Abstract
Ru(OH)x/Al2O3 is among the more versatile catalysts for aerobic alcohol oxidation and dehydrogenation of nitrogen heterocycles. Here, we describe the translation of batch reactions to a continuous-flow method that enables high steady-state conversion and single-pass yields in the oxidation of benzylic alcohols and dehydrogenation of indoline. A dilute source of O2 (8% in N2) was used to ensure that the reaction mixture, which employs toluene as the solvent, is non-flammable throughout the process. A packed bed reactor was operated isothermally in an up-flow orientation, allowing good liquid-solid contact. Deactivation of the catalyst during the reaction was modeled empirically, and this model was used to achieve high conversion and yield during extended operation in the aerobic oxidation of 2-thiophene methanol (99+% continuous yield over 72 h).
Introduction
Conversion of alcohols to carbonyl compounds is one of the most common transformations in organic chemistry. Process-scale alcohol oxidations are often done using stoichiometric or catalytic reagents, such as pyridine•SO31-3 and NaOCl/TEMPO (TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxyl);4-7 however, there has been long-standing interest in the development of aerobic methods that generate essentially no by-products. Applications of aerobic alcohol oxidation in the pharmaceutical and fine-chemical industries have been limited, often because the performance of existing catalytic methods do not match or exceed that of traditional oxidation methods and/or because mixtures of oxygen gas and organic solvents represent a potential safety hazard.8
There has been considerable recent progress in the development of improved homogeneous and heterogeneous catalysts for aerobic alcohol oxidation.9 Homogeneous Pd and Cu catalysts are particularly effective in these applications, and, in collaboration with Eli Lilly, we have demonstrated safe and scalable flow-based processes for aerobic alcohol oxidation using Pd(OAc)2/pyridine10 and Cu/TEMPO11 catalyst systems.12-14 These reactions were demonstrated up to kilogram scale. The Cu/TEMPO methods show especially broad scope and exhibit reactor residence times as low as 5 min. During the course of these studies, we became interested in exploring analogous continuous-flow applications of heterogeneous catalysts that could lower the catalyst loading and facilitate product purification and/or direct coupling of alcohol oxidation with downstream synthetic steps.
Ruthenium-based heterogeneous catalysts,15 especially the Ru(OH)x/Al2O3 catalyst developed by Mizuno and coworkers,15c-f exhibit broad substrate scope, including tolerance to heterocycles and other functional groups commonly encountered in pharmaceuticals and fine chemicals. These methods have been studied extensively in batch format and activated substrates exhibit turnover frequencies as high as 100 h−1. Hii and coworkers recently adapted an XCube™ reactor for use with the Ru(OH)x/Al2O3 catalyst to oxidize alcohols under aerobic conditions.16 High product yields were obtained by using a semi-batch operation method in which the reactant was recirculated continuously through the catalyst bed to replace dissolved oxygen and obtain high conversions for substrate:Ru ratios varying from 10–70 mol substrate/mol Ru. In previous studies, the Ru(OH)x/Al2O3 catalyst was found to deactivate during use, potentially limiting the utility of this catalyst under continuous operating conditions.15c In the present study, we have investigated the heterogeneous Ru(OH)x/Al2O3 catalyst for aerobic alcohol oxidation in a continuous-flow process using a packed-bed reactor (PBR). Characterization of the catalyst deactivation kinetics provides the basis for identification of process conditions that enable high single-pass yields (>95 %) of aldehydes and ketones via oxidation of the corresponding alcohols.
Results and Discussion
Catalyst Performance in Batch Reactions
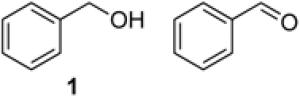
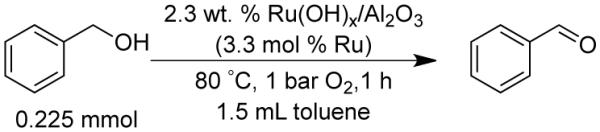
In order to benchmark the flow process described below, we initiated our study with the oxidation of benzyl alcohol as a model substrate under batch conditions (Scheme 1). Previous studies15c-f have employed trifluorotoluene or toluene as the solvent. We observed similar rates with both solvents and elected to proceed with toluene. Batch-reuse experiments show that the catalyst loses activity upon reaction with an alcohol (Table 1). An initial reaction with 3.3 mol % Ru resulted in near quantitative yield of benzaldehyde. Recovery of the catalyst and attempted reuse in a second reaction, however, resulted in only 17% yield of benzaldehyde (entry 2). As described previously,15c essentially full activity could be recovered upon stirring the catalyst in aqueous NaOH, washing with water and drying under vacuum (entry 3).
Scheme 1.
Ru(OH)x/Al2O3-Catalyzed Oxidation of Benzyl Alcohol.
Table 1.
Loss of Catalyst Activity During Oxidation of Benzyl Alcohol.a
0.15 M benzyl alcohol, 3.3 mol % Ru as Ru(OH)x/Al2O3, 80 °C, 1 h, 1 bar O2, 1.5 mL toluene.
GC yield using tetradecane as an internal standard.
Used catalyst was stirred in 0.1 M NaOH for 16 hours then washed with water and dried in vacuum prior to reuse.
Description of Flow Reactor
The three main sections of the flow reactor used for the aerobic oxidation reactions (Figure 1) are the gas and liquid feeds, the packed-bed reactor, and a gas/liquid separation unit. The gas feed consists of a premixed cylinder containing 8 % O2 in N2. Several mass flow controllers in parallel enable consistent control over the gas flow rate from 0.5 to 150 sccm. The alcohol and solvent (liquid feed) are delivered to the reactor via a syringe pump capable of flow rates ranging from 1 µL/min to 100 mL/min. The liquid and gas are mixed at a tee and fed into the reactor. A preheated coil and the reactor are submerged in ethylene glycol heat transfer fluid for isothermal operation. The reactor consists of ¼" or ½" o.d. stainless steel tubing with sufficient volume to contain the desired catalyst charge. The reactor is oriented in a vertical direction, to minimize complications from settling of the catalyst and/or channeling through the catalyst bed. The liquid and gas are co-fed in an upflow direction to achieve full catalyst wetting in flooded-bed mode in preference to trickle-bed operation in downflow17. At these low flow rates neither bed fluidization nor compaction is expected or observed. Liquid and gas exiting the reactor are separated in a large diameter (1") tube that permits gas-liquid disengagement. The gas is vented to a fume hood through a relief valve set at the desired operating pressure, and the liquid is removed through automatic cycling of two pneumatic valves. This arrangement permits ready control of reactor temperature, pressure, and gas and liquid flow rates.
Figure 1.
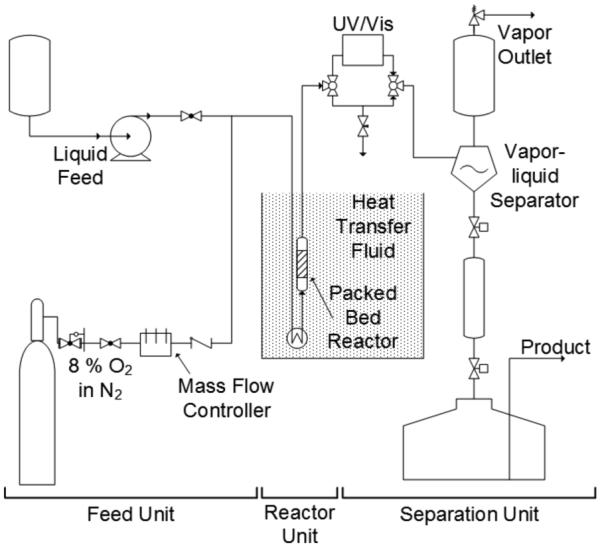
Schematic diagram of the flow reactor used for heterogeneous aerobic oxidation reactions.
Reactor Safety Considerations
Several features of the reactor were designed to address concerns about safety hazards (such as explosions and/or fires) associated with the use of O2 with an organic solvent. The reactor is operated within the slug flow regime such that small regions of vapor space are isolated from remaining vapors by the liquid (flames only propagate in the vapor phase). The ancillary tubing is all 1/16" or 1/8" o.d., and has an i.d. below the flame propagation threshold.18 The O2 source is prepared as an 8% mixture in N2 to ensure that the oxygen concentration remains below the limiting O2 concentration (LOC) of toluene, which has been reported to be 11.6% O2 at 1 atm.19-21 Taken together, these features provide several layers of safety in the process design. The reactor is operated at 11 atm total pressure, and the resulting 0.9 atm partial pressure of O2 is comparable to the 1 atm pure O2 used for the batch reactions in Table 1.
Reactor Characterization
We studied the flow patterns in the reactor by monitoring the liquid residence time both with and without gas flow. In the small diameter ancillary tubes (1/16" or 1/8" o.d.) the flow pattern is confined bubble flow,22,23 with the volume ratio of liquid to gas being the same as the 1:8 ratio of the volumetric feed flow rates. The resulting residence time distribution (RTD) shows near plug-flow behavior with a small amount of broadening from mixing or axial dispersion (Figure 2) and demonstrates that the bed is static and filled with well-distributed liquid. After subtraction of catalyst and ancillary tubing volume, the residence time (τ) indicates that the reactor void volume is 80-90 % liquid-filled, and the gas percolates or bubbles through at a higher linear velocity. The RTD curve in Figure 2 is readily fit to a standard local-mixing model with tanks in series (nCSTR)24 with ~100 mixing stages. Reactor kinetics calculations for reactors with more than 20 stages result in conversions indistinguishable from ideal plug flow models. Therefore the reactor is treated as an ideal plug-flow PBR for kinetic analysis and rate determinations.
Figure 2.
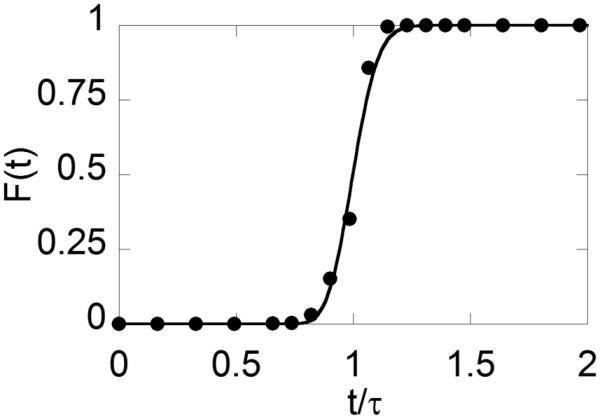
Residence time distribution curve showing the step change response associated with the flow of benzyl alcohol starting at time t=0 through the 1/2" packed-bed reactor loaded with the Ru(OH)x/Al2O3 catalyst. The reaction was monitored by GC, with F(t) indicating the fraction of total [benzyl alcohol + benzaldehyde] detected from the reactor outlet as a function of time and τ corresponding to the mean residence time. Conditions: 20.8 g Ru(OH)x/Al2O3, 0.5 mL/min liquid flow and 40 sccm gas flow (8 % O2 in N2) at 11 bar and 80 °C. The curve reflects a fit to an nCSTR model, with τ = 61 min and n = 137. See Experimental section for further explanation.
Analysis of Ru(OH)x/Al2O3 activity under continuous-flow conditions
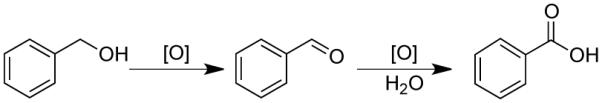
The kinetics of benzyl alcohol oxidation to benzaldehyde over the heterogeneous catalyst are modeled as a first-order reaction. The effective contact time of the substrate with the catalyst is inversely related to the weight hourly space velocity (WHSV) (eq 1), which provides a value that can be translated to different reactor sizes and configurations. Catalyst deactivation during the oxidation reaction was monitored in the PBR over several days at a constant WHSV. The yield of benzaldehyde was converted to an initial rate based on the first order substrate dependence as reported by Mizuno15d using eq 2. The decrease in rate is then reported as a function of the quantity of reactants that have been exposed to the catalyst (ratio defined as ρ = mol alcohol/mol Ru). After 30 equivalents the reaction rate has decreased by 90 % in the PBR, consistent with the loss of activity observed in batch reactions (see Table 1). After this initial decrease in catalytic activity, the subsequent decrease in catalyst activity proceeds at a slower rate. The decay of activity (keff) is modeled well empirically by an extended exponential (eq 3), where k0 = 26, β = 2.5, and n = 0.156.
| (1) |
| (2) |
| (3) |
Previous work12c has suggested that catalyst deactivation is caused by active site poisoning from benzoic acid arising from the over-oxidation of benzyl alcohol (Scheme 2). In normal use at high conversion, trace amounts (0-2%) of benzoic acid from sequential oxidation in presence of by-product water are detected in the reactor effluent, and larger amounts of the carboxylic acid over-oxidation product accumulate during extended reaction times (see Figure S6 in Supporting Information). The benzoic acid by-product is a catalyst poison. Upon regenerating the used catalyst from a batch reaction with aqueous NaOH, sodium benzoate is detected in the NaOH wash solution by UV/visible spectroscopy. The amount of benzoate removed (0.90 mole benzoate/mole Ru) correlates closely with the loss of catalyst activity (86%), suggesting that one benzoate group binds to each Ru center. Additional experiments show that benzoate is generated from both substrate and solvent (toluene) oxidation. For example, in the oxidation of 4-methylbenzyl alcohol in toluene, 5% of the carboxylic acid recovered from the NaOH wash step originates from toluene (i.e., benzoic acid rather than 4-methylbenzoic acid).
Scheme 2.
Sequential Oxidation of Benzyl Alcohol to Benzoic Acid.
Efforts to identify different solvents or basic additives that improve catalyst stability and sustain activity resulted in only minor improvements (see Supporting Information). Therefore, we elected to use the catalyst deactivation kinetics (cf. Figure 3) as a basis for the development of continuous-flow conditions that could achieve high single-pass yields. As shown in Figure 3, the catalyst undergoes a rapid decrease in activity during the first 50 turnovers but the activity stabilizes substantially beyond this point. The empirical fit of the data (eq 3) can be used to determine conditions to achieve high steady state yields of product for an extended period of time at a constant WHSV. In principle, more sophisticated process conditions could be developed in which the flow rate is adjusted according to the changing catalytic activity, but this approach was not taken in the present study.
Figure 3.
Decrease in catalyst activity of Ru(OH)x/Al2O3 under continuous flow conditions during the oxidation of benzyl alcohol (ρ is the dimensionless ratio of reactants exposed to the catalyst). 0.9 g of 2.3 wt% Ru(OH)x/Al2O3, 0.15 M benzyl alcohol in toluene at 0.05 mL/min, 80 °C, 11 bar 8 % O2 in N2 at 4 sccm.
Aerobic oxidation of diverse alcohols under continuous flow conditions
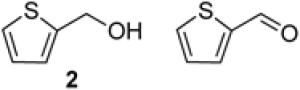
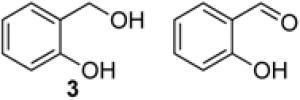
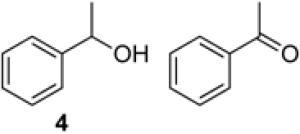
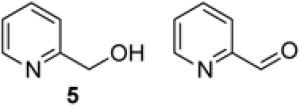
To demonstrate use of a partially deactivated catalyst in flow, we tested five different alcohols that undergo efficient and selective oxidation with fresh catalyst under batch reaction conditions (see Scheme 1 for conditions): benzyl alcohol (1), 2-(hydroxylmethyl)thiophene (2), 2-(hydroxymethyl)phenol (3), 1-phenylethanol (4), and 2-(hydroxymethyl)pyridine (5). The successful reactivity of these substrates in batch (and flow, as described below) show that the Ru(OH)x/Al2O3 catalyst is not poisoned by heteroatom functional groups.
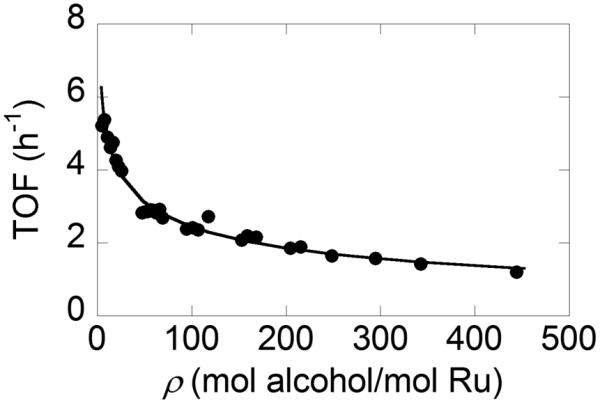
Preliminary reactivity of each substrate was measured over a catalyst bed that had been exposed to at least 350 equivalents of benzyl alcohol (i.e., ρ = 350; cf. Figure 3), and an effective rate constant was determined at low to moderate conversion. These rate constants show a good relative correlation with rate constants determined from batch reactions with fresh Ru(OH)x/Al2O3 (eq 4 and Figure 4). The correlation suggests that the poisoning associated with accumulated benzoic acid from over-oxidation of benzyl alcohol is non-selective and affects other alcohol substrates in the same proportions.25
Figure 4.
Comparison of reaction rates observed in the aerobic oxidation of alcohols 1–5 (cf. Table 2) under flow and batch conditions relative to the rate of benzyl alcohol (BA).
| (4) |
Batch data from benzylic and related heteroaryl methanol oxidations showed aldehyde/ketone yields of >95% and high initial TOF of 10-110 hr−1 over fresh catalysts. With the relative rate data from batch reactions (cf. Figure 4), an appropriate WHSV was estimated for high steady-state yields of the desired product over the deactivated catalyst. Following optimization of the reaction around the estimated WHSV, excellent steady state yields were obtained for each of the alcohol oxidations, as shown in Table 2.
Table 2.
Steady State Yields Obtained from Deactivated Ru(OH)x/Al2O3-Catalyzed Aerobic Alcohol Oxidation Under Continuous Flow Conditions.
All reactions proceed with >99 % selectivity. Yields determined by GC (internal standard = tetradecane). Reaction conditions: 0.15 M substrate in toluene, Ru(OH)x/Al2O3 (2.3 wt. %), 11 bar 8 % O2 in N2, 2:1 mol O2:mol substrate, 80 °C.
9:1 toluene:CH3CN
0.5 M substrate, 125 °C.
Extended operation at scale
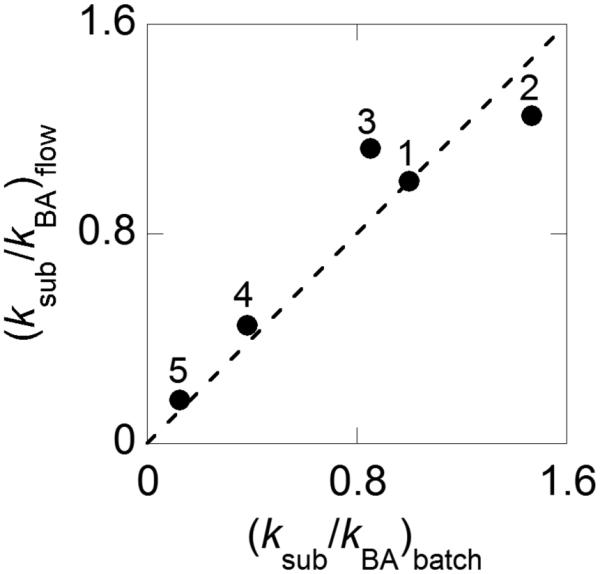
By understanding the catalyst activity profile (cf. Figure 3), it was possible to demonstrate longer-term, sustained catalyst performance in a 72 h continuous oxidation with 2-thiophene methanol as the substrate. The steady-state yields of 2-thiophene carboxaldehyde remained above 99% throughout the reaction (Figure 5). There is no significant change in the Ru content of the catalyst, and minimal leaching of Ru is detected in the reactor effluent as well. After 500 turnovers and a month of use, the catalyst was recovered from the PBR and dissolved in aqueous HCl for ICP-AES analysis. The ICP-AES data for the original catalyst and the recovered catalyst show no change in the Ru content within experimental error (wt % Ru fresh catalyst = 2.3 ± 0.1; wt % Ru aged catalyst = 2.4 ± 0.1). Only trace amounts of Ru were detected in the product stream, which was analyzed by concentrating the solution to provide increased sensitivity. The measured Ru content revealed 3 ppb in 770 ml of an accumulated product solution, which corresponds to 5 ppm of the original catalyst Ru content.
Figure 5.
Yield of 2-thiophene carboxaldehyde obtained during continuous operation over 72 h. 0.15 M 2-thiophene methanol in toluene at 0.16 mL/min, 80 °C, 11 bar 8 % O2 in N2 at 12.8 sccm, 20.8 g Ru(OH)x/Al2O3.
The rather low WHSVs associated with the steady-state catalyst (cf. Table 2) mean that quite large quantities of catalyst would be required to achieve good mass throughput in large-scale applications, and the Ru(OH)x/Al2O3 catalyst system may not be practical for process scale aerobic alcohol oxidation unless off-line catalyst reactivation is incorporated into the process. The kinetic modeling approach described here to achieve high steady-state product yields should, however, be applicable to other heterogeneous catalysts that undergo systematic loss of activity under continuous-flow conditions.
Oxidation of Indoline in Flow
In addition to alcohol oxidation, the Ru(OH)x/Al2O3 catalyst also promotes oxidative dehydrogenation of amines.15e We demonstrated this reaction under flow conditions for the dehydrogenation of indoline to indole. Following optimization of the conditions, the indole was obtained in 95% yield (Scheme 3). Once again, however, the WHSV is quite low. This reaction does not follow the same activity relation shown in Figure 4 {(kindoline/kBA)batch = 1.9 and (kindoline/kBA)flow = 0.74}, suggesting that the site requirement or adsorption geometry may require more access to the Ru surface sites and thus be more strongly inhibited following partial catalyst deactivation by benzoic acid.
Scheme 3.
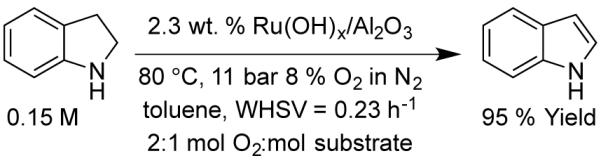
Oxidation of Indoline in Flow.
Conclusion
The present report demonstrates the use of a Ru(OH)x/Al2O3 catalyst for the continuous oxidation of alcohols in a packed bed reactor, which enables high single-pass steady-state yields. The catalyst is shown to deactivate through the binding of carboxylic acids to the Ru catalyst but it remains capable of achieving high steady-state yields, provided the operating conditions are adjusted to account for the decrease in catalyst activity. The catalyst tolerates diverse functional groups and shows a strong correlation between the relative rates of different alcohols under batch and flow conditions.
Experimental
General Considerations
The catalyst was prepared from RuCl3 according to literature procedures15c using basic γ-Al2O3 (155 m2g−1). Commercially available reagents were obtained from Aldrich and used as received. Toluene was obtained from commercial sources (Aldrich, ACS grade). No special measures were taken to exclude air or water from the solvent or reaction mixtures.
GC Method and Retention Times
GC analyses were performed using a DB-Wax column installed in a Shimadzu GC-17A equipped with flame-ionization detector. A 10 min GC method was used consisting of a ramp at 20 °C/min from 70 °C to 200 °C (6.5 min), and 3.5 min at 200 °C. The injector and detector were held at 300 °C and the column flow was 3.2 mL/min of He with a split ratio of 34:1. Retention times were as follows: benzyl alcohol (4.0 min), benzaldehyde (1.7 min), 2-thiophene methanol (4.1 min), 2-thiophenecarboxaldehyde (2.6 min), salicyl alcohol (4.2 min), salicylaldehyde (2.5 min),1-phenylethanol (3.4 min), acetophenone (2.3 min), 2-pyridine methanol (4.5 min), 2-pyridinecarboxaldehyde (1.9 min), indoline (3.7 min), indole (6.1 min), tetradecane (1.1 min).
Procedure for the Batch Reaction Oxidation of Alcohols
A 1.5 mL solution of 0.15 M substrate and 0.05 M tetradecane (used as internal standard) in toluene are added to 30 mg of Ru(OH)x/Al2O3 (3.3 mol % Ru). The reaction mixture is placed on a shaker and mixed under 1 atm pure O2 for 30 min. The reaction mixture is then heated to the reaction temperature. The post reaction solution is injected onto a GC to determine product and reactant concentrations. The catalyst is recovered by filtration.
Flow Reactions
Representative Packed Bed Reactor
The packed bed reactor is made from a stainless steel tube 0.25” o.d. × 3” long with 1 cm of glass wool inside a Swagelok fitting with a 200 mesh stainless steel screen. Powdered Ru(OH)x/Al2O3 (1.25 g) was added leaving 1 cm of open space for more glass wool to be retained by another 200 mesh stainless steel screen and a Swagelok fitting.26
Procedure for the Alcohol Oxidation in Flow
A solution of 0.15 M substrate and 0.05 M tetradecane in toluene is added to a 260 mL syringe pump (Teledyne ISCO 260D), and a 1 gal gas reservoir is filled with O2 and N2 to a 86 bar mixture of 8 % O2 (8 % O2 in N2 is used to stay below the LOC flammability limit of the toluene). 20-21 The gas is regulated down to a pressure of 14.5 bar and flows through a mass flow controller with a controlled O2 to substrate molar ratio of 2:1. The gas and liquid are mixed in a 1/16” tee and sent through a preheat zone before passing through the packed bed reactor. The preheat zone and PBR are submerged in a Paratherm HE heat transfer fluid kept at 80 °C. The weight hourly space velocity (WHSV) is controlled by adjusting the gas and liquid flow rates. 100-500 µL of reaction product can be removed through a small tee for GC analysis, and the remaining liquid and gas are separated using a large tee with the liquids collected out the bottom using 2 valves in series and the gases vented out the top through a pressure relief valve. The pressure relief valve controls the reaction pressure and is maintained at 11 bar.
Procedure for the Deactivation of the Packed Bed Reactors
The deactivation of the catalyst is done by flowing a 0.15 M solution of benzyl alcohol in toluene through the packed bed reactor at 0.05 mL/min, 80 °C, and 11 bar 8 % O2 in N2 at 4 sccm. Samples are collected twice a day and the resulting yield of benzaldehyde determined by GC.
Residence Time Distribution (RTD)
Representative Procedure for the Determination of Liquid-Only RTD
A solution of 0.01 M phenanthrene in toluene is added to a syringe pump, and a second syringe pump is charged with pure toluene. An HPLC UV/Vis detector (Waters 2487) is attached to the reactor outlet, set at 330 nm. A 2 mM solution of phenanthrene is pumped through the reactor until the UV/Vis detector exhibits a constant output voltage. At t = 0 the pump flow rates are adjusted to afford a 4 mM solution of phenanthrene and the UV/Vis output is monitored. The output signals are normalized to an initial value of 0 and a final value of 1, and a dimensionless time is generated by dividing the time by the calculated residence time (τ). The resulting data are fit with an nCSTR-in-series model by adjusting the number of CSTRs (n) and the residence time (τ) in Matlab (release 2011a, Mathworks).
Representative Procedure for the Determination of Gas and Liquid RTD
A solution of 0.01 M phenanthrene and 8 % O2 in N2 are pumped through the reactor at the same rates used for catalytic reactions (0.5 mL/min liquid, 40 sccm gas for 20.8 g PBR) for 16 h. At t = 0 the phenanthrene flow is stopped and replaced with a solution of 0.15 M benzyl alcohol in toluene at the same flow rate. The outlet is monitored by collecting GC samples every 5 min. A normalized response curve is generated using eq 5.
| (5) |
A dimensionless time is generated by dividing the time by the average residence time. The resulting data are fit with a nCSTR in series model24 by adjusting the number of CSTRs (n) and the residence time (τ) in Matlab (release 2011a, Mathworks).
Representative Procedure for ICP-AES Analysis
Around 10 mg of Ru(OH)x/Al2O3 are added to 10 mL of HCl in a 100 mL volumetric flask. The HCl and solid catalyst are heated to 40 °C for 5 h. The resulting solution is cooled and diluted to 100 mL with DI water. The solution is analyzed on a Perkin Elmer Instruments Optima 2000 DV ICP AES.
Supplementary Material
Acknowledgements
We are grateful to Eli Lilly for the donation of the reactor and Drs. Martin Johnson and Matthew Yates for training on its operation. Financial support of this work was provided by the NIH (RC1-GM091161 and R01-GM100143) and a consortium of pharmaceutical companies (Eli Lilly, Pfizer, and Merck).
Footnotes
Supporting Information Available. Additional RTD curves, catalyst characterization, and reactor pictures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Parikh JR, Doering WVE. J. Am. Chem. Soc. 1967;89:5505–5507. [Google Scholar]
- (2).Liu C, Ng JS, Behling JR, Yen CH, Campbell AL, Fuzail KS, Yonan EE, Mehrotra DV. Org. Process Res. Dev. 1997;1:45–54. [Google Scholar]
- (3).Ripin DHB, Abele S, Cai WL, Blumenkopf T, Casavant JM, Doty JL, Flanagan M, Koecher C, Laue KW, McCarthy K, Meltz C, Munchhoff M, Pouwer K, Shah B, Sun JM, Teixeira J, Vries T, Whipple DA, Wilcox G. Org. Process Res. Dev. 2003;7:115–120. [Google Scholar]
- (4).Anelli PL, Biffi C, Montanari F, Quici S. J. Org. Chem. 1987;52:2559–2562. [Google Scholar]
- (5).Ciriminna R, Pagliaro M. Org. Process Res. Dev. 2010;14:245–251. [Google Scholar]
- (6).Fritz-Langhals E. Org. Process Res. Dev. 2005;9:577–582. [Google Scholar]
- (7).Bogdan A, McQuade DT. Beilstein J. Org. Chem. 2009;5 doi: 10.3762/bjoc.5.17. http://dx.doi.org/10.3762/bjoc.5.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer JL, Linderman RJ, Lorenz K, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY. Green Chemistry. 2007;9:411–420. For context, see the following: [Google Scholar]; (b) Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337–2347. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]; (c) Caron S, Dugger RW, Ruggeri SG, Ragan JA, Ripin DHB. Chem. Rev. 2006;106:2943–2989. doi: 10.1021/cr040679f. [DOI] [PubMed] [Google Scholar]
- (9).(a) Mallat T, Baiker A. Chem. Rev. 2004;104:3037–3058. doi: 10.1021/cr0200116. For relevant reviews, see: [DOI] [PubMed] [Google Scholar]; (b) Markó IE, Giles PR, Tsukazaki M, Chellé-Regnaut I, Gautier A, Dumeunier R, Philippart F, Doda K, Mutonkole J-L, Brown SM, Urch CJ. Adv. Inorg. Chem. 2004;56:211–240. [Google Scholar]; (c) Zhan B-Z, Thompson A. Tetrahedron. 2004;60:2917–2935. [Google Scholar]; (d) Schultz MJ, Sigman MS. Tetrahedron. 2006;62:8227–8241. [Google Scholar]; (e) Matsumoto T, Ueno M, Wang N, Kobayashi S. Chem. Asian J. 2008;3:196–214. doi: 10.1002/asia.200700359. [DOI] [PubMed] [Google Scholar]; (f) Parmeggiani C, Cardona F. Green Chem. 2012;14:547–564. [Google Scholar]; (g) Ryland BL, Stahl SS. Angew. Chem. Int. Ed. 2014;53:8824–8838. doi: 10.1002/anie.201403110. http://dx.doi.org/10.1002/anie.201403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ye XA, Johnson MD, Diao TN, Yates MH, Stahl SS. Green Chem. 2010;12:1180–1186. doi: 10.1039/c0gc00106f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Greene JF, Hoover JM, Mannel DS, Root TW, Stahl SS. Org. Proc. Res. Devel. 2013;17:1247–1251. [Google Scholar]
- (12).(a) Bavykin DV, Lapkin AA, Kolaczkowski ST, Plucinski PK. Appl. Catal., A. 2005;288:175–184. For other examples of flow-based aerobic oxidation methods, see the following leading references: [Google Scholar]; (b) Wang NW, Matsumoto T, Ueno M, Miyamura H, Kobayashi S. Angew. Chem. Int. Ed. 2009;48:4744–4746. doi: 10.1002/anie.200900565. [DOI] [PubMed] [Google Scholar]; (c) Chapman AO, Akien GR, Arrowsmith NJ, Licence P, Poliakoff M. Green Chem. 2010;12:310–315. [Google Scholar]; (d) Aellig C, Scholz D, Hermans I. ChemSusChem. 2012;5:1732–1736. doi: 10.1002/cssc.201200438. [DOI] [PubMed] [Google Scholar]; (e) Hamano M, Nagy KD, Jensen KF. Chem Commun. 2012;48:2086–2088. doi: 10.1039/c2cc17123f. [DOI] [PubMed] [Google Scholar]; (f) Aellig C, Scholz D, Conrad S, Hermans I. Green Chem. 2013;15:1975–1980. [Google Scholar]; (g) Liu X, Jensen KF. Green Chem. 2013;15:1538–1541. [Google Scholar]; (h) Obermayer D, Balu AM, Romero AA, Goessler W, Luque R, Kappe CO. Green Chem. 2013;15:1530–1537. [Google Scholar]; (i) Gutmann B, Elsner P, Roberge D, Kappe CO. ACS Catal. 2013;3:2669–2676. [Google Scholar]; (j) Ushakov DB, Gilmore K, Kopetzki D, McQuade DT, Seeberger PH. Angew. Chem. Int. Ed. 2014;53:557–561. doi: 10.1002/anie.201307778. [DOI] [PubMed] [Google Scholar]; (k) He Z, Jamison TF. Angew. Chem. Int. Ed. 2014;53:3353–3357. doi: 10.1002/anie.201310572. [DOI] [PubMed] [Google Scholar]
- (13).(a) Kawaguchi T, Miyata H, Ataka K, Mae K, Yoshida J. Angew. Chem. Int. Ed. 2005;44:2413–2416. doi: 10.1002/anie.200462466. For continuous process examples, see: [DOI] [PubMed] [Google Scholar]; (b) Baxendale IR, Deeley J, Griffiths-Jones CM, Ley SV, Saaby S, Tranmer GK. Chem. Commun. 2006:2566–2568. doi: 10.1039/b600382f. [DOI] [PubMed] [Google Scholar]; (c) Johnson MD, May SA, Calvin JR, Remacle J, Stout JR, Diseroad WD, Zaborenko N, Haeberle BD, Sun WM, Miller MT, Brennan J. Org. Process Res. Dev. 2012;16:1017–1038. [Google Scholar]
- (14).(a) Anderson NG. Org. Process Res. Dev. 2001;5:613–621. For continuous processing scale-up reviews, see: [Google Scholar]; (b) Wiles C, Watts P. Green Chem. 2012;14:38–54. [Google Scholar]
- (15).(a) Yamaguchi K, Mori K, Mizugaki T, Ebitani K, Kaneda K. J. Am. Chem. Soc. 2000;122:7144–7145. [Google Scholar]; (b) Ji HB, Mizugaki T, Ebitani K, Kaneda K. Tetrahedron Lett. 2002;43:7179–7183. [Google Scholar]; (c) Yamaguchi K, Mizuno N. Angew. Chem. Int. Ed. 2002;41:4720–4724. doi: 10.1002/1521-3773(20021202)41:23<4538::AID-ANIE4538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (d) Yamaguchi K, Mizuno N. Chem. Eur. J. 2003;9:4353–4361. doi: 10.1002/chem.200304916. [DOI] [PubMed] [Google Scholar]; (e) Mizuno N, Yamaguchi K. Catal. Today. 2008;132:18–26. [Google Scholar]; (f) Yamaguchi K, Kim JW, He JL, Mizuno N. J. Catal. 2009;268:343–349. [Google Scholar]
- (16).Zotova N, Hellgardt K, Kelsall GH, Jessiman AS, Hii KK. Green Chem. 2010;12:2157–2163. [Google Scholar]
- (17).Wu YX, Khadilkar MR, Al-Dahhan MH, Dudukovic MP. Ind. Eng. Chem. Res. 1996;35:397–405. [Google Scholar]
- (18).Lewis B, Elbe G. v. Combustion, Flames and Explosions of Gases. 2nd Academic Press Inc.; New York: 1961. pp. 228–261. [Google Scholar]
- (19).Schroder V, Molnarne M. J. Hazard. Mater. 2005;121:37–44. doi: 10.1016/j.jhazmat.2005.01.032. [DOI] [PubMed] [Google Scholar]
- (20).Brooks MR, Crowl DA. J. Loss Prev. Proc. Ind. 2007;20:144–150. [Google Scholar]
- (21). Ongoing studies suggest the LOC of toluene decreases by 1-2% at elevated pressures. More extensive analysis of LOC values for different solvents at different pressures will be the focus of a forthcoming publication.
- (22).Chen L, Tian YS, Karayiannis TG. Int. J. Heat Mass Transfer. 2006;49:4220–4230. [Google Scholar]
- (23). Slug flow in the small diameter tubing at these low flow rates was observed using a small segment of glass tubing at Eli Lilly. Slug flow has also been observed using Teflon tubing and a colored solution.
- (24). The nCSTR model is a simplified two-parameter reactor model that treats local mixing as being produced by multiple equal-volume CSTRs in series. For example, behavior of a 50 mL tubular reactor might be approximated by 100 CSTRs (0.50 ml volume each) in series to model how the actual RTD deviates from ideal plug flow behavior.
- (25). Independent deactivation studies were not performed with each alcohol, but one would expect that different alcohols might lead to different deactivation parameters (cf. eq 3).
- (26). Photos are available in the supporting information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.