Abstract
Background
Temporary interruption of oral anticoagulation (OAC) for procedures is often required and some propose using bridging anticoagulation. However, the use and outcomes of bridging during OAC interruptions in clinical practice are unknown.
Methods and Results
The ORBIT-AF registry is a prospective, observational registry study of US outpatients with AF. We recorded incident temporary interruptions of OAC for a procedure, including use and type of bridging therapy. Outcomes included multivariable-adjusted rates of myocardial infarction (MI), stroke or systemic embolism (SSE), major bleeding, cause-specific hospitalization, and death within 30 days. Of 7,372 patients treated with OAC, 2,803 overall interruption events occurred in 2,200 patients (30%) at median follow-up of 2 years. Bridging anticoagulants were used in 24% (n=665), predominantly with low-molecular weight heparin (73%, n=487) and unfractionated heparin (15%, n=97). Bridged patients more likely had prior cerebrovascular events (22% vs. 15%, p=0.0003) and mechanical valve replacements (9.6% vs. 2.4%, p<0.0001); however there was no difference in CHA2DS2-VASc scores (94% ≥2 vs. 95%, p=0.5). Bleeding events were more common in bridged patients than non-bridged (5.0% vs. 1.3%, adjusted OR 3.84, p<0.0001). Incidence of MI, SSE, major bleeding, hospitalization, or death within 30 days was also significantly more common in patients receiving bridging (13% vs. 6.3%, adjusted OR 1.94, p=0.0001).
Conclusions
Bridging anticoagulation is used in one-quarter of anticoagulation interruptions and is associated with higher risk for bleeding and adverse events. These data do not support the use of routine bridging and additional data are needed to identify best practices around anticoagulation interruptions.
Keywords: atrial fibrillation, anticoagulation, outcomes research, temporary interruption, bridging
Oral anticoagulation (OAC) significantly reduces the risk of stroke in patients with atrial fibrillation (AF). However, many AF patients on chronic anticoagulation undergo procedures that require temporary interruption of OAC.1, 2 Some have advocated that patients receive short-acting anticoagulants during these temporary interruptions to ‘bridge’ the patient and potentially reduce the risk of embolic events during the interruption.3 While guidelines have been published regarding when and how to initiate bridging therapy,4 they are based on limited data. Thus it remains unclear as to whether patients who temporarily interrupt their anticoagulation should receive bridging anticoagulation or not.
We assessed the incidence of temporary interruption of oral anticoagulation for procedures among a national, outpatient AF registry. We specifically examined (1) causes for interruption of anticoagulation; (2) the patterns of use of bridging anticoagulation agents (relative to underlying risk and current guidelines); and (3) the outcomes among patients who were bridged versus not bridged.
Methods
The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORIBT-AF) is a national, community-based registry of outpatients with AF. Eligible patients were enrolled by nationally-representative sample primary care, cardiology, and/or electrophysiology sites. An adaptive design was used to ensure heterogeneity of practice-type and geography. Study coordination was managed the Duke Clinical Research Institute. Major inclusion criteria were age 18 years or older and electrocardiographically-documented AF that was not due to a reversible cause and follow-up was out to a maximum of 3 years. The ORBIT-AF registry has been described in detail previously.5 The present analysis includes patient data out to 2 years of follow-up.
Data collection was primarily derived from the patient's medical record, and included demographics, medical history, and AF history at baseline. Additionally, at baseline and every 6 months, investigators recorded medical and surgical therapies, vital signs, laboratory measurements, and echocardiographic data. The collection of medication data included use and monitoring of OAC therapies. Sites were also instructed to enter which OAC treatment was used, as well as values for international normalized ratio (INR) monitoring, where applicable. At each follow-up, investigators were queried as to whether the patient temporarily interrupted their OAC in order to undergo a procedure. Only interruptions for procedures were recorded; interruptions due to bleeding or other reasons are not captured. All medical management around the procedure was guided entirely by the patient's treatment team. For such interruptions we collected: the date and type of procedure; use of bridging anticoagulant (defined as an anticoagulant temporarily administered in place of chronic therapy, for the purpose of stroke prevention before, during or after the periprocedural period); and adverse events occurring during the interruption (bleeding event, thrombotic event, or other event; no further specification was reported). Type of procedure was categorized as cardiac catheterization, catheter ablation, endoscopy (gastrointestinal, bronchoscopic, or genitourinary), cardiac surgery, non-cardiac surgery (not further specified), device implantation, dental procedures, or other (not further specified). Bridging anticoagulant was categorized as low-molecular weight heparin (LMWH), unfractionated heparin (UFH), fondaparinux, or other (not further specified).
Separately at each follow-up, investigators recorded the incidence and dates of any adverse events, including death, cause-specific hospitalization (cardiovascular, bleeding, or other, as determined by the investigator), incident heart failure, myocardial infarction (MI), stroke or systemic embolism (adjudicated by the coordinating center, from primary source documentation), or major bleeding as defined by the International Society of Thrombosis and Haemostasis criteria.6
Analyzing Temporary Interruptions
The present analysis included only patients on an oral anticoagulant at baseline, and who had at least 1 follow-up visit. The study population was subsequently divided by incidence of interruption during follow-up: none versus any (≥1). The baseline characteristics of these patients were compared.
Subsequently, all interruption events were queried for the type of procedure requiring interruption and use of bridging anticoagulant. Additionally, the use of bridging anticoagulation was compared among high-risk subgroups. Among patients using warfarin, time to resumption of therapeutic INR (≥2) was calculated. The use of bridging anticoagulation in the subgroup of patients receiving dabigatran was also described.
Adverse events occurring during the interruption of chronic anticoagulation (bleeding, thrombotic, or other [not further detailed]) are described, and stratified by use of any bridging anticoagulant versus none. The incidence and timing of adverse events occurring within 30 days after the date of the procedure for which there was an interruption are also described (and may overlap with those occurring during interruption); these include cause specific hospitalization, and the composite of MI, stroke, major bleeding, hospitalization, or death. The association of bridging with adverse events was assessed in a multivariable model of the composite outcome.
Statistical Methods
Comparisons between groups with no interruption versus any interruption are performed at the patient level. Comparisons between procedure types, bridging anticoagulant, and adverse events are performed at the interruption level (a patient may have had more than 1 interruption during follow-up). In univariate analyses, categorical variables are presented as frequencies and percentages, and differences between 2 groups are assessed by the Chi-Square test. Continuous variables are presented as median (Q1-Q3) or mean (standard deviation) and differences between 2 groups are assessed by the Wilcoxon rank sum test.
In analysis of adverse events within 30 days following interruption, multiple interruption events from the same patient were included unless the interruptions occurred within 30 days of a prior interruption. However, interruption events were excluded if the date was missing. In order to identify the association between use of any bridging anticoagulant and adverse events, a multivariable model was developed. Co-variates included age, estimated glomerular filtration rate, sex, prior cerebrovascular events, the presence of significant valvular disease or prior mechanical valve replacement, prior gastrointestinal bleeding, the presence of congestive heart failure, type of AF at baseline (new onset, paroxysmal, persistent, longstanding persistent), left atrial diameter size, patient level of education, CHADS2 score, procedure requiring interruption (with non-cardiac surgery as the referent), and type of oral anticoagulation at baseline (warfarin versus dabigatran; neither rivaroxaban nor apixaban was used in this cohort). The outcomes examined included: any bleeding events (major bleeding or bleeding hospitalization); cardiovascular events (stroke, systemic embolism, MI, or cardiovascular hospitalization); and the composite of any MI, stroke or systemic embolism, any hospitalization, or death, all within 30 days following the date of the procedure requiring interruption. Adjusted odds ratios were calculated using logistic regression with generalized estimating equation, which also accounted for correlations within the same patient.
The ORBIT-AF registry was approved by the institutional review board of Duke University, and each site received institutional review board approval subject to local requirements. All patients signed written, informed consent and analyses of the aggregate, de-identified data were performed by the Duke Clinical Research Institute using SAS software (version 9.3, SAS Institute, Cary, North Carolina, USA).
Results
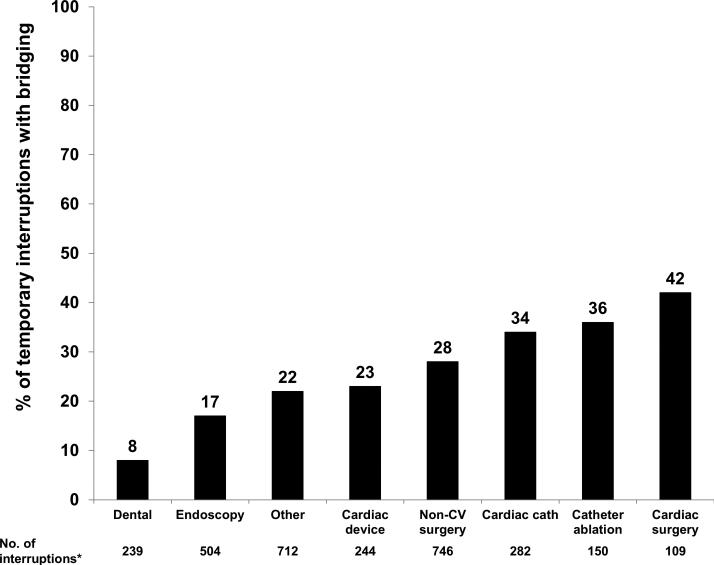
The overall ORBIT-AF population included 10,132 patients from 176 sites; 9,642 patients had at least 1 follow-up visit. Excluding patients not on oral anticoagulation at baseline (n=2,270) yielded a final study cohort of 7,372 patients. The median follow-up duration was 24 months. Overall, there were 2803 reported interruptions; the majority in non-cardiac surgery (n=746, 27%), other procedures (n=712, 25%); and endoscopy (n=504 18%). Overall, 2138 (76%) interruptions did not use bridging anticoagulation, while 665 (24%) did. Distribution of bridging use, by procedure, is shown in Figure 1.
Figure 1.
Proportion of interruptions involving anticoagulant bridging, by procedure. Endoscopy includes gastrointestinal, genitourinary, or bronchoscopic. CV: cardiovascular; cath: catheterization.
Among the 665 interruption events that involved bridging anticoagulation, LMWH was used in 487 (73%), UFH in 97 (15%), fondaparinux in 7 (1.1%), and another anticoagulant was used in 76 (11%). Twenty-three interruptions involving bridging were in patients treated with dabigatran at baseline: 12 used LMWH, 6 UFH, and 5 used other agents (none used fondaparinux). Comparison of baseline characteristics between patients with no interruption (n=5172, 70%) versus those with ≥1 interruption during follow-up (n=2,200, 30%), stratified by bridging use, are shown in Table 1. Compared with patients that did not have any interruption, those experiencing at least 1 interruption were slightly younger (median age 75 vs. 76, p=0.0002), more likely White (92% vs. 89%, p=0.005), less likely to have new onset AF (2.6% vs. 4.3%, p=0.0001), and had higher median calculated creatinine clearance (71 vs. 69 mL/min/1.72 m2, p=0.002)7. Rates of prior coronary vascular or cerebrovascular disease, as well as mean CHADS2 scores, were all similar (p=NS for each). Of patients with at least 1 interruption, patients with any bridging interruption were statistically younger (median age 74 vs. 75, p=0.009), and were more likely to have congestive heart failure (44% vs. 34%, p<0.0001), prior cerebrovascular events (22% vs. 15%, p=0.0003), any valve disease (34% vs. 27%, 0.0006), and prior mechanical valve (9.6% vs. 2.4%, p<0.0001), compared with patients that had at least 1 interruption but none with bridging. Baseline oral anticoagulant also differed significantly (dabigatran in 3.7% vs. 6.8%, p=0.02). While mean CHADS2 (2.53 vs. 2.34, p=0.004) and CHA2DS2-VASc scores (4.25 vs. 4.03, p=0.01) were higher in bridged patients, there were no differences in rates of CHADS2 score ≥2 (78% vs. 76%, p=0.4) or CHA2DS2-VASc score ≥2 (94% vs. 95%, p=0.5). Use of additional antiplatelet therapy was similar for concomitant single antiplatelet (39% vs. 36%) and dual antiplatelet therapy (3.0% vs. 2.2%; p=0.2 across antiplatelet categories).
Table 1.
Baseline demographics, past medical history, and laboratory studies by incidence of temporary interruption.
| No temporary interruption (n=5172) | ≥1 Temporary Interruption (n=2200) |
|||
|---|---|---|---|---|
| Patients with ≥1 interruption, none with bridging (N=1608) | Patients with ≥1 interruption with bridging (N=592) | P-value (no bridging vs. bridging) | ||
| Age (years) | 76 (68-82) | 75 (68-81) | 74 (67-80) | 0.009 |
| Female | 43 | 41 | 42 | 0.7 |
| Race/ethnicity | 0.1 | |||
| White | 89 | 92 | 91 | |
| Black or African American | 5.0 | 3.5 | 5 | |
| Hispanic | 4.6 | 3.7 | 2.7 | |
| Other | 1.5 | 1.2 | 0.5 | |
| AF Type | 0.5 | |||
| New onset | 4.3 | 2.7 | 2.2 | |
| Paroxysmal | 46 | 46 | 48 | |
| Persistent | 19 | 16 | 17 | |
| Longstanding persistent | 31 | 35 | 32 | |
| CHADS2 scores, mean (SD) | 2.4 (1.3) | 2.34 (1.21) | 2.53 (1.31) | 0.004 |
| CHA2DS2-VASc scores, mean (SD) | 4.0 (1.7) | 4.03 (1.62) | 4.25 (1.74) | 0.01 |
| ATRIA scores, mean (SD) | 2.78 (1.89) | 2.74 (1.94) | 2.72 (1.95) | 0.9 |
| Prior cerebrovascular event | 17 | 15 | 22 | 0.0003 |
| Coronary artery disease | 36 | 36 | 41 | 0.05 |
| Congestive heart failure | 34 | 34 | 44 | <0.0001 |
| Significant valve disease | 27 | 27 | 34 | 0.0006 |
| Moderate/severe mitral stenosis | 1.7 | 1.1 | 2.5 | 0.01 |
| Prior mechanical valve replacement | 3.6 | 2.4 | 9.6 | <0.0001 |
| Prior GI bleeding | 0.97 | |||
| Never | 92 | 91 | 91 | |
| >6 months prior | 6.9 | 1.4 | 1.5 | |
| ≤6 months prior | 0.8 | 7.3 | 7.1 | |
| Baseline oral anticoagulant | 0.02 | |||
| Warfarin | 93 | 93 | 96 | |
| Dabigatran | 6.5 | 6.8 | 3.7 | |
| Most recent INR prior to procedure, mean (SD) | - | 2.34 (0.76) | 2.28 (0.71) | 0.3 |
| % time INR 2-3, pre-procedure†† | - | 67% | 62% | 0.0002 |
| Concomitant antiplatelet‡ | ||||
| Aspirin | 36 | 36 | 38 | 0.4 |
| Clopidogrel | 4.5 | 4.2 | 6.9 | 0.01 |
| Prasugrel | 0.03 | 0.06 | 0 | 0.5 |
| Calculated creatinine clearance† (mL/min/1.73m ) | 69 (49-95) | 71 (54-97) | 70 (51-96) | 0.3 |
| LVEF (%) | 55 (50-60) | 55 (50-60) | 55 (45-60) | <0.001 |
As calculated by the Cockcroft-Gault formula.7
As calculated using the Rosendaal method.8
Including aspirin, clopidogrel, or prasugrel; no patient was on ticagrelor.
Values are presented as % or median (interquartile range), unless noted otherwise.
SD: standard deviation; GI: gastrointestinal; BMI: body mass index; LVEF: left-ventricular ejection fraction.
Among patients treated with warfarin who had at least 1 follow-up INR after the procedure (n=1452), time to the achievement of therapeutic range (first INR ≥2) following the procedure was significantly shorter for interruptions using bridging, versus those without bridging (median 17 days vs. 23, p<0.001).8
Outcomes
Unadjusted rates of individual outcomes during and after interruption are displayed in Table 2. Events during interruption were relatively infrequent overall. Event rates were higher for interruptions in which bridging anticoagulation was used, including any adverse event during interruption (5.3% vs. 2.8%, p=0.01), major bleeding (3.6% vs. 1.2%, p=0.0007), bleeding hospitalization (2.2% vs. 0.7%, p=0.006), and cardiovascular hospitalization (4.2% vs. 2.2%, p=0.02). Event counts and rates across different procedure types, stratified by bridging, are shown in Table 3.
Table 2.
Unadjusted outcomes during and after temporary interruption of oral anticoagulation.
| Overall (N=2280) | No Bridging (N=1766) | Bridging (N=514) | P-value | |
|---|---|---|---|---|
| Any adverse event during interruption | 3.4 (77) | 2.8 (50) | 5.3 (27) | 0.01 |
| Bleeding Event | 2.2 (50) | 1.8 (3l) | 3.7 (19) | 0.02 |
| Thrombotic Event | 0.6 (l3) | 0.5 (9) | 0.8 (4) | 0.5 |
| Other Adverse Event | 0.6 (l4) | 0.6 (10) | 0.8 (4) | 0.6 |
| Events within 30 days following procedure requiring interruption* | ||||
| Myocardial infarction | 0.2 (5) | 0.2 (4) | 0.2 (1) | 0.9 |
| Stroke or systemic embolism | 0.4 (8) | 0.3 (5) | 0.6 (3) | 0.3 |
| Major bleeding | 1.7 (38) | 1.2 (20) | 3.6 (18) | 0.0007 |
| Hospitalization | ||||
| Cardiovascular | 2.7 (59) | 2.2 (38) | 4.2 (21) | 0.02 |
| Bleeding | 1.0 (23) | 0.7 (12) | 2.2 (11) | 0.006 |
| Other | 3.1 (69) | 2.8 (49) | 4.0 (20) | 0.2 |
| Death | 0.2 (4) | 0.2 (3) | 0.2 (1) | 0.9 |
Values are presented as % (n).
Denominators exclude interruptions missing date, or those that occurred within 30 days of a previous interruption (n=2227 overall, 1724 without bridging, 503 with bridging). Events within 30 days of the procedure requiring interruption may overlap with those during interruption.
Table 3.
Adverse events within 30 days by procedure type and bridging anticoagulation.
| Cardiovascular Events* | Bleeding Events† | |||
|---|---|---|---|---|
| No Bridging (n=1724) | Bridging (n=503) | No Bridging (n=1724) | Bridging (n=503) | |
| Catheterization/PCI | 9/139 (6.5%) | 3/65 (4.6%) | 2/139 (1.4%) | 1/65 (1.5%) |
| Catheter Ablation | 1/66 (1.5%) | 5/41 (12.2%) | 1/66 (1.5%) | 0/41 (0%) |
| Endoscopic Procedure | 9/343 (2.6%) | 2/64 (3.1%) | 5/343 (1.5%) | 5/64 (7.8%) |
| Cardiac Surgery | 3/48 (6.3%) | 2/28 (7.1%) | 2/48 (4.2%) | 2/28 (7.1%) |
| Non-cardiac Surgery | 6/410 (1.5%) | 2/149 (1.3%) | 5/410 (1.2%) | 12/149 (8.1%) |
| Device Implantation | 9/139 (6.5%) | 2/38 (5.3%) | 0/139 (0%) | 0/38 (0%) |
| Dental Work | 1/166 (0.6%) | 0/16 (0%) | 0/166 (0%) | 0/16 (0%) |
| Other | 5/413 (1.2%) | 7/102 (6.9%) | 7/413 (1.7%) | 5/102 (4.9%) |
Excluding interruptions missing date, or those that occurred within 30 days of a previous interruption.
Includes stroke, systemic embolism, myocardial infarction, or cardiovascular hospitalization within 30 days of procedure requiring interruption.
Includes major bleeding or bleeding hospitalization within 30 days of procedure requiring interruption.
The association between bridging and adverse events persisted in multivariate-adjusted analysis (Table 4) – the use of bridging anticoagulation during interruption was significantly associated with an increase in bleeding events (adjusted OR 3.84 for major bleeding or bleeding hospitalization, 95% CI 2.07-7.14, p<0.0001) and showed a trend towards increased cardiovascular events (adjusted OR 1.62, 95% CI 0.95-2.78, p=0.07). Overall, bridging was associated with an increased risk of adverse events, including the composite of MI, bleeding, stroke or systemic embolism, hospitalization, or death within 30 days (adjusted OR 1.94, 95% CI 1.38-2.71, p=0.0001). The procedure for which the patient required interruption appeared to minimally influence composite adverse outcomes (p=0.2 across all procedures); however, adverse events were significantly less common for dental procedures (adjusted OR 0.19 vs. non-cardiac surgery, 95% CI 0.06-0.63, ppairwise=0.0063). Baseline anticoagulant (warfarin vs. dabigatran) was not significantly associated with outcomes following temporary interruption in the adjusted model.
Table 4.
Adjusted 30-day outcomes, by use of bridging anticoagulation.
| Unadjusted, % (n) | Adjusted* | ||||
|---|---|---|---|---|---|
| No Bridging (N=1724) | Bridging (N=503) | P-value | Adjusted OR (95% CI), bridging vs. no bridging | P-value | |
| Cardiovascular events† | 2.5 (43) | 4.6 (23) | 0.02 | 1.62 (0.95-2.78) | 0.07 |
| Bleeding events‡ | 1.3 (22) | 5.0 (25) | <0.0001 | 3.84 (2.07-7.14) | <0.0001 |
| Overall composite^ | 6.3 (108) | 13 (64) | <0.0001 | 1.94 (1.38-2.71) | 0.0001 |
Denominators exclude interruptions missing date, or those that occurred within 30 days of a previous interruption. Events within 30 days of the procedure requiring interruption may overlap with those during interruption.
Adjustment model co-variates included age, estimated glomerular filtration rate, sex, prior cerebrovascular events, the presence of significant valvular disease or prior mechanical valve replacement, prior gastrointestinal bleeding, the presence of congestive heart failure, type of AF at baseline (new onset, paroxysmal, persistent, longstanding persistent), left atrial diameter size, patient level of education, CHADS2 score, procedure requiring interruption (with non-cardiac surgery as the referent), and type of oral anticoagulation at baseline (warfarin versus dabigatran; neither rivaroxaban nor apixaban was used in this cohort).
Includes stroke, systemic embolism, myocardial infarction, or cardiovascular hospitalization within 30 days of procedure requiring interruption.
Includes major bleeding or bleeding hospitalization within 30 days of procedure requiring interruption.
Includes the composite of stroke, myocardial infarction, major bleeding, hospitalization, or death within 30 days of procedure requiring interruption.
In a sensitivity analysis that included baseline, concomitant antiplatelet use (none, single, double), there remained a consistent, significant association between bridging and adverse outcome.
Discussion
There are three major findings from this study. First, interruptions of OAC are common in contemporary patients with AF in clinical practice, often for cardiac procedures and non-cardiac surgery, as well as minimally-invasive procedures. Second, of those having temporary interruptions, bridging anticoagulation was used in approximately one-quarter of patients and the decision to use bridging or not appears guided by patient factors related to bleeding or thromboembolic risk. Finally we found that the use of bridging anticoagulation was significantly associated with higher overall bleeding and adverse event rates.
The rate of bridging anticoagulation was higher than that reported in contemporary trials.9 Patients with prior cerebrovascular events, those with mechanical valves, and patients receiving warfarin (compared with dabigatran) were more likely to receive bridging anticoagulation, as would be expected. Additionally, bridging varied by type of procedure. These data generally reflect the limited guideline support for bridging – specifically, that the decision for bridging in moderate- or high-risk patients be patient- and procedure-specific, and to avoid bridging in patients at low risk of thromboembolism.4 Furthermore, the guidelines recommend more conservative management of bridging medications, and also call attention to scenarios where OAC could be continued without interruption (e.g., dental procedures). While this appears to demonstrate improvement in the previously-described practice variability,10 there remains room for further improvement based upon the data in this study. Bridging anticoagulation appeared to be used more commonly than the guidelines would suggest. For example, we observed that a significant number of OAC interruptions were for dental procedures (n=239, 9% of all interruptions), and 8% of these temporary interruptions involved use of a bridging anticoagulant. Furthermore, there were excess adverse events in bridged patients undergoing specific procedures (e.g., catheter ablation, endoscopy,), indicating particularly unfavorable risk in these cases. Such management may contribute to worse clinical outcomes overall, and our data do not support the routine use of bridging in AF patients requiring temporary interruption of anticoagulation.
Our data show that the risks associated with interruptions, and the risk of bridging during them, are not limited to the periprocedural period. Adverse events in patients interrupting OAC persist out as late as 30 days, and include bleeding events, thrombotic events, and recurrent hospitalizations. While the use of bridging has been shown to be safe in closely-controlled clinical trials,3, 11 outcomes in the community, where protocols are often absent or inconsistent, have been more limited. They included heterogeneous patient cohorts, anticoagulated for a variety of indications, and only bleeding and thromboembolic outcomes were reported.1, 2 The most recent US national guidelines highlight the dearth of evidence for the practice;12 furthermore, there is mounting evidence that certain procedures may be more-safely performed with anticoagulation uninterrupted.13, 14 Importantly, there is less experience with uninterrupted, direct-acting oral anticoagulants in this setting.15, 16 The risks of bridging likely highlight the challenges in managing patients on OAC in the periprocedural period. In the patient receiving bridging agents, both of the most common drugs (UFH and LMWH) require attention to dosing to prevent bleeding and provide anticoagulant effect (UFH on a continuous basis, LMWH with changes in weight, kidney function, or in pregnancy). Additionally, many patients require transitions in anticoagulants at the same time they are experiencing a transition in care (e.g., on admission, from ICU to the floor, or during discharge to another facility or home). Such circumstances likely contribute to an increased risk associated with the use of short-term anticoagulants. Close attention to anticoagulant transitions and dosing is vital to minimizing risk.17 Properly identifying the group of patients, if any, in whom the risk of these pitfalls is outweighed by the benefit of OAC interruption and bridging remains a challenge. They are likely to include patients at extremely high risk of periprocedural thromboembolic events (e.g., those with mechanical mitral valve prostheses), undergoing procedures for which uninterrupted, periprocedural anticoagulation is prohibitively dangerous (e.g., neurological procedures). Some have speculated that in patients at lower risk of bleeding, bridging may be worthwhile.11 However, in our cohort of AF patients, most of whom with low-risk ATRIA bleeding scores, we found bridging anticoagulation was still significantly associated with worse clinical events at 30 days, particularly bleeding and bleeding hospitalizations. This said, the results here are observational and we cannot rule of the beneficial role of bridging in select circumstances. The ongoing Effectiveness of Bridging Anticoagulation for Surgery (BRIDGE) study, which randomized nearly 2,500 warfarin-treated patients undergoing surgery to either LMWH or placebo during the perioperative period, will provide additional insight (NCT00786474). Importantly, we also observed the use of bridging anticoagulation in patients receiving the oral, direct thrombin inhibitor dabigatran. While guidelines are limited on the use of novel oral anticoagulants in the setting of procedures,18 their pharmacokinetics are such that bridging is likely redundant (although this remains to be proven in patients at high risk of thromboembolic events). In contrast to warfarin, which requires several days to both take effect and to wash out, direct-acting anticoagulants demonstrate short time-to-onset, and are cleared relatively quickly, similar to LMWHs. Thus, the use of bridging anticoagulants in such patients has been cautioned, however, additional studies are needed.9
Limitations
This analysis is derived from the ORBIT-AF registry, which is an observational study of real-world patients in community, clinical practice. Limitations of such a study include enrollment and/or sampling biases and reporting bias. Because patients were not randomized either to the occurrence of an interruption or to the use of bridging, a causal relationship between these events and adverse outcomes cannot be confirmed. Furthermore, it is possible that post-procedure parenteral anticoagulation is a requirement of the procedure, thus use of such an agent would occur whether or not a patient is on long-term oral anticoagulation. Data for patients who undergo procedures without interruption and for those who interrupt anticoagulation due to reasons other than procedures are not available; thus, we cannot comment on the implications of our findings for these groups. Lastly, despite statistical methods aimed at adjusting for baseline differences in the population, we cannot exclude residual and/or unmeasured confounding of the results.
Conclusions
Temporary interruptions are common in patients receiving OAC for AF, and occur even for minimally-invasive procedures. Many patients receive bridging anticoagulation, and its use varies by procedure type and certain patient characteristics. Use of bridging anticoagulation was associated with increased risk of bleeding and adverse events following interruption. These data do not support the use of routine bridging in anticoagulated patients with AF, and additional data are needed to identify best practices around anticoagulation interruptions.
Supplementary Material
Acknowledgments
Contributors: ORBIT-AF Investigators: R. Mendelson, A. Nahhas, J. Neutel, B. Padanilam, D. Pan, J. Poock, J. Raffetto, R. Greengold, P. Roan, F. Saba, M. Sackett, R. Schneider, Z. Seymour, J. Shanes, J. Shoemaker, V. Simms, N. Smiley, D. Smith, C. Snipes, R. Sotolongo, C. Staniloae, S. Stoltz, D. P. Suresh, T. Tak, A. Tannenbaum, S. Turk, K. Vora, P. Randhawa, J. Zebrack, E. Silva, E. Riley, D. Weinstein, T. Vasiliauskas, S. Goldbarg, D. Hayward, C. Yarlagadda, D. Laurion, A. Osunkoya, R. Burns, T. Castor, D. Spiller, C. Luttman, S. Anton, J. McGarvey, R. Guthrie, G. Deriso, R. Flood, L. Fleischer, J. S. Fierstein, R. Aggarwal, G. Jacobs, N. Adjei, A. Akyea-Djamson, A. Alfieri, J. Bacon, N. Bedwell, P. Berger, J. Berry, R. Bhagwat, S. Bloom, F. Boccalandro, J. Capo, S. Kapadia, R. Casanova, J. E. Morriss III, T. Christensen, J. Elsen, R. Farsad, D. Fox, B. Frandsen, M. Gelernt, S. Gill, S. Grubb, C. Hall, H. Harris, D. Hotchkiss, J. Ip, N. Jaffrani, A. Jones, J. Kazmierski, F. Waxman, G. L. Kneller, A. Labroo, B. Jaffe, M. Lebenthal, D. Lee, M. Lillestol, K. LeClerc, P. Maccaro, N. Mayer, J. Kozlowski, S. Benjamin, R. Detweiler, P. Igic, T. Jackson, J. Pappas, R. Littlefield, A. Frey, R. Vranian, W. Long, P. Grena, A. Arouni, J. Quinn, K. Browne, S. Forman, M. Ebinger, R. Blonder, H. Snyder, S. Slabic, D. Williams, R. Stein, S. Kirkland, K. Cohen, W. Walthall, K. Davis, B. Snoddy, O. Alvarado, C. Leach, S. Rothman, A. Sharma, A. Olatidoye, S. AlMahameed, S. Rosenthal, G. Sutter, W. Reiter, T. Thompson, S. Thew, J. Kobayashi, M. Williams, J. Kramer, S. A. Latif, B. Rhee, A. Adler, D. Ruiz-Serrano, S. Stringam, K. Wolok, A. Focil, S. Butman, H. Ingersoll, R. Borge, Y. Al-Saghir, P. Coats, N. Farris, K. Shore, M. B. Schwartz, C. Gornick, P. Eilat, E. Quinlan, Y. Paliwal, R. Mitra, A. Jingo, A. A. Aslam, L. Allen, R. Watson, S. Voyce, M. Turakhia, D. Goytia-Leos, M. Lurie, G. Mallis, B. Atwater, J. Strobel, J. Murray, D. Fisher, M. Atieh, R. Landes, A. Drabick, E. Harman, B. Ashcraft, M. Krista, A. Videlefsky, E. Rivera-Zayas, A. E. Tan.
Funding Sources: The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ. Dr. Steinberg was funded by NIH T-32 training grant #5 T32 HL 7101-38.
Footnotes
Clinical Trial Registration Information: clinicaltrials.gov. Identifier: NCT01165710.
Disclosures: The following relationships exist related to this presentation: BAS, SK, LT, BJG, and MWS report no disclosures. GCF reports research support from AHRQ (significant), consultant to Janssen (modest), Medtronic (modest); PRK reports consultant/advisory board (modest): Johnson & Johnson, Daiichi Sankyo, Sanofi, Boehringer Ingelheim, Merck, Bristol Myers Squibb, and Portola. KWM reports financial disclosures prior to August 1, 2013, can be viewed at https://www.dcri.org/about-us/conflict-of-interest/Mahaffey-COI_2011-2013.pdf; disclosures after August 1, 2013, can be viewed at http://med.stanford.edu/profiles/kenneth_mahaffey. PC reports employment, significant: Janssen Pharmaceuticals, Inc. EDP reports research grant, significant: American Heart Association, American College of Cardiology, Janssen Pharmaceutical Products, Eli Lilly & Company, and Society of Thoracic Surgeons; consultant/advisory board, modest: Merck & Co; consultant/advisory board, significant: Boehringer Ingelheim, Genentech, Sanofi-Aventis, and Janssen Pharmaceutical Products. JPP reports research grant, significant: Johnson & Johnson/Janssen Pharmaceuticals, and Boston Scientific Corporation; other research support, significant: Johnson & Johnson/Janssen Pharmaceuticals; consultant/advisory board, modest: Forest Laboratories, Inc. and Medtronic Inc.; and consultant/advisory board, significant: Johnson & Johnson/Janssen Pharmaceuticals. JA reports modest Consultant/Advisory Board from Bristol Myers Squibb, Pfizer, Janssen, Daiichi, Boehringer Ingelheim, and Alere.
References
- 1.Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630–1639. doi: 10.1161/CIRCULATIONAHA.112.105221. [DOI] [PubMed] [Google Scholar]
- 2.Spyropoulos AC, Turpie AG, Dunn AS, Spandorfer J, Douketis J, Jacobson A, Frost FJ, Investigators R. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4:1246–1252. doi: 10.1111/j.1538-7836.2006.01908.x. [DOI] [PubMed] [Google Scholar]
- 3.Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med. 2004;164:1319–1326. doi: 10.1001/archinte.164.12.1319. [DOI] [PubMed] [Google Scholar]
- 4.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R. American College of Chest P. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S–350S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162:606–612. e601. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 9.Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y, Spyropoulos AC, Hankey GJ, Singer DE, Nessel CC, Mahaffey KW, Fox KA, Califf RM, Becker RC. on behalf of the RAFI. Outcomes of Temporary Interruption of Rivaroxaban Compared with Warfarin in Patients with Nonvalvular Atrial Fibrillation: Results from ROCKET AF. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.005754. doi 10.1161/CIRCULATIONAHA.1113.005754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douketis JD, Crowther MA, Cherian SS. Perioperative anticoagulation in patients with chronic atrial fibrillation who are undergoing elective surgery: results of a physician survey. Can J Cardiol. 2000;16:326–330. [PubMed] [Google Scholar]
- 11.Dunn AS, Spyropoulos AC, Turpie AG. Bridging therapy in patients on long-term oral anticoagulants who require surgery: the Prospective Peri-operative Enoxaparin Cohort Trial (PROSPECT). J Thromb Haemost. 2007;5:2211–2218. doi: 10.1111/j.1538-7836.2007.02729.x. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, FIeld ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014:129. doi: 10.1016/j.jacc.2014.03.022. doi:10.1161/CIR.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 13.Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, Simpson CS, Ayala- Paredes F, Coutu B, Leiria TL, Essebag V. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–2093. doi: 10.1056/NEJMoa1302946. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed I, Gertner E, Nelson WB, House CM, Dahiya R, Anderson CP, Benditt DG, Zhu DW. Continuing warfarin therapy is superior to interrupting warfarin with or without bridging anticoagulation therapy in patients undergoing pacemaker and defibrillator implantation. Heart Rhythm. 2010;7:745–749. doi: 10.1016/j.hrthm.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Lakkireddy D, Reddy YM, Di Biase L, Vanga SR, Santangeli P, Swarup V, Pimentel R, Mansour MC, D'Avila A, Sanchez JE, Burkhardt JD, Chalhoub F, Mohanty P, Coffey J, Shaik N, Monir G, Reddy VY, Ruskin J, Natale A. Feasibility and Safety of Dabigatran Versus Warfarin for Periprocedural Anticoagulation in Patients Undergoing Radiofrequency Ablation for Atrial Fibrillation Results From a Multicenter Prospective Registry. J Am Coll Cardiol. 2012;59:1168–1174. doi: 10.1016/j.jacc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Lakkireddy D, Reddy YM, Di Biase L, Vallakati A, Mansour MC, Santangeli P, Gangireddy S, Swarup V, Chalhoub F, Atkins D, Bommana S, Verma A, Sanchez JE, Burkhardt JD, Barrett CD, Baheiry S, Ruskin J, Reddy V, Natale A. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2014;63:982–988. doi: 10.1016/j.jacc.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Mahaffey KW, Hellkamp AS, Patel MR, Hannan KL, Schwabe K, Nessel CC, Berkowitz SD, Halperin JL, Hankey GJ, Becker RC, Piccini JP, Breithardt G, Hacke W, Singer DE, Califf RM, Fox KA. End of study transition from study drug to open-label vitamin K antagonist therapy: the ROCKET AF experience. Circ Cardiovasc Qual Outcomes. 2013;6:470–478. doi: 10.1161/CIRCOUTCOMES.113.000132. [DOI] [PubMed] [Google Scholar]
- 18.Spyropoulos AC, Douketis JD, Gerotziafas G, Kaatz S, Ortel TL, Schulman S. Subcommittee on Control of Anticoagulation of the SSCotI. Periprocedural antithrombotic and bridging therapy: recommendations for standardized reporting in patients with arterial indications for chronic oral anticoagulant therapy. J Thromb Haemost. 2012;10:692–694. doi: 10.1111/j.1538-7836.2012.04630.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.