Abstract
Since the first report in 2005, Roux-en-Y gastric bypass (RYGB) surgery has been linked to a variety of metabolic changes that alter kidney stone risk. The studies with the highest level of evidence, performed in non-stone forming patients before and after RYGB, cite a number of kidney stone risk factors, including a 25% increase in urinary oxalate, a 30% decrease in urinary citrate, and reduction in urine volume by half a liter. In addition to these, recent clinical and experimental studies have contributed to our understanding of the pathophysiology of stone disease in this unique population. This review summarizes the current RYGB urinary chemistry profiles and epidemiological studies, outlines known and theoretical mechanisms of hyperoxaluria and hypocitrituria, and provides some standard recommendations for reducing stone risk in RYGB stone formers as well as some novel ones, including correction of metabolic acidosis and use of probiotics.
Keywords: Morbid obesity, hyperoxaluria, gastric bypasssurgery, hypocitrituria, nephrolithiasis, calcium oxalate stones
Introduction
Obesity in the US is an overwhelming clinical problem, with recent estimates suggesting over a third of American adults are obese with a body mass index (BMI) >30 kg/m2, including more than 15 million who are considered morbidly obese (BMI >40 kg/m2) (1–3). Most medical weight loss results are either temporary or completely ineffective. Bariatric surgery, in particular, Roux-en-Y gastric bypass (RYGB), has proven to be the only effective, long-term weight reduction treatment option, curing diabetes and hypertension and lowering both cardiovascular and overall mortality in this population (4,5). These successes have led to a 6-fold increase in bariatric surgery over the last 10 years (36,700 procedures in 2000 rising to 220,000 procedures in 2009) with estimates that over 1.5 million Americans now have bypassed intestinal tracts (4,6).
In 2005, Nelson et al. first described the renal complications of hyperoxaluria, calcium oxalate stones, and oxalate nephropathy in a select group of patients following RYGB (7). Since that report, a number of publications have examined the risk factors that may predispose RYGB patients to form kidney stones. This review summarizes urinary chemistry profiles and kidney stone incidence in both stone-forming and non-stone forming patients after RYGB. Additionally, recently published clinical and experimental literature focusing on pathophysiology of stone risk will be reviewed along with recommendations that may reduce stone risk in stone-forming RYGB patients.
Types of bariatric surgery
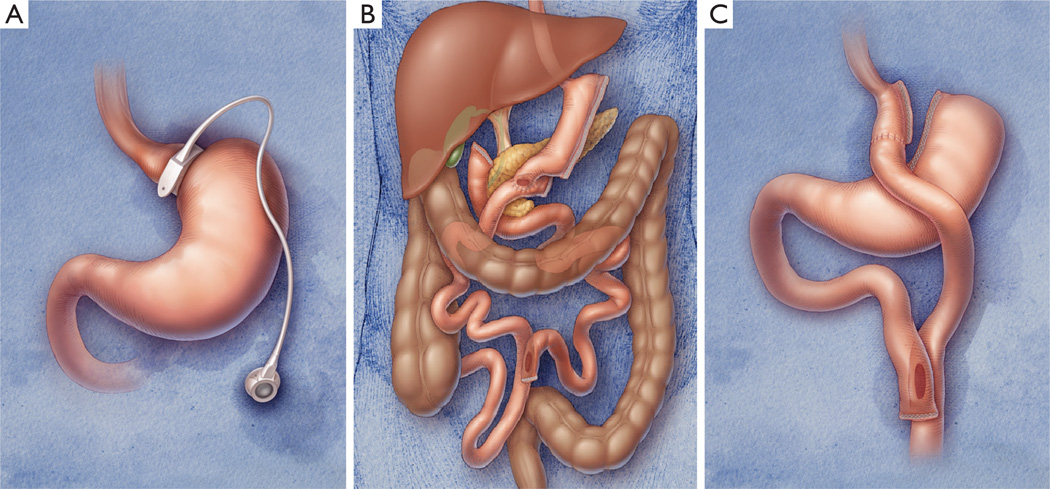
Although this review will focus on outcomes following RYGB, several authors use patients with other types of bariatric surgery as control groups. Therefore, a focused review of different types of weight-loss surgery is appropriate. There are three general ways to lose weight through bariatric surgery: (I) restrict the amount of food that can be ingested (“restrictive procedure”); (II) decrease the amount of intestine available for nutrient absorption (“malabsorptive procedure”); or (III) combine both restrictive and malabsorptive procedures. The mostcommonly used restrictive procedures are the adjustable gastric band (GB) and the vertical sleeve gastrectomy. As there have been no comparisons of sleeve gastrectomy to RYGB in regards to stone risk, sleeve gastrectomy will only be discussed in the context of biliopancreatic diversion (BPD). The laparoscopic GB procedure, commonly referred to as “lap band,” involves placing an inflatable silicon band around the upper portion of the stomach to create a small pouch (Figure 1A). The band is attached to a subcutaneous saline reservoir which can be adjusted by percutaneous injection, depending on tolerability and the amount of satiety the bariatric surgeon wants to create. Long-term weight loss for this procedure is patient- and surgeon-dependent but is considered to be traditionally less (~10% total body weight) than a malabsorptive procedure (25–30% total body weight) (5).
Figure 1.
(A) Cartoon depiction of laparoscopic adjustable gastric band; (B) vertical sleeve gastrectomy with duodenal switch; and (C) Roux-en-Y gastric bypass surgery. Images courtesy of Covidien®.
Modern malabsorptive procedures have evolved since the 1950’s to include both a restrictive and a malabsorptive component. BPD with duodenal switch involves surgical excision of ~70% of the lateral stomach, creating a long narrow tubular gastric remnant (sleeve gastrectomy). The distal small bowel is then divided 250 cm proximal to the ileocecal valve, and the duodenum is transected. This distal ileal limb is anastomosed 2 cm distal to the pylorus while the biliary-pancreatic limb is reanastomosed ~100 cm proximal to the ileocecal valve (Figure 1B). Similarly, RYGB combines the principles of restrictive and malabsorptive procedures. Unlike BPD, the lesser curvature of the stomach is stapled, creating a 20–30 mL pouch for RYGB (Figure 1C). The jejunum is then transected 60–70 cm distal to the ligament of Treitz, and the portion distal to this is sewn to the small stomach pouch. The transected jejunum is then anastomosed anywhere from 80–120 cm down the jejunum, creating two limbs in the shape of a “Y” that effectively separate ingested food until they meet at a distal jejunal common channel (8,9). Total length of these limbs ultimately affects outcomes, with shorter common channel (very long or very very long Roux limb) portending higher malabsorption and weight loss.
Changes in urinary chemistry after RYGB
Over the last ten years, a variety of reports have linked RYGB to metabolic changes that alter the urinary milieu and kidney stone risk. Table 1 summarizes the studies that contain nine or more patients and report urine chemistry profiles before and/or after RYGB procedure. Of the studies reported, 8/13 contain retrospective components with highly variable inclusion criteria, use of controls, timing of 24-hour urine collection, non-standardized diets or dietary supplements, and methodological quality. Some studies include bariatric non-stone formers, bariatric stone formers both pre- and post-op, or bariatric patients who develop stones de novo post-op. Despite these limitations, a review of these studies gives the practicing urologist a broad sense of urinary changes that may be seen with each procedure.
Table 1.
Urinary profiles following Roux-en-Y gastric bypass surgery, grouped by stone history and study design
| Article | Procedures [n] |
F/U (mo.) |
Urinary oxalate (CaOx SS) |
Other urinary changes and study comments |
|---|---|---|---|---|
| Primarily non-stone formers, prospectively collected | ||||
| Park 2009 (10) | RYGB [45] | 9.6 | Pre-op: 32 (1.27) Post-op: 40 (2.23) |
De novo hyperoxaluria occurred in 90%. No symptomatic stone events during study |
| Duffey 2010 (11) | RYGB [21] | 24 | Pre-op: 33 (1.73) Post-op: 63 (2.2) |
De novo hyperoxaluria occurred in 52%. Hypocitraturia increased from 10% at baseline to 48% but relative CaOx SS was unchanged |
| Kumar 2011 (12) | RYGB [9] BPD [2] |
6, 12 | Pre-op: 26 (1.0) Post-op: 32 (1.8) |
Decreased total urine volume and higher fecal fat excretion at 6 and 12 months. Oral oxalate loading test at 6 and 12 months resulted in higher urine oxalate excretion |
| Wu 2013 (13) | RYGB [38] | 6 | Pre-op: 38 (4.9) Post-op: 48 (10.5) |
Urine calcium increased by 43 mg/day (perhaps due to higher supplementation) while urine volume decreased by ½ liter/day. Stone formation or passage events were not recorded |
| Agrawal 2013 (14) | RYGB [13] | 6 | Pre-op: 12.6 (1.4) Post-op: 28.4 (5.7) |
Prospective 24-hr urine study that included one stone former. Urinary oxalate was elevated 2, 4, and 6 months after RYGB. Both citrate and urine volume decreased by 30% |
| Valezi 2013 (15) | RYGB [151] | 12 | Pre-op: 24 (NR) Post-op: 41 (NR) |
Prospective 24-hr urine study that included 16 stone formers. Mean urinary uric acid levels increased 30% while volume decreased 40%. A total of 100% of patients were hypocitrituric at year 1 |
| Non-stone formers, retrospectively collected | ||||
| Nelson 2005 (7) | RYGB [13] LL-RYGB [9] |
NR | RYGB =88 (2.38) LL-RYGB =95 (2.69) |
CaOx SS was reported in µmL/L (normal range <1.77). Long limb RYGB results in a shorter common channel resulting in more malabsorption |
| Patel 2009 (16) | RYGB [52] BPD [6] |
14.2 | RYGB =62 (NR) BPD =90 (NR) |
Comparisons made to healthy and stone forming adults from a commercial database |
| Penniston 2009 (17) | RYGB [27] GB [12] |
32 | RYGB =48 (1.89) GB =41 (2.78) |
Urine calcium decreased ~50% in RYGB versus GB, and 52% RYGB had urinary citrate <370 mg/day vs. 9% in GB. Urine volume decreased in both groups |
| Maalouf 2010 (18) | RYGB [19] Con [19] |
42 | RYGB =45 (7.0) Con =30 (5.0) |
~50% reduction in urinary citrate level compared to controls (mean 358 vs. 767 mg/day) |
| Froeder 2012 (19) | RYGB [58] BPD [3] Con [30] |
48 | RYGB/BPD =26 (NR) Con =29 (NR) |
Oxalate loading (RYGB =22, Con =21) showed higher urine oxalate in RYGB. No difference in O. formigenes colonization between subgroups (RYGB =10, Con =13). 6 patients had stones pre-op. No difference in CaOx SS, reported as Tiselius index |
| Primarily stone formers, retrospectively or prospectively collected | ||||
| Sinha 2007 (20) | RYGB [31] | N/A | Post-op: 60 (2.23) | Post-RYGB data compared to normal population references for urinary oxalate excretion |
| Asplin 2007 (21) | JIB [27] GB/RYGB [132] Con [2,210] |
N/A | JIB =102 (NR) GB/RYGB =83 (NR) Con =34 (NR) |
GB and RYGB surgeries were not separated for analysis. Mean time from procedure to stone event of 3.6 years. Stone formers identified in a corporate stone database |
| Pang 2012 (22) | JIB [1] RYGB [6] BPD [2] |
N/A | Entire cohort Free Diet =65 (1.97) Met Diet =62 (1.13) |
Recurrent stone forming bariatric patients mean 11 years after surgery had increased pH, urine volume, and citrate on Met (controlled metabolic) diet. No significant changes in urine oxalate excretion was noted, even on low oxalate diet |
F/U, follow-up in months, some means are number of months post-procedure; CaOx SS, calcium oxalate supersaturation; RYGB, Roux-en-Y gastric bypass; NR, not recorded; LL-RYGB, Long-limb Roux-en-Y gastric bypass; BPD, biliopancreatic diversion with duodenal switch; JIB, jejunoileal bypass; GB, gastric band; Con, control.
Neslon et al. [2005] first proposed the link between hyperoxaluria and stone risk in a group of 23 patients who developed either calcium oxalate stones (n=21) or oxalate nephropathy (n=2) following RYGB, sparking a flurry of research in post-operative urinary changes after bariatric surgery (7). Park et al. [2009] described in a prospective, longitudinal study (n=45) significant increases in urinary oxalate excretion, calcium oxalate supersaturations (CaOx SS), and decreases in urinary citrate, calcium, and total urine volume after RYGB when compared to preoperative urine samples (10). Although there were no symptomatic stone events after a mean of 9.6 study months, they contended that chronic acidosis in these patient may lead to decreases in urinary citrate (a known stone inhibitor), further increasing stone risk in addition to hyperoxaluria (10). Similarly, Duffey et al. [2010] described a doubling of urinary oxalate excretion and significant decreases in urinary citrate excretion in a two-year, prospective study in RYGB non-stone forming patients (11). Furthermore, their study importantly showed that risk of post-operative hyperoxaluria appears to increase over time, not decrease or remain stable (11).
Recently, three groups have described the temporal course of CaOx SS in the early post-operative period after RYGB. Wu et al. [2013] noted urinary changes 6 months after RYGB (n=38) from those baseline, including significant increases in urinary oxalate excretion, calcium, and CaOx SS and decreases in total urine volume (13). The lack of hypocitrituria and presence of hypercalciuria in this cohort, compared to previous studies, was felt to be due to increased utilization of CaOx SS in their patients post-operatively (13). Agrawal et al. [2013] evaluated 24-hour urine in 13 patients before and at time points 1, 2, 4, and 6 months after RYGB (14). Using a variety of standardized in-house assays and one private hospital-based laboratory, they noted a doubling of urinary oxalate starting at month 2 through month 6 (P=0.005), a 40% reduction in urinary citrate at month 6 (P=0.4), and 30–60% reduction in urinary volume (P<0.001) that started in the immediate post-operative month (14). Last, Valezi et al. [2013] studied the pre- to post-operative changes in urinary metabolites in 151 patients after RYGB, 16 of which had history of a priori stone disease (15). At one year, urinary oxalate levels increased 37% (mean 24 to 41 mg/day, P<0.001) while decreased in both urine citrate (36%; mean 268 to 170 mg/day, P<0.001) and urine volume (29%; 1.3 to 0.9 liters/day, P<0.001) were noted. Unlike Duffey et al. who found that increasing age was a predictor for post-operative hyperoxaluria (11), this group found that presence of pre-operative stones was the only predictor of hyperoxaluria (15). Overall, across all three studies, RYGB increased CaOx SS 3–4 fold compared to patients’ baseline studies with almost 80% of all patients having with CaOx SS >2 (considered high).
Interventional diet or dietary supplement studies in the post-bariatric surgery setting have also provided insights into the mechanisms involved in recurrent stone disease. Pang et al. [2012] looked at the effect changing diet in a small number of recurrent stone formers who were, on average, 11 years out from RYGB (n=6), jejunoileal bypass (JIB) (n=2) or BPD (n=1) (22). The authors performed 24-hour urine on these patients on baseline diets and then placed them on a metabolic diet consisting of 1,000 mg calcium, reduced oxalate (70–80 mg), 20% protein, <25% fat, and 3,000 mg sodium diet. They found that, although urine oxalate levels remained high, the metabolic diet positively affected urinary CaOx SS by increasing urine volume and raising urine pH and urinary citrate (22). Although not supported by their data, the authors also recommended additional strategies, such as oral calcium supplements, oxalate binders, and lower fat meals to reduce future stone risk.
These recommendations were further investigated by Froeder et al. [2012], who observed similar decreases in urine citrate and total urinary volume in RYGB and BPD patients compared to morbidly obese controls (19). Interesting, they found that bariatric patients had similar rates of hyperoxaluria (13–20%) compared to controls at baseline. However, when group of patients (n=43) were given a 375 mg oxalate load (spinach juice), bariatric patients had a two-fold elevation in urinary oxalate at all urine time-points compared to controls, suggesting that excessive oxalate absorption was occurring (19). The authors went on to test a subset of these patients (n=21) for Oxalobacter formigines (a commensal pathogen in the GI tract that uses oxalate as an energy source) colonization and found no difference in rates, again suggesting that the differences in hyperoxaluria between these two populations was due to oxalate over-absorption, not lack of bacterial consumption. Lastly, Sakhaee and colleagues published two recent 2-phase, placebo crossover studies evaluating the short-term effect of effervescent potassium citrate and calcium citrate (40 meq potassium, 800 mg calcium, 100 meq citrate/day) in 24 patients at a mean 4.7 years after RYGB (23) and in 15 patients at a mean 4.2 years after RYGB (24). From a bone standpoint, the more soluble delivery of calcium lowered markers of bone resorption but had no effect on serum parathyroid hormone (23). The potassium and citrate raised urine pH and lowered calcium oxalate agglomeration by direct crystal testing. Additionally, this product had more pronounced effects on acute serum calcium (measured hourly ×6 hours) after ingestion compared to calcium citrate, suggesting superior calcium uptake and availability (24). Although this product is not commercially available, these two placebo-based, interventional trials convincingly contends that calcium citrate reduces stone risk in bariatric stone formers and may be a more suitable supplement that the standard calcium citrate.
Changes in stone incidence after RYGB
Table 2 focuses on incidence of stone events after RYGB surgery. Durrani et al. [2006] was the first to report an increased prevalence of stones (3.2% de novo stones from chart review) in a cohort of RYGB patients (n=972) compared with expected rates derived from a control population (25). Matlaga et al. [2009] then reported claims data in a case-control study of 4,639 patients post-RYGB surgery compared to obese controls, determining a stone diagnosis in 7.65% of RYGB versus 4.63% of controls (26). Compared to restrictive procedures (such as the GB) which yield an estimated person-time stone incidence rate of 3.40 stones per 1,000 person-years, Matlaga et al. estimated person-time stone incidence rate of 16.62 stones per 1,000 person-years for RYGB and 11.3 stones per 1,000 person-years for routine obesity (26). To see if this stone risk phenomenon remained true in non-obese patients, Shimizu et al. [2012] reviewed computed tomography (CT) scans from gastric cancer patients who had either distal gastrectomy with Bilroth I/Roux-en-Y versus total gastrectomy with Roux-en-Y reconstruction (28). In this population, patients with total gastrectomy were more likely to have renal stones by CT (21/85, 25%) than patients with some portion of the stomach remaining (10/141, 7%). Although no urinary chemistries were available, the authors hypothesized that total gastrectomy may lead to more fat malabsorption than partial gastrectomy, perhaps furthering hyperoxaluria. Furthermore, they did not find a significant difference in stone incidence between distal gastrectomy patients with either Bilroth I or Roux-en-Y reconstruction, again suggesting that extent of stomach resection may be more important than length of the common channel from an absorptive standpoint. This study does not address potential confounders such as fluid intake differences.
Table 2.
Kidney stone incidence following bariatric surgery
| Article | Procedure [n] |
F/U (mo.) |
Post-procedural stone incidence |
Comments |
|---|---|---|---|---|
| Durrani 2006 (25) | RYGB =972 | NR | 26/85 (31%): recurrent 32/887 (3.6%): de novo |
Stones identified by patient chart review. Mean time to stone formation was 2.8 years (de novo) and 1.9 years (recurrent) |
| Matlaga 2009 (26) | RYGB [4,639] Con [4,639] |
18.6 | RYGB =355/4,639 (7.7%) Con =215 (4.6%) |
Stones identified by CPT code within claims data versus matched obese controls |
| Costa-Matos 2009 (27) | RYGB [58] | 42† | RYGB =0/58 (0%) | Stones identified by RUS. One patient had a stone pre-RYGB which remained unchanged post-op |
| Shimizu 2012 (28) | DTGx [226] | NR | 31/226 (13.7%) | Stones identified by CT in gastric cancer patients. Incident stones occurred in 25% (21/85) of total vs. 7% (10/141) distal gastrectomy. Mean time to first stone 17.6 months post-surgery |
| Valezi 2013 (15) | RYGB [151] | 12 | 27/151 (18%): total 11/135 (8%): de novo |
A total of 16 patients had pre-existing stones by RUS or by stone history. Post-operative stone formation was predicted by elevated urinary oxalate and uric acid levels |
F/U, follow-up in months, some means are number of months post-procedure;
, follow-up time reported as median; RYGB, Roux-en-Y gastric bypass; GB, gastric band; Con, ontrol; CPT, common procedural terminology; RUS, renal ultrasound; BPD, biliopancreatic diversion with duodenal switch; DTGx, distal or total gastrectomy with Bilroth I or Roux-en-Y gastric bypass.
In a large, prospective case series (n=151) of laparoscopic RYGB patients, Valezi [2013] reported de novo stone incidence of 8% one year post-procedure (15). Using multivariate analysis, post-operative hyperoxaluria (OR 1.41; 95% CI, 1.101–1.803; P=0.006) and hyperuricosuria (OR 1.009; 95% CI, 1.002–1.016; P=0.013) were found to be the only predictors of developing RYGB-related kidney stones. Although these epidemiological studies are not perfect, it appears that RYGB procedure increases future stone risk at least 2–3 fold, primarily driven by hyperoxaluria. More long-term studies are needed to identify other risks, such as food preference changes (increased leafy green foods) and increased animal protein (potentially leading to higher uric acid intake) in order to solidify mechanisms behind this conclusion. Additionally, predictive stone risk calculators may help urologists and bariatric surgeons counsel patients who are considering RYGB or screen for metabolic abnormalities in patients who are in the post-operative setting, especially as it appears that since stone risk increases over time in this population.
Pathophysiology in experimental models of bariatric surgery
To date, clinical data suggest that malabsorptive bariatric procedures have higher incident kidney stone risk through changes in urinary volume, oxalate, and citrate. These procedures, therefore, fall into the spectrum of gastrointestinal disorders characterized by malabsorption of bile salts and/or fatty acids—termed “enteric hyperoxaluria”. Mechanistically, as fat is malabsorbed, fat-soluble vitamins and calcium ions are saponified by intraluminal free fatty acids, leading to steatorrhea with subsequent nutrient loss. As a consequence of this reduced calcium availability in the intestinal lumen, there is decreased calcium-oxalate binding and increased unbound oxalate within the small and large intestine. This increased free oxalate load, otherwise destined for fecal excretion, has a higher chance of either passive or active gut absorption. Once absorbed, oxalate is an end-product of metabolism and must be filtered and excreted by the kidney (12,19,29,30). Concurrently, permeability of the GI tract to oxalate can be dramatically increased by exposure to unconjugated bile salts and long chain fatty acids, both of which have been shown to be increased in the GI tract of RYGB patients (12,22,31–33). While this hypothesis fits in well for hyperoxaluria and malabsorption, only a small number of human studies have been published in this area with limited results (12,19,34). In an attempt to better understand these mechanisms, our group established a diet-induced obese rodent model of RYGB surgery and tested the long-term effect of high dietary fat and oxalate on fecal fat excretion and 24-hour urine parameters (35).
In a cohort of 19 experimental animals and 16 sham-operated controls, we tested two different diets (40% versus 10% fat) with high (1.5%) and no oxalate content. In all age-matched, sham-operated control animals, we saw no appreciable change in urinary oxalate or fecal fat on any of these regimens (35). However, RYGB animals on high fat and oxalate diets had 8-fold higher fecal fat excretion and heavier stools, a 5-fold increase in urine oxalate excretion, and 4-fold increase in CaOx SS. In this setting, just lowering dietary fat resulted in a 40% decrease in oxalate excretion. For RYGB animals on a no-oxalate added diet, urine oxalate was consistently higher than controls, suggesting that other mechanisms may have resulted in excess oxalate excretion. These include increased systemic oxalate production, enhanced renal oxalate excretion, slow GI transit times causing increased GI uptake, or perhaps even changes in gut flora as a direct result of the bypass.
For years, the anaerobic bacterium Oxalobacter formigenes, a gut commensal capable of metabolizing oxalic acid, has been implicated as a possible etiology for increased urinary oxalate levels in calcium oxalate stone formers (36). In 2005, subjects with fat malabsorption, hyperoxaluria, and calcium oxalate stones caused by a variety of GI diseases (including 6 RYGB patients) were given the probiotic “Oxadrop®” (Lactobacillus acidophilus and brevis, Streptococcus and Bifidobacterium) (37). Over a period of 3 months, mean urinary oxalate levels decreased by 20% in 10 patients (37). In the RYGB population, it has been suggested that higher rates of O. formigenes colonization could also result in decreased levels of oxalate in the gut and subsequently, the urine (13,19). To test this hypothesis, our group has begun colonizing hyperoxaluric RYGB animals with this bacterium, and preliminary data in 4 animals has shown that persistent colonization results in a 75% reduction in urinary oxalate levels (unpublished). These exciting results require more validation with larger experimental numbers but may offer a future potential therapy for RYGB patients who suffer from hyperoxaluria and recurrent oxalate stones.
In addition to hyperoxaluria, the other commonly cited urinary abnormalities in bariatric surgical patients include low urinary volume and low urine citrate, both of which can significantly increase calcium oxalate risk and supersaturation (11–13,16,18,20,21). Low urine citrate is likely reflective of systemic metabolic acidosis. Although scant data exists in humans, Abegg et al. noted mild elevations in serum lactate levels and confirmed metabolic acidosis by arterial blood gas in obese Wistar rats 14 weeks after experimental RYGB surgery (38). We also confirmed that our Sprague-Dawley RYGB model has significantly lower serum bicarbonate that sham operated controls (unpublished). Taken together with the effervescent potassium calcium citrate data, the current literature seems to suggest that chronic metabolic acidosis could play a larger role in hypocitrituria and stone disease than previously expected. In a broader context, chronic metabolic acidosis can also affect the bone-renal axis resulting in increased bone resorption secondary to chronic GI bicarbonate loss. This phenomenon has been previously documented in a small group of RYGB patients prior to administration of potassium alkali therapy (23).
As previously reviewed, we agree with the dietary recommendations to reduce stone risk that have been advocated for by so many other authors in this area: reduction in oxalate-rich and fatty foods to minimize enteric absorption, increased hydration to increase total urine volume and decrease urine supersaturation, calcium supplementation using calcium citrate instead of calcium carbonate, and citric salts (potassium citrate) to correct metabolic acidosis and hypocitrituria (11,20,26,28). Probiotics may also be beneficial, although existing data is limited (37). Future directions for study or therapy in these patients include a more critical appraisal of vitamin deficiencies, including pyridoxine (vitamin B6—an important co-factor in liver metabolism of glyoxalate), clarification of the role of gut hormones in renal and GI oxalate transport, and publication of results from investigators who use cholestyramine to bind bile acids in this population.
Conclusions
RYGB surgery and calcium oxalate stone disease appear to be irrevocably linked through mechanisms of hyperoxaluria, low urine volume, and hypocitrituria. Further investigations in the RYGB animal model as well as stone-centered human pharmaceutical trials are needed to better define the etiology of and the best means to prevent this renal complication, especially in light of growing obesity and bariatric surgical trends.
Acknowledgements
This project was supported by funds from the NIH (K08 DK089000-4), AUA Foundation Rising Star in Urology Research Award, Astellas Global Development, Inc., and Ethicon Endo-Surgery.
Footnotes
Disclosure: The authors have written review articles for other journals involving stone risk in bariatric surgery. As the literature in these areas overlap, many of the same references, summaries of literature, discussion points, and even tables are similar. Although attempts have been made to design each review article differently, the articles may contain sentences that have been rephrased or only slightly revised. Although the experimental gastric bypass project, discussed in the text, was supported by a supplies grant through Ethicon Endo-Surgery (staplers and reloads), the authors have no financial conflicts of interest with any of their disclosures.
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 3.Demaria EJ, Jamal MK. Surgical options for obesity. Gastroenterol Clin North Am. 2005;34:127–142. doi: 10.1016/j.gtc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn GL. The 2008 Edward E. Mason Founders lecture: interdisciplinary teams in the development of “best practice” obesity surgery. Surg Obes Relat Dis. 2008;4:679–684. doi: 10.1016/j.soard.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Nelson WK, Houghton SG, Milliner DS, et al. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481–485. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Semins MJ, Matlaga BR, Shore AD, et al. The effect of gastric banding on kidney stone disease. Urology. 2009;74:746–749. doi: 10.1016/j.urology.2009.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 10.Park AM, Storm DW, Fulmer BR, et al. A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182:2334–2339. doi: 10.1016/j.juro.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 11.Duffey BG, Alanee S, Pedro RN, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211:8–15. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R, Lieske JC, Collazo-Clavell ML, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654–661. doi: 10.1016/j.surg.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JN, Craig J, Chamie K, et al. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2013;9:83–87. doi: 10.1016/j.soard.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal V, Liu XJ, Campfield T, et al. Calcium oxalate supersaturation increases early after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2014;10:88–94. doi: 10.1016/j.soard.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Valezi AC, Fuganti PE, Junior JM, et al. Urinary evaluation after RYGBP: a lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg. 2013;23:1575–1580. doi: 10.1007/s11695-013-0916-0. [DOI] [PubMed] [Google Scholar]
- 16.Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181:161–166. doi: 10.1016/j.juro.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penniston KL, Kaplon DM, Gould JC, et al. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol. 2009;182:2340–2346. doi: 10.1016/j.juro.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 18.Maalouf NM, Tondapu P, Guth ES, et al. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183:1026–1030. doi: 10.1016/j.juro.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froeder L, Arasaki CH, Malheiros CA, et al. Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol. 2012;7:2033–2040. doi: 10.2215/CJN.02560312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 21.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Pang R, Linnes MP, O’Connor HM, et al. Controlled metabolic diet reduces calcium oxalate supersaturation but not oxalate excretion after bariatric surgery. Urology. 2012;80:250–254. doi: 10.1016/j.urology.2012.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakhaee K, Griffith C, Pak CY. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis. 2012;8:67–72. doi: 10.1016/j.soard.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Sakhaee K, Pak C. Superior calcium bioavailability of effervescent potassium calcium citrate over tablet formulation of calcium citrate after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9:743–748. doi: 10.1016/j.soard.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Durrani O, Morrisroe S, Jackman S, et al. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol. 2006;20:749–752. doi: 10.1089/end.2006.20.749. [DOI] [PubMed] [Google Scholar]
- 26.Matlaga BR, Shore AD, Magnuson T, et al. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181:2573–2577. doi: 10.1016/j.juro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Costa-Matos A, Guidoni LR, Carvalho KA, et al. Is there an association between urolithiasis and Roux-en-y gastric bypass surgery? Int Braz J Urol. 2009;35:432–435. doi: 10.1590/s1677-55382009000400006. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu H, Ichikawa D, Tamagaki K, et al. Evaluation of postoperative nephrolithiasis and renal dysfunction in gastric cancer patients. Gastric Cancer. 2013;16:338–344. doi: 10.1007/s10120-012-0192-z. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann AF, Laker MF, Dharmsathaphorn K, et al. Complex pathogenesis of hyperoxaluria after jejunoileal bypass surgery. Oxalogenic substances in diet contribute to urinary oxalate. Gastroenterology. 1983;84:293–300. [PubMed] [Google Scholar]
- 30.Andersson H, Jagenburg R. Fat-reduced diet in the treatment of hyperoxaluria in patients with ileopathy. Gut. 1974;15:360–366. doi: 10.1136/gut.15.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobbins JW, Binder HJ. Effect of bile salts and fatty acids on the colonic absorption of oxalate. Gastroenterology. 1976;70:1096–1100. [PubMed] [Google Scholar]
- 32.Siener R, Petzold J, Bitterlich N, et al. Determinants of urolithiasis in patients with intestinal fat malabsorption. Urology. 2013;81:17–24. doi: 10.1016/j.urology.2012.07.107. [DOI] [PubMed] [Google Scholar]
- 33.Freel RW, Hatch M, Earnest DL, et al. Oxalate transport across the isolated rat colon. A re-examination. Biochim Biophys Acta. 1980;600:838–843. doi: 10.1016/0005-2736(80)90486-1. [DOI] [PubMed] [Google Scholar]
- 34.Odstrcil EA, Martinez JG, Santa Ana CA, et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704–713. doi: 10.3945/ajcn.2010.29870. [DOI] [PubMed] [Google Scholar]
- 35.Canales BK, Ellen J, Khan SR, et al. Steatorrhea and hyperoxaluria occur after gastric bypass surgery in obese rats regardless of dietary fat or oxalate. J Urol. 2013;190:1102–1109. doi: 10.1016/j.juro.2013.02.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allison MJ, Dawson KA, Mayberry WR, et al. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985;141:1–7. doi: 10.1007/BF00446731. [DOI] [PubMed] [Google Scholar]
- 37.Lieske JC, Goldfarb DS, De Simone C, et al. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 2005;68:1244–1249. doi: 10.1111/j.1523-1755.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 38.Abegg K, Gehring N, Wagner CA, et al. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R999–R1009. doi: 10.1152/ajpregu.00038.2013. [DOI] [PubMed] [Google Scholar]