Abstract
Objectives
To examine the prevalence of seizures, epilepsy, and seropositivity to cysticercosis in rural villagers (cysticercosis-endemic setting), rural-to-urban migrants into a non-endemic urban shantytown, and urban inhabitants of the same non-endemic shanty town.
Methods
Three Peruvian populations (n=985) originally recruited into a study about chronic diseases and migration, were studied. These groups included rural inhabitants from an endemic region (n=200), long-term rural-to-urban migrants (n=589), and individuals living in the same urban setting (n=196). Seizure disorders were detected by a survey and a neurologist examined positive respondents. Serum samples from 981/985 individuals were processed for cysticercosis antibodies on immunoblot.
Results
Epilepsy prevalence (per 1,000 people) was 15.3 in the urban group, 35.6 in migrants, and 25 in rural inhabitants. A gradient in cysticercosis antibody seroprevalence was observed: urban 2%, migrant 13.5%, and rural group 18% (p<0.05). A similarly increasing pattern of higher seroprevalence was observed among migrants by age at migration. In rural villagers, there was strong evidence of an association between positive serology and having seizures (p=0.011) but such an association was not observed in long-term migrants or in urban residents. In the entire study population, compared to seronegative participants, those with strong antibody reactions (≥4 antibody bands) were more likely to have epilepsy (p<0.001).
Conclusions
It is not only international migration that affects cysticercosis endemicity; internal migration can also affect patterns of endemicity within an endemic country. The neurologic consequences of cysticercosis infection likely outlast the antibody response for years after rural-to-urban migration.
Keywords: Cysticercosis, neurocysticercosis, seizures, migration, Taenia solium, Peru
INTRODUCTION
Neurocysticercosis (NCC) is the most frequent helminthic infection of the human central nervous system, caused by the larvae of the pork tapeworm Taenia solium. A growing global public health problem,1 NCC is endemic in most developing countries, where it constitutes the main cause of secondary epilepsy. NCC is also emerging in industrialized countries because of migration from endemic zones.2
Closely linked to poverty as for most zoonotic infections, the bulk of cysticercosis transmission occurs in rural villages where domestic pig raising coexists with poor sanitation and lack of sewage and potable water facilities. There are multiple reports of NCC in travelers and migrants to industrialized countries.2-7 Migration also occurs within countries, usually mobilizing people from disease-endemic regions to more urban centers. However, data on the variations in prevalence and expression of NCC following migration within endemic regions is scarce or nonexistent.
In the particular case of Peru, between the 1970s and the 1990s, prolonged terrorism-induced instability and insecurity (“Shining Path”) led thousands in rural areas to abandon their communities and move to large urban areas, primarily Lima, where they established shantytowns.8 Taking advantage of, and expanding upon a study on migration and chronic diseases already ongoing in Peru,9,10 we examined the prevalence of seizures, epilepsy, and seropositivity to cysticercosis in three populations of individuals older than 30 years: rural villagers from a cysticercosis-endemic setting, long-term rural-to-urban migrants into a non-endemic urban shantytown, and urban inhabitants born and living in the same non-endemic shanty town.
MATERIALS AND METHODS
Study design and study populations
This cross-sectional study assessed the prevalence of seizures and the prevalence of specific antibodies to cysticercosis in serum of three population-based groups: Rural, people born in Ayacucho who had always lived in a rural environment; Rural-to-urban Migrants, people born in Ayacucho who migrated from rural to urban areas and currently living in Lima, an 8-million metropolis; and Urban, people born and currently living in Lima.
The study expanded an ongoing study, reported elsewhere, that aimed to determine the relationship between migration and chronic diseases.9,10 For that parent study, the populations were San Jose de Secce, population 7,215 inhabitants, a village in Ayacucho located in the Central/Southern Peruvian Highlands, Figure 1;11 and a shanty-town, Pampas de San Juan de Miraflores in Lima, population 362,643 inhabitants.
Figure 1.
Peru map showing the locations of Secce (Ayacucho) and Lima (modified from reference 11)
Setting
San Jose de Secce, rural site in Ayacucho, is located in one of the most severely affected areas during the period of terrorist violence,12 where a large proportion of the population had migrated to urban areas. By the time of the study, 91% of Ayacucho's inhabitants lived in a rural area and 97% spoke Quechua, a native language.13
Pampas de San Juan de Miraflores was the urban area for the study. It is a typical shantytown where migrants from rural areas have moved into Lima, Peru's capital, and settled over the years. Migrants from the Southern provinces, including Ayacucho, are more likely to settle in San Juan de Miraflores. Both urban and rural-to-urban migrant participants were selected from this 100% urban area. Only 11% learnt a native language during childhood and, based on place of birth, 50% were classified as migrants.13 Travel from San Jose de Secce to Lima (310 km) takes 12 to 15 hours by bus due to the mountainous geography of the Andean region.
Sampling
In the parent study, a single-stage random sampling method was used in all groups. For all study groups, individuals from both sexes aged 30 years and older, to allow the evaluation of the effects of time from migration, and permanently living in their residence were considered eligible. The sampling frames of adults >30 years were 398, 1,785, and 4,621 for the rural (Ayacucho), rural-to-urban migrants (Ayacucho to Lima) and urban (Lima) groups, respectively. The selection of participants was stratified by 5-year age groups and sex. The original study enrolled a total of 989 people, 201 people in the rural group, 199 in the urban group, and 589 migrants.9,10
Evaluations and exams
The parent study gathered information in different modules including a socio demographic, migration and chronic disease's risk factors surveys, a clinical examination and laboratory tests, as reported elsewhere.9 For this study, all consenting individuals were screened for seizures using a standardized 9-question survey.14-16 A trained neurologist interviewed positive survey's respondents to confirm or rule out a diagnosis of seizures or epilepsy according to definitions provided below.
An aliquot of 100 uL of serum was separated from archive samples collected during the parent study and processed for specific antibodies against cysticercosis on enzyme-linked immunoelectrotransfer blot (EITB, western blot) assay using purified glycoprotein antigens.17
Operational definitions
Positive survey's respondent refers to any individual with an affirmative response to one or more of the 9 questions.14-16 Individual with seizures refers to any individual describing one or more seizure events, provoked or unprovoked. A diagnosis of seizures does not depend on confirmatory testing but it is based on interview data from the patient or from witnesses. A normal clinical examination or a negative EEG does not exclude the diagnosis. Seizures were typified according to the classification of seizure types of the International League Against Epilepsy.18 Individual with epilepsy refers to any individual describing two or more unprovoked seizures in a period of more than 24 hours.19,20 Active epilepsy refers to an individual with epilepsy and at least one epileptic seizure in the last 5 years preceding the interview.19,20 Inactive epilepsy refers to an individual with a history of epilepsy but no epileptic seizures in the last 5 years preceding the interview.19,20 Positive EITB refers to antibody response to at least one of the seven LL-GP purified antigens used in the assay.17 Positive EITB responses were categorized as weak, responses to 1 or 2 bands; intermediate, 3 antibody bands; or strong, 4 to 7 antibody bands.
Data Analysis
Differences in proportions were assessed using Chi-square or Fisher's exact test. Prevalences of seizures and epilepsy (per 1,000 people) are presented as minimal estimates since they represent confirmed cases, even when only a proportion of survey-positive individuals attended the neurological examination for case confirmation. Cysticercosis antibody seroprevalence was calculated for each study group by age strata. In the case of migrants, seroprevalence was also calculated by time from migration. Multivariable analysis was performed using logistic regression and having epilepsy as outcome of interest. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated. All analyses were conducted using Stata v12.1 (Stata Corporation, College Station, TX, USA). Two-sided p-values <0.05 were considered statistically significant.
ETHICAL APPROVAL
This ancillary study and consent forms were reviewed and approved by Institutional Review Board of the Universidad Peruana Cayetano Heredia, Lima, Peru.
RESULTS
This study enrolled a total of 987 of the 989 participants from the parent study, 522 females (53%), mean age 48 years (range 30 to 93). As expected, due to the stratified sampling method, there were no differences in age or sex between the three studied populations.
Seizure survey and neurological evaluation
From 987 participants enrolled in this study, almost all (n=985) responded to the seizure survey and 278/985 were found to be positive survey respondents. Of these, 68% (188/278) were further evaluated by a neurologist. The proportions of positive respondents to the survey and the coverage of the neurological evaluation were similar between study groups, except for a slightly lower proportion of urban inhabitants attending the neurological evaluation (Table 1).
Table 1.
Studied populations, coverage of evaluations, and minimal prevalence of seizures and epilepsy
| Group | Urban | Migrant | Rural |
|---|---|---|---|
| N | 196 | 589 | 200 |
| Male | 89 (45.4%) | 280(47.5%) | 95 (42.5%) |
| Mean age | 49.0 | 48.6 | 49.3 |
| Age range | 31 - 68 | 31 - 89 | 30 - 93 |
| Positive surveys (all) | 46 (23%) | 165 (28%) | 68 (34%) |
| Males | 15/89 (16.9%) | 78/280 (27.9%) | 33/95 (34.7%) |
| Females | 31/107 (29.0%) | 87/309 (28.2%) | 35/105 (33.3%) |
| Examined by neurologist (all) | 27/46 (59%) | 117/165 (71%) | 44/68 (65%) |
| Males | 7/15 (46.7%) | 52/78 (66.7%) | 15/33 (45.5%) |
| Females | 20/31 (64.5%) | 65/87 (74.7%) | 29/35 (82.9%) |
| Active epilepsy | 1 | 13 | 4 |
| Inactive epilepsy | 2 | 8 | 1 |
| All epilepsies | 3 (15.3/1000) | 21 (35.6/1000) | 5 (25.0/1000) |
| Single seizure | 1 | 3 | 1 |
| All seizures | 4 (20.4/1000) | 24 (40.7/1000) | 6 (29.9/1000) |
The prevalence of epilepsy (per 1,000 people) was higher in migrants (35.6) than in rural inhabit ants (25), and lower in the urban group (15.3), although these differences did not reach statistical significance (p=0.191 and p=0.160 respectively). There was no evidence of a difference in overall prevalence of epilepsy or seizures between males and females (13/464 versus 21/521, p=0.292). All six cases of seizures in the rural population, as well as three out of four in the urban group, were females. This led us to evaluate the proportions of positive survey respondents by sex in each study population, and how many of these completed the neurological consultation for case confirmation (Table 1). More positive female respondents attended the neurological consultation in the rural group (29/35, 83% versus 15/33 males, 46%, OR 5.8, 95% CI 1.9 - 17.7, p=0.001).
The age of onset of epilepsy was under 10 years in only 2 cases (7%), from 11 to 20 years in 8 (29%), from 20 to 30 years in 4 (14%), and from 31 to 60 years in the remaining 14 (50%). In one case we were not able to define an age of onset of seizures.
Serology according to migration exposure
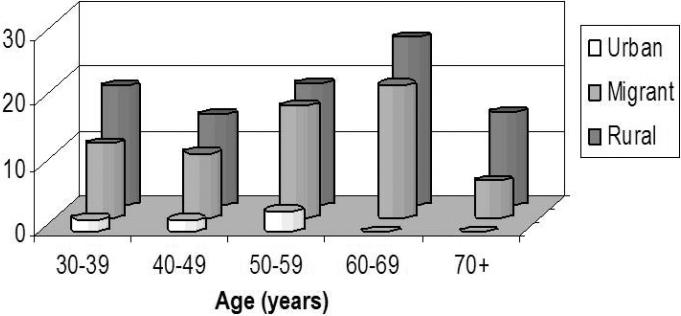
Of 985 survey respondents, 981 had an archived serum sample, all of which were processed for cysticercosis antibodies on EITB. A gradient in cysticercosis antibody seroprevalence was observed, being higher in individuals living in rural villages, less so in migrants, and much lower in urban residents (Table 2). The curves of seroprevalence by age demonstrated parallel trends between individuals living in rural villages and migrants, with a much lower seroprevalence in the urban group (Figure 2).
Table 2.
Seroprevalence and number of reactive bands on EITB by population origin
| Group | Urban | Migrant | Rural |
|---|---|---|---|
| N | 195 | 587 | 199 |
| Seropositive | 4 (2.1%) | 79 (13.5%) | 36 (18.1%) |
| 1 to 2 bands | 3 (1.5%) | 32 (5.5%) | 8 (4.0%) |
| 3 bands | 1 (0.5%) | 45 (7.7%) | 23 (11.6%) |
| 4 to 7 bands | 0 (0%) | 2 (0.3%) | 5 (2.5%) |
| Seroprevalence in individuals with seizures | 0/4 (0%) | 3/24 (12.5%) | 4/6 (66.7%) |
| Seroprevalence in negative survey respondents | 1/149 (0.7%) | 55/423 (13.0%) | 20/131 (15.3%) |
Figure 2.
Seroprevalence of antibodies to cysticercosis by age group in rural, migrant and urban populations in Peru.
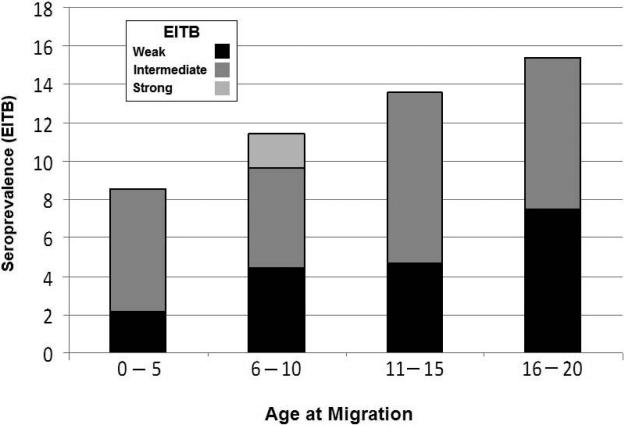
Intra-group analysis of the migrant population showed that seroprevalence consistently increased according to the age the individual had at the time of leaving their rural villages, between age 0 and 15 years or older. These findings suggest cumulative exposure while in their rural villages: the older the age at migration from the rural area, the higher the seroprevalence of antibodies (Figure 3).
Figure 3.
Current serum antibody responses by age at migration in individuals who moved (~30 years ago) from an endemic rural region into a non-endemic city.
Given that the study participants migrated a long time ago, we also assessed the effect of the time elapsed since migration. Seroprevalence in the migrant group decreased with time away from their native villages (16.5% [46/279] in the initial 25 years, 12.2% [25/205] between 25 and 40 years, and 8.0% [8/103] in those away for more than 40 years), suggesting that antibodies wane over along time in a sizable proportion of seropositive individuals. Adjusting by time away from the rural area did not affect the increasing pattern of serology by age at migration.
Relation between seroprevalence and seizures
In rural villagers, there was strong evidence of an association between positive EITB and having seizures (4/6 versus 32/193, p=0.011, Fisher's exact test). This association was not observed in long-term migrants or in urban residents.
Of 7 individuals reacting to 4 or more specific antibody bands on EITB, 3 had active epilepsy (2 of 5 in rural group, and 1 of 2 migrants). There was evidence of an association between strong response (≥4 antibody bands) on EITB with seizures (3/29 versus 4/952, p<0.001, Fisher's exact test). Logistic regression analysis for epilepsy confirmed this observation: compared to seronegative participants, those with a strong positive serology response were 12 times more likely to have epilepsy, after adjusting by study group (urban, migrant, rural) (Table 3).
Table 3.
Factors associated with epilepsy in urban, migrant and rural individuals in Peru (n=985.
| Odds Ratio | 95% C.I. | p-value | |
|---|---|---|---|
| Endemicity | |||
| No (urban) | Reference | ||
| Endemic (migrant, rural) | 2.09 | 0.62 - 7.08 | 0.235 |
| Serology response | |||
| Seronegative | Reference | ||
| 1 or 2 antibody bands | 0.80 | 0.10 - 6.06 | 0.826 |
| 3 antibody bands | 0.97 | 0.22 - 4.23 | 0.966 |
| 4 or more antibody bands | 12.79 | 3.16 - 51.62 | <0.001 |
DISCUSSION
We found strong evidence of differences in antibody profiles and weaker evidence of higher seizure prevalence in rural villagers and long-term migrants compared to residents of a non-endemic urban shantytown in Lima, Peru. The prevalence of serum antibodies was higher in rural habitants and moderately high in migrants but much lower in the urban population. In the context where most studies of cysticercosis from non-endemic areas focus on international migration as a source of transmission – we were unable to find any publication related to changes in cysticercosis prevalence related to within-country migration – this report demonstrates that within-country rural-to-urban migration also carries a considerable burden, in terms of symptoms and immunological responses that last over time. As such our results confirm the impact of the disease in rural areas and contribute to defining high-risk populations in apparently non-endemic settings.
In rural villagers there was a significant association between antibody seropositivity and seizures, yet this association did not hold in long-term migrants. This most likely reflects the chronic nature of seizures in relation to waning of antibodies along the years in a sizable proportion of individuals with NCC – those who resolve the infection with or without residual brain calcifications. However, a significant association between epilepsy and having a strong serological reaction, ≥4 antibody bands, was found in the overall study population, and persisted after adjusting by population of origin - suggesting that epilepsy is present in individuals with heavier or more severe cysticercosis infections.
Seizures were more frequent in individuals with a strong positive serological response, and in people born in a rural endemic region, both in rural residents and long-term migrants. The unexpected finding of all six cases of seizures in rural villagers being female seems associated to male positive survey respondents failing to attend the neurological consultation for case definition, suggesting a sub-diagnosis of seizure cases in male rural villagers due to shame or fear to stigma. Also the data on age of onset of seizures shows a very low proportion of seizure disorders beginning in childhood. This is most likely affected by recall bias since our population was evaluated many years after (all participants were 30 years or older). The sample size, calculated for the parent study and powered to yield differences on cardiovascular outcomes, did not allow confirming the increased odds ratios for seizures (1.9) and epilepsy (2.2) found in this study for the rural, rural-resident and rural-to-urban migrant, population groups.
Another limitation of this study is the lack of brain imaging studies. Computed tomography (CT) scans were not feasible due to the rural nature of San Jose de Secce, without access to a CT scanner or even less to a MRI machine, which is a typical situation for heavily endemic rural regions. This limitation also impeded to assess the presence or frequency of individuals with acute symptomatic seizures. Chances for selection bias are less likely. Because migration is often driven by economic and other factors that are likely to be related to health, migrants rarely are representative of the rural area they come from. Terrorism and political violence led to mass rural-to-urban migration in Peru. A large proportion of the population migrated because of the need to escape from violence rather than effects of economic forces or need for healthcare. Thus the selection bias among migrants was reduced.21
Both, rural residents and rural-to-urban migrants had a much higher frequency of antibodies to cysticercosis, with stronger reactions in rural villagers, seemingly the strongest associated factor to seizures among those studied here. The association between seizures and serology disappears in the migrant population, yet the overall seizure and epilepsy prevalence were still high in this group. In addition to this, a pattern towards a higher seroprevalence of serum antibodies was observed among rural-to-urban migrants by age at migration: the higher the age at migration from the rural area the higher the seroprevalence of antibodies.
The comparisons between rural, migrant and urban populations presented in this study confirm the association between cysticercosis and seizures in rural endemic communities, expose clear differences in endemicity within an endemic country, and suggest that the neurologic consequences of cysticercosis infection likely outlast the antibody response for years.
ACKNOWLEDGEMENTS
We are grateful to Yesenia Castillo for her dedicated laboratory work, and to Juan Francisco Chiroque, Can-dice Romero and Lilia Cabrera who coordinated the fieldwork phase of this study. Partial support from the Fogarty International Center/NIH, NHLBI/NIH and the Wellcome Trust is acknowledged. HG and LS are supported by Wellcome Trust Senior Research Fellowships in Public Health and Clinical Science, respectively. The funders had no role in study design, data collection, analysis, or interpretation, in writing the report, or in the decision to submit the article for publication.
REFERENCES
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–61. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Schantz PM, Wilkins PP, Tsang VCW. Immigrants, imaging and immunoblots: the emergence of neurocysticercosis as a significant public health problem. In: Scheld WM, Craig WA, Hughes JM, editors. Emerging infections 2. ASM Press; Washington D.C.: 1998. pp. 213–41. [Google Scholar]
- 3.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004;63:1559–64. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 4.del la Garza Y, Graviss EA, Daver NG, et al. Epidemiology of neurocysticercosis in Houston, Texas. Am J Trop Med Hyg. 2005;73:766–70. [PubMed] [Google Scholar]
- 5.Leshem E, Kliers I, Bakon M, et al. Neurocysticercosis in travelers: a nation-wide study in Israel. J Travel Med. 2011;18:191–7. doi: 10.1111/j.1708-8305.2011.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Chatel G, Gulletta M, Scolari C, et al. Neurocysticercosis in an Italian traveler to Latin America. Am J Trop Med Hyg. 1999;60:255–6. doi: 10.4269/ajtmh.1999.60.255. [DOI] [PubMed] [Google Scholar]
- 7.Roca C, Gascon J, Font B, et al. Neurocysticercosis and population movements: analysis of 23 imported cases in Spain. Eur J Clin Microbiol Infect Dis. 2003;22:382–4. doi: 10.1007/s10096-003-0936-0. [DOI] [PubMed] [Google Scholar]
- 8.Coral I. Desplazamiento por violencia politica en el Peru, 1980-1992. Documentos de Trabajo N°58 Serie Politica N°6. Instituto de Estudios Peruanos; Lima: 1994. p. 35. [Google Scholar]
- 9.Miranda JJ, Gilman R, Garcia H, et al. The effect on cardiovascular risk factors of migration from rural to urban areas in Peru: PERU MIGRANT Study. BMC Cardiovascular Disorders. 2009;9:23. doi: 10.1186/1471-2261-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda JJ, Gilman RH, Smeeth L. Differences in cardiovascular risk factors in rural, urban and rural-to-urban migrants in Peru. Heart. 2011;97:787–96. doi: 10.1136/hrt.2010.218537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizo-Patron P. Peru – A brief geographic and touristic view. The Ene river basin. 2007 [Google Scholar]
- 12.Pedersen D, Tremblay J, Errázuriz C, et al. The sequelae of political violence: Assessing trauma, suffering and dislocation in the Peruvian highlands. Soc Sci Med. 2008;67:205–17. doi: 10.1016/j.socscimed.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Instituto Nacional de Estadística e Informática . Sistema de Consulta de Resultados Censales. INEI; Lima, Perú: 2007. Censos Nacionales 2007: XI de Población y VI de Vivienda. [Google Scholar]
- 14.Montano SM, Villaran MV, Ylquimiche L, et al. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 15.Placencia M, Sander JW, Shorvon SD, et al. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain. 1992;115:783–94. doi: 10.1093/brain/115.3.783. [DOI] [PubMed] [Google Scholar]
- 16.Placencia M, Suarez J, Crespo F, et al. A large-scale study of epilepsy in Ecuador: methodological aspects. Neuroepidemiology. 1992;11:74–84. doi: 10.1159/000110915. [DOI] [PubMed] [Google Scholar]
- 17.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. 1989;159:50–9. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 18.From the Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 19.Rudzinski LA, Shih JJ. The classification of seizures and epilepsy syndromes. Continuum (Minneap Minn) 2010;16:15–35. doi: 10.1212/01.CON.0000368230.11492.d5. [DOI] [PubMed] [Google Scholar]
- 20.Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry. 1996;61:433–43. doi: 10.1136/jnnp.61.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda JJ. Migration from Rural to Urban Areas in Peru: Impact on Health Outcomes. Georgia State University; Atlanta, GA: 2009. Working Paper Available at: http://aysps.gsu.edu/ECON_MA_MonteroJJ.pdf. [Google Scholar]