requiring emergency department visits or inpatient admissions were identified in the 30 days following either procedure: lower gastrointestinal bleeding or administration of blood transfusions (excluding patients with transfusions performed 90 days prior to either procedure), gastrointestinal perforation, other gastrointestinal events (paralytic ileus, nausea, vomiting and dehydration, abdominal pain), and cardiovascular events (myocardial infarction or angina; arrhythmias; congestive heart failure [CHF]; cardiac or respiratory arrest; or syncope, hypotension, or shock). These adverse events were chosen as they have been associated with complications of OC, which is the traditional method of colorectal evaluation. Similar adverse events in the 90 days preceding CTC or OC were also recorded. Death within 30 days was evaluated, although cause of death cannot be inferred from claims data.
We accounted for common comorbidities associated with these adverse events using claims data from the year preceding CTC or OC including: atrial fibrillation / flutter, congestive heart failure, chronic pulmonary disease, stroke, diabetes, renal failure, diverticulosis, and obesity.7,9,21
Sociodemographic characteristics including gender, age, and race were obtained from beneficiary summary files. Age was categorized into three groups (66–74, 75–84, and ≥ 85 years) and race into five groups (White, Black, Hispanic, Asian, and other).
Statistical Analysis
Chi-square analysis was used to compare sociodemographic and clinical characteristics, unadjusted risks of adverse events, and mortality within 30 days between patients who received initial CTC and OC. Unadjusted risks of adverse events were included as these data have not been previously reported using claims data. Given the cohort study design a generalized linear regression with a logit link and binomial distribution was used to estimate the odds ratios (OR) of adverse events between patients undergoing CTC and OC by indication (i.e. symptomatic versus asymptomatic) controlling for differences in patient characteristics including gender, age, race, comorbidities associated with studied adverse events, and adverse events in the preceding 90 days. Due to low event rates for the targeted adverse events there is limited concern of overestimation of the risk ratio, which is approximated by the OR in cohort studies. Statistical significance was declared for results with a two-sided p-value of < 0.05. We used STATA, version 11(STATA Corp) for all statistical analyses.
Results
The final cohort included 6,114 initial CTC outpatients with a mean age of 76.7 years (age range, 66 – 103 years; 2,223 males [mean age 77; age range 66-96]; 3,891 females [mean age 77; age range 66-103]) and 149,202 initial OC outpatients with a mean age of 74.4 years (age range, 66-104 years; 67,586 males [mean age 74; age range 66-101]; 81,616 females [mean age 75; age range 66-104]). Women, patients > 75 years of age and Whites were more likely to receive CTC as their initial method of colorectal evaluation than OC (Table 1). Patients undergoing initial CTC had a higher prevalence of comorbidties than patients undergoing initial OC, except for diabetes, diverticulosis and obesity. Among patients referred to initial CTC, with or without OC in the subsequent 30 days, the unadjusted risk of lower gastrointestinal bleeding per 1000 patients was 12.4, of perforation was 0.7, of other gastrointestinal events was 18.0 and of cardiovascular events was 45.5. Of note, perforation rates were low in both cohorts (4/6,114 [0.7%] initial CTC and 181/ 149,202 [1.2%], initial OC). Similar proportions of patients died within 30 days in both cohorts.
Table 1.
Frequency of Medicare outpatient characteristics and unadjusted adverse events within 30 days of receiving initial CT colonography (CTC) or initial optical colonoscopy (OC) between January 2007 and December 2008, (%)
| Initial CTC (n = 6,114) | Initial OC (n = 149,202) | p value | |
|---|---|---|---|
| Gender | <.001 | ||
| Male | 2223 (36.4) | 67586 (45.3) | |
| Female | 3891 (63.6) | 81616 (54.7) | |
| Age | <.001 | ||
| 66-74 | 2500 (40.9) | 82411 (55.2) | |
| 75-84 | 2700 (44.2) | 55893 (37.5) | |
| ≥85 | 914 (14.9) | 10898 (7.3) | |
| Race | <.001 | ||
| White | 5688 (93.0) | 130481 (87.5) | |
| Black | 239 (3.9) | 10133 (6.8) | |
| Other a | 78 (1.3) | 2946 (2.0) | |
| Asian | 52 (0.9) | 3197 (2.1) | |
| Hispanic | 57 (0.9) | 2445 (1.6) | |
| Comorbidities associated with Adverse Events | |||
| Atrial fibrillation / flutter | 1610 (26.3) | 18813 (12.6) | <.001 |
| Congestive heart failure | 1298 (21.2) | 19735 (13.2) | <.001 |
| Chronic pulmonary disease | 1759 (28.8) | 34149 (22.9) | <.001 |
| Diabetes | 1729 (28.3) | 45040 (30.2) | 0.001 |
| Stroke | 1419 (23.2) | 24457 (16.4) | <.001 |
| Renal disease | 636 (10.4) | 12827 (8.6) | <.001 |
| Diverticulosis | 2617 (42.8) | 85437 (57.3) | <.001 |
| Obesity | 298 (4.9) | 8010 (5.4) | 0.092 |
| Unadjusted adverse events within 30 days (risk per 1000 Medicare beneficiaries) | |||
| Gastrointestinal bleeding / transfusion | 76 (12.4) | 2471 (16.6) | 0.013 |
| Perforation | 4(0.7) | 181 (1.2) | 0.214 |
| Other GI | 110 (18.0) | 2692 (18.0) | 0.997 |
| --- Paralytic ileus | 11 (1.8) | 573 (3.8) | 0.011 |
| --- Nausea, Vomiting or Dehydration | 75 (12.3) | 1721 (11.4) | 0.623 |
| --- Abdominal pain | 34 (5.6) | 695 (4.7) | 0.311 |
| Cardiovascular disease | 278 (45.5) | 5167 (34.6) | <.001 |
| --- MI or angina b | 49 (8.0) | 1112 (7.5) | 0.617 |
| --- Arrhythmias | 148 (24.2) | 2754 (18.5) | 0.001 |
| --- CHF | 119 (19.5) | 1874 (12.6) | <.001 |
| --- Cardiac or respiratory arrest c | 28 (4.6) | 544 (3.6) | 0.238 |
| ---Syncope, Hypotension or Shock | 49 (8.0) | 945 (6.3) | 0.106 |
| Mortality | |||
| Death within 30 days | 21 (0.3) | 499 (0.3) | 0.905 |
Other includes Unknown and Native American
including chest pain
not including shortness of breath
Multivariate regression was performed to account for differences in gender, age, race, comorbidities associated with adverse events, and adverse events in the preceding 90 days. This revealed higher risk of lower gastrointestinal bleeding, other gastrointestinal events and cardiovascular events among patients who underwent initial OC compared to initial CTC in the subsequent 30 days (OR 1.91 95CI [1.47,2.49], OR 1.35 95CI [1.07,1.69] and OR 1.38 95CI [1.18,1.62], respectively), but no difference in the risk of perforation (Table 2). Similarly, even with adjustment for age, patients greater than 75 years who received either CTC or OC demonstrated a higher risk of lower gastrointestinal bleeding, other gastrointestinal events, and cardiovascular events than patients less than 75 years (OR 2.98 95CI [2.61,3.41], OR 1.39 95CI [1.26,1.53], and OR 1.44 95CI [1.33,1.55], respectively).
Table 2.
Regression analysis of adverse events among Medicare outpatients who received initial CT colonography (CTC) or initial optical colonoscopy (OC) between January 2007 and December 2008*
| Gastrointestinal Bleed | Perforation | Other Gastrointestinal events | Cardiovascular Events | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Total population (n = 155,316) | |||||||||
| Type of Examination | |||||||||
| Initial CTC (n = 6,114) | |||||||||
| Initial OC (n = 149,202) | 1.91 | [1.47,2.49] | 2.62 | [0.84,8.22] | 1.35 | [1.07,1.69] | 1.38 | [1.18,1.62] | |
| Age | 66-74 (ref) | ||||||||
| 75-84 | 1.38 | [1.24,1.53] | 1.18 | [0.84,1.66] | 1.39 | [1.26,1.53] | 1.44 | [1.33,1.55] | |
| ≥ 85 | 2.98 | [2.61,3.41] | 0.75 | [0.38,1.46] | 1.87 | [1.63,2.15] | 2.54 | [2.29,2.80] | |
Regression analyses are adjusted for gender, age, race, comorbidities associated with adverse events (i.e. atrial fibrillation / flutter, congestive heart failure, chronic pulmonary disease, diabetes, stroke, renal disease, diverticulosis, obesity), and adverse events in preceding 90 days.
We also examined whether this pattern differed depending on the presence of symptoms. Higher unadjusted adverse event rates per 1,000 patients were demonstrated among those with symptoms relative to those without symptoms within both cohorts (Table 3). Following adjustment for gender, age, race, comorbidities associated with adverse events, and adverse events in the preceding 90 days, symptomatic patients referred to initial OC were found to have higher adjusted rates of lower gastrointestinal bleeding, other gastrointestinal events and cardiovascular events compared to symptomatic patients referred to initial CTC, with or without OC in the subsequent 30 days (OR 1.92 95CI [1.47, 2.51], OR 1.35 95CI [1.07,1.70], OR 1.43 95CI [1.22, 1.68], respectively )(Table 4). Again, even with adjustment for age, higher rates of lower gastrointestinal bleeding, other gastrointestinal events and cardiovascular events were demonstrated among symptomatic patients greater than 75 years compared to patients less than 75 years (OR 2.70 95CI [2.43,3.21], OR 1.77 95CI [1.53,2.05], OR 2.37 95CI [2.14,2.63], respectively )(Table 4). A similar pattern was observed within the asymptomatic cohort, but did not reach significance.
Table 3.
Unadjusted risk per 1000 Medicare outpatients for adverse events within 30 days of initial CT colonography (CTC) or initial optical colonoscopy (OC) received between January 2007 and December 2008 by indication
| Initial CTC | Initial OC | |||||||
|---|---|---|---|---|---|---|---|---|
| Asymptomatic (n=1384) | Symptomatic (n=4,730) | Asymptomatic (n=54,039) | Symptomatic (n=95,163) | |||||
| Events (n) | Risk | Events (n) | Risk | Events (n) | Risk | Events (n) | Risk | |
| Gastrointestinal bleeding | 4 | 2.9 | 72 | 15.2 | 371 | 6.9 | 2100 | 22.1 |
| Perforation | 1 | 0.7 | 3 | 0.6 | 46 | 0.9 | 135 | 1.4 |
| Other GI | 5 | 3.6 | 105 | 22.2 | 311 | 5.8 | 2381 | 25 |
| --- Ileus | 0 | 0 | 11 | 2.3 | 76 | 1.4 | 497 | 5.2 |
| --- Nausea, Vomiting or Dehydration | 4 | 2.9 | 71 | 15 | 169 | 3.1 | 1552 | 16.3 |
| --- Abdominal pain | 2 | 1.4 | 32 | 6.8 | 107 | 2 | 588 | 6.2 |
| CVD events | 26 | 18.8 | 252 | 53.3 | 610 | 11.3 | 4557 | 47.9 |
| --- MI or angina a | 4 | 2.9 | 45 | 9.5 | 176 | 3.3 | 936 | 9.8 |
| --- Arrhythmias | 14 | 10.1 | 134 | 28.3 | 329 | 6.1 | 2425 | 25.5 |
| --- CHF | 5 | 3.6 | 114 | 24.1 | 94 | 1.7 | 1780 | 18.7 |
| --- Cardiac or respiratory arrest b | 1 | 0.7 | 27 | 5.7 | 43 | 0.8 | 501 | 5.3 |
| 9 | 6.5 | 40 | 8.5 | 149 | 2.8 | 796 | 8.4 | |
including chest pain
not including shortness of breath
Table 4.
Regression analysis of adverse events among Medicare outpatients who received initial CT colonography (CTC) or initial optical colonoscopy (OC) between January 2007 and December 2008 by indication*
| Gastrointestinal Bleed | Perforation | Other Gastrointestinal events | Cardiovascular Events | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Asymptomatic population (n = 55,423) | |||||||||
| Type of Examination | |||||||||
| Initial CTC (n = 1,384) | |||||||||
| Initial OC (n = 54,039) | 2.29 | [0.76, 6.90] | 3.53 | [0.20, 63.54] | 2.36 | [0.89, 6.30] | 1.22 | [0.74, 2.01] | |
| Age | 66-74 (ref) | ||||||||
| 75-84 | 1.14 | [0.87,1.48] | 1.02 | [0.49, 2.14] | 0.92 | [0.70, 1.22] | 1.35 | [1.10,1.64] | |
| ≥ 85 | 1.49 | [0.81, 2.75] | 1.99 | [0.45, 8.69] | 1.22 | [0.68, 2.20] | 1.56 | [1.05, 2.32] | |
| Symptomatic population (n = 99,893) | |||||||||
| Type of Examination | |||||||||
| Initial CTC (n = 4,730) | |||||||||
| Initial OC (n = 95,163) | 1.92 | [1.47, 2.51] | 2.55 | [0.73, 8.94] | 1.35 | [1.07,1.70] | 1.43 | [1.22, 1.68] | |
| Age | 66-74 (ref) | ||||||||
| 75-84 | 1.36 | [1.21,1.52] | 1.20 | [0.81,1.79] | 1.40 | [1.26, 1.56] | 1.38 | [1.27, 1.50] | |
| ≥ 85 | 2.79 | [2.43, 3.21] | 0.67 | [0.33, 1.37] | 1.77 | [1.53, 2.05] | 2.37 | [2.14, 2.63] | |
Regression analyses are adjusted for gender, age, race, comorbidities associated with adverse events (i.e. atrial fibrillation / flutter, congestive heart failure, chronic pulmonary disease, diabetes, stroke, renal disease, diverticulosis, obesity), and adverse events in preceding 90 days.
Discussion
Our study demonstrates that patients ≥ 66 years of age who undergo initial CTC, with or without subsequent OC within 30 days, have lower risks of lower gastrointestinal bleeding, other gastrointestinal events and cardiovascular events compared to patients who undergo initial OC. However, the risk of perforation does not differ between initial CTC and OC. This pattern is similar among symptomatic, asymptomatic and older patients, although it did not reach significance for asymptomatic patients.
The findings in this population based study differ somewhat from prior case series demonstrating lower unadjusted rates of perforation among patients who undergo CTC (0.00 – 0.05%) 3,22 and of cardiovascular events (0.05- 0.06 per 1000 diagnostic CTC procedures) 3,4. Differences in our results may reflect limitations of prior study populations, follow up interval, or measurement approach. Alternatively, these findings may reflect the small number of perforations identified in our study; particularly within the CTC cohort. Although we used the same methodology to identify perforations in both cohorts (i.e., emergency department visits and hospitalizations), CTC is more sensitive to sub-clinical perforation than OC due to the ability to visualize small amount of pneumoperitoneum.23 It is reassuring that our results are similar to perforation rates from a study where patients with pneumoperitoneum on CTC were referred to the emergency department (0.6 per 1,000 CTC procedures) 6, which resembles our measurement approach. Our results also extend the previously published literature about the risks of adverse events from CTC among Medicare patients in that we are the first study to assess the risk of lower gastrointestinal bleeding following CTC.
Given that clinicians and patients desire data on the risks of initial CTC relative to initial OC, we have provided comparative data between these two procedures. Our analyses suggest that patients who undergo initial CTC, with or without subsequent OC, may experience lower rates of serious gastrointestinal, other gastrointestinal and cardiovascular events compared to patients who receive initial OC. This finding is intuitively reasonable, given that CTC does not require sedation and the intention of CTC is to selectively refer the approximately 8-15% of patients with suspected clinically significant polyps (> 6mm) and masses to OC for further evaluation.17,18,22 However, it is important to recognize that we were unable to fully adjust for differences between the groups based upon the information available in claims data. Patients who receive CTC are generally sicker than patients who receive OC.20 Furthermore, given standard CTC technique, which does not include intravenous contrast, the use of a 30 day time interval is conservative and may include adverse events not directly related to this procedure. As such, our results may underestimate the differences between these two examinations.
Even after adjustment, we found that the risk of adverse events following CTC depends upon patient age (comparing patients 75 and older to patients under 75). Higher risk of lower gastrointestinal bleeding and cardiovascular events among patients older than 75 years who undergo colorectal evaluation are concordant with prior studies evaluating OC 7-10, but has not previously been demonstrated for CTC. Both the US Preventive Services Task Force and the American College of Physicians recommend against routine screening of patients older than 75 due to increased risk of complications, competing causes of mortality and demonstrated benefit at least 7 years after screening.24,25 Our findings support the inclusion of CTC in these recommendations.
Our study has several limitations. We used billing codes from Medicare claims rather than medical record review to determine adverse events. No studies validating the use of billing claims for CTC have been reported. However, prior studies have demonstrated high sensitivity and specificity for the assessment of procedures, including endoscopy, using Medicare claims compared to medical charts 26-28 and high likelihood of identifying diagnoses and select adverse events in Medicare claims 29. It is reassuring that our unadjusted adverse event risks among the OC cohort are similar to prior studies.9 We did not perform a randomized control trial, and there are likely to be unmeasured confounders or selection effects that we were unable to include in our adjustments. The technique utilized for CTC cannot be derived from claims data but is potentially important given that manual insufflation, luminal disease and use of a rectal balloon are associated with perforation.3,4,6,23 Some of the adverse events specific to CT, such as radiation risk, cannot be measured with claims data. Although our findings may not generalize to younger patients, colorectal cancer is predominantly a disease of the elderly.
In conclusion, our study demonstrates that adverse events rates following initial CTC among the elderly are low, with the greatest risk for cardiovascular events. Rates of lower gastrointestinal bleeding, other gastrointestinal events and cardiovascular events are lower following initial CTC, with or without subsequent OC, than following initial OC; similar rates of perforation may stem from the small number of perforations in both cohorts. A similar pattern was demonstrated among patients > 75 years of age and those with symptoms referable to colorectal cancer. These data can help primary care providers and patients seeking to understand complications associated with these methods of colorectal cancer screening and targeted diagnostic evaluation.
Supplementary Material
Highlights.
We evaluated complication risk 30 days following initial CT colonography (CTC) in the elderly.
A cohort of elderly initial optical colonoscopy (OC) patients was selected for comparison.
Adverse events, except perforation, are lower following initial CTC than OC.
Similar perforation rates may reflect the small number of perforations overall.
Adverse events were higher in patients >75 years of age for both modalites.
Acknowledgments
None
Funding / Support: Data for this study was purchased through Grant #IRG-78-002-31 from the American Cancer Society. Data analysis was funded through Grant #: 1-KM-CA156715-01 from the National Institutes of Health
Abbreviations
- CTC
Computed Tomographic Colonography
- OC
Optical Colonoscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper has not been presented at any meeting nor has it been accepted for presentation at a future meeting.
Conflicts of Interest: None of the authors have any conflicts of interest to report.
References
- 1.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008 Sep 18;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003 Dec 4;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 3.Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006 May;239(2):313–316. doi: 10.1148/radiol.2392052002. [DOI] [PubMed] [Google Scholar]
- 4.Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006 May;239(2):464–471. doi: 10.1148/radiol.2392051101. [DOI] [PubMed] [Google Scholar]
- 5.Atkin W, Dadswell E, Wooldrage K, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013 Apr 6;381(9873):1194–1202. doi: 10.1016/S0140-6736(12)62186-2. [DOI] [PubMed] [Google Scholar]
- 6.Sosna J, Blachar A, Amitai M, et al. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology. 2006 May;239(2):457–463. doi: 10.1148/radiol.2392050287. [DOI] [PubMed] [Google Scholar]
- 7.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008 Dec;135(6):1899–1906. 1906, e1891. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Penfold RB, De Coster C, Au W, Bernstein CN, Moffatt M. Predictors of serious complications associated with lower gastrointestinal endoscopy in a major city wide health region. Can J Gastroenterol. 2010 Jul;24(7):425–430. doi: 10.1155/2010/714591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009 Jun 16;150(12):849–857, W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 10.Rutter CM, Johnson E, Miglioretti DL, Mandelson MT, Inadomi J, Buist DS. Adverse events after screening and follow-up colonoscopy. Cancer Causes Control. 2012 Feb;23(2):289–296. doi: 10.1007/s10552-011-9878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2011 Oct;74(4):885–896. doi: 10.1016/j.gie.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007 Jul;66(1):27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population. Journal of the National Cancer Institute. 2010 Aug 18;102(16):1238–1252. doi: 10.1093/jnci/djq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MEDCAC transcript Computed Tomography Colonography [November 20 2013];2008 http://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=45&year=All&bc=AAAIAAAAAAAAAA%3d%3d&.
- 15.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006 Dec 19;145(12):880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 16.Center RDA. [February 28 2013];Strengths and Limitations of CMS Administrative Data in Research. 2012 http://www.resdac.org/resconnect/articles/156.
- 17.Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL. CT colonography: performance and program outcome measures in an older screening population. Radiology. 2010 Feb;254(2):493–500. doi: 10.1148/radiol.09091478. [DOI] [PubMed] [Google Scholar]
- 18.Macari M, Nevsky G, Bonavita J, Kim DC, Megibow AJ, Babb JS. CT colonography in senior versus nonsenior patients: extracolonic findings, recommendations for additional imaging, and polyp prevalence. Radiology. 2011 Jun;259(3):767–774. doi: 10.1148/radiol.11102144. [DOI] [PubMed] [Google Scholar]
- 19.Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Utilization and predictors of early repeat colonoscopy in Medicare beneficiaries. Am J Gastroenterol. 2010 Dec;105(12):2670–2679. doi: 10.1038/ajg.2010.344. [DOI] [PubMed] [Google Scholar]
- 20.Zafar HM, Yang J, Harhay M, Lev-Toaff A, Armstrong K. Predictors of CT Colonography Utilization Among Asymptomatic Medicare Beneficiaries. J Gen Intern Med. 2013 Mar 29; doi: 10.1007/s11606-013-2414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohsiriwat V. Colonoscopic perforation: incidence, risk factors, management and outcome. World J Gastroenterol. 2010 Jan 28;16(4):425–430. doi: 10.3748/wjg.v16.i4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007 Oct 4;357(14):1403–1412. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 23.Pendse DA, Taylor SA. Complications of CT colonography: A Review. Eur J Radiol. 2012 May 15; doi: 10.1016/j.ejrad.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Qaseem A, Denberg TD, Hopkins RH, Jr., et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012 Mar 6;156(5):378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 25.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008 Nov 4;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 26.Schenck AP, Klabunde CN, Warren JL, et al. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev. 2007 Oct;16(10):2118–2127. doi: 10.1158/1055-9965.EPI-07-0123. [DOI] [PubMed] [Google Scholar]
- 27.Ko CW, Dominitz JA, Green P, Kreuter W, Baldwin LM. Accuracy of Medicare claims for identifying findings and procedures performed during colonoscopy. Gastrointest Endosc. 2011 Mar;73(3):447–453. e441. doi: 10.1016/j.gie.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javitt JC, McBean AM, Sastry SS, DiPaolo F. Accuracy of coding in Medicare part B claims. Cataract as a case study. Arch Ophthalmol. 1993 May;111(5):605–607. doi: 10.1001/archopht.1993.01090050039024. [DOI] [PubMed] [Google Scholar]
- 29.Fowles JB, Lawthers AG, Weiner JP, Garnick DW, Petrie DS, Palmer RH. Agreement between physicians' office records and Medicare Part B claims data. Health Care Financ Rev. 1995;16(4):189–199. Summer. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.