Abstract
Bisphosphonates are commonly prescribed for treatment of osteoporosis. Long-term use of bisphosphonates has been correlated to atypical femoral fractures (AFF). AFFs arise from fatigue damage to bone tissue that cannot be repaired due to pharmacologic treatments. Despite fatigue being the primary damage mechanism of AFFs, the effects of osteoporosis treatments on fatigue properties of cortical bone are unknown. To examine if fatigue-life differences occur in bone tissue after different pharmacologic treatments for osteoporosis, we tested bone tissue from the femurs of sheep given a metabolic acidosis diet to induce osteoporosis, followed by treatment with a selective estrogen reception modulator (raloxifene), a bisphosphonate (alendronate or zoledronate), or parathyroid hormone (teriparatide, PTH). Beams of cortical bone tissue were created and tested in four-point bending fatigue to failure. Tissues treated with alendronate had reduced fatigue life and less modulus loss at failure compared to other treatments, while tissue treated with PTH had a prolonged fatigue life. No loss of fatigue life occurred with zoledronate treatment despite its greater binding affinity and potency compared to alendronate. Tissue mineralization measured by microCT did not explain the differences seen in fatigue behavior. Increased fatigue life with PTH suggests that current treatment methods for AFF could have beneficial effects for restoring fatigue life. These results indicate that fatigue life differs with each type of osteoporosis treatment.
Introduction
Osteoporotic fractures are a substantial public health concern with total fractures and associated costs estimated to continue to rise through 2025(1). Bisphosphonates are a commonly prescribed class of anti-resorptive drug that increase bone mineral density between 0–8% while reducing the risk of fracture by up to 50% in osteoporotic patients(2,3). The large decrease in fracture risk despite the modest increase in bone mineral density suggests a material property change in bisphosphonate-treated tissue. Suppression of bone remodeling with bisphosphonates has led to concern over inability to repair damaged and older tissue(4). To fully understand the reduction in fracture risk, all fracture properties and mechanisms should be examined.
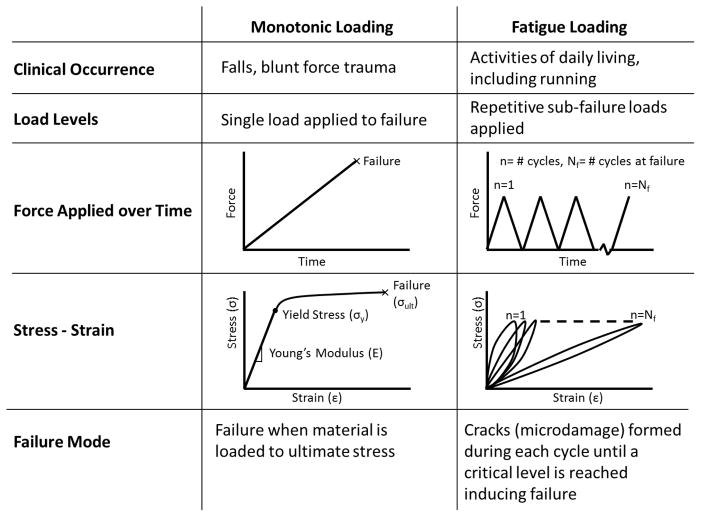
Fracture of osteoporotic bone typically occurs through one of two mechanisms, a single overload (traumatic failure), or repetitive sub-fracture loads (fatigue failure; Figure 1). Typical osteoporotic hip fractures are due to mechanical overload, in which the femoral head and neck are subjected to loads that the bone cannot withstand due to reduced bone mass. Fatigue loads are repetitive, sub-failure forces applied to the tissue. Activities of daily living create fatigue loads that in turn create microdamage in the tissue(5). Healthy individuals are unlikely to experience fatigue fractures under normal loading conditions since damage to the bone is typically repaired before fracture can occur. However, tissue properties may be altered in individuals using anti-resorptive treatments(6–9). Knowledge of fatigue on bone tissue has been primarily gained from testing of machined sections of bones and has shown fatigue dependence with temperature, stress amplitude, and bone microstructure(10–12). Studies examining fatigue of osteoporotic and treated tissue have focused on microdamage accumulation rather than the material properties of the tissue(4).
Figure 1.
Comparison of monotonic and fatigue loading. In monotonic loaded samples, force is increased until the sample fails. In fatigue, a repetitive sub-failure load is applied creating damage that eventually coalesces to cause failure.
Bisphosphonates act through osteoclast inhibition, which leads to reduced bone turnover, increased bone mass and increased mineralization(13). However, injury within tissue cannot be remodeled leading to an accumulation of microdamage(14–17). Reduced bone turnover with bisphosphonate treatment increases mineralization and collagen maturity in bone tissue as measured by Fourier transform infrared spectroscopy (FTIR)(18). Tests on whole bones after bisphosphonate therapy indicate an increase in monotonic strength and stiffness at corticocancellous sites without concomitant changes to the tissue-level modulus or ultimate strength(4,17). A loss of toughness and energy dissipation in cortical and cancellous tissue has been found with bisphosphonate treatment(4). Fatigue properties are likely altered with bisphosphonate treatment; however, minimal data regarding these properties have been published(4). Increased microdamage in both cortical and cancellous tissue with bisphosphonate treatment may reflect an inability to repair damage within the tissue(14–17). Alendronate reduced the fatigue life in beams created from rib bones from healthy canines; however, the dosing was supraphysiological and osteoporosis was not induced prior to treatment(19).
Long-term bisphosphonate use is associated with atypical femoral fractures (AFF)(20,21). AFF incidence with bisphosphonate use is relatively low, but is associated with considerable morbidity(22). The mechanics of these fractures indicate critical differences from typical osteoporotic fractures(23,24). Association with low loads indicates AFFs result from repetitive (fatigue) loading rather than a single traumatic incident. The transverse nature of the fractures suggests altered material properties with tissue becoming more brittle.
Bisphosphonates are the most common therapy prescribed for osteoporosis treatment, but other treatments exist. Selective Estrogen Receptor Modulators (SERM) reduce osteoporotic vertebral fracture risk by 30–50%(25). SERMs bind to the estrogen receptors with an affinity similar to estradiol(26). Teriparatide (PTH) has been beneficial in patients who experience AFFs by inducing increased bone remodeling, removal of older more fully mineralized tissue and replacement with new less fully mineralized tissue(27). Mechanical property data for SERM and PTH treatments of bone have focused on monotonic failure properties and have not included fatigue.
The purpose of this study was to examine the fatigue and fracture properties of bone tissue after different osteoporosis treatments using a sheep model of osteopenia to determine if a correlation exists between fatigue life and treatment type. Osteopenia was induced in sheep and followed by an osteoporosis treatment or vehicle. Beams of known geometry created from the femoral diaphysis of these sheep were loaded in four-point bending fatigue to failure. Given the inhibition of remodeling, and increased mineralization and collagen maturity reported with bisphosphonate treatment, we theorized that a shorter fatigue life will occur with bisphosphonate treatment.
Materials and Methods
Animal Model
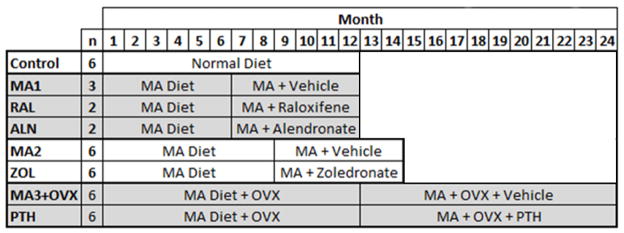
Samples used in this study were from remaining femur tissue from previously published and in progress studies(28). For all studies we fed a metabolic acidosis (MA) diet to skeletally mature sheep to induce osteopenia(29). In the first study, sheep fed a normal diet served as healthy controls for the experiment (C, n=6). In the second study, sheep were fed the MA diet for 12 months and given Alendronate (ALN; n=2), Raloxifene (RAL; n=2) or a vehicle (MA1; n=3) treatment during months 7–12. The low sample sizes were not planned and reflect factors beyond our control in the experiment. To further examine bisphosphonate treatment, a third experiment was performed with sheep fed a MA diet for 8 months followed by 6 months of treatment with Zoledronate (Reclast, ZOL; n=6) or vehicle (MA2; n=6) while continuing the MA diet. The longer initial MA term in experiment three was due a delay in procuring the zoledronate. In the second study, alendronate (0.15 mg/kg) and raloxifene (0.8 mg/kg) were administered daily via a cannula placed into the duodenum, whereas zoledronate (5 mg/sheep) was administered as a single intravenous injection. This schedule replicates the clinical dosing in which alendronate is taken orally daily or weekly and zoledronate is administered intravenously once a year(30,31). All animal procedures were reviewed and approved by the Colorado State University IACUC and the Hospital for Special Surgery IACUC.
A fourth set of skeletally mature sheep were fed an MA diet for one year after ovariectomy, which has been shown to induce osteopenia(32). The sheep were then maintained on the MA diet and were administered teriparatide (PTH, n=6) or vehicle (MA3+OVX, n=6) for one year. Treatment was administered daily via subcutaneous injection (5 mcg/kg). All animal procedures were reviewed and approved by the University of Minnesota IACUC and the Hospital for Special Surgery IACUC.
Sample Preparation
Sheep were euthanized at the end of the specified treatment period. Femurs were removed and stored at −20°C in saline-soaked gauze until the time of sample preparation. Beams were cut out of the medial diaphysis of the femurs using a low speed diamond saw (Buehler Isomet; Lake Bluff, IL, USA). Beams were then polished using 15, 5 and 1 micron lapping films with ethylene glycol used as a lubricant to prevent mineral leaching(33,34). Samples were polished to a final size of 2 × 2 × 25 mm. After polishing, samples were stored at −20°C in hydroxyapatite-buffered saline-soaked gauze until testing.
Fatigue Testing
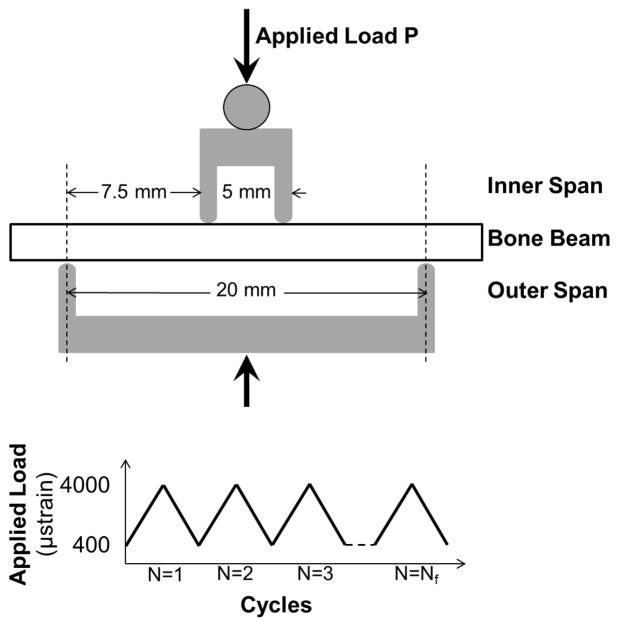
Beams were tested in four-point bending fatigue(35,36) (Bose Electroforce LM-1, Eden Prairie, Minnesota, USA). The bottom supports were placed 20 mm apart, and the top loading points placed 5 mm apart (Figure 2). These positions created a constant bending moment between the loading points and limited the effects of crushing at the load points(37). Preconditioning was completed by 20 cycles of loading from 2 to 20 N. These values were chosen through preliminary testing that demonstrated that these loads induced normal surface strains below the 2500 με necessary to create microdamage and alter fatigue life(10). The initial flexural modulus was measured from the 10th cycle and calculated using the assumptions of linear elastic beam theory. Initial modulus values were used to calculate the force necessary to achieve desired values of strain of 400 to 4000 με on the tensile and compressive surfaces. Samples were loaded in force control from 400 to 4000 με (R=0.1) to failure with peak-to-peak force and displacement measured at each cycle. Cycles–to-failure, Nf, was defined as the number of cycles experienced before the sample broke. Modulus loss at failure was defined as the percent change in modulus from the 10th cycle to Nf, and was calculated with linear elastic beam theory(38). All testing was completed at physiologic temperature (37°C) in hydroxyapatite-buffered PBS (1g HA added per 1L PBS and allowed to sit overnight until solution was supersaturated) with temperature monitored continuously.
Figure 2.
Set up for four point bending fatigue loading. P = applied load. The span between the inner supports was 5 mm, and for the outer supports 20 mm. A cyclic load was applied to failure with strain levels between 400 and 4000 μstrain.
Microcomputed Tomography (microCT)
Tissue mineral density (TMD) was measured with microCT at a 50 micron voxel size (eXplore CT 120, GE Healthcare, Waukesha, WI, USA). A mineral phantom was used for calibration with analysis completed in Microview (version ABA 2.2, GE Healthcare, Waukesha, WI, USA).
Statistical Analysis
The purpose of the experiment was to determine differences in Nf among the different treatment groups and correlate the differences to TMD data. A standard least squares analysis was used to compare each group to the grand mean of the data. The four separate experiments limited the ability to compare data across experiments. A log transform was performed on the cycles-to-failure data to meet the assumption of equal variance between groups.
Different treatments and durations of MA controls can influence the results. Low sample sizes also limited comparisons among groups. For comparison of the MA1, raloxifene and alendronate groups, a Students t-test was used to compare each group with a Bonferroni post hoc correction applied. A Students t-test was completed also for the MA2 and zoledronate data, MA3+OVX data and PTH. The Bonferroni correction and t-test comparisons were necessary due to low sample sizes and not meeting the assumptions for an ANOVA.
Results
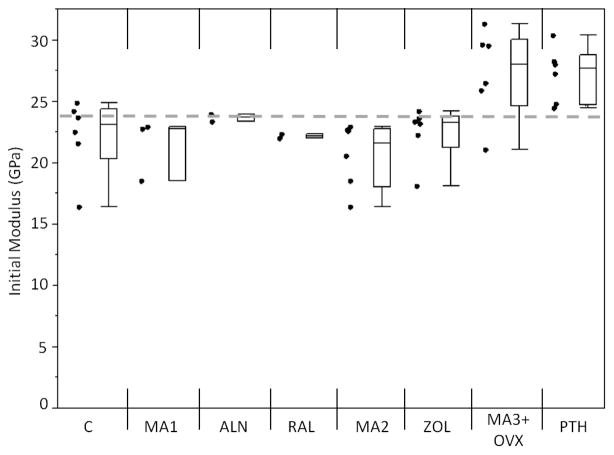
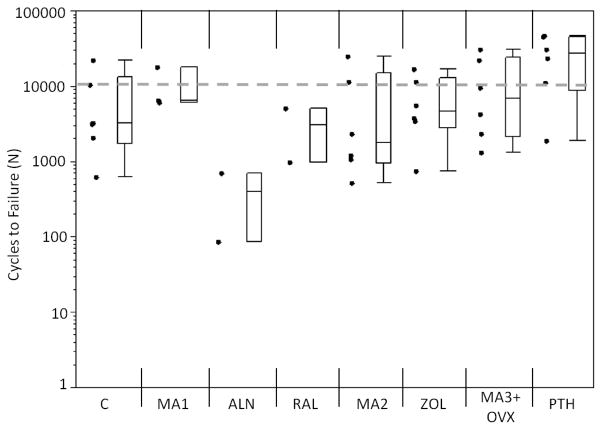
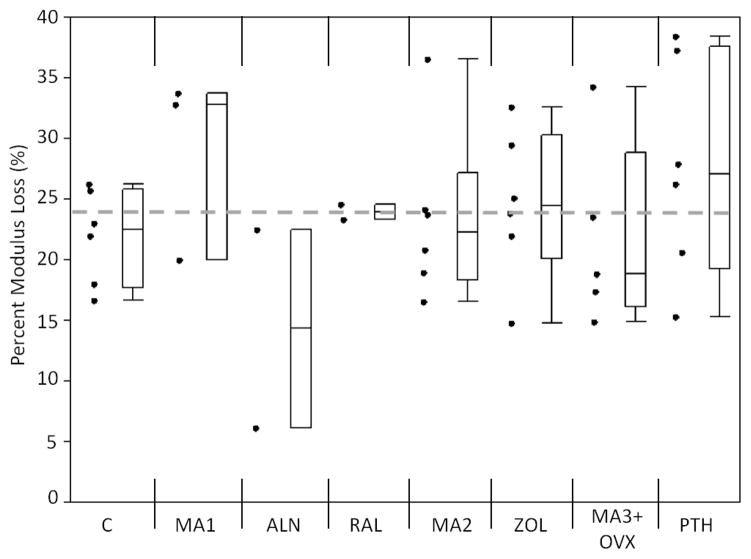
An increase in the initial flexural modulus was seen in the MA3+OVX and PTH groups as compared to the grand mean of all groups (Figure 3). Samples treated with alendronate had a significantly lower Nf compared to the grand mean of all groups (p<0.01), while PTH samples had significantly greater Nf compared to the grand mean (p<0.01; Figure 4). A loss of fatigue life occurred between alendronate (ALN) and its metabolic acidosis control (MA1; p<0.01). Modulus loss at failure was significantly lower in the alendronate-treated groups compared to the grand mean (p<0.05; Figure 5).
Figure 3.
Initial modulus values for each group. MA3+OVX and PTH had higher initial moduli than the other groups. Markers to the left represent individual sample data points. Box and whisker plots on right show the minimum, maximum, mean, and 25th and 75th quartiles for each group. Dashed line is the grand mean.
Figure 4.
Cycles to failure for each group. Alendronate (ALN) had fewer cycles to failure compared to the grand mean (p<0.01). Teriparatide (PTH) had more cycles to failure compared to the grand mean (p<0.01). Markers to the left represent individual sample data points. Box and whisker plots on the right show the minimum, maximum, mean, and 25th and 75th quartiles. Dashed line represents the grand mean.
Figure 5.
Modulus loss at failure for each group. Alendronate had a lower modulus loss at failure compared to the grand mean (p<0.05). Markers to the left represent individual sample data points. Box and whisker plots on the right show the minimum, maximum, mean, and 25th and 75th quartiles. Dashed line represents the grand mean.
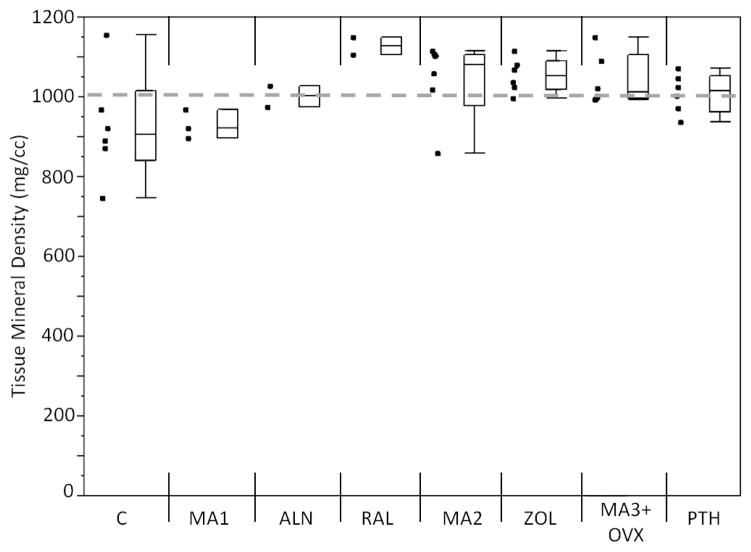
Mineralization measures (TMD) did not account for the differences in fatigue behavior. Control samples had a lower TMD compared to the grand mean, while raloxifene raised the TMD above the grand mean (p<0.05; Figure 6).
Figure 6.
Mineralization measure for each group. Control samples had a lower TMD as compared to the grand mean while raloxifene samples had increased TMD as compared to the grand mean (p<0.05). Markers to the left represent individual sample data points. Box and whisker plots on the right show the minimum, maximum, mean, and 25th and 75th quartiles. Dashed line represents the grand mean.
Discussion and Conclusions
Fatigue properties were examined in cortical bone tissue from sheep treated by anti-resorptive drugs after induction of osteoporosis. Four-point bending fatigue testing to failure was completed at physiologic temperature on bone beams created from the femoral diaphysis. Osteoporosis treatments had differing effects on the fatigue life of cortical bone tissue. Alendronate treatment caused a significant loss in fatigue life as compared to the grand mean and its MA control; however, zoledronate-treated specimens did not experience any change in fatigue life from the grand mean or MA control. Greater changes might be expected with zoledronate than alendronate given zoledronate’s greater binding affinity and potency(39). Alendronate has a relative potency of 1–2×103, whereas zoledronate has a relative potency of 104 compared to etidronate(40). Raloxifene did not change the fatigue life of the tissue while PTH increased fatigue life over the grand mean of the data. Differences in the fatigue life indicate material property changes caused by binding affinity, dosing, chemical structure or collagen changes.
Differences in the administration of the bisphosphonates may contribute to the altered fatigue properties. Alendronate was administered daily via cannula while zoledronate was given once over the course of the experiment via intravenous injection following clinical dosing regimens(30,31). With daily dosing of alendronate the bisphosphonate is present in the serum continuously affecting biomarkers of bone turnover, whereas a single dose of zoledronate may allow the serum biomarker levels to return to pre-treatment homeostasis. Serum CTX is known to be reduced with bisphosphonate dosing and increase with time since last administration(41,42). Increased collagen maturity occurs with bisphosphonate treatment, and suppression of serum biomarkers such as CTX may indicate differences between the two bisphosphonate types.
Bisphosphonate molecular structure and distribution throughout the tissue are also theorized to have an effect on the tissue properties. Regions of higher mineralization were surface-based on trabeculae with zoledronate treatment(28), which supports the idea that zoledronate has a more surface-based effect. Distribution of bisphosphonates throughout cortical bone tissue has only been reported with the use of ibandronate and differences in distribution between proximal and distal cortices were noted(43). Higher-affinity bisphosphonates have less diffusion into the bone, which could cause differences between alendronate and zoledronate(44).
Alendronate-treated samples had lower modulus loss at failure indicating a more brittle material. Microdamage quantities in these samples were not analyzed, so differences in this parameter among groups are unknown. Microdamage is associated with fatigue loading, and increased microdamage is correlated to loss of modulus in trabecular bone(45). Greater microdamage created by activities of daily living occurs in both cortical and cancellous tissues with alendronate treatment compared to untreated control tissues(14,16).
In this study, applied loads created maximum normal strains from 400 to 4000 με. In laboratory fatigue conditions in bending, damage creation starts at 2500 με in regions under tension; however, greater than 4000 με is necessary in the regions under compression(10). The 4000 με applied in our study would, therefore, create damage in the tensile region with the compressive region receiving little damage. Greater damage in the tensile region is similar to AFF progression, in which the stress fracture develops from the lateral cortex that is under tension during normal weight-bearing activities.
Results of this study are limited by several factors including the underpowered sample sizes for both alendronate and raloxifene treatments. As previously stated, small sample sizes were unplanned and due to factors beyond our control in the experiment; however, recent studies in a different animal model have also demonstrated a reduction in fatigue life with alendronate treatment(19). The lack of fatigue life change with raloxifene treatment may indicate fatigue life preservation; however, this result may be due to lack of power from a small sample size. SERM therapies also have the side effect of increased risk of thromboembolic problems(46) indicating that an increased fatigue life alone may not make this therapy more appropriate. Four-point bending fatigue is not a typical method for fatigue measurements. Tensile and compressive fatigue are more commonly used for material characterization(10,11); however, limited tissue from repurposed samples prevented the possibility of analyzing tissues by this method. Finally, although having samples from four separate studies enhanced our ability to make comparisons, this situation was less than ideal as sheep are known to experience seasonal differences in BMD(47). Nevertheless, we did include untreated control samples to compare with treatment group samples.
In this study fatigue life differences with osteoporosis treatments depended on both the class of treatment, type of drug, and mode of delivery. Alendronate caused a reduction in bone tissue fatigue life while PTH caused an increase in fatigue life. Raloxifene and zoledronate did not change fatigue life. Material property alterations may be due to differences in chemical structure, mechanisms of actions of these drugs, or dosing regimens by which the drugs are administered. Under the confines of this study, drug uptake or the effect of dosing regimen were not possible to examine; however, these variables are avenues for future research that may help explain the occurrence of AFF.
Table 1.
Samples used were four different studies: [1] Age-matched control sheep fed a normal diet (Control); [2] Sheep were fed a metabolic acidosis (MA) diet for six months followed by the MA diet and twelve months of MA diet and treated by vehicle (MA1), raloxifene (RAL) or alendronate (ALN); [3] Sheep were fed an MA diet for eight months followed by six months of the MA diet combined with vehicle (MA2) or zoledronate (ZOL); [4] Sheep had an ovariectomy and were fed an MA diet for a year, followed by a year of the MA diet and vehicle (MA3+OVX) or parathyroid hormone (PTH). Control, MA1, RAL and ALN were euthanized after 12 months, MA2 and ZOL at 14 months and MA3+OVX and PTH at 24 months.
|
Highlights.
Cortical bone beams from sheep with osteoporosis treatments were tested in fatigue
Alendronate lowered fatigue life while zoledronate did not alter fatigue life
PTH treatment increased fatigue life of cortical bone tissue
Effect on fatigue life differs by type of osteoporosis treatment in cortical bone
Acknowledgments
Funding provided by NIH AR053571, NIH AR041325, NIH EB004321, and the NSF GRFP. We thank Laura Nielsen for her help preparing the PTH samples.
Footnotes
Author’s Roles: Study design: GRB, JM, ARI, ALB, MCV; Study conduct: GRB, TC, JM, GEP; Data collection: GRB, TC; Data analysis: GRB, TC; Data interpretation: GB, ALB, MCV; Drafting manuscript content: GRB; Approving final version of manuscript: GRB, TC, ARI, JM, GEP, ALB, MCV. GRB, MCV take responsibility for the integrity of the data analysis.
Disclosures:
Garry R. Brock: No Disclosures
Julia T. Chen: No Disclosures
Anthony R. Ingraffea: No Disclosures
Jennifer MacLeay: current address 3631 sw stonybrook drive, Topeka KS
G. Elizabeth Pluhar: No Disclosures
Adele Boskey: Grant Paid to Institution: NIH AR041325; Stock: Pfizer, Lilly, Amgen, <$5000 each
Marjolein C.H. van der Meulen: Grant Paid to Institution: NIH AR053571, NIH EB004321; Board Membership: Relievant Medsystems, Scientific Advisory Board
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Garry R. Brock, Email: grb54@cornell.edu.
Julia T. Chen, Email: tc468@cornell.edu.
Anthony R. Ingraffea, Email: ari1@cornell.edu.
Jennifer MacLeay, Email: jmmacleay@gmail.com.
G. Elizabeth Pluhar, Email: pluha006@umn.edu.
Adele L. Boskey, Email: boskeya@hss.edu.
Marjolein C.H. van der Meulen, Email: mcv3@cornell.edu.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002 Mar;112:281–9. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 3.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-portales JA, Downs RW, Dequeker J, Favus MJ, Seeman E, Recker RR, Capizzi T, Santora AC, Lombardi A, Shah RV, Hirsch LJ, Karpf DB. Effect of Oral Alendronate on Bone Mineral Density and the Incidence of Fractures in Postmenopausal Osteoporosis. N Engl J Med. 1995;333(22):1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 4.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone Elsevier Inc. 2011 Jul;49(1):56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 5.Taylor D, Hazenberg JG, Lee TC. Living with cracks: damage and repair in human bone. Nat Mater. 2007 Apr;6(4):263–8. doi: 10.1038/nmat1866. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly E, Meredith DS, Nguyen JT, Gladnick BP, Rebolledo BJ, Shaffer AD, Lorich DG, Lane JM, Boskey AL. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J bone Miner Res. 2012 Mar;27(3):672–8. doi: 10.1002/jbmr.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bala Y, Depalle B, Farlay D, Douillard T, Meille S, Follet H, Chapurlat R, Chevalier J, Boivin G. Bone micromechanical properties are compromised during long-term alendronate therapy independently of mineralization. J bone Miner Res. 2012 Apr;27(4):825–34. doi: 10.1002/jbmr.1501. [DOI] [PubMed] [Google Scholar]
- 8.Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011 Aug;469(8):2170–8. doi: 10.1007/s11999-010-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008 Mar;42(3):456–66. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Pattin CA, Caler WE, Carter DR. Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech. 1996 Jan;29(1):69–79. doi: 10.1016/0021-9290(94)00156-1. [DOI] [PubMed] [Google Scholar]
- 11.Carter DR, Hayes WC. Fatigue life of compact bone-I: Effects of stress amplitude, temperature and density. J Biomech. 1976 Jan;9(1):27–34. doi: 10.1016/0021-9290(76)90136-6. [DOI] [PubMed] [Google Scholar]
- 12.Carter DR, Hayes WC, Schurman DJ. Fatigue life of compact bone-II. Effects of microstructure and density. J Biomech Eng. 1976;9:211–8. doi: 10.1016/0021-9290(76)90006-3. [DOI] [PubMed] [Google Scholar]
- 13.Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003 Feb;5(1):65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 14.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000 Apr;15(4):613–20. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 15.Yamagami Y, Mashiba T, Iwata K, Tanaka M, Nozaki K, Yamamoto T. Effects of minodronic acid and alendronate on bone remodeling, microdamage accumulation, degree of mineralization and bone mechanical properties in ovariectomized cynomolgus monkeys. Bone Elsevier Inc. 2013 May;54(1):1–7. doi: 10.1016/j.bone.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001 May;28(5):524–31. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 17.Allen MR, Burr DB. Mineralization, Microdamage, and Matrix: How Bisphosphonates Influence Material Properties of Bone. BoneKEy-Osteovision. 2007;4(2):49–60. [Google Scholar]
- 18.Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone Elsevier Inc. 2010 Mar;46(3):666–72. doi: 10.1016/j.bone.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajaj D, Geissler JR, Allen MR, Burr DB, Fritton JC. The resistance of cortical bone tissue to failure under cyclic loading is reduced with alendronate. Bone Elsevier Inc. 2014 Apr 1;64:57–64. doi: 10.1016/j.bone.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res. 2010 Dec;468(12):3384–92. doi: 10.1007/s11999-010-1535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate Use and Atypical Fractures of the Femoral Shaft. N Engl J Med. 2011;364(18):1728–37. doi: 10.1056/NEJMoa1010650. [DOI] [PubMed] [Google Scholar]
- 22.Lo JC, Huang SY, Lee Ga, Khandelwal S, Khandewal S, Provus J, Ettinger B, Gonzalez JR, Hui RL, Grimsrud CD. Bone. 1. Vol. 51. Elsevier Inc; 2012. Jul, Clinical correlates of atypical femoral fracture; pp. 181–4. [DOI] [PubMed] [Google Scholar]
- 23.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler Ra, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn Ta, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MCH, Weinstein RS, Whyte M. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010 Nov;25(11):2267–94. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 24.Shane E, Burr D, Abrahamsen B, Adler Ra, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn Ta, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen Howe T, van der Meulen MC, Weinstein RS, Whyte MP. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the american society for bone and mineral research. J Bone Miner Res. 2014 Jan;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Stakkestad J, Glu CC, Krueger K, Cohen FJ, Eckert S, Avioli LV. Reduction of Vertebral Fracture Risk in Postmenopausal Women with Osteoporosis Treated with Raloxifene Results From a 3-Year Randomized Clinical Trial. J Am Ceram Soc. 1999;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 26.Rey JRC, Cervino EV, Rentero ML, Crespo EC, Alvaro AO, Casillas M. Raloxifene: mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. Open Orthop J. 2009 Jan;3:14–21. doi: 10.2174/1874325000903010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang CY, Zebaze RMD, Ghasem-Zadeh A, Iuliano-Burns S, Hardidge A, Seeman E. Bone. 1. Vol. 52. Elsevier B.V; 2013. Jan, Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy; pp. 360–5. [DOI] [PubMed] [Google Scholar]
- 28.Burket JC, Brooks DJ, MacLeay JM, Baker SP, Boskey AL, van der Meulen MCH. Variations in nanomechanical properties and tissue composition within trabeculae from an ovine model of osteoporosis and treatment. Bone Elsevier Inc. 2013 Jan;52(1):326–36. doi: 10.1016/j.bone.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLeay JM, Olson JD, Enns RM, Les CM, Toth CA, Wheeler DL, Turner AS. Dietary-induced metabolic acidosis decreases bone mineral density in mature ovariectomized ewes. Calcif Tissue Int. 2004 Nov;75:431–7. doi: 10.1007/s00223-004-0217-7. [DOI] [PubMed] [Google Scholar]
- 30.Bone HG, Adami S, Rizoli R, Favus M, Ross PD, Santora A, Prahalada S, Daifotis A, Orlofl J, Yates J. Weekly Administration of Alendronate: Rationale and Plan for Clinical Assessment. Clin Ther. 2000;22(1):15–28. doi: 10.1016/s0149-2918(00)87974-6. [DOI] [PubMed] [Google Scholar]
- 31.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Sc DM, Cummings SR. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 32.Macleay JM, Olson JD, Turner AS. Effect of dietary-induced metabolic acidosis and ovariectomy on bone mineral density and markers of bone turnover. J Bone Miner Metab. 2004 Jan;22(6):561–8. doi: 10.1007/s00774-004-0524-0. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly E, Baker SP, Boskey AL, van der Meulen MCH. Effects of surface roughness and maximum load on the mechanical properties of cancellous bone measured by nanoindentation. J Biomed Mater Res. 2006 May;77A(2):426–35. doi: 10.1002/jbm.a.30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brock GR, Kim G, Ingraffea AR, Andrews JC, Pianetta P, van der Meulen MCH. Nanoscale examination of microdamage in sheep cortical bone using synchrotron radiation transmission x-ray microscopy. PLoS One. 2013 Jan;8(3):e57942. doi: 10.1371/journal.pone.0057942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res. 1998 May;16(3):322–9. doi: 10.1002/jor.1100160308. [DOI] [PubMed] [Google Scholar]
- 36.Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005 Jul;37(1):96–102. doi: 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Zhai T, Xu YG, Martin JW, Wilkinson AJ, Briggs GAD. A self-aligning four-point bend testing rig and sample geometry effect in four-point bend fatigue. Int J Fatigue. 1999;21:889–94. [Google Scholar]
- 38.Beer FP, Johnston ER, DeWolf JT. Mechanics of Materials. 4. New York, NY: McGraw Hill; 1996. pp. 308–63. [Google Scholar]
- 39.Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008 Jun;19(6):733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 40.Shaw NJ, Bishop NJ. Bisphosphonate treatment of bone disease. Arch Dis Child. 2005 May;90(5):494–9. doi: 10.1136/adc.2003.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000 Feb;66(2):100–3. doi: 10.1007/pl00005830. [DOI] [PubMed] [Google Scholar]
- 42.Marx RE, Cillo JE, Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg. 2007 Dec;65(12):2397–410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Smith SY, Recker RR, Hannan M, Müller R, Bauss F. Intermittent intravenous administration of the bisphosphonate ibandronate prevents bone loss and maintains bone strength and quality in ovariectomized cynomolgus monkeys. Bone. 2003 Jan;32(1):45–55. doi: 10.1016/s8756-3282(02)00923-7. [DOI] [PubMed] [Google Scholar]
- 44.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood a, Russell RGG, Ebetino FH. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006 May;38(5):617–27. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Lambers FM, Bouman AR, Rimnac CM, Hernandez CJ. Microdamage caused by fatigue loading in human cancellous bone: relationship to reductions in bone biomechanical performance. PLoS One. 2013 Jan;8(12):e83662. doi: 10.1371/journal.pone.0083662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gennari L, Merlotti D, Valleggi F, Martini G, Nuti R. Selective estrogen receptor modulators for postmenopausal osteoporosis: current state of development. Drugs Aging. 2007 Jan;24(5):361–79. doi: 10.2165/00002512-200724050-00002. [DOI] [PubMed] [Google Scholar]
- 47.Arens D, Sigrist I, Alini M, Schawalder P, Schneider E, Egermann M. Seasonal changes in bone metabolism in sheep. Vet J. 2007 Nov;174(3):585–91. doi: 10.1016/j.tvjl.2006.10.001. [DOI] [PubMed] [Google Scholar]