Abstract
BACKGROUND AND PURPOSE
Posterior fossa syndrome is a severe postoperative complication occurring in up to 29% of children undergoing posterior fossa tumor resection; it is most likely caused by bilateral damage to the proximal efferent cerebellar pathways, whose fibers contribute to the triangle of Guillain and Mollaret. When the triangle is disrupted, hypertrophic olivary degeneration develops. We hypothesized that MRI patterns of that reflect patterns of damage to the proximal efferent cerebellar pathways and show association with clinical findings, in particular the presence or absence of posterior fossa syndrome.
MATERIALS AND METHODS
We performed blinded, randomized longitudinal MRI analyses of the inferior olivary nuclei of 12 children with and 12 without posterior fossa syndrome after surgery for midline intraventricular tumor in the posterior fossa. Fisher’s exact test was performed to investigate the association between posterior fossa syndrome and hypertrophic olivary degeneration on MRI. Sensitivity and specificity of MRI findings of bilateral hypertrophic olivary degeneration for posterior fossa syndrome were measured.
RESULTS
Of the 12 patients with posterior fossa syndrome, 9 had bilateral inferior olivary nucleus abnormalities. The 12 patients without posterior fossa syndrome had either unilateral or no inferior olivary nucleus abnormalities. The association of posterior fossa syndrome and hypertrophic olivary degeneration was statistically significant (P < .0001).
CONCLUSIONS
Hypertrophic olivary degeneration may be a surrogate imaging indicator for damage of the contralateral proximal efferent cerebellar pathway. In the appropriate clinical setting, bilateral hypertrophic olivary degeneration may be a sensitive and specific indicator of posterior fossa syndrome.
INTRODUCTION
Posterior fossa syndrome (PFS), a complication of posterior fossa surgery 1, 2, occurs in 11%–29% of patients undergoing posterior fossa tumor resection 3. Although the definition of the “all-inclusive” PFS is broad and comprises complex neurobehavioral and motor symptoms, cerebellar mutism is at the core of the diagnosis 4.
Growing evidence suggests that PFS is the result of bilateral damage to the proximal efferent cerebellar pathways (pECP) along the dentato-rubro-thalamo-cortical pathway 5–13. This relationship was initially observed as cerebellar mutism after stereotactic ablation of the bilateral dentate nuclei 14. Reversed cerebello-cerebral diaschisis, in which deprivation of the cerebral cortex from cerebellar input due to bilateral pECP damage results in a frontally predominant drop of cerebral cortical perfusion, has been proposed to be the mechanism of PFS, and cerebellar mutism is thought to be a form of speech apraxia9. During the months following surgery speech and the associated neurological deficits usually improve, but those rarely if ever completely normalize, which suggests a profound disturbance of complex neural systems with significant implications for the long-term quality of life of the steadily increasing number of survivors15
Damage anywhere along the dentato-rubro-thalamo-cortical pathway may lead to a speech disorder, and that to the dentate nuclei in particular has repeatedly been cited as being a cause of cerebellar mutism 5, 10, 16, 17, which can occur after injury along the superior cerebellar peduncles 8, brachium pontis/conjunctivum 6, 18, bilateral thalamic tracts 11, or the frontal lobes 19–21. Given that the dentate nuclei, superior cerebellar peduncles, and mesencephalic tegmental decussation often lie adjacent to and may, therefore, be invaded by midline intraventricular posterior fossa tumors, these structures are the ones most prone to injury during aggressive tumor resection.
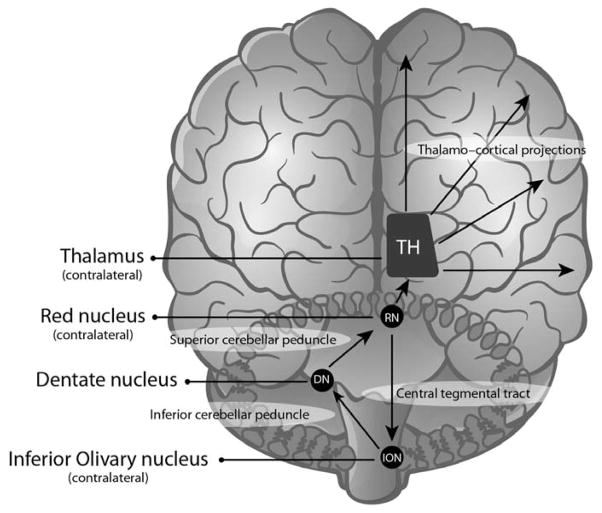
The efferent cerebellar tracts that pass through the superior cerebellar peduncles 3 are adjacent to and involve fibers associated with the dentato-rubral segment of the Guillain-Molaret triangle (GMT) 22. The GMT is composed of an ipsilateral red nucleus and inferior olivary nucleus (ION) that are connected by the central tegmental tract and a contralateral dentate nucleus that is connected through the superior (dentato-rubral) and inferior cerebellar (dentato-olivary) peduncles (Figure 1).
FIG 1.
The Guillain-Mollaret triangle and dentato-rubro-thalamo-cortical projections.
Disrupting the GMT leads to degeneration of the ION, 23–26 resulting in visible changes in both pathologic evaluation and MRI findings 27, 28. Specifically, damage to the dentate nucleus, superior cerebellar peduncle, or both lead to contralateral hypertrophic olivary degeneration (HOD), but damage to the tegmental tracts leads to ipsilateral HOD.
The purpose of this study was to determine whether MRI findings of children with PFS could be used to validate the clinical diagnosis of PFS. We hypothesized that patterns of hypertrophic degeneration in the inferior olivary nuclei reflect patterns of damage to the pECPs and that these patterns would show association with clinical findings, in particular with the presence or absence of PFS.
MATERIALS AND METHODS
Patients and Study Design
We retrospectively evaluated ION changes on MRI of children with and without PFS after posterior fossa surgery for midline intraventricular tumor. Patients were selected from an Institutional Review Board–approved, prospectively designed clinical trial of patients with newly diagnosed medulloblastoma, supratentorial primitive neuroectodermal, or atypical teratoid rhabdoid tumors. Patient/guardian consent was obtained prior to enrollment in the clinical trial. A total of 24 patients were selected: All patients were boys, and their mean age was 9 ± 3.2 years. Of the 24 patients, 12 had a recorded clinical diagnosis of PFS, which required the presence of complete mutism in the early postoperative period that lasted at least 24 hours and was not explained by any other cause such as medication. For comparison, 12 age- and gender-matched patients without PFS were selected as controls. All patients, regardless of clinical PFS status, were evaluated for associated cerebellar signs and symptoms. Of the 12 patients with PFS, 11 had medulloblastoma, and 1 had an atypical teratoid rhabdoid tumor. Of the 12 patients without PFS, all had medulloblastomas.
MR Imaging
Postoperative follow-up studies included standardized axial long TR imaging (proton density and T2-weighted), in addition to multiplanar non-enhanced and contrast enhanced T1-weighted, axial contrast-enhanced FLAIR and diffusion-weighted imaging. The geometrical parameters for the long-TR imaging sequences (used for the evaluation of the ION) were identical: number of slices=31, slice thickness=5mm, FoV read=210mm, FoV phase=100%. Other parameters for the proton density and T2-weighted sequence were: TR=4500ms, TE1=13ms, TE2=105ms, turbo factor=5, bandwidth=98Hz/Px, and for FLAIR: TR=10000ms, TI=2500ms, TE=103ms, turbo factor=21, bandwidth=130Hz/Px In compliance with protocol requirements, all studies were performed on 1.5T magnets (MAGNETOM Avanto, Siemens, Erlangen, Germany) at the same center using a 4-channel, circularly polarized head array coil. Average follow up was 38 ± 20 months and each patient had an average of 14 ± 5 follow up studies.
Image Analysis
Blinded, randomized analysis of MR images was performed by a senior attending neuroradiologist. Specific attention was given to the bilateral IONs on long TR images. The IONs were evaluated for volume and signal changes independently. Abnormality was deemed to represent HOD if it met the following criteria: 1) both hypertrophy and signal changes (T2 prolongation) were present; 2) the hypertrophic component was modest or absent, but the signal changes were unequivocal. The findings within each ION for both T2- and proton density-weighted sequences were classified as follows: 0 = No visible abnormality; 1 = Questionable abnormally high signal within the hilum or entire nucleus with or without volume changes; or 2 = Definite abnormally high signal within the hilum or entire nucleus with or without volume changes. A score of 2 at any point during the postoperative follow-up qualified the respective ION for HOD status. Patients were subsequently categorized as having definite bilateral HOD, definite unilateral HOD, or no HOD.
Statistical analysis
Statistical analysis was performed by using SAS 9.2 software (SAS Institute, Cary, NC). Fisher’s exact test was performed to investigate the association of HOD-related MRI abnormalities with PFS. The sensitivity and specificity of HOD as an indicator of PFS were calculated by using the clinical diagnosis of PFS as the gold standard (true-positive).
RESULTS
Of the 12 patients with clinically diagnosed PFS, 9 had bilateral HOD on MRI; 2 had definitive unilateral HOD changes (1 with questionable contralateral involvement), and 1 had no abnormal MRI findings that were consistent with HOD. Conversely, 10 of the 12 patients without PFS had no HOD-suggestive findings on MRI, and 2 had unilateral HOD. In cases of unilateral HOD, patients with PFS had HOD on the right side, and patients without PFS had on the left. The earliest time definite bilateral HOD was seen in our patients was 1 month from surgery and the longest interval between surgery and the appearance of definite bilateral HOD was 5.5 months (mean 3.5 months).
The clinical features of all 24 patients are summarized in Table 1. All 12 patients with postoperative mutism had severe cerebellar syndrome with dysmetria and ataxia. All non-PFS patients had some degree of cerebellar dysfunction without mutism, and 5 were quite severely affected. The 2 patients with clinical PFS but only unilateral HOD had mild postoperative dysmetria and severe ataxia. The 2 patients who had unilateral HOD without clinical PFS had mild postoperative dysmetria and mild ataxia.
Table 1.
Clinical features of patients with and without posterior fossa syndrome
| Variable | PFS (n=12) | Non-PFS (n=12) |
|---|---|---|
|
| ||
| Mutism >24 hours | 12 | 0 |
|
| ||
| Immediate post-operative | ||
| Mild to moderate dysmetria | 7 | 2 |
| Severe dysmetria | 5 | 0 |
| Mild to moderate ataxia | 0 | 7 |
| Severe ataxia | 12 | 2 |
|
| ||
| At last follow-up* | ||
| Mild to moderate dysmetria | 2 | 0 |
| Severe dysmetria | 1 | 0 |
| Mild to moderate ataxia | 7 | 5 |
| Severe ataxia | 5 | 1 |
The facts that 100% of patients with bilateral HOD changes had PFS, and 0% of patients without PFS had such changes suggest that the presence of bilateral HOD is associated with PFS. The results of Fisher’s exact test confirmed the strong association between bilateral HOD and clinical PFS (p <.0001). These findings also indicate a false-positive MRI rate of 0% (0/9) for bilateral HOD, as all patients with bilateral HOD indeed had PFS. The false-negative MRI rate was 25% (3/12). In this clinical setting, MRI findings of bilateral HOD were 75%-sensitive and 100%-specific for the diagnosis of PFS. Using bilateral HOD as a putative indicator of PFS, the positive predictive value of bilateral HOD is 100%, and the negative predictive value of the absence of HOD is 80%.
DISCUSSION
As previously stated, damage to the bilateral pECPs is the most widely accepted cause of postoperative PFS and cerebellar mutism 5, 8–12. Because of the anatomic overlap between the first segments of the dentato-rubro-thalamo-cortical pathway and the GMT, damage to the pECP leads to contralateral HOD 23–28. Therefore, damage to the bilateral pECPs should lead to the development of bilateral HOD by MRI, which may serve as a delayed surrogate imaging indicator of PFS. Indeed, the 100%-positive predictive value yields a high confidence that a positive result (obtained by using bilateral HOD as a classifier) is truly indicative of PFS.
Although bilateral HOD has previously been associated with linguistic pathway abnormalities 29–32, we could not find any report of cerebellar mutism and linguistic abnormalities attributed to bilateral damage of the pECPs and with subsequent HOD in the ION. Most of the described cases in which unilateral HOD was observed on imaging do not have associated linguistic category findings 33–37 although this was described in at least one study 38.
Bilateral HOD was not present in every case of clinically diagnosed PFS in our cohort. This divergence could be due to inconsistencies in clinical diagnostic criteria because, despite all ongoing efforts and research, the diagnosis of PFS is sometimes still a judgment call, dependent on the investigator’s experience. Our criteria of prolonged mutism and severe associated cerebellar syndrome with ataxia were used to differentiate PFS from other causes of postoperative speech disorders, including damage to lower cranial nerves 13, psychological issues 39, and medication-induced deficits. Indeed, at least 1 of our patients with PFS without bilateral HOD on MRI was described in the medical records as having immediate mutism following anesthesia recovery, a presentation that is not typical of cerebellar mutism, which generally appears an average of 1.7 days after surgery 3, 5, 40. In fact, immediate onset of mutism may indicate a bulbar abnormality from direct, inadvertent surgical injury to lower cranial nerve nuclei 13, rather than true cerebellar mutism in the context of PFS. We, therefore, recognize the challenges of differentiating cerebellar mutism from other forms of transient speech arrests in these patients during the postoperative period when other compounding clinical considerations occur concurrently.
Another reason that bilateral HOD was not always present in patients with PFS is that surgical damage is not necessarily a binary, all-or-none phenomenon. In other words, the magnitude of damage to the pECP that is needed to cause a critical drop of cerebellar input to the supratentorial brain may be different from the amount of damage leading later to visible MRI changes of HOD. For example, one may hypothesize that the majority of the bilateral pECP fibers need to be destroyed to result in bilateral HOD but somewhat less damage may be sufficient to cause a global frontal lobe dysfunction and resultant speech apraxia (i.e., cerebellar mutism). Indeed, the 2 mechanisms causing PFS and HOD are likely different: one leading to frontal lobe dysfunction and mutism through reversed cerebello-cerebral diaschisis, the other to a peculiar form of degeneration associated with initial hypertrophy through a trans-synaptic mechanism with yet poorly understood neurological correlates. Another unique clinical aspect of bilateral HOD in our patient cohort was the absence of palatal myoclonus in all patients, although this neurological sign is commonly seen to be associated with HOD. The reason for this discrepancy is unclear. However, given that our cohort’s lesions occurred in the specific circumstance of posterior fossa surgery and consistently in a specific location (i.e., pECP), but others in the literature often involved injury to the second, descending (rubro-olivary) segment of the GMT, an unidentified anatomical explanation may exist, such as involvement of brainstem structures immediately adjacent to the descending tegmental tracts in the development of palatal myoclonus.
The MRI signs and evolution of HOD-type changes are well described, with pathologic evaluation first revealing 6 distinct stages after destruction of the central tegmental tracts. These were described as no olivary changes (within 24 hours), olivary amiculum degeneration (typically 2–7 days), olivary hypertrophy (approximately 3 weeks), culminant olivary enlargement (approximately 8.5 months), olivary pseudohypertrophy (9.5 months), and olivary atrophy (after a few years) 28. These findings were later classified into 3 stages on the basis of MRI of other cases and analysis of cases in the literature 27, 34. The first is seen with T2 and proton density signal hyperintensity without hypertrophy and may occur as early as 4 weeks after injury. The second stage demonstrates hypertrophy starting about 4 months after injury extending until hypertrophy resolves approximately 3–4 years later. The third and final stage exhibits resolved hypertrophy with persistent high T2 and proton density signal intensity and is noted to persist indefinitely. These concur with our observations.
We were fortunate that our retrospective analysis was performed on patients whose clinical imaging protocol had included both axial T2 and proton density imaging. The proton density sequence was often the most conclusive imaging sequence, especially on the earlier postoperative follow-up studies, as previously reported by others 41. However, our imaging protocol was not optimized to evaluate the ION, an area which is somewhat difficult to visualize on routine imaging and is often prone to artifacts. Our protocol was also limited by slice thickness, which was routinely 5 mm. Because the olivary body measures approximately 1.25 cm, with the ION being in the bottom portion, only 1 or 2 slices could generally be obtained to evaluate the region-of-interest on traditional 5-mm imaging with the possibility of significant partial volume averaging effects.
Our finding of unilateral HOD in patients with and without PFS may be due to the proposed process of one cerebellar hemisphere normally providing asymmetrical input toward speech. There is no consensus in the literature as to which side provides more input, with an earlier paper implicating the left cerebellar hemisphere, 42 and more recent papers implicating the right 43–47. In cases in which we detected unilateral HOD, patients with PFS had unilateral right HOD, and patients without PFS had unilateral left HOD. Other papers describe inconclusive findings 38, 48. Given the inconsistency between literature reports, it is possible that inter-individual variations determine the dominant cerebellar hemisphere for speech and language production. Alternatively, complete damage to the dominant side may lead to PFS even if damage to the subdominant pECP is partial, hence insufficient to cause corresponding HOD, which would explain the few exceptions of clinical-imaging discrepancies (i.e., clinical PFS syndrome in conjunction with unilateral HOD only).
Although bilateral HOD is not 100%-sensitive for detecting PFS, these findings suggest that a threshold of damage to the pECPs is required for both MRI and clinical perceptibility. Future studies evaluating the degree of damage to the pECPs and defining PFS diagnostic criteria in a larger patient cohort with detailed clinical evaluation will be the next step in understanding this complex syndrome. Our data demonstrate that changes of HOD on MRI may be used in the future as an objective, although a posteriori, criterion in the diagnosis of PFS and help clarify the definition of the clinical syndrome. Future evaluation will include prospective analysis of a larger cohort of posterior fossa surgery patients who have PFS, with more robust diagnostic information (optimized imaging of the GMT and pECPs) and structured clinical information acquired and evaluated by experienced investigators in order to formulate standardized clinical and imaging diagnostic criteria for this complex, challenging syndrome.
CONCLUSION
Damage to the pECP manifests as contralateral HOD through disruption of the GMT and subsequent trans-synaptic degeneration. We provide objective imaging data demonstrating that damage to the bilateral pECPs manifests as bilateral HOD. Our false-positive MRI rate for bilateral HOD was 0%; hence, bilateral HOD may be a delayed, but reliable surrogate imaging marker for PFS. Therefore, MRI findings, especially those obtained by using imaging protocols that are optimized for the visualization of the ION, can be useful criteria in the diagnosis of PFS as a more robust definition is developed. Our findings contribute to the growing body of evidence supporting the role of bilateral pECP damage in the pathogenesis of postoperative PFS.
FIG 2.
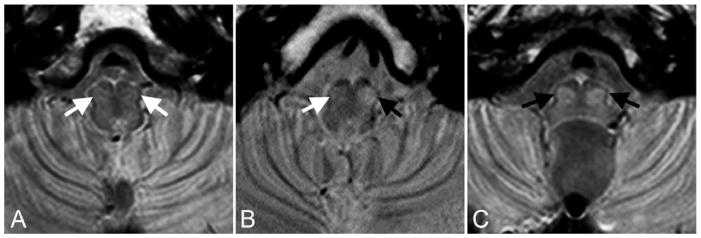
Transverse proton density–weighted images illustrating the MRI appearance of (A) normal IONs (white arrows), (B) unilateral (black arrow) and (C) bilateral HOD (black arrows) in patients approximately 4 months after surgery for midline posterior fossa tumors. Note that with this imaging technique normal IONs (white arrows) are barely recognizable, but in abnormal conditions their conspicuity is markedly increased allowing for confident identification of HOD in most cases.
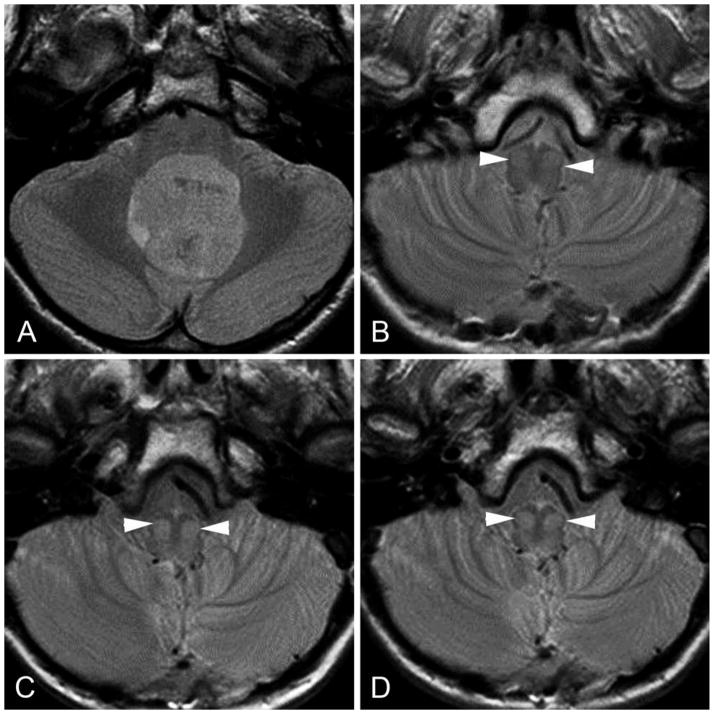
FIG 3.
Transverse proton density–weighted images showing chronological changes (increasing conspicuity) within the bilateral inferior olivary nuclei (white arrowheads) in a patient with PFS during the first year after surgery. A, preoperative study. B, at 1-month follow-up. C, at 6-months follow-up. D, at 10-months follow-up.
Acknowledgments
The authors thank Cherise M. Guess, PhD, ELS for reviewing and editing the manuscript.
ABBREVIATIONS
- GMT
Guillain-Mollaret triangle
- HOD
hypertrophic olivary degeneration
- ION
inferior olivary nucleus
- pECP
proximal efferent cerebellar pathway
- PFS
posterior fossa syndrome
References
- 1.Hirsch JF, Renier D, Czernichow P, et al. Medulloblastoma in childhood. Survival and functional results. Acta Neurochir (Wien) 1979;48:1–15. doi: 10.1007/BF01406016. [DOI] [PubMed] [Google Scholar]
- 2.Stein BM, Fraser RA, Tenner MS. Normal pressure hydrocephalus: complication of posterior fossa surgery in children. Pediatrics. 1972;49:50–58. [PubMed] [Google Scholar]
- 3.Gudrunardottir T, Sehested A, Juhler M, et al. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27:355–363. doi: 10.1007/s00381-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 4.Gudrunardottir T, Sehested A, Juhler M, et al. Cerebellar mutism: definitions, classification and grading of symptoms. Childs Nerv Syst. 2011;27:1361–1363. doi: 10.1007/s00381-011-1509-7. [DOI] [PubMed] [Google Scholar]
- 5.Ersahin Y, Mutluer S, Cagli S, et al. Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery. 1996;38:60–65. doi: 10.1097/00006123-199601000-00015. discussion 66. [DOI] [PubMed] [Google Scholar]
- 6.Pollack IF, Polinko P, Albright AL, et al. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37:885–893. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123 (Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- 8.Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132:3087–3095. doi: 10.1093/brain/awp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol. 2010;31:288–294. doi: 10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puget S, Boddaert N, Viguier D, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009;115:1338–1347. doi: 10.1002/cncr.24150. [DOI] [PubMed] [Google Scholar]
- 11.Crutchfield JS, Sawaya R, Meyers CA, et al. Postoperative mutism in neurosurgery. Report of two cases. J Neurosurg. 1994;81:115–121. doi: 10.3171/jns.1994.81.1.0115. [DOI] [PubMed] [Google Scholar]
- 12.Koh S, Turkel SB, Baram TZ. Cerebellar mutism in children: report of six cases and potential mechanisms. Pediatr Neurol. 1997;16:218–219. doi: 10.1016/s0887-8994(97)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105:444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 14.Fraioli B, Guidetti Effects of stereotactic lesions of the dentate nucleus of the cerebellum in man. Appl Neurophysiol. 1975;38:81–90. doi: 10.1159/000102647. [DOI] [PubMed] [Google Scholar]
- 15.Steinbok P, Cochrane DD, Perrin R, et al. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39:179–183. doi: 10.1159/000072468. [DOI] [PubMed] [Google Scholar]
- 16.Kusano Y, Tanaka Y, Takasuna H, et al. Transient cerebellar mutism caused by bilateral damage to the dentate nuclei after the second posterior fossa surgery. Case report. J Neurosurg. 2006;104:329–331. doi: 10.3171/jns.2006.104.2.329. [DOI] [PubMed] [Google Scholar]
- 17.Rekate HL, Grubb RL, Aram DM, et al. Muteness of cerebellar origin. Arch Neurol. 1985;42:697–698. doi: 10.1001/archneur.1985.04060070091023. [DOI] [PubMed] [Google Scholar]
- 18.Pollack IF. Posterior fossa syndrome. Int Rev Neurobiol. 1997;41:411–432. doi: 10.1016/s0074-7742(08)60362-1. [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 20.Nagaratnam N, Nagaratnam K, Ng K, et al. Akinetic mutism following stroke. J Clin Neurosci. 2004;11:25–30. doi: 10.1016/j.jocn.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Tahta K, Cirak B, Pakdemirli E, et al. Postoperative mutism after removal of an anterior falcine meningioma. J Clin Neurosci. 2007;14:793–796. doi: 10.1016/j.jocn.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Guillain GMP. Deux de myoclonies synchrones et rhythmees velopharyngolaryngo-oculo-dia-phragmatiques. Rev Neurol. 1931;12:545–566. [Google Scholar]
- 23.Gautier JC, Blackwood W. Enlargement of the inferior olivary nucleus in association with lesions of the central tegmental tract or dentate nucleus. Brain. 1961;84:341–361. doi: 10.1093/brain/84.3.341. [DOI] [PubMed] [Google Scholar]
- 24.Jellinger K. Hypertrophy of the inferior olives. Report on 29 cases. Z Neurol. 1973;205:153–174. doi: 10.1007/BF00316018. [DOI] [PubMed] [Google Scholar]
- 25.Krings T, Foltys H, Meister IG, et al. Hypertrophic olivary degeneration following pontine haemorrhage: hypertensive crisis or cavernous haemangioma bleeding? J Neurol Neurosurg Psychiatry. 2003;74:797–799. doi: 10.1136/jnnp.74.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchino A, Hasuo K, Uchida K, et al. Olivary degeneration after cerebellar or brain stem haemorrhage: MRI. Neuroradiology. 1993;35:335–338. doi: 10.1007/BF00588362. [DOI] [PubMed] [Google Scholar]
- 27.Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol. 2000;21:1073–1077. [PMC free article] [PubMed] [Google Scholar]
- 28.Goto N, Kaneko M. Olivary enlargement: chronological and morphometric analyses. Acta Neuropathol. 1981;54:275–282. doi: 10.1007/BF00697000. [DOI] [PubMed] [Google Scholar]
- 29.Gerace C, Fele MR, Luna R, et al. Neurological picture. Bilateral hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry. 2006;77:73. doi: 10.1136/jnnp.2005.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieder CR, Reboucas RG, Ferreira MP. Holmes tremor in association with bilateral hypertrophic olivary degeneration and palatal tremor: chronological considerations. Case report. Arq Neuropsiquiatr. 2003;61:473–477. doi: 10.1590/s0004-282x2003000300028. [DOI] [PubMed] [Google Scholar]
- 31.Vaidhyanath R, Thomas A, Messios N. Bilateral hypertrophic olivary degeneration following surgical resection of a posterior fossa epidermoid cyst. Br J Radiol. 2010;83:e211–215. doi: 10.1259/bjr/27446907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shepherd GM, Tauboll E, Bakke SJ, et al. Midbrain tremor and hypertrophic olivary degeneration after pontine hemorrhage. Mov Disord. 1997;12:432–437. doi: 10.1002/mds.870120327. [DOI] [PubMed] [Google Scholar]
- 33.Akar S, Drappatz J, Hsu L, et al. Hypertrophic olivary degeneration after resection of a cerebellar tumor. J Neurooncol. 2008;87:341–345. doi: 10.1007/s11060-008-9523-7. [DOI] [PubMed] [Google Scholar]
- 34.Birbamer G, Buchberger W, Kampfl A, et al. Early detection of post-traumatic olivary hypertrophy by MRI. J Neurol. 1993;240:407–409. doi: 10.1007/BF00867352. [DOI] [PubMed] [Google Scholar]
- 35.Macht S, Hanggi D, Turowski B. Hypertrophic olivary degeneration following pontine cavernoma hemorrhage: a typical change accompanying lesions in the Guillain-Mollaret triangle. Klin Neuroradiol. 2009;19:235–237. doi: 10.1007/s00062-009-9001-4. [DOI] [PubMed] [Google Scholar]
- 36.Phatouros CC, McConachie NS. Hypertrophic olivary degeneration: case report in a child. Pediatr Radiol. 1998;28:830–831. doi: 10.1007/s002470050475. [DOI] [PubMed] [Google Scholar]
- 37.Salamon-Murayama N, Russell EJ, Rabin BM. Diagnosis please. Case 17: hypertrophic olivary degeneration secondary to pontine hemorrhage. Radiology. 1999;213:814–817. doi: 10.1148/radiology.213.3.r99dc43814. [DOI] [PubMed] [Google Scholar]
- 38.Ash L, Srinivasan A. Case of the season: hypertrophic olivary degeneration. Semin Roentgenol. 2008;43:171–172. doi: 10.1053/j.ro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Ferrante L, Mastronardi L, Acqui M, et al. Mutism after posterior fossa surgery in children. Report of three cases. J Neurosurg. 1990;72:959–963. doi: 10.3171/jns.1990.72.6.0959. [DOI] [PubMed] [Google Scholar]
- 40.Gelabert-Gonzalez M, Fernandez-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg. 2001;103:111–114. doi: 10.1016/s0303-8467(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 41.Yokota T, Hirashima F, Furukawa T, et al. MRI findings of inferior olives in palatal myoclonus. J Neurol. 1989;236:115–116. doi: 10.1007/BF00314408. [DOI] [PubMed] [Google Scholar]
- 42.Lechtenberg R, Gilman S. Speech disorders in cerebellar disease. Ann Neurol. 1978;3:285–290. doi: 10.1002/ana.410030402. [DOI] [PubMed] [Google Scholar]
- 43.Gebhart AL, Petersen SE, Thach WT. Role of the posterolateral cerebellum in language. Ann N Y Acad Sci. 2002;978:318–333. doi: 10.1111/j.1749-6632.2002.tb07577.x. [DOI] [PubMed] [Google Scholar]
- 44.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 45.Baillieux H, De Smet HJ, Paquier PF, et al. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Ackermann H, Mathiak K, Ivry RB. Temporal organization of “internal speech” as a basis for cerebellar modulation of cognitive functions. Behav Cogn Neurosci Rev. 2004;3:14–22. doi: 10.1177/1534582304263251. [DOI] [PubMed] [Google Scholar]
- 47.Fiez JA, Petersen SE, Cheney MK, et al. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(Pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 48.Bruno MK, Wooten GF. Hypertrophic olivary degeneration. Arch Neurol. 2012;69:274–275. doi: 10.1001/archneurol.2011.657. [DOI] [PubMed] [Google Scholar]