Abstract
Background
Trachoma is a major cause of avoidable blindness. It is responsible for about six million blind people worldwide, mostly in the poor communities of developing countries. One of the major strategies advocated for the control of the disease is the application of various environmental sanitary measures to such communities.
Objectives
To assess the evidence for the effectiveness of environmental sanitary measures on the prevalence of active trachoma in endemic areas.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2011, Issue 9), MEDLINE (January 1950 to September 2011), EMBASE (January 1980 to September 2011), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to September 2011), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) and ClinicalTrials.gov (www.clinicaltrials.gov). There were no date or language restrictions in the electronic searches for trials. The electronic databases were last searched on 23 September 2011. We checked the reference list of included trials and the Science Citation Index. We also contacted agencies, experts and researchers in trachoma control.
Selection criteria
We included randomised and quasi-randomised controlled trials comparing any form of environmental hygiene measures with no measure. These hygiene measures included fly control, provision of water and health education. Participants in the trials were people normally resident in the trachoma endemic areas.
Data collection and analysis
Two authors independently extracted data and assessed the quality of the included trials. Study authors were contacted for additional information. Six trials met the inclusion criteria but we did not conduct meta-analysis due to heterogeneity of the studies.
Main results
We included six studies with a total of 12,294 participants from 79 communities. Two studies that assessed insecticide spray as a fly control measure found that trachoma is reduced by at least 55% to 61% with this measure compared to no intervention. However, another study did not find insecticide spray to be effective in reducing trachoma. One study found that another fly control measure, latrine provision, reduced trachoma by 29.5% compared to no intervention; this was, however, not statistically significantly different and findings have not been confirmed by a more recent study. Another study revealed that health education reduced the incidence of trachoma. These findings were not confirmed by a second study, however, which found that a modest health education programme with modest water supply did not reduce trachoma. However, all the studies have some methodological concerns.
Authors’ conclusions
There is some evidence from two trials that insecticides are effective in reducing trachoma, however, this effect was not demonstrated in another trial that used insecticides. Two trials on latrine provision as a fly control measure have not demonstrated significant trachoma reduction. Health education had shown significant reduction of trachoma in one study but another study did not demonstrate similar findings. Generally there is a dearth of data to determine the effectiveness of all aspects of environmental sanitation in the control of trachoma.
PLAIN LANGUAGE SUMMARY
Environmental sanitation measures to reduce trachoma transmission
Trachoma is the commonest cause of preventable vision loss and is common in poor communities. Repeated bouts of conjunctivitis caused by chlamydia infection lead to scarring and turning in of the eyelid. The lashes rub the cornea causing opacification and blindness. Environmental sanitation is a package of measures aimed at eliminating factors that encourage proliferation of flies and the spread of the disease. Some of these interventions include provision of water and latrines as well insecticide spray to control flies and health education programmes to improve the personal and environmental hygienic practices of the people. We included six studies involving 12,294 participants of different ages and both sexes in this review. The trials were conducted in The Gambia, Mali, Tanzania, Niger and Ethiopia. Two studies looked at insecticide spray, one looked at insecticide spray and provision of latrines, one study looked at provision of latrines, and two studies looked at health education with one of them having health education combined with water supply. Prevalence of active trachoma, prevalence of Chlamydia trachomatis and fly count measures were the main outcomes assessed. Two studies conducted in the same area found insecticide spray effective in reducing active trachoma but one study in a different setting found the spray ineffective. A separate study found health education on personal and environmental hygiene to be effective in reducing active trachoma, however, another study found that a modest health education programme combined with a modest water supply was not effective in reducing active trachoma. One study on latrine provision found no impact on trachoma. However, more research is needed.
BACKGROUND
Description of the condition
Trachoma is a chronic infective condition of the eye caused by the micro-organism Chlamydia trachomatis. The disease is more prevalent in the poor underprivileged communities of sub Saharan Africa, Asia, South America and the Middle East (WHO 1997a; WHO 1997b). It is estimated that there are over 146 million people, mostly children, with active trachoma, a proportion of whom may progress to blindness. About 10 million others have trichiasis (turned-in eyelashes) and are at risk of going blind. Trachoma is responsible for over six million blind people worldwide (WHO 1997a). The organism Chlamydia trachomatis is transmitted from one person to another mostly children who are the reservoir of the disease, by close contact and through contaminated fingers and cloths used to wipe discharge on the faces of children (Mariotti 2000). Flies are believed to be major transmitters of the disease (Pruss 2000).
The disease begins in early childhood. It is characterised by redness of the eye and discharge, with inflammatory thickening of the upper tarsal conjunctiva (mucous membrane lining the inner surface of the upper lid) and development of follicles, whitish inflammatory elevations within the conjunctiva. Repeated inflammation from cycles of infection and reinfection causes entropion (distortion of the eyelids), trichiasis, and corneal abrasion. Blindness can subsequently occur due to corneal opacity (loss of corneal clarity).
Description of the intervention
The World Health Organization (WHO) and partners have developed the SAFE strategy as a comprehensive strategy for control of the disease. This entails eyelid surgery to correct in-turned eyelashes to prevent corneal abrasion and blindness, antibiotics to treat active trachoma so as to prevent scarring of the tarsal conjunctiva, facial cleanliness and environmental sanitation to break transmission of the disease (WHO 1997a). Cochrane systematic reviews on antibiotics (Evans 2011) and face washing (Ejere 2004) have already been published.
Environmental sanitation is a package of measures aimed at eliminating factors that encourage proliferation of flies and the spread of the disease in the environment. Some of these factors include poor faecal and refuse disposal, presence of animal pens within human households and inadequate water supply. Thus, environmental sanitary interventions include: provision of water; latrines; refuse dumps; insecticide spray to control flies; relocating animal pens away from human households; and health education to improve personal and environmental hygiene (Mariotti 2000).
Why it is important to do this review
It is believed that improving the environment can reduce the incidence of trachoma (Bailey 2000) and is likely to lead to more sustainable control of the disease (Pruss 2000). However, this component of the SAFE strategy is not well defined. Improving the environment covers a wide variety of environmental control measures as described above. These have been implemented in various forms in different communities as part of a global effort to control trachoma. Some traditional reviews of the impact of environmental interventions have been reported. However, the reviews were mostly based on observational studies (Emerson 2000; Pruss 2000). Where controlled trials were included, the methodological quality and selection criteria were not adequately specified. A systematic review is needed to summa rise the best available evidence from trials of the impact of environmental interventions on active trachoma in endemic communities.
OBJECTIVES
To assess the evidence for the effectiveness of environmental sanitary interventions on the prevalence of active trachoma in endemic communities.
METHODS
Criteria for considering studies for this review
Types of studies
We included randomised and quasi-randomised controlled trials.
Types of participants
Participants in these trials were people normally resident in trachoma endemic communities. There were no age restrictions on the participants in the trials.
Types of interventions
We considered the following interventions.
Fly control interventions versus no intervention. Fly control interventions included all or any of the following: insecticide sprays, provision of latrines, provision of refuse dumps or provision of animal pens away from households.
Water provision versus no intervention. Water provision included any measure(s) aimed at improving the availability, distribution or utilisation of water to individuals, households or communities.
Health education versus no intervention. Health education refers to any programme aimed at improving personal and environmental hygiene and delivered by any means appropriate to local settings such as radio or television, posters, group discussion, leaflets, role play, religious gatherings, etc.
Any combination of the above mentioned interventions versus no intervention.
Types of outcome measures
Primary outcomes
The primary outcome for this review was active trachoma measured as the number of participants with trachoma follicular inflammation (TF) or trachoma intense inflammation (TI), as defined below; at any follow-up period reported in the trials. Active trachoma was defined using the Thylefors (1987) scale (Thylefors 1987). On this scale, active trachoma is categorised as trachoma follicular inflammation (TF) or trachoma intense inflammation (TI). Trachoma follicular inflammation is defined as the presence of five or more follicles, each of which is at least 0.5 mm in diameter, on the flat surface of the upper tarsal conjunctiva. Trachoma intense inflammation is defined as the presence of marked inflammatory thickening of the upper tarsal conjunctiva that obscures more than half of the deep conjunctival vessels.
We planned to include trials that used other trachoma grading scales provided the scales used could be related to the Thylefors (1987) scale.
Secondary outcomes
Secondary outcomes for this review were:
fly density measures such as ’fly-eye contact’ or as reported in the studies;
latrine utilisation as measured and reported in the studies;
water utilisation as measured and reported in the studies;
adverse effects i.e. any reported adverse effects on the use of insecticides for fly control.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 9, part of The Cochrane Library. www.thecochranelibrary.com (accessed 23 September 2011), MEDLINE (January 1950 to September 2011), EM-BASE (January 1980 to September 2011), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to September 2011), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) and ClinicalTrials.gov (www.clinicaltrials.gov). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 23 September 2011.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 5), mRCT (Appendix 5) and ClinicalTrials.gov (Appendix 6).
Searching other resources
We contacted organisations and persons related to trachoma research and control activities such as International Trachoma Initiative (ITI), International Agency for the Prevention of Blindness (IAPB), International Centre of Eye Health (ICEH), London School of Hygiene & Tropical Medicine, John Hopkins School of Public Health and some individuals working in the field. Existing reviews were identified and their citations were checked for relevant trials. We used the Science Citation Index to search for references that cited the studies that were included in the review.
Data collection and analysis
Selection of studies
Two authors independently screened titles and abstracts found by the electronic searches. Disagreements between the authors were resolved by the third author. We retrieved hard copies of trials that were thought to be potentially relevant to the review for further assessment. The trials were independently assessed for inclusion into the review by two authors. There was 10% disagreement in the trials selection. This was resolved by the third author. Trials that met the agreed selection criteria were included and assessed for methodological quality.
In the 2011 studies selection there was no disagreement in the selection of the studies.
Data extraction and management
Two authors independently extracted data onto a standardised data extraction form and entered the data into RevMan (Review Manager 2011). We compared the extracted data for differences. Disagreements were also resolved by the third author at this stage.
Assessment of risk of bias in included studies
Two authors independently assessed the included studies and disagreements between them were resolved by the third author. All included studies were assessed using the Cochrane Collaboration tool for assessing the risk of bias (Higgins 2011a) modified to take into account the assessment of risk of bias in cluster-randomised trials (Higgins 2011b). We graded the following criteria as low risk of bias or high risk of bias or unclear:
Recruitment bias: whether or not recruitment to the trial could have been affected by knowledge of the intervention.
Baseline imbalance: whether or baseline imbalances between communities randomised to the different interventions could explain any differences in effect.
Performance bias: whether or not participants and personnel were masked to the study interventions. We considered active trachoma and other outcomes separately.
Detection bias: whether or not the outcome assessors were masked to the study interventions. Again we considered active trachoma and other outcomes separately.
Attrition bias: whether or not all clusters were followed up and the percentage of the community assessed at different time-points.
Reporting bias: whether or selective outcome reporting was likely to have occurred.
Measures of treatment effect
We calculated risk ratios for dichotomous outcomes.
Unit of analysis issues
In general we report the findings of the trials as reported because we did not pool data from different studies (see below). In the protocol, we specified the following: if we encounter trials where the units of allocation and analysis are different (i.e. the unit of allocation was the community and the unit of analysis was individuals in the community) and have not been accounted for in the analysis, we will contact primary investigators for additional data to develop estimates of intra-cluster correlation coefficients or design effect to calculate more appropriate confidence intervals.
Data synthesis
Due to the six trials included in this review having significant clinical heterogeneity we presented a narrative summary of the results of the trials. If additional trials become available in future we will analyse them as follows: data will be combined in a meta-analysis if appropriate using the random-effects model. If there are fewer than three studies we will use a fixed-effect model. In analysing cluster-randomised trials, if a meta-analysis is not possible a tabulated summary of results will be presented.
Subgroup analysis and investigation of heterogeneity
We planned to assess heterogeneity by visual examination of the forest plot. In the protocol we pre-specified the following subgroups of interest however at present there are not enough data to explore these fully: communities with intense active trachoma versus communities with less intense active trachoma. Intense active trachoma is defined in this review as communities with a baseline prevalence of TF and/or TI equal to or greater than 20% among children less than 10 years, while less intense is defined as communities with a prevalence of TF and/or TI less than 20% amongst children less than 10 years (WHO 1997b).
Sensitivity analysis
In the protocol we planned the following sensitivity analysis: if possible we will conduct a sensitivity analysis to investigate the influence of studies with quasi-randomised methods and those without concealment of allocation on the overall estimates of effect. At present there are not enough data to conduct this sensitivity analysis.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The initial electronic searches generated 335 citations and abstracts. These were independently screened by two authors and the full texts of 11 potentially relevant articles were retrieved. Two authors again independently assessed these articles. Many of these articles were observational studies, reviews and overviews of studies or journal editorials. We considered three trials for inclusion Emerson 1999; Resnikoff 1995; Sutter 1983). A third author resolved any disagreements in the selection of the three studies. Of the three studies, we excluded one study (Sutter 1983) after contacting the investigators as it was confirmed to be an observational study. One other ongoing study (Emerson 2004) was finally published and after assessment we included it. Thus three studies were included in the original published version of this review (Emerson 1999; Emerson 2004; Resnikoff 1995).
Updated searches 2006/2007
Updated searches were done in November 2006 and July 2007. For the 2006 search the Trials Search Co-ordinator (TSC) scanned the search results (a total of 80 reports) and removed any references which were not relevant to the scope of the review. Nineteen reports were identified for potential inclusion in the review and the abstracts of these articles were assessed independently by two authors. One new trial West 2006 met the inclusion criteria and was included in the review. The 2007 search identified 19 new reports of studies but none met the inclusion criteria for the review.
Updated searches 2011
Updated searches were conducted on 23 September 2011. After deduplication the searches identified a total of 148 references. The TSC scanned the search results and removed 80 references which were not relevant to the scope of the review. We assessed 68 references which were made up of 13 abstracts from clinical trial registers and 55 abstracts from journals. These abstracts were independently assessed by two authors. We obtained full text copies of two studies and have included them in the review (Abdou 2010; Stoller 2011). The remaining 53 references did not meet the inclusion criteria for the review.
Included studies
See the ’Characteristics of included studies’ table for further details of the six included studies.
Setting and participants
Resnikoff 1995 was a cluster-randomised study conducted in the Oulessebougou district of Mali. A total of 1810 people of all ages in four villages were randomised into three intervention groups and one control group. Of these, 1334 people in three villages were assigned to different intervention groups and 476 people in one village were assigned to the control group.
Emerson 1999 was a community based cluster- (quasi) randomised study conducted in Sangal area of The Gambia. A total of 1134 people of all ages in four villages (clusters) were allocated to intervention (insecticide spray for fly control) or control (no intervention) in sets of two villages for wet and dry seasons. Two villages with a population of 588 people received insecticide spray, while the remaining two villages with a population of 546 people did not receive any intervention.
Emerson 2004 was a community based cluster-randomised controlled trial conducted in the North Bank and Central River division of The Gambia. A total of 7080 people (aged four months and older) in 21 clusters (one or more close neighbouring rural communities) were randomised in sets of three clusters to receive insecticide spray, latrines or control. As such, all the seasons in the study area were covered in seven stages. Seven clusters, with a total of 2244 people, received insecticide spray, seven other clusters, with a population of 2230, received latrines; while the remaining seven clusters, with a population of 2606, received no intervention.
West 2006 was a community based randomised controlled trial undertaken in Kongwa Tanzania in which 302 children one to seven years old in 16 Balozi (clusters) were randomised in two years. Each year eight Balozi were randomised into four intervention and four control clusters. In total 119 children in eight intervention Balozi and 183 children in eight control Balozi were enrolled. The households in the intervention clusters were sprayed with insecticide throughout the year, while the households in the control Balozi did not receive any intervention. At baseline all residents of both intervention and control Balozi were treated with one dose of azithromycin.
Abdou 2010 was a community based cluster-randomised study in Maradi district of Niger in West Africa. A total of 557 children aged one to five years old in 12 villages were randomised into six villages for intervention and six villages as control, although data were only collected on 10 of these villages. The intervention villages had at least one clean water well constructed and a three month modest health education programme was executed three months prior to the two year survey. The control villages had no well constructed and no specific health education programmes. But villages in both arms of the study had access to the regular trachoma radio messages.
Stoller 2011 was a cluster-randomised trial in Ethiopia investigating the effects of intensive latrine promotion on emergence of infection with ocular C. trachomatis after mass treatment with antibiotics. A total of 24 communities were included in the study and followed up for 24 months. The construction of a simple pit latrine by participating households using locally available materials was currently in progress in the study area; in the intervention villages health workers and additional sanitation volunteers intensified the promotion and provided free latrine slabs and training on the construction of the latrine.
Interventions
In Resnikoff 1995, people in each intervention village were assigned to antibiotics and health education, health education alone, or antibiotics alone. They were compared with people from the control village who did not receive any intervention. We were interested specifically in the comparisons between health education alone versus no intervention. The health education programme was based on community participation and consisted of repeated information concerning personal and family hygiene, including household sanitation. The information also concerned trachoma and its complications as well as elements of primary health care. The programme was specifically directed towards women and school children. Posters and booklets were specially designed for this. The programme was conducted at a frequency of one week per month for the six-month period of the survey.
In Emerson 1999, the insecticide spray villages had 0.175% volume to volume deltamethrin applied by ultra-low-volume application within and up to 20 metres outside each village. The spray consisted of an attack phase of spraying every two days for two weeks followed by a maintenance phase of spraying twice weekly in the wet season and once weekly in the dry season.
In Emerson 2004, the insecticide spray clusters had space spraying with permethrin for six months. The spray was based on an attack phase of spraying every two days for two weeks to kill the adult fly population followed by a maintenance phase of spraying twice a week. The clusters assigned to latrine provision had Gambian improved household pit latrines (non-ventilated). One latrine was allocated per household or 20 people, whichever allowed the most latrines. Latrines were located less than six metres from the households. The control clusters did not receive any intervention.
In West 2006, in each intervention Balozi (neighbourhood), a solution of 10% permethrin in water was sprayed inside houses, compounds, cattle pens, around yards, latrines and in between houses using a sprayer machine. The spraying was commenced with an attack phase of spraying every two days for two weeks and then a maintenance of once per week for the rest of the study period.
In Abdou 2010 the intervention villages had at least one hand pump well constructed (range of one to three wells) over the two year period. However, all villages at the start of the trial were not far from the source of water but it was not easily portable. The new wells provided much safer water than the existing ones.
The health education programme was implemented three months prior to the two year survey. A male village health worker was given the role of health educator; and provided a two day training programme on the spread of trachoma through lack of hygiene and flies. The health worker used flip charts and interactive discussions in one or two village meetings to highlight the importance of using portable water, latrines, environmental sanitation, garbage control and washing faces to minimise trachoma transmission.
In Stoller 2011 the construction of a simple pit latrine by participating households using locally available materials was currently in progress in the study area; in the intervention villages health workers and additional sanitation volunteers intensified the promotion and provided free latrine slabs and training on the construction of the latrine.
Outcome measures
In Resnikoff 1995, outcome was assessed in the study as incidence of active trachoma determined by the cumulative number of new cases of active trachoma within the six-month study period. Active trachoma was defined using the Thylefors (1987) grading scheme. Incidence as an outcome was not in our protocol but post-hoc we realised that it could be a valuable outcome in assessing impact of trachoma intervention programmes.
In Emerson 1999, outcome measures recorded in the study included prevalence of active trachoma, fly density measures (fly-eye contact, fly population) and adverse effects of insecticides. Active trachoma was graded using the WHO (Thylefors) simplified grading scheme. Fly-eye contact was measured only in the dry season by hand-net collection of flies that touched the eyes of 10 seated children for 15 minutes, measured fortnightly. Fly population was measured by determining the number of flies caught by four fish-baited traps placed in each village at an animal-tethering area, in a latrine, at the centre of a domestic compound and at the main meeting point, measured for 24 hours every two weeks. How adverse effects of insecticides were determined was not stated.
In Emerson 2004, outcome measures included prevalence of active trachoma, fly-eye contact (a measure of fly density) and latrine utilisation. Active trachoma was defined by using the Thylefors (1987) simplified grading scheme. Fly density was determined by measuring the number of flies making contact with the eyes of volunteer children of less than five years i.e. fly-eye contact. This was achieved by catching all the flies making contact with the eyes of the children using eight hand nets. Contact with the eyes was defined as flies touching the eye, lid margins or lashes. The fly catch was done once every two weeks in each cluster. The catch was done on the same days, same time and locations for each cluster. Latrine utilisation was determined by visual inspection once a week for the first month and once a month thereafter. The inspection involved monitoring presence of adequate screening, faeces in the pit, flies around the latrine slab and a path worn to the latrine.
In West 2006, outcome measures were prevalence of active trachoma in children under eight years at baseline, six months and one year after mass antibiotic treatment, infection prevalence rates, fly count in each Balozi. Active trachoma was defined by using the Thylefors (1987) WHO simplified grading scheme. Infection prevalence rates referred to presence of Chlamydia trachomatis from an ocular swab as measured with a qualitative polymerase chain reaction (PCR) assay. While the fly count was mean number of flies captured per day in the intervention versus the control Balozi. The flies were captured by two fly paper strips placed in every Balozi at the same spot every week over the course of the year.
In Abdou 2010, outcome measures used were prevalence of active trachoma (presence of TF and or TI) and infection rates from a randomly selected sample of one to five year olds at baseline, one year and two year periods. Active trachoma was graded by assessing both eyes using the WHO simplified grading scheme (Thylefors 1987), while infection rate was assessed by taking a right eye swab using Dacron swab and analysing for Chlamydia trachomatis using Amplicor qualitative PCR. Infection was defined as a positive laboratory result.
In Stoller 2011 the main outcome measures were ocular C. trachomatis infection and active trachoma in children aged 0 to 9 years. Household latrine coverage and use were also estimated.
Excluded studies
See the ’Characteristics of excluded studies’ table for further details.
Risk of bias in included studies
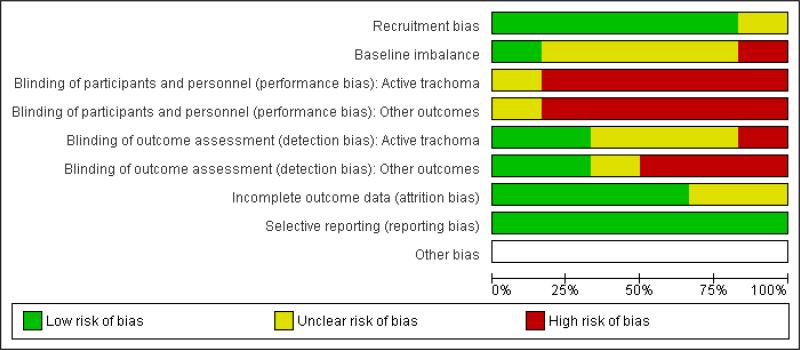
Figure 1.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
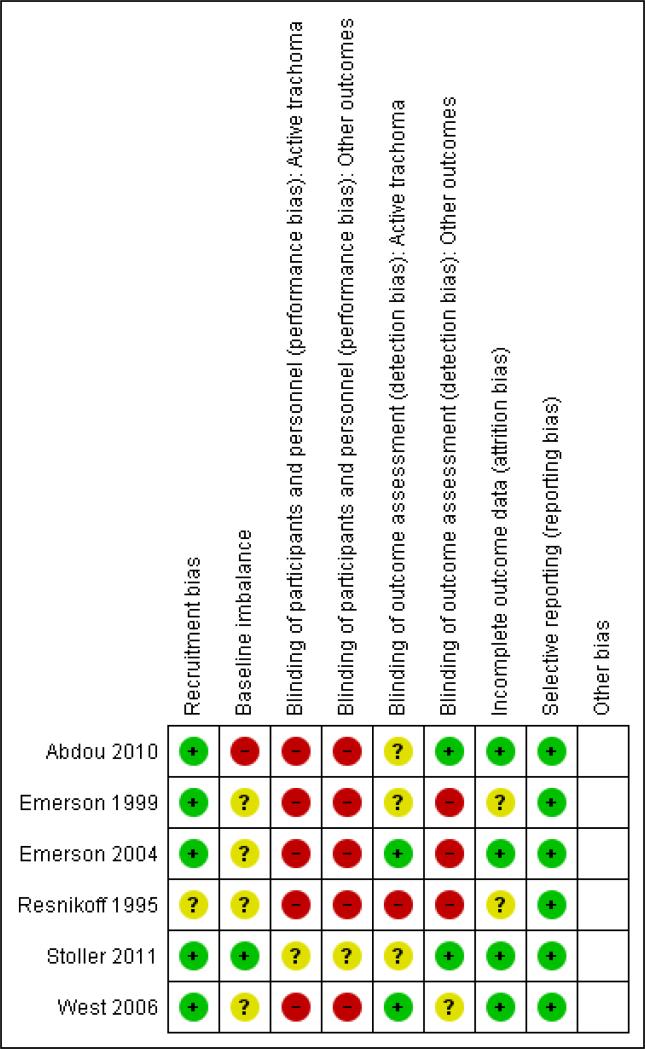
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Blinding
It is difficult to mask participants and caregivers to community-based interventions and all the included studies were considered to be at high risk of performance bias. In Resnikoff 1995 the outcome assessors were not masked either so was considered to be at high risk of detection bias.
In the other studies, attempts were made to mask outcome assessment. For outcomes such as clinical grading of active trachoma we graded risk of bias as unclear in some studies (Abdou 2010; Emerson 1999; Stoller 2011) because even though the outcome assessor may not know the intervention status of the community, it is possible that the participants could have provided this information. However, other studies (Emerson 2004; West 2006) used photographic grading of active trachoma for which it was possible to mask the outcome assessors properly and we graded these as low risk of bias. Similarly, for laboratory outcomes such as measuring C.trachomatis infection, these could be masked successfully (Abdou 2010; Stoller 2011).
Measurements of the number of flies again were probably diffi-cult to mask and none of the studies that reported these outcomes (Emerson 1999 Emerson 2004 West 2006) mentioned any attempts to mask assessment so this was considered to be high risk of bias.
Incomplete outcome data
Three studies were graded at low risk of attrition bias (Abdou 2010; Emerson 2004; West 2006; Stoller 2011) because all clusters completed the trial and loss to follow-up was similar between intervention and control clusters. In Abdou 2010 two clusters “outliers” were removed from the analysis but this was at the outset and one from each arm of the study. For Emerson 1999 and Resnikoff 1995 it was unclear as to the risk of attrition bias. In Emerson 1999 there were some differences in follow-up between intervention and control clusters and in Resnikoff 1995 there was not enough information to assess this properly.
Selective reporting
It is probably difficult to address this conclusively without access to the trial protocols, however, we graded all the studies at low risk because they all reported appropriate outcome measures and there was no evidence from the study report that data were collected and not reported.
Other potential sources of bias
We considered two other potential sources of bias relevant to cluster-randomised trials: recruitment bias and baseline imbalance.
None of the studies discussed recruitment bias. In general we felt that these trials were at low risk of recruitment bias because the community-level interventions, such as fly spraying and latrine provision would be unlikely to affect response to the study assessments. The exception to this was Resnikoff 1995 where the intervention was health education and we felt that the effect of this on recruitment would be unclear.
There were some baseline imbalances that might have affected the study results in Abdou 2010 and West 2006. Although the other studies did not report significant imbalances the numbers of clusters randomised was low so we felt that it was possible that there could be imbalances in other important variables so in general we graded these as unclear.
Effects of interventions
Of the trials included in this review, Emerson 2004 assessed the effect of two different fly control measures i.e. insecticide spray and latrine provision; Emerson 1999 assessed insecticide spray only; and Resnikoff 1995 assessed the impact of health education on trachoma.
However, the two trials involving insecticide spray had significant clinical heterogeneity and, therefore, conducting a meta-analysis was not appropriate. The studies were conducted for different durations as Emerson 1999 had the intervention applied for three months, while Emerson 2004 had the intervention applied for six months. The studies must have been carried out at different seasons of the year (a factor known to affect fly population and likely trachoma transmission). We have, therefore, presented a narrative summary of these trials.
Also the two trials involving health education i.e. Resnikoff 1995 and Abdou 2010 are widely heterogeneous in their interventions as one used health education only (Resnikoff 1995) and the other used a combined health education and water supply (Abdou 2010), thus the two trials cannot be combined for meta-analysis, we have therefore presented a narrative summary of the trials.
Primary outcome - active trachoma
Health education versus no intervention
In Resnikoff 1995, health education (one village) was compared to no intervention (one village). The incidence of active trachoma was lower in the health education village than in the control village (4.2% versus 7.1%) at six months. The odds of reducing trachoma in villages receiving health education was about twice that of control villages (odds ratio 2.4; 95% CI 1.1 to 5.1).
Heath education and improved water supply versus no intervention
In Abdou 2010 health education and water supplied in six trachoma endemic villages were compared to no intervention in six other similar villages. There was no difference in active trachoma rates between the intervention villages and control villages at one year (39% versus 34%) and two years (54% versus 49%) periods. On the prevalence of Chlamydia trachomatis infection there was a more pronounced reduction of infection rates over the two years in intervention villages (26% to 15%) compared to control villages (15% to 11%). This difference, however, was not statistically significant (P = 0.39 at one year and P = 0.11 at two years).
Fly control interventions versus no intervention
i. Insecticide spray
In Emerson 2004, seven clusters that had insecticide spray were compared to seven other clusters with no intervention. There was a mean prevalence reduction of 3.47% active trachoma in the insecticide spray clusters compared to the control clusters (no intervention clusters). This meant a reduction of 55.8% of active trachoma in the intervention clusters compared to the control. In Emerson 1999, the mean reduction of active trachoma in spray villages compared to control villages was 61% (prevalence of 6.2% in the intervention villages versus 15.7% in the control villages). In West 2006, eight Balozi (neighbourhoods) that received insecticide spray throughout the year were compared with eight other Balozi with no spray. But the residents of both intervention and control Balozi received azithromycin at baseline. There was no difference in prevalence of trachoma at six months (20% versus 33%, P = 0.07) and one year (43% versus 44%, P = 0.09) between the groups. The Chlamydia trachomatis infection rates (by PCR) were also not different between the intervention and control groups at six months (9.4% versus 6.7%, P = 0.45).
ii. Latrine provision
In Emerson 2004, seven clusters provided with latrines were compared to seven others with no intervention (control). There was a mean active trachoma prevalence reduction of 1.26% in the latrine clusters compared to the control clusters with no intervention. This meant a reduction of 29.5% of active trachoma in the intervention clusters compared to the control. This difference was not significant statistically.
Emerson 1999 did not assess latrine provision.
Stoller 2011 provided intensive latrine promotion and demonstrated that this lead to higher latrine coverage and use in intervention communities (80.8% and 61.7% respectively) compared to control communities (30.0% and 25.0%). However, at 24 months they could not demonstrate a difference between intervention and control communities in the prevalence of ocular infection and active trachoma in children.
Secondary outcomes
Fly density
i. Insecticide spray
• Fly-eye contact
Emerson 2004 which compared insecticide spray to control showed an 88% (95% CI 64 to 100) reduction of Musca Sorbens flies, and 92% (95% CI 26.1 to 100) fewer Musca Domestica flies in the insecticide clusters than for the no intervention clusters. In Emerson 1999, there was 96% fewer flies caught in the eyes of children in the intervention villages than in the control villages, for the dry season.
• Fly population
In Emerson 1999, there was 75.5% fewer Musca Sorbens flies and 64% fewer Musca Domestica flies in the intervention villages compared to the control villages.
The Emerson 2004 trial did not measure fly population.
In West 2006 comparing insecticide spray to control showed a significantly lower fly count in the intervention group than control for all monitored weeks consistently except for some weeks within the year (P < 0.05) .
ii. Latrine provision
In Emerson 2004, which compared latrine provision and no intervention, there was 30% (95% CI 7.2 to 52.3) reduction of Musca Sorbens than in the control.
Emerson 1999 did not assess latrine provision.
Stoller 2011 provided intensive latrine promotion and demonstrated that this lead to higher latrine coverage and use in intervention communities (80.8% and 61.7% respectively) compared to control communities (30.0% and 25.0%).
Latrine utilisation
In Emerson 2004 which compared latrine provision versus no intervention, latrine utilisation in the intervention clusters was assessed to be 98%.
Emerson 1999 did not assess latrine provision.
Stoller 2011 provided intensive latrine promotion and demonstrated that this lead to higher latrine coverage and use in intervention communities (80.8% and 61.7% respectively) compared to control communities (30.0% and 25.0%).
DISCUSSION
Summary of main results
Health education
Two trials assessed heath education but one assessed health education versus no intervention while the other assessed health education and water supply versus no intervention. The former trial suggested that health education reduced transmission of active trachoma as well as reduced the prevalence of active trachoma at six months with the odds of reducing trachoma about double in the health education village. However, this study has only one cluster (village) for each trial arm. A single village per intervention has no variability. As such it is difficult to determine whether differences between villages were due to intervention or inherent differences in the villages. Furthermore the outcome assessor was not masked and the analysis may not have considered the differences in the unit of allocation and analysis.The second trial using health education and water supply versus no intervention demonstrated that there were no significant differences between the intervention and control villages in terms of active trachoma rate and infection rate as determined by the presence of Chlamydia trachomatis. However, in this trial the interventions provided may not have been sufficient enough to result in a difference between the intervention and control arms considering the fact that health education was only provided three months before the end of the trial. Health education is expected to change the attitudes and practices of the people to enhance personal and environmental hygiene which will reduce transmission of the disease. However, for such a change to take place and translate into reduced infection, longer periods of time are needed, especially as the incubation period of the diseases can be as long as 28 days (Grassly 2008). Furthermore it was stated that both trial arm villages had access to regular trachoma control messages on the radio. As such both study groups might have had almost similar health education messages. Even the water supply provided to the intervention villages, may seem inadequate because only one to three wells were built in each of the intervention villages with a population of 600 to 1200 people each. Also it was stated that intervention and control villages at the beginning of the survey were not far from the source of water. So the hand pump water well constructed in the intervention villages may not have made a significant difference to the control villages as far as water availability is concerned. The methodological quality of the study is also inadequate. At baseline the intervention villages had a significantly higher prevalence of Chlamydia trachomatis infection rates, a higher proportion of three to four year olds and a higher proportion of children living in compounds where garbage is observed within. This may suggest that there was higher risk factors and burden of disease in the intervention villages to which a wider margin of reduction is needed to be achieved to demonstrate difference with the control villages.
Fly control interventions
Two trials that assessed fly control measures included in this review agreed that insecticide spray significantly reduced the magnitude of active trachoma, by at least 55%, as well as markedly reducing the density of house flies by as much as 88% to 92%.One trial, however, did not find a significant effect of fly control by insecticide spray in the reduction of active trachoma.
The difference in these trials is difficult to explain. However, factors that may have influenced the variation in the result may include the fact that the magnitude of the disease at baseline varies significantly in the two study areas. The Gambian study may be said to have been done in a trachoma hypoendemic area while the Tanzania trial was done in a hyperendemic area. Thus the role of flies in the transmission of the disease as well as the effect of fly control may differ in the two settings. In Tanzania the role of flies in transmission of trachoma may be limited as an earlier study in similar Tanzanian communities had shown that face washing promotion is effective in reducing active trachoma in communities with a higher burden of the disease. Also the Tanzanian study at baseline applied mass antibiotics treatment in both the intervention and control groups. This might have significantly cleared the trachoma infection in the communities such that transmission was stalled. As such fly control may not show any effect on the disease.
The role of insecticide spray for the control of trachoma remains unclear. It is pertinent that more studies in different settings are undertaken to ascertain the significance of fly and fly control in prevention of trachoma and possibly other diseases in different settings.
As it is, flies are known vectors not only for trachoma but for other diseases, especially childhood diarrhoeal diseases. As such, insecticide spray is likely to have wider public health relevance in this regard. These studies did not seem to adequately assess the possibility of any untoward effects from such space spraying with insecticides for a prolonged period of time (years). Emerson 1999 reported no adverse effect for the three-month study period, although it was not clear how this conclusion was reached. Another concern for the application of this intervention is its sustainability. Community insecticide spray intervention will require continuous engagement of human and material resources which are likely to be unsustainable in many poor trachoma communities.
Latrine provision as an interventional measure for trachoma control produced less reduction of active trachoma and house flies than insecticide spray. In fact, the trials included in this review failed to demonstrate a reduction in ocular infection active trachoma with increased latrine provision and use. A likely explanation may be that absence of latrines is only one of the factors responsible for fly proliferation in such trachoma communities. Other factors such as poor garbage disposal, poor personal hygiene, presence of animals and their dung etc., if not tackled as well, may interfere with trachoma reduction. In addition, these studies were conducted in communities with a lower prevalence of active trachoma (6% to 18%); the result may be different when latrines are provided in communities with higher prevalence of active trachoma. Furthermore, the short period of time (three to six months) for the intervention and follow up in two of the studies may have been inadequate to demonstrate the impact of the latrine provision however in a more recent study follow-up continued for 24 months.
Agreements and disagreements with other studies or reviews
We found three traditional reviews. The reviews included several observational studies on this topic. Prost 1989 reviewed 15 observational studies relating to the effect of water on trachoma and concluded that provision of water seemed to reduce trachoma within the general context of behavioural and environmental factor improvements. However, neither the search procedures nor the inclusion and exclusion criteria used were mentioned in the report. The methodological quality of studies was not assessed.
Esrey 1991 reviewed 16 observational studies relating to the effects of improved water supply and sanitation on trachoma and reported that there was significant reduction of trachoma in communities that were closer to the source of water (30% median reduction in trachoma). Studies elsewhere (Bailey 1991; West 1989) have demonstrated that closeness to the source of water may not translate into use of water for hygiene purposes. The review did not include non-English papers and technical/agency reports. There were no details of quality assessments of included studies. Furthermore, the pooling of data from different observational studies with differing confounding factors to determine the median reduction of disease may be subject to bias.
Pruss 2000 reviewed 19 studies of which four were stated to be clinical trials and the remainder observational studies. The different studies reported on different environmental parameters ranging from water availability, garbage collection, absence of latrines/toilets, personal hygiene, presence of animals within households and fly control. The authors concluded that both reducing fly densities and hygiene education decreased transmission of trachoma. Personal and domestic hygiene also appeared to have great potential for a sustainable reduction in trachoma transmission. However, the Pruss review did not mention how many assessors selected the included studies. The methods employed in assessing the quality of the studies were not mentioned or how many authors did the assessment. The methods of data extraction from studies and the number of authors that extracted the data were not mentioned. Finally, as in most observational studies the authors attested to the fact that the various environmental confounding factors that had not been adequately controlled may have seriously affected the results.
AUTHORS’ CONCLUSIONS
Implications for practice
Evidence from two trials suggests insecticide spray can reduce transmission of active trachoma, but one trial did not find insecticide spray effective in reduction of active trachoma. Thus the role of insecticide spray in the control of trachoma remains uncertain. On health education one trial suggests that health education may reduce transmission of active trachoma. But another study concluded that provision of modest short-term heath education with improved water supply does not reduce the prevalence of the disease.
Non-Cochrane reviews, which included mostly observational studies, also suggest a potential benefit of environmental interventions for reducing trachoma in communities. However, it is diffi-cult to rely on this evidence because of validity issues. As we await trials that assess the individual contribution of each component of environment sanitation to the control of trachoma it is difficult to be certain which component of environmental sanitation is more effective. Therefore, all available interventions need to be applied in communities with trachoma, within the context of the SAFE strategy. These interventions include health education on personal and environmental hygiene; water supply and education on water use for hygiene; and fly control measures such as provision of latrines, refuse dumps and insecticide spray.
Implications for research
Randomised controlled trials (RCTs) are needed to assess the effects of the various components of environmental sanitation in the control of trachoma and to give a quantitative measure of the impact of each intervention. Future research needs to consider issues in the design, conduct and analysis of such studies. The study should be a RCT with an adequate number of villages in each arm of the intervention groups. Villages should be allocated to groups by concealed random allocation. Ideally, and in view of the varying responses of people to community interventions, as well as differing mode of transmission of the disease which may vary in different settings, it would be best to undertake trials in different regions of the world and in different seasons of the year. When investigating the impact of health education it is important to consider differing behavioural patterns and attitudes in the uptake of such messages in different communities. Also such interventions should be provided for a reasonable period of time sufficient enough to possibly result in attitudinal changes that can affect the disease, before assessing the impact. In assessing the impact of water supply it is important to measure not only the water availability but also water use for hygiene purposes. The possibility of masking the outcome assessors (by taking pictures of everted lids of participants and assessing them elsewhere, by different people) should be considered. Outcome measures may include both prevalence and incidence of the disease but, due to the lack of association between TF/TI and infection; outcome measures should also include Chlamydia trachomatous infection. Analysis of the data should be based on intention-to-treat analysis and include statistical corrections for correlation among individuals within villages if cluster-randomisation was used.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Abdou 2010 | |
|---|---|
| Methods | Randomisation of 10 villages using simple random number table Outcome assessors were partially masked Losses to follow up was same for the both groups (11% versus 12%) Some ofthe baseline variables were not equal for both groups for example the intervention villages had significantly higher prevalence of Chlamydia trachomatis infection rates (26% versus 14%), higher proportion of 3 to 4 year olds and higher proportion of children living in compounds where garbage is observed within |
| Participants | 557 children: aged 1 to 5 years old in 10 villages in Niger republic |
| Interventions | 1.The intervention villages had a health education programme which was implemented 3 months prior to the 2 year survey. A dedicated health educator used flip charts and interactive discussions in one or two village meetings to highlight the importance of personal hygiene 2.Also all intervention villages had at least one hand pump well constructed (range of 1 to 3 wells) over the 2 year period |
| Outcomes | Prevalence of active trachoma, prevalence of Chlamydia trachomatis from conjunctival swab |
| Notes | Both group of villages had access to an ongoing radio programme on trachoma, also it was reported both village groups were not far from the source of water |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Recruitment bias | Low risk | “Within villages, we aimed to randomly select 60 children ages 1 to 5 years as sentinel markers of infection and trachoma. The census data from the house-to house survey that we collected was the basis for selection of children. Stratified random sampling was applied to select no more than one child per mother to minimize clustering of children within households. Of 591 children selected, 557 were examined (94%) at baseline. The same sample of children was surveyed for infection one year (January 2007) and two years (January 2008) later.” Methods, page 2 “At one year, we re-surveyed 91% of the original sample (91% in intervention and 91 % in the control villages). At two years, we re-surveyed 89% of the original sample (89% in the intervention and 88% in the control villages). The primary reason for loss to follow-up at both times was death of the child or child having left the village.” Results, page 4 |
| Baseline imbalance | High risk | “The study populations in the two arms were mostly similar. The overall baseline prevalences of trachoma were similar in the intervention (43%) and control arms (40%, p = 0.75). However, the prevalence of infection with C. trachomatis at baseline was 26% in the intervention villages and 14% in the control villages, significantly different (p = 0.02) (Table 1). There was no difference by intervention arm in the proportion of female sentinel children, the number of children in the compound younger than 8 years, time to walk and wait to get water, or the size of the village (Table 1). However, there was imbalance in the ages of the sentinel children, with more 1-2 year-olds in the control villages, and more 3-4 year-olds in the intervention villages. The children in the intervention villages were also more likely to live in a compound with waste inside, 70%, compared to children in the control villages, 51% (Table 1).” Results, page 4 |
| Blinding of participants and personnel (performance bias) Active trachoma |
High risk | For such community based interventions such as health education and provision of clean water supply it was not feasible to blind participants and personnel |
| Blinding of participants and personnel (performance bias) Other outcomes |
High risk | For community based interventions such as health education and provision of clean water supply it was not feasible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) Active trachoma |
Unclear risk | “The trachoma grader was masked to the intervention status of the village they were working in, although we cannot exclude their hearing from village residents.” Methods, page 3 |
| Blinding ofoutcome assessment (detection bias) Other outcomes |
Low risk | Ocular C. trachomatis infection: “The laboratory personnel were masked to intervention and control status of the swabs received from the field.” Methods, page 3 |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “Two villages were extreme outliers: one had a small population and a low trachoma rate of 3% of children aged 5 years and younger; the other had a very high rate of 82%. These villages were removed from the trial, one from each arm, as they led to extreme imbalance at the outset (Figure 1).” Methods, page 2 “At one year, we re-surveyed 91% of the original sample (91% in intervention and 91% in the control villages). At two years, we re-surveyed 89% of the original sample (89% in the intervention and 88% in the control villages). The primary reason for loss to follow-up at both times was death of the child or child having left the village.” Results, page 4 |
| Selective reporting (reporting bias) | Low risk | The pre-specified outcomes were infection with C. trachomatis and active trachoma and these were reported |
| Emerson 1999 | |
|---|---|
| Methods | Quasi-randomisation of 4 villages Losses to follow up was 18%, but not similar in the study groups Outcome assessor was masked |
| Participants | 1134 people of all ages in 4 villages in The Gambia |
| Interventions | 1. Insecticide spray (588 people in 2 villages) versus no intervention (546 people in 2 villages) for 3 months Insecticide spray with 0.175% deltamethrin |
| Outcomes | Prevalence of trachoma, fly-eye contact, fly population |
| Notes | |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Recruitment bias | Low risk | “1124 people of all ages were screened for trachoma at baseline, of whom 924 (82%) were also screened at 3 months. Loss to follow-up, mainly owing to inclusion oftemporary migrants in the baseline data, was similar for intervention and control groups (rate ratio for intervention vs control 1-13 [0-83-1-54]).” Results, page 1402 |
| Baseline imbalance | Unclear risk | Although there was some evidence to suggest that the villages were similar (see quotes below) only 4 villages were randomised “arbitrarily” so other differences in other important confounders cannot be excluded “Village communities were of similar size, age composition (table), and ethnicity (Wolof)”. Results, page 1402 “Data on trachoma prevalence (figure) shows that there was no difference in the community prevalence ofactive trachoma at baseline in either village pair (wet season intervention 26/295 [8·8%] vs control 33/271 [12·2%]; dry season 34/189 [18·0] vs 27/169 [16·0]).” Results, page 1402 |
| Blinding of participants and personnel (performance bias) Active trachoma |
High risk | Community based interventions like spray of insecticide in the villages cannot be masked from the villagers |
| Blinding of participants and personnel (performance bias) Other outcomes |
High risk | Community based interventions like spray of insecticide in the villages cannot be masked from the villagers |
| Blinding of outcome assessment (detection bias) Active trachoma |
Unclear risk | “The whole of each village community was screened for trachoma at baseline and at 3 months by the same community ophthalmic nurse, who was unaware of the treatment status of each village.” Methods, page 1401 Although the assessor did not know the status of the villages with respect to interventions, the assessor may have heard the status of villages from the people and may have noticed the fly traps set in the villages |
| Blinding of outcome assessment (detection bias) Other outcomes |
High risk | Fly related outcome measures: “Fly populations were monitored by four fish-baited traps placed in each village at an animal-tethering area, in a latrine, at the centre of a domestic compound, and at the main meeting point for 24 h every 2 weeks. To measure fly-eye contact in the dry season, hand-net collections of eye-seeking flies were made fortnightly from ten seated children for 15 min. Flies that touched the children's eyes were collected and taken to the laboratory for identification”. Methods, page 1401 No mention of blinding for this outcome |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | % with ocular examination at follow-up varied in the different villages. Wet season control village 85%, wet season intervention village 77%; dry season control village 91%, dry season intervention village 74%. This was attributed to temporary migrants being examined at baseline |
| Selective reporting (reporting bias) | Low risk | The study reported active trachoma but did not report ocular infection, however, the study report did not give any indication that data on ocular infection was recorded. The focus of the report was entomological |
| Emerson 2004 | |
|---|---|
| Methods | Randomisation by drawing pieces of folded paper from a hat Outcome assessment was masked Losses to follow up was not different between treatment groups and the control group |
| Participants | 7080 people 4 months and above of all sexes in 21 clusters of The Gambia |
| Interventions | 1.Insecticide spray (2244 people) versus no intervention (2606 people) for 6 months Spray with water soluble permethrin 2.Latrine provision (2230) versus no intervention (2606) for 6 months One latrine per household or 20 people whichever gave the most latrines |
| Outcomes | 1.Prevalence of active trachoma 2.Fly-eye contact (fly density) 3.Latrine utilisation |
| Notes | |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Recruitment bias | Low risk | “Everybody over 4 months of age was recruited to the study provided that informed consent was obtained and they intended to stay in the village for 6 months.” Methods, page 1094 There was no discussion of recruitment bias in the paper but the review authors made the judgement that the provision of community-level interventions in this study (fly control/latrines) was unlikely to influence the recruitment of participants to the survey of active trachoma and fly-eye contact |
| Baseline imbalance | Unclear risk | Analysis done on pairs based on recruitment to the study but “Clusters were at least 1·5 km apart but were not matched since this would have reduced the interpretability and statistical power of the study”. Methods, page 1093 Clusters and study populations appeared similar with respect to sanitation, access to water, housing quality, age, sex and ethnicity. There were some differences in trachoma status and fly numbers but unclear as to how important these would be. As only 21 clusters randomised baseline differences in other important confounders cannot be excluded |
| Blinding of participants and personnel (performance bias) Active trachoma |
High risk | “They [the clusters] were recruited in sets of three and randomly assigned to insecticide spray, latrines, or control by drawing from a hat at a meeting of village heads held at the district chief's office.” Methods, page 1094 |
| Blinding of participants and personnel (performance bias) Other outcomes |
High risk | “They [the clusters] were recruited in sets of three and randomly assigned to insecticide spray, latrines, or control by drawing from a hat at a meeting of village heads held at the district chief's office.” Methods, page 1094 |
| Blinding of outcome assessment (detection bias) Active trachoma |
Low risk | “Both eyes were inspected for trichiasis and the right eyelid everted and examined with 2·5 magnification. If trachomatous follicles were present that did not qualify as grade TF (fewer than five, or <0·5 mm in diameter) then the left eyelid was also examined. A single photograph using either slide film (Fujichrome 100ASA) or a digital image (696405 pixels) of the everted eyelid was taken to verify field grades.” Methods, page 1095 “Photographs of eyes from study participants were graded by clinicians who were unaware of the field diagnosis, whether the photograph was from the baseline or followup survey, or if the participant was from an intervention or control cluster”. Methods, page 1095 “The kappa values were also similar for each of the treatment groups in both baseline and follow-up surveys: control group at baseline 0·76, follow-up 0·63; spray group, 0·60 and 0·84; latrine group, 0·63 and 0·95, suggesting that there was no systematic bias in the field diagnoses.” Results, page 1097 |
| Blinding of outcome assessment (detection bias) Other outcomes |
High risk | “We monitored fly-eye contact once every 2 weeks in each cluster by use of eight 15 min hand-net catches of eyeseeking flies from the faces of volunteer children younger than 5 years of age. A contact was defined by the feet or proboscis of a fly touching the eye, lid margin, or lashes. The fly making the contact was caught in a hand-net; which was passed to an assistant who transferred the fly to a tube. Flies were identified by magnification.” Methods, page 1094 No mention of masking for this outcome |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All clusters completed trial and loss to follow-up similar in the clusters “All 21 clusters were recruited and visited at follow-up; 7080 people were recruited from these clusters, and 6087 (86%) were seen at follow-up (figure 1). The number of participants lost to follow-up did not differ between either the spray and control groups (p = 0·08) or between the latrine and control groups (p = 0·55). The proportion lost because of travelling also did not differ between these groups (p = 0·84 and p = 0·57, respectively). Participants with active trachoma at baseline were 1·38 (95% CI 1·01-1·88) more likely to be lost to follow-up than were those without active trachoma, but the proportions with active trachoma lost to follow-up did not differ between the spray and control groups (p = 0·71) or between the latrine and control groups (p = 0·57). Results, page 1095/1096 |
| Selective reporting (reporting bias) | Low risk | “The primary outcome measures were fly-eye contact and prevalence ofactive trachoma.” Methods, page 1094 These outcomes were reported. |
| Resnikoff 1995 | |
|---|---|
| Methods | Paper reports “Randomisation” (How randomisation was done could not be ascertained) Assessor not masked |
| Participants | 1810 people of all ages in 4 villages of Mali |
| Interventions | 1) Health education (424) versus none (476) for 6 months Health education was given by repeated information on personal, family hygiene and trachoma, at a frequency of one week per month |
| Outcomes | Incidence of active trachoma Incidence was determined by expressing the cumulative number of new cases of active trachoma over the follow up period of 6 months |
| Notes | The study had 4 arms, but we only used 2 arms i.e. Health education versus no intervention Age and sex distribution in the 2 villages were identical The baseline prevalence of active trachoma in the 2 villages was not significantly different (21% versus 19%) The follow up period in all the villages was identical - 6 months |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Recruitment bias | Unclear risk | “With the permission of administrative and traditional authorities, all inhabitants of these four villages were surveyed”. Patients and methods, page 102 There was no discussion of recruitment bias in the paper and little information on response rates. It was unclear whether the community-level intervention here - provision of health education (based on community participation) and antibiotic distribution - would have affected recruitment to the study assessments |
| Baseline imbalance | Unclear risk | Although there was some evidence to suggest that the villages were similar (see quotes and data below) only 4 villages were randomised so other differences in other important confounders cannot be excluded “Four villages, matched for size and epidemiological, economic and social conditions, were included in the study. All villages were situated the same distance from the health centre and each village possessed a school and was equipped with boreholes.” Patients and methods, page 102 “The age and sex distribution was identical in all four villages” Results, page 103 Table 2 (page 109) shows the sex distribution (46% male in treatment community and 51% male in control community). No data on age distribution Baseline prevalence of active trachoma (figure 1, page 109) just over 20% in treatment community and just under 20% in control community |
| Blinding of participants and personnel (performance bias) Active trachoma |
High risk | For community based interventions such as health education it was not feasible to mask participants and personnel |
| Blinding of participants and personnel (performance bias) Other outcomes |
High risk | For community based interventions such as health education it was not feasible to mask participants and personnel |
| Blinding of outcome assessment (detection bias) Active trachoma |
High risk | For community based interventions such as health education it would have been difficult to mask outcome assessors and this was not mentioned in the report |
| Blinding of outcome assessment (detection bias) Other outcomes |
High risk | For community based interventions such as health education it would have been difficult to mask outcome assessors and this was not mentioned in the report |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | “At the initial examination, 1810 subjects were enrolled and examined” Results, page 104. Of these, 424 were from the community treated with topical antibiotics (village 2) and 476 were from the control community (village 4) (table 2 page 109) “A total of 347 subjects with active trachoma were included in the clinical trial. Two hundred and sixty five (76%) of these subjects were successfully followed for 6 months and were included in the analysis of the results.” Results, page 105) However, the distribution of these cases by village is not reported. Using figure 1 (page 109) we can estimate that there were 89 cases of active trachoma in treatment community and 90 cases in control community. The “cure rate” in treatment village was 82% (estimated 73 people cured) and 36% in control community (estimated 33 people cured) No information was given on possible reasons for loss to follow up |
| Selective reporting (reporting bias) | Low risk | Only clinical outcomes reported but no indication that microbiological data collected |
| Stoller 2011 | |
|---|---|
| Methods | Cluster-randomised trial of 24 communities in Ethiopia. A random selection of 60 children aged 0-9 years in each was monitored for clinical signs of trachoma and ocular chlamydial infection at baseline, 12 and 24 months |
| Participants | Children resident in trachoma endemic communities |
| Interventions | Mass treatment with azithromycin or topical tetracycline. 12 communities were randomised to intensive latrine promotion |
| Outcomes | Active trachoma and ocular infection |
| Notes | |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Recruitment bias | Low risk | The subkebeles were randomly selected and the children to be examined in each sentinel team were randomly selected at all measurement intervals |
| Baseline imbalance | Low risk | Random selection of subkebeles and children to be examined Baseline variables reported and were comparable except for antibiotics coverage which was higher in control arm Table 1 page 79 |
| Blinding of participants and personnel (performance bias) Active trachoma |
Unclear risk | Latrine provision is difficult to mask but unclear the effect this would have had on the participants |
| Blinding of participants and personnel (performance bias) Other outcomes |
Unclear risk | Latrine provision is difficult to mask but unclear the effect this would have had on the participants |
| Blinding of outcome assessment (detection bias) Active trachoma |
Unclear risk | For clinical trachoma grading assessors could not be effectively masked. Outcome assessors were from outside the area |
| Blinding of outcome assessment (detection bias) Other outcomes |
Low risk | For the primary outcome measure - ocular chlamydial infection using PCR, the assessors in the lab were masked |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | A random sample of 60 participants sampled from each community at each time point |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported. Authors have reported all outcomes measures they assessed |
| West 2006 | |
|---|---|
| Methods | Cluster randomisation of 16 neighbourhoods (Balozi) by using a table of random number for allocation Similar follow up periods and similar lost to follow up in the study groups, but lost to follow up 25 to 30% Outcome assessors were masked |
| Participants | 302 children 1 to 7 years in 16 Balozi in Kongwa, Tanzania |
| Interventions | 1. Insecticide spray (119 children in 8 Balozi) versus no intervention (183 children in 8 Balozi) for 1 year Insecticide spray with 10% permethrin |
| Outcomes | Prevalence of active trachoma, Chlamydia trachomatis infection rate (PCR), fly count |
| Notes | NCT00347763 |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Recruitment bias | Low risk | “Follow-up rates of children in the intervention balozi were 77% at 6 months and 67% at 1 year, and 75% and 69% in controls, respectively. Children lost to follow-up were either temporarily out of their balozi, had died, or had moved away.” Results, page 598 There was no discussion of recruitment bias in the paper but the review authors made the judgement that the provision of community-level interventions in this study (fly control) was unlikely to influence the recruitment of participants to the study |
| Baseline imbalance | Unclear risk | Although there was some evidence to suggest that the clusters (balozi) were similar (see quotes and data below) only 16 balozi were randomised so differences in other important confounders cannot be excluded “The mean household size did not differ between the balozi randomised to intervention and the control neighbourhoods.” Results, page 598 “The mean number of flies in the balozi per day at baseline (measured 5 weeks before the start of spraying) did not differ between the intervention and control groups.” Results, page 598 Mean prevalence of trachoma: - 63% intervention; 68% control active trachoma - 29% intervention; 35% control ocular infection “Trachoma andinfection prevalence rates adjusted for clustering at the balozi level, period of enrolment, and potentially confounding factors of age, sex, baseline trachoma status, and antibiotic treatment.” Statistical analysis, page 598 |
| Blinding of participants and personnel (performance bias) Active trachoma |
High risk | For community based interventions such as fly control it was not feasible to mask participants and personnel and this was not described in the report |
| Blinding of participants and personnel (performance bias) Other outcomes |
High risk | For community based interventions such as fly control it was not feasible to mask participants and personnel and this was not described in the report |
| Blinding of outcome assessment (detection bias) Active trachoma |
Low risk | “Two graders assessed the photographs independently, masked to the intervention status and time of the examination. [...] Outcomes are reported on the basis of masked photographic gradings” Procedures, page 597 |
| Blinding of outcome assessment (detection bias) Other outcomes |
Unclear risk | For community based interventions such as fly control it was not feasible to mask the entomological outcome assessors and this was not described in the report However, for laboratory assessment of ocular C. trachomatis infection masking should be relative straightforward however this was not described in the report |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “All 16 balozi initially selected were included in the trial. [...] Follow-up rates of children in the intervention balozi were 77% at 6 months and 67% at 1 year, and 75% and 69% in controls, respectively. Children lost to follow-up were either temporarily out of their balozi, had died, or had moved away.” Results, page 598 |
| Selective reporting (reporting bias) | Low risk | Active trachoma and ocular infection were reported; no indication of any outcomes for which data collected and not reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bailey 1991 | The study was an observational study (case-control), thus not a controlled clinical/community trial |
| Esrey 1991 | The paper was a ‘traditional’ review/overview of studies relating to improved water supply and sanitation |
| Potter 1993 | The article was an editorial of the BMJ, not a controlled trial |
| Pruss 2000 | The article was a review of studies relating to environmental sanitary interventions |
| Sutter 1983 | The allocation of intervention and control villages was decided long after intervention had started. Thus it was not a controlled trial |
| Taylor 2002 | The article was an editorial not a study/clinical trial |
| West 1988 | The article was a review/overview of community intervention programs for trachoma control; it was not a clinical/community trial |
| West 1989 | The study was an observational survey (cross-sectional study), not a controlled clinical trial |
| West 1996 | The intervention in this community based clinical trial was face washing, not environmental sanitary measures (as defined in the review) |
DATA AND ANALYSES
This review has no analyses.
ACKNOWLEDGEMENTS
The Cochrane Eyes and Vision Group editorial team prepared and executed the electronic searches (Iris Gordon, Anupa Shah). We are grateful to Paul Courtright, Catey Bunce, Marie Diener-West and Roberta Scherer for their peer review comments. We are also grateful to the UK Cochrane Centre for providing their facilities to the main review author during the review process (through The Cochrane Collaboration Aubrey Sheiham Public Health and Primary Care Scholarship).
Richard Wormald (Co-ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
SOURCES OF SUPPORT
Internal sources
National Eye Centre, Kaduna, Nigeria.
NIHR/Department of Health, UK.
Funded JE to assist in updating the version published in Issue 2, 2012.
External sources
Cochrane Health Promotion and Public Health Field, Australia.
UK Cochrane Centre, UK.
APPENDICES
Appendix 1. CENTRAL search strategy
-
#1
MeSH descriptor Trachoma
-
#2
MeSH descriptor Chlamydia trachomatis
-
#3
trachom* or tracom*
-
#4
(#1 OR #2 OR #3)
-
#5
MeSH descriptor Health Education
-
#6
MeSH descriptor Environmental Health
-
#7
MeSH descriptor Insect Control
-
#8
MeSH descriptor Insecticides
-
#9
MeSH descriptor Hygiene
-
#10
MeSH descriptor Water Supply
-
#11
water* or sanit* or educat* or latrine* or spray* or hygien*
-
#12
(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
-
#13
(#4 AND #12)
Appendix 2. MEDLINE (OVID) search strategy
randomized controlled trial.pt
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1-7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp trachoma/
exp chlamydia-trachomatis/
trac?oma$.tw.
or/13-15
exp health education/
exp environmental health/
exp insect control/
exp insecticides/
exp hygiene/
exp water supply/
(water$ or sanita$ or educat$).tw.
(latrine$ or spray$ or hygien$).tw.
or/17-24
16 and 25
12 and 26
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OVID) search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1-5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12-21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25-28
29 not 10
30 not (11 or 23)
11 or 24 or 31
exp trachoma/
exp chlamydia-trachomatis/
trac?oma$.tw.
or/33-35
exp health education/
exp environmental health/
exp insect control/
exp insecticide/
exp hygiene/
exp sanitation/
exp water management/
(water$ or sanita$ or educat$).tw.
(latrine$ or spray$ or hygien$).tw.
or/37-45
36 and 46
32 and 47
Appendix 4. LILACS search strategy
trachoma$ or tracom$ and water or sanit$ or educat$ or latrine$ or spray$ or hygiene$
Appendix 5 metaRegister of Controlled Trials search strategy
trachoma or tracoma or trachomatis
Appendix 6. ClinicalTrials.gov search strategy
Trachoma OR Trachomatis
WHAT'S NEW
Last assessed as up-to-date: 23 September 2011.
| Date | Event | Description |
|---|---|---|
| 5 January 2012 | New search has been performed | Issue 2, 2012: Electronic searches were updated, risk of bias tables have been completed for all included trials and text modified. A new author joined the review team to assist with updating the review |
| 5 January 2012 | New citation required but conclusions have not changed | Issue 2, 2012: Two new trials were included in the update (Abdou 2010; Stoller 2011). |
HISTORY
Protocol first published: Issue 1, 2003
Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 23 October 2008 | Amended | Converted to new review format. |
| 6 June 2007 | New citation required and conclusions have changed | Substantive amendment. Issue 4 2007: 1 new trial (West 2006) has been included. |
Footnotes
Citation: Rabiu M, Alhassan MB, Ejere HOD, Evans JR. Environmental sanitary interventions for preventing active trachoma. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No.: CD004003. DOI: 10.1002/14651858.CD004003.pub4.
CONTRIBUTIONS OF AUTHORS
MR and HE came up with the review question.
MR and MA screened the title and abstracts from the initial search, screened retrieved papers against inclusion criteria, appraised the quality of papers, extracted data from papers, entered data into RevMan and conducted data analysis.
MR wrote to authors of papers for additional information and obtained and screened data on unpublished studies.
MR, MA and HE provided methodological, clinical and policy perspectives.
MR and HE wrote the review.
HE resolved disagreements between review authors.
JE assisted the review authors with updating the review for Issue 2, 2012.
DECLARATIONS OF INTEREST
None known.
INDEX TERMS
Medical Subject Headings (MeSH)
Diptera; Endemic Diseases [* prevention & control]; Health Education [methods]; Insect Control; Insecticides; Randomized Controlled Trials as Topic; Sanitation [* methods]; Toilet Facilities; Trachoma [* prevention & control; transmission]
MeSH check words
Animals; Humans
REFERENCES
- Abdou A, Munoz BE, Nassirou B, Kadri B, Moussa F, Baarè I, et al. How much is not enough? A community randomized trial of a Water and Health Education programme for Trachoma and Ocular C. trachomatis infection in Niger. Tropical Medicine & International Health. 2010;15(1):98–104. doi: 10.1111/j.1365-3156.2009.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson PM, Lindsay SW, Walraven GE, Faal H, Bogh C, Lowe K, et al. Effect of fly control on trachoma and diarrhoea. Lancet. 1999;353(9162):1401–3. doi: 10.1016/S0140-6736(98)09158-2. [DOI] [PubMed] [Google Scholar]
- Emerson PM, Lindsay SW, Alexander N, Bah M, Dibba SM, Faal HB. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet. 2004;363(9415):1093–8. doi: 10.1016/S0140-6736(04)15891-1. [DOI] [PubMed] [Google Scholar]
- Resnikoff S, Peyramaure F, Bagayogo CO, Hugnet P. Health education and antibiotic therapy in trachoma control. Revue internationale du trachome et de pathologie oculaire tropicale et subtropicale et de sante publique. 1995;72:89–98. [PubMed] [Google Scholar]
- Stoller NE, Gebre T, Ayele B, Zerihun M, Assefa Y, Habte D, et al. Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial. International Health. 2011;3(2):75–84. doi: 10.1016/j.inhe.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SK, Emerson PM, Mkocha H, McHiwa W, Munoz B, Bailey R, et al. Intensive insecticide spraying for fly control after mass antibiotic treatment for trachoma in a hyperendemic setting: a randomised trial. Lancet. 2006;368(9535):596–600. doi: 10.1016/S0140-6736(06)69203-9. [DOI] [PubMed] [Google Scholar]
- Bailey R, Downes B, Downes R, Mabey D. Trachoma and water use; a case control study in a Gambian village. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85(6):824–8. doi: 10.1016/0035-9203(91)90470-j. [DOI] [PubMed] [Google Scholar]
- Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bulletin of the World Health Organization. 1991;69(5):609–62. [PMC free article] [PubMed] [Google Scholar]
- Potter AR. Combating blinding trachoma. BMJ. 1993;307(6898):213–4. doi: 10.1136/bmj.307.6898.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss A, Mariotti SP. Preventing trachoma through environmental sanitation: a review of the evidence base. Bulletin of the World Health Organization. 2000;78(2):258–66. [PMC free article] [PubMed] [Google Scholar]
- Sutter E, Ballard R. Community participation in the control of trachoma in Gazankulu. Social Science and Medicine. 1983;17(22):1813–7. doi: 10.1016/0277-9536(83)90397-0. [DOI] [PubMed] [Google Scholar]
- Taylor H. Flies and trachoma. Clinical & Experimental Ophthalmology. 2002;30(2):65. doi: 10.1046/j.1442-6404.2002.00506.x. [DOI] [PubMed] [Google Scholar]
- West S, Taylor HR. Community-based intervention programs for trachoma control. International Ophthalmology. 1988;12(1):19–23. doi: 10.1007/BF00133776. [DOI] [PubMed] [Google Scholar]
- West S, Lynch M, Turner V, Munoz B, Rapoza P, Mmbaga BB, et al. Water availability and trachoma. Bulletin of the World Health Organization. 1989;67(1):71–5. [PMC free article] [PubMed] [Google Scholar]
- West SK, Munoz B, Lynch M, Kayongoya A, Mmbaga BB, Taylor HR. Risk factors for constant, severe trachoma among preschool children in Kongwa, Tanzania. American Journal of Epidemiology. 1996;143(1):73–8. doi: 10.1093/oxfordjournals.aje.a008659. [DOI] [PubMed] [Google Scholar]
- Bailey R, Lietman T. The SAFE strategy for the elimination of trachoma by 2020: will it work?. Bulletin of the World Health Organization. 2001;79(3):233–6. [PMC free article] [PubMed] [Google Scholar]
- Ejere HOD, Alhassan MB, Rabiu M. Face washing promotion for preventing active trachoma. Cochrane Database of Systematic Reviews. Issue. 2004;3 doi: 10.1002/14651858.CD003659.pub2. [DOI: 10.1002/14651858.CD003659.pub2] [DOI] [PubMed] [Google Scholar]
- Emerson PM, Cairncross S, Bailey RL, Mabey DC. Review of the evidence base for the ’F’ and ’E’ components of the SAFE strategy for trachoma control. Tropical Medicine and International Health. 2000;5(8):515–27. doi: 10.1046/j.1365-3156.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Evans JR, Solomon AW. Antibiotics for trachoma. Cochrane Database of Systematic Reviews. 2011;(3) doi: 10.1002/14651858.CD001860.pub3. [DOI: 10.1002/14651858.CD001860.pub3] [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Grassly NC, Ward ME, Ferris S, Mabey DC, Bailey RL. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS Neglected Tropical Diseases. 2008;2(21):e341. doi: 10.1371/journal.pntd.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Higgins JPT, Deeks JJ, Altman DG, Higgins JPT, Green S, editors. Chapter 16: Special topics in statistics. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Mariotti SP, Pruss A. Preventing trachoma. A guide for environmental sanitation and improved hygiene. [19 November 2002];The SAFE strategy. http://whqlibdoc.who.int/hq/2000/WHO.PBD.GET.00.7.pdf. [Google Scholar]
- Prost A, Negrel AD. Water, trachoma and conjunctivitis. Bulletin of the World Health Organization. 1989;67(1):9–18. [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bulletin of the World Health Organization. 1987;65(4):477–83. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Blindness and visual disability. Part II of VII: major causes worldwide. [10 October 2002]; apps.who.int/inf-fs/en/fact144.html. [Google Scholar]
- World Health Organization Future approaches to trachoma control. [10 October 2002];Report of a global scientific meeting. whqlibdoc.who.int/hq/1996/WHO.PBL.96.56.pdf.
- Rabiu M, Alhassan M, Ejere H. Environmental sanitary interventions for preventing active trachoma. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD004003.pub2. [DOI: 10.1002/14651858.CD004003.pub2] [DOI] [PubMed] [Google Scholar]
- Rabiu M, Alhassan MB, Ejere HOD. Environmental sanitary interventions for preventing active trachoma. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD004003.pub3. [DOI: 10.1002/14651858.CD004003.pub3] [DOI] [PubMed] [Google Scholar]