Abstract
Background
Decisions between medical and surgical management of Crohn’s disease (CD) incorporate risk assessments for potential complications of each therapy. Analytic morphomics is a novel method of image analysis providing quantifiable measurements of body tissue composition, characterizing body fat more comprehensively than body mass index (BMI) alone. The aim of this study was to determine risk factors associated with post-operative complications in CD, incorporating fat composition analysis using analytic morphomics.
Methods
We performed a retrospective review of adults undergoing bowel resection for CD between 2004–2011 at a single center. CT scans within 30 days prior to surgery underwent morphomic analysis of body geometry and fat characterization. Post-operative infectious complications were defined as the need for a post-operative abdominal drain, intravenous antibiotics, or re-operation within 30 days. Bivariate and multivariate analysis using logistic regression were used to generate a prediction model of infectious complications.
Results
A total of 269 subjects met selection criteria; 27% incurred post-operative infectious complications. Bivariate analysis showed hemoglobin, albumin, surgical urgency, high-dose prednisone use, and subcutaneous-to-visceral fat volume distribution as predictors of complications. BMI, anti-TNF-alpha-therapies, and immunomodulator use were not predictors of complication. Multivariate modeling demonstrated a c-statistic of 0.77 and a NPV of 81.1% with surgical urgency (OR 2.78, 95% CI 1.46–6.02, P=.004), subcutaneous-to-visceral fat distribution (OR 2.01, 95% CI 1.20–3.19, P=.006), and hemoglobin (OR 0.69, 95% CI 0.55–0.85, P =.001) as predictors of infectious complication.
Conclusions
Fat subtype and distribution are predictive of post-operative infectious complications following bowel resection for Crohn’s disease. Analytic morphomics provides additional body composition detail not captured by BMI.
Keywords: Analytic morphomics, operative risk, inflammatory bowel disease, surgery
Introduction
Crohn’s disease is an immune-mediated inflammatory bowel disease with a prevalence of 136–318/100,000 in the North America1. Modern immunomodulator and biologic therapies have improved clinical outcomes, yet nearly 50% of patients with Crohn’s disease will require surgical management within 10 years of diagnosis2,3. In some urgent and emergent situations, such as the development of complete intestinal obstruction, large abscess or penetrating disease, surgery is unavoidable. However, in other instances the decision to pursue medical vs. surgical management is less clear, especially in the case of partially obstructing strictures which are almost invariably composed of a mixture of inflammatory and fibrotic elements4. Management decisions to either intensify medical therapy or proceed with surgical approaches hinge upon several data points, including objective measures of existing inflammatory disease activity. Further, risks related to surgery, including poor anastomotic and incisional wound healing following surgery are important considerations when counseling patients for surgery.
Several factors have been shown to impact the risk of complication in general abdominal surgeries including poor nutritional status and obesity5. Crohn’s disease has not been spared from the obesity epidemic, as several studies examining IBD have demonstrated rates of obesity approaching that of the general population6. While the general surgical literature has demonstrated an association between BMI and post-operative complications, the association of BMI with post-operative complications in the Crohn’s disease population has been inconsistent7, 8. BMI is a course measurement that fails to capture details of body composition. Body characteristics of lean muscle mass, fat subtypes (visceral and subcutaneous), and tissue distribution can be measured using data from standard computed tomography (CT) scans9. Analytic morphomics provides reproducible measures of body composition, which have been associated with surgical morbidity and mortality in both liver transplant recipients and those undergoing colectomies for colon cancer10, 11. Morphomic assessment offers quantitation of body composition factors related to surgical complication in Crohn’s disease, improving the counseling provided to patients regarding their individual operative risk.
In this study, we aimed to identify risk factors for infectious post-operative complication following bowel resection for Crohn’s disease, including morphometric assessments of body composition. We hypothesized that fat composition characteristics and variation of fat distribution would correlate with the incidence of infectious surgical complications.
Methods
Selection Criteria
This study was approved by the University of Michigan Institutional Review Board. Patients with Crohn’s disease undergoing surgical bowel resection between January 1, 2004 and December 31, 2011 at the University of Michigan Health System (UMHS) were identified. Crohn’s disease diagnosis was verified using one inpatient and outpatient ICD-9 code of 555.X with confirmation by review of medical records12. Subject selection criteria included the availability of a computer tomography scan (CT) of the abdomen using standard or enterography-protocol performed within 1 month prior to abdominal surgery. Subjects undergoing surgeries where the primary indication was abscess or phlegmon were excluded considering the composite outcome of infectious post-operative complication. In addition, patients using parenteral nutrition prior to surgery were also excluded as their nutritional status could interact with the primary outcome assessment.
Clinical Data Collection
UMHS electronic medical records were reviewed using a combination of automated data extraction (University of Michigan Data Warehouse Service), natural language search engines (EMERSE), and manual chart review13. Patient demographics, active medications, smoking history and laboratory data immediately prior to surgery were collected. For medications, immunomodulator use was considered active if thiopurine or methotrexate use was documented within 2 weeks of surgery. Anti-tumor necrosis alpha therapy was considered active if patients were exposed to infliximab, adalimumab, or certolizumab within 8 weeks of surgery. Corticosteroid use was categorized as none, 1–20mg, 21–40mg, and >40mg. Surgical records were manually reviewed for surgical indication, type of surgery performed and American Society of Anesthesiologists (ASA) score. Surgical urgency was classified as emergent for surgery within 24 hours of admission, urgent for surgery during hospitalization, and elective for patients presenting for scheduled preoperative hospital admission.
Definition of Post-Operative Complication
The composite endpoint of post-operative infectious complication included the following criteria: (1) Use of intravenous antibiotics for an intra-abdominal infection source for at least 10 days, (2) Post-operative abdominal drain placement within 30 days of index surgery, and (3) re-operation within 30 days of index surgery. Medical records were reviewed to determine the indications of the interventions used in our composite endpoint. Infectious complications potentially related to perioperative interventions, but remote from the surgical site, including Clostridium difficile colitis, urinary tract infections, and pneumonia, were not included in the composite post-operative complication endpoint.
Analytic Morphomics
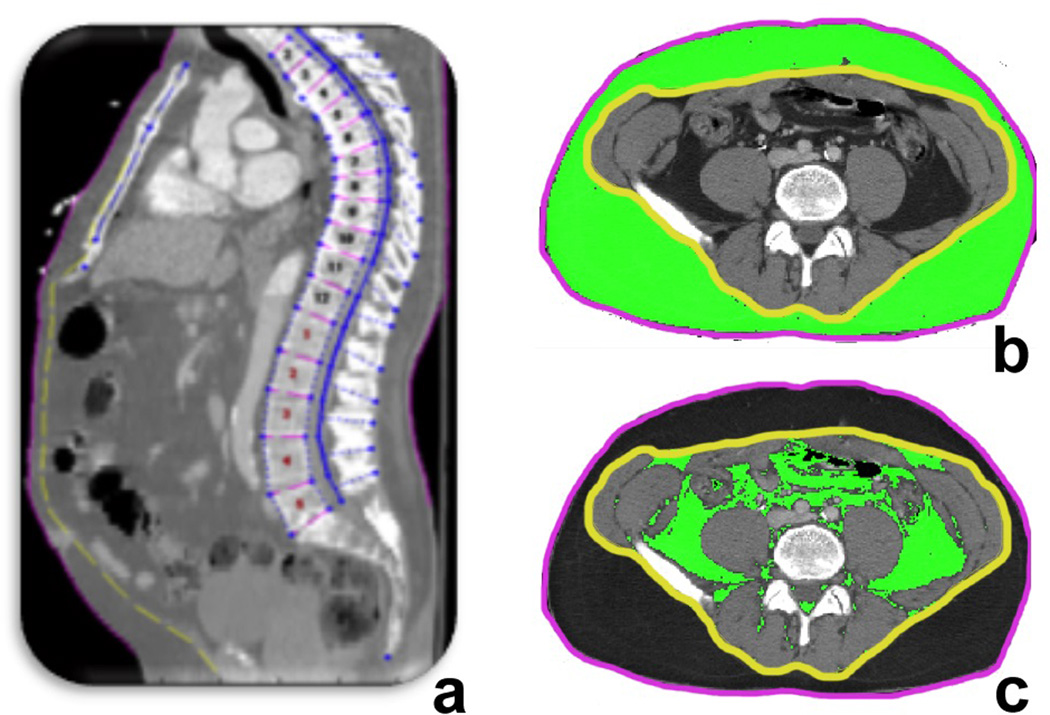
The cross-sectional areas of subcutaneous and visceral fat were measured at multiple spinal levels, from the 10th thoracic (T10) vertebral body to the 5th lumbar (L5) vertebral body. CT scans underwent semi-automated vertebral and soft tissue identification using image processing algorithms developed by the University of Michigan Morphomic Analysis Group in MATLAB 13.0 (MathWorks, Natick MA)14. First, individual vertebral levels were identified, serving as spatial landmarks for correlating other structures and tissues. Second-level processing included automated determination of the body perimeter defined by the transition from air (0 Hounsfield units) to skin. Definition of the fascial plane is achieved by using a nearest neighbor subtraction algorithm to delineate the zone of Hounsfield unit density change from the relatively homogenous subcutaneous fat densities to intra-abdominal non-fat tissues with higher densities. Following these regional definitions, subcutaneous and visceral fat were quantified by area at each spinal level (Figure 1). To control for variations in body size, subcutaneous and visceral fat measurements were standardized to total body cross sectional area at each spinal level. Fat volumes were calculated by multiplying fat area by vertebral body height at each spinal level. To assess the impact of variation in fat distribution by body region, we assessed the relative degree of subcutaneous and visceral fat (standardized to total body volume) over several regions. Total fat volumes within a spinal range were calculated by summation of individual level volumes (e.g. T10:L5). The relative change in fat distribution across spinal levels was defined as the ratio of subcutaneous to visceral fat measurements between the most cephalad and caudal spinal levels within the abdominal field (e.g. subcutaneous fat to visceral fat ratio at T10 divided by the subcutaneous fat to visceral fat ratio at L5). In addition to fat characteristics, other morphomic body parameters, including body geometries of anterior-posterior distances and body eccentricity, were measured. All morphomic measurements underwent quality control assessment. A single reviewer (GS) reviewed the results of automated spinal level, fascial plane, and fat area identification, as occasionally imaging artifact led to inaccurate automated tissue identification.
Figure 1. Subcutaneous and visceral fat definitions by automated morphometric analysis methodology.
Computed tomography scans are processed by image boundary definition routines written in Matlab. Vertebral body definitions are verified by a human operator and corrected when necessary (a). Fascial plane identification (b, yellow line) serves as boundary between subcutaneous (b) and visceral fat (c) at midpoints of spinal levels. Fat volumes are the product of fat area and vertebral body height as individual spinal levels.
Statistical Analysis
Demographic, clinical, and laboratory features were compared between patients with and without complications using the Fisher’s exact test and the Mann–Whitney Wilcoxon rank-sum test for categorical and continuous variables, respectively. Simple linear regression was used to compare BMI to morphomic fat measurements. Bivariate analysis by logistic regression was performed using the composite post-operative complication endpoint as the dependent variable. Regression models were developed using variables identified with a P < .05 on bivariate analysis, as well as those determined a priori to be clinical relevant. Multicollinearity was assessed by reviewing the correlation matrix and variance inflation factor analysis of covariates (VIF) included in the model. The discriminatory power of the regression models were evaluated using receiver operating characteristic (ROC) curve analysis. C-statistics in ROC analysis range from 0.5 to 1.0, with values of 0.5 indicating the model is no better than chance and 1.0 indicating the model perfectly discriminates the two groups. Models are considered reasonable if the c-statistic exceeds 0.70 and strong if greater than 0.80. All analyses were conducted using STATA statistical software 13.0 (College Station, TX) and R statistical package 2.14.0.
Results
Cohort characteristics
Within the 7-year period reviewed, 364 patients with Crohn’s disease underwent surgical bowel resection for obstructive disease or medication non-responsiveness at the University of Michigan Health System. Ninety-five (95) patients were excluded because of unsuitable CT scans, typically due to abbreviated CT scans terminating at L2–L3 spinal levels or the use of imaging formats unable to be converted to the DICOM standard. A total of 269 patients were analyzed. Subjects were grouped by the occurrence of an infectious surgical complication, with an observed complication rate of 27%. Patient mean age was 35.9±15.8 years, 45.9% were male and the cohort was overwhelmingly Caucasian (89.6%). When BMI was categorized as underweight (≤18.5 kg/m2), normal (>18.5–24.5 kg/m2), overweight (>24.5–30 kg/m2), or obese (≥30 kg/m2), there was no significant difference in complications among BMI categories using Fisher’s exact test (P = .193).
When considering medication usage across all patients, mean immunomodulator use was 37.5%, mean anti-TNF use within 8 weeks of surgery was 33.1%, and use of any corticosteroid was 44.9%. A corticosteroid dose of 40mg or more was more frequently observed in those with surgical complications (7.7% vs. 13.7%, P = .041), however doses less than 40mg were not associated with complications in this study. Outside of high-dose prednisone exposure, no other patient characteristics significantly differed between those with and without surgical complications, including gender, age, ethnicity, smoking status, diabetes mellitus, immunomodulator and anti-TNF use, and American Society of Anesthesiologists (ASA) score (Table 1).
Table 1.
Patient characteristics at the time of surgery
| Characteristic | No Complication (n=196) | Complication (n=73) | P value | |
|---|---|---|---|---|
| Male (%) | 90 (45.9) | 33 (45.2) | 0.51 | |
| Age | 35.9±15.8 | 35.8 (17.3) | 0.919 | |
| Ethnicity (%) | 0.823 | |||
| Caucasian | 175 (89.3) | 66 (90.4) | ||
| African Amer. | 18 (9.1) | 6 (8.2) | ||
| Other | 2 (1.0) | 1 (1.4) | ||
| BMI Category | 0.193 | |||
| Underweight | (BMI <18.5) | 43 (21.9) | 14 (19.2) | |
| Normal | (BMI 18.5–25) | 87 (44.4) | 43 (58.9) | |
| Overweight | (BMI 25–30) | 46 (26.5) | 11 (15.1) | |
| Obese | (BMI >30) | 20 (10.2) | 5 (6.8) | |
| Medication Use (%) | ||||
| Prednisone | 83(42.3) | 38 (52.0) | 0.361 | |
| <20mg | 30 (15.3) | 11 (15.1) | 0.432 | |
| 20–40mg | 38 (19.4) | 17 (23.2) | 0.124 | |
| >40mg | 15 (7.7) | 10 (13.7) | 0.041 | |
| Immunomodulator | 75 (38.3) | 26 (35.6) | 0.249 | |
| Anti-TNF Use | 66 (33.6) | 23 (31.5) | 0.284 | |
| Smoking, Active (%) | 45 (23.0) | 13 (17.8) | 0.224 | |
| Diabetes mellitus (%) | 15 (7.6) | 6 (8.2) | 0.899 | |
| ASA Score, mean | 2.40±0.5 | 2.45±0.5 | 0.557 | |
| Laboratory Values | ||||
| White blood cell count (k3/mL) | 9.85 (±5.42) | 9.67 (±5.86) | 0.835 | |
| Hemoglobin (g/dL) | 11.96 (±1.91) | 11.13 (±2.21) | <0.001 | |
| Platelets (k2/mL) | 279.5 (±159.6) | 294.19 (±141.4) | 0.140 | |
| Albumin (g/dL) | 3.60 (±0.70) | 3.30 (±0.78) | 0.007 | |
Complete blood counts and comprehensive metabolic panels were collected within 48 hours prior to surgery for all patients. On bivariate analysis, lower hemoglobin values were a predictor of surgical complication (11.96 g/dL vs. 11.13g/dL, P < .001). In addition, lower serum albumin levels were observed in those with surgical complications (3.60 g/dL vs. 3.30 g/dL, P = .007). White blood cell count, platelet count, blood urea nitrogen, and serum creatinine measurements did not differ significantly between those with and without surgical complications. C-reactive protein (CRP) was not included in the analysis, as values were only available for 36% of subjects.
Surgical characteristics
Ileocecectomy was the most common surgery performed (62.5%), followed by hemicolectomy (20.0%). The type of surgery performed was not associated with an increased rate of complications. Unsurprisingly, patients undergoing a primary anastomosis without diversion had a statistically significant increased probability of infectious complications (68.4% vs. 52.1%, P = .022). Surgical urgency and the timing of surgery were associated with an increased risk of surgical complication (Table 2). Those undergoing emergent (within 24 hours of admission) and urgent surgery (during hospitalization) developed surgical complications more frequently than those undergoing elective surgery (36.7% vs. 18.4%, P, < .001). Of those where surgical complication occurred (n = 73, 27%) 71% received IV antibiotics for an intra-abdominal or wound infection, 41% required a post-operative drain placement, and 32% underwent repeat surgery within 30 days. Examination of the surgeries performed on a weekend date (Saturday, Sunday) did not significantly increase risk of surgical complication on bivariate analysis.
Table 2.
Operative characteristics in patients undergoing bowel resection for Crohn's disease
| Bivariate Analysis | |||||
|---|---|---|---|---|---|
| No Complication (n=196) |
Complication (n=73) |
Odds Ratio | 95% CI | p value | |
| Surgical Urgency (%) | 2.80 | 1.73–4.51 | <0.001 | ||
| Elective | 115 (58.7) | 26 (35.6) | 1.00 | Reference | |
| Urgent | 74 (37.8) | 38 (52.1) | 2.72 | 1.45–5.10 | 0.002 |
| Emergent | 7 (3.8) | 9 (12.3) | 8.12 | 2.70–24.39 | <0.001 |
| Weekend Surgery | 35 (17.9) | 14 (19.1) | 1.43 | 0.71–2.88 | 0.312 |
| Surgical Type (%) | 0.215 | ||||
| Ileocecectomy | 124 (62.2) | 46 (63.0) | 1.00 | Reference | |
| Hemicolectomy | 34 (17.3) | 20 (27.4) | 1.38 | 0.71–2.72 | 0.345 |
| Small Bowel Resection | 38 (19.4) | 7 (9.6) | 0.44 | 0.13–1.54 | 0.198 |
| Anastomosis Without Diversion | 134 (68.4) | 38 (52.1) | 1.95 | 1.10–3.49 | 0.022 |
Body mass, geometry, and fat distribution analysis
Traditional measurements of body mass were not associated with the development of surgical complications. BMI measurement within 30 days of CT imaging for those without and with surgical complication was 22.5±7.9 kg/m2 vs. 21.9±6.0 kg/m2, P = .919 (Table 3).. Standardized subcutaneous fat volume ratios (volume of fat standardized to body volume) between T10 and L5 spinal levels did not demonstrate significant differences between those with and without complications (0.38 vs. 0.40, P = .54). Further, standardized visceral fat volume between T10:L5 showed no significant associate with surgical complication rate. However, the relative proportion of fat subtype between the T10 and L5 was significant. Subcutaneous to visceral fat ratios were significantly greater the in caudal region in those with post-operative complication (0.95 vs. 0.62, P = .012). The subcutaneous-to-visceral fat ratio was very weakly correlated with BMI (R2 = 0.0239, P = .007). BMI was not correlated with standardized subcutaneous fat measurements, (P = .869, R2 = 0.0001). To determine if the distribution of fat subtype was merely a surrogate of body geometry, central adiposity shape information was assessed using anterior to posterior distance and body eccentricity; neither were predictors of complication status.
Table 3.
Morphometric measurements of body fat distribution
| No Complication (n=196) |
Complication (n=73) |
Bivariate Analysis | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Odds Ratio |
95% CI | p value | |
| BMI, kg/m2 | 22.5 (±7.9) | 21.9 (±6.0) | 0.99 | 0.96–1.04 | 0.919 |
| Total Ant-Post Distance – T10, mm | 237.4 (±38.5) | 230.7 (±38.1) | 0.99 | 0.99–1.00 | 0.334 |
| Body Eccentricity – T10 | 0.68 (±0.06) | 0.68 (±0.06) | 2.8 | 0.02–443.70 | 0.691 |
| Percent Visceral Fat Volume – T10:L5, % | 0.67 (±0.67) | 0.56 (±0.36) | 0.58 | 0.26–1.29 | 0.179 |
| Percent Subcutaneous Fat Volume – T10:L5,% | 0.38 (±0.20) | 0.40 (±0.27) | 1.49 | 0.42–5.31 | 0.537 |
| Subcutaneous-to-Visceral Fat Ratio – T10:L5 | 0.62 (±0.41) | 0.95 (±0.69) | 1.73 | 1.13–2.66 | 0.012 |
Multivariate model of post-operative complication
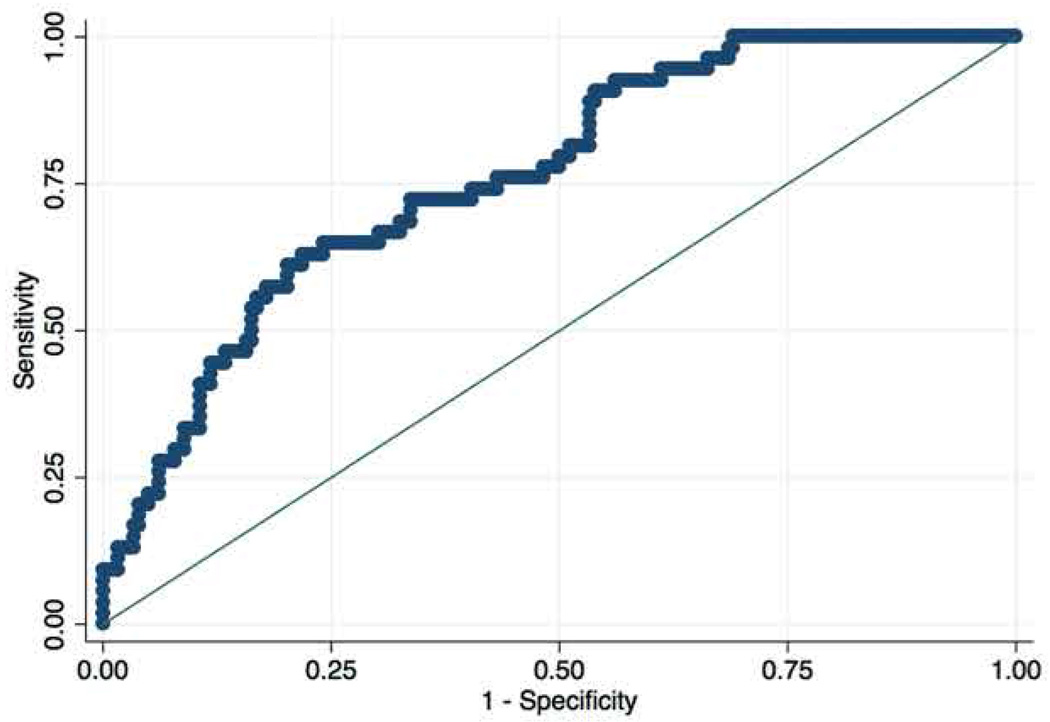
A multivariate model of risk for post-operative infectious complications following bowel resection was constructed using subcutaneous-to-visceral fat ratio between T10:L5, hemoglobin, albumin, recent exposure to prednisone >40mg/day, and surgical urgency, with additional adjustment for gender, age, diabetes, smoking, and ASA scores (Table 4). Emergent or urgent surgical bowel resection increased the odds of complication (OR 2.78 95% CI 1.48–6.02, P = .004). Patients with a higher ratio of subcutaneous to visceral fat in the central region (T10) relative to the mid-lower abdominal region (L5) were more likely to experience a complication in the multivariate model (OR 2.01, 95% CI 1.20–3.19, P = .006). Albumin and prednisone dosage were not significant contributors to the risk of surgical complications after adjusting for covariates in this multivariate model. Multicollinearity of covariates was not detected and VIF values were less than 3.0. Receiver operating curve of model performance yielded a c-statistic of 0.77, NPV of surgical complication of 81.1% (Figure 2).
Table 4.
Multivariate model for surgical complications in Crohn's disease
| Odds Ratio |
95% CI | p value | |
|---|---|---|---|
| Surgical Urgency | 2.78 | 1.48–6.02 | 0.004 |
| Subcutaneous-to-Visceral Fat Ratio – T10:L5 | 2.01 | 1.20–3.19 | 0.006 |
| Hemoglobin | 0.69 | 0.55–0.85 | 0.001 |
| Albumin | 1.30 | 0.72–2.35 | 0.372 |
| Prednisone >40mg/day | 1.05 | 0.76–1.44 | 0.792 |
| Gender | 1.50 | 0.71–3.17 | 0.283 |
| Age | 1.00 | 0.78–3.14 | 0.926 |
| Diabetes | 0.67 | 0.11–3.73 | 0.618 |
| Active Smoking | 0.69 | 0.29–1.64 | 0.406 |
| ASA Score | 0.86 | 0.45–1.63 | 0.635 |
Figure 2. Receiver operating curve for the multivariate model predicting post-operative complications.
Multivariate model for post-operative surgical complications following Crohn’s disease-related bowel resection demonstrated an OR 1.83, 95% CI 1.19–2.34 (P = .021). Reduced hemoglobin, surgical urgency, and elevated subcutaneous fat ratio between T10:L5 were the most predictive variables in the model. The c-statistic of this model was 0.77 with a NPV of 81.1% for post-operative complication.
Discussion
In this study, we show that an increased degree of subcutaneous relative to visceral fat increases the risk of infectious complication following bowel resection in Crohn’s disease. Despite this finding, BMI was not predicative of surgical complication in this single-center cohort. The discrepancy between BMI and subcutaneous-visceral fat measurements ability to predict complications suggests that careful measurement of both fat subtype and distribution provides more useful data than the relatively coarse measure of BMI. Further, there was very little correlation between BMI and the morphomic measurements of standardized fat volumes. In addition to providing a model for post-operative complications in Crohn’s disease, this study demonstrates the utility of quantitative measures more representative of tissue composition and distribution in inflammatory bowel disease.
Our multivariate model for predicting post-surgical infectious complication includes several individual parameters previously shown to increase risk of surgical complications in Crohn’s disease. Surgical urgency has been reported to increase the rate of surgical complications, likely the result of more severe disease phenotypes combined with the potential for limited availability of colorectal expertise in emergent presentations15. It is important to emphasize that patients with a known abscess or perforation were excluded from this study, as preoperative peritoneal contamination could confound outcome assessment. Considering the laboratory parameters studied, low hemoglobin levels were a strong component in the model, potentially reflecting the chronicity of poorly controlled disease16. Serum albumin levels less than 3.5 g/dL have been previously reported to predict post-operative morbidity in both general abdominal surgery and in IBD-related surgery17–19. However, albumin was significant in the adjusted multivariate model, potentially because disease chronicity and subsequent malnutrition impacting wound healing and infection risk are better captured by hemoglobin or fat distribution. Corticosteroids at doses greater than 40mg are known to increase post-operative infectious complications in IBD patients20, 21. While high dose corticosteroids were associated with post-operative complications on bivariate analysis, high dose prednisone was not significant in this multivariate model. Because intermediate and long term corticosteroids are likely to impact body fat, the relatively low contribution of steroids in this risk model may be due to collinearity of fat changes and steroid use.
Increased proportion of subcutaneous relative to visceral fat volume distributed more in the thoracic vs. lumbar spinal regions was a significant predictor of post-operative complications in our multivariate model. Khoury et al. found that obese patients (defined as BMI >30kg/m2) undergoing laparoscopic bowel resection (not limited to IBD) had significantly higher rates of wound infection, conversion to open procedures, and lengthened hospital stay relative to non-obese patients5. Studies assessing the impact of obesity on surgical outcomes in Crohn’s disease have had variable results. A retrospective study of outcomes following Crohn’s disease related surgery (n = 2319) found that obesity assessed by BMI conferred an increased risk of wound and intrabdominal infection7. However, outside of the Causey et al. study, both our data and several other groups have not found an association between traditional measures of body mass including weight, BMI, and body geometry with surgical complications in IBD16, 22, 23. BMI is unable to capture changes in fat distribution and overall body composition, including variations in subcutaneous and visceral fat. Variations in fat volume and distribution may impact surgical outcomes simply by making tissue approximation more difficult. Morphomics allows careful quantitation of body composition changes typically assessed by subjective physician assessments.
Outside of the role of fat composition and distribution, adiposity itself may affect disease activity and outcomes in IBD. Patients with IBD are not immune from the obesity epidemic. In North America and Europe, BMI values in CD patients have steadily increased from 20.8 in 1991 to 27.0 in 2001, mirroring weight gain trends in the general population6, 24–26. Further, increased adiposity has been associated with systemic inflammation, increased intestinal permeability, and alterations in the intestinal microbiome27–29. These findings suggest that adiposity may have a role in the development, phenotype, and severity of inflammatory bowel disease. A few retrospective studies have demonstrated an elevated BMI (>25kg/m2) was associated with shorter time from IBD diagnosis to first surgery and a higher incidence of perianal penetrating disease30, 31.
BMI is a simple, zero-cost tool to measure mass distribution based on height, however BMI fails to characterize fat composition. Despite the biologic plausibility and connections between obesity and inflammation, BMI itself has not been directly associated with clinical disease activity scores, disease phenotype, or incidence of IBD32. Studies in both adult and pediatric populations have demonstrated that BMI is a poor indicator of nutritional status, body fat, and lean mass content when compared to anthropometric measures of triceps skin fold thickness and bioelectrical impedance analysis33, 34. A systematic review reported that over a third of patients with Crohn’s disease had altered body composition compared to control35. Other objective measures of body fat composition including hydrodensitrometry and bioelectrical impedance testing provide reproducible results, but can be time consuming and costly. Morphomic assessment may highlight the body composition changes occurring in IBD that are not apparent when assessing patients using BMI36. Considering the frequency of imaging in subjects with IBD, analytic morphomic analysis provides a quantitative, high throughput, value-added measurement of body composition which may aid investigations examining the impact of obesity in IBD.
Several limitations must be considered when interpreting the results of our work. First, our composite outcome of post-operative infectious complication was designed to have high specificity over sensitivity, which may result in failing to capture all post-operative events. Second, we were unable to accurately differentiate isolated wound infection versus anastomotic leak or direct bowel-related infectious complication. The risk factors identified may further segregate between these two regions of infection. Third, decisions on surgical timing, pre-operative optimization strategies and surgical methods were not standardized among providers, introducing selection bias into the results. Surgical approach and techniques were likely to have varied based on patient condition; this cannot be controlled in the context of a retrospective cohort study design. Further, we did not stratify our subjects based on underlying disease severity or activity, as insufficient information was available to meaningfully characterize cumulative disease activity. Finally, the impact of anti-TNF therapies on post-operative infection remains debated37. Insufficient data was available to determine proximity of anti-TNF administration to surgery in our cohort; time to exposure may prove to be an important variable in anti-TNF associated surgical risk.
This multivariate model of post-operative infectious complication in Crohn’s disease may help physicians better individualize operative risk when discussing surgery with patients. Certainly several situations, regardless of risk, demand surgical management including bowel obstruction, large abscesses, or penetrating disease.. While modification of the factors in this model are unlikely to immediately reduce their risk of operative complication, such models can serve as a clinical guide to better quantify the factors contributing to individual surgical risk.
As we continue to examine the interplay between intestinal inflammation and obesity, tools providing more granular measures of body composition, like analytic morphomics, will be important for studying the role of adiposity on disease outcomes. While subject to the limitations of a retrospective study design, this data indicates that more detailed fat assessment may aid in the prediction of post-operative infectious complications in Crohn’s disease. Quantitative body composition assessments using analytic morphomic techniques may provides a new quantitative tool for studying the impact of adiposity on Crohn’s and other inflammatory diseases.
Acknowledgments
Grant Support: Crohn’s and Colitis Foundation of America Career Development Award #3775 (Stidham).
Veterans Affairs HSR&D CDA-2 Career Development Award 1IK2HX000775 (Waljee)
Footnotes
Disclosures: No potential conflicts of interest relevant to this manuscript are present.
Writing Assistance: No writing assistance was provided.
Author Contributions
Ryan W. Stidham (co-first author and corresponding author): study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision
Akbar K. Waljee (co-first author): study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision.
Nicholas M. Day: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Carrie L. Bergmans: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Katelin M. Zahn: acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Peter D. R. Higgins: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis.
Stewart C. Wang: critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative and technical support.
Grace L. Su: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; administrative and technical support; study supervision.
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, et al. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970–2004) The American Journal of Gastroenterology. 2012;107:1693–1701. doi: 10.1038/ajg.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2014;39:660–671. doi: 10.1111/apt.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rieder F, Fiocchi C. Mechanisms of tissue remodeling in inflammatory bowel disease. Dig Dis. 2013;31:186–193. doi: 10.1159/000353364. [DOI] [PubMed] [Google Scholar]
- 5.Khoury W, Stocchi L, Geisler D. Outcomes after laparoscopic intestinal resection in obese versus non-obese patients. The British journal of surgery. 2011;98:293–298. doi: 10.1002/bjs.7313. [DOI] [PubMed] [Google Scholar]
- 6.Nic Suibhne T, Raftery TC, McMahon O, et al. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. Journal of Crohn's & Colitis. 2013;7:e241–e248. doi: 10.1016/j.crohns.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Causey MW, Johnson EK, Miller S, et al. The impact of obesity on outcomes following major surgery for Crohn's disease: an American College of Surgeons National Surgical Quality Improvement Program assessment. Diseases of the colon and rectum. 2011;54:1488–1495. doi: 10.1097/DCR.0b013e3182342ccb. [DOI] [PubMed] [Google Scholar]
- 8.Uchino M, Ikeuchi H, Matsuoka H, et al. Risk factors for surgical site infection and association with infliximab administration during surgery for Crohn's disease. Diseases of the colon and rectum. 2013;56:1156–1165. doi: 10.1097/DCR.0b013e31829f682c. [DOI] [PubMed] [Google Scholar]
- 9.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Annals of surgery. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 10.Cruz RJ, Dew MA, Myaskovsky L, et al. Objective radiologic assessment of body composition in patients with end-stage liver disease: going beyond the BMI. Transplantation. 2013;95:617–622. doi: 10.1097/TP.0b013e31827a0f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabel MS, Terjimanian M, Conlon ASC, et al. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. Journal of surgical oncology. 2013;108:169–175. doi: 10.1002/jso.23366. [DOI] [PubMed] [Google Scholar]
- 12.Hou JK, Tan M, Stidham RW, et al. Accuracy of Diagnostic Codes for Identifying Patients with Ulcerative Colitis and Crohn's Disease in the Veterans Affairs Health Care System. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanauer DA. EMERSE: The Electronic Medical Record Search Engine. AMIA Annu Symp Proc. 2006:941. [PMC free article] [PubMed] [Google Scholar]
- 14.Miller BS, Ignatoski KM, Daignault S, et al. A quantitative tool to assess degree of sarcopenia objectively in patients with hypercortisolism. Surgery. 2011;150:1178–1185. doi: 10.1016/j.surg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Ananthakrishnan AN, McGinley EL. Weekend hospitalisations and post-operative complications following urgent surgery for ulcerative colitis and Crohn's disease. Alimentary Pharmacology and Therapeutics. 2013;37:895–904. doi: 10.1111/apt.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bautista MC, Otterson MF, Zadvornova Y, et al. Surgical Outcomes in the Elderly with Inflammatory Bowel Disease are Similar to Those in the Younger Population. Digestive Diseases and Sciences. 2013;58:2955–2962. doi: 10.1007/s10620-013-2754-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang SS, Yu CS, Yoon YS, et al. Risk factors for complications after bowel surgery in Korean patients with Crohn's disease. Journal of the Korean Surgical Society. 2012;83:141–148. doi: 10.4174/jkss.2012.83.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisar PJ, Appau KA, Remzi FH, et al. Preoperative hypoalbuminemia is associated with adverse outcomes after ileoanal pouch surgery. Inflammatory Bowel Diseases. 2012;18:1034–1041. doi: 10.1002/ibd.21842. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Archives of surgery (Chicago, Ill.: 1960) 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian V, Saxena S, Kang J-Y, et al. Preoperative Steroid Use and Risk of Postoperative Complications in Patients With Inflammatory Bowel Disease Undergoing Abdominal Surgery. The American Journal of Gastroenterology. 2008;103:2373–2381. doi: 10.1111/j.1572-0241.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 21.Aberra FN, Lewis JD, Hass D, et al. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology. 2003 doi: 10.1016/s0016-5085(03)00883-7. [DOI] [PubMed] [Google Scholar]
- 22.Iesalnieks I, Kilger A, Glass H, et al. Intraabdominal septic complications following bowel resection for Crohn's disease: detrimental influence on long-term outcome. International journal of colorectal disease. 2008;23:1167–1174. doi: 10.1007/s00384-008-0534-9. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn's disease. Diseases of the colon and rectum. 2000;43:1141–1145. doi: 10.1007/BF02236563. [DOI] [PubMed] [Google Scholar]
- 24.Moran GW, Dubeau M-F, Kaplan GG, et al. The Increasing Weight of Crohn's Disease Subjects in Clinical Trials: A Hypothesis-generatings Time-trend Analysis. Inflammatory Bowel Diseases. 2013 doi: 10.1097/MIB.0b013e31829936a4. [DOI] [PubMed] [Google Scholar]
- 25.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflammatory Bowel Diseases. 2011;17:2162–2168. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obesity facts. 2009;2:370–372. doi: 10.1159/000262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gummesson A, Carlsson LMS, Storlien LH, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring, Md.) 2011;19:2280–2282. doi: 10.1038/oby.2011.251. [DOI] [PubMed] [Google Scholar]
- 29.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends in immunology. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Hass DJ, Brensinger CM, Lewis JD, et al. The impact of increased body mass index on the clinical course of Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:482–488. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Blain A, Cattan S, Beaugerie L, et al. Crohn's disease clinical course and severity in obese patients. Clinical nutrition (Edinburgh, Scotland) 2002;21:51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 32.Chan SSM, Luben R, Olsen A, et al. Body Mass Index and the Risk for Crohn's Disease and Ulcerative Colitis: Data From a European Prospective Cohort Study (The IBD in EPIC Study) The American Journal of Gastroenterology. 2013;108:575–582. doi: 10.1038/ajg.2012.453. [DOI] [PubMed] [Google Scholar]
- 33.Wiskin AE, Wootton SA, Hunt TM, et al. Body composition in childhood inflammatory bowel disease. Clinical nutrition (Edinburgh, Scotland) 2011;30:112–115. doi: 10.1016/j.clnu.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Bin CM, Flores C, Alvares-da-Silva MR, et al. Comparison between handgrip strength, subjective global assessment, anthropometry, and biochemical markers in assessing nutritional status of patients with Crohn's disease in clinical remission. Digestive Diseases and Sciences. 2010;55:137–144. doi: 10.1007/s10620-008-0692-1. [DOI] [PubMed] [Google Scholar]
- 35.Bryant RV, Trott MJ, Bartholomeusz FD, et al. Systematic review: body composition in adults with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics. 2013;38:213–225. doi: 10.1111/apt.12372. [DOI] [PubMed] [Google Scholar]
- 36.Erhayiem B, Dhingsa R, Hawkey CJ, et al. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:684–687. e1. doi: 10.1016/j.cgh.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Syed A, Cross RK, Flasar MH. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn's disease patients. The American Journal of Gastroenterology. 2013;108:583–593. doi: 10.1038/ajg.2012.464. [DOI] [PubMed] [Google Scholar]