Abstract
Objectives
We assessed 2 pathways through which dietary antioxidants may counter adverse effects of exposure to particulate matter less than 2.5 micrometers in diameter (PM2.5) on blood pressure (BP): main (compensatory) and modifying (protective) models.
Methods
We used 2002 to 2003 data from the Detroit Healthy Environments Partnership community survey conducted with a multiethnic sample of adults (n = 347) in low- to moderate-income, predominantly Hispanic and non-Hispanic Black neighborhoods in Detroit, Michigan. We used generalized estimating equations to test the effects of ambient exposure to PM2.5 and dietary antioxidant intake on BP, with adjustment for multiple confounders.
Results
Dietary antioxidant intake was inversely associated with systolic BP (b = −0.5; P < .05) and pulse pressure (b = −0.6; P < .05) in neighborhoods closest to major sources of air pollutants. Adverse effects of PM2.5 remained significant after accounting for antioxidant intakes. Exploratory analyses suggested potential modifying effects of antioxidant intake on associations between ambient PM2.5 exposure and BP.
Conclusions
Interventions to improve access to antioxidant-rich foods in polluted urban areas may be protective of cardiovascular health. However, efforts to reduce PM2.5 exposure remain critical for cardiovascular health promotion.
Regulatory actions reducing fine particulate matter less than 2.5 micrometers in diameter (PM2.5) are associated with improvements in life expectancy in the United States.1,2 However, levels of PM2.5 remain high and continue to be positively associated with risk of high blood pressure (BP), a precursor for many adverse cardiovascular outcomes, including coronary heart disease, myocardial infarction, and heart failure.3-6 In the United States overall, medical expenses associated with the nearly 1 in 3 adults with hypertension7 are estimated at approximately $131 billion annually.8 Cardiovascular disease is the leading cause of death in the United States and accounts for one third of the excess risk of death experienced by non-Hispanic Black in comparison with non-Hispanic White Americans.9,10 Non-Hispanic Blacks, Hispanics, and individuals of low income in the United States are disproportionately likely to reside in communities with excess exposure to environmental hazards, including PM2.5.11-13 Continued investigation of strategies to reduce exposure to PM2.5, and its adverse effects on BP, are essential to efforts to reduce racial and ethnic disparities in cardiovascular risk.
Oxidative stress may be one molecular pathway linking PM2.5 to BP.14-17 PM2.5 compounds, whose composition largely depends on their source (e.g., industry, transportation), typically contain organic chemicals, metals, soot, soil, dust, allergens, and acids on their surface. When inhaled, these particles, alone or through chemical reactions, may initiate the creation of reactive oxygen species (ROS), commonly referred to as free radicals, resulting in various physiological responses in lung, heart, and vascular tissue.18 Specifically, ROS can contribute to vasoconstriction, endothelial dysfunction, and hypertrophy, among other mechanisms that can ultimately contribute to hypertension.19
Oxidative stress may be mitigated when antioxidants absorb ROS in the airways and inhibit oxidation.20 Antioxidants are available through dietary intake of foods or supplements (e.g., vitamins A, C, and E and selenium) and may protect against adverse effects of oxidative stress. The majority of studies addressing the effects of antioxidants on cardiovascular health have examined the modifying (protective) or main (compensatory) role of antioxidant intake from supplements, rather than from whole foods captured through dietary intakes. These effects remains unsettled, however, with several meta-analyses reporting minimal or no main effects of supplements on the incidence of major cardiovascular effects across study designs.21,22 Romieu et al. conducted a substantial review of air pollution, oxidative stress, and various health outcomes and concluded that antioxidant supplements may modify air pollution’s adverse effects on cardiovascular health.23
A few clinical studies have noted deleterious effects of antioxidant supplement use.24,25 Many factors compromise or complicate comparison of these studies’ outcomes. For example, study design varies by antioxidant type, dose, duration, and the health status of study participants.26-28 Reflecting these inconclusive findings, the American Heart Association’s scientific position recommends against antioxidant supplement use.29
By contrast, on the basis of modest evidence of reductions in aging-related illnesses,30 the Institute of Medicine provides recommended dietary allowances for many well-known anti-oxidants, including selenium (400 mg) and vitamins A (900 μg), C (90 mg), and E (15 mg). Despite the uncertainties in the evidence base, several scholars recommend direct dietary intake of antioxidants through healthy food (i.e., fruit, vegetables, whole grains) or beverage sources to mitigate the adverse effects of ROS on cardiovascular health.30-34
Antioxidant intakes are not consistent across diverse populations. Chun et al.31 used food consumption and supplement use data from National Health and Nutritional Examination Survey (1999–2002)35 to estimate overall antioxidant intake in the United States, deriving antioxidant values from the US Department of Agriculture Database for the Flavonoid Content of Selected Food.36 They concluded that overall intake appeared to be higher among women, older adults, non-Hispanic Whites, and higher-income and physically active individuals. For some antioxidants, including vitamin C and carotenes, intake appeared to be higher among nonsmokers and those who did not consume alcohol.31 Researchers have used various clinical indicators to detect antioxidant deficiency among those with chronic illnesses, including asthma, chronic obstructive lung diseases, diabetes, and cardiovascular disease,13,23,37,38 which have well-established disparities by race, ethnicity, and income.39,40
The unequal distribution of exposure to PM2.5 and unequal access to antioxidant-rich foods41 raise questions about their contributions to racial, ethnic, and socioeconomic health inequities. Residents of urban communities of color and low-income communities are more likely to experience excess exposure to PM2.5.11,42 Emerging research also suggests racial differences in oxidative stress, with persons of color experiencing higher levels.43-45 Access to stores that sell fresh produce, an important source of dietary antioxidants, is low in some urban communities, particularly lower-income communities composed predominantly of people of color.46-50 Together, excess exposure to air pollutants and psychosocial stress may increase levels of oxidative stress in low-income, urban communities of color, at the same time that these communities experience reduced access to foods rich in protective antioxidants. Few studies have examined the question of whether dietary anti-oxidant intake (DAI) may counter the adverse effects of exposure to PM2.5 on blood pressure in a community sample.
We previously reported adverse effects of PM2.5 on blood pressure4,51 and associations between neighborhood availability of fruits and vegetables and dietary intakes of those foods.41,50 We built on those findings to specifically examine, in data from Detroit, Michigan, the extent to which DAI is inversely associated with BP and whether it may partially compensate for or counter adverse effects of PM2.5 on BP. If higher levels of DAI inhibit oxidation through absorption of ROS, thus reducing levels of oxidative stress, adverse effects of PM2.5 on BP may be contingent on DAI levels. Thus, we also examined protective models, exploring the extent to which DAI modifies adverse effects of exposure to PM2.5 on BP. We considered the implications of our findings for understanding and intervening to reduce excess risk of cardiovascular disease among residents of predominantly non-Hispanic Black and Hispanic low- to moderate-income urban communities. Our research questions were (1) Is DAI associated with reduced BP? (2) Does DAI reduce adverse effects of PM2.5 on BP? and (3) Does DAI modify the association between PM2.5 and BP?
METHODS
The Healthy Environments Partnership (HEP) is a community-based participatory research collaboration established in 2000 to investigate and address social and environmental factors that contribute to disparities in cardiovascular disease.52 HEP examines racial and socioeconomic inequalities in cardiovascular risk and the role of social and physical environmental exposures in this process, as well as disseminating and translating findings to inform new and established intervention and policy efforts. HEP engages academic researchers and representatives from health service organizations, community-based organizations, and the community at large in a collaborative effort to address these questions. Representatives of these partner organizations compose the HEP Steering Committee, which meets monthly to oversee all aspects of the research process.
Data
Our data came from 3 sources: (1) the HEP 2002 to 2003 community survey53; (2) a modified Block Food Frequency Questionnaire (Berkeley Nutrition Services, Berkeley, CA), implemented as part of the community survey; and (3) community-level ambient exposure measures collected in 2002 to 2003.
The HEP community survey had a stratified 2-stage probability sample of occupied housing units, designed for 1000 completed interviews with adults aged 25 years or older in 3 parts of Detroit, allowing for comparisons across geographic areas of the city.52 The survey collected self-reported demographic and health data, including age, gender, race, ethnicity, household income, education, smoking behavior, hypertension medication use, and dietary intake. The survey also collected anthropometric clinical measures (height, weight, BP) during the interviews. For a subset of 347 participants, the survey measured BP a second time, along with additional clinical measures (e.g., triglycerides, fasting blood glucose). All survey participants completed the Block Food Frequency Questionnaire.
Measures
Dependent variables were systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP). Certified phlebotomists measured BP by the method used by the National Health and Nutritional Examination Survey,35 with a portable cuff device (Omron model HEM 711AC, Omron Healthcare Inc, Lake Forest, IL) that passed Association for the Advancement of Medical Instrumentation standards.54 Phlebotomists used a large cuff for participants whose arm circumference was greater than 15 inches. They took 3 consecutive measures of SBP and DBP, separated by about 1 minute, at each of the 2 time points, with the mean of the second and third measures used for all data analysis. PP, an indicator of arterial stiffness, was calculated as the difference between SBP and DBP.55
Independent variables were DAI and PM2.5. We created DAI from self-reported dietary intakes in the Block Food Frequency Questionnaire. We assigned antioxidant levels according to estimates for specific foods and quantities established by Halvorsen et al.56 Between 2002 and 2003, we assessed daily community-level PM2.5 in study communities with tapered element oscillating microbalances (TEOM model 1400, Rupprecht and Patashnick Inc, East Greenbush, NY).57 We used a monitoring site established by the Michigan Department of Environmental Quality and 2 additional sites to capture PM2.5 levels in each of the 3 study communities. All participants in the 2002 HEP survey resided within 5 kilometers of 1 of 3 monitors.4 We also collected the following meteorological data: daily temperature, atmospheric pressure, relative humidity, wind speed, and wind direction, at each site.
Covariates were age, gender, race/ethnicity, household income, education, body mass index (defined as weight in kilograms divided by the square of height in meters), smoking behavior, doctor-diagnosed diabetes, total cholesterol, and medication use for hypertension. We also estimated models that controlled for meteorological variables (temperature, atmospheric pressure, relative humidity).
Analyses
Our study built on previously reported findings demonstrating associations between PM2.5 and BP in a multiethnic urban community.4 We used the same statistical modeling technique, the PROC SURVEYREG procedure of SAS for Windows version 9.13 (SAS Institute, Cary, NC), to test for associations between DAI and BP and for the joint effect of ambient exposure PM2.5 and DAI on BP. These procedures are specifically designed for analysis of complex sample survey data and incorporate the complex sample weights (final weights, strata, and primary sampling unit) for standard error estimates.
To temporally align PM2.5 measures with BP measures, we examined lagged exposure with individual 24-hour daily spans from 1 day before (lag 1) through 4 days before (lag 4) and larger spans of 48 (2 days average), 72 (3 days average), up to 120 (5 days average) hours average prior. After removing outliers, the final sample for these analyses ranged from 270 to 300, depending on lag of exposure considered.
To test for mediation effects, we used the method described by Judd and Kenny, which involves computing the difference between 2 parameter estimates (with and without the mediator) and then testing for the significance of the difference.58 To assess whether the slope of the association between DAI and BP varied by area, we ran models that incorporated an interaction between area and DAI. Similarly, in models assessing the joint effects of DAI and PM2.5, we included interaction terms for DAI and area and for PM2.5 and area. Results reported are from models with these interaction terms. All models adjusted for covariates.
RESULTS
Table 1 summarizes baseline demographic and health data for study participants (n = 347). The mean SBP was 129.7 millimeters of mercury (SE = 1.3 mm/Hg), mean DBP was 78.9 millimeters of mercury (SE = 0.07 mm/Hg), and mean PP was 50.9 millimeters of mercury (SE = 1.1 mm/Hg). A majority (22%) of participants had been prescribed medication to treat hypertension. The mean level of PM2.5 was 15.7 micrograms per cubic meter (SE = 0.7 μg/m3), at the US Environmental Protection Agency’s former standard (15 μg/m3) and above the new annual National Ambient Air Quality Standards attainment level (12 μg/m3). Mean DAI was 7.11 millimoles per day (SE = 0.3 mmol/day), with average intake of 6.1 millimoles per day (SE = 4.1 mmol/day), in eastside, 6.9 millimoles per day (SE = 4.2 mmol/day) in northwest, and 7.9 millimoles per day (SE = 5.8 mmol/day) in southwest Detroit.
TABLE 1.
Baseline Demographic and Health Characteristics of Study Participants: Detroit Healthy Environments Partnership; Detroit, MI; 2002–2003
| Characteristic | % or Mean ±SE (95% CI) |
|---|---|
| Age, y | 21.3 ±1.1 (19.1, 23.5) |
| Female | 55.6 |
| Race/ethnicity | |
| Hispanic | 18.0 |
| White | 20.1 |
| Black | 58.5 |
| Annual household income, $ | |
| < 10 000 | 35.0 |
| 10 000–19 999 | 27.9 |
| 20 000–34 999 | 22.3 |
| ≥ 35 000 | 14.8 |
| Education | |
| < high school diploma | 27.3 |
| High school diploma | 22.3 |
| Some college | 29.5 |
| ≥ college diploma | 20.9 |
| BMI | 30.9 ±0.5 (30, 31.9) |
| Hypertension medication | 22.2 |
| Smoking status | |
| Never | 34.0 |
| Current | 43.3 |
| Former | 22.7 |
| Antioxidant dietary intake, mmol/d | 7.11 ±0.29 (6.5, 7.7) |
| Baseline blood pressure measures | |
| Systolic | 128.8 ±1.3 (126.2, 131.5) |
| Diastolic | 80.1 ±0.7 (78.6, 81.5) |
| Pulse | 48.8 ±0.9 (46.9, 50.6) |
| Blood pressure measures at time 2 | |
| Systolic | 129.7 ±1.3 (127.0, 132.4) |
| Diastolic | 78.9 ±0.7 (77.4, 80.4) |
| Pulse | 50.9 ±1.1 (48.6, 53.2) |
| Ambient exposure | |
| PM2.5 (lag 1) | 309 15.7 ±0.7 (14.4, 17.1) |
| PM2.5 (at time 2, lag 1) | 291 14 ±0.4 (13.1, 14.9) |
Note. BMI = body mass index; CI = confidence interval; PM2.5 = particulate matter < 2.5 micrometers in diameter. The sample size was n = 347.
The results for associations between DAI and BP indicated an inverse association of DAI with SBP (b = −0.42; 95% confidence interval [CI] = −0.83, −0.01; P = .049) and PP (b = −0.55; 95% CI = −0.88, −0.22; P = .003), but not DBP (b = 0.12; 95% CI = −0.27, 0.51; P = .548).
Results from models testing the joint effects of PM2.5 and DAI on BP are shown in Table 2. Results are presented for each of four 24-hour lags of PM2.5. Because differences in associations between PM2.5 and BP by area of the city were reported previously,4,52 we also tested for differences across areas of the city in the joint effects of PM2.5 and DAI. These models showed that associations between PM2.5 and BP remained significant after accounting for DAI in southwest Detroit, the area of the city with the greatest proximity to multiple stationary and mobile sources of PM2.5. Associations were not significant for eastside and northwest Detroit. For residents of southwest Detroit, DAI was significantly and inversely associated with SBP at lags 2 (b = −0.52; 95% CI = −1.0, −0.1; P = .03), 3 (b = −0.59; 95% CI = −1.1, −0.1; P = .02), and 4 (b = −0.49; 95% CI = −0.9, −0.1; P = .03) and with PP at lags 1 (b = −0.57; 95% CI = −1.0, −0.1; P = .01), 2 (b = −0.59; 95% CI = −1.1, −0.1; P = .02), 3 (b = −0.74; 95% CI = −1.2, −0.1; P = .01), and 4 (b = −0.56; 95% CI = −1.1, −0.1; P = .05), after accounting for the effect of ambient exposure of PM2.5 (results not shown). We also observed antioxidant effects combined with effects of multiday averaged exposure to PM2.5 on BP outcomes in the models. Results were similar, with significant antioxidant effects on SBP (2-, 3-, 4-, and 5-day averages) and PP (2-, 3-, 4-, and 5-day averages; results not shown).
TABLE 2.
Joint Effects of Particulate Air Pollution and Dietary Antioxidant Intake on Blood Pressure Outcomes: Detroit Healthy Environments Partnership; Detroit, MI; 2002–2003
| b | Lag 1, b (95% CI) | Lag 2, b (95% CI) | Lag 3, b (95% CI) | Lag 4, b (95% CI) |
|---|---|---|---|---|
| Systolic BP | ||||
| PM2.5 | −2.6 (−3.0, −2.2) | 4.2 (3.9, 4.5) | 3.0 (2.7, 3.3) | 7.3 (6.7, 7.9) |
| DAI | −0.5 (−1.0, 0.0) | −0.5* (−1, −0.1) | −0.6* (−1.1, −0.1) | −0.5* (−0.9, −0.1) |
| Diastolic BP | ||||
| PM2.5 | −2.1 (−2.3, −1.8) | −1.1 (−1.4, −0.8) | 0.6 (0.3, 0.9) | 2.7 (2.1, 3.3) |
| DAI | 0.1 (−0.7, 0.8) | 0.0 (−0.7, 0.8) | 0.1 (−0.6, 0.8) | 0.0 (−0.7, 0.8) |
| Pulse pressure | ||||
| PM2.5 | −0.4 (−0.8, 0.0) | 5.4 (5, 5.8) | 2.5 (2.2, 2.7) | 4.8 (4.5, 5.2) |
| DAI | −0.6* (−1.0, −0.1) | −0.6* (−1.1, −0.1) | −0.7** (−1.2, −0.2) | −0.6* (−1.1, 0) |
Note. BP = blood pressure; CI = confidence interval; DAI = dietary antioxidant intake; PM2.5 = particulate matter < 2.5 micrometers in diameter.
P < .05;
P < .01;
P < .001.
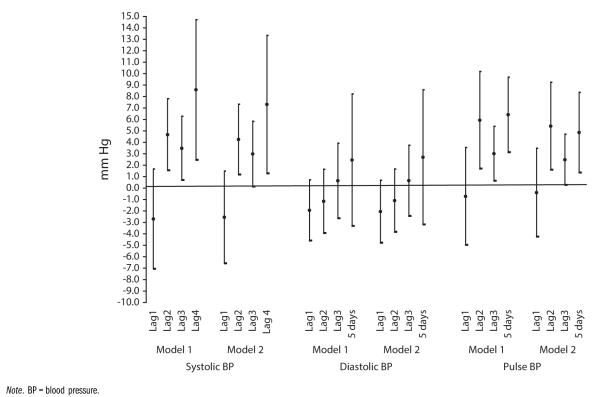
Parameter estimates for PM2.5 in Table 2 were somewhat reduced from those previously reported in models that did not account for DAI.4 Figure 1 shows these differences for each measure of BP, with model 1 showing previously reported levels not accounting for DAI4 and model 2 showing estimates for associations between PM2.5 and SBP and PP after adjustment for DAI.
FIGURE 1.
Associations between particulate matter < 2.5 micrometers in diameter and blood pressure without (model 1) and with (model 2) dietary antioxidant intake: Detroit Healthy Environments Partnership, 2002–2003.
To assess whether the reductions in associations between PM2.5 and BP, with adjustment for DAI in model 2, were statistically significant, we ran formal tests of mediation, with methods proposed by Friedman and McAdam59 (see also Zhang et al.60). Results from these analyses suggested that DAI exerted a small but statistically significant effect, reducing adverse effects of PM2.5 on SBP and PP. The test statistics for this comparison were notable for lags 2 to 4 for SBP (P < .001) and for PP (P = .001). These findings were consistent with a hypothesized reduction in ROS through absorption by antioxidants.
Finally, we ran exploratory models assessing whether associations between PM2.5 exposure and blood pressure differed among participants with high and low DAI. Although not statistically significant, our results suggested a potential modifying effect of DAI on associations between PM2.5 and BP. Specifically, we found some suggestion that, at higher levels of DAI, the adverse effects of PM2.5 on SBP were dampened somewhat. Because of our relatively small sample size and the multiple interaction terms in these final models, our confidence in reporting these results is relatively low. Further study is needed on this effect.
DISCUSSION
Two key findings emerged from our examination of whether antioxidant dietary intakes counter adverse effects of exposure to PM2.5 on BP in a multiethnic community sample. First, our findings were generally consistent with the hypothesis that DAI offers some protection against adverse effects of PM2.5 on BP. Our finding of an inverse association between DAI and SBP and PP was consistent and extended results reported elsewhere, in studies that used dietary supplements rather than our DAI measures.26,61 This effect was significant in the study community that hosts the greatest number of point and mobile sources of PM2.5. The inclusion of antioxidants in the model only slightly attenuated the main effect of PM2.5 on SBP and PP in southwest Detroit.
Our second finding, on whether DAI modifies associations between PM2.5 and BP, although exploratory, was suggestive that adverse effects of PM2.5 on BP may be weakened for those with higher DAI. However, these analyses were underpowered, and further analyses with larger data sets are warranted.
Effects of Dietary Antioxidant Intake
Our results supported the hypothesis that DAI is inversely associated with indicators of SBP and PP. Associations remained statistically significant in models that included PM2.5, suggesting that these effects occurred above and beyond effects of PM2.5 and may serve to partially compensate for adverse effects of PM2.5 on SBP. An individual with average DAI in our sample (7.4 mmol/d) would realize a 3.5–millimeters of mercury decrease in SBP. We detected no significant associations with DBP, but dietary antioxidants similarly reduced adverse effects of PM2.5 on PP. In other words, residents who reported higher dietary intakes of antioxidant-rich foods slightly reduced adverse effects of PM2.5 on SBP and on PP. Our results were consistent with the idea that PM2.5 influences BP through the production of ROS and that DAI may reduce these adverse effects through absorption of free radicals. PM2.5 retained a significant adverse association with SBP and PP, even after accounting for DAI. Thus, our findings suggest that DAI may reduce but, at the level of DAI we found, not eradicate adverse effects of PM2.5 on BP.
Our tests of whether DAI modifies associations between PM2.5 and BP must be considered exploratory, because of the limited sample size and number of covariates in our models. Our findings are suggestive of reductions in associations between PM2.5 and BP for individuals reporting higher levels of DAI, but require further study.
Our tests of both main and modifying effects suggested that DAI is likely insufficient to protect against adverse effects of PM2.5 on BP. Our findings support the importance of continued efforts to strengthen the existing monitoring network to include near-roadway monitoring of PM2.5 as well as reductions in the National Ambient Air Quality Standards for fine particles from 15 to 12 micrograms per cubic meter to promote health. Such efforts may be particularly important to protect the health of residents in neighborhoods near point and mobile sources of pollution, who are disproportionately likely to be members of racial and ethnic groups that experience excess vulnerability caused by cumulative exposures to adverse social and economic conditions.11,12,42
Limitations and Strengths
We relied on self-reported indicators of dietary intake, to which we assigned estimated antioxidant values. Although it is unlikely these biases were systematically patterned so as to skew results, these measures had a degree of imprecision. Our data set did not allow assessment of biological indicators of oxidative stress, individual sensitivity to oxidative stress, or gene–environment interactions that may moderate antioxidant levels present in blood and tissues.23,62,63 Our study focused on low- to moderate-income communities of color, which may experience higher baseline levels of oxidative stress43-45 as well as higher exposures to PM2.5. Such communities have been underrepresented in previous studies of anti-oxidant intake.
Levels of DAI in our sample were low relative to estimates from other investigations. For example, the Health Professionals Follow-Up Study reported daily DAI of approximately 10.76 millimoles64; our sample averaged 7.4 millimoles. Chun et al. estimated DAI as well as supplemental antioxidant intake from National Health and Nutritional Examination Survey data.31 Their reported consumption of vitamins A, C, and E and selenium translates to about 32 millimoles per day, substantially higher than our estimates. Thus, the DAI derived from our data may have underestimated the compensatory or protective effects that may operate in populations with higher antioxidant intake from diet or supplements.
Despite these limitations, our study had several unique strengths and contributions. It was among only a handful of studies to examine the joint effects of PM2.5 and DAI in a community-dwelling population, rather than in a controlled, clinical setting. Our data provided measured (rather than self-reported) BP and ambient measures of air quality recorded over a 3-year period. We used measures of daily intake of antioxidants derived from whole foods, rather than supplements. Our study also highlighted the potential of a long-term community–academic partnership to advance new research questions that address cumulative impacts of community environmental conditions on health.
Conclusions
Our findings are consistent with, and build upon, previously reported results suggesting that residents of some Detroit neighborhoods experience excess cardiovascular risk in part through exposure to poor air quality.4,51,65-67 Our finding that DAI was associated with reduced blood pressure and may partially mitigate adverse effects of PM2.5 on hemodynamic indicators is particularly relevant in light of previous research reporting limited access to healthy food in some Detroit neighborhoods48,50,68 and linking food access to dietary intakes.41,53 Aligning with extensive, ongoing work to improve equity of food environments and nutrition throughout the United States,69-72 our findings emphasize the need to ensure availability of foods rich in antioxidants in food stores, with particular attention to such availability in areas in which residents are exposed to air pollution.
Although our findings suggested beneficial effects of DAI, large and adverse effects of PM2.5 on SBP and PP remained. Our findings suggest that these potential protective effects, although helpful, are unlikely to eliminate adverse effects of PM2.5 exposure on cardiovascular health or the disproportionate risk of such exposures on the health of low- to moderate-income urban communities. Attention to land-use decisions that shape the exposures of residents of low- to moderate-income communities and communities of color to particulate pollutants is critical to efforts to reduce health inequities.73,74 Such efforts should consider these cumulative effects and devise strategies to address underlying social, political, and economic dynamics that may place marginalized communities at disproportionate risk. In recognition of the disproportionate effects of such cumulative exposures for residents of low- to moderate-income urban communities, continued investment should be made to improve mechanisms to better quantify the cumulative effects of social, economic, and chemical exposures and to incorporate these assessment tools into regulatory decision-making processes.75-77
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences (NIEHS; grants RO1-ES10936-0, R01-ES14234, and P30ES017885) and the National Institute of Minority Health and Health Disparities (NIMHD) (grant P60 MD002249). Work by the Michigan Department of Environmental Quality and funding from the Michigan Center for the Environment and Children’s Health (grants US EPA-R826710-01, NIEHS-P01-ES09589-01, and R01-ES10688-03) helped support collection of air quality data.
We thank the members of the HEP Steering Committee for their contributions to this work, including representatives from Detroit Institute of Population Health, Detroit Hispanic Development Corporation, Friends of Parkside, Henry Ford Health System, Warren Conner Development Coalition, University of Michigan School of Public Health, and community members. We also thank the following members of the Stakeholder Advisory Board of the University of Michigan Environmental Health Sciences Core Center for their contributions: Jaye Clement, Paul Harbin, Yolanda Hill, Erminia Ramirez, Sheryl Shellman Weir, Sherita Smith, Donele Wilkins, and Guy Williams.
Note. The results presented here are solely the responsibility of the authors and do not necessarily represent the views of NIEHS or NIMHD.
Footnotes
Contributors A. J. Schulz conceptualized the study and oversaw the data analysis. G. B. Mentz helped analyze data, oversaw the compilation of data for the antioxidant scales, and created the antioxidant scales. N. R. Sampson helped analyze data and conducted relevant literature reviews. J. T. Dvonch oversaw the portions of the analysis related to air quality amd helped review and interpret findings. A. G. Reyes helped conceptualize the study. B. Izumi helped construct the antioxidant scale, review the literature, and interpret findings. All authors contributed to and reviewed the article.
Human Participant Protection The University of Michigan institutional review board approved the HEP study. The survey was conducted in accordance with ethical standards for treatment of human participants and the Helsinki Declaration of 1975, as revised in 2000. All survey participants gave written informed consent prior to inclusion in the study.
Contributor Information
Natalie R. Sampson, University of Michigan, Dearborn..
J. Timothy Dvonch, Department of Environmental Health Science, School of Public Health, University of Michigan, Ann Arbor..
Angela G. Reyes, Detroit Hispanic Development Corporation, Detroit, MI..
Betty Izumi, School of Community Health, Portland State University, Portland, OR..
References
- 1.Correia AW, Pope CA, III, Dockery DW, Wang Y, Ezzati M, Dominici F. Effect of air pollution control on life expectancy in the United States: an analysis of 545 US counties for the period from 2000 to 2007. Epidemiology. 2013;24(1):23–31. doi: 10.1097/EDE.0b013e3182770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120(7):965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD, Rajagopalan S. Chronic air pollution exposure and endothelial dysfunction: what you can’t see—can harm you. J Am Coll Cardiol. 2012;60(21):2167–2169. doi: 10.1016/j.jacc.2012.08.974. [DOI] [PubMed] [Google Scholar]
- 4.Dvonch JT, Kannan S, Schulz AJ, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53(5):853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibald-Mulli A, Stieber J, Wichmann H-E, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91(4):571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110(15):2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Vital signs: prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60(4):103–108. [PubMed] [Google Scholar]
- 8.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 9.Cruickshank JK, Mzayek F, Liu L, et al. Origins of the “Black/White” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation. 2005;111(15):1932–1937. doi: 10.1161/01.CIR.0000161960.78745.33. [DOI] [PubMed] [Google Scholar]
- 10.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 11.American Lung Association Urban air pollution and health inequities: a workshop report. Environ Health Perspect. 2001;109(suppl 3):357–374. [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda ML, Edwards SE, Keating MH, Paul CJ. Making the environmental justice grade: the relative burden of air pollution exposure in the United States. Int J Environ Res Public Health. 2011;8(6):1755–1771. doi: 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 14.Behndig AF, Mudway IS, Brown JL, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27(2):359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 15.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 16.Cross CE, Valacchi G, Schock B, et al. Environmental oxidant pollutant effects on biologic systems: a focus on micronutrient antioxidant-oxidant interactions. Am J Respir Crit Care Med. 2002;166(12 pt 2):S44–S50. doi: 10.1164/rccm.2206015. [DOI] [PubMed] [Google Scholar]
- 17.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 18.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassègue B, Griendling KK. Reactive oxygen species in hypertension; an update. Am J Hypertens. 2004;17(9):852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Kelly FJ. Dietary antioxidants and environmental stress. Proc Nutr Soc. 2004;63(4):579–585. doi: 10.1079/pns2004388. [DOI] [PubMed] [Google Scholar]
- 21.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361(9374):2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 22.Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS ONE. 2013;8(2):e56803. doi: 10.1371/journal.pone.0056803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31(1):179–197. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- 24.Albanes D, Heinonen OP, Taylor PR, et al. α-Tocopherol and β-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 25.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 26.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv. 2010;10(6):354–362. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
- 27.Viña J, Gomez-Cabrera MC, Borras C. Fostering antioxidant defences: up-regulation of antioxidant genes or antioxidant supplementation? Br J Nutr. 2007;98(suppl 1):S36–S40. doi: 10.1017/S0007114507839596. [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara D, Fujiwara N, Suzuki K. Antioxidants: benefits and risks for long-term health. Maturitas. 2010;67(2):103–107. doi: 10.1016/j.maturitas.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 29.American Heart Association Scientific Position [Accessed August 29, 2014]; Available at: http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Vitamin-and-Mineral-Supplements_UCM_306033_Article.jsp.
- 30.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook N. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 31.Chun OK, Floegel A, Chung S-J, Chung CE, Song WO, Koo SI. Estimation of antioxidant intakes from diet and supplements in U. S. adults. J Nutr. 2010;140(2):317–324. doi: 10.3945/jn.109.114413. [DOI] [PubMed] [Google Scholar]
- 32.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Melnyk JP, Tsao R, Marcone MF. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res Int. 2011;44(1):14–22. [Google Scholar]
- 34.Ye Z, Song H. Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15(1):26–34. doi: 10.1097/HJR.0b013e3282f11f95. [DOI] [PubMed] [Google Scholar]
- 35.National Health and Nutrition Examination Staff Procedures Manual for the NHANES Survey 1971—73— part 15a. National Center for Health Statistics; Washington, DC: 1972. [Google Scholar]
- 36.Bhagwat S, Haytowitz DB, Holden JM. [Accessed August 28, 2014];USDA database for the flavonoid content of selected foods. (Release 3.1). 2013 Available at: http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Flav/Flav_R03-1.pdf.
- 37.Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15(2):143–149. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- 38.Boots AW, Haenen GRMM, Bast A. Oxidant metabolism in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;22(46 suppl):14s–27s. doi: 10.1183/09031936.03.00000403a. [DOI] [PubMed] [Google Scholar]
- 39.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention Forward: CDC health disparities and inequalities report—United States, 2011. MMWR Surveill Summ. 2011;60(suppl):1–2. [PubMed] [Google Scholar]
- 41.Izumi BT, Zenk S, Schulz AJ, Mentz GB, Wislon C. Associations between neighborhood availability and individual consumption of dark-green and orange vegetables among ethnically diverse adults in Detroit. J Am Diet Assoc. 2011;111(2):274–279. doi: 10.1016/j.jada.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill MS, Jerrett M, Kawachi I, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111(16):1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris AA, Zhao L, Patel RS, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team Up to Eliminate Health Disparities (META-Health) study. Metab Syndr Relat Disord. 2012;10(4):252–259. doi: 10.1089/met.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szanton S, Rifkind JM, Mohanty JG, et al. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med. 2012;19(4):489–495. doi: 10.1007/s12529-011-9188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warolin J, Coenen KR, Kantor JL, et al. The relationship of oxidative stress, adiposity and metabolic risk factors in healthy Black and White American youth. Pediatr Obes. 2014;9(1):43–52. doi: 10.1111/j.2047-6310.2012.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mari Gallagher Research and Consulting Group [Accessed December 2, 2013];Good food: examining the impacts of food deserts on public health in Chicago. 2006 Available at: http://www.marigallagher.com/site_media/dynamic/project_files/Chicago_Food_Desert_Report.pdf.
- 47.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Treuhaft S, Hamm MJ, Litjens C. [Accessed December 2, 2013];Healthy food for all: building equitable and sustainable food systems in Detroit and Oakland. 2009 Available at: http://www.policylink.org/atf/cf/%7B97C6D565-BB43-406D-A6D5-ECA3BBF35AF0%7D/Healthy%20Food%20For%20All-8-19-09-FINAL.pdf.
- 49.Zenk SN, Schulz AJ, Israel BA, et al. Neighborhood poverty and inequitable exposure to stressful social environments: results from a community-based participatory research partnership in Detroit. Paper presented at 4th International Conference on Urban Health; Toronto, Ontario. October 26–28, 2005. [Google Scholar]
- 50.Zenk SN, Schulz AJ, Israel BA, et al. Fruit and vegetable access differs by community racial composition and socioeconomic position in Detroit, Michigan. Ethn Dis. 2006;16(1):275–280. [PubMed] [Google Scholar]
- 51.Kannan S, Dvonch JT, Schulz A, et al. Exposure to fine particulate matter and acute effects on blood pressure: Effect modification by measures of obesity and location. J Epidemiol Community Health. 2010;64(1):68–74. doi: 10.1136/jech.2008.081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: the Healthy Environments Partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zenk SN, Schulz AJ, Lachance LL, et al. Multilevel correlates of satisfaction with neighborhood availability of fresh fruits and vegetables. Ann Behav Med. 2009;38(1):48–59. doi: 10.1007/s12160-009-9106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yarows SA, Brook RD. Measurement variation among 12 electronic home blood pressure monitors. Am J Hypertens. 2000;13(3):276–282. doi: 10.1016/s0895-7061(99)00182-x. [DOI] [PubMed] [Google Scholar]
- 55.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34(3):375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 56.Halvorsen BL, Carlsen MH, Phillips KM, et al. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84(1):95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 57.Keeler GJ, Dvonch JT, Yip FY. Assessment of personal and community-level exposures to particulate matter among children with asthma in Detroit, Michigan, as part of Community Action Against Asthma (CAAA) Environ Health Perspect. 2002;110(suppl 2):173–181. doi: 10.1289/ehp.02110s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981;5(5):602–619. [Google Scholar]
- 59.Friedman D, McAdam D. Collective identity and activism: networks, choices, and the life of a social movement. In: Morris AD, Mueller CM, editors. Frontiers in Social Movement Theory. Yale University Press; New Haven, CT: 1992. pp. 156–173. [Google Scholar]
- 60.Zhang Z, Zyphur MJ, Preacher KJ. Testing multilevel mediation using hierarchical linear models: problems and solutions. Organ Res Methods. 2009;12(4):695–719. [Google Scholar]
- 61.Marchioli R, Barzi F, Bomba E, et al. Antioxidant vitamins and prevention of cardiovascular disease: epidemiological and clinical trial data. Lipids. 2001;36(suppl):S53–S63. doi: 10.1007/s11745-001-0683-y. [DOI] [PubMed] [Google Scholar]
- 62.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minelli C, Wei I, Sagoo G, Jarvis D, Shaheen S, Burney P. Interactive effects of antioxidant genes and air pollution on respiratory function and airway disease: a HuGE review. Am J Epidemiol. 2011;173(6):603–620. doi: 10.1093/aje/kwq403. [DOI] [PubMed] [Google Scholar]
- 64.Mekary RA, Wu K, Giovannucci E, et al. Total antioxidant capacity intake and colorectal cancer risk in the Health Professionals Follow-Up Study. Cancer Causes Control. 2010;21(8):1315–1321. doi: 10.1007/s10552-010-9559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fann N, Roman H, Fulcher C, et al. Maximizing health benefits and minimizing inequality: incorporating local-scale data in the design and evaluation of air quality policies. Risk Anal. 2011;31(6):908–922. doi: 10.1111/j.1539-6924.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 66.Li S, Batterman S, Wasilevich E, Elasaad H, Wahl R, Mukherjee B. Asthma exacerbation and proximity of residence to major roads: a population-based matched case-control study among the pediatric Medicaid population in Detroit, Michigan. Environ Health. 2011;10:34. doi: 10.1186/1476-069X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu YC, Batterman SA. Proximity of schools in Detroit, Michigan to automobile and truck traffic. J Expo Sci Environ Epidemiol. 2006;16(5):457–470. doi: 10.1038/sj.jes.7500484. [DOI] [PubMed] [Google Scholar]
- 68.Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Neighborhood racial composition, neighborhood poverty, and the spatial accessibility of supermarkets in metropolitan Detroit. Am J Public Health. 2005;95(4):660–667. doi: 10.2105/AJPH.2004.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glanz K, Sallis JF, Frank LD. Healthy nutrition environments: concepts and measures. Am J Health Promot. 2005;19(5):330–333. ii. doi: 10.4278/0890-1171-19.5.330. [DOI] [PubMed] [Google Scholar]
- 70.Lytle LA. Measuring the food environment: state of the science. Am J Prev Med. 2009;36(4, suppl):S134–S144. doi: 10.1016/j.amepre.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKinnon M, Reedy J, Morrisette M, Lytle L, Yaroch AL. Measures of the food environment: a compilation of the literature, 1990-2007. Am J Prev Med. 2009;36(4, suppl):S124–S133. doi: 10.1016/j.amepre.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Odoms-Young AM, Zenk S, Mason M. Measuring food availability and access in African-American communities: implications for intervention and policy. Am J Prev Med. 2009;36(4, suppl):S145–S150. doi: 10.1016/j.amepre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Maantay J. Zoning, equity, and public health. Am J Public Health. 2001;91(7):1033–1041. doi: 10.2105/ajph.91.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson S, Hutson M, Mujahid M. How planning and zoning contribute to inequitable development, neighborhood health, and environmental injustice. Environ Justice. 2008;1(4):211–216. [Google Scholar]
- 75.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114(8):1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis AS, Sax SN, Wason SC, Campleman SL. Nonchemical stressors and cumulative risk assessment: an overview of current initiatives and potential air pollutant interactions. Int J Environ Res Public Health. 2011;8(6):2020–2073. doi: 10.3390/ijerph8062020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.National Environmental Justice Advisory Council [Accessed August 29, 2014];Ensuring risk reduction in communities with multiple stressors: environmental justice and cumulative risks/impacts. 2004 Available at: http://www.epa.gov/environmentaljustice/resources/publications/nejac/nejac-cum-risk-rpt-122104.pdf.