Tantalizing connections between autoimmune rheumatic diseases and cancer have become increasingly evident over the past several decades. These connections are complex, with different relationships in frequency, timing and types of cancers observed in different diseases or disease subgroups. Several recent advances from disparate fields have begun to illuminate the dynamic and bidirectional interactions occurring at the cancer-immune system interface which may be relevant to understanding the origins of autoimmunity (1). These interactions include the existence of potent anti-cancer immune responses which limit tumor growth, as well as multiple immune and inflammatory pathways that can contribute to tumor growth and robustness. The striking ability of immune checkpoint inhibitors to reveal powerful anti-cancer immune responses in patients with cancer highlight that natural immune responses to cancers exist, and may regulate the emergence of cancer (2). Recent data in systemic sclerosis (SSc, scleroderma) patients suggests that, in some cases, autoimmunity may be initiated by autoantigen mutation in that patient’s cancer (3, 4). Interestingly, there exist patients with the same form of scleroderma and an identical autoimmune response who do not have a detectable cancer, raising the possibility that in these patients, the disease mechanism is the same except that the anti-tumor immune response has successfully eliminated the cancer. Similar striking associations with cancer are also apparent in other rheumatic phenotypes, particularly dermatomyositis (DM). The autoimmune rheumatic diseases therefore provide an exceptional opportunity to study cancer-immune interactions, and interrogate the mechanisms of the autoimmune rheumatic diseases, as well as the natural immune response to cancers in humans.
This review highlights the relationships between cancer and rheumatic diseases, focused on kinetics (how closely in time the cancer and rheumatic disease present) and immune response (the frequency of cancer in rheumatic disease patients with different autoantibody specificities). We will highlight similarities to various paraneoplastic, immune-mediated processes, and will introduce important new tumor immunoediting concepts. While space constraints require that this review focus on specific immune responses associated with cancer in SSc and DM, the principles outlined are likely relevant to other autoimmune rheumatic syndromes.
An increased risk of cancer, and a temporal clustering of cancer with rheumatic disease onset, is present in DM and SSc
Patients with DM and SSc have an increased risk of cancer after adjusting for age and gender compared to general population-based controls (SIRs or RRs ranging from 3.0–7.7 for DM and 1.4–3.2 for SSc) (5–22). Table 1 highlights cancer sites for which these patients are at an elevated risk. While men (8, 14, 26), older patients developing myositis and SSc (5, 17, 18, 22, 27–30), and patients with rapid and severe onset of disease (29, 30), poor response to therapy, or diffuse cutaneous SSc may also have a higher risk of malignancy, these have not been consistently identified as risk factors for cancer.
Table 1.
Increased risk of particular tumor types among patients with dermatomyositis and systemic sclerosis.
| Dermatomyositis | Systemic Sclerosis | ||
|---|---|---|---|
| Cancer site | Reference | Cancer site | Reference |
| Ovary | (20, 21, 23) | Lung | (5, 7–9, 11, 13–16) |
| Lung | (20, 21, 23, 24) | Hematologic | (8, 12–16) |
| Pancreas | (21, 23) | Esophagus | (6) |
| Stomach | (21) | Oropharynx | (6, 16) |
| Colorectal | (21, 23) | Skin | (8, 9) |
| Breast | (21, 23, 24) | Cervix | (8) |
| Non-Hodgkin’s lymphoma | (21) | Liver | (9, 14, 25) |
| Nasopharynx (SE Asians) | (24) | Bladder | (14) |
In both diseases, there is a close temporal relationship between malignancy and autoimmunity onset. This is most striking in DM where the majority of patients with a malignancy have cancer preceding myositis diagnosis (19, 21, 31), often within 2 years (21). The risk of malignancy is highest in the first year after myositis diagnosis, then gradually decreases over time (19–21). In SSc, a similar temporal relationship has been observed in patients with breast cancer (32, 33). This temporal clustering, in conjunction with reports suggesting that cancer therapy may improve myositis (34) or SSc (35, 36) outcomes, suggests a possible mechanistic relationship between malignancy and rheumatic disease. Investigating this relationship is complex because of the significant heterogeneity in clinical phenotypes, age of rheumatic disease onset, tumor types, and cancer and rheumatic disease therapies employed in these patients. However, the strong associations between unique autoantibodies and the temporal clustering of cancer diagnosis with rheumatic disease onset suggest that immunological subsets may be a critical filter in understanding the cancer-autoimmunity relationship.
Unique autoantibodies associate with a temporal clustering of cancer and rheumatic disease
Autoantibodies have important diagnostic and prognostic power across the spectrum of the autoimmune rheumatic diseases. Within a given phenotype, different autoantibodies may be associated with distinct clinical phenotypes. Myositis and SSc autoantibodies illustrate this well; we have therefore focused on these below.
Myositis
Interestingly, within the spectrum of myositis (37), well-characterized myositis-specific autoantibodies are associated with distinct phenotypes. For example, antibodies against the aminoacyl tRNA synthetases (especially anti-Jo-1) are found in myositis patients with a common set of clinical features including interstitial lung disease, mechanics hands, non-erosive arthritis and fever (the “antisynthetase syndrome”). Mi-2 antibodies are found exclusively in DM patients; these patients frequently have more severe skin rashes and respond better to steroid therapy. While antibodies against melanoma differentiation-associated gene5 (MDA5) are also DM-specific, patients with this specificity are typically amyopathic and frequently have interstitial lung disease (38). Yet another distinct clinical part of the myositis spectrum is associated with antibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase - these are a feature of patients with an immune-mediated necrotizing myopathy (39, 40).
While the clinical phenotypes associated with known autoantibodies are widely recognized, myositis autoantibodies have not, until recently, been meaningfully associated with cancer. The usefulness of autoantibodies as predictors of cancer-associated myositis was examined by Chinoy et al (41) in a study of 282 myositis/connective tissue disease overlap patients. They showed that patients with myositis-specific and myositis-associated antibodies that can be assayed by routinely available clinical tests have a significantly lower risk of an associated cancer than autoantibody-negative DM patients.
Recently emerging data has shown that while this may be the case for the “well-established/historic” myositis autoantibodies, two new specificities do indeed appear to be associated with cancer. These, and evidence of their cancer association, are discussed below.
TIF1γ antibodies
A new DM-specific autoantibody, found in 13–21% of adult DM patients, was recently reported by 2 groups (42, 43). In both cases, cohorts of patient sera were screened by immunoprecipitation using radiolabeled cell lysates, enabling detection of a 155kDa protein. Although the cohort sizes and the numbers of antibody-positive patients were small, both studies noted that these antibodies were frequently detected in patients with an associated malignancy. The target of this new antibody specificity was identified as transcription intermediary factor 1 gamma (TIF1γ) (44). This multifunctional protein is a member of the tripartite-motif containing protein family, and has complex effects on various cellular pathways. For example, TIF1γ has a critical role in tissue differentiation through interactions with SMAD proteins (45). Thus, in embryonic stem cells, TIF1γ interacts with SMAD2/3, allowing this complex to activate specific differentiation genes by promoting transcriptional elongation (46). TIF1γ is also required for proper development of mammary glands, where it inhibits SMAD4 by ubiquitinylation (47). To evaluate the usefulness of TIF1γ antibodies for diagnosing cancer-associated DM, Trallero-Araguas et al (48) performed a systematic review and meta-analysis using data from six published studies; in each, immunoprecipitation from lysates was used for antibody detection. The meta-analysis showed that anti-TIF1γ positive DM patients have a 27-fold higher odds of having cancer-associated myositis than their anti-TIF1γ negative counterparts (48).
NXP-2 antibodies
In 2007, antibodies against a 140 kD protein (“anti-MJ”) were reported in 18% of juvenile DM patients (49). The targeted autoantigen was subsequently found to be NXP-2 (50), a protein that localizes to the promyelocytic leukemia nuclear bodies and the nucleoplasm (41). There are 3 structurally separated, conserved domains (51) with important roles in various functions. For example, Takahashi et al (41) showed that NXP-2 recruits and activates p53, inducing cellular senescence, thereby preventing cell proliferation. Initial studies of this specificity were performed on pediatric DM cohorts (52, 53), confirming a prevalence of ~23–25%. They are notable for the lack of malignancy reports amongst the >200 young patients studied. More recent studies in adult myositis populations report prevalences ranging from 1.6 – 30% of adult DM and 1.6 – 8% of PM patients (54, 55). Interestingly, Ichimura et al (55) noted that associated cancers were present in 3/7 (43%) of the anti-NXP-2 positive DM patients in their cohort, with all of the carcinomas being advanced stage. Intriguingly, 6/7 (86%) of the anti-NXP-2 positive DM patients were male, and 3/3 (100%) of the cancers in this group were in male patients. These were not restricted to male-specific cancer sites.
>80% of patients with cancer-associated myositis have antibodies against NXP-2 or TIF1γ
Readout of specific antibodies in the 140–155 kD range following immunoprecipitation from lysates is challenging as there are multiple specificities in this size range (including TIF1γ, NXP-2 and MDA-5). Fiorentino and colleagues (56) therefore recently developed sensitive, specific assays that unequivocally detect antibodies against NXP-2 and TIF1γ. Using these, antibodies against TIF1γ and NXP-2 were evaluated in 213 patients from two separate, well-defined DM cohorts (111 patients from Stanford University Dermatology and 102 from the Johns Hopkins Myositis Center).
Antibodies against TIF1γ and NXP-2 were detected in 82/213 (38%) and 37/213 (17%), respectively, of DM patients. The antibody groups were mostly non-overlapping, with only 2 patients having both specificities. Cancer-associated DM was detected in 29/213 (14%) of DM patients, with 24/29 (83%) having antibodies against either TIF1γ or NXP-2. The overall frequency of cancer in TIF1γ/NXP2-positive patients was 20.5%. In the remaining patients with neither TIF1γ nor NXP-2 antibodies, there were only 5/96 (5%) cases of cancer. An important relationship of age and cancer frequency in DM patients was noted across all antibody groups: for patients >60 years old, cancer was found in 55% of patients with anti-NXP-2 antibodies, 31% of anti-TIF1γ-positive patients, and 17% of patients without either of these antibodies. Additionally, antibodies against NXP-2 were specifically associated with cancer in males (7/9, 78%). This observation is similar to that made in 3 patients by Ichimura et al (55). While the numbers in both of these studies are small, this finding is intriguing and awaits confirmation.
These observations make several important points about the relationship between DM and cancer: (i) Where cancers occur, they generally manifest around the time of DM diagnosis, irrespective of antibody response; (ii) the prevalence of cancers within 3 years of myositis diagnosis is higher in anti-TIF1γ and NXP2 autoantibody groups; (iii) even in patients with TIF1γ or NXP2 antibodies, a sizable proportion of patients do not manifest cancer; (iv) the frequency of cancers associated with DM increases at age >60 years, irrespective of serology; (v) the association of NXP2 antibodies and cancer in DM may be enhanced in males. Additional data is needed to confirm whether these findings are generalizable (particularly to different ethnic populations). The association of DM and cancer therefore does not appear to be binary, but rather to be influenced strongly by several parameters, including autoantibody targets and age. It is possible that a binary parameter remains to be defined. Of note, current data regarding immune responses examines overall autoantibody responses, but we do not yet understand the nuances of these specificities in terms of epitopes and magnitude, nor the specificity of other immune effector pathways for these antigens. It is also possible that no individual parameter will be the key determinant of whether a cancer emerges clinically, but that the additive effects of multiple parameters will be explicative. The close temporal clustering of DM and cancer, and the elevated frequency of cancer in patients with TIF1γ/NXP2 antibodies irrespective of age, suggests that additional important parameters remain to be defined (see below).
Scleroderma
As in myositis, scleroderma-specific autoantibodies are associated with distinct clinical phenotypes and are useful for risk stratification and assessing long-term prognosis (57–60). The three most common scleroderma specific autoantibodies are anti-centromere, anti-topoisomerase 1 and anti-RNA polymerase III (POL3). Patients targeting centromere proteins B/C tend to have limited cutaneous disease with features of the CREST (calcinosis, Raynaud’s, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome, major ischemic digital loss, pulmonary hypertension and overlap features with Sjogren’s syndrome or primary biliary cirrhosis. In contrast, patients with anti-topoisomerase 1 antibodies have a higher risk of diffuse cutaneous disease and interstitial lung disease, and those with anti-POL3 antibodies usually have aggressive, rapidly progressive diffuse cutaneous disease and a significantly higher risk of scleroderma renal crisis, myopathy, cardiac disease, and gastric antral vascular ectasia. Careful study of patients in these autoantibody subsets has demonstrated the usefulness of autoantibodies as predictors of cancer-associated scleroderma.
POL3 antibodies identify patients at risk for cancer-associated scleroderma
Many investigations probing the relationship between cancer and scleroderma have excluded cancer cases diagnosed around the time of scleroderma onset to avoid a potential detection bias. Consequently, patients with a close temporal relationship between cancer diagnosis and scleroderma onset have not been studied until recently. We investigated whether clinical characteristics differed by autoantibody status among patients with scleroderma and cancer (4). We demonstrated that patients with POL3 antibodies had a close temporal relationship between malignancy and the clinical onset of scleroderma, and a unique nucleolar POL3 expression pattern in their cancerous tissues (4). These data suggested that expression of scleroderma antigens in cancers might be associated with scleroderma-specific autoantibody responses.
The association between POL3 antibodies and a close cancer-scleroderma interval has subsequently been confirmed by others (27, 33, 61). In an Italian cohort, patients with POL3 antibodies had a higher prevalence of cancer and were more likely to have cancer concurrent with scleroderma onset than patients with other autoantibodies (61). However, this study had only 16 anti-POL3 patients, 7 of whom had cancer (61). In a study of 451 Australian scleroderma patients, those with POL3 antibodies had a 4.2-fold increased odds of having malignancy diagnosed within 5 years of scleroderma onset compared to patients without this specificity (27). The overall prevalence of cancer (~13%) was similar between patients with and without POL3 antibodies (27). Another investigation in a cohort of 2177 UK scleroderma patients demonstrated that the prevalence of malignancy was higher in patients with POL3 antibodies (14.2%) than in patients with topoisomerase 1 (6.3%, p<0.0001) or centromere (6.8%, p<0.001) antibodies (33). Among those with cancer within 36 months of scleroderma onset, 55.3% of patients were positive for POL3 antibodies compared with 13.6% of patients with topoisomerase 1 antibodies (p<0.002) and 23.5% of patients with centromere antibodies (p<0.008) (33). These data suggest that patients with new onset scleroderma and POL3 antibodies may benefit from more aggressive evaluation for an underlying malignancy given their heightened cancer risk.
Interestingly, while a close cancer-scleroderma interval is most frequent amongst patients with POL3 antibodies, there were patients with a short cancer-scleroderma interval and other autoantibody specificities in all cohorts. Since an anti-cancer immune response may be an important feature in some patients with scleroderma and POL3 antibodies (see below), the co-occurrence (though infrequent) of cancer and scleroderma in these other serologic subgroups suggests that cancer may be an important initiator of the immune response in many scleroderma patients, but that an immune response against specific targets might exert more potent anti-cancer effects. Indeed, centromere proteins and topoisomerase 1 play important roles in cancer fitness and survival. These pathways are targets of potent anti-cancer therapeutics, including inhibitors of topoisomerase and the mitotic spindle, suggesting that immune responses to some pathways may have more deleterious effects on cancer growth and survival than others. It is possible that the other targets of the immune response in rheumatic autoimmune diseases – where cancer incidence is low – may be effective therapeutic targets in cancers. It is also possible that distinct mechanisms (unrelated to neoplastic transformation) may underlie the immune targeting of centromere and topoisomerase 1 including infections and other cellular states.
Genetic alteration of autoantigens in cancer may be an antigen source in the rheumatic diseases
It has been hypothesized that patients with cancer and the rheumatic diseases may develop cancer secondary to (i) target tissue damage from the autoimmune disease, (ii) cytotoxic therapies used to treat aggressive manifestations, or (iii) as a consequence of a defective immune system that predisposes patients to develop both cancer and autoimmunity (3, 62). However, data demonstrating unique nucleolar POL3 expression in cancerous tissues from scleroderma patients with POL3 antibodies (4), and the co-occurrence of cancer and scleroderma in this patient subset raised the intriguing possibility that genetic alterations in the POLR3A locus in tumors may trigger autoimmunity (3).
To address this, we studied tumors from 16 scleroderma patients, 8 with POL3 antibodies and 8 with either centromere or topoisomerase 1 antibodies. The POLR3A locus had genetic alterations in 6/8 cancers from patients with POL3 antibodies, but not in tumors from patients with other scleroderma antibodies. Interestingly, these genetic alterations took two forms: (i) Somatic mutations - 3 patients had somatic mutations in POLR3A; in each, the mutation caused a change in a single amino acid (different in all 3 patients). The mutations were present at a diminished frequency in the cancers, suggesting that they arose quite late in cancer development, or that the mutations arose early and were negatively selected during cancer evolution (see Loss of Heterozygosity (LOH) below). It is noteworthy that mutations in POLR3A are very uncommon in cancer, with a frequency of 0.7% in the COSMIC database (p<10−20), suggesting that these mutations help to initiate the immune response to POL3. Indeed, 2 of the 3 patients with mutated forms of POL3 demonstrated mutation-specific T cell immune responses. The fact that mutations in specific autoantigens such as POLR3A are infrequent in cancers in general suggests that even infrequent mutations, if they are in the right genes (i.e. autoantigens), may initiate autoimmunity when presented to the immune system in the context of the appropriate MHC framework. A deeper understanding of these relationships will require much more extensive data on frequency of mutations in additional autoantigens. (ii) LOH – 5/8 patients exhibited LOH at the POLR3A locus. This was not a feature in cancers of scleroderma patients with other immune responses, including antibodies to topoisomerase 1 or centromere proteins, strongly indicating that the POL3-specific immune response might be shaping the molecular evolution of the cancer, consistent with immunoediting (see below).
As ~85% of patients with scleroderma and POL3 antibodies do not manifest a cancer clinically, we hypothesize that potent anti-tumor immune responses successfully eradicate an underlying malignancy in most patients with scleroderma who manifest an autoantibody response against POL3.
Although the immune response to POL3 may be initiated against mutated proteins in the patient’s cancer, the presence of autoimmune injury to self tissues (which do not express the mutated version) suggests that the immune response spreads to the wild type (WT) version of the antigen present in self tissue. Consistent with this, autoantibodies in the patients with mutated POL3 were cross-reactive with both WT and mutant POL3 (3). There is significant data demonstrating that a modified version of a self antigen can initiate a T cell immune response to the altered antigen, and that the resulting B cell response recognizes the altered and WT antigens similarly (63). When WT and altered antigens are present at the time of immunization, a T cell response to the WT antigen can be initiated (64). We propose a similar mechanism here, with mutated antigen initiating a mutant-specific T cell response, cross-reactive B cell response, and upon release and autoantibody-mediated uptake of WT antigen, a T cell response directed against the WT molecule (Figure 1). While one patient in our study had CD4 T cells recognizing the WT POLR3A antigen, defining CD4 T cells directed against the WT POL3 antigen in scleroderma patients with and without cancer remains a high priority.
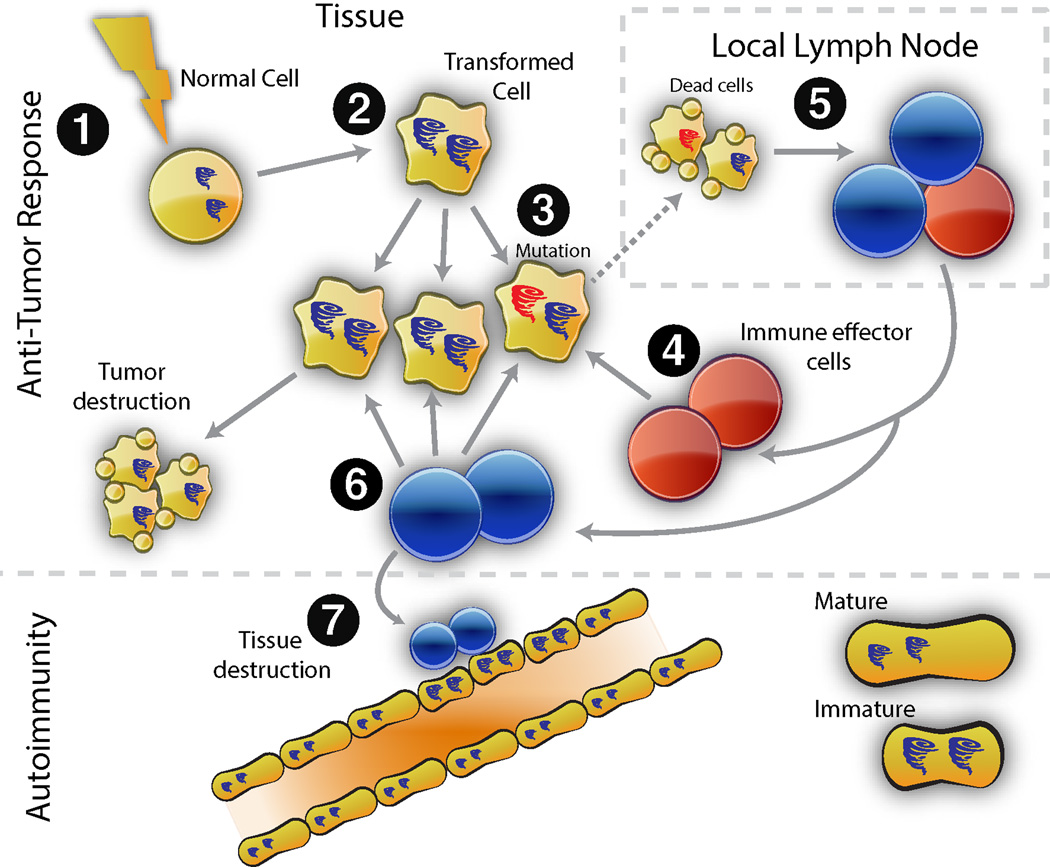
Figure 1. Model for cancer-induced autoimmunity.
Transformation of normal cells (1) may result in gene expression patterns which resemble immature cells involved in tissue healing (2). Occasionally, autoantigens become mutated (3); these are not driver mutations, and not all cancer cells have them. The first immune response is directed against the mutated form of the antigen (4), and may spread to the wild type version (5). Immune effector cells directed against the mutant (depicted in red) delete exclusively cancer cells containing the mutation (6). Immune effector cells directed against the wild type (blue cells) delete cancer cells without the mutation, and also cross-react with the patient’s own tissues (particularly immature cells expressing high levels of antigen, found in damaged/repairing tissue). Once autoimmunity has been initiated, the disease is self-propagating. Immature cells (expressing high antigen levels) that repair the immune-mediated injury themselves become the targets of the immune response, sustaining an ongoing cycle of damage/repair that provides the antigen source fueling the autoimmune response.
Other cancer-induced autoimmune syndromes suggest that tumor antigen expression triggers unique autoimmune phenotypes and perhaps improved cancer outcomes
There is significant evidence for activation of the immune response in various cancers. Several tumor antigens have been defined in these circumstances; in most instances where antibodies to tumor antigens have been defined, these are not associated with damage of normal tissues. However, there are numerous examples of paraneoplastic autoimmune syndromes, where there is immune damage of specific target tissues (e.g. paraneoplastic pemphigus, paraneoplastic neurological degenerations, Lambert-Eaton syndrome) (65). Additionally, during cancer immunotherapy, activation of autoimmunity often predicts a beneficial anti-cancer effect (e.g. vitiligo in melanoma, autoantibodies appearing during IFN treatment of metastatic melanoma) (66, 67). Tumor-infiltrating lymphocytes are also predictive of better cancer prognosis. Patients with autoimmune paraneoplastic disease often have smaller tumors than patients without paraneoplastic syndromes (68). Indeed, some tumors may not be evident at diagnosis, likely due to a robust immune response. However, the amount of damage to normal tissue that can accrue over time can be high, causing significant morbidity and even mortality. The finding that severe autoimmunity affecting the nervous system sometimes significantly delays tumor diagnosis resembles the autoimmune rheumatic diseases, where cancers may not appear for prolonged periods. Interestingly, in several cases, there is shared antigen expression in tumors and in target tissues (68). In one investigation, myositis autoantigen expression was increased in cancer types associated with myositis and in regenerating muscle cells in myositis muscle (69). These data suggest that while malignancies may be an antigen source initiating the immune response, regenerating cells in target tissues may be an antigen source propagating a feed-forward loop of tissue damage and autoimmune disease (69).
Principles of cancer immunosurveillance and immunoediting may provide insight into the kinetics and pathogenesis of the rheumatic diseases
Several important immunological principles that were suggested during development of the discipline of immunology (e.g. cancer immunosurveillance and suppressor T cells) preceded the ability to address their underlying molecular mechanisms, and resulted in an initial mistaken conclusion that the principle was incorrect. This was true of the immunosurveillance hypothesis of Burnet in the 1950s (70), which proposed that mutations in cancer might provoke an effective immune response causing regression of the tumor without it ever making its existence known. The hypothesis was difficult to address directly in these early years, due to limited knowledge about molecular mechanisms of immunity, the specific cells mediating immune responses, and an inability to define specific cancer antigens. When an extensive series of experiments in the 1970s failed to show any increase in cancer initiation in immunodeficient mice exposed to mutagen (71), the field of immunosurveillance began to fall out of favor. As the cells, molecules and pathways of immunology became better defined, enabling investigation of the roles of specific pathways in vivo in various mouse models using genetic approaches, several observations strongly suggested that various immune pathways (e.g. IFNγ, perforin) played central roles in regulating cancer emergence. Additionally, a growing body of knowledge (reviewed in (1)) showed that the immune system could target specific antigens in different cancers, demonstrating that cancers were not immunologically silent but rather were actively recognized by the immune system.
The immunosurveillance hypothesis underwent an important revision in the early 2000s, when it was recognized that the tumor does not remain constant in the presence of immune pressure, but is rather shaped by the immune response such that the resulting tumor is less capable of stimulating the immune system. Schreiber and colleagues proposed that immunosurveillance be renamed immunoediting, acknowledging the dual “host-protective and tumor-promoting actions of immunity on developing cancers” (72). One form of editing involves loss of cancer-specific antigen expression (similar to the LOH described for POLR3A above). Another form involves the expression of immune checkpoints by the cancer (2). The recent success of immune checkpoint inhibitors (e.g. anti-PD1) in activating durable tumor-specific immune responses, with clinically significant anti-cancer effects underscores the relevance of pre-formed immune responses which become silenced during tumor evolution, and emphasize that the clinical emergence of cancer likely represents a distinct phase of immunoediting.
Indeed, Schreiber and colleagues have proposed that cancer immunoediting has 3 stages: (i) Elimination; (ii) Equilibrium and (iii) Escape (1). Although not all stages have been observed in vivo, significant indirect evidence suggests their existence. There is likely considerable heterogeneity amongst cancers and individuals in terms of whether each stage occurs, and its duration. Conceptually, the stages are defined by the relative dominance of the cancer or the immune response. In the first stage (Elimination), the immune response dominates, and antigens in the nascent cancer initiate innate and adaptive immune responses, resulting in elimination of the cancer. In the second stage (Equilibrium), the anti-cancer immune response and the cancer are balanced – the cancer does not grow significantly, nor is the host immune system fully effective at eradication. This highly dynamic stage depends on ongoing matching of the immune response and the cancer. If significant changes occur in either (e.g. immune system weakening, or changes in the cancer that allow it to be less affected by the immune response, or acquired resistance to an immune effector pathway through expression of a checkpoint molecule), the cancer could escape and grow. Stage three (Escape) represents the cancer dominating the immune response, evidenced by unregulated cancer growth. Cancers in this stage are likely significantly immunoedited, and are much poorer immune targets than nascent, unedited tumors. Of note, almost all cancers that present clinically are already in stage 3; current cancer immunotherapies targeting immune checkpoints focus on this stage.
A model of cancer-induced autoimmunity in the rheumatic diseases
The causes of the autoimmune rheumatic diseases remain unclear. Although a minority of patients with autoimmune rheumatic diseases manifest cancers, some intriguing observations about this association suggest that cancers may play important roles in disease initiation. The evolving understanding of cancer immunoediting, clearer definition of the targets of the immune responses in cancer-associated rheumatic diseases, and the important co-clustering of cancer and rheumatic diseases in a subgroup of patients suggest a model in which mutations in autoantigens in cancers initiate an autoimmune response against highly specific targets. The initial mutation-specific immune response subsequently spreads to the WT version of the protein, inducing tissue damage, focused on tissues in which the function and expression of that antigen is prominent. In some cases, this immune response challenges the cancer, either eliminating it or maintaining it in equilibrium. Such patients present with the autoimmune rheumatic phenotype, but no cancer. In other cases, the anti-cancer response actively immunoedits the cancer, and the cancer may eventually lose expression of the mutant antigen, and may also evolve to avoid the anti-cancer effects through other mechanisms. In this minority of patients, cancer emerges. The critical immune effector pathways, which effect damage and dysfunction of normal tissue or the cancer expressing the WT allele, are not yet defined but likely include CD4 T cells, CD8 T cells, B cells, and autoantibodies. In addition to non-tolerized structure, initiation of a primary immune response to cancer autoantigens would require the appropriate MHC scaffold, co-stimulation, and absence of immune checkpoints. The recent recognition of non-malignant somatic mutation as a cause of chronic tissue dysfunction (73) also raises the question of whether mutations occurring in benign lesions which become visible to the immune system in the setting of danger might also be relevant to autoimmunity (74).
This model provides numerous testable hypotheses related to early detection of cancer, and possible therapy of cancer as an approach in autoimmune diseases. Since the immune response to the autoantigen is initiated and driven by the cancer and cross-reacts with antigens in normal tissue, effective therapy to remove the cancer could rid the host of the apical immune stimulus, and allow the peripheral immune mediated damage to wane once resolution and tissue healing occur. The striking examples of autoimmune diseases disappearing after effective anti-cancer therapy are consistent with this model (34–36). This also focuses attention on the immune response in rheumatic autoimmune diseases as a potentially positive force, and suggests that approaches regulating antigen expression in the target tissue may allow the beneficial anti-cancer effects of the immune system to remain focused on the cancer, while avoiding damage to self tissues.
The next decade will be an exciting time in understanding the rheumatic autoimmune diseases. It is also likely that these diseases will reveal critical secrets about natural anti-tumor immunity, and how this might be harnessed for treating cancers.
Acknowledgements
We thank John Hall, PhD for assistance with making Figure 1, and Paul Rosen for assistance with editing.
Financial support: This work was supported by funding from NIH/NIAMS K23 AR061439 (AS), RO1 AR44684 (LCR), R37 DE12354 (AR), R56 AR062615-01A1 (LCR) and the Dorothy and Donald Stabler Foundation.
Footnotes
Conflicts of Interest: None
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343(6167):152–157. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AA, Rosen A, Hummers L, Wigley F, Casciola-Rosen L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis and rheumatism. 2010;62(9):2787–2795. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Shakra M, Guillemin F, Lee P. Cancer in systemic sclerosis. Arthritis and rheumatism. 1993;36(4):460–464. doi: 10.1002/art.1780360405. [DOI] [PubMed] [Google Scholar]
- 6.Derk CT, Rasheed M, Artlett CM, Jimenez SA. A cohort study of cancer incidence in systemic sclerosis. The Journal of rheumatology. 2006;33(6):1113–1116. [PubMed] [Google Scholar]
- 7.Hill CL, Nguyen AM, Roder D, Roberts-Thomson P. Risk of cancer in patients with scleroderma: a population based cohort study. Annals of the rheumatic diseases. 2003;62(8):728–731. doi: 10.1136/ard.62.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olesen AB, Svaerke C, Farkas DK, Sorensen HT. Systemic sclerosis and the risk of cancer: a nationwide population-based cohort study. The British journal of dermatology. 2010;163(4):800–806. doi: 10.1111/j.1365-2133.2010.09861.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal AK, McLaughlin JK, Gridley G, Nyren O. Incidence of cancer among patients with systemic sclerosis. Cancer. 1995;76(5):910–914. doi: 10.1002/1097-0142(19950901)76:5<910::aid-cncr2820760528>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal AK, McLaughlin JK, Linet MS, Persson I. Scleroderma and malignancy: an epidemiological study. Annals of the rheumatic diseases. 1993;52(7):531–533. doi: 10.1136/ard.52.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters-Golden M, Wise RA, Hochberg M, Stevens MB, Wigley FM. Incidence of lung cancer in systemic sclerosis. The Journal of rheumatology. 1985;12(6):1136–1139. [PubMed] [Google Scholar]
- 12.Siau K, Laversuch CJ, Creamer P, O'Rourke KP. Malignancy in scleroderma patients from south west England: a population-based cohort study. Rheumatology international. 2011;31(5):641–645. doi: 10.1007/s00296-009-1348-y. [DOI] [PubMed] [Google Scholar]
- 13.Bonifazi M, Tramacere I, Pomponio G, Gabrielli B, Avvedimento EV, La Vecchia C, et al. Systemic sclerosis (scleroderma) and cancer risk: systematic review and meta-analysis of observational studies. Rheumatology. 2013;52(1):143–154. doi: 10.1093/rheumatology/kes303. [DOI] [PubMed] [Google Scholar]
- 14.Onishi A, Sugiyama D, Kumagai S, Morinobu A. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis and rheumatism. 2013;65(7):1913–1921. doi: 10.1002/art.37969. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JQ, Wan YN, Peng WJ, Yan JW, Li BZ, Mei B, et al. The risk of cancer development in systemic sclerosis: a meta-analysis. Cancer epidemiology. 2013;37(5):523–527. doi: 10.1016/j.canep.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CF, Luo SF, Yu KH, Chou IJ, Tseng WY, Chang HC, et al. Cancer risk among patients with systemic sclerosis: a nationwide population study in Taiwan. Scandinavian journal of rheumatology. 2012;41(1):44–49. doi: 10.3109/03009742.2011.618145. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL, Chang YT. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis research & therapy. 2010;12(2):R70. doi: 10.1186/ar2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Airio A, Pukkala E, Isomaki H. Elevated cancer incidence in patients with dermatomyositis: a population based study. The Journal of rheumatology. 1995;22(7):1300–1303. [PubMed] [Google Scholar]
- 19.Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Annals of internal medicine. 2001;134(12):1087–1095. doi: 10.7326/0003-4819-134-12-200106190-00008. [DOI] [PubMed] [Google Scholar]
- 20.Chow WH, Gridley G, Mellemkjaer L, McLaughlin JK, Olsen JH, Fraumeni JF., Jr Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer causes & control : CCC. 1995;6(1):9–13. doi: 10.1007/BF00051675. [DOI] [PubMed] [Google Scholar]
- 21.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 22.Stockton D, Doherty VR, Brewster DH. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. British journal of cancer. 2001;85(1):41–45. doi: 10.1054/bjoc.2001.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigurgeirsson B, Lindelof B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. The New England journal of medicine. 1992;326(6):363–367. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- 24.Huang YL, Chen YJ, Lin MW, Wu CY, Liu PC, Chen TJ, et al. Malignancies associated with dermatomyositis and polymyositis in Taiwan: a nationwide population-based study. The British journal of dermatology. 2009;161(4):854–860. doi: 10.1111/j.1365-2133.2009.09274.x. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee S, Dombi GW, Severson RK, Mayes MD. Risk of malignancy in scleroderma: a population-based cohort study. Arthritis and rheumatism. 2005;52(8):2415–2424. doi: 10.1002/art.21225. [DOI] [PubMed] [Google Scholar]
- 26.Zahr ZA, Baer AN. Malignancy in myositis. Current rheumatology reports. 2011;13(3):208–215. doi: 10.1007/s11926-011-0169-7. [DOI] [PubMed] [Google Scholar]
- 27.Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis research & therapy. 2011;13(6):R211. doi: 10.1186/ar3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derk CT. Associations of breast cancer development in patients with systemic sclerosis: an exploratory study. Clinical rheumatology. 2007;26(10):1615–1619. doi: 10.1007/s10067-007-0546-9. [DOI] [PubMed] [Google Scholar]
- 29.Fardet L, Dupuy A, Gain M, Kettaneh A, Cherin P, Bachelez H, et al. Factors associated with underlying malignancy in a retrospective cohort of 121 patients with dermatomyositis. Medicine. 2009;88(2):91–97. doi: 10.1097/MD.0b013e31819da352. [DOI] [PubMed] [Google Scholar]
- 30.Ponyi A, Constantin T, Garami M, Andras C, Tallai B, Vancsa A, et al. Cancer-associated myositis: clinical features and prognostic signs. Annals of the New York Academy of Sciences. 2005;1051:64–71. doi: 10.1196/annals.1361.047. [DOI] [PubMed] [Google Scholar]
- 31.Zantos D, Zhang Y, Felson D. The overall and temporal association of cancer with polymyositis and dermatomyositis. The Journal of rheumatology. 1994;21(10):1855–1859. [PubMed] [Google Scholar]
- 32.Colaci M, Giuggioli D, Vacchi C, Lumetti F, Iachetta F, Marcheselli L, et al. Breast cancer in systemic sclerosis: results of a cross-linkage of an Italian Rheumatologic Center and a population-based Cancer Registry and review of the literature. Autoimmunity reviews. 2014;13(2):132–137. doi: 10.1016/j.autrev.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Moinzadeh P, Fonseca C, Hellmich M, Shah AA, Chighizola C, Denton CP, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis research & therapy. 2014;16(1):R53. doi: 10.1186/ar4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andras C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. The Journal of rheumatology. 2008;35(3):438–444. [PubMed] [Google Scholar]
- 35.Hasegawa M, Sato S, Sakai H, Ohashi T, Takehara K. Systemic sclerosis revealing T-cell lymphoma. Dermatology. 1999;198(1):75–78. doi: 10.1159/000018070. [DOI] [PubMed] [Google Scholar]
- 36.Juarez M, Marshall R, Denton C, Evely R. Paraneoplastic scleroderma secondary to hairy cell leukaemia successfully treated with cladribine. Rheumatology. 2008;47(11):1734–1735. doi: 10.1093/rheumatology/ken367. [DOI] [PubMed] [Google Scholar]
- 37.Mammen AL. Dermatomyositis and polymyositis: Clinical presentation, autoantibodies, and pathogenesis. Annals of the New York Academy of Sciences. 2010;1184:134–153. doi: 10.1111/j.1749-6632.2009.05119.x. [DOI] [PubMed] [Google Scholar]
- 38.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinoy H, Fertig N, Oddis CV, Ollier WE, Cooper RG. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Annals of the rheumatic diseases. 2007;66(10):1345–1349. doi: 10.1136/ard.2006.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura K, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology. 2007;46(1):25–28. doi: 10.1093/rheumatology/kel161. [DOI] [PubMed] [Google Scholar]
- 43.Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O'Hanlon TP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis and rheumatism. 2006;54(11):3682–3689. doi: 10.1002/art.22164. [DOI] [PubMed] [Google Scholar]
- 44.Targoff IN, Trieu EP, Levy-Neto M, Prasertsuntarasai T, Miller FW. Autoantibodies to Transcriptional Intermediary Factor 1-gamma (TIF1-g) in Dermatomyositis. Arthritis Rheum. 2006;54(Suppl.9):S518. [Google Scholar]
- 45.Massague J, Xi Q. TGF-beta control of stem cell differentiation genes. FEBS Lett. 2012;586(14):1953–1958. doi: 10.1016/j.febslet.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xi Q, Wang Z, Zaromytidou AI, Zhang XH, Chow-Tsang LF, Liu JX, et al. A poised chromatin platform for TGF-beta access to master regulators. Cell. 2011;147(7):1511–1524. doi: 10.1016/j.cell.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hesling C, Lopez J, Fattet L, Gonzalo P, Treilleux I, Blanchard D, et al. Tif1gamma is essential for the terminal differentiation of mammary alveolar epithelial cells and for lactation through SMAD4 inhibition. Development. 2013;140(1):167–175. doi: 10.1242/dev.085068. [DOI] [PubMed] [Google Scholar]
- 48.Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O'Callaghan A, Martinez-Gomez X, Bosch X, Labrador-Horrillo M, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis and rheumatism. 2012;64(2):523–532. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 49.Oddis CV, Fertig N, Goel A, Espada G, Confalone Gregorian M, Maldonado Cocco JA, et al. Clinical and serological characterization of the anti-MJ antibody in childhood myositis. Arthritis and rheumatism. 1997;40(9):S139. [Google Scholar]
- 50.Targoff IN, Trieu EP, Levy-Neto M, Fertig N, Oddis CV. Sera with Autoantibodies to the MJ Antigen React with NXP-2. Arthritis Rheum. 2007;56(Suppl.9):S787. [Google Scholar]
- 51.Kimura Y, Sakai F, Nakano O, Kisaki O, Sugimoto H, Sawamura T, et al. The newly identified human nuclear protein NXP-2 possesses three distinct domains, the nuclear matrix-binding, RNA-binding, and coiled-coil domains. J Biol Chem. 2002;277(23):20611–20617. doi: 10.1074/jbc.M201440200. [DOI] [PubMed] [Google Scholar]
- 52.Gunawardena H, Wedderburn LR, Chinoy H, Betteridge ZE, North J, Ollier WE, et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis and rheumatism. 2009;60(6):1807–1814. doi: 10.1002/art.24547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espada G, Maldonado Cocco JA, Fertig N, Oddis CV. Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. The Journal of rheumatology. 2009;36(11):2547–2551. doi: 10.3899/jrheum.090461. [DOI] [PubMed] [Google Scholar]
- 54.Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M, et al. Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis research & therapy. 2012;14(2):R97. doi: 10.1186/ar3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichimura Y, Matsushita T, Hamaguchi Y, Kaji K, Hasegawa M, Tanino Y, et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Annals of the rheumatic diseases. 2012;71(5):710–713. doi: 10.1136/annrheumdis-2011-200697. [DOI] [PubMed] [Google Scholar]
- 56.Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis and rheumatism. 2013;65(11):2954–2962. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris ML, Rosen A. Autoimmunity in scleroderma: the origin, pathogenetic role, and clinical significance of autoantibodies. Curr Opin Rheumatol. 2003;15(6):778–784. doi: 10.1097/00002281-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34(1):1–15. v. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Graf SW, Hakendorf P, Lester S, Patterson K, Walker JG, Smith MD, et al. South Australian Scleroderma Register: autoantibodies as predictive biomarkers of phenotype and outcome. Int J Rheum Dis. 2012;15(1):102–109. doi: 10.1111/j.1756-185X.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- 60.Shah AA, Wigley FM. My approach to the treatment of scleroderma. Mayo Clin Proc. 2013;88(4):377–393. doi: 10.1016/j.mayocp.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Airo P, Ceribelli A, Cavazzana I, Taraborelli M, Zingarelli S, Franceschini F. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. The Journal of rheumatology. 2011;38(7):1329–1334. doi: 10.3899/jrheum.101144. [DOI] [PubMed] [Google Scholar]
- 62.Shah AA, Rosen A. Cancer and systemic sclerosis: novel insights into pathogenesis and clinical implications. Current opinion in rheumatology. 2011;23(6):530–535. doi: 10.1097/BOR.0b013e32834a5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin RH, Mamula MJ, Hardin JA, Janeway CA., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. The Journal of experimental medicine. 1991;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mamula MJ, Lin RH, Janeway CA, Jr, Hardin JA. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. Journal of immunology. 1992;149(3):789–795. [PubMed] [Google Scholar]
- 65.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. The New England journal of medicine. 2003;349(16):1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 66.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. The New England journal of medicine. 2006;354(7):709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 67.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. The Journal of experimental medicine. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albert ML, Darnell RB. Paraneoplastic neurological degenerations: keys to tumour immunity. Nature reviews Cancer. 2004;4(1):36–44. doi: 10.1038/nrc1255. [DOI] [PubMed] [Google Scholar]
- 69.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. The Journal of experimental medicine. 2005;201(4):591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burnet M. Cancer; a biological approach. I. The processes of control. British medical journal. 1957;1(5022):779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stutman O. Immunodepression and malignancy. Advances in cancer research. 1975;22:261–422. doi: 10.1016/s0065-230x(08)60179-7. [DOI] [PubMed] [Google Scholar]
- 72.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 73.Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341(6141):1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matzinger P. Tolerance, danger, and the extended family. Annual review of immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]