Abstract
Background
Normal visual development occurs when the brain is able to integrate the visual input from each of the two eyes to form a single three-dimensional image. The process of development of complete three-dimensional vision begins at birth and is almost complete by 24 months of age. The development of this binocular vision is hindered by any abnormality that prevents the brain from receiving a clear, similar image from each eye, due to decreased vision (e.g. amblyopia), or due to misalignment of the two eyes (strabismus or squint) in infancy and early childhood. Currently, practice patterns for management of a child with both strabismus and amblyopia are not standardized.
Objectives
To study the functional and anatomic (ocular alignment) outcomes of strabismus surgery before completion of amblyopia therapy as compared with surgery after completion of amblyopia therapy in children under seven years of age.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 6), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2014), EMBASE (January 1980 to July 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to July 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 24 July 2014. A manual search for articles from a review of the references of the selected publications and conference abstracts was completed to identify any additional relevant studies.
Selection criteria
We searched for randomized controlled trials (RCTs) that provided data on strabismus surgery in children less than seven years of age, performed after initiation of, but before completion of amblyopia therapy, as compared with strabismus surgery after completion of amblyopia therapy.
Data collection and analysis
Two authors independently assessed studies identified from the electronic and manual searches.
Main results
There were no RCTs that fit our inclusion criteria and so no analysis was possible.
Authors’ conclusions
As there are no RCTs currently available and the best existing evidence is only from non-randomized studies, there is a need for prospective RCTs to investigate strabismus surgery in the presence of strabismic amblyopia. The optimal timing of when to perform strabismus surgery in children with amblyopia is unknown.
PLAIN LANGUAGE SUMMARY
Is it better to do strabismus (squint) surgery before or after completing amblyopia (lazy eye) treatment?
Background
In normal development of vision, children are able to use the visual information from each of their two eyes to form a single image. When an infant or child’s eyes are not properly aligned, this is called strabismus (also known as squint). With misalignment, the two eyes do not work together to relay visual information. The brain may suppress the image that comes from the squinting (weaker) eye in order to perceive a single image. When this happens, the weaker eye may develop into a lazy eye. This is a condition known as amblyopia. Lazy eye can be treated by covering the normal eye (called occlusion therapy) for varying periods of time. This is so that the child is forced to use and strengthen the weaker eye. Treatment continues until the weaker eye reaches normal vision or no change in vision is seen over several follow-up visits. In the past, we have believed that good, long lasting results could be achieved by surgically aligning the eyes, but only if both eyes had equally good vision before the surgery. So, in children with both squint and lazy eye, this meant delaying squint surgery until the lazy eye had been corrected. However, we also know that for a child to develop three-dimensional vision with a normal ability to recognize depth (depth perception), the eyes have to be aligned from a very early age. After the age of two to three years, proper three-dimensional vision fails to develop completely. Although this is not necessarily a major handicap, it can be important because some professions require perfect depth perception.
Review question
So, there is a question over the timing of squint surgery - Is it better to do surgery before completion of lazy eye treatment or after completion of lazy eye treatment? In the present review, we attempted to find evidence from studies that have compared these two approaches to try to resolve this question.
Key results
After conducting a broad search for studies, as of 24 July 2014, we found no randomized clinical studies that had addressed this question.
Treatment of lazy eye cannot be ignored, while at the same time each child needs to be given the best possible chance for seeing three-dimensional images. Each child with squint and lazy eye must be treated with both of these goals in mind. Further research is needed to determine the ideal timing for squint surgery in children with squint and lazy eye.
BACKGROUND
Description of the condition
Normal visual development occurs when the brain is able to integrate the visual input from each of the two eyes to form a single image. This state is called binocular vision and usually is present by three to six months of age (Thorn 1994; Wong 2008). There are several levels of binocularity, of which stereopsis is the highest and refers to the ability to perceive a three-dimensional image from information received by the brain from both eyes.
The development of stereoscopic vision starts at birth, the moment both eyes start receiving visual input, and occurs at the level of the visual cortex. The development of stereoscopic vision depends on:
the precise motor alignment of the two eyes such that almost identical visual stimulation occurs on corresponding points on the retina of each eye;
the presence of an anatomically normal visual pathway (the pathway taken by the visual signal that starts at the retina of each eye and culminates at the visual cortex).
If either one of the above conditions is not present, normal binocular vision will fail to develop (although subnormal stereopsis is still possible) unless corrective action is taken (Von Noorden 1976; Von Noorden 1988).
When the visual axes of the two eyes fail to meet at the point of fixation, ocular misalignment, known as strabismus or ’squint’, is present. This condition may be due to an extraocular muscle imbalance (motor) defect or due to a sensory (abnormal visual stimulation) defect.
If the misalignment is not corrected and both eyes are used alternately and to an equal extent, visual acuity (VA) usually develops equally in both eyes (Sireteanu 1982). In this situation, however, the development of stereopsis at the cortical level cannot occur if the misalignment was present before the age of about two years (Banks 1975; Von Noorden 1976). However, when one eye is used preferentially and the other eye is suppressed, vision in the suppressed eye becomes reduced as amblyopia (also known as lazy eye) develops.
Amblyopia is a unilateral or bilateral decrease in VA caused by either or both of the following:
formed vision deprivation (unclear images falling on the retina of one or both eyes due to conditions such as uncorrected refractive errors or congenital cataract);
abnormal binocular interaction (inability of the two eyes to work together due to misalignment with preferential fixation of one eye, or grossly unequal refractive errors).
No organic cause in the visual pathway is found on physical examination in these children (Von Noorden 1998).
Amblyopia is reversible in certain situations by interventions such as correction of refractive errors with spectacles, or occlusion therapy, or both (Von Noorden 2002). The critical period for amblyopia reversal currently is thought to be from birth to about 10 years of age, beyond which anatomical changes in the visual cortex may be less likely to occur (Campos 1995; Epelbaum 1993). Although less responsive to treatment compared with children under seven years of age, a meta-analysis of randomized amblyopia treatment trials found that older children, aged seven to less than 13 years, still showed mean improvement in visual acuity (Holmes 2011). Recent studies also have shown that adults can respond to binocular therapy for amblyopia (Hess 2013; Hess 2014; Mansouri 2014). Amblyopia is classified into five types (Taylor 2014):
strabismic amblyopia (reduction in vision due to ocular misalignment);
stimulus-deprivation amblyopia (obstruction in the visual axis causing a decrease in vision);
anisometropic amblyopia (reduced visual acuity due to difference between refractive error of the two eyes);
ametropic amblyopia (reduced vision resulting from uncorrected refractive error);
meridional amblyopia (reduced vision due to uncorrected astigmatism).
In this review we considered only strabismic amblyopia; interventions for other types of amblyopia have been evaluated in a series of Cochrane reviews (Antonio-Santos 2014; Li 2009; Shotton 2012).
Description of the intervention
Treatment for amblyopia involves methods of exercising the amblyopic (weaker) eye by encouraging its increased use. This training is achieved by temporarily reducing the vision in the normal (more preferred) eye. Several methods exist to reduce the vision of the stronger eye temporarily, including penalization with cycloplegic or dilating eye drops, optical blurring of vision with high plus lenses, or physical occlusion of the normal eye. Of these methods, occlusion therapy (e.g. patching) is considered standard treatment of amblyopia (AAO PPP Amblyopia 2012). Occlusion therapy may be part-time or full-time depending on the duration that the normal eye is kept occluded (AAO PPP Amblyopia 2012). Binocular training approaches for amblyopia treatment are also available, such as viewing perceptual learning programs with handheld devices (Hess 2011). Binocular training aims to treat amblyopia by restoring the underlying issue of reduced or absent binocularity.
Surgical correction of ocular misalignment involves operating on the appropriate extraocular muscles of one or both eyes, to either strengthen or weaken them as required, so that the eyes are mechanically pulled into alignment. This mechanical effect is thought to be greatly helped and stabilized by the presence of good and equal vision in both eyes, thus stimulating fusion (locking the eyes together in good alignment) in the newly aligned position.
How the intervention might work
Classic teaching dictates that amblyopia must be corrected to the maximum extent possible before realignment surgery is under-taken (Von Noorden 2002). This belief seems to make good physiological sense since proper fixation of each eye, possible only when vision is almost equal in both eyes, would be expected to enhance and maintain the effect of any realignment surgery that is undertaken. However, it is also well known that development of binocular vision and stereopsis is dependent on correct eye alignment in early infancy (Tychsen 1992). Furthermore, amblyopia therapy often can take months or years to complete. There have been an increasing number of reports suggesting that for better physiological results, surgery should not be delayed until the completion of amblyopia therapy (Ing 1966; Taylor 1963).
Some studies show that the development of stereopsis may be normal if realignment surgery is performed by 12 months of age (Ing 2002). Beyond this age, development of stereopsis is usually defective, although fusion (perception of a single image with input from both eyes) may still develop normally up to the age of 21 months (Ing 2002). Some observational and non-randomized prospective studies suggest that the best results, albeit with a greater number of reoperations, are in children in whom the first surgery is done by six months of age (Ing 2004; Shauly 1994; Spiegel 1997). Stereoacuity also can be achieved in those undergoing surgery after six months of age, again with better results associated with earlier surgery: the ELISS Study, a large multi-center, non-randomized trial, reported that children who underwent strabismus surgery at 20 months of age had better gross stereopsis than those who had surgery at 49 months of age; the first group had a larger number of reoperations to get to the end point of good alignment (Simonsz 2005). However, a Cochrane review examining interventions for infantile esotropia (crossing of the eyes) did not identify any randomized controlled trials (RCTs) conducted to inform the optimal age for performing strabismus surgery, highlighting the need for further research to improve the evidence for timing of surgical intervention to correct strabismus in childhood (Elliott 2013).
In summary, there are potential advantages to each of the two possible approaches to managing amblyopia in a child who needs strabismus surgery. With the approach of overcoming amblyopia first using occlusion therapy, which often takes a number of years to resolve, good visual acuity can be established before any surgery is performed. This approach is thought to improve the physiological mechanisms which fine-tune and stabilize ocular alignment once surgery has been done (Figueira 2006; Von Noorden 2002). With the second approach, to proceed with surgery as early as possible, even when amblyopia therapy has not been completed, the child’s opportunity to develop normal binocular vision and stereopsis at a younger age can be maximized. Studies of binocular training approaches also suggest that aligning the eyes earlier, thereby optimizing conditions for binocularity, may lead to improvements in amblyopia (Hess 2011). Thus, there are two very valid physiological approaches to the management of a child with strabismic amblyopia.
Why it is important to do this review
Strabismus and amblyopia put people at a disadvantage. Aside from the visibly noticeable nature of strabismus and resulting social stigma in some cultures, visual criteria (including stereopsis) have to be met for certain professions (e.g. airline pilots, surgeons, armed forces personnel, seamen). This review was intended to inform clinicians regarding the timing of strabismus surgery while treating children with strabismus and co-existing amblyopia in order to achieve the best possible treatment outcomes. The importance of this issue is underscored by the short timeframe available for treatment, and the fact that treatment occurs in early childhood, which means that any intervention should have the desired impact.
Traditionally in the treatment of strabismic amblyopia, amblyopia therapy is completed prior to strabismus surgery. In this review, we considered strabismus realignment surgery performed during the course of the amblyopia therapy (i.e. surgery after amblyopia therapy was initiated, but before completion of therapy) compared with the conventional approach of completing amblyopia therapy before surgical alignment. This review aimed to analyze evidence from RCTs to establish which of the above approaches achieved the best clinical outcomes in children with strabismic amblyopia.
OBJECTIVES
To study the functional and anatomic (ocular alignment) outcomes of strabismus surgery before completion of amblyopia therapy as compared with surgery after completion of amblyopia therapy in children under seven years of age.
METHODS
Criteria for considering studies for this review
Types of studies
We planned to include RCTs in this review. We also considered studies that had not used randomization to allocate participants to treatment groups, but which had instead used techniques intended to allocate participants in an unbiased fashion, such as quasi-randomized clinical trials. We planned to include studies in which participants with conditions other than those considered for this review were enrolled, but in which randomization was stratified and results were reported separately based on the subgroup(s) that met our participant selection criteria. For these studies, we planned to analyze only the participant subgroups in the scope of this review. We did not include studies when subgroups that met our inclusion criteria were non-randomized.
Types of participants
We planned to include trials that enrolled children with the following characteristics:
aged less than seven years;
any type of strabismic amblyopia requiring both occlusion therapy for amblyopia as well as strabismus surgery.
We excluded studies involving children with the following characteristics:
sensory amblyopia, co-existing ocular problems that might contribute to decreased vision (e.g. retinal disorders, glaucoma) or neurological developmental problems;
incomplete amblyopia therapy (amblyopia therapy was initiated before, but discontinued after strabismus surgery).
We originally intended to include children two years and younger; however, due to difficulties in diagnosing children under three years and because amblyopia is more reliably diagnosed at three years of age and older (Friedman 2008), we expanded the inclusion criteria up to seven years of age. We selected seven years of age as a cut-off point based on research showing different treatment effects between children less than seven years of age and children seven years and older (Holmes 2011).
Types of interventions
We planned to include trials that compared strabismus surgery before completion of amblyopia therapy with strabismus surgery after completion of amblyopia therapy. The type of amblyopia therapy of interest was occlusion therapy (part-time or full-time), performed in accordance with established practice patterns (AAO PPP Amblyopia 2012).
Surgical alignment included any type or number of surgeries on the extraocular muscles needed to achieve ocular alignment. In cases where multiple surgeries had been performed to achieve eventual alignment, the status of amblyopia treatment for the randomized surgery was the defining criterion for determining treatment groups (surgery before or after completion of amblyopia treatment). Acceptable surgical interventions included any procedure used to strengthen or weaken appropriate extraocular muscles with the goal of achieving ocular alignment.
Types of outcome measures
Primary outcomes
The primary outcome for this review was the proportion of children with orthotropia or microtropia at three years follow-up. We defined orthotropia as having no manifest deviation on cover test and microtropia as small angle squint (10 prism diopters or less) with some fusion ability. We considered a composite outcome of orthotropia or microtropia as appropriate because most children with early onset strabismus will never achieve perfect ocular alignment.
Secondary outcomes
Secondary outcomes for comparison of interventions were:
mean best-corrected visual acuity (BCVA) of the amblyopic eye at three years follow-up, as measured by LogMAR visual acuity charts (or equivalent);
proportion with presence of motor fusion, defined as a fusion response using a base out or base in prism test, synoptophore, or other standard clinical test at three years of follow-up;
proportion with presence of sensory fusion, defined as integration of visual signals from both eyes into a single perception, as determined by clinical testing (e.g. Bagolini glasses, Worth’s 4 dot test) at three years of follow-up;
proportion with at least 3000 seconds of arc of stereopsis (e.g. tip of the wings of the Titmus fly test or better) at three years of follow-up.
We planned to summarize any adverse effects of amblyopia therapy or strabismus surgery reported by the included trials.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 6), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2014), EMBASE (January 1980 to July 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to July 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (IC-TRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 24 July 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5) and ClinicalTrials.gov (Appendix 6).
Searching other resources
We tried to contact trial investigators (by email or telephone numbers provided in publications) and other experts to identify ongoing or unpublished studies, including studies submitted for publication or in press. However, we did not receive a response from any of the authors we tried to contact.
We inspected the citation lists from potentially eligible studies for additional studies. We reviewed the following conference proceedings from the last five years for relevant studies:
American Association of Pediatric Ophthalmology and Strabismus;
Strabismological Society of India;
British Isles Paediatric Ophthalmology and Strabismus Association.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts identified from the searches to ascertain which studies met the inclusion criteria for the review. We classified each record as ’definitely relevant’, ’possibly relevant’, or ’definitely not relevant’. We obtained the full-text reports of all records assessed as ’definitely relevant’ or ’possibly relevant’ and two review authors independently assessed them to determine inclusion for the review. We resolved all discrepancies through discussion. Studies excluded after the full text assessment are described in the ’Characteristics of excluded studies’ table.
CHARACTERISTICS OF STUDIES.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Birch 1990 | Not a randomized or quasi-randomized trial |
| Birch 2000 | Not a randomized or quasi-randomized trial |
| Birch 2006 | Not a randomized or quasi-randomized trial |
| Buck 2009 | Not a randomized or quasi-randomized trial |
| Dadeya 2001 | Method of treatment allocation not reported, though comparison is of interest |
| Elliott 2013 | Not a randomized or quasi-randomized trial; review article, intervention comparison not of interest |
| Figueira 2006 | Not a randomized or quasi-randomized trial |
| Lam 1993 | Not a randomized or quasi-randomized trial |
| Meyer 1998 | Not a randomized or quasi-randomized trial |
| NCT01791946 | Included adult participants only (ages 18 to 60 years old) |
| Shauly 1994 | Not a randomized or quasi-randomized trial |
| Simonsz 2005 | Not a randomized or quasi-randomized trial |
| Spiegel 1997 | Not a randomized or quasi-randomized trial |
| Spierer 1988 | Not a randomized or quasi-randomized trial |
| Weakley 1997 | Not a randomized or quasi-randomized trial |
| Zak 1982 | Not a randomized or quasi-randomized trial |
Three studies (Dadeya 2001; Lam 1993; Weakley 1997) included outcome measures that we planned to evaluate for our review, but for two studies (Lam 1993; Weakley 1997) the participants were not randomized and for one study (Dadeya 2001) the method of allocation to treatment groups was not available - either in the articles published or by trying to contact the study investigators.
We identified no trials that met our eligibility criteria in the searches. Should eligible trials be found in future updates to this review, we will follow the steps below.
Data extraction and management
No data could be extracted for this review as there were no trials that met our criteria. However, the following methods will apply to future updates of the review whenever we find studies eligible for inclusion.
Two review authors independently will extract information relating to study methods, participant characteristics, interventions, and outcomes using paper data collection forms developed by the Cochrane Eyes and Vision Group. We will resolve discrepancies through discussion.
We will extract the following details from the studies:
methods: design, inclusion and exclusion criteria, and follow-up period;
participants: age, type of strabismus, previous surgeries or treatment if any;
interventions: amblyopia therapy and number of ocular realignment surgeries;
outcomes: orthotropia, orthophoria +/− 10 prism diopters, BCVA in the amblyopic and fellow eyes and presence of sensory fusion and stereopsis;
adverse events: failure of surgery, i.e. inability to fuse, reversal of amblyopia, or both;
quality of life outcomes, when available.
One review author will enter data into Review Manager software (RevMan 2012) and a second author will verify all values.
Assessment of risk of bias in included studies
For this review, there were no risk of bias assessments. If eligible trials are found for the review update, two review authors will assess the risk of bias in included trials as per the guidelines provided in Chapter 8 of the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011a). We will evaluate the following criteria: methods of randomization (random sequence generation), allocation concealment, masking of outcome assessors, incomplete outcome data, selective outcome reporting (reporting bias) and bias from other sources such as methods used to measure strabismus.
We will consider randomization and allocation concealment to be adequate when the process is clearly described in the trial report(s) and indeed to be at low risk of bias. If this information is not available, we will contact the authors and obtain details. Masking of participants and physicians is not possible with the interventions considered for this review. However, it would be possible to mask the assessment of treatment outcomes by having vision and orthoptic testing completed by two separate orthoptists.
We will grade each risk of bias domain, as ’low risk of bias’, ’high risk of bias’ or ’unclear risk of bias’. Trials may be graded as unclear if all the domains are not clearly mentioned and there is a possibility that it was a quasi-randomized trial. For studies with insufficient information to assess risk of bias, we will contact the trial authors. We will not exclude studies based on risk of bias assessments, but will conduct sensitivity analyses to assess the impact on the summary effect from studies with unclear or high risk of bias. We will resolve discrepancies through discussion.
Measures of treatment effect
We will perform meta-analysis according to the guidelines provided in Chapter 9 of the Cochrane Handbook for Systematic Review of Interventions (Deeks 2011). We will calculate summary relative risks with 95% confidence intervals for dichotomous outcomes such as orthotropia and orthophoria within 10 diopters, success or failure of surgery at the end of follow-up and presence or absence of stereopsis. For continuous outcomes, such as mean change in visual acuity, we will report the mean differences with 95% confidence intervals.
Unit of analysis issues
The unit of analysis for the purpose of this review will be the individual participant. In management of strabismus, the eyes are not considered individually, but rather as a single working unit to achieve fusion, single binocular vision and stereopsis.
Dealing with missing data
For missing data, we will contact the study investigators. We will allow a period of one month for response and will consider no response as permanently missing data.
For missing data, we will use methods described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions as appropriate (Higgins 2011b).
Assessment of heterogeneity
We will assess studies for statistical heterogeneity using the I2 statistic, with a value above 60% suggesting substantial statistical heterogeneity. We also will examine the result of the Chi2 test for heterogeneity and the degree of overlap in confidence intervals of included studies. Poor overlap may suggest the presence of heterogeneity. We will examine clinical and methodological heterogeneity across included trials based on the inclusion and exclusion criteria in the trials and the risk of bias in each trial.
Assessment of reporting biases
We will assess selective outcome reporting for each included study as part of the risk of bias assessment.
When 10 or more trials are included in a meta-analysis, we will use a funnel plot to examine publication bias.
Data synthesis
We will conduct a fixed-effect meta-analysis when the included trials are homogeneous with respect to participants and interventions, and when the I2 value indicates no or minimal statistical heterogeneity in effect estimates from the trials (I2 ≤ 60%). If we find substantial statistical heterogeneity indicated by I2 values > 60%, we will explore possible reasons by examining the characteristics of the included trials, including methodological and participant details, rechecking data extracted from the included trials and conducting subgroup analyses that may indicate possible reasons for heterogeneity as described below. We will report the results of the investigation of heterogeneity in a narrative summary. We will use a random-effects model if there is substantial statistical heterogeneity and our investigation provides inadequate evidence for possible reasons contributing to heterogeneity.
Subgroup analysis and investigation of heterogeneity
Heterogeneity among studies may be related to variability in the participants or outcomes and variability in trial design and quality. The treatment effects may be different with different populations and treatment characteristics such as types and numbers of surgeries.
Should findings from one or more RCTs become available at a later date, we propose to undertake the following subgroup analyses.
Type of tropia: children with esotropia vs. exotropia.
- Age at which surgery was performed. Age categories will be:
- less than one year of age;
- one or more years of age.
- Level of visual acuity in the worse eye at the time of surgery. The visual acuity subgroups will be:
- better than 20/40 (or its equivalent);
- 20/40 to 20/60 (or its equivalent);
- worse than 20/60 (or its equivalent).
Sensitivity analysis
We will conduct sensitivity analyses to determine the impact of excluding studies of lower methodological quality, industry-funded studies, and unpublished studies when there are an adequate number of included studies. We will conduct sensitivity analyses to examine the impact of any assumptions made regarding missing data, unit of analysis issues, or both.
RESULTS
Description of studies
Results of the search
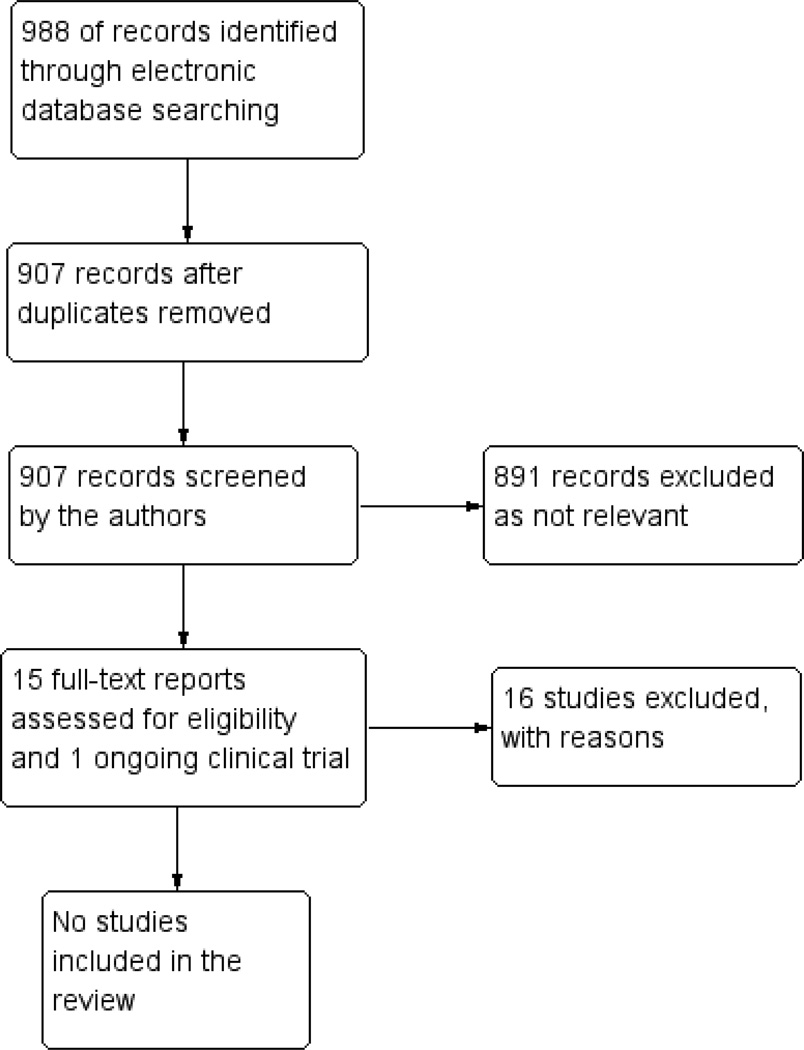
The electronic searches identified a total of 988 references (Figure 1). After duplicates were removed we assessed 907 records for potential inclusion in the review. We excluded 891 records and obtained full-text reports of 16 studies for further assessment. We excluded 15 studies see Characteristics of excluded studies table. We identified one potentially relevant ongoing study (NCT01791946); however, this was also excluded as only adult participants (ages 18 to 60 years old) were eligible for the study.
Figure 1.
Results from searching for studies for inclusion in the review
Included studies
We found no studies meeting the inclusion criteria for this systematic review.
Excluded studies
See: ’Characteristics of excluded studies’ table.
We excluded 15 full-text articles because none of these studies were randomized (or quasi-randomized) trials. One ongoing study was not eligible for this review because it included adults only. Of the 16 studies excluded, three were non-randomized studies investigating interventions and outcomes related to the timing of strabismus surgery in children with amblyopia (Dadeya 2001; Lam 1993; Weakley 1997). These three studies evaluated the effect of timing of amblyopia treatment in children with esotropia with no other neurological or sensory defects (Table 1). The study authors concluded that it is reasonable to proceed with early surgery and continue occlusion therapy after surgery in certain forms of esotropia (acquired esotropia and infantile esotropia with no or mild amblyopia).
Table 1.
Select sample of non-randomized studies
| Study | Participants | Age | Follow up time | Results for motor success |
Results for sen- sory success |
Study investiga- tors’ conclusions |
|---|---|---|---|---|---|---|
| Dadeya 2001 | Group 1 (50 par- tic- ipants): surgery AFTER comple- tion of ambly- opia therapy Group 2 (50 par- tic- ipants): surgery BEFORE com- pletion of ambly- opia therapy |
< 9 years | 3 months after surgery |
Group 1: 84% Group 2: 75% |
Group 1: 55% Group 2: 50% |
“There was no sig- nificant differ- ence in motor suc- cess (84% vs 75%) and sensory suc- cess (55% vs 50%) whether amblyopia was fully treated or partially treated.” |
| Lam 1993 | Group 1 (26 par- tic- ipants): surgery AFTER comple- tion of ambly- opia therapy Group 2 (21 par- tic- ipants): surgery BEFORE com- pletion of ambly- opia therapy |
< 8 years | 6 months after surgery |
Group 1: 14/26 (54%) Group 2: 12/21 (57%) |
Group 1: 8/14 (57%) Group 2: 5/12 (42%) |
“There was no sig- nificant difference detected in motor or sensory outcome whether amblyopia was fully or only partially treated be- fore surgery.” It was noted that five of the 21 patients who had surgery before amblyopia therapy was complete re- portedly had spon- taneous re- versal of amblyopia after surgery |
| Weakley 1997 | Group 1 (51 par- ticipants) : surgery in chil- dren with no am- blyopia, includ- ing those previ- ously treated for amblyopia Group 2 (51 par- tic- ipants): surgery BEFORE com- pletion of ambly- opia therapy |
< 9 years | 4 to 6 weeks after surgery |
Group 1: 43/51 (84%) Group 2: 39/51 (76%) |
Not assessed | “The presence of mild or moderate amblyopia does not appear to have an influence on surgi- cal outcome for pa- tients with acquired esotropia. The effect of am- blyopia on sensory outcome was not studied as most patients were too young for re- liable sensory test- ing” |
Risk of bias in included studies
We found no studies meeting the inclusion criteria for this systematic review; thus, no risk of bias assessments were conducted.
Effects of interventions
We found no studies meeting the inclusion criteria for this systematic review; thus, no effects of interventions are available.
DISCUSSION
Summary of main results
No studies met our inclusion criteria. Many of the studies we identified from our searches were not eligible because they were non-randomized, retrospective studies. Our searches were not designed to systematically identify non-randomized studies; however, in our search for RCTs we did identify three studies that compared strabismus surgery before versus after completion of amblyopia therapy in children (Dadeya 2001; Lam 1993; Weakley 1997).
Overall completeness and applicability of evidence
Since no RCTs were identified, we have elected to discuss the applicability of the evidence reported in the three non-randomized studies we excluded from this review. These studies only included participants with esotropia (Dadeya 2001; Lam 1993; Weakley 1997).
The natural history of and development of strabismic amblyopia has been found to be different in esotropia and exotropia. In infantile esotropia, amblyopia develops in about 40 to 50% of children (Spiegel 1997; Von Noorden 2002). However, it appears that amblyopia develops in a much smaller proportion and to a lesser degree in exotropic children. In a study of 235 children with exotropia who had not received any surgical treatment in the United States, amblyopia was present in only 4.5% of participants (Mohney 2003). In a Canadian study of 109 children with intermittent exotropia, the highest level of amblyopia found in two (1.8%) children was 6/12 and 15 (14%) children had amblyopia with visual acuities of 6/9; in total there were 17 (16%) children in the study treated for amblyopia (Romanchuk 2006).
Physiologically, in esotropia, the fovea of the deviating eye competes with the strong temporal hemi-field of the normal eye. This leads to efforts by the brain to suppress the image from the deviated eye, ultimately leading to development of amblyopia (Von Noorden 2002). In exotropia, the fovea of the deviated eye competes with the relatively weaker contralateral nasal hemi-field. Thus, the effort to suppress the image of the deviating eye is not as great as in esotropia, presumably leading to a lesser risk or degree of amblyopia. Additionally, children with exotropia frequently have intermittent deviations (so that they are still binocular when no deviation is present), or they are frequently able to alternate fixation, so that each eye is used equally (though not simultaneously), thus, greatly reducing the risk of amblyopia developing.
Quality of the evidence
We did not identify any RCTs comparing strabismus surgery before versus after completion of amblyopia therapy. As our search strategy was designed to identify RCTs and not other study design types (e.g. cohort studies, case-control studies), the non-randomized studies we identified from our searches represent only a select sample. Potential underlying differences between groups in non-randomized studies, such as age, severity of disease, and reasons for not completing follow-up, may lead to confounding.
Potential biases in the review process
We attempted to minimize selection bias in the review process by developing a sensitive search strategy to identify relevant studies. We searched multiple sources including several electronic literature databases, clinical trial registries, conference proceedings, and citation lists. In order to minimize bias and errors in the review process, we performed the steps of the systematic review in duplicate (e.g. two people independently screened titles and abstracts).
Agreements and disagreements with other studies or reviews
There is no new evidence provided by this review. Regarding current thought and existing non-randomized evidence, it has been taught, until recently, that amblyopia therapy must be completed before surgery for the best results (Friendly 1985). Von Noorden stated that “the need for eliminating amblyopia, if present, before surgery is not controversial” (Von Noorden 1972). However, as our understanding of the visual development processes have advanced with basic science as well as clinical research, it has become apparent that this clinical dogma may actually now be controversial. Guidelines by the Royal College of Ophthalmologists state that “provided that suitable orthoptic supervision is carried out, it is not necessary to delay surgery until completion of amblyopia therapy” (RCO Guidelines 2000). This recommendation also is reflected in the current Prefered Practice Patterns by the American Academy of Ophthalmology, which now state that “amblyopia therapy should be initiated before esotropia surgery” (AAO PPP Esotropia and Exotropia 2012).
It is clear that there is no rigorous evidence with regard to preferring surgery before completing amblyopia therapy versus after completing amblyopia therapy. The aim of this review was to look at management options for strabismic amblyopia in children. Unfortunately, most of the research has been done on infantile esotropia, with some retrospective studies looking at acquired forms of esotropia. There were no studies found addressing this question in children with other forms of strabismus. However, it is also known that amblyopia is most frequently seen in patients with esotropic deviations, and so this is probably the most relevant group for this review.
The perceived advantage in delaying surgery until amblyopia has been completely treated is to attain a stable and long-lasting motor alignment.
However, some advantages of early surgery may include the following.
Earlier expansion of the binocular peripheral field. This has been documented regardless of the amblyopic state of the eye (Wortham 1989).
Early alignment potentially increasing the chances of development of some degree of binocularity.
Better development of fine motor skill, and visually directed tasks (Rogers 1982).
Spontaneous reversal of amblyopia by restoring binocularity (Dadeya 2001; Lam 1993).
Lesser incidence of amblyopia, nystagmus, inferior oblique over action and dissociated vertical deviation (Zak 1982).
Reduces school stigma of “cross eyes”.
Potential disadvantages of early surgery may include the following.
Development of a false sense of security in the parents and so that continuation of amblyopia therapy may be compromised.
Difficulty with amblyopia and visual acuity assessments in young children; fixation preference testing is not accurate (Friedman 2008).
Increased number of surgeries may be required (Simonsz 2011).
AUTHORS’ CONCLUSIONS
Implications for practice
There are no RCTs available that provide evidence for either one of these management options in children with strabismic amblyopia: 1) amblyopia is treated completely and then alignment surgery is performed versus (2) continued treatment of amblyopia following early surgical alignment. Guidelines by both the Royal College of Ophthalmologists as well as the American Academy of Ophthalmology indicate that strabismus surgery may be done prior to completion of amblyopia therapy (AAO PPP Esotropia and Exotropia 2012; RCO Guidelines 2000). However, clinicians should continue to practice on a case-by-case basis drawing on their past experience and conferring with their patients.
Implications for research
Although we conducted a sensitive search of several electronic literature databases to identify RCTs evaluating the outcome of strabismus surgery before completing amblyopia therapy versus after completing amblyopia therapy, no eligible studies were identified. Further, no planned or ongoing trials on this important topic were found by searching clinical trial registries.
Well-designed RCTs are needed to determine the treatment strategy which achieves the best clinical outcomes in children with strabismic amblyopia. Data from non-randomized studies, such as Dadeya 2001 and Weakley 1997, could be used as a starting point to estimate an adequate sample size and hence to examine the feasibility of conducting an RCT for this management problem. Both of these studies presented data on motor success in participants under nine years of age who had surgery before or after completion of amblyopia therapy. Additionally, outcomes of final sensory success, visual acuity, and motor success ideally should be ascertained after visual maturity is reached (9 to 10 years of age).
In addition to conventional treatment for amblyopia, namely occlusion therapy, other management methods may also be considered for future trials. Recent data showing advantages with binocular training approaches provide promising implications for the treatment of strabismic amblyopia (Hess 2011).
ACKNOWLEDGEMENTS
We would like to acknowledge Iris Gordon for designing and undertaking the electronic searches, and the Cochrane Eyes and Vision Group US Project for their help in retrieving full texts of the articles we had requested. We also would like to acknowledge the resources and time provided for the development of the review during the Cochrane Review Development workshop held in November 2012 at the Christian Medical Colege, Vellore. We thank Sarah Hatt, Barbara Hawkins, and Kate Taylor for reviewing and providing comments to this review.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
Contract N-01-EY-2-1003 and Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
National Institute for Health Research (NIHR), UK.
Richard Wormald, Co-ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Amblyopia
#2 amblyop*
#3 MeSH descriptor Strabismus
#4 strabism* or esotrop*
#5 MeSH descriptor Oculomotor Muscles
#6 MeSH descriptor Accommodation, Ocular
#7 MeSH descriptor Convergence, Ocular
#8 MeSH descriptor Fixation, Ocular
#9 MeSH descriptor Vision, Binocular
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Ophthalmologic Surgical Procedures
#12 surg* or operat* or recess* or resect* or excis* or incis*
#13 (#11 OR #12)
#14 newborn:kw
#15 infant:kw
#16 child:kw
#17 MeSH descriptor Pediatrics
#18 newborn* or neonate* or infant* or child* or paediatric* or pediatric*
#19 nurser* or kindergarten* or preschool* or toddler* or offspring
#20 school*
#21 boy* or girl* or prepubescen*
#22 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21)
#23 (#10 AND #13 AND #22)
Appendix 2. MEDLINE (OvidSP) search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1–7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
amblyopia/
amblyop$.tw.
exp strabismus/
(strabism$ or esotrop$).tw.
oculomotor muscles/
Accommodation, Ocular/
convergence, ocular/
fixation, ocular/
vision, binocular/
or/13–21
exp Ophthalmologic Surgical Procedures/
(surg$ or operat$ or recess$ or resect$ or excis$ or incis$).tw.
or/23–24
exp child/
exp infant/
exp pediatrics/
(infant$ or child$ or juvenile$).tw.
(paediatric$ or pediatric$).tw.
(nurser$ or kindergarten$ or preschool$ or pre school$ or school$).tw.
or/26–31
22 and 25 and 32
12 and 33
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1–5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12–21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25–28
29 not 10
30 not (11 or 23)
11 or 24 or 31
amblyopia/
amblyop$.tw.
exp strabismus/
(strabism$ or esotrop$).tw.
Extraocular Muscle/
Accommodation/
Binocular Convergence/
Eye Fixation/
Binocular Vision/
or/33–41
Eye Surgery/
(surg$ or operat$ or recess$ or resect$ or excis$ or incis$).tw.
or/43–44
exp newborn/
exp infant/
exp child/
exp pediatrics/
(newborn$ or neonate$ or infant$ or child$ or paediatric$ or pediatric$).tw.
(nurser$ or kindergarten$ or preschool$ or pre school$).tw.
or/45–51
42 and 45 and 52
32 and 53
Appendix 4. LILACS search strategy
amblyop$ or strabism$ or esotrop$ and surg$ or operat$ or recess$ or resect$ or excis$ or incis$ and paediatric$ or pediatric$ or infant$ or child$ or juvenile$ or nurser$ or kindergarten$ or preschool$ or pre school$ or school$
Appendix 5. metaRegister of Controlled Trials search strategy
amblyop* AND strabism* AND surg*
Appendix 6. ClinicalTrials.gov search strategy
Amblyopia AND Strabismus AND Surgery
Footnotes
DATA AND ANALYSES
This review has no analyses.
CONTRIBUTIONS OF AUTHORS
Conceiving the review: SK, SP, SJ, AB
Designing the review: SK, SP, SJ, AAS, AB
Co-ordinating the review: SK
-
-Designing search strategies: SK, SP, SJ, AAS, IG*
-
-Undertaking searches: IG*
-
-Screening search results: SK, SP
-
-Organizing retrieval of papers: SK, SP, SJ, CEVG@US**
-
-Screening retrieved papers against inclusion criteria: SK, SP
-
-Appraising quality of papers: SK, SP
-
-Extracting data from papers: SK, SP, SJ
-
-Writing to authors of papers for additional information: SK, SP, SJ
-
-Providing additional data about papers: SK, SP, SJ
-
-Obtaining and screening data on unpublished studies: SK, SP, SJ
-
-Entering data into RevMan: SJ
-
-Analysis of data: SK, SP, SJ, AB
-
-Providing a methodological perspective: SK, SP, SJ, AAS, AB
-
-Providing a clinical perspective: SK, SP, SJ, AAS
-
-Providing a policy perspective: SK, SP, SJ, AAS
-
-Providing a consumer perspective: SK, SP, SJ, AAS
Writing the review: SK, SP, SJ, AAS, AB
Providing general advice on the review: SK, SP, SJ, AAS, AB
Guarantor for review: SK
*Iris Gordon - Cochrane Eyes and Vision Group Trials Search Co-ordinator
**CEVG@US - Cochrane Eyes and Vision Group US Project
DECLARATIONS OF INTEREST
None known.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
Based on substantial feedback during the peer review process, we revised the inclusion criteria for this review from the published protocol to include children older than two years of age (up to seven years of age). We feel this change was warranted based on published findings that detecting strabismic amblyopia in children under three years of age is problematic and many cases may not be detected under later. We also revised the outcomes for this review from the published protocol. This included switching the primary outcome from best-corrected visual acuity to proportion of participants with orthotropia or microtropia at three years follow-up and changing the threshold for presence of at least 2000 seconds of arc of stereopsis at the end of the follow-up (tip of the wings of the Titmus fly test or better). These changes were made in order to better assess the effectiveness of treating strabismic amblyopia in young children.
REFERENCES
* Indicates the major publication for the study
References to studies excluded from this review
- Birch EE, Stager DR, Berry P, Everett ME. Prospective assessment of acuity and stereopsis in amblyopic infantile esotropes following early surgery. Investigative Ophthalmology and Visual Science. 1990;31(4):758–765. {published data only} [PubMed] [Google Scholar]
- Birch EE, Fawcett S, Stager DR. Why does early surgical alignment improve stereoacuity outcomes in infantile esotropia? Journal of AAPOS. 2000;4(1):10–14. doi: 10.1016/s1091-8531(00)90005-3. {published data only} [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR. Long-term motor and sensory outcomes after early surgery for infantile esotropia. Journal of AAPOS. 2006;10(5):409–413. doi: 10.1016/j.jaapos.2006.06.010. {published data only} [DOI] [PubMed] [Google Scholar]
- Buck D, Powell C, Cumberland P, Davis H, Dawson E, Rahi J, et al. Presenting features and early management of childhood intermittent exotropia in the UK: inception cohort study. British Journal of Ophthalmology. 2009;93(12):1620–1624. doi: 10.1136/bjo.2008.152975. {published data only} [DOI] [PubMed] [Google Scholar]
- Dadeya S, Kamlesh MS. Is it mandatory to treat amblyopia prior to surgery in esotropia? Acta Ophthalmologica Scandinavia. 2001;79(1):28–30. doi: 10.1034/j.1600-0420.2001.079001028.x. {published data only} [DOI] [PubMed] [Google Scholar]
- Elliott S, Shafiq A. Interventions for infantile esotropia. Cochrane Database of Systematic Reviews. 2013;(Issue 7) doi: 10.1002/14651858.CD004917.pub3. {published data only} [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira EC, Hing S. Intermittent exotropia: comparison of treatments. Clinical and Experimental Ophthalmology. 2006;34(3):245–251. doi: 10.1111/j.1442-9071.2006.01199.x. {published data only} [DOI] [PubMed] [Google Scholar]
- Lam GC, Repka MX, Guyton DL. Timing of amblyopia therapy relative to strabismus surgery. Ophthalmology. 1993;100(12):1751–1756. doi: 10.1016/s0161-6420(13)31403-1. {published data only} [DOI] [PubMed] [Google Scholar]
- Meyer K, Breitschwerdt H, Kolling GH, Simonsz HJ. The Early vs Late Infantile Strabismus Surgery Study: do sources for bias exist in this non-randomised trial? British Journal of Ophthalmology. 1998;82(8):934–938. doi: 10.1136/bjo.82.8.934. {published data only} [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01791946. [accessed 2 October 2013];Binocular treatment of amblyopia before and after strabismus surgery. clinicaltrials.gov/show/NCT01791946 {unpublished data only}
- Shauly Y, Prager TC, Mazow ML. Clinical characteristics and long-term postoperative results of infantile esotropia. American Journal of Ophthalmology. 1994;117(2):183–189. doi: 10.1016/s0002-9394(14)73075-2. {published data only} [DOI] [PubMed] [Google Scholar]
- Simonsz HJ, Kolling GH, Unnebrink K. Final report of the early vs late infantile strabismus surgery study (ELISSS), a controlled, prospective, multi-centre study. Strabismus. 2005;13(4):169–199. doi: 10.1080/09273970500416594. {published data only} [DOI] [PubMed] [Google Scholar]
- Spiegel PH, Wright KW. Optimum timing for surgery for congenital esotropia. Seminars in Ophthalmology. 1997;12(4):166–170. {published data only} [Google Scholar]
- Spierer A. Binocular function after surgical alignment of infantile esotropia. Metabolic, Pediatric and Systemic Ophthalmology. 1988;11(1–2):35–36. {published data only} [PubMed] [Google Scholar]
- Weakly DR, Holland DR. Effect of ongoing treatment of amblyopia on surgical outcome in esotropia. Journal of Pediatric Ophthalmology and Strabismus. 1997;34(5):275–278. doi: 10.3928/0191-3913-19970901-04. {published data only} [DOI] [PubMed] [Google Scholar]
- Zak TA, Morin JD. Early surgery for infantile esotropia: results and influence of age upon results. Canadian Journal of Ophthalmology. 1982;17(5):213–218. {published data only} [PubMed] [Google Scholar]
Additional references
- American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel. Preferred Practice Pattern® Guidelines. Amblyopia; [accessed 2 October 2013]. www.aao.org/ppp. [Google Scholar]
- American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel. Preferred Practice Pattern® Guidelines. [accessed 2 October 2013];Esotropia and Exotropia. www.aao.org/ppp.
- Antonio-Santos A, Vedula SS, Hatt SR, Powell C. Occlusion for stimulus deprivation amblyopia. Cochrane Database of Systematic Reviews. 2014;(Issue 2) doi: 10.1002/14651858.CD005136.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MS, Aslin RN, Letson RD. Sensitive period for the development of human binocular vision. Science. 1975;190(4215):675–677. doi: 10.1126/science.1188363. [DOI] [PubMed] [Google Scholar]
- Campos E. Amblyopia. Survey of Ophthalmology. 1995;40(1):23–39. doi: 10.1016/s0039-6257(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Chapter 9: Analysing data and undertaking meta-analyses. Higgins JPT, Green S (editors). Available from www.cochrane-handbook.org. [Google Scholar]
- Epelbaum M, Milleret C, Buisseret P, Dufier JL. The sensitive period for strabismic amblyopia in humans. Ophthalmology. 1993;100(3):323–327. doi: 10.1016/s0161-6420(13)32170-8. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Katz J, Repka MX, Giordano L, Ibironke J, Hawse P, et al. Preference and HOTV optotype visual acuity in preschool children: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2008;115(10):176–179. doi: 10.1016/j.ophtha.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friendly DS. Management of infantile esotropia. International Ophthalmology Clinics. 1985;25(4):37–52. doi: 10.1097/00004397-198502540-00005. [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–136. [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011;19(3):110–118. doi: 10.3109/09273972.2011.600418. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B. New insights into amblyopia: binocular therapy and noninvasive brain stimulation. Journal of AAPOS. 2013;17(1):89–93. doi: 10.1016/j.jaapos.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic and Physiological Optics. 2014;34(2):146–162. doi: 10.1111/opo.12123. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Available from www.cochrane-handbook.org. [Google Scholar]
- Higgins JPT, Deeks JJ, Altman DG, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Chapter 16: Special topics in statistics. Higgins JPT, Green S (editors). Available from www.cochrane-handbook.org. [Google Scholar]
- Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, et al. Effect of age on response to amblyopia treatment in children. Archives of Ophthalmology. 2011;129(11):1451–1457. doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing M, Costenbader FD, Parks MM, Albert DG. Early surgery for congenital esotropia. American Journal of Ophthalmology. 1966;61(6):1419–1427. doi: 10.1016/0002-9394(66)90480-6. [DOI] [PubMed] [Google Scholar]
- Ing MR, Okino LM. Outcome study of stereopsis in relation to duration of misalignment in congenital esotropia. Journal of AAPOS. 2002;6(1):3–8. doi: 10.1067/mpa.2002.120172. [DOI] [PubMed] [Google Scholar]
- Ing MR, Rezentes K. Outcome study of the development of fusion in patients aligned for congenital esotropia in relation to duration of misalignment. Journal of AAPOS. 2004;8(1):35–37. doi: 10.1016/j.jaapos.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Li T, Shotton K. Conventional occlusion versus pharmacologic penalization for amblyopia. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD006460.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri B, Singh P, Globa A, Pearson P. Binocular training reduces amblyopic visual acuity impairment. Strabismus. 2014;22(1):1–6. doi: 10.3109/09273972.2013.877945. [DOI] [PubMed] [Google Scholar]
- Mohney B, Huffaker RK. Common forms of childhood exotropia. Ophthalmology. 2003;110(11):2093–2096. doi: 10.1016/j.ophtha.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Royal College of Ophthalmologists. [accessed 25 October 2013];Guidelines for the management of strabismus and amblyopia in childhood - February 2000. www.mrcophth.com/focus1/Strabismus.htm.
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. [Google Scholar]
- Rogers GL, Chazan S, Fellows R, Tsou BH. Strabismus surgery and its effect upon infant development in congenital esotropia. Ophthalmology. 1982;89(5):479–483. doi: 10.1016/s0161-6420(82)34766-1. [DOI] [PubMed] [Google Scholar]
- Romanchuk KG, Dotchin SA, Zurevinsky J. The natural history of surgically untreated intermittent exotropia-looking into the distant future. Journal of AAPOS. 2006;10(3):225–231. doi: 10.1016/j.jaapos.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Taylor K, Powell C, Hatt SR, Stewart C. Interventions for unilateral and bilateral refractive amblyopia. Cochrane Database of Systematic Reviews. 2012;(Issue 4) doi: 10.1002/14651858.CD005137.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsz HJ, Kolin GH. Best age for surgery for infantile esotropia. European Journal of Paediatric Neurology. 2011;15(3):205–208. doi: 10.1016/j.ejpn.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Sireteanu R. Binocular vision in strabismic humans with alternating fixation. Vision Research. 1982;22(8):889–896. doi: 10.1016/0042-6989(82)90025-6. [DOI] [PubMed] [Google Scholar]
- Taylor DM. How early is early surgery in the management of strabismus? Archives of Ophthalmology. 1963;70:752–756. doi: 10.1001/archopht.1963.00960050754006. [DOI] [PubMed] [Google Scholar]
- Taylor K, Elliott S. Interventions for strabismic amblyopia. Cochrane Database of Systematic Reviews. 2014;(Issue 7) doi: 10.1002/14651858.CD006461.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn F, Gwiazda J, Cruz AA, Bauer JA, Held R. The development of eye alignment, convergence, and sensory binocularity in young infants. Investigative Ophthalmology and Visual Science. 1994;35(2):544–553. [PubMed] [Google Scholar]
- Tychsen L. Binocular vision. In: Hart W Jr., editor. Adler’s physiology of the eye: clinical application. 9th Edition. Mosby: 1992. pp. 806–808. [Google Scholar]
- Von Noorden GK, Jsaze A, Parks ME. Surgical treatment of congenital esotropia. Transactions - American Academy of Ophthalmology and Otolaryngology. 1972;76:1465–1478. [PubMed] [Google Scholar]
- Von Noorden GK, Crawford ML, Middle-Ditch PR. The effects of monocular visual deprivation: disuse or binocular interaction? Brain Research. 1976;111(2):277–285. doi: 10.1016/0006-8993(76)90772-1. [DOI] [PubMed] [Google Scholar]
- Von Noorden GK. A reassessment of infantile esotropia. XLIV Edward Jackson memorial lecture. American Journal of Ophthalmology. 1988;105(1):1–10. doi: 10.1016/0002-9394(88)90113-4. [DOI] [PubMed] [Google Scholar]
- Von Noorden GK. Treatment of amblyopia and surgical outcome. Journal of Pediatric Ophthalmology and Strabismus. 1998;35(1):5–6. doi: 10.3928/0191-3913-19980101-04. [DOI] [PubMed] [Google Scholar]
- Von Noorden GK. Binocular vision and ocular motility: theory and management of strabismus. 5th Edition. Mosby: St. Louis; 2002. Esodeviations. [Google Scholar]
- Wong AM. Timing of surgery for infantile esotropia: sensory and motor outcomes. Canadian Journal of Ophthalmology. 2008;43(6):643–651. doi: 10.3129/i08-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortham E, Greenwald MJ. Expanded binocular peripheral visual fields following surgery for esotropia. Journal of Pediatric Ophthalmology and Strabismus. 1989;26(3):109–112. doi: 10.3928/0191-3913-19890501-04. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Korah S, Philip S, Jasper S, Antonio-Santos A, Braganza A. Strabismus surgery before versus after completion of amblyopia therapy in children. Cochrane Database of Systematic Reviews. 2011;(Issue 8) doi: 10.1002/14651858.CD009272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]