Abstract
As the resident immune cells of the central nervous system, microglia rapidly respond to brain insults, including stroke and traumatic brain injury. Microglial activation plays a major role in neuronal cell damage and death by releasing a variety of inflammatory and neurotoxic mediators. Their activation is an early response that may exacerbate brain injury and many other stressors, especially in the acute stages, but are also essential to brain recovery and repair. The full range of microglial activities is still not completely understood, but there is accumulating knowledge about their role following brain injury. We review recent progress related to the deleterious and beneficial effects of microglia in the setting of acute neurological insults, and the current literature surrounding pharmacological interventions for intervention.
Keywords: Microglia, Brain injury, Inflammation, Neurotoxic mediator
Introduction
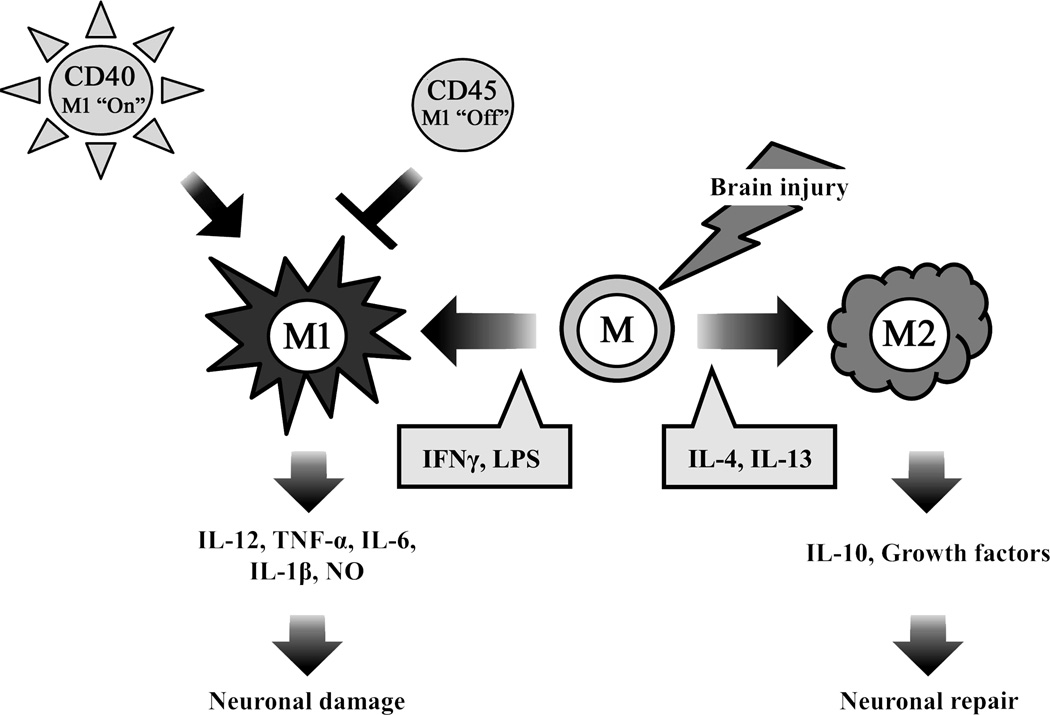
Inflammation within the central nervous system (CNS) can produce a variety of acute neurological insults including stroke, traumatic brain injury and others that lead to brain cell damage and death [1]. Microglia are the resident macrophages of the brain, and they are a key player in its immune responses [2–4]. Microglia constitute 15% of the total glial population, with predominance in the grey matter [5]. They respond to extracellular signals and are involved in clearing debris and toxic substances by phagocytosis, thereby retaining normal cellular homeostasis in the central nervous system (CNS) [6]. Consequently, under nonpathological states there is continuous, low-level microglial activity in the CNS which is primarily involved in activity-dependent synaptic pruning and repair [2]. In the event of acute brain insults such as trauma, ischemia, and neurodegeneration, microglia quickly activate by undergoing morphologic transformation from a “ramified” resting state, characterized by many branching processes, to an active, motile “amoeboid” state. In the amoeboid state, microglia are difficult to distinguish from circulating macrophages and monocytes, not only morphologically, but also with regard to surface markers and function [7,8]. When activated, microglia have been shown to release a variety of inflammatory and cytotoxic mediators contributing to cell damage and cell death leading to exacerbated brain injury [9,10]. Harmful effects appear to be predominant particularly in the acute stages of brain injury, while beneficial activities emerge in later stages. Current studies indicate that different signals lead to two primary activation phenotypes: classically activated (M1) and alternatively activated (M2) [11–13] (Fig. 1). The M1 phenotype, activated by lipopolysaccharide (LPS) and the pro-inflammatory cytokine interferonγ (IFNγ), promote transcriptional activation of nuclear factor-κB (NF-κB) and generate high levels of pro-inflammatory cytokines and oxidative metabolites such as interleukin (IL)-12, tumor necrosis factor (TNF)-α, IL-6, IL-1β, and nitric oxide (NO), formerly indicated to cause additional damage. In contrast, the M2 phenotype is triggered by anti-inflammatory cytokines such as IL-4 or IL-13 [14,15], which are thought to inhibit inflammation and enhance tissue repair and wound healing [16]. Microglia activation may initiate with an M1 phenotype that mediated an innate or an adaptive immune response and ultimately exacerbates neuronal damage [17,18]. Microglia may then adopt the M2 phenotype and facilitate repair-oriented functions by secreting growth factors and by clearing cellular debris via phagocytosis. M2 microglia also tend to limit proinflammatory signal production. This highlights the significance of identifying and understanding the different microglial phenotypes and their unique functions [19,20]. Furthermore, microglia present a potential target for therapeutic intervention with various time windows depending on the functions they contribute. Recent work has also shown that microglia can switch from the M1 phenotype to the M2 phenotype. In HIV associated dementia [21], spinal cord injury [22], microglia have been shown to initiate and maintain a M1 phenotype in the presence of CD40 ligation by CD40L and tumor necrosis factor (TNF), but may switch or ‘polarize’ to the M2 phenotype through upregulation of CD45. In a study of aged rodents subjected to TBI, histological studies indicated that aged brains of injured mice had not only larger lesions and worsened outcome, but microglial polarization towards M1, compared to younger rodents [23]. The challenge will be to discover methods for selectively suppressing the detrimental effects of microglial activation without compromising the restorative properties such as repair and remodeling. In related areas such as Alzheimer’s and HIV related dementia, strategies to turn ‘off’ CD40 or turn ‘on’ CD45 and favoring a M2 phenotype appear to improve neurological outcome [21], and suggest potential therapeutic targets for stroke and brain trauma. However, very little work in this area has been carried out in stroke and brain trauma.
Figure 1.
Microglial activation in brain jury. Microglia are activated by brain injury such as ischemic stroke and traumatic brain injury, which affect the polarization status of the cell. In brain injury, resting microglia (M) can be polarized to the M1 phenotype, or alternatively to the anti-inflammatory, pro-phagocytic M2 phenotype by the some of factors shown in the grey boxes. M1 microglia take part in the neuronal damage by elaborating pro-inflammatory molecules, whereas M2 microglia promote neuronal protection, and through phagocytic functions, set the stage for recovery and repair. While not extensively studied in brain injury, CD40 lead to the M1 phenotype, whereas CD45 turns this ‘off.’
Microglial receptors for activation
Microglia respond to specific receptors in the extracellular space and adjacent cell surfaces that modulate their activation and effector function. Microglial phagocytosis may require different types of receptors to initiate function [24]. Microglial receptors are able to bind foreign microbial pathogens as well as cellular debris. Such receptors include the Toll-like receptors (TLRs), the purinergic receptors, which bind nucleotides, and a relatively newly recognized family, triggering receptor expressed on myeloid cells (TREM).
TLRs
The exact initiating stimulus of acute brain damage has not been fully characterized, although some recent research has now identified a number of candidate factors. Several studies have shown that brain damage does induce activation of the TLRs [25], a family of transmembrane proteins involved in the identification of and defense against microbials. TLRs are observed in several cell types in the CNS and are a critical activating mechanism of innate immunity. They have been found on microglia and perivascular macrophages. Microglia are activated by stimulation of TLR4, which in turn leads to the upregulation of several proinflammatory genes. Lehnardt and others have shown that TLR4 is necessary for microglia activation after hypoxia [26] and TLR4-knockout mice have better neurological outcome following ischemic stroke [27–30].
Moreover, reports investigating the real trigger of microglial activation in brain injury have hypothesized that the ischemic brain produces substances which can bind TLRs. Substances released by dying cells which then bind immune receptors have been categorized as damage-associated molecular pattern molecules (DAMPs). There are also studies implicating the high mobility group box-1 (HMGB-1) [31,32], which is an endogenous TLR agonist and is related to cytokines. HMGB-1 is released by neurons as well as other immune cell types that contribute to cell death; HMGB-1 effectively functions as a pro-inflammatory cytokine [33]. Other examples of DAMPs are hyaluronan, surfactant protein, uric acid and heat shock proteins. These substances can bind to and stimulate microglia and other immune cells, resulting in the upregulation of many immune mediators by activation of several pro-inflammatory transcription factors, including NF-κB [34], hypoxia inducible factor 1 (HIF-1), interferon regulator factor 1 and signal transducer and activator of transcription 3 (STAT3) [35].
Current research suggests that the presence of upstream TLR4 activators may indicate adverse outcomes after brain injury. In experimental stroke, HSP60 has been shown to activate TLR4 and thereby worsen brain injury [36]. Toll-like receptors are also involved in the phenomenon of tolerance, whereby stimulation of one or more of these receptors with ligands, such as lipopolysaccharide (TLR4) or CpG (TLR9), triggers protection from subsequent lethal insults [37–39]. Mice deficient in these receptors did not achieve tolerance [37].
Purinergic receptors
Purinergic receptors have emerged as important sensors of brain injury. Of the numerous purinergic receptors identified, P2X7 and P2Y12 have been characterized as such, but P2×4 and adenosine receptors also contribute [40]. P2X7 signaling generates the production and release of other cytokines including TNF-α and IL-1β via activation of several mitogen-activated protein (MAP) kinase family members [41–43], microglia proliferation [44,45], and superoxide production [46]. Furthermore, activated P2X7 can escalate plasminogen release [47] and activation of the transcription factors NFAT (nuclear factor of activated T cells) and NF-κB, which are key regulators of pro-inflammatory genes [48,49]. In contrast, microglia also show decreased phagocytosis during P2X7 receptor stimulation [50]. A few recent studies have demonstrated that P2X7 antagonists reduce injury and post-injury inflammation when administered acutely after experimental CNS injury model [51,52]. However, other reports have described experiments where stroke appeared to be exacerbated by P2X7 antagonists [53]. These investigations are intriguing, but their results are difficult to interpret because these antagonists may also influence other purinergic receptors at concentrations found in vivo, and cell types other than microglia express P2X7, as well as other purinergic receptors.
The earliest reports of P2Y12 receptors described their involvement in microglial membrane ruffling and chemotaxis [54]. Activation of P2Y12 contributes to process extension and subsequent microglia migration toward injury and involves the activation of the PI-3 kinase [55] and Akt [56] signaling pathway. Interestingly, just as P2X4 is beginning to be associated with microglial movement, P2Y12 has recently been implicated in neuropathic pain [57]. Clopidogrel, a widely used antiplatelet agent, is also a P2Y12 receptor antagonist. Recent findings from our lab demonstrated that clopidogrel was protective in an experimental model of global cerebral ischemia [58]. In addition, P2Y12 knockout mice showed neuroprotection and reduced microglial chemotaxis. Nevertheless, it is important to remember that some antagonists such as cangrelor, previously believed to be P2Y12-specific, can also antagonize P2Y13 [58,59]. Since both receptors also have common agonists, and both are Gi-coupled, a role for P2Y13 may not have been eliminated by these pharmacological investigations. Researchers continue to identify novel P2 receptor agonists and antagonists; with an increasing number of available pharmacological agents, there are many more opportunities to study the therapeutic potential of purinergic receptors in regulating microglial activities.
Both P2×7 and P2×12 receptors react to adenosine triphosphate (ATP) as an endogenous agonist. It has been hypothesized that ATP is released extracellularly as a result of tissue injury, but it is unlikely that this occurs simply as a passive result of membrane disruption. For instance, ischemic injury produces energy deficiency and ATP depletion, and ATP released into brain extracellular matrix is rapidly degraded by exonucleases [60]. Therefore, an active, ongoing release of ATP is more likely to be the stimulus source acting on microglial purinergic receptors. A feed forward, ATP-induced release of ATP from astrocytes is one feasible mechanism [61].
In microglia, P2X4 receptors are primarily related to pain; their expression is markedly increased in spinal cord microglia following peripheral nerve injury which directly contributes to tactile allodynia [46]. P2X4 is thought to modulate pain by inducing increased production and release of brain-derived neurotrophic factor (BDNF) [62,63], a neurotrophin increased in pain models, which regulates neurotransmitter release and interneuron activity [41]. A recent study has also reported a role for P2X4 in microglial chemotaxis [55], which formerly had been mainly associated with P2Y12 [54].
PPARγ
Peroxisome proliferator activated receptors (PPARs) belong to the superfamily of nuclear hormone receptor, which mediates gene expression related to reproduction, metabolism, development, and immune responses. There are three different PPAR isotypes (α, β/δ, and γ), and each isotype exhibits distinct tissue distributions and ligand specificities [64, 65]. In the CNS, PPAR β/δ is generally expressed, while PPARα only appears in astrocytes, and PPARγ is the dominant isoform in microglia [66]. In ischemic stroke, inflammation induces the release of cytokines such as TNF-α and IL-1β, and adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule (VCAM). These mediators drive the accumulation of macrophages and activated microglia in the ischemic tissue regions [67,68]. Infiltrating inflammatory cells express inducible nitric oxide synthases (iNOS) and produce generate large amounts of nitric oxide (NO), with the subsequent formation of peroxynitrite [1]. The activation of PPARγ can antagonize these harmful effects, suggesting a promising neuroprotective role for PPARγ agonists in ischemic stroke [69,70].
TREM
TREM was initially identified on account of its ability to bind pathogens such as bacteria and viruses, as well as for its role in initiating phagocytosis [71]. Recent studies show the TREM family consists of two members, TREM1 and TREM2. Both receptors appear on myeloid cells, including microglia. After crosslinking, TREM recruits engages its adapter protein, DNAX-activating protein of 12 (DAP12) and activates signaling, which differs depending on which TREM receptor is activated. In addition to binding anionic patterns on various microbials, TREM was also observed binding an as yet unknown ligand in the CNS [72–74].
TREM1 signaling is largely pro-inflammatory. The ligand is constitutively expressed on macrophages, neutrophils and microglia and is upregulated by various mediators such as the TLR ligands LPS and lipoteichoic acid (LTA) and the pro-inflammatory cytokine TNF [75,76]. Activation of TREM1 on monocytes and neutrophils boosts secretion of pro-inflammatory cytokines and chemokines, and mediates the upregulation of cell-surface molecules involved in extravasation, cell activation, and costimulation [77]. These phenomena have not been studied extensively. However TREM1 is gaining recognition as a potentially important molecule in brain injury responses.
Contrary to TREM1, TREM2 signaling is primarily anti-inflammatory, while also promoting phagocytosis. Activation of TREM-2 activates downstream signaling through pathways including phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ1, and p44-p42 extracellular signal regulated kinase (ERK), but not by classical inflammatory pathways such as NF-κB and the p38 stress-activated protein kinase [78,79]. In addition, TREM2 inhibits secretion of proinflammatory factors such as cytokines and ROS [72,73].
Considerable efforts to identify the endogenous TREM ligand have been unsuccessful in definitively determining what causes TREM activation in the brain. One recent study using a model of autoimmune encephalomyelitis demonstrated that HSP60 is one possible TREM ligand [80]. A mitochondrial stress protein, HSP60 can translocate to the cell surface under appropriate conditions [81]. Activation of microglia by HSP60 was shown to trigger phagocytosis in TREM2 expressing microglia, but not among microglia that were deficient in TREM2 [80]. In another study, microglia that appeared to bind TREM2 was activated to phagocytose injured cells without stimulating a typical inflammatory response or the release of reactive oxygen species. Conversely, loss of TREM2 blocked phagocytosis and triggered inflammation [82]. TREM2 deficiency in cultured microglia was shown to interrupt phagocytosis and expression of microglial proinflammatory responses such as TNFα, while overexpression of TREM2 increased phagocytosis and TNF-α [82]. Restriction of TREM2 using a monoclonal antibody in experimental autoimmune encephalomyelitis resulted in exacerbation of immune responses with increased demyelination and worsened neurological function [83]. These findings indicate that TREM2 may play an important role in regulating microglial phagocytosis and inflammatory responses in brain injury.
Activated microglia become highly motile, migrating to the site of injury and phagocytosing remnants from dead or dying neurons. Relevant to brain injury, TREM2 seems to mediate phagocytosis of apoptotic neurons [84]. While there is yet no definitive conclusion as to whether this behavior is beneficial or harmful during various stages of brain injury, the common consensus appears to be that microglial and macrophage phagocytosis are important effectors in facilitating recovery and remodeling by clearing necrotic debris as well as infiltrating neutrophils that otherwise exacerbate damage [85,86]. Searches showed two recent papers reporting the effects of TREM2 after ischemic stroke. One study using a model of therapeutic hypothermia in experimental stroke showed that TREM2 positive microglia were increased under conditions of protection, indicating that TREM2 may be correlated to improved outcomes after ischemia [87]. However, in a study of TREM2 deficient mice, the knockouts had similar lesion sizes compared to wild type animals, but also fewer activated microglia and overall reduction in inflammatory responses [88] - observations which seem to contradict those seen in a neuroinflammation model [83]. While the therapeutic potential of TREM2 and microglial phagocytosis yet requires further research, these early studies show that TREM2 could be an important target for treating inflammatory injuries at sub-acute time points.
Mechanisms of microglial cytotoxicity
Microglia, like macrophages, respond to invading pathogens by promoting rapid sequestration and inoculation of microorganisms, and by restricting the effects of cell damage and necrosis [89]. These acute responses include migration, proliferation, and the release of a variety of effector substances including superoxide, nitric oxide (NO), proteases, and cytokines. This is accompanied by phagocytosis of damaged cells. Some of these responses may exacerbate brain injury and thus provide potential therapeutic targets.
Superoxide
Superoxide is a reactive species which interacts with other molecules to form more highly reactive oxygen species, such as peroxynitrite, hypocholorus acid, carbonyl radical, and hydroxyl radical, all of which are directly cytotoxic to neurons and glia. In immune cells such as microglia, superoxide is generated by the partial reduction of molecular oxygen. The result is the production of O2●− that are formed by the 1 electron reduction of oxygen using NADPH as the electron donor: 2O2 + NADPH → 2O2- + NADP + H+ [90]. Superoxide and other reactive species are also pro-inflammatory signaling molecules that stimulate microglial activation in a feed-forward manner [91,92]. The production of superoxide in microglia occurs mainly by NADPH oxidase (NOX), of which a number of isoforms have been identified [93,94]. The isoform predominantly detected in immune cells such as microglia is NOX2, or professional NOX. NOX expression and activation has been strongly linked to ischemic stroke and related brain disorders, and its inactivation or deficiency has been reported to be protective [95]. While NOX2 exists in both microglia and circulating immune cells, one study using a bone marrow chimera model indicated that the toxic effects of NOX-produced superoxide was specifically attributable to NOX expressed in brain cells [96]. Work from our own group using a similar experiment showed ischemic brain injury worsened after increases in superoxides that were produced by NOX from both microglia and circulating immune cells [97,98]. The involvement of NOX from circulating immune cells appeared to cause more severe damage than with microglial NOX alone.
The introduction of microglia to endothelial cell and astrocyte cocultures is also known to exacerbate ischemia-like injury. However, interrupting the production of superoxide protected these elements of the BBB in in vitro models of ischemia [98]. In addition, Walder el al. [96] reported that mice genetically modified to be deficient in the gp91 subunit of NOX2 were protected after experimental ischemia. A few others have investigated the therapeutic potential of treatment with the pharmacological NOX antagonists apocynin [99–102] and honokiol [103,104]. Their findings characterize NOX as a promising target for treating stroke and brain injury, but it is possible that the efficacy of this therapeutic intervention may be attributed largely or in part to inhibition of NOX in cell types other than microglia [105].
NO and NOS
The free radical gas nitric oxide (NO) is another substance generated by activated microglia which has been implicated in a wide range of functions following brain injury, including neuronal synaptic activity, host defense, modulating vascular tone, and as an inhibitor of platelet aggregation and leukocyte adhesion [106]. Nitric oxide is produced from L-arginine through nitric oxide synthases (NOS) [107]. At this time, three NOS have been closely examined in brain injury models: endothelial NOS (eNOS, NOS-3), neuronal NOS (nNOS, NOS-1), and inducible NOS (iNOS, NOS-2). Of these isoforms, iNOS is thought to be the most relevant to inflammation. iNOS is expressed mainly by microglia and other macrophages but has also been observed in astrocytes [108–111]. Active microglia increase the inducibility of iNOS and the consequent production of NO. NO generated in microglia may also react with superoxide to produce peroxynitrite, an even more reactive oxygen species that can damage cellular DNA [112,113]. Microglial NO may also be neuroprotective against brain injury [114,115], but more often, it has proven to be cytotoxic, especially at high levels. In an oligodendrocyte cell culture model of ischemia, one study showed considerable cell death occurred after exposure to microglia-derived NO [116]. NO is also toxic to blood-brain barrier (BBB) constituents [117,118].
Studies investigating potential therapeutic applications have reported that pharmacological suppression of iNOS with aminoguanidine in mice decreases infarct volume after experimental stroke, and iNOS deficient mice also showed better outcomes after injury [110,119]. Moreover, therapeutic hypothermia and neuroprotection by estrogen and progesterone have been linked to reduced iNOS production, suggesting that NO/iNOS play a detrimental role in brain injury [120–122].
MMPs
The matrix metalloproteinases (MMPs) are a family of at least 28 zinc-dependent endopeptidases that, when active, degrade the extracellular matrix. MMPs can cause disruption of the BBB, leading to further infiltration of circulating immune cells, serum proteins and hemorrhage [123]. They are an important part of the inflammatory responses to brain injury. Resting MMPs are typically found in the cytosol in uninjured conditions, but in pathologic states, they transport extracellularly. Once situated outside the cell, they are cleaved to an active form and can degrade substrates of the extracellular matrix [1]. MMP-2, −3 and −9 have been extensively characterized in stroke and, to a lesser extent, in traumatic brain injury.
Microglia are the major main source of MMP release following various forms of brain injury, particularly MMP-3 and MMP-9 [124,125]. Fibronectin and vitronectin, substances commonly located in the plasma, can stimulate microglial cells to produce pro-MM-9 [126]. Neutrophils can also produce and secrete MMP-9 [127] and studies experiments of bone marrow chimeras have suggested that MMP-9 derived from circulating immune cells contribute to worsened ischemic injury. The damage contributed by MMPs secreted by leukocytes may even be more significant than those from microglia [128].
Cytokines and chemokines
Cytokines and chemokines are two classes of proteins that function as signaling molecules for inter- and extracellular communication during inflammation. Inactive microglia release many different types of cytokines and chemokines at low levels. The pattern of this production changes drastically in the injured brain [129]. During early stages of injury, these factors act primarily as intercellular signaling molecules, and many have feed forward effects in driving the inflammatory response. TNF-α, IFN-γ, IL-1, and IL-6 are some of the best-studied cytokines. They have been shown to simulate microglia [130] and to have direct cytotoxic effects [131]. They are also capable of affecting BBB integrity [132]. These molecules have been observed as early as 1 day after experimental stroke ischemic stroke [133,134].
Though thought to be deleterious in the acute phases of injury, cytokines may have more beneficial effects at a later stage. IL-10 signaling ultimately inhibits proinflammatory cytokines such as IL-1 and TNF-α, as well as other cytokine receptor expression and downstream signals that are upregulated in pathologic states [135]. Another study showed that TGF-β1 overexpression by microglia improved neurobehavioral recovery after experimental ischemia, and these outcomes were correlated with a reduction in the inflammatory response, also attributed to TGF-β1 overexpression [136,137]. There have also been instances of in vitro work that have observed microglia protecting cultured neurons from ischemia-like injuries by releasing TGF-β1 [138]. In addition, microglia also generate a number of neurotrophic factors, such as TGFβ1, BDNF, and GDNF, and these cytokines are considered important in protecting neuronal integrity after brain injury [139–142].
Microglia are also activated by and are able to produce chemokines that perform a broad range of functions in the injured brain [143–146]. The expression of monocyte chemoattractant protein-1 (MCP-1, CCL2) is triggered by the proinflammatory stimuli, such as IFN-γ, TNF-α, and IL-1β [147]. Chemokines can also lead to the disruption of the BBB and are involved in the recruitment of mononuclear leukocytes into the CNS [147–149].
Mechanisms of microglial protection
Microglia play key roles in the tissue repair, remodeling, growth, and homeostasis by releasing anti-inflammatory cytokines and growth factors and by clearing cell debris [150]. Less has been studied in this area, as it pertains to affecting outcome in stroke and brain trauma. However, recent studies demonstrate that microglia may be beneficial after stroke [151,153]. Transgenic mice in which proliferating microglia were ablated, suffered increased lesion size and higher numbers of apoptotic cells after experimental ischemic stroke [152]. Moreover, Mac-2+ resident microglia release neurotrophic molecules such as insulin-like growth factor (IGF-1), which leads to neuroprotection [152]. In addition, organotypic hippocampal slice cultures exposed to ischemia-like injury led to decreased neuronal death when co-cultured with microglial BV2 cells [150]. Other studies suggest that microglia may be beneficial because they engulf neutrophils [153] and release TNF-α [154] after brain injury. Microglia may also be beneficial through their phagocytic functions. Recent reports demonstrate that dead cells release nucleic acid remnants into the extracellular space where they associate with appropriate receptors on phagocytes. Some of these phagocytosis initiating signals have been referred to as danger associated molecular patterns (DAMP) [155]. Those identified in stroke and related injury models include purines such as UTP, ADP and ATP, and signal through purinergic receptor systems to lead to chemotaxis and phagocytosis [156,157]. However, phagocytosis through these signaling systems, while leading to the clearance of injured cells, may also worsen cell death either by causing microglia to phagocytose viable cells or generate more neurotoxic substances [158]. Preliminary work in our lab suggests that TREM2 may also bind purines, and TREM2 signaling in microglia, which leads to phagocytosis [159], tends to be anti-inflammatory [160]. Deficiency of TREM2 led to worsened recovery following stroke [161]. Finally, no published works are yet available in stroke or brain trauma models regarding the microglial M1/M2 switch, and whether its modulation improves neurological outcome. These beneficial effects of microglia should be explored, as they may provide new therapeutic avenues in brain injury.
Microglia in Ischemia and Traumatic Brain Injury
Activated microglia are key players in neuroinflammation, which is present in many types of neurologic disorders. Research in microglia research point to many promising therapeutic targets for a broad range of neuroinflammatory diseases, including acute injuries such as ischemic stroke and traumatic brain injury [162]. A comparative review of microglial activation in stroke and brain trauma shows considerable overlap. In both injury models, neuroinflammation develops over a time period of hours to days after onset, presenting an ideal time window for therapeutic intervention during which microglia play a central and nuanced role in responding to injury, sustaining inflammatory signaling and gene transcription, and mediating cytotoxic mechanisms [163,164]. Identifying parallels and distinctions between stroke and TBI may also provide useful clues for future research in microglial response to acute injury, as well as help isolate the most robust microglial targets for therapeutic intervention.
Ischemic Stroke
Stroke is the second leading cause of death worldwide and the fourth leading cause of death in the US. While improvements in acute stroke care have considerably reduced stroke death rates, post-ischemic morbidity and disability are yet a major and costly public health concern [165]. During ischemic stroke, major blood vessels in the brain are occluded, causing oxygen and glucose deprivation in brain tissues. This primary insult initiates subsequent secondary brain damage involving necrotic and apoptotic neuron death as well as the release of cytotoxic and proinflammatory substances such as glutamate, superoxide, nitric oxide, chemokines and cytokines [163]. Secondary injury mechanisms are largely responsible for the high rates of post-stroke morbidity and disability. Therefore, it is important to identify targets for treatments that can lessen the impact of secondary brain damage. Microglial activation occurs in the early stages of neuroinflammation [98,166]; activated microglia can be detected in lesions as early as 2 hours post-ischemia and can be detected up to 1 week after injury [85]. Many studies have shown that the direct application of activated microglia has been shown to effect cell death in neurons [26,166,167]. The cytotoxic effects begin shortly after insult and can continue to exacerbate injury for a few days afterwards. It is thought that the later effects of activated microglia may be important for tissue repair and wound healing [168,169]. Thus, timing is an important element to consider when manipulating microglial activation in stroke. Studies in therapeutic microglial targets will also need to find ways to suppress cytotoxic mechanisms without disrupting beneficial effects.
Traumatic Brain Injury
Brain trauma is another significant cause of morbidity and mortality worldwide, hospitalizing 1.7 million Americans each year [170]. As in stroke, microglial activation and neuroinflammation have been implicated as important mediators of secondary damage by releasing proinflammatory cytokines, cytotoxic superoxide, NO, and proteases such as MMP-9 [163,171]. Traumatic brain injury begins with a primary physical insult causing brain deformation [172]. Within seconds to minutes, acute injury can trigger a variety of secondary injury mechanisms, including ischemia, hemorrhage, CNS hypoxia, free radical formation, excitotoxicity, blood-brain-barrier disruption, necrotic and apoptotic cell death, and inflammation [164]. Microglia activation plays a central role in the inflammatory branch of secondary injury by promoting secretion of proinflammatory cytokines and recruiting circulating immune cells, thereby exacerbating injury [173,174]. One mechanism observed in brain trauma but not in stroke, is that activated microglia rapidly migrate to the site of injury, where they fuse to form a barrier between damaged and healthy tissues by about half an hour after injury. This phenomenon appears to be mediated by ATP and P2Y–receptor-dependent mechanisms [175]. In humans, active microglia appear 72 hours after injury, at which time they have been shown to localize at the site of injury [176,177]. These secondary mechanisms can continue long after the time of injury. Rat studies have shown that elevated levels of activated microglia persist long after initial trauma [178,179], and have been observed in human brains for up to 16 years after injury [176].
Prolonged microglial activation may be among the most neurotoxic consequences of neuroinflammation whether in stroke or TBI [180]. The presence of chronically activated microglia is associated with sustained increase in the expression of pro-inflammatory cytokines such as IL-1β and TNF-α [181]. Furthermore, a number of clinical studies have shown that brain trauma may increase the risk for dementia and Alzheimer’s [182–184]. Based on these findings, experts have hypothesized that microglial activation may link TBI to these neurodegenerative conditions which tend to emerge later in life [171]. Identifying microglial targets for treating acute injury may additionally provide greater insight into preventing or mitigating chronic neurodegenerative diseases.
Microglial Targets for Treatment
To date, much work has been done to identify microglial targets for therapy. The research has produced a considerable body of literature describing a broad repertory of potential pharmacological inducers and suppressors. Most of these approaches protect against the deleterious effects of neuroinflammation, whether by upregulating repressors of microglial activation or by blocking microglia-mediated proinflammatory signals or substances. Fewer studies focus on upregulating microglial repair and remodeling. While many of these drugs have produced promising preclinical results, there has been little headway in pharmacological treatments for acute injury at the clinical level [185,186]. The difficulty in identifying effective therapy is understandable given the complex, extensive web of immune responses in the injured brain. Successful intervention will most likely require a compound or combination of compounds that can modulate multiple targets. Tailoring such a drug or cocktail demands a detailed understanding of the secondary injury processes [187]. A central initiator and mediator of these processes, microglia - its activators, functions, and secretions - can serve as a useful focal point for organizing the current repertoire of known anti-inflammatory drugs, highlighting key targets for future investigation.
Pharmacological Inhibition of Microglial Activation
One of the best-studied inhibitors of microglial activation, minocycline is a second-generation tetracycline known for its ancillary function as an effective suppressor of brain inflammation. The earliest studies in minocycline neuroprotection reported the drug’s ability to inhibit microglial activation in stroke, and were studied as a promising anti-inflammatory drug in many other neurodegenerative diseases, including brain trauma [186–194]. Minocycline inhibits microglia-mediated neuron death as well as several pro-inflammatory cytokines secreted by injury-activated microglia, thereby suppressing both downstream cytotoxic mechanisms in addition to disrupting the inflammatory feed-forward loop [195,196]. Minocycline is additionally known to block MMP-9, released by activated microglia, and reduces blood-brain-barrier disruption in stroke and TBI [123,124,197,198]. Studies have shown that minocycline treatment can reduce lesion sizes and improves neurobehavioral outcomes after injury [199].
Apocynin has anti-oxidant and anti-inflammatory capabilities, specifically to block the activity of NOX. NOX exists on immune cells (microglia and leukocytes), and apocynin inhibits the release of superoxide through NOX by blocking migration of p47phox to the membrane, thus interfering with assembly of the functional NOX complex [200]. Immune cell generated NOX also appears important in the maintenance of vascular integrity. The addition of microglia to endothelial cell and astrocyte cocultures worsens ischemia-like injury, and inhibiting superoxide production with apocynin preserved these BBB constituents in vitro [98]. Thus, NOX contributes to BBB disruption downstream events in ischemic stroke. In fact, apocynin attenuated brain edema formation MMP-9 expression [201], BBB disruption and hemorrhagic transformation [100] as well as inhibiting immune cell responses [99].
TLR4
Upon exposure to signals released at the site of injury, the transmembrane receptor TLR4 activates microglia and triggers the upregulation of pro-inflammatory genes [163]. Thus TLR4 may be a critical first step in microglial activation and injury response. Considerable work has been done to more closely understand TLR4 mechanisms as well as its potential as a therapeutic target. In stroke, several groups have shown that TLR4 may be necessary for microglial activation, and animal studies describe better functional outcomes for TLR4-deficient mice following experimental stroke [26–29]. Work in trauma continues to compile a range of different neuroprotective TLR4 inhibitors that confer neuroprotection after TBI including resatorvid, curcumin, and ethyl pyruvate [202–204]. Studies have also associated TLR2 and TLR4 with increased inflammation and poorer outcomes among patients admitted for ischemic stroke, and support a clinical need for developing a therapy targeting TLRs [203,205].
TNF-α
Tumor necrosis factor (TNF) is an important immune signaling molecule released by activated microglia in acute injury and an especially promising therapeutic target for stroke and TBI [206]. Current findings in TNF studies using pharmacological inhibitors such as etanercept suggest that the therapeutic time window for treatments targeting components of microglia-mediated inflammation may extend long after injury [206]. Groups have demonstrated the efficacy of etanercept fused with a decoy BBB-receptor in mouse models of stroke, and still others have described the anti-inflammatory and neuroprotective properties of TNF-α antagonist such as thalidomides and thalidomide derivatives [207–209].
PPARγ
PPAR controlled transcription pathways have shown to be anti-inflammatory in the brain in both stroke and TBI models. Upon dimerizing with an activating receptor, the PPARγ transcription factor translocates to the nucleus where it activates transcription [210]. Though PPARγ expression increases in microglia after stroke and trauma, a reduction in available ligand leads to fewer instances of DNA binding such that PPAR transcription is suppressed [211,212]. PPARγ suppression can be reversed by introducing endogenous ligand or by treatment with agonists such as rosiglitazone and pioglitazone [213,214]. Treatment with PPARγ ligand after stroke decreases microglia and macrophage activation, and curbs the expression of inflammatory molecules such as ICAM-1, MMP-9, IL-1β, COX-2, TNFα, iNOS, and reactive oxygen species [215–2117]. PPAR γ agonists have also been shown to reduce inflammation and confer neuroprotection in animal models of traumatic injury [213,218,219].
Conclusion
The role of inflammation in brain injury has become an increasingly popular area of investigation, given its pleotropic roles in both acute damage and long term recovery. Microglia are key players in neuroinflammation and are considered the major immunocompetent cells of the brain. For this reason, much effort has been directed towards an understanding of how microglia become activated and how they mediate neuroinflammation. These efforts have led to many studies which have shown the beneficial and detrimental effects of microglial functions in brain ischemia and trauma. Modulation of microglial behaviors is clearly an important topic of investigation, and represents a huge potential for the development of new therapeutic strategies in acute brain injury.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH) (NS40516 to MY), the Veteran’s Merit Awards to MY, and American Hearts Association (13POST14810019) to JYK. Grants to MY and JYK were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
References
- 1.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1998;19:S81–S84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 3.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 4.Thomas WE. Brain macrophages. evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992;17:61–74. doi: 10.1016/0165-0173(92)90007-9. [DOI] [PubMed] [Google Scholar]
- 5.Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 6.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 7.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 8.Appel SH, Zhao W, Beers DR, Henkel JS. The microglial-motoneuron dialogue in ALS. Acta Myol. 2011;30:4–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol. 2006;84:49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- 10.Wood PL. Microglia as a unique cellular target in the treatment of stroke: potential neurotoxic mediators produced by activated microglia. Neurol Res. 1995;17:242–248. doi: 10.1080/01616412.1995.11740321. [DOI] [PubMed] [Google Scholar]
- 11.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 19.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Salemi J, Obregon DF, Cobb A, Reed S, Sadic E, Jin J, Fernandez F, Tan J, Giunta B. Flipping the switches: CD40 and CD45 modulation of microglial activation states in HIV associated dementia (HAD) Mol Neurodegener. 2011;6:3. doi: 10.1186/1750-1326-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 25.Trendelenburg G. Acute neurodegeneration and the inflammasome: central processor for danger signals and the inflammatory response? J Cereb Blood Flow Metab. 2008;28:867–881. doi: 10.1038/sj.jcbfm.9600609. [DOI] [PubMed] [Google Scholar]
- 26.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, Li C. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 31.Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 32.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem. 2009;109:287–294. doi: 10.1111/j.1471-4159.2009.05972.x. [DOI] [PubMed] [Google Scholar]
- 38.Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperlagh B, Illes P. Purinergic modulation of microglial cell activation. Purinergic Signal. 2007;3:117–127. doi: 10.1007/s11302-006-9043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2×7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morigiwa K, Quan M, Murakami M, Yamashita M, Fukuda Y. P2 Purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci Lett. 2000;282:153–156. doi: 10.1016/s0304-3940(00)00887-9. [DOI] [PubMed] [Google Scholar]
- 43.Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 44.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2×7 receptor drives microglial activation and proliferation: a trophic role for P2×7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di Virgilio F, Abbracchio MP, Verderio C. A role for P2×7 in microglial proliferation. J Neurochem. 2006;99:745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- 46.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2×7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 47.Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, Kohsaka S. ATP stimulation of Ca2+ -dependent plasminogen release from cultured microglia. Br J Pharmacol. 1998;123:1304–1310. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrari D, Stroh C, Schulze-Osthoff K. P2×7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- 49.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang KM, Yang CS, Sun SH, Tzeng SF. Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J Neurochem. 2009;111:1225–1237. doi: 10.1111/j.1471-4159.2009.06409.x. [DOI] [PubMed] [Google Scholar]
- 51.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2×7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, Pedata F, Sancesario G. P2×7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26:974–982. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- 53.Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2×7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull. 2008;31:1121–1130. doi: 10.1248/bpb.31.1121. [DOI] [PubMed] [Google Scholar]
- 54.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2×4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 56.Irino Y, Nakamura Y, Inoue K, Kohsaka S, Ohsawa K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J Neurosci Res. 2008;86:1511–1519. doi: 10.1002/jnr.21610. [DOI] [PubMed] [Google Scholar]
- 57.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster CM, Hokari M, McManus A, Tang XN, Ma H, Kacimi R, Yenari MA. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS One. 2013;8:e70927. doi: 10.1371/journal.pone.0070927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 61.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 62.Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1beta in microglial cells. Crit Rev Immunol. 2009;29:335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- 63.Brough D, Le Feuvre RA, Iwakura Y, Rothwell NJ. Purinergic (P2×7) receptor activation of microglia induces cell death via an interleukin-1-independent mechanism. Mol Cell Neurosci. 2002;19:272–280. doi: 10.1006/mcne.2001.1054. [DOI] [PubMed] [Google Scholar]
- 64.Kielian T, Drew PD. Effects of peroxisome proliferator-activated receptor-gamma agonists on central nervous system inflammation. J Neurosci Res. 2003;71:315–325. doi: 10.1002/jnr.10501. [DOI] [PubMed] [Google Scholar]
- 65.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 66.Basu-Modak S, Braissant O, Escher P, Desvergne B, Honegger P, Wahli W. Peroxisome proliferator-activated receptor beta regulates acyl-CoA synthetase 2 in reaggregated rat brain cell cultures. J Biol Chem. 1999;274:35881–35888. doi: 10.1074/jbc.274.50.35881. [DOI] [PubMed] [Google Scholar]
- 67.Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- 68.Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol. 1998;18:503–513. doi: 10.1161/01.atv.18.4.503. [DOI] [PubMed] [Google Scholar]
- 69.Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34:1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 70.Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Liou JY, Shyue SK, Wu KK. 15d–prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- 71.N’Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA, Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J Cell Biol. 2009;184:215–223. doi: 10.1083/jcb.200808080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol. 2010;225:24–28. doi: 10.1016/j.expneurol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 73.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 74.Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 75.Schenk M, Bouchon A, Birrer S, Colonna M, Mueller C. Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine. J Immunol. 2005;174:517–524. doi: 10.4049/jimmunol.174.1.517. [DOI] [PubMed] [Google Scholar]
- 76.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 77.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 78.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 79.Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001;194:1111–1122. doi: 10.1084/jem.194.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, Meldolesi J. The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem. 2009;110:284–294. doi: 10.1111/j.1471-4159.2009.06130.x. [DOI] [PubMed] [Google Scholar]
- 81.Soltys BJ, Gupta RS. Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective. Int Rev Cytol. 2000;194:133–196. doi: 10.1016/s0074-7696(08)62396-7. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–1301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- 84.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis. 2014 doi: 10.1007/s11011-014-9531-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49:1422–1434. doi: 10.1007/s12035-013-8620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawabori M, Hokari M, Zheng Z, Kim JY, Calosing C, Hsieh CL, Nakamura MC, Yenari MA. Triggering Receptor Expressed on Myeloid Cells-2 Correlates to Hypothermic Neuroprotection in Ischemic Stroke. Ther Hypothermia Temp Manag. 2013;3:189–198. doi: 10.1089/ther.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sieber MW, Jaenisch N, Brehm M, Guenther M, Linnartz-Gerlach B, Neumann H, Witte OW, Frahm C. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLoS One. 2013;8:e52982. doi: 10.1371/journal.pone.0052982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 90.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 91.Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mander PK, Jekabsone A, Brown GC. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol. 2006;176:1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- 93.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 95.Tang XN, Cairns B, Kim JY, Yenari MA. NADPH oxidase in stroke and cerebrovascular disease. Neurol Res. 2012;34:338–345. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 97.Tang XN, Zheng Z, Giffard RG, Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol. 2011;70:606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 99.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 103.Chen CM, Liu SH, Lin-Shiau SY. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic Clin Pharmacol Toxicol. 2007;101:108–116. doi: 10.1111/j.1742-7843.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- 104.Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–166. doi: 10.1016/j.brainres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 105.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 107.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 108.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29:1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 110.Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. Am J Physiol. 1995;268:R286–R292. doi: 10.1152/ajpregu.1995.268.1.R286. [DOI] [PubMed] [Google Scholar]
- 111.Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- 112.Cui J, Holmes EH, Liu PK. Oxidative damage to the c-fos gene and reduction of its transcription after focal cerebral ischemia. J Neurochem. 1999;73:1164–1174. doi: 10.1046/j.1471-4159.1999.0731164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, Kim LJ, Protopsaltis TS, Zhang Y, Pinsky DJ, Connolly ES., Jr. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31:3047–3053. [PubMed] [Google Scholar]
- 114.Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- 115.Colton CA. Induction of nitric oxide in cultured microglia: evidence for a cytoprotective role. Adv Neuroimmunol. 1995;5:491–503. doi: 10.1016/0960-5428(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 116.Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 117.Kacimi R, Giffard RG, Yenari MA. Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J Inflamm. 2011;8:7. doi: 10.1186/1476-9255-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thiel VE, Audus KL. Nitric oxide and blood-brain barrier integrity. Antioxid Redox Signal. 2001;3:273–278. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- 119.Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872:215–218. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]
- 120.Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- 121.Coughlan T, Gibson C, Murphy S. Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem. 2005;93:932–942. doi: 10.1111/j.1471-4159.2005.03068.x. [DOI] [PubMed] [Google Scholar]
- 122.Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. 2002;22:3921–3928. doi: 10.1523/JNEUROSCI.22-10-03921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- 126.del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, Wang X, Hosomi N, Mabuchi T, Koziol JA. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32:919–932. doi: 10.1038/jcbfm.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez MA, Monasterio J. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32:1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 128.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 129.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. ScientificWorldJournal. 2008;8:1119–1147. doi: 10.1100/tsw.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 132.Vexler ZS, Tang XN, Yenari MA. Inflammation in adult and neonatal stroke. Clin Neurosci Res. 2006;6:293–313. doi: 10.1016/j.cnr.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 134.Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 135.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 136.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 137.Flanders KC, Ren RF, Lippa CF. Transforming growth factor-betas in neurodegenerative disease. Prog Neurobiol. 1998;54:71–85. doi: 10.1016/s0301-0082(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 138.Lu YZ, Lin CH, Cheng FC, Hsueh CM. Molecular mechanisms responsible for microglia-derived protection of Sprague-Dawley rat brain cells during in vitro ischemia. Neurosci Lett. 2005;373:159–164. doi: 10.1016/j.neulet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 139.Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y. Expression disparity of brain-derived neurotrophic factor immunoreactivity and mRNA in ischemic hippocampal neurons. Neuroreport. 2002;13:2271–2275. doi: 10.1097/00001756-200212030-00020. [DOI] [PubMed] [Google Scholar]
- 140.Suzuki S, Tanaka K, Nogawa S, Nagata E, Ito D, Dembo T, Fukuuchi Y. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1256–1262. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 141.Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24:437–448. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 143.Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y. Macrophage inflammatory protein-3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neurosci Res. 2009;64:75–82. doi: 10.1016/j.neures.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 144.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 146.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 147.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 149.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Miroglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- 152.Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferation microglial cell exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–25605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Neumann J, Sauerzweig S, Rönicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, Leak RK, Gao Y, Sun BL, Zheng P, Chen J. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglia phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 158.Emmrich JV, Hornik TC, Neher JJ, Brown GC. Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 2013;280:5030–5038. doi: 10.1111/febs.12401. [DOI] [PubMed] [Google Scholar]
- 159.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–1301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- 161.Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh C, Nakamura M, Yenari M. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. Stroke. 2014;45:A9. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81:1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- 163.Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 166.Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36:681–693. doi: 10.1002/jnr.490360609. [DOI] [PubMed] [Google Scholar]
- 167.Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol. 2010;43:325–331. doi: 10.5115/acb.2010.43.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 169.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 170.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- 171.Smith C. Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol Appl Neurobiol. 2013;39:35–44. doi: 10.1111/nan.12006. [DOI] [PubMed] [Google Scholar]
- 172.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 173.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 175.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 176.Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, Nicoll JA. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 177.Engel S, Schluesener H, Mittelbronn M, Seid K, Adjodah D, Wehner HD, Meyermann R. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol. 2000;100:313–322. doi: 10.1007/s004019900172. [DOI] [PubMed] [Google Scholar]
- 178.Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- 179.Smith DH, Chen XH, Pierce JE, Wolf JA, Trojanowski JQ, Graham DI, McIntosh TK. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–727. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- 180.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 181.Holmin S, Mathiesen T. Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport. 1999;10:1889–1891. doi: 10.1097/00001756-199906230-00017. [DOI] [PubMed] [Google Scholar]
- 182.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 183.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 184.Mayeux R, Ottman R, Tang MX, Noboa-Bauza L, Marder K, Gurland B, Stern Y. Genetic susceptibility and head injury as risk factors for Alzheimer’s disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993;33:494–501. doi: 10.1002/ana.410330513. [DOI] [PubMed] [Google Scholar]
- 185.Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke. 2013;44:2942–2950. doi: 10.1161/STROKEAHA.113.000731. [DOI] [PubMed] [Google Scholar]
- 186.Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 7:115–126. doi: 10.1016/j.nurt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2010;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 188.Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32:2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- 189.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 191.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- 193.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 194.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- 196.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, Opdenakker G, Koistinaho J. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25:460–467. doi: 10.1038/sj.jcbfm.9600040. [DOI] [PubMed] [Google Scholar]
- 198.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1401. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 200.Touyz RM. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 2008;51:172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- 201.Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, Miyake M, Liu KJ. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014;11:59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang D, Li H, Li T, Zhou M, Hao S, Yan H, Yu Z, Li W, Li K, Hang C. TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: Implication in the treatment of human brain injury. Neurochem Int. 2014;75:11–18. doi: 10.1016/j.neuint.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 204.Su X, Wang H, Zhao J, Pan H, Mao L. Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-kappaB pathway after traumatic brain injury in the rat. Mediators Inflamm. 2011:807142. doi: 10.1155/2011/807142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brea D, Blanco M, Ramos-Cabrer P, Moldes O, Arias S, Perez-Mato M, Leira R, Sobrino T, Castillo J. Toll-like receptors 2 and 4 in ischemic stroke: outcome and therapeutic values. J Cereb Blood Flow Metab. 2011;31:1424–1431. doi: 10.1038/jcbfm.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–343. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 207.Yoon JS, Lee JH, Tweedie D, Mughal MR, Chigurupati S, Greig NH, Mattson MP. 3,6′-dithiothalidomide improves experimental stroke outcome by suppressing neuroinflammation. J Neurosci Res. 2013;91:671–680. doi: 10.1002/jnr.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sumbria RK, Boado RJ, Pardridge WM. Brain protection from stroke with intravenous TNFalpha decoy receptor-Trojan horse fusion protein. J Cereb Blood Flow Metab. 2012;32:1933–1938. doi: 10.1038/jcbfm.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 211.Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- 212.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 213.Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- 214.Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- 215.Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 216.Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- 217.Pereira MP, Hurtado O, Cardenas A, Alonso-Escolano D, Bosca L, Vivancos J, Nombela F, Leza JC, Lorenzo P, Lizasoain I, Moro MA. The nonthiazolidinedione PPARgamma agonist L-796,449 is neuroprotective in experimental stroke. J Neuropathol Exp Neurol. 2005;64:797–805. doi: 10.1097/01.jnen.0000178852.83680.3c. [DOI] [PubMed] [Google Scholar]
- 218.Yonutas HM, Sullivan PG. Targeting PPAR isoforms following CNS injury. Curr Drug Targets. 2013;14:733–742. doi: 10.2174/1389450111314070003. [DOI] [PubMed] [Google Scholar]
- 219.Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]