Abstract
Background
After anterior cruciate ligament reconstruction (ACLR), motivation to return to previous levels of activity is high. Very few studies have used return-to-activity criteria to determine when to permit athletic play. Return-to-activity measures objectively evaluate functional limb symmetry; however, previous biomechanical studies have found gait deviations in these individuals that persist up to 2 years after surgery.
Purpose
To evaluate gait biomechanics in a specific cohort of ACL patients 1 year after surgery and retrospectively compare individuals who pass return-to-activity criteria 6 months after surgery with those who fail.
Study Design
Prospective analysis.
Methods
A total of 40 athletes who participated regularly (>50 h/y) in cutting, jumping, and pivoting activities and who sustained an isolated, unilateral ACL rupture were included in this study. All participants underwent reconstruction by the same surgeon and received individualized postoperative rehabilitation. Performance-based and self-report data were measured 6 months after surgery to assess readiness to return to activity (90% outcome required to pass); 20 subjects passed return-to-activity criteria and 20 subjects did not. Motion analysis was performed 1 year after surgery, and knee flexion angles, moments, and excursions were measured during gait and evaluated for all subjects.
Results
There was no limb × group interaction or effect of group for all measures. Decreased knee measures were seen on the involved limb compared with the uninvolved limb for all subjects, and failed subjects demonstrated larger differences between limbs.
Conclusion
Patients continued to demonstrate biomechanical limb asymmetries 1 year after ACLR, regardless of performance-based measures at 6 months. Early return to activity did not ensure limb symmetry at 1 year.
Clinical Relevance
Gait asymmetries were seen in all subjects 1 year after surgery regardless of status at 6 months. Potentially prolonging athlete’s timelines for returning to activity may prove beneficial for a successful return to activity as well as for long-term knee function.
Keywords: return to activity, anterior cruciate ligament, knee flexion angle, gait biomechanics
After anterior cruciate ligament reconstruction (ACLR), there is a strong desire for the athlete to return to high-level activities as quickly as possible. One of the challenges of postoperative management has been to determine a patient’s readiness to safely and successfully return to activity, as multiple factors can influence return after ACLR.24 The research on readiness for return to activity after ACLR shows that 60% of research reports use time from surgery to determine clearance, with 6 months as a common time point.3 From a clinical perspective, time from reconstruction does not take patient performance into account, which can vary greatly after ACLR.5 Only 15% of studies report using 1 or 2 objective criteria to determine clearance to return to activity.3 This lack of clear objective criteria may place the ACL-reconstructed athlete at increased risk for reinjury or suboptimal performance. A battery of tests incorporating performance-based and patient-reported outcomes may be useful in accurately characterizing a patient’s readiness to return to activity after ACLR. Hartigan et al10 found that half of athletes were able to pass these return-to-activity criteria at or before 6 months after ACLR, regardless of preoperative physical therapy intervention. Passing rates improved 1 year after surgery, with more than 75% of athletes passing these return-to-activity criteria, suggesting that large improvements in functional performance occur from 6 months to 1 year after surgery. Objective, measurable criteria are critical to ensure that athletes are fully rehabilitated and their knees are ready to meet the demands of their sport.3,10
Movement asymmetries are pervasive following ACL injury and reconstruction, and have been reported to exist up to 2 years after surgery.19,20 Altered movement patterns have been suggested to be an instigating factor in the initiation and development of osteoarthritis in the ACL-injured knee as well as a risk factor for future reinjury.4,19,25 The first year after ACLR is a vulnerable time for athletes attempting to return to activity,14 and the rate for a second knee injury is as high as 49%.3 Athletes with multiplane biomechanical asymmetries at the hip and knee at the time of return to sport were at least 3 times more likely to incur a second ACL injury within the next year than those without these asymmetries.19 Using specific return-to-activity criteria 6 months after ACLR, subjects that passed these criteria demonstrate smaller limb-to-limb differences during gait compared with those who failed, supporting a relationship between clinical and functional measures and biomechanical findings.6 Large improvements in functional measures from 6 months to 1 year have been reported; however, biomechanical asymmetries have been reported to persist up to 2 years after ACLR. Further classifying ACL-reconstructed patients as passing or failing these return-to-activity criteria will allow us to determine if limb symmetry is maintained from 6 months to 1 year or if differences between limbs deteriorate over time. The purpose of this study was to determine if patients who are ready to return to activity at 6 months based on clinical and functional measures demonstrate symmetrical movement patterns 1 year after ACLR. It is hypothesized that subjects who pass strict return-to-activity criteria at 6 months will continue to demonstrate small limb-to-limb differences during gait at 1 year, while those who fail will continue to demonstrate significant limb-to-limb differences at 1 year.
MATERIALS AND METHODS
A total of 40 athletes who suffered an isolated, unilateral ACL rupture (30 males, 10 females; mean age, 30.3 ± 10 years; range, 20.6–43.9 years) were included in this study. All subjects were regular participants (≥50 h/y) in jumping, cutting, and pivoting activities prior to their injury.11 All subjects were classified as having poor dynamic knee stability according to a preoperative screening examination.8 These subjects were part of a larger randomized control trial that evaluated preoperative physical therapy interventions up to 2 years after ACLR. All testing sessions were completed by a licensed physical therapist. This study was approved by the Human Subjects Review Board, and patients provided informed consent.
All subjects underwent hamstring autograft (n = 13; mean age, 27 ± 5.7 years) or soft tissue allograft (n = 27; mean age, 29.7 ± 4.3 years) ACLR by the same orthopaedic surgeon. After reconstruction, all subjects received the same criterion-based postoperative rehabilitation program.1,17 Clinical and functional data including quadriceps strength and single-legged hop measures, and patient-reported outcomes were collected 6 months and 1 year after surgery.
Quadriceps strength measures were obtained during a maximal voluntary isometric contraction (MVIC)22 using an electromechancial dynamometer (KIN-COM; Chattanooga Corp, Chattanooga, Tennessee, USA). Subjects were seated in an upright position with their hip and knee at 90° of flexion.15 Testing was completed initially on the uninvolved limb followed by the involved limb. A ratio of quadriceps index (QI) was calculated as the quotient of the involved quadriceps MVIC to the uninvolved quadriceps MVIC multiplied by 100.
Four single-legged hop measures were completed as previously described18 with the patient wearing a functional knee brace. Testing was completed on the uninvolved limb followed by the involved limb and consisted of the single hop for distance, crossover hop for distance, triple hop for distance, and 6-minute timed hop tests.15,18 A limb symmetry index (LSI) was calculated for the distance hops from the mean of 2 measures, as the quotient of involved limb hop distance to the uninvolved limb hop distance multiplied by 100. The 6-minute timed hop was calculated as the quotient of uninvolved limb hop time to the involved limb hop time multiplied by 100. Subjects who did not achieve ≥80% QI or who demonstrated increased knee joint effusion did not complete single-legged hop tests, as this was determined to be unsafe.
Patient-reported outcomes were completed after functional measures. The Knee Outcome Survey–Activities of Daily Living Scale (KOS-ADLS)13 and the Global Rating Score of Perceived Knee Function (GRS) were used to determine patients’ perceptions of their knee function. The KOS-ADLS is a patient-reported measure of current symptoms and how these symptoms affect the knee during activities of daily living. Total scores are expressed as a percentage from 0% to 100%, with higher scores representing better knee function and fewer symptoms.13 The GRS is a single question that asks patients to rate their current knee function, including sports activities, on a scale from 0 to 100, with 0 being the inability to perform any activity and 100 being the level of knee function prior to injury.8,13
A score of ≥90% was required on all test measures (QI, 4 single-legged hop tests plus LSI, KOS-ADLS, GRS) to meet our return-to-activity criteria. Subjects who met these criteria 6 months after ACLR were classified as passing subjects, while those who did not meet these criteria were classified as failing subjects.
Biomechanical variables were collected 1 year after ACLR. Kinematic data were collected with an 8-camera 3-dimensional motion capture system (VICON; Oxford Metrics Ltd, London, England) sampled at 120 Hz. Twenty static retroreflective markers were placed on the pelvis and lower extremities to identify joint centers and segment positions. Kinetic data were collected simultaneously with an embedded force plate (Bertec, Worthington, Ohio, USA) and were also used to determine timing variables during gait. Five walking trials were collected on each limb while the subjects maintained a self-selected walking speed with ±5% variability. Postprocessing of these data was completed using rigid-body analysis and inverse dynamics with custom software programming (Visual3D; C-Motion Inc, Germantown, Maryland, USA; LabVIEW 8.2; National Instruments Corp, Austin, Texas, USA). Kinematic and kinetic variables were low-pass filtered at 6 Hz and 40 Hz, respectively. Initial contact and toe off were determined using a 50-N force-plate threshold. All walking trials were normalized to 100% of stance before being averaged for statistical analysis.
All data were analyzed using SPSS version 19.0 (IBM, Armonk, New York, USA). Paired t tests were used to determine subject demographics and differences between groups. Knee kinematics and kinetics were evaluated for all subjects 1 year after surgery using a repeated-measures analysis of variance (ANOVA) with a between-subjects factor of return-to-activity status (pass or fail). Post hoc tests were used to determine where differences between limbs existed. Variables analyzed included knee angles and moments at initial contact, peak knee flexion (PKF), and peak knee extension (PKE). Knee excursions were also measured during weight acceptance (WA; from initial contact to PKF) and during midstance (MS; PKF to PKE). The a priori significance level was set at P = .05. Clinically meaningful asymmetries were determined to be present if values met or exceeded minimal clinically important differences (MCIDs). Motion capture data were collected from 10 healthy athletes and used to determine MCIDs between limbs (knee angles ≥3°, knee moments ≥0.04 N·m/kg·m) irrespective of statistical significance.6,7
RESULTS
Six months after surgery, 20 subjects were classified as passing (10 autograft, 10 allograft) and 20 subjects were classified as failing (3 autograft, 17 allograft). Passing subjects were more than 3 times more likely to have had an autograft (positive likelihood ratio= 3.33) than were failing subjects. Passing subjects demonstrated significantly higher QI, single hop, crossover hop, timed hop, and GRS scores compared with failing subjects at 6 months (Table 1). One year after surgery, 29 subjects were classified as passing (12 improved from 6 months, 17 maintained status) and 11 were classified as failing (3 declined from 6 months, 8 maintained status). Based on classification at 6 months, 1-year functional testing showed significantly increased QI and GRS scores in passing subjects compared with failing subjects (Table 2). No other clinical or functional measures were different between the groups at 1 year. One year after surgery, there was no difference between groups regarding age at the time of surgery (P = .14), body mass index (BMI) (P= .62), or time from surgery to 1-year testing (P = .96) (Table 3). Though not statistically significantly, passing subjects were younger than failing subjects.
TABLE 1.
Six-Month Functional Measuresa
| Pass (n = 20)
|
Fail (n = 20)
|
P Value | |||
|---|---|---|---|---|---|
| Mean (SD), % | n | Mean, (SD), % | n | ||
| QI | 100.34 (7.02) | 20 | 91.32 (15.21) | 20 | .02 |
| Single hop | 96.94 (4.70) | 20 | 87.39 (7.97) | 19 | <.001 |
| Crossover hop | 97.21 (6.60) | 20 | 91.26 (8.18) | 18 | .02 |
| Triple hop | 95.16 (3.84) | 20 | 92.73 (6.75) | 18 | .17 |
| 6-minute timed hop | 99.57 (4.86) | 20 | 95.28 (7.21) | 18 | .04 |
| KOS-ADLS | 97.51 (1.84) | 20 | 95.64 (3.97) | 19 | .06 |
| GRS | 95.20 (3.64) | 20 | 89.79 (6.21) | 19 | .002 |
Significant differences appear in boldface.
GRS, Global Rating Score; KOS-ADLS, Knee Outcome Survey–Activities of Daily Living Scale; QI, quadriceps index; SD, standard deviation.
TABLE 2.
One-Year Functional Measures Based on 6-Month Classificationa
| Pass (n = 20)
|
Fail (n = 20)
|
P Value | |||
|---|---|---|---|---|---|
| Mean (SD), % | n | Mean (SD), % | n | ||
| QI | 103.96 (12.92) | 20 | 92.69 (7.84) | 20 | .002 |
| Single | 103.72 (6.69) | 19 | 98.74 (11.73) | 19 | .145 |
| Cross | 103.35 (8.81) | 19 | 97.07 (11.35) | 19 | .061 |
| Triple | 98.59 (4.57) | 19 | 95.49 (7.72) | 19 | .153 |
| Timed | 99.93 (7.20) | 19 | 97.59 (6.82) | 19 | .356 |
| KOS-ADLS | 97.16 (4.12) | 20 | 96.65 (4.39) | 20 | .707 |
| GRS | 97.50 (2.72) | 20 | 93.20 (5.97) | 20 | .007 |
Significant differences appear in boldface.
GRS, Global Rating Score; KOS-ADLS, Knee Outcome Survey–Activities of Daily Living Scale; QI, quadriceps index; SD, standard deviation.
TABLE 3.
Subject Demographicsa
| Pass | Fail | P Value | |
|---|---|---|---|
| Age, y | 27.83 (10.45) | 32.74 (10.03) | .14 |
| BMI, kg/m2 | 28.45 (4.93) | 29.24 (4.91) | .62 |
| Testing, wk | 54.20 (3.91) | 54.25 (2.81) | .96 |
Values are expressed as mean (standard deviation).
BMI, body mass index.
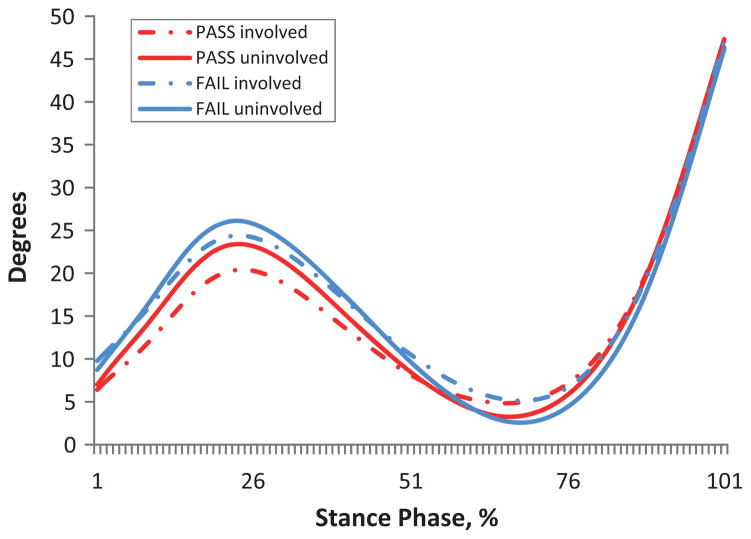
There was no significant limb × group interaction (P > .13) and no effect of group (P > .054) for all kinematic and kinetic measures. There was a main effect of limb for knee flexion angles at PKF (P=.02) and PKE (P=.01)(Table 4). Knee flexion angles at PKF were smaller on the involved limb compared with the uninvolved limb in both groups (pass, P = .16; fail, P = .07). The involved limb of all subjects was more flexed at PKE compared with the uninvolved limb (pass, P = .10; fail, P = .051); however, differences between limbs did not exceed MCID at both PKF and PKE (Table 4, Figure 1).
TABLE 4.
Knee Flexion Angles (in Degrees) During Gaita
| Subjects | PKF, Mean (SD)
|
Difference | P Value | PKE, Mean (SD)
|
Difference | P Value | ||
|---|---|---|---|---|---|---|---|---|
| Involved | Uninvolved | Involved | Uninvolved | |||||
| All | 22.66 (8.82) | 25.04 (6.52) | 2.4 | .02 | 4.50 (4.53) | 2.58 (4.43) | 1.9 | .01 |
| Pass | 21.27 (8.82) | 23.36 (6.52) | 2.1 | .16 | 4.54 (4.51) | 2.82 (5.4) | 1.7 | .10 |
| Fail | 24.05 (10.28) | 26.72 (6.36) | 2.6 | .07 | 4.47 (4.67) | 2.35 (3.31) | 2.1 | .051 |
Significant differences appear in boldface.
PKE, peak knee extension; PKF, peak knee flexion; SD, standard deviation.
Figure 1.
Knee flexion angle during stance.
There was a main effect of limb for knee moments at initial contact (P = .004), PKF (P < .001), and PKE (P = .002) (Table 5). Measures at PKF and PKE exceeded MCID for all subjects. Limb differences were greater in failing subjects at PKF (pass, P=.002; fail, P=.030) and in passing subjects at PKE (pass, P = .010). Differences between limbs at PKE for failing subjects, while exceeding MCID, were not statistically significant (fail, P = .052) (Table 5).
TABLE 5.
Knee Moments (in N·m/kg·m) During Gaita
| Subjects | PKF, Mean (SD)
|
Difference | P Value | PKE, Mean (SD)
|
Difference | P Value | ||
|---|---|---|---|---|---|---|---|---|
| Involved | Uninvolved | Involved | Uninvolved | |||||
| All | 0.42 (0.16) | 0.50 (0.14) | 0.08 | <.001 | 0.09 (0.07) | 0.14 (0.10) | 0.05 | .002 |
| Pass | 0.42 (0.14) | 0.49 (0.13) | 0.07 | .002 | 0.08 (0.07) | 0.13 (0.09) | 0.05 | .010 |
| Fail | 0.42 (0.18) | 0.51 (0.14) | 0.09 | .030 | 0.10 (0.08) | 0.14 (0.11) | 0.04 | .052 |
Significant differences appear in boldface.
PKE, peak knee extension; PKF, peak knee flexion; SD, standard deviation.
There was a main effect of limb for knee excursion measures during WA and MS (P < .001) (Table 6). In both groups, knee angles were decreased on the involved limb compared with the uninvolved limb. Differences between limbs during WA exceeded MCID for failing subjects (pass, P = .053; fail, P = .001), and all subjects demonstrated MCID during MS, with failing subjects having larger differences between limbs (pass, P = .002; fail, P = .001) (Table 6, Figure 1).
TABLE 6.
Knee Excursion Measuresa
| Subjects | Knee Exc WA, Mean (SD)
|
Difference | P Value | Knee Exc MS, Mean (SD)
|
Difference | P Value | ||
|---|---|---|---|---|---|---|---|---|
| Involved | Uninvolved | Involved | Uninvolved | |||||
| All | 14.57 (5.67) | 17.16 (4.56) | 2.6 | <.001 | 18.16 (7.14) | 22.46 (5.43) | 4.3 | <.001 |
| Pass | 14.41 (5.12) | 16.39 (4.08) | 2.0 | .053 | 16.74 (5.39) | 20.54 (4.07) | 3.8 | .002 |
| Fail | 14.73 (6.29) | 17.92 (4.98) | 3.2 | .001 | 19.58 (8.44) | 24.37 (6.02) | 4.8 | .001 |
Significant differences appear in boldface.
Exc, excursion; MS, midstance; SD, standard deviation; WA, weight acceptance.
Mean interlimb differences for knee flexion angles at PKF and PKE did not exceed MCID; however, more than one-half of subjects in both groups demonstrated clinically meaningful asymmetries at PKF (Table 7). Individuals with clinically meaningful asymmetries were further found to demonstrate significantly decreased knee flexion angles of the involved limb compared with the uninvolved limb at PKF (P = .03) and PKE (P = .01). There was no limb × group interaction (PKF, P = .74; PKE, P = .84), and no effect of group (PKF, P = .9; PKE, P = .91) for these knee flexion measures.
TABLE 7.
Subjects With Differences ≥3 Degreesa
| Subjects | IC | PKF | PKE |
|---|---|---|---|
| Pass (n = 20) | 7 (35) | 11 (55) | 6 (30) |
| Fail (n = 20) | 6 (32) | 11 (55) | 10 (50) |
Values are expressed as n (%).
IC, initial contact; PKE, peak knee extension; PKF, peak knee flexion.
DISCUSSION
Gait asymmetries were seen in all subjects 1 year after ACLR, regardless of their return-to-activity status at 6 months. Smaller knee angles, moments, and excursions were seen on the involved limb compared with the uninvolved limb in all subjects at 1 year. Knee angles did not exceed MCIDs for all subjects. Meaningful differences between limbs were seen in all subjects for knee moments at PKF and in passing subjects at PKE. Mean knee excursion measures during WA for failing subjects and MS for all subjects were clinically meaningful based on an interlimb difference of ≥3°. Based on these data, failing subjects continue to demonstrate greater limb-to-limb asymmetries 1 year after surgery; however, passing subjects demonstrated meaningful kinetic limb asymmetries and knee joint excursions during MS.
All subjects demonstrated statistically significant asymmetries for knee flexion angles and moments during gait at 1 year after ACLR. Continued limb asymmetry after ACLR may put these individuals at risk for reinjury in the future, as biomechanical asymmetries have been found to predict reinjury. Paterno et al19 evaluated biomechanical variables of dynamic landing tasks and postural stability balance measures of individuals at the time that they returned to sport activities. Within the first year of play, 13 of 56 subjects (23%) suffered a second ACL injury, and those who were reinjured demonstrated altered movement patterns at the time of return to sport. Even though our study did not evaluate the same biomechanical tasks, both studies found sagittal plane asymmetries. Biomechanical asymmetries seen in activities of daily living, such as gait, may be magnified during dynamic tasks such as a drop landing.12 Asymmetries 1 year after surgery may potentially increase the risk for a second ACL injury. Continued tracking of these subjects for reinjury will allow us to further quantify this risk.
Individuals who passed return-to-activity criteria 6 months after surgery demonstrated a less than 10% deficit in quadriceps strength, hop performance measures, and self-reported knee function—all commonly used clinical and functional measures.10 Six months after surgery, half of subjects met these criteria. To date, these criteria are the most stringent published guidelines to determine return-to-activity readiness.3 From this study, it was noted that individuals who failed these criteria 6 months after surgery demonstrated meaningful limb-to-limb asymmetries 1 year after surgery. These subjects may benefit from a targeted neuromuscular rehabilitation program that addresses these movement asymmetries and better prepares individuals for a safe and successful return to activity.26
Di Stasi et al6 evaluated return-to-activity status and gait biomechanics 6 months after surgery. A relationship was seen between poor clinical and functional measures and greater limb-to-limb asymmetries. Failing subjects demonstrated greater kinematic and kinetic limb differences at the hip and knee compared to passing subjects, with clinically meaningful differences and moderate to large effect sizes in failing subjects, suggesting that these criteria are useful in discriminating the presence of meaningful gait asymmetries at the same time point. Similar to their findings, based on 6-month functional performance, greater limb differences at 1 year were seen in failing subjects compared with those who passed. The difference between their results and the present study is the lack of discrimination of 6-month clinical and functional measures to identify clinically meaningful limb-to-limb asymmetries at 1 year. Though differences existed in the present study for knee angles, they were not clinically meaningful, and kinetic differences were present for both groups. Overall, the findings of this study support the results of Di Stasi et al,6 with greater asymmetries in failing subjects 1 year after surgery.
With further evaluation of the raw knee flexion values, the involved limb of passing subjects demonstrated smaller knee flexion angles, moments, and excursions compared with the uninvolved limbs of passing subjects and with both limbs of failing subjects. This pattern is typically seen in ACL-deficient individuals acutely after injury.21 Rudolph et al21 found that there was a difference between limbs of ACL-deficient patients with poor dynamic knee stability compared with ACL-deficient patients with good dynamic knee stability and control subjects. The involved limb of ACL-deficient patients with poor dynamic knee stability demonstrated significantly less knee flexion during gait compared with their uninjured limb and with both limbs of ACL-deficit patients with good dynamic knee stability as well as controls, a finding that they described as a stiffening strategy. They also found asymmetrical kinetic measures between limbs of ACL-deficient patients, with smaller moments on the involved limb. It has been suggested that truncated movement patterns result in altered loading patterns during gait. Failure to resolve this altered loading and stiffening strategy after surgery may be a potential mechanism for the progression of knee joint degeneration and poor long-term knee function.9
Despite mean knee flexion angles at PKF failing to exceed MCID, more than half of subjects demonstrated clinically meaningful asymmetries. Return-to-activity status was not able to discriminate individuals with knee angle differences ≥3° between limbs. Limb asymmetries during gait may become more pronounced during participation in athletic activities and may potentially predispose an athlete to a greater risk for reinjury.12,19 Persistent movement asymmetries in both the frontal and sagittal planes during walking, running, and jumping activities have been suggested as a risk factor for long-term detrimental effects on the knee joint and have the potential to contribute to joint degeneration.2,4,23,25 This subgroup of patients who fail return-to-activity criteria may require a prolonged period of rehabilitation prior to returning to activity to normalize movement patterns to ensure successful return to activity and long-term knee joint function and health.12
Several limitations need to be addressed in this study. A sampling bias toward male subjects was present in this study resulting in unequal groups (30 men, 10 women). While females are more likely to tear their ACL, a greater number of males participate in more high-risk activities.16 Potential differences between sexes may not have been accounted for with this unequal distribution. Despite statistical significance, the mean knee flexion values were not clinically meaningful; however, more than half of subjects had ≥3° differences between limbs at PKF. Clinical and functional data regarding athletic participation for these subjects were not thoroughly evaluated 1 year after surgery. It is possible that not all subjects were participating in athletic activities at the time of 1-year follow-up testing.
CONCLUSION
Altered movement patterns were present in this cohort 1 year after ACLR in subjects who both passed and failed return-to-activity criteria, with greater differences between limbs in failing subjects. Failure to resolve these altered movement patterns may predispose a higher risk for reinjury as well as impact long-term knee joint health.12,19,25 Determining safe return-to-activity criteria is currently not standardized among clinicians3; however, it is evident from this work that time-based criteria may not be appropriate since many patients continue to demonstrate functional deficits at 6 months and biomechanical asymmetries at 1 year. Early clearance to return to activities may be related to poor outcomes after surgery. Further work is needed to establish clinical and functional measures along with biomechanical criteria as a means to determine readiness to return to activities in an effort to allow a more safe and successful return.
Acknowledgments
These data were part of a larger randomized clinical trial funded by the National Institute of Health (R01-AR048212). The authors thank Drs Dan Ramsay, Wendy Hurd, Erin Hartigan, and Stephanie Di Stasi for their assistance with clinical and biomechanical data collection and Martha Callahan for research coordination.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42:601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriacchi T, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 3.Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1697–1705. doi: 10.1016/j.arthro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43:366–370. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 5.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 6.Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med. 2013;41:1310–1318. doi: 10.1177/0363546513482718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech (Bristol, Avon) 2012;27:360–365. doi: 10.1016/j.clinbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 9.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Altered loading in the injured knee after ACL rupture. J Orthop Res. 2013;31:458–464. doi: 10.1002/jor.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartigan EH, Axe MJ, Snyder-Mackler L. Time line for noncopers to pass return-to-sports criteria after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2010;40:141–154. doi: 10.2519/jospt.2010.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3–4):226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 12.Hewett TE, Di Stasi SL, Myer GD. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:216–224. doi: 10.1177/0363546512459638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Laboute E, Savalli L, Puig P, et al. Analysis of return to competition and repeat rupture for 298 anterior cruciate ligament reconstructions with patellar or hamstring tendon autograft in sportspeople [in English, French] Ann Phys Rehabil Med. 2010;53:598–614. doi: 10.1016/j.rehab.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Logerstedt D, Lynch A, Axe MJ, Snyder-Mackler L. Symmetry restoration and functional recovery before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21:859–868. doi: 10.1007/s00167-012-1929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: a 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Manal TJ, Snyder-Mackler L. Practice guidelines for ACL rehabilitation: a criterion-based rehabilitation progression. Oper Tech Orthop. 1996;6:190–196. [Google Scholar]
- 18.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after ACL rupture. Am J Sports Med. 1991;19:513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 19.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38:1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011;44:1948–1953. doi: 10.1016/j.jbiomech.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9(2):62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 22.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901–907. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 23.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 24.Thomeé R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:1798–1805. doi: 10.1007/s00167-011-1669-8. [DOI] [PubMed] [Google Scholar]
- 25.Webster KE, Feller JA. The knee adduction moment in hamstring and patellar tendon anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2012;20:2214–2219. doi: 10.1007/s00167-011-1835-z. [DOI] [PubMed] [Google Scholar]
- 26.White K, Di Stasi SL, Smith AH, Snyder-Mackler L. Anterior cruciate ligament- specialized post-operative return-to-sports (ACL-SPORTS) training: a randomized control trial. BMC Musculoskelet Disord. 2013;14:108. doi: 10.1186/1471-2474-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]