Abstract
Scandium-44 (t½ = 3.9 h) is a relatively new radioisotope of potential interest for use in clinical positron emission tomography (PET). Herein, we report for the first time the room temperature radiolabeling of proteins with 44Sc for in vivo PET imaging. For this purpose, the Fab fragment of Cetuximab, a monoclonal antibody that binds with high affinity to epidermal growth factor receptor (EGFR) was generated and conjugated with N-[(R)-2-Amino-3-(p-isothiocyanato-phenyl) propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N′,N″,N″-pentaacetic acid (CHX-A″-DTPA). The high purity of Cetuximab-Fab was confirmed by SDS-PAGE and mass spectrometry. The potential of the bioconjugate for PET imaging of EGFR expression in human glioblastoma (U87MG) tumor-bearing mice was investigated after 44Sc labeling. PET imaging revealed rapid tumor uptake (maximum uptake of ~12 %ID/g at 4 h post-injection) of 44Sc-CHX-A″-DTPA-Cetuximab-Fab with excellent tumor-to-background ratio, which might allow for same day PET imaging in future clinical studies. Immunofluorescence staining was conducted to correlate tracer uptake in the tumor and normal tissues with EGFR expression. This successful strategy for immunoPET imaging of EGFR expression using 44Sc-CHX-A″-DTPA-Cetuximab-Fab can make clinically translatable advances to select the right population of patients for EGFR-targeted therapy and also monitor the therapeutic efficacy of anti-EGFR treatments.
Keywords: Antibody Fab, Cetuximab, ImmunoPET imaging, Radiolabeling, Scandium, Theranostic
INTRODUCTION
Scandium-44 is a relatively new radioisotope with excellent nuclear decay characteristics [t½ = 3.9 h, Eβ+ (max) = 1.47 MeV, β+ branching ratio = 94.3 %] for PET imaging. 1 The radioisotope can be conveniently produced in a cyclotron by irradiation of Ca targets with protons by the nuclear reaction 44Ca(p,n)44Sc.2, 3 The longer half-life of 44Sc compared to other more commonly used PET radioisotopes such as 18F (t½ = 109 min) or 68Ga (t½ = 68 min) potentially allows centralized production and cost-efficient distribution of 44Sc-based radiopharmaceuticals regionally. Another advantage lies in the availability of 47Sc (t½ = 3.35 days), which is a moderate energy (0.6 MeV, 100%) β− emitter suitable for targeted therapy. 3 By coupling to the same biological vector, 44Sc can be used as an imaging surrogate for 47Sc and might also aid in estimating dosimetry for therapy with 47Sc, thereby representing an ideal ‘theranostic’ pair.
Despite these excellent attributes, only limited number of 44Sc radiopharmaceuticals based on peptides and other small biomolecules have been reported till date. 4-7 In all these studies, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was used as the bifunctional chelator (BFC) and radiolabeling was carried out at ~95 °C. Although 44Sc-DOTA complexes are formed with high in vivo stability, 1, 8 the relatively slow complexation kinetics and requirement of elevated temperature for complex formation impede the preparation of radiotracers comprising temperature sensitive and fragile macromolecules such as proteins. In view of this, it is essential to identify a suitable BFC which can be radiolabeled with 44Sc at room temperature within a reasonable period of time and also demonstrate high in vitro and in vivo stability. The feasibility to radiolabel 44Sc with proteins such as antibody fragments would enhance the scope of 44Sc-radiopharmacy and facilitate utilization of this radioisotope for immunoPET imaging.
Over the last few decades, EGFR has been investigated as a major target for uncontrolled tumor growth in various types of cancers.9 Cetuximab is a chimeric human-murine IgG1 monoclonal antibody that binds specifically to EGFR with high affinity.10, 11 The delayed tumor uptake and prolonged circulation half-life are the major limitations towards the use of intact antibodies as molecular imaging probes. 12-14 In order to accelerate targeting of EGFR, Cetuximab-F(ab′)2 fragments were earlier generated and radiolabeled with 111In. 15, 16 However, it took several hours to obtain satisfactory contrast between the tumor and normal tissues, after administration of the radiolabeled agent. 16 This is especially a disadvantage when repeated imaging is required within short time intervals, for example when studying the dynamics of EGFR expression during treatment. Therefore, we aimed to further improve EGFR targeting kinetics by using monovalent (Fab) fragments of Cetuximab for PET imaging. Cetuximab-Fab fragments, comprising both VH and VL domains, are expected to retain the specificity and antigen-binding affinity of the parent antibody, while demonstrating improved pharmacokinetics for tissue penetration. 12 The decay half-life of 44Sc matches the biological half-life of Fab fragments, which is another desirable feature for successful immunoPET imaging. 12
Herein, we report the generation of Cetuximab-Fab fragment and its radiolabeling with 44Sc at room temperature using CHX-A″-DTPA (N-[(R)-2-Amino-3-(p-isothiocyanato-phenyl) propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N′,N″,N″-pentaacetic acid) as the BFC. The in vitro and in vivo characteristics of 44Sc-CHX-A″-DTPA-Cetuximab-Fab were investigated for PET imaging of EGFR expression in a human glioblastoma (U87MG) tumor model. The present study is the first report, to the best of our knowledge, on the radiolabeling and preclinical evaluation of 44Sc-labeled protein molecules. This strategy can be extended for radiolabeling other temperature sensitive biomolecules with 44Sc for PET imaging.
RESULTS
Generation of Cetuximab-Fab and its characterization
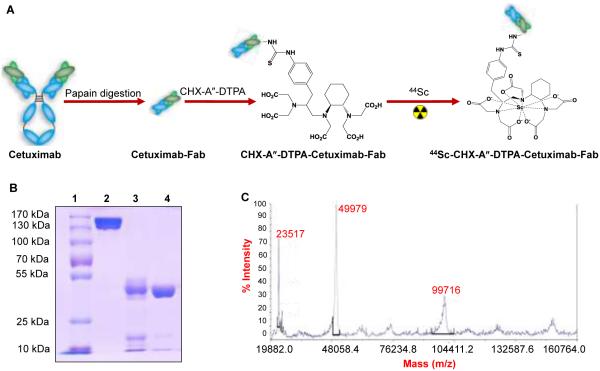
Cetuximab-Fab was generated from the intact antibody upon papain digestion for 4 h (Figure 1A). Protein A columns were used for separation of Cetuximab-Fab from the intact antibody and Fc fragments. The Protein A resin binds specifically to the Fc region of immunoglobulin molecules, especially IgGs,17-19 allowing intact antibody and the Fc fragments generated during papain digestion to be trapped in the column and purified Cetuximab-Fab to pass through. Purified Cetuximab-Fab solution was further concentrated and buffer exchanged into PBS by ultrafiltration. SDS-PAGE showed the disappearance of the intact Cetuximab band (~ 150 kDa) and the appearance of band corresponding to Cetuximab-Fab (~ 50 kDa),indicating complete digestion of Cetuximab by papain to yield high quality Fab fragment (Figure 1B). The molecular weight of Cetuximab-Fab as determined by mass spectrometry was ~49.9 kDa (Figure 1C). The purified Cetuximab-Fab was further used for bioconjugation and preclinical investigation in targeted, blocking and negative control groups. For non-targeted groups, the purified Fab fragments were denatured by high energy ultrasonication for over one hour
Figure 1.
Generation of Cetuximab-Fab and its characterization. (A) Schematic diagram for Cetuximab-Fab generation from intact antibody, conjugation and radiolabeling. The figures are not drawn to scale. (B) SDS-PAGE to confirm the purity of Cetuximab-Fab (lane 1: ladder, lane 2: intact Cetuximab; lane 3: unpurified Cetuximab-Fab after papain digestion and lane 4: purified Cetuximab-Fab after passing through Protein A column). (C) Mass spectrometry of Cetuximab-Fab (~ 49.9 kDa).
Flow cytometry
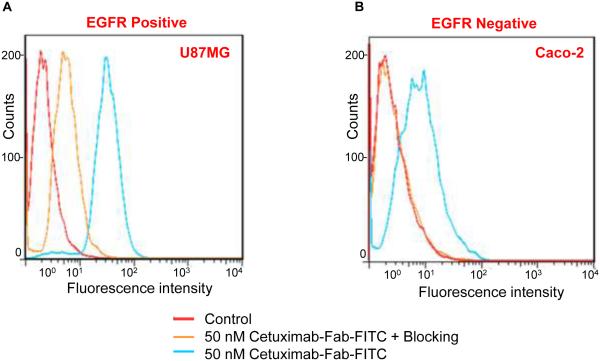
To confirm that the generated Cetuximab-Fab retained the EGFR binding characteristics of the intact antibody, in vitro targeting experiments were carried out using U87MG (high EGFR expression) and Caco-2 (low EGFR expression) cells for flow cytometry. Fluorescein isothiocynate (FITC; Excitation = 494 nm / Emission = 521 nm) conjugated Cetuximab-Fab (50 nM) significantly enhanced the mean fluorescence intensity of U87MG cells (~ 20-fold higher than the unstained cells), whereas treatment with a “blocking” dose of Cetuximab (1 μM) reduced the cell fluorescence by about 10-fold (Figure 2A). These results demonstrate that FITC-Cetuximab-Fab specifically binds to EGFR on the U87MG cells. Meanwhile, the fluorescence signal from Caco-2 cells was minimal, indicating low non-specific binding of FITC-Cetuximab-Fab (Figure 2B). The differences in the mean fluorescence intensities of U87MG and Caco-2 cells for targeted and blocking groups are shown in Figure S1. The control groups for both cell types show similar fluorescence background which confirms that FITC-Cetuximab-Fab exhibits strong and specific binding to EGFR with negligible non-specific binding, in vitro. Papain digestion therefore, does not compromise the EGFR binding specificity of Cetuximab-Fab, thereby encouraging further in vivo studies.
Figure 2.
Flow cytometry in U87MG (high EGFR expression) and Caco-2 (low EGFR expression) cells confirm the EGFR specificity and affinity of Cetuximab-Fab.
44Sc-labeling of Cetuximab-Fab and serum stability evaluation
Both intact and denatured Cetuximab-Fab fragments were labeled with 44Sc for in vivo studies (see Experimental Section). The labeling conditions were carefully optimized to give the highest radiolabeling yields (see Supporting Information; experimental section and figure S2). The radiolabeled bioconjugates were purified using PD-10 columns with PBS as the mobile phase. The radioactivity fractions which typically elute between 3 and 4 mL were collected for further experiments, and a typical size exclusion column chromatography profile can be seen in Figure S3A. The unreacted 44Sc starts eluting from the column after 6.0 mL. The decay-corrected radiochemical yields of Cetuximab-Fab conjugated with different BFCs are summarized in Table S1. Only DTPA analogs were found to be suitable for complexation with 44Sc at room temperature with appreciable yields for in vivo studies. Since the in vivo stability of CHX-A″-DTPA complexes is expected to be better than conventional DTPA analogs 20, 21, 44Sc-CHX-A″-DTPA-Cetuximab-Fab was used in all further studies. The specific activity of 44Sc-CHX-A″-DTPA-Cetuximab-Fab was ~63 GBq/µmol, assuming complete recovery of the radiolabeled agent after size exclusion chromatography. The whole procedure of 44Sc labeling and purification of the radiolabeled Fab fragment could be completed within 45 min.
Before in vivo investigation in mice, serum stability studies were carried out to assess the stability of 44Sc-CHX-A″-DTPA-Cetuximab-Fab. High serum stability is a prerequisite for in vivo applications of a radiolabeled agent. If the radiolabeled complexes are not stable in serum, 44Sc may be transchelated by serum proteins, resulting in accumulation of the radioactivity in non-target organs and faulty interpretation of the imaging data obtained. It was found that >92% of 44Sc remained within the CHX-A″-DTPA-Cetuximab-Fab conjugates over a 6 h incubation period (Figure S3B), indicating high stability of the 44Sc-CHX-A″-DTPA complex.
In vivo PET imaging and biodistribution studies
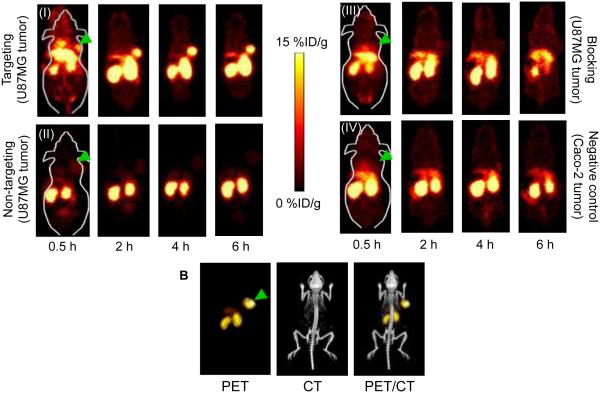
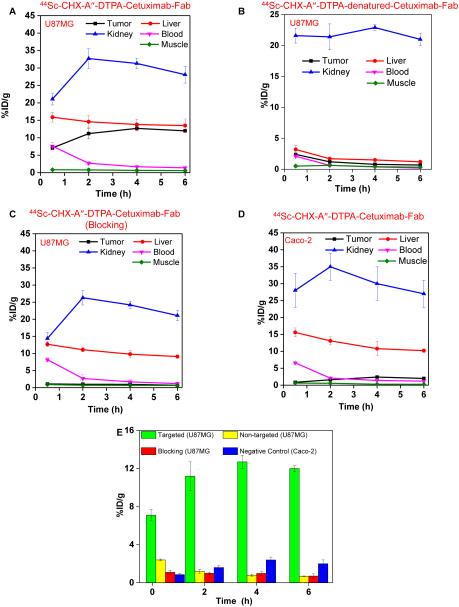
The time points of 0.5, 2, 4 and 6 h post injection (p.i.) were chosen for serial PET scans. The coronal slices that contain the U87MG (high EGFR expression) or Caco-2 (low EGFR expression) tumors are shown in Figure 3A. In addition, representative microPET, microCT and fused images of a mouse at 4 h p.i. of 44Sc-CHX-A″-DTPA-Cetuximab-Fab are shown in Figure 3B for direct visual comparison. Quantitative data obtained from ROI analysis of the PET images are shown in Figure 4A-D. 44Sc-CHX-A″-DTPA-Cetuximab-Fab accumulated rapidly in the tumor and was clearly visible as early as 0.5 h p.i.; peaked at 4 h p.i., and remained prominent over time (7.1±0.6, 11.2±1.5, 12.7±0.7, and 12.0±0.3 %ID/g at 0.5, 2, 4, and 6 h p.i., respectively; n=3; Figure 3A(I) and Figure 4A). The uptake of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in liver was found to be 15.9±1.4, 14.6±1.8, 13.8±1.6, and 13.5±1.9 %ID/g at 0.5, 2, 4, and 6 h p.i., respectively. Moreover, considerable uptake was observed in the kidneys, 21.1±1.7, 32.7±2.8, 31.3±1.5, and 28.1±2.4 %ID/g at 0.5, 2, 4, and 6 h p.i., respectively (n=3; Figure 4A). This indicates successful clearance of 44Sc-CHX-A″-DTPA-Cetuximab-Fab fragment through both hepatobiliary and renal pathways, which can be attributed to the much smaller size of the Fab fragment compared to the intact Cetuximab antibody (49.9 kDa vs. 150 kDa). Radioactivity levels in the blood were found to be 7.6±1.1, 2.7±0.5, 1.7±0.3, and 1.4±0.2 %ID/g at 0.5, 2, 4, and 6 h p.i., respectively (n=3; Figure 4A), indicating significantly faster clearance from the blood than radiolabeled intact Cetuximab, which had high (~ 10 %ID/g) blood radioactivity levels even at 48 h p.i. 16, 22 Furthermore, to confirm that the uptake of the radiolabeled probes was specifically due to EGFR targeting and not a result of enhanced permeability and retention (EPR) effect in U87MG tumor, serial PET scans with intravenous injections of 44Sc-CHX-A″-DTPA-denatured-Cetuximab-Fab were also carried out in U87MG tumor bearing mice ( n = 3) as a non-targeted control (Figures 3A(II) and 4B).
Figure 3.
Serial PET imaging of EGFR expression. (A) Serial coronal PET images at 0.5, 2, 4, and 6 h p.i. of (I) 44Sc-CHX-A″-DTPA-Cetuximab-Fab in U87MG tumor bearing mice (targeted), (II) 44Sc-CHX-A″-DTPA-denatured-Cetuximab-Fab in U87MG tumor bearing mice (non-targeted), (III)44Sc-CHX-A″-DTPA-Cetuximab-Fab after treatment with a 2 mg blocking dose of Cetuximab before injection in U87MG tumor bearing mice, and (IV) 44Sc-CHX-A″-DTPA-Cetuximab-Fab in Caco-2 tumor bearing mice (negative control). (B) Representative PET/CT images of U87MG tumor bearing mouse at 4 h p.i. of 44Sc-CHX-A″-DTPA-Cetuximab-Fab. Arrowheads indicate tumors.
Figure 4.
Quantitative region-of-interest (ROI) analysis of the PET data. Time–activity curves of the liver, tumor, blood, kidney, and muscle following intravenous injection of (A) 44Sc-CHX-A″-DTPA-Cetuximab-Fab in U87MG tumor bearing mice (targeted), (B) 44Sc-CHX-A″-DTPA-denatured-Cetuximab-Fab in U87MG tumor bearing mice (non-targeted), (C) 44Sc-CHX-A″-DTPA-Cetuximab-Fab in U87MG tumor bearing mice after treatment with a 2 mg blocking dose of Cetuximab (blocking), (D) 44Sc-CHX-A″-DTPA-Cetuximab-Fab in Caco-2 tumor bearing mice (negative control). (E) Comparison of tracer uptake in the tumors between all four groups.
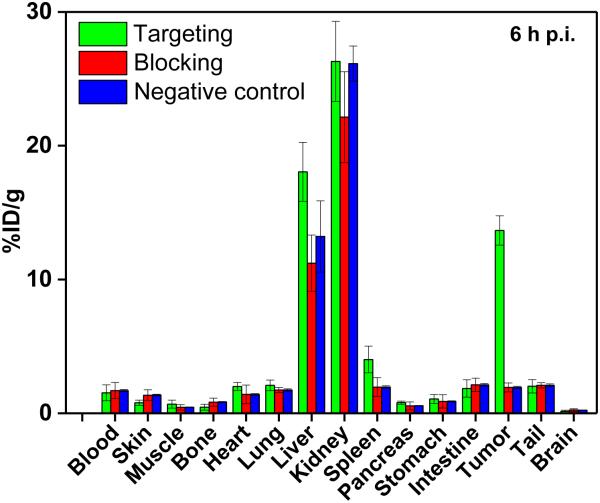
Administering a blocking dose of Cetuximab significantly reduced the tumor uptake of 44Sc-CHX-A″-DTPA-Cetuximab-Fab to 1.1±0.2, 0.98±0.21, 1.0±0.1, and 0.71±0.23 %ID/g at 0.5, 2, 4, and 6 h p.i., respectively (n=3; Figures 3A(III) and 4C), further demonstrating the specificity of 44Sc-CHX-A″-DTPA-Cetuximab-Fab towards EGFR in vivo. The specificity of 44Sc-CHX-A″-DTPA-Cetuximab-Fab was further validated by in vivo studies in mice bearing Caco-2 (EGFR negative) tumors, where the tumor uptake was minimal (0.86±0.07, 1.6±0.2, 2.4±0.3, and 2.0±0.4 %ID/g at 0.5, 2, 4, and 6 h p.i., respectively; n = 3) as shown in Figures 3A(IV) and 4D. The radioactivity uptake in all other organs was similar to that of U87MG tumor bearing mice. Figure 4D summarizes and compares the tumor uptake of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in all four groups. The differences in tumor %ID/g values were statistically significant (P < 0.05; n=3) at all-time points examined. After the last PET scans at 6 h p.i., mice were sacrificed and ex vivo biodistribution studies were performed. Good agreement was observed between the biodistribution data and that obtained from ROI quantification of tracer uptake based on PET images at the last time point. (Figure 5) The results were further validated by ex vivo histological examination (see Supporting Information, figure S4)
Figure 5.
Biodistribution of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in U87MG tumor bearing mice (targeted), of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in U87MG tumor bearing mice after treatment with a blocking dose of Cetuximab (blocking) and of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in Caco-2 tumor bearing mice (negative control), at 6 h p.i. (n=3).
DISCUSSION
The EGFR is a glycosylated transmembrane protein that contributes in several tumorigenic mechanisms including tumor survival, invasion, angiogenesis and metastatic spread. 11, 23 It is also involved in the pathogenesis of many tumors. In many cases, EGFR expression may act as a prognostic indicator predicting poor survival and/or more advanced disease stages. 9, 23 Overexpression of EGFR has been found in several human malignancies such as cancers of head and neck, esophagus, stomach, pancreas, ovary, cervix, breast, lung, kidney and bladder. 11 To target EGFR-mediated tumor cell proliferation or growth, a chimeric human-murine IgG1 monoclonal antibody, Cetuximab, has been developed which specifically binds to the EGFR with a high affinity. 11 The United States Food and Drug Administration (US FDA) has approved this antibody for treatment of patients with EGFR expressing metastatic colorectal carcinomas. 11 However, not much is known about patient-specific tumor uptake and the relationship between dosage and efficacy of Cetuximab therapy. 22 Moreover, delayed uptake in tumor and extended circulation times are some major disadvantages associated with the use of intact antibodies for immunoPET studies. 12, 14
Antibody Fab fragments with lower molecular weight compared to the intact antibodies are expected to display faster blood-/tissue-clearance kinetics, thereby exhibiting a high tumor contrast within 2-3 h after intravenous injection.12 Moreover, engineered antibody fragments like Fab have been shown to be much less immunogenic than intact antibodies, which is further advantageous for molecular imaging. 14 Despite the well documented use of Cetuximab in molecular imaging and therapy, 10, 22, 24 there are surprisingly no reports on use of its Fab fragment. The present study showed that 44Sc-CHX-A″-DTPA-Cetuximab-Fab can be an effective tracer for early non-invasive imaging of EGFR expression in human glioblastoma (U87MG) tumor model, reported to demonstrate high EGFR expression. 22 44Sc was chosen due to its favorable nuclear decay characteristics [Eβ+ (max) = 1.47 MeV, β+ branching ratio = 94.3 %] and its short half-life (~ 3.9 h) which is very well suited for imaging applications with antibody fragments with comparable biological half-lives. The high branching ratio of 44Sc allows lower amount of activity to be administered for PET imaging, resulting in lower radiation dose to normal tissues.
There are two main routes for production of 44Sc. In the first method, 44Sc can be obtained as a daughter product of 44Ti via 44Ti/44Sc generator. 1 The cyclotron-independent availability of 44Sc from 44Ti/44Sc generator provides the obvious logistic advantages for usage of this excellent radioisotope at remote PET facilities. Owing to the long half-life of 44Ti (t½ = 59.3 y), ideally this generator should be able to provide 44Sc for several decades. 1 However, 44Ti is produced through the nuclear reaction 45Sc(p,2n)44Ti, which requires long-term irradiation of Sc targets at a high proton flux (25 MeV proton, 200 μA) for production of sufficient quantities of 44Ti. 3 Presently, 44Ti can be produced only at a few facilities in the world, with limited yields and at high costs. 1,6 Also, 44Sc availed from this generator is not directly amenable for radiopharmaceutical preparation and requires post-elution concentration and purification procedures which make the process cumbersome. 25 These undesirable features make the 44Ti/44Sc generator impractical for use in clinical context. A more prudent approach is the direct cyclotron production of 44Sc by proton irradiation of natural calcium targets. 2, 6, 26, 31 There are numerous cyclotron facilities all over the world which can be utilized for cost-effective production of 44Sc with adequate yields and radionuclidic purity suitable for clinical studies.
A critical component of a 44Sc-based radiopharmaceuticals is the BFC that binds the radiometal ion in a stable coordination complex and also conjugates with a suitable biomolecule for in vivo tumor targeted imaging.8, 21, 27 In order to use 44Sc for labeling Cetuximab Fab, it is essential to ensure that the radiolabeling is carried out within a reasonable period of time at room temperature to prevent denaturation of the antibody. Though the thermodynamic stability constant of Sc3+ complex with DOTA is much higher than with other polyaminopolyacetate ligands (Table S1),27 adequate radiolabeling yield could not be achieved at room temperature while using this BFC. In view of this, use of acyclic DTPA analogs for radiolabeling Cetuximab-Fab appears to be an ideal choice as they demonstrate rapid complexation kinetics with 44Sc3+ at room temperature. However, DTPA complexes with 90Y3+ (which has similar chemical properties as 44Sc3+, being same group elements in the periodic table) have been reported to be kinetically labile when administered in vivo.20, 21 The structurally reinforced CHX-A″-DTPA is a significant improvement over the traditional DTPA chelators in terms of increased in vivo stability without sacrificing the rapid complexation kinetics. 20, 21 This is probably due to the presence of the cyclohexyl group which increases the rigidity of the structure of the complex thereby reducing the rate of dissociation.20 CHX-A″-DTPA-Cetuximab-Fab could be radiolabeled with 44Sc at room temperature with >60% yield and appreciable specific activity (~ 63 GBq/µmol). 44Sc-CHX-A″-DTPA-Cetuximab-Fab could retain its integrity even when incubated in excess volume of mouse serum at 37 °C for 6 h, demonstrating its suitability for in vivo PET imaging.
In vivo PET imaging of EGFR expression in U87MG tumor bearing mice showed a good uptake of 44Sc-CHX-A″-DTPA-Cetuximab-Fab as early as 0.5 h after injection of the tracer, with excellent imaging contrast within 2 h. The maximum tumor uptake (~12 %ID/g) of the radiolabeled Cetuximab-Fab was slightly lower than the maximum tumor uptake (~13 %ID/g) reported for the intact antibody. 22 This is expected since the Fab fragment lacks the Fc region which has been shown to play an important role in internalization of the antibody. 12, 14 However, the lack of Fc reduces non-specific binding between Fc and its receptors on various types of cells (e.g., macrophages, dendritic cells, neutrophils, natural killer cells, B cells etc.) and thus improves the tumor-to-normal tissue ratio. 12
Rapid clearance of 44Sc-CHX-A″-DTPA-Cetuximab-Fab by renal and hepatobiliary routes enables fast and repeated imaging which might be helpful in monitoring tumor dynamics. Also, the high tumor-to-blood ratios 44Sc-CHX-A″-DTPA-Cetuximab-Fab at early time points would allow same-day PET imaging following radiotracer injection. Thus, 44Sc-CHX-A″-DTPA-Cetuximab-Fab could overcome the inherent shortcomings of Cetuximab based immunoPET imaging because of its rapid tumor accumulation and fast background clearance, without compromising significantly on the tumor uptake. This strategy can be a valuable asset in selecting patients for anti-EGFR therapy and also monitoring the therapeutic efficacy of the process.
CONCLUSIONS
In the present study, we reported the successful production and characterization of Cetuximab-Fab. We further demonstrated the feasibility of using cyclotron produced 44Sc for radiolabeling the Fab fragments at room temperature in order to image EGFR expression in glioblastoma xenografts in mice. Among the various BFCs studied, CHX-A″-DTPA was identified as the most promising choice as it permits 44Sc labeling at room temperature within a reasonable period of time. 44Sc-CHX-A″-DTPA-Cetuximab-Fab could be prepared with high radiolabeling yield and appreciable specific activity suitable for in vivo studies. Serum stability studies revealed that the radiolabeled bioconjugate was remarkably stable in mouse serum maintained at 37 ºC. Rapid, prominent, and target-specific uptake in the U87MG tumor was observed for 44Sc-CHX-A″-DTPA-Cetuximab-Fab. The radiolabeled agent was rapidly cleared from the biological system by both hepatobiliary as well as renal routes. This study may further inspire development of new 44Sc/47Sc based radiopharmaceuticals for immunoPET imaging and personalized cancer management.
EXPERIMENTAL SECTION
Chemicals
Erbitux® (Cetuximab) was obtained from ImClone LLC, NJ. Fluorescein-labeled secondary antibodies were purchased from Jackson Immunoresearch Laboratories, Inc. (West Grove, CA). p-isothiocyanato benzyl derivatives of diethylenetriaminepentacetic acid (p-SCN-Bn-DTPA), CHX-A″-DTPA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (p-SCN-Bn-DOTA) and 1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) were procured from Macrocyclics, Inc. (Dallas, TX). Fluorescein isothiocyanate (FITC) and Chelex-100 resin (50–100 mesh) were purchased from Sigma-Aldrich (St. Louis, MO). AlexaFluor350-NHS ester (NHS denotes N-hydroxysuccinimide) was acquired from Invitrogen (Grand Island, NY). PD-10 columns were purchased from GE Healthcare (Piscataway, NJ). Pierce® immobilized papain, Pierce® Protein A columns, Protein A IgG binding and elution buffers, and all other chemicals were purchased from Thermo Fisher Scientific (Fair Lawn, NJ). Amicon Ultra-15 centrifugal filter units with Ultracel-30 membrane (30K centrifugal filters) of 5 mL capacity were obtained from Millipore (Merck, Germany). Matrigel was purchased from BD Biosciences, Franklin lakes, NJ. Tissue-Tek OCT Compound (embedding medium for frozen tissue specimen) was procured from Torrance, CA. Water and all buffers were of Millipore grade and pretreated with Chelex-100 resin to ensure that the aqueous solution was free of heavy metals.
Generation and characterization of Cetuximab-Fab
Cetuximab (2 mg/mL) was digested with immobilized papain (Cetuximab: papain ratio ~ 1:40 in a reaction buffer (20 mM sodium phosphate monobasic, 10 mM disodium ethylenediaminetetraacetic acid (EDTA), and 80 mM cysteine-HCl; pH ~7) for 4 h at 37 °C. 28 Subsequently, the reaction mixture was centrifuged at 5000×g for 1 min to remove the immobilized papain. The reaction mixture containing Cetuximab-Fab was subsequently purified by passing through Protein A column. The concentration of Cetuximab-Fab in the purified solution was determined from UV absorbance at 280 nm using NanoDrop UV/Visible spectrophotometer (Thermo Scientific, USA). The purity of Cetuximab-Fab was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 5 % stacking gel and 8 % resolving gel; non-reducing conditions) using Coomassie brilliant blue R-250 staining. The molecular weight of Cetuximab-Fab was determined by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry, which served as a reference for the Cetuximab-Fab band in SDS-PAGE.
BFC/FITC/AlexaFluor350 conjugation of Cetuximab-Fab
CHX-A″-DTPA conjugation with denatured and intact Cetuximab-Fab was carried out at pH 9.0, maintaining the reaction ratio of CHX-A″-DTPA to Cetuximab-Fab at 10:1. CHX-A″-DTPA-Cetuximab-Fab was purified using PD-10 columns with PBS as the mobile phase. CHX-A″-DTPA-Cetuximab-Fab eluted between 3.0 – 4.0 mL. Same procedure was adopted for conjugation of other BFCs (p-SCN-Bn-DTPA, p-SCN-Bn-DOTA and p-SCN-Bn-NOTA). FITC (for flow cytometry analysis) or AlexaFluor350-NHS ester (for histology applications) were also conjugated onto Cetuximab-Fab using similar reaction and purification procedures. However, the reaction ratio of FITC or AlexaFluor350 NHS ester to Cetuximab-Fab was 3:1 to limit the number of dyes per Cetuximab-Fab and avoid self-quenching of the fluorescence signal.
Flow cytometry
The EGFR specificity of Cetuximab-Fab was evaluated by fluorescence-activated cell sorting (FACS) analysis in two cell lines: human glioblastoma cells (U87MG; high EGFR expression 22) and human epithelial colorectal adenocarcinoma cells (Caco-2; low EGFR expression 29). Cells were harvested and suspended in cold PBS (pH 7.4) with 2 % bovine serum albumin at a concentration of 5×106 cells/mL, and were incubated with FITC-Cetuximab-Fab at 50 nM concentration for 30 min at room temperature, washed and centrifuged at 1,000 rpm for 5 min. Afterwards, the cells were analyzed by FACS using a BD FACSCalibur four-color analysis cytometer (Becton-Dickinson, San Jose, CA) and FlowJo analysis software (Tree Star, Inc., Ashland, OR).
Radiolabeling of Cetuximab-Fab
Cyclotron produced 44Sc (74 MBq) 31 was diluted in 500 μL of 0.5 M sodium acetate buffer (pH 6.5) and added to 40 μg of BFC conjugated Cetuximab-Fab. The pH of the reaction mixture was carefully adjusted to ~4.5 and incubated for 30 min at room temperature (25 °C) with constant shaking. The radiolabeled agent was purified using PD-10 columns with PBS as the mobile phase. The radioactive fractions containing 44Sc-labeled-Cetuximab-Fab (which typically elute between 3-4 mL) were collected and used for further in vivo PET imaging studies. To determine the radiolabeling yield, the fractions containing Cetuximab-Fab were pooled together; combined activity was measured and compared with the total activity passed through the column. The radiolabeled agent was passed through a 0.2-μm syringe filter before in vivo studies.
Serum stability of 44Sc-CHX-A″-DTPA-Cetuximab-Fab
In order to determine the in vitro stability of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in mouse serum, 200 µL of the reaction mixture was added to 1.8 mL of mouse serum pre-warmed at 37 °C and incubated at the same temperature for different time intervals. As a control, equivalent activity of free 44ScCl3 (maintained at pH 4.5 in acetate buffer) was incubated with mouse serum under the same conditions. Aliquots were withdrawn at intervals of 15 min, 30 min, 1 h, 2 h, 4 h and 6 h and their activities were measured. The aliquot at each time point was taken in a 30 kDa centrifugal filter tube and centrifuged at 5000 radial centrifugal force for 15 minutes. The activity of the filtrate was measured, corrected for decay and compared with the activity of the serum solution (before ultrafiltration) to determine the percentage of free 44Sc (that detaches from 44Sc-CHX-A″-DTPA-Cetuximab-Fab) at each time point.
Animal models
All animal studies were conducted under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. U87MG cells and Caco-2 cells were used for tumor inoculation when they reached ~80% confluence. Four- to five-week-old female athymic nude mice were purchased from Harlan (Indianapolis, IN) and tumors were established by subcutaneously injecting 5 × 106 cells, suspended in 100 µL of 1:1 mixture of DMEM medium and matrigel, into the front flank of the mice. 22 The tumor sizes were monitored every alternate day and in vivo experiments were carried out when the diameter of the tumors reached 6-8 mm (typically 3 weeks after inoculation).
PET imaging and biodistribution studies
PET and PET/CT scans at various time points p.i., image reconstruction, and region-of-interest (ROI) analyses were performed using a microPET/microCT Inveon rodent model scanner (Siemens Medical Solutions USA, Inc.) and Inveon Research Workplace [IRW] vendor software, respectively, as described previously. 30 Each tumor-bearing mouse was injected with 1.85-3.7 MBq of 44Sc-CHX-A″-DTPA-Cetuximab-Fab via the tail vein and static PET scans were performed. In order to improve the detection statistics and minimize inter-scan variability due to radioactive decay, 20 million coincidence events per mouse were acquired for every static PET emission scan (energy window: 350-650 keV; time window 3.432 ns; resolution 1.5 mm). Quantitative data is presented as percentage injected dose per gram (%ID/g) of tissue. Blocking studies were carried out to evaluate EGFR specificity of 44Sc-CHX-A″-DTPA-Cetuximab-Fab in vivo, in which a group of three mice bearing U87MG tumors were each injected with 2 mg of Cetuximab, 24 h before 44Sc-CHX-A″-DTPA-Cetuximab-Fab administration. Biodistribution studies were carried out after the last PET scans to validate the PET results. The radioactivity in the tissue was measured using a gamma-counter (Perkin Elmer) and presented as %ID/g.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported, in part, by the University of Wisconsin - Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365 and P30CA014520), the Department of Defense (W81XWH-11-1-0644), the American Cancer Society (125246-RSG-13-099-01-CCE), US Department of Energy (DOE-SC0008384) and the Fulbright Scholar Program (1831/FNPDR/2013).
Footnotes
SUPPORTING INFORMATION
Data related to 44Sc-labeling yields of Cetuximab-Fab conjugates, 44Sc-labeling yields in different pH conditions, elution profile of 44Sc-CHX-A″-DTPA-Cetuximab-Fab from PD-10 column, serum stability of 44Sc-CHX-A″-DTPA-Cetuximab-Fab, and immunofluorescence staining of tumor and normal tissues are provided in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Roesch F. Scandium-44: benefits of a long-lived PET radionuclide available from the (44)Ti/(44)Sc generator system. Curr Radiopharm. 2012;5:187–201. doi: 10.2174/1874471011205030187. [DOI] [PubMed] [Google Scholar]
- (2).Severin GW, Engle JW, Valdovinos HF, Barnhart TE, Nickles RJ. Cyclotron produced (4)(4)gSc from natural calcium. Appl Radiat Isot. 2012;70:1526–30. doi: 10.1016/j.apradiso.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Cutler CS, Hennkens HM, Sisay N, Huclier-Markai S, Jurisson SS. Radiometals for combined imaging and therapy. Chem Rev. 2013;113:858–83. doi: 10.1021/cr3003104. [DOI] [PubMed] [Google Scholar]
- (4).Koumarianou E, Loktionova NS, Fellner M, Roesch F, Thews O, Pawlak D, Archimandritis SC, Mikolajczak R. 44Sc-DOTA-BN[2-14]NH2 in comparison to 68Ga-DOTA-BN[2-14]NH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? Appl Radiat Isot. 2012;70:2669–76. doi: 10.1016/j.apradiso.2012.08.004. [DOI] [PubMed] [Google Scholar]
- (5).Eigner S, Vera DR, Fellner M, Loktionova NS, Piel M, Lebeda O, Rosch F, Ross TL, Henke KE. Imaging of protein synthesis: in vitro and in vivo evaluation of (44)Sc-DOTA-puromycin. Mol Imaging Biol. 2013;15:79–86. doi: 10.1007/s11307-012-0561-3. [DOI] [PubMed] [Google Scholar]
- (6).Muller C, Bunka M, Reber J, Fischer C, Zhernosekov K, Turler A, Schibli R. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent beta--emitters: in vitro and in vivo study of a 44Sc-DOTA-folate conjugate. J Nucl Med. 2013;54:2168–74. doi: 10.2967/jnumed.113.123810. [DOI] [PubMed] [Google Scholar]
- (7).Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, Barnhart TE, Cai W. (44)Sc: An Attractive Isotope for Peptide-Based PET Imaging. Mol Pharm. 2014;11:2954–61. doi: 10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Majkowska-Pilip A, Bilewicz A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J Inorg Biochem. 2011;105:313–20. doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- (9).Corcoran EB, Hanson RN. Imaging EGFR and HER2 by PET and SPECT: a Review. Med Res Rev. 2014;34:596–643. doi: 10.1002/med.21299. [DOI] [PubMed] [Google Scholar]
- (10).Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today (Barc) 2005;41:107–27. doi: 10.1358/dot.2005.41.2.882662. [DOI] [PubMed] [Google Scholar]
- (11).Sihver W, Pietzsch J, Krause M, Baumann M, Steinbach J, Pietzsch HJ. Radiolabeled Cetuximab Conjugates for EGFR Targeted Cancer Diagnostics and Therapy. Pharmaceuticals (Basel) 2014;7:311–38. doi: 10.3390/ph7030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40:167–81. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Romer T, Leonhardt H, Rothbauer U. Engineering antibodies and proteins for molecular in vivo imaging. Curr Opin Biotechnol. 2011;22:882–7. doi: 10.1016/j.copbio.2011.06.007. [DOI] [PubMed] [Google Scholar]
- (14).Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–36. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- (15).van Dijk LK, Hoeben BA, Stegeman H, Kaanders JH, Franssen GM, Boerman OC, Bussink J. 111In-cetuximab-F(ab')2 SPECT imaging for quantification of accessible epidermal growth factor receptors (EGFR) in HNSCC xenografts. Radiother Oncol. 2013;108:484–8. doi: 10.1016/j.radonc.2013.06.034. [DOI] [PubMed] [Google Scholar]
- (16).van Dijk LK, Hoeben BA, Kaanders JH, Franssen GM, Boerman OC, Bussink J. Imaging of epidermal growth factor receptor expression in head and neck cancer with SPECT/CT and 111In-labeled cetuximab-F(ab')2. J Nucl Med. 2013;54:2118–24. doi: 10.2967/jnumed.113.123612. [DOI] [PubMed] [Google Scholar]
- (17).Bork C, Holdridge S, Walter M, Fallon E, Pohlscheidt M. Online integrity monitoring in the protein A step of mAb production processes-increasing reliability and process robustness. Biotechnol Prog. 2014;30:383–90. doi: 10.1002/btpr.1849. [DOI] [PubMed] [Google Scholar]
- (18).Shamashkin M, Godavarti R, Iskra T, Coffman J. A tandem laboratory scale protein purification process using Protein A affinity and anion exchange chromatography operated in a weak partitioning mode. Biotechnol Bioeng. 2013;110:2655–63. doi: 10.1002/bit.24955. [DOI] [PubMed] [Google Scholar]
- (19).Wang L, Dembecki J, Jaffe NE, O'Mara BW, Cai H, Sparks CN, Zhang J, Laino SG, Russell RJ, Wang M. A safe, effective, and facility compatible cleaning in place procedure for affinity resin in large-scale monoclonal antibody purification. J Chromatogr A. 2013;1308:86–95. doi: 10.1016/j.chroma.2013.07.096. [DOI] [PubMed] [Google Scholar]
- (20).Chakravarty R, Chakraborty S, Dash A. A systematic comparative evaluation of 90Y-labeled bifunctional chelators for their use in targeted therapy. J Labelled Comp Radiopharm. 2014;57:65–74. doi: 10.1002/jlcr.3140. [DOI] [PubMed] [Google Scholar]
- (21).Brechbiel MW. Bifunctional chelates for metal nuclides. Q J Nucl Med Mol Imaging. 2008;52:166–73. [PMC free article] [PubMed] [Google Scholar]
- (22).Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34:850–8. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- (23).Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- (24).Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, Lambin P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50:123–31. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- (25).Pruszynski M, Loktionova NS, Filosofov DV, Rosch F. Post-elution processing of (44)Ti/(44)Sc generator-derived (44)Sc for clinical application. Appl Radiat Isot. 2010;68:1636–41. doi: 10.1016/j.apradiso.2010.04.003. [DOI] [PubMed] [Google Scholar]
- (26).Hoehr C, Oehlke E, Benard F, Lee CJ, Hou X, Badesso B, Ferguson S, Miao Q, Yang H, Buckley K, Hanemaayer V, Zeisler S, Ruth T, Celler A, Schaffer P. (44g)Sc production using a water target on a 13MeV cyclotron. Nucl Med Biol. 2014;41:401–6. doi: 10.1016/j.nucmedbio.2013.12.016. [DOI] [PubMed] [Google Scholar]
- (27).Price EW, Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem Soc Rev. 2014;43:260–90. doi: 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- (28).Andrew SM, Pimm MV, Perkins AC, Baldwin RW. Comparative imaging and biodistribution studies with an anti-CEA monoclonal antibody and its F(ab)2 and Fab fragments in mice with colon carcinoma xenografts. Eur J Nucl Med. 1986;12:168–75. doi: 10.1007/BF00256915. [DOI] [PubMed] [Google Scholar]
- (29).Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther. 2005;4:435–42. doi: 10.1158/1535-7163.MCT-04-0280. [DOI] [PubMed] [Google Scholar]
- (30).Zhang Y, Hong H, Orbay H, Valdovinos HF, Nayak TR, Theuer CP, Barnhart TE, Cai W. PET imaging of CD105/endoglin expression with a (6)(1)/(6)(4)Cu-labeled Fab antibody fragment. Eur J Nucl Med Mol Imaging. 2013;40:759–67. doi: 10.1007/s00259-012-2334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Valdovinos HF, Hernandez R, Barnhart TE, Graves S, Nickles RJ. Separation of cyclotron-produced 44Sc from a natural calcium target using a dipentyl pentylphosphonate functionalized extraction resin. Appl Radiat Isot. 2014;95:23–9. doi: 10.1016/j.apradiso.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.