Abstract
Cells of specialized secretory organs expand their secretory pathways to accommodate the increased protein load necessary for their function. The endoplasmic reticulum (ER), the Golgi apparatus and the secretory vesicles, expand not only the membrane components but also the protein machinery required for increased protein production and transport. Increased protein load causes an ER stress response akin to the Unfolded Protein Response (UPR). Recent work has implicated several bZip transcription factors in the regulation of protein components of the early secretory pathway necessary to alleviate this stress. Here, we highlight eight bZip transcription factors in regulating secretory pathway component genes. These include components of the three canonical branches of the UPR–ATF4, XBP1, and ATF6, as well as the five members of the Creb3 family of transcription factors. We review findings from both invertebrate and vertebrate model systems suggesting that all of these proteins increase secretory capacity in response to increased protein load. Finally, we propose that the Creb3 family of factors may have a dual role in secretory cell differentiation by also regulating the pathways necessary for cell cycle exit during terminal differentiation.
Keywords: bZip transcription factors, endoplasmic reticulum, Golgi, secretion, secretory capacity, secretory vesicles
The early secretory pathway
An estimated one third of the open reading frames encoded by eukaryotic genomes is predicted to travel through the secretory pathway (Dancourt and Barlowe, 2010; Suh and Hutter, 2012). Trafficked proteins include the secreted and membrane-bound components of the plasma membrane and endomembrane compartments. Classical genetic screens, biochemical reconstitution studies and live cell imaging, largely in yeast and mammalian tissue culture cells, have revealed critical components of the secretory machinery and have provided insight into the molecular mechanisms by which proteins enter the secretory pathway, undergo post-translational modifications and ultimately target to their correct final cellular compartments. Recent genome-wide approaches continue to reveal new components and uncover their roles in secretion. It is clear that the machinery of secretion is largely conserved, allowing for unprecedented advances in our understanding of the molecules and mechanisms driving secretion and secretory organelle homeostasis (Barlowe and Miller, 2013). Excellent comprehensive reviews have been recently published addressing each of the known steps in the secretory pathway (D’Arcangelo et al., 2013; Delic et al., 2012; Denic, 2012; Moore and Hollien, 2012; Aebi, 2013; Ast and Schuldiner, 2013; Barlowe and Miller, 2013; Brandizzi and Barlowe, 2013; Chen et al., 2013; Denic et al., 2013; Gidalevitz et al., 2013; Johnson et al., 2013; Miller and Schekman, 2013; Nyathi et al., 2013; Oka and Bulleid, 2013; Venditti et al., 2014). These steps and the major players (Supplemental Table 1) are discussed below along with the introduction to the secretory genes whose expression is under the control of the transcription factors that are the major emphasis of this review.
Entry into the secretory pathway
Proteins enter the secretory pathway at the endoplasmic reticulum (ER) and are subsequently moved by vesicular trafficking through the different secretory compartments to their ultimate destinations (Mandon et al., 2013). Most proteins enter the secretory pathway by a mechanism known as co-translational translocation (Ng and Walter, 1994; Walter and Johnson, 1994; Nyathi et al., 2013) (Fig. 1, step 1). As precursor proteins are synthesized on ribosomes, a “signal sequence” – an N-terminal α-helical stretch of approximately 20 hydrophobic residues (von Heijne, 1983) or the first transmembrane domain of a membrane protein (Friedlander and Blobel, 1985) – emerges from the exit tunnel of the ribosome, and is bound by the signal recognition particle (SRP) (Rapoport, 2007; Cross et al., 2009; Janda et al., 2010). The SRP is an RNA-protein complex composed of a short RNA and six polypeptides (Walter and Blobel, 1982; Keenan et al., 2001; Egea et al., 2005; Saraogi and Shan, 2011). Upon engagement of the SRP with the ribonucleoprotein complex (RNC), translation is arrested (Walter and Blobel, 1981). The SRP-RNC then binds a receptor on the ER surface – an α,β heterodimer known as the Signal Recognition Particle Receptor (SR) (Gilmore et al., 1982; Gilmore and Blobel, 1983). The SR transfers the ribosome-bound nascent polypeptide chain to the Sec61 translocon channel (composed of a single membrane spanning protein [Sec61β] (Johnson and van Waes, 1999; Raden et al., 2000; Song et al., 2000; Osborne et al., 2005; Jiang et al., 2008), which forms the translocation tunnel, and two smaller subunits–Sec61α and Sec61γ), through which secreted proteins are fed into the ER and transmembrane domains are partitioned into the lipid bilayer as translation resumes (Schnell and Hebert, 2003; Mandon et al., 2009).
Figure 1.
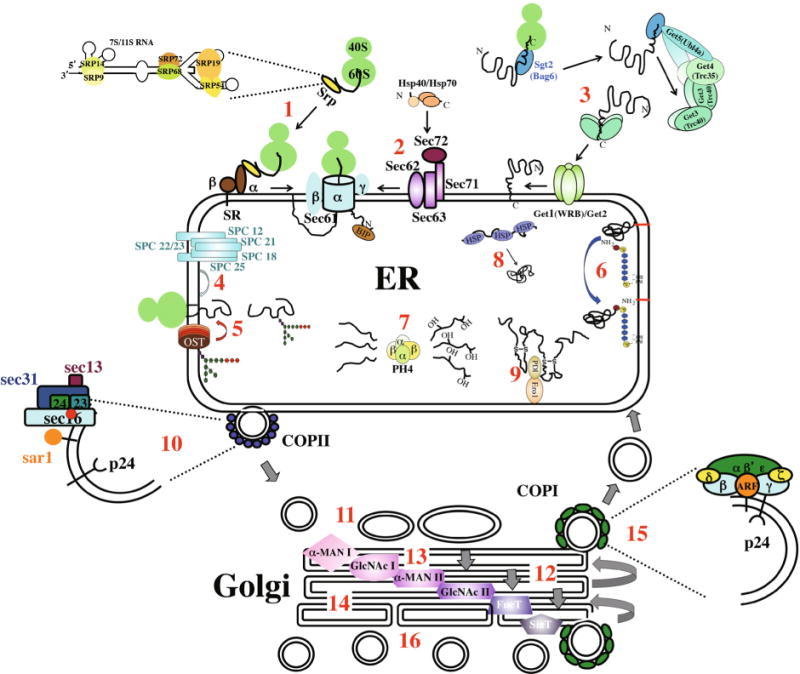
Major components of the secretory system are illustrated, from ER entry (steps 1–3) to post-Golgi sorting of vesicles containing fully processed cargo molecules (step16). Co-translational translocation involves the signal recognition particle (SRP), the signal recognition particle receptor (SR) and the Sec61 translocon complex (Sec61) (1). Post-translational translocation involves cytosolic chaperones (Hsp40s and Hsp70s), the Sec63 complex and the Sec61 translocon (2). Insertion of tail-anchored proteins into the ER membrane requires the Get pathway (3). Removal of N-terminal signal sequences requires the SPase complex (4). N-linked glycosylation requires oligosaccharide transferase (OST) to recognize the consensus sequence and attach the precursor glycan to the protein (5). Both steps 4 and 5 occur as protein is still being synthesized. GPI anchor addition involves cleavage of a C-terminal, membrane-spanning hydrophobic domain and covalent attachment of the lipid moiety (6). The hydroxylation of proline residues in ECM proteins, such as collagens, is mediated by Prolyl-4-hydroxylase (7). Protein folding in the ER is assisted by a large number of chaperone proteins, many of them HSPs (8). Many secreted cargo and surface proteins form disulfide bonds between cysteine residues (9). Anterograde trafficking between the ER and Golgi is mediated by COPII vesicles (10), which dock and fuse to either the cis-Golgi or ERGIC (11). Trafficking through the Golgi is likely to occur by a Golgi maturation mechanism (12), where earlier Golgi compartments are reformed by fusion of retrograde trafficking of COPI vesicles containing compartment-specific enzymes (13). O-linked glycosylation – the addition of N-acetyl-galactosamine sugars to oxygen atoms in polypeptides – also occurs in the Golgi (14 – not illustrated). COPII vesicles are also involved in retrograde trafficking from the Golgi to ER (15). Cargo is sorted for transport to specific final destinations (lysosomes, endosomes, plasma membrane) in the trans-Golgi (16). Some evidence suggests some compartmentalization of the trans-Golgi with respect to the ultimate destinations of cargo proteins.
At least three groups of proteins-representing as much as 20% of the yeast secretome-enter the ER by a post-translational mechanism. The groups include short secreted proteins of less than 70–80 residues, proteins with a C-terminal transmembrane domain whose N-terminal domain is cytosolic – the so-called tail-anchored (TA) proteins (Kutay et al., 1993; Shao and Hegde, 2011b; Ast and Schuldiner, 2013; Johnson et al., 2013) – and proteins whose signal sequences are either not hydrophobic enough or that fail to form an alphα-helical structure (Ng et al., 1996). GPI-anchored proteins are highly represented in this last group (Ast et al., 2013). Translocation into the ER is SRP-independent since the proteins are fully translated before the hydrophobic stretch targeted by the SRP clears the ribosome exit tunnel. With the exception of TA proteins (Borgese and Fasana, 2011; Shao and Hegde, 2011b), proteins that enter the ER post-translationally still enter the ER through the core Sec61 channel, aided by the Sec62-Sec63 complex, which includes the Sec71 and Sec72 proteins (Feldheim and Schekman, 1994; Panzner et al., 1995; Young et al., 2001) (Fig. 1, step 2). Sec63 is a transmembrane domain (TMD) protein whose lumenal portion binds and recruits Kar2/BiP, a lumenal HSP70 protein that functions to ratchet proteins into the ER lumen (Matlack et al., 1999). All proteins that enter the ER post-translationally must be kept in an unfolded state by the action of cytosolic chaperones, including members of the HSP70 (4 in yeast, 40 in humans) and HSP40 (22 in yeast, 100s in humans) families of proteins (Johnson et al., 2013). These chaperones also keep secreted proteins from forming large insoluble aggregates in the cytosol. Interestingly, calmodulin binds the signal sequences of short secreted proteins as well as the C-terminal membrane spans of the mammalian TA proteins, likely functioning in a similar capacity to the HSP chaperones (Shao and Hegde, 2011a).
The TA proteins, which represent an estimated 5% of membrane proteins, are inserted into the ER membrane independently of the Sec61 translocon through what is known as the GET (guided entry of TA proteins) pathway in yeast and the related Trc40 pathway in mammals (Stefanovic and Hegde, 2007; Schuldiner et al., 2008; Denic, 2012; Denic et al., 2013) (Fig. 1, step 3). As the C-terminal TMD of a TA protein emerges from the exit tunnel of the ribosome, it is bound and shielded by Sgt2 in yeast and Bag6 in mammals (Mariappan et al., 2010; Wang et al., 2010; Chartron et al., 2011). The TMD bound by Sgt2/Bag6 is, in turn, passed to the ATPase Get3 (Trc40 in mammals) through a pre-targeting complex that includes two other proteins, Get4 and Get5 (Trc35 and Ubl4a, respectively, in mammals) (Gristick et al., 2014). This complex is proposed to bring together two activated Get3 dimers to form a tetramer that cradles the TMD. ATP hydrolysis occurs as the tetramer forms around the hydrophobic TMD, releasing the TMD-bound Get3 tetramer from the Get4/Get5 complex (Wereszczynski and McCammon, 2012; Rome et al., 2013). TMD-bound Get3 then interacts with a heterodimeric ER receptor – Get1 and Get2 (Stefer et al., 2011). The cytosolic domains of the Get1 (WRB in mammals) and Get2 multi-span transmembrane proteins bind and pry open the Get3 protein, freeing both the TMD and Get3, releasing ADP. Through a poorly understood mechanism, the exposed TMD is then inserted into the ER membrane. As mentioned, the human Trc40 pathway has many of the same components, although the mammalian ortholog to Get2 awaits discovery. Interestingly, although mammalian Bag6 seems to have a similar function to yeast Sgt2, it has no sequence similarity and the closest mammalian ortholog to Sgt2 – SGTA – binds TMDs only weakly (Mariappan et al., 2010; Wang et al., 2010; Chartron et al., 2011).
Processing in the ER
Post-translational modifications and the folding of both membrane and secreted proteins begin as proteins enter the ER (Barlowe and Miller, 2013; Chen et al., 2013; Delic et al., 2013). Among the post-translational modifications that occur even as proteins are being translocated into the ER are removal of the N-terminal signal peptide (Fig. 1, step 4) and protein glycosylation (Fig. 1, step 5). Removal of the signal peptide is done by the signal peptidase complex (SPC), which in yeast is composed of four ER membrane spanning proteins known as Spc1, Spc2, Spc3, and the protease Sec11 (YaDeau et al., 1991; Fang et al., 1996; Mullins et al., 1996; Fang et al., 1997; Meyer and Hartmann, 1997). The SPC in mammals has five transmembrane proteins – SPase 12 (Spc1 equivalent) and SPase 25 (SPc2 homolog) (Fujimoto et al., 1984; Kalies and Hartmann, 1996), and three with significant ER lumenal domains: SPase 18 and SPase21 (yeast Sec11 functional homologs (Greenburg et al., 1989; Liang et al., 2003)) and SPase 22/23 (Spc3 homolog (Fang et al., 1997; Meyer and Hartmann, 1997)). The complex sits very close to (perhaps in direct contact with) the translocon, with the active site of the enzyme close to the lumenal surface (Antonin et al., 2000).
The majority of secretory proteins are glycosylated (Spiro, 2002). N-linked glycosylation involves the transfer of a 14-sugar – Glucose3Mannose9N-acetyl glucosamine2 (Glc3Man9GlcNAc2) – from dolichol to asparagine residues found in consensus sequences Asn-X- Ser or Asn-X- Thr (X is any non-proline residue) as proteins enter the ER through the translocation machinery (Fig. 1, step 5). The enzyme complex that carries out N-linked glycosylation–Oligosaccharyltrans-ferase or OST–is often found physically associated with the Sec61 translocon. Indeed, the timing of this modification makes it more of a co-translational rather than post-translational modification. Trimming of the sugar residues added by Ost is intimately linked to protein folding, quality control and exit from the ER. O-linked glycosylation occurs in the Golgi (Roth et al., 1994; Hirschberg et al., 1998; Naim et al., 1999) and is the addition of N-acetyl-galactosamine sugars to oxygen atoms in polypeptides.
Glycosylphosphatidylinositol (GPI) anchors are added en bloc post-translationally to many types of proteins entering the secretory pathway, including enzymes, adhesion molecules, receptors, and prion proteins (Menon and Vidugiriene, 1994; Vidugiriene and Menon, 1994) (Fig. 1, step 6). The GPI addition at the C terminus anchors the proteins in the outer leaflet of the lipid bilayer facing the extracellular space. Precursor proteins typically have the canonical N-terminal signal sequence as well as a C-terminal signal sequence for GPI anchor addition, which is a hydrophobic stretch long enough to span a lipid bilayer preceded by a shorter hydrophobic spacer adjacent to the GPI attachment site ω (Orlean and Menon, 2007). The ω attachment site and the two C-terminal residues are typically small amino acid residues (Ala, Asn, Asp, Cys, Gly or Ser). The ER membrane localized GPI- transamidase complex (composed of GPI8p and Gaa1p), with its cysteine-protease-like catalytic subunit, simultaneously removes the C-terminal hydrophobic region and attaches preformed GPI to the ω consensus site.
Prolyl hydroxylation is the most prevalent protein modification that occurs to the human proteome (Fig. 1, step 7). The process is mediated in most species by a hetero-tetramer Prolyl-4-hydroxylase composed of two α and two β subunits (Kivirikko et al., 1989). These ER lumenal enzymes transfer a hydroxyl group to the fourth position of proline using 2-oxoglutarate, Fe2+, and ascorbate as cofactors. Substrates for prolyl hydroxylation include major components of the extracellular matrix (ECM), such as collagen and elastin. Prolyl hydroxylation stabilizes collagen by raising the melting temperature of the protein, allowing collagen to be stable at body temperature (Gorres and Raines, 2010). Interestingly, whereas only two different ER prolyl-4-hydroxylase α subunits (PH4α) are encoded in the mammalian genome, the Drosophila genome encodes at least 19, almost all of which show tissue-specific expression patterns (Abrams and Andrew, 2002). This is in contrast with the variation of their substrates – > 30 collagens are encoded in vertebrates and only two in Drosophila (Hulmes, 2008; http://flybase.org/cgi-bin). Collagen and other secreted proteins are also modified by lysyl oxidation, a reaction mediated by membrane-bound homodimeric lysyl hydroxylase enzymes found in the lumen of the ER (Guzman et al., 1976). Both prolyl and lysyl hydroxylation are irreversible modifications that increase protein stability (Berg and Prockop, 1973; Quinn and Krane, 1976).
Other events that occur in the ER include protein folding and disulfide bond formation (Braakman and Bulleid, 2011; Gidalevitz et al., 2013). Folding of proteins in the ER environment has unique challenges: The ER is an oxidizing environment with huge redox potential. There is far greater crowding than in the cytosol and unique machinery exists for protein modifications (glycosylation and disulfide bond formation) (Csala et al., 2012) (Fig. 1, step 8). In keeping with the unusual conditions for protein folding in this environment, the most abundant ER proteins are involved in folding: chaperones, protein disulfide isomerases and peptidylprolyl isomerases (collectively referred to as foldases) and glycosylation enzymes (Gidalevitz et al., 2013; Luo and Lee, 2013). Indeed, Kar2/Bip, the chaperone that ratchets proteins into the ER during translocation, prevents unfavorable interactions between the protein and the ER membrane, and channels proteins down more favorable folding pathways (Hamman et al., 1998).
Disulfide bond formation occurs in the ER and is the covalent attachment of two cysteine residues (often quite widely separated along the polypeptide chain) through a disulfide bridge (Bulleid and Ellgaard, 2011; Bulleid, 2012; Oka and Bulleid, 2013) (Fig. 1, step 9). The PDI family of dithiol-disulfide oxidoreductases (of which there are about 20 different proteins) catalyzes disulfide bond formation in the ER. Once PDIs introduce disulfides into newly synthesized proteins, PDIs are re-oxidized by ER-specific oxidases, such as yeast Ero1p (vertebrate Ero1α and Ero1β) (Frand et al., 2000).
Unfolded protein structures can be recognized as exposed hydrophobic regions, unpaired cysteine residues or immature glycans; these proteins are removed from the ER by the ER-associated protein degradation (ERAD) pathway (Thibault and Ng, 2012; Merulla et al., 2013; Olzmann et al., 2013). As mentioned earlier, Glc3Man9GlcNac2 is added to proteins as they emerge from the translocon into the lumen of the ER. Enzymatic trimming of these oligosaccharides indicates proper protein folding and allows exit from the ER (Määttänen et al., 2010). The terminal α1,2 glucose residue is removed by glucosidase I and the second α1,3 glucose residue is removed by glucosidase II (Deprez et al., 2005). Calnexin (membrane proteins) or calreticulin (lumenal proteins) binds Glc1Man9GlcNac2 (Williams, 2006). The protein is released from calnexin or calreticulin as the last glucose is removed and it is able to move through the secretory pathway. If a protein is misfolded, an enzyme known as UGGT/UGT functions as a folding sensor that adds one α1,3 glucose (Sousa et al., 1992), allowing calnexin or calreticulin to rebind (D’Alessio et al., 2010). Correctly folded proteins, free of calnexin and calreticulin, are directed to ER exit sites (Ellgaard and Helenius, 2003). After a few cycles of calnexin/calreticulin binding, misfolded proteins are targeted for ERAD, which involves ubiquitylation, unfolding, and removal of the protein from the ER and subsequent targeting to the proteasome (Meusser et al., 2005).
ER and Golgi anterograde and retrograde trafficking
The next major organelle in the secretory pathway is the Golgi, which further modifies, sorts and packages proteins for their final destinations either within or outside the cell (Nakamura et al., 2012). The Golgi comprises stacks of membrane-bound cisternae organized into functional domains – cis, medial and trans. Within each Golgi domain are distinct arrays of enzymes that sequentially modify secretory cargo. Proteins traffic in coated vesicles from the ER to the cis-Golgi – the earliest Golgi compartment (Gillon et al., 2012; D’Arcangelo et al., 2013; Miller and Schekman, 2013; Venditti et al., 2014). The protein coats on vesicles function to recruit cargo (inner coat) and to bend the membrane to form vesicles of specific sizes and shapes (outer coat) (Miller and Schekman, 2013). Anterograde COPII coated vesicles form in the ER in ER Exit Sites – ERES – and directly fuse with the cis-Golgi, in the case of yeast, or to a sub-compartment known as the ER/Golgi Intermediate Compartment– ERGIC–in the case of higher eukaryotes (Brandizzi and Barlowe, 2013). The ERGIC is a stable tubular-vesicular membranous sub-compartment characterized by the presence of ERGIC53 as well as a number of other proteins distinct from those of the ER or Golgi (Appenzeller-Herzog and Hauri, 2006). The ERGIC is proposed to be the first post-ER sorting station, where cargo destined for further anterograde transport to the Golgi is separated from cargo destined to return to the ER. The ERGIC may also function as a last place to retrieve unfolded proteins for return to the ER and subsequent ERAD processing.
The COPII vesicle coats include five highly conserved core proteins: Sar1, Sec23, Sec24, Sec13 and Sec31 (Zanetti et al., 2012; D’Arcangelo et al., 2013; Miller and Schekman, 2013). Coat assembly begins with the recruitment of the Sar1 GTPase to the ER membrane by Sec12, a Sar1 GEF that localizes to the ER membrane (Fig. 1, step 10). Insertion of Sar1-GTP into the ER membrane leads to the sequential recruitment of the inner (Sec23 and Sec24) then outer (Sec13 and Sec31) coat components. Sec23 is a Sar1-GAP and Sec24 is a transmembrane protein whose lumenal domain binds cargo either directly or indirectly through the p24 and p24-related cargo binding proteins (Strating et al., 2009; Dancourt and Barlowe, 2010). Sec13 and Sec31 form a lattice-like cage that deforms the membrane allowing for the COPII vesicles to pinch off from the ER membrane. Fusion of COPII-coated vesicles with either the cis-Golgi or the ERGIC requires proteins that tether the vesicles to their target membranes, proteins that remove the COPII coat, proteins that bring the vesicle and target membrane in close enough contact for membrane fusion, as well as cytoskeletal motor proteins involved in active movement of the vesicles to their target membranes if the distance is too great for passive diffusion (Brandizzi and Barlowe, 2013) (Fig. 1, step 11).
In yeast, anterograde trafficking of proteins from the early cis-Golgi compartment to the late trans Golgi compartment most likely occurs through a maturation type mechanism (Fig. 1, step 12), a model based on the absence of anterograde transport vesicles (Martinez-Menárguez et al., 2001) and on simultaneous live imaging of both cis- and trans- Golgi components, which allowed direct visualization of Golgi compartment maturation (Losev et al., 2006; Matsuura-Tokita et al., 2006). In this model, ER- or ERGIC-derived vesicles containing newly synthesized secretory proteins fuse to form cisternae in the cis-Golgi, which then mature into the medial and trans-Golgi (Bonfanti et al., 1998; Glick and Luini, 2011; Luini, 2011; Mironov et al., 2001). Resident Golgi enzymes, such as those involved in sequential glycosylation and other processing events (Fig. 1, steps 13 and 14), are returned to their appropriate earlier compartments by retrograde vesicular transport.
COPI coated vesicles mediate both the retrograde transport among the different Golgi compartments and between the Golgi and ER (Cottam and Ungar, 2012) (Fig. 1, step 15). Similar to the structure of COPII coated vesicles, COPI vesicles include a GTPase (Arf1) that in its active GTP-bound form initiates vesicle assembly, as well as both inner (γ, δ, ζ, and β-COP) and outer coat components (α, β’ and ε-COP). As with transport of COPII vesicles, the appropriate transport, targeting, and fusion of COPI vesicles require a number of distinct cargo binding proteins, adaptors, Golgi structural proteins, fusion proteins and cytoskeletal proteins (Ungar et al., 2002; Willett et al., 2013a; Willett et al., 2013b).
In mammals, protein traffic through the Golgi is more complicated and seems to occur by multiple mechanisms. Larger proteins, such as procollagen, are thought to traffic by a Golgi maturation-type mechanism, whereas smaller cargo appears to move either by vesicular transport or by diffusion through intercisternal tubular structures – narrow tunnel-like structures that connect individual cisternae (Beznoussenko et al., 2014). Labeling cargo proteins as well as Golgi resident proteins either green or red in two different cells that were subsequently fused revealed mixing of small Golgi proteins but not of large. Moreover, the small proteins appeared to move in COPI sized vesicles in an Arf1-dependent manner (Pellett et al., 2013), supporting COPI-mediated anterograde transport of cargo through the Golgi cisternae. Recent work also suggests that different regions of the Golgi (the central region versus the rim) may be linked to trafficking of different sized cargo through this organelle (Cobbold et al., 2004; Lavieu et al., 2013).
The details of how ER resident proteins are retrieved from the Golgi are a bit better understood than those targeting proteins to different Golgi compartments (Cosson et al., 1998). Retrieval of many ER proteins requires either of two well characterized sorting signals: a C-terminal HDEL/KDEL in soluble ER proteins (Semenza et al., 1990; Capitani and Sallese, 2009) and a C-terminal, membrane proximal K(X) KXX motif in transmembrane ER proteins (Gaynor et al., 1994; Townsley and Pelham, 1994). The KDEL/HDEL sequences in soluble proteins bind to the Erd2 transmembrane receptor protein (also known as the KDEL receptor), linking the proteins to COPI vesicles, whereas the K(X)KXX motif is directly bound by the COPI coat proteins (Cosson et al., 1997). Other ER proteins are retrieved through di-basic signals that are also directly bound by proteins in the Cop1 coat (McBride et al., 2007). Finally, some resident ER proteins bind to a transmembrane cargo-adaptor protein known as Rer1 (Nishikawa and Nakano, 1993; Sato et al., 2003).
Post-Golgi trafficking
Secreted and transmembrane proteins are sorted for delivery in the trans-Golgi network (TGN), with proteins targeted to the lysosome, either directly or through endosomes, to secretory vesicles, for constitutive or regulated secretion, or to distinct domains in the plasma membrane (Kienzle and von Blume, 2014) (Fig. 1, step 16). As with all steps in the secretory pathway, signals on the cargo proteins themselves play a key role in determining their ultimate destination. These signals, which range from specific sugar modifications (Kaluza et al., 1990), tyrosine residues in a specific sequence context (Alconada et al., 1996), di-aromatic residues (Schweizer et al., 2000), specific phosphorylation events (Johnston et al., 2005) and even disulfide bonds (Zanna et al., 2008), bind adaptor proteins (Guo et al., 2013a; Guo et al., 2013b) (Hirst et al., 2013), which connect them to the coat proteins required to form and pinch off vesicles (Miller et al., 2007; Kametaka et al., 2010). The coat proteins, in turn, interact with specific target signals, tethering proteins and molecules that bring both the vesicular membrane and target membrane into close enough proximity for fusion (Fölsch et al., 2001; Jacob and Naim, 2001; Chapuy et al., 2008; Pols et al., 2013). Lipid molecules also appear to function in sorting proteins to their final correct destinations (Carlton et al., 2004). Recent evidence even suggests that cargo destined for different locations may be sorted into distinct sub-domains within the TGN (Gleeson et al., 2004).
As should be clear from this abbreviated description of the events required to bring proteins into the secretory pathway, to modify those same proteins and deliver them to their final destinations requires the orchestration of a huge number of distinct complexes containing protein, lipid, carbohydrate and RNA components. How are the appropriate levels of each component achieved and how do professional secretory cells, such as those of the cartilage, bone, mammary glands or pancreas, adjust to huge increases in the levels of protein going through the system? How do cells respond to stress conditions that overwhelm the secretory machinery? Studies over the past couple of decades suggest that a large portion of regulation is at the level of transcription and that the transcription factors regulating secretory capacity are poised to both sense and respond to the volume of proteins trafficking through the system.
The Unfolded Protein Response (UPR) and the regulation of secretory capacity
As mentioned earlier, the endoplasmic reticulum (ER) is where secreted and transmembrane proteins enter the secretory pathway, and it is where protein folding occurs and post-translational processing begins. Whereas “housekeeping” levels of components of the molecular machinery appear sufficient for the functional demands of most cell types, there are times when demands on the system require some adjustment in component levels. Drug treatment, disease or even normal physiologic changes in protein load can affect ER function, leading to the accumulation of unfolded proteins. To restore ER homeostasis, the cell activates a pathway commonly known as the unfolded protein response (UPR). The UPR alleviates ER stress by increasing transcription of the chaperone proteins and lipids that increase folding capacity in the ER, as well as upregulating other components of the secretory machinery. The UPR also decreases protein load by increasing production of the ERAD machinery that degrades misfolded proteins (Travers et al., 2000). Finally, the UPR reduces protein load through the attenuation of protein translation (Harding et al., 1999; Hollien and Weissman, 2006; Hollien et al., 2009). If ER homeostasis is not restored, the UPR then triggers the execution of cytotoxic programs leading to cell death.
The canonical UPR consists of three parallel “branches,” each activating one of a set of related bZip transcription factors (Fig. 2). An excellent recent review by Gardner et al. provides a detailed description of the UPR pathway induction (Gardner et al., 2013), which will not be discussed in detail here. Instead, we provide a brief discussion of what is known about the roles of each of these bZip transcription factors in UPR, findings largely based on studies of cultured cells. We then discuss what is known about the in vivo roles of each transcription factor, which have been revealed through more recent loss-of-function and overexpression studies in animal systems.
Figure 2.
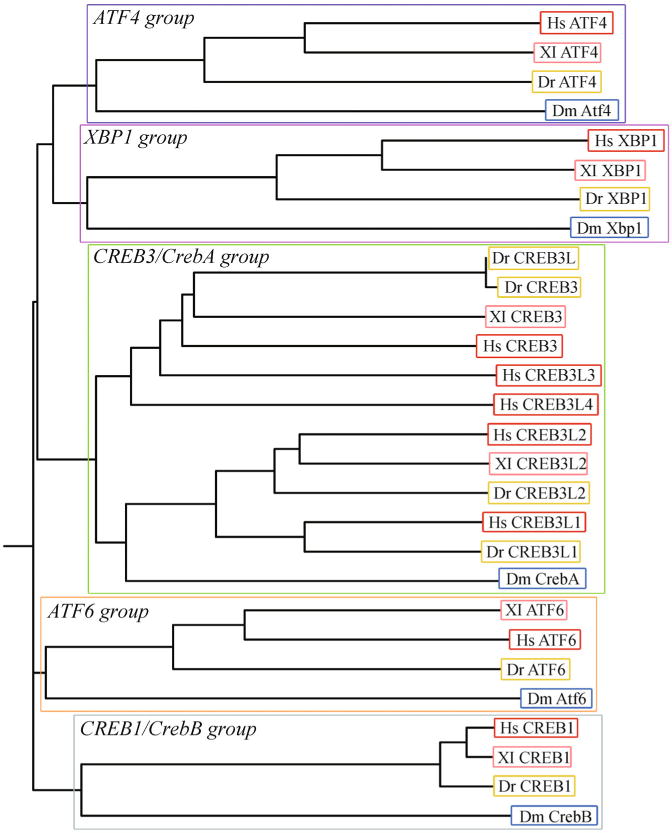
The bZip proteins implicated in modulating secretory capacity either during drug-induced or physiologic ER stress. Alignments and Phylip rooted tree were generated using the N-terminal processed and active forms of the ATF6 and Creb3 proteins, using the ClustalW program and Drawgram program available at the Biology Workbench 3.2 (http://seqtool.sdsc.edu/CGI/BW.cgi).
Ire-1/XBP1
The most highly conserved branch of the UPR is regulated by the transmembrane kinase, Ire-1, which has orthologs in all eukaryotes (Mori, 2009). Under normal conditions in the ER, Ire-1 is bound to the ER lumenal chaperone BiP. In response to ER stress, which is often induced during experimentation by the drug tunicamycin, a glycosylation inhibitor that leads to huge increases in unfolded ER proteins, BiP is recruited away from Ire-1 (Fig. 3). In the absence of BiP binding, Ire-1 oligomerizes, thereby inducing a conformational change that activates its RNAse domain (Kimata et al., 2007; Aragón et al., 2009; Korennykh et al., 2009). The primary target of the Ire-1 RNAse, which catalyzes an unconventional splicing event, is the HAC1 mRNA in yeast (Cox and Walter, 1996) and the related Xbp1 mRNA in metazoan cells (Yoshida et al., 2001). The unconventional Ire-1 splicing of the Xbp1 mRNA removes either 23 [worms (Calfon et al., 2002) and flies (Ryoo et al., 2007; Souid et al., 2007)] or 26 nucleotides [mammals (Yoshida et al., 2001)], causing a shift in the downstream open reading frame, and resulting in the production of a more stable and more active form of the bZip Xbp1 transcription factor. Expression of the active stable form of Xbp1 in NIH 3T3 cells, even in the absence of ER stress, significantly expands the ER. Xbp1 does this, in part, by upregulating the activity of enzymes that synthesize ER-specific lipid pools, specifically the CDP choline membrane biogenesis pathway enzymes (Sriburi et al., 2004; Bommiasamy et al., 2009). Xbp1 transcriptional targets, identified by genome-wide approaches, include genes involved in co-translational translocation into the ER (i.e. Srp9, SR, Sec61α, β, γ) disulfide bond formation (Ero1), foldases, glycosylation enzymes, vesicle trafficking components (i.e. COPI and COPII vesicle components), the KDEL receptor and proteins involved in the retrograde trafficking, docking and fusion of Golgi vesicles (Lee et al., 2003; Shaffer et al., 2004; Sriburi et al., 2004).
Figure 3.
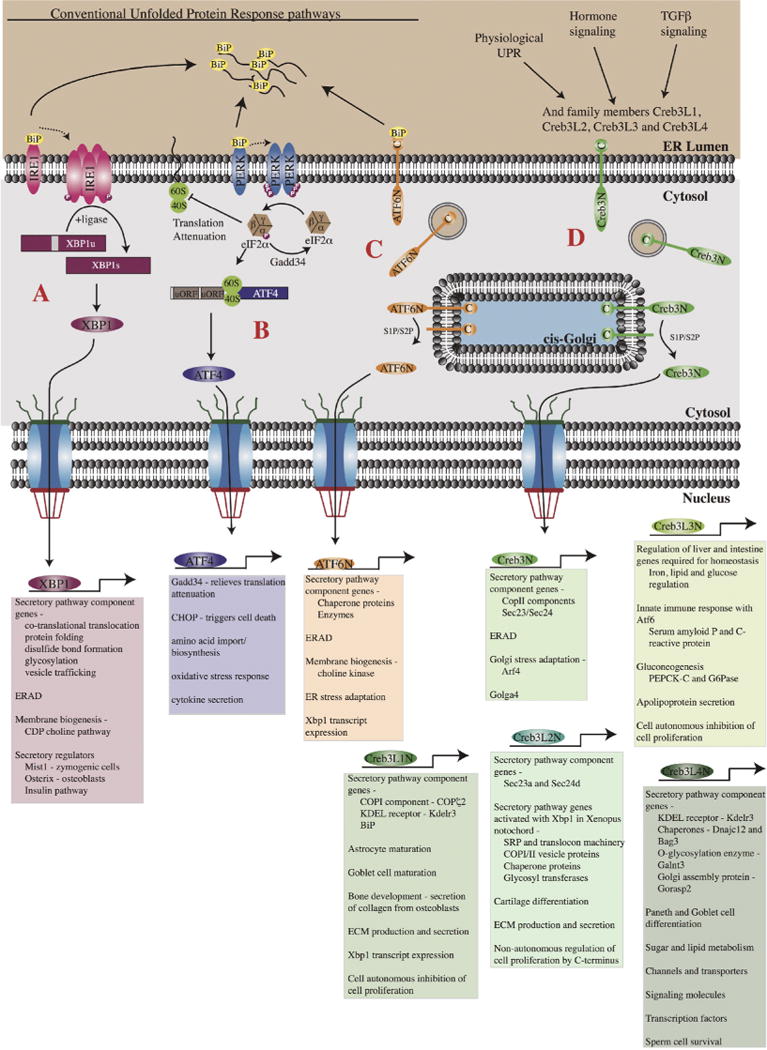
Regulation and function of the bZip transcription factors that upregulate secretory capacity during the UPR and physiological ER stress. (A). The Ire-1 kinase is held inactive by the binding of the chaperone protein BiP. Accumulation of unfolded proteins leads to the release of BiP allowing it to oligomerize and autophosphorylate thereby activating the RNAse domain. The primary substrate for Ire-1 in mammalian cells is the Xbp1 mRNA, which gets spliced by Ire-1. Following translation, the active Xbp1 transcription factor translocates to the nucleus where it activates target genes involved in the secretory pathway, membrane biogenesis, ERAD, and secretory cell differentiation. (B). PERK also binds to BiP and is only activated following the release of BiP in the presence of unfolded proteins. BiP release leads to dimerization of PERK and the subsequent phosphorylation of eIF2α, leading to translational attenuation. This event also leads to the preferential translation of the ATF4 mRNA. ATF4 then functions in the nucleus to regulate genes involved in secretory activity as well as UPR dependent cell death. (C). ATF6 is an ER membrane bound transcription factor that during the UPR traffics to the Golgi and is cleaved by the Site-1 and Site-2 proteases releasing its N-terminal bZip domain. The active ATF6 transcription factor then translocates to the nucleus where it regulates genes involved in protein folding, membrane biogenesis and ERAD. (D). The Creb3 transcription factors are also ER-membrane bound and similar to ATF6 undergo cleavage by the Site-1 and Site-2 proteases. Unlike ATF6 (or the other canonical members of the UPR), Creb3 proteins do not bind BiP but are instead activated by physiological changes in secretory demand. Following cleavage, the N-terminal transcription factor domain translocates to the nucleus to activate gene transcription. Creb3 family members are largely involved in regulating the protein machinery of the early secretory pathway.
During normal development, Xbp1 mRNA is highly expressed in secretory tissues. In the fly, the Xbp1 transcript is detected to very high levels in several secretory organs, including the salivary glands, the proventriculus, the Malpighian tubules, the nervous system and the epidermis (Ryoo et al., 2007; Souid et al., 2007; Ryoo et al., 2013; Sone et al., 2013). Xbp1 expression in the fly embryo is regulated by CrebA (R.F. and D.A., unpublished), another bZip transcription factor that plays a key role in the secretory capacity of Drosophila tissues. Mutations in Drosophila Xbp1 are larval lethal (Souid et al., 2007) but the specific role of Xbp1 during normal fly development is not fully understood.
Xbp1’s role in secretory function is probably best characterized in the B cells of the mammalian immune system, which differentiate to become antibody secreting plasma cells (Shaffer et al., 2004). Upon activation, B cells upregulate the secretory machinery and begin producing and secreting high levels of antibodies. Xbp1 is not required for the differentiation of B cells into plasma cells (Todd et al., 2008; Hu et al., 2009; Taubenheim et al., 2012). Instead, Xbp1 loss leads to a significant reduction in secretory activity (Shaffer et al., 2004; Sriburi et al., 2004). This reduced secretion is largely due to a decrease in the activity of genes required for membrane biogenesis and in the expression of genes encoding secretory pathway machinery, thereby limiting ER expansion and allowing for accumulation of unfolded proteins (Sriburi et al., 2004; McGehee et al., 2009). A similar phenotype is observed in the pancreatic cells of Xbp1 mutant mice. Xbp1 deficient β-cells fail to increase secretory capacity and consequently fail to secrete enough insulin to regulate blood glucose levels (Lee et al., 2011a).
Xbp1 also affects secretory cell development and function indirectly by regulating downstream transcription factors such as Osterix and Mist1 (Huh et al., 2010; Tohmonda et al., 2011). Osterix is essential for the differentiation of osteoblasts that occurs in response to BMP2 signaling and is absolutely dependent on Xbp1 for expression (Tohmonda et al., 2011). Mist1 and one of its downstream targets, the E3 ubiquitin ligase Mindbomb, are required for zymogenic cell differentiation in the stomach (Huh et al., 2010; Capoccia et al., 2013). In these cells, Xbp1 is both necessary and sufficient for MIST1 activation (Huh et al., 2010). Finally, in the pancreas, Xbp1 indirectly regulates the levels of genes involved in the insulin secretion pathway as evidenced by the failure of Xbp1 to bind the enhancers of the target genes in chromatin-immunoprecipitation experiments (Lee et al., 2011a). The insulin secretion genes indirectly affected by Xbp1 include the proprotein convertases PC1 and PC2, carboxypeptidase E (CPE) and synaptophysin.
Xbp1 signaling has been implicated in the pathogenesis of several human diseases. Importantly, overexpression of Xbp1 has been used to develop a mouse model of multiple myeloma, a plasma cell cancer (Carrasco et al., 2007) and, correspondingly, blocking Xbp1 or its upstream regulator Ire1 can induce myeloma cell toxicity (Papandreou et al., 2011; Volkmann et al., 2011; Cross et al., 2012). Loss of Xbp1, on the other hand, has been implicated in diseases whose hallmarks are protein misfolding and aggregation. Specifically, targeted knockdown of Xbp1 in dopaminergic neurons triggers chronic ER stress and, consequently, neuronal degeneration, a phenotype characteristic of Parkinson’s disease (Valdés et al., 2014). Moreover, selective expression of Xbp1 in neurons can provide protection from degeneration during forced ER stress (Valdés et al., 2014). These studies highlight the importance in identifying Xbp1 regulated genes as potential key targets for therapies to treat secretory diseases.
In metazoans, Ire-1 also induces degradation of mRNAs encoding secretory cargo through the Regulated Ire-1-Dependent Decay (RIDD) pathway, thus also decreasing protein influx into the ER (Hollien and Weissman, 2006; Hollien et al., 2009).
PERK/ATF4
The second branch of the UPR is activated by the transmembrane kinase– PRKR-like endoplasmic reticulum kinase or PERK– which initially promotes survival of cells undergoing ER stress, and then activates apoptosis in cells unable to overcome the accumulation of unfolded proteins. As with Ire-1, BiP is normally bound to the inactive PERK receptor. In response to ER stress, BiP is released, PERK autophosphorylates, and subsequently phosphorylates and inactivates eIF2α, thereby reducing general protein translation and decreasing secretory protein load (Harding et al., 1999). PERK increases the translation of mRNA transcripts that contain inhibitory upstream open reading frames, including those of the bZip transcription factor ATF4 (Harding et al., 2000) (Fig. 3). Unlike many of the other transcription factors that are discussed in this review, ATF4 is not a membrane bound transcription factor and therefore does not undergo Regulated Intramembranous Proteolysis (RIP) for activation. Instead, ATF4 has many dimerization partners and, upon dimerization, the ATF4 transcription factor becomes active. ATF4 target genes have important roles in the UPR – the downstream target GADD34 reverses the translational attenuation induced by PERK and the downstream target CHOP activates the pro-apoptotic response should ER stress not be alleviated. Microarray and ChIP-seq experiments conducted in Atf4−/− cells revealed that ATF4 has a much broader role in secretory function than previously thought. Target genes include those encoding the proteins that import amino acids into the cell, as well as those encoding proteins that respond to oxidative stress (Han et al., 2013; Harding et al., 2003). During increased secretory function, the production and secretion of proteins both depletes amino acids and increases reactive oxygen species. ATF4 appears to be the major factor, through its target genes, that allows for the replenishment of amino acids, either through import or biosynthesis, and the alleviation of oxidative stress in secretory cells. More recently, ATF4 has been shown to dimerize with different partners to regulate secretory function, both positively and negatively, in several different cell types. Downstream of the Toll-like receptor 4 (TLR4), ATF4 partners with phosphorylated c-Jun to activate genes necessary for the secretion of inflammatory cytokines from monocytes (Zhang et al., 2013). In osteoblasts, ATF4 and FoxO1 physically interact to suppress the secretion of osteocalcin, a hormone that increases insulin secretion from the pancreas (Yoshizawa et al., 2009; Kode et al., 2012). Hence, in the absence of ATF4 or FoxO1, osteocalcin secretion is enhanced and blood glucose levels rise, resulting in reduced glucose tolerance (Kode et al., 2012). Thus, identifying new partners for this widely expressed transcription factor will help elucidate novel pathways with critical roles in the function of secretory organs and could reveal new strategies for combating diabetes and other metabolic diseases.
ATF6
The final branch of the canonical UPR pathway is mediated through the bZip transcription factor ATF6, of which there are two ubiquitously expressed isoforms in mammals, ATF6α and ATF6β (Haze et al., 1999, 2001). During ER stress, both isoforms translocate to the Golgi where they undergo RIP by the Site-1 and Site-2 proteases, releasing the N-terminal bZip domain to enter the nucleus where it upregulates the expression of genes through the well-characterized ER stress response elements (ERSE) found in the enhancer regions of many UPR target genes (Yoshida et al., 1998; Haze et al., 1999) (Fig. 3). ATF6α appears to be the predominant isoform of active ATF6 and its targets encode a wide range of proteins involved in mediating ER homeostasis, including those required for protein folding, such as ER chaperones (GRP94/HSP90B1, GRP78/BiP, calreticulin), folding enzymes (ERp72, PDI), as well as those required for ERAD (EDEM, Derlin-3) (Okada et al., 2002; Wu et al., 2007; Yamamoto et al., 2007; Adachi et al., 2008; Bommiasamy et al., 2009; Belmont et al., 2010).
Neither ATF6α nor ATF6β are essential; mice harboring null mutations in either gene are completely viable (Wu et al., 2007). Single mutants are more sensitive to ER stress, as injection of tunicamycin can induce acute liver and kidney damage. ATF6 is required for normal development, since double knockout mice do not survive, indicating some level of functional redundancy between the two isoforms. ATF6α has recently been shown to increase membrane biogenesis in an Xbp1-independent manner. Overexpression of ATF6α in Chinese hamster ovary (CHO) cells lead to a dramatic enlargement of the ER, a result that was subsequently replicated in two human cell lines. Moreover, ATF6 was able to increase ER membrane in the absence of Xbp1, suggesting that it has the capability to upregulate membrane biogenesis genes on its own. Interestingly, ATF6 regulates different genes than XBP1 in the CDP-choline membrane biogenesis pathway. Xbp1 largely regulates the activity of the transferases CCT, CPT and CEPT, whereas ATF6 overexpression results in only a slight increase in CCT activity and a robust increase in choline kinase activity. Transcriptome analysis, however, revealed that the increases in transferase activity are largely controlled at the post-transcriptional level; mRNAs for each gene are not increased in XBP1 or ATF6 over-expressing cells (Bommiasamy et al., 2009).
The Creb3 family of UPR sensors
Recently, an additional family of UPR responsive transcription factors has been described, which we will refer to as the Creb3 family of bZip transcription factors. The Creb3 family is highly conserved, with orthologs identified in species ranging from sponges to humans (Barbosa et al., 2013). Creb3 proteins are distinguished by a conserved domain of ~30 amino acids adjacent to the bZip DNA binding domain, the ATB domain (Adjacent To BZip) (Bailey and O’Hare, 2007; Barbosa et al., 2013). Whereas, the ATB domain is found in all Creb3 orthologs, it is absent from all other bZip transcription factors, of which there are 55 in humans. The Creb3 proteins can be classified into three different groupings, with classes A and B being ER-bound factors that undergo the same RIP processing that activates ATF6 during the UPR (Liang et al., 2006; Murakami et al., 2006; Stirling and O’Hare, 2006; Zhang et al., 2006; Kondo et al., 2007). The major differences between class A and class B Creb3 proteins are residues within the transmembrane domain that are likely to reflect some variability in the proteolytic processing of each class (Barbosa et al., 2013). Class C Creb3 proteins completely lack the transmembrane domain and are constitutively nuclear (Barbosa et al., 2013).
The Creb3 family and transcriptional regulation of secretory capacity
Most of the initial studies on mammalian Creb3 proteins were done in tissue culture cells using pharmacological agents to induce ER stress, since their regulation by RIP suggested that they were likely to function primarily in the UPR. As mouse knockouts have been characterized over the past five years, it is clear that these factors function during normal organ development. Their relative contributions are likely to be underestimated, however, due to potential functional redundancy among the multiple members of this family. Thus, experiments in genetic model systems–with only one or two Creb3 proteins–have been key to revealing how these factors function during normal physiology in the regulation of secretory capacity.
Drosophila CrebA is the major regulator of secretory capacity
Drosophila encodes only a single Creb3 family member– CrebA, which, unlike its mammalian orthologs, does not contain a transmembrane domain, does not undergo processing by RIP and is constitutively nuclear (Fox et al., 2010). CrebA is expressed in multiple secretory organs in Drosophila embryos, larvae and adults, with the highest levels of expression in the embryonic and larval salivary glands (Smolik et al., 1992), epidermis, larval imaginal discs and the adult male accessory gland (ModEncode data). Indeed, CrebA was first discovered to regulate the secretory pathway in a screen to identify the transcription factors regulating the elevated levels of secretory pathway component genes (SPCGs) observed in the embryonic salivary glands (SG) (Abrams and Andrew, 2005). Abrams et al. (Abrams and Andrew, 2005) found that although both CrebA and the FoxA factor, Fork head (Fkh), were required for wild-type expression levels of all 34 SPCGs that were tested, Fkh only indirectly regulates SPCG expression by maintaining CrebA expression (Abrams and Andrew, 2005; Fox et al., 2010). CrebA directly regulates SPCG expression through a consensus motif that closely resembles the previously identified Creb Response Element (CRE). Subsequent microarray experiments identified close to 400 genes that were downregulated in CrebA mutant embryos (Fox et al., 2010). Of these, nearly one-third were annotated by Gene Ontology to be components of the secretory pathway. Close examination of the target genes revealed that CrebA has a major role in regulating secretion in that it not only regulates the protein components of the secretory machinery (Table 1), but it also upregulates expression of genes encoding secreted cargo proteins (Fox et al., 2010). In the SG, the regulation of secretory cargo may be indirect, since CrebA boosts expression of a SG-specific bHLH factor, Sage. Sage, with Fkh, directly activates SG genes that encode secreted proteins and the enzymes that modify secreted proteins (Fox et al., 2013). Thus, CrebA increases secretory capacity of tissues both directly, through upregulation of SPCGs, and indirectly, by boosting expression of the transcription factors that activate tissue-specific secretory cargo genes and their modifying enzymes. Importantly, not only is CrebA required for upregulation of the general secretory machinery, it is also sufficient (Fox et al., 2010).
Table 1.
Secretory pathway component genes regulated by CrebA bZip transcription factor
| General functional category | Human or (yeast) gene | D. melanogaster gene name and/or CG # | E-value for relatedness | Fold change in CrebA null | Requires CrebA based on in situ | ||
|---|---|---|---|---|---|---|---|
| Srp9 | Srp9/CG8268 | 2.250e-13 | −2.00 | Yes | |||
| Srp68 | Srp68/CG5064 | 1.19e-128 | −2.04 | Yes | |||
| Signal recognition particle- SRP | Srp72 | Srp72/CG5434 | 3.97e-124 | −1.81 | Yes | ||
| Srp54 | Srp54k/CG4659 | 0 | −1.44 | Yes | |||
| Srp14 | Srp14/CG5417 | 3.705e-13 | −1.59 | Yes | |||
| Srp19 | Srp19/CG4457 | 4.836e-29 | −1.89 | Yes | |||
| SrpRα | Gtp-bp/CG2522 | 1.99e-177 | Not on array | Yes | |||
| SRP Receptor- R | SrpRβ | SrpRβ/CG33162 | 1.859e-37 | −3.31 | Yes | ||
| Sec61α | Sec61α/CG2522 | 0 | −1.78 | Yes | |||
| Sec61 translocon complex | Sec61β | Sec61β/CG10130 | 2.531e-27 | −1.99 | Yes | ||
| Sec61γ | Sec61γ/C14214 | 2.530e-29 | −2.08 | Yes | |||
| Sec62 | Trp1/CG4785 | 1.049e-55 | −1.62 | Yes | |||
| Sec63 complex: post-translational translocatio | Sec63 | Sec63/CG8583 | 1.02e-176 | −2.03 | Yes | ||
| Sec71 (yeast) | Sec71/CG7578 | 0 | −1.24 | Yes | |||
| TRAM | TRAM/CG11642 | 2.429e-65 | −5.21 | Yes | |||
| TRAPδ | TA P δ/CG9035 | 1.798e-21 | −2.12 | Yes | |||
| BiP | Hsc70–3/CG4147 | 0 | −1.32* | n.d. | |||
| ER lumenal Hsp70s | Jem1 | CG9356 | 1.119e-16 | −1.50 | n.d. | ||
| Sil1 | CG10420 | 4.567e-31 | −1.87 | n.d. | |||
| Lhs1 (yeast) | CG2918 | 1.226e-44 | −1.45 | n.d. | |||
| Spastin | spas/CG5977 | 3.19e-138 | −1.41 | n.d. | |||
| ER morphology proteins | Atlastin | atl/CG6668 | 3.38e-178 | −1.23* | n.d. | ||
| Reep3 | CG8331 | 1.978e-20 | −1.84 | n.d. | |||
| Rtn1/Rtn2 | Rtnl1/CG33113 | 4.606e-58 | −1.33 | n.d. | |||
| Spase 22–23 | Spase 22–23/CG5677 | 1.138e-56 | −1.81* | Yes | |||
| Signal peptidase complex | Spase 12 | Spase12/CG11500 | 1.755e-16 | −2.5 | Yes | ||
| Spase 18–21 | twr/CG2358 | 2.639e-77 | −2.3 | Yes | |||
| Spase 25 | Spase25/CG1751 | 2.074e-40 | −2.2 | Yes | |||
| MagT1 | Ostγ/CG7830 | 6.43e-101 | −1.43 | Yes | |||
| N-linked glycosylation | Ost4 | CG33774 | 2.177e-07 | Not on array | n.d. | ||
| Stt3 (yeast) | OstStt3/CG7748 | 0 | −1.72 | n.d. | |||
| Pdi1 (yeast) | Pdi/CG6988 | 3.701e-52 | −1.40 | n.d. | |||
| Disulfide bond formation | Mpd1 (yeast) | CaBP1/CG5809 | 2.393e-18 | −1.52* | n.d. | ||
| Mpd2 (yeast) | ERp60/CG8983 | 3.340e-06 | −1.30 | n.d. | |||
| Eps1 (yeast) | prtp/CG1837 | 3.572e-08 | −1.29* | n.d. | |||
| Ph4αPV | 3.000e-88 | −1.40 | n.d. | ||||
| Prolyl hydroxylation | Ph4α (isoform 2) | Ph4αMP | 2.000e-96 | −1.54 | n.d. | ||
| PH4αSG1 | 2.000e-90 | −1.54 | n.d. | ||||
| Sugar trimming and protein folding | UGGT | Ugt/CG6850 | 0 | −1.54 | n.d. | ||
| Calreticulin | Crc | 0 | −1.42 | n.d. | |||
| CHOp24 | CHOp24/CG3564 | 2.648e-68 | −2.2 | Yes | |||
| ER cargo receptors | TMED7 | P24-1/CG1967 | 1.951e-51 | −1.60 | Yes | ||
| TMED4 | P24-2/CG33105 | 3.647e-77 | −1.9 | Yes | |||
| Sar1 | Sar1/CG7073 | 2.606e-79 | −1.28 | Yes | |||
| Sec23 | Sec23/CG1250 | 0 | −1.73* | Yes | |||
| COPII components | Sec24B | Sec24/CG1472 | 0 | −1.18* | Yes | ||
| Sec13 | Sec13/CG6773 | 1.10e-100 | −2.44 | Yes | |||
| Sec31 | Sec31/CG8266 | 0 | −1.8 | Yes | |||
| Sec12 (yeast) | Smu1/CG5451 | 2.178e-04 | −1.21 | n.d. | |||
| COPII regulators | PREB | CG9175 | 4.163e-54 | −1.49 | n.d. | ||
| Sec16A | Sec16/CG32654 | 8.358e-60 | −1.54 | n.d. | |||
| COPII vesicle-Golgi membrane fusion | Bos1 | eya/CG9554 | 1.28e-122 | −1.50 | n.d. | ||
| SCFD1 | Slh/CG3539 | 0 | −1.46 | n.d. | |||
| Grasp65 | Grasp65/CG7809 | 5.305e-61 | −2.38 | n.d. | |||
| Golgi structural proteins | GCC88 | GCC88/CG10703 | 1.048e-58 | −1.20 | n.d. | ||
| Golgin-84 | Golgin84/CG17785 | 1.177e-25 | −1.35 | n.d. | |||
| O-linked glycosylation | Pmt1,2,3,5,6 (yeast) | tw/CG12311 | 1.757e-88 | −1.76 | n.d. | ||
| Pmt4,7 (yeast) | rt/CG6097 | 5.727e-89 | −1.31 | n.d. | |||
| α-Cop | α-Cop/CG7961 | 0 | −2.11 | n.d. | |||
| β-Cop | β-Cop/CG6223 | 0 | −1.9 | n.d. | |||
| β’-Cop | β’-Cop/CG6699 | 0 | −1.9 | n.d. | |||
| COPI vesicle components (Golgi – ER) | γ-Cop | γ-Cop/CG1528 | 0 | −1.74 | Yes | ||
| δ-Cop | δ-Cop/CG14813 | 1.70e-168 | −1.68* | n.d. | |||
| ɛ-Cop | ɛ-Cop/CG9543 | 1.470e-50 | −1.77* | Yes | |||
| ξ-cop | ξ-Cop/CG3948 | 1.878e-62 | −2.7/−1.95 | Yes | |||
| Arf1 | ARF79F/CG8385 | 8.49e-101 | −1.2* | Yes | |||
| Arf-1 GAPs | Gcs1 (yeast) | ArfGAP1/CG4237 | 2.380e-36 | −1.95 | n.d. | ||
| Glo3 (yeast) | ArfGAP3/CG6838 | 4.538e-29 | −1.58 | n.d. | |||
| ER retrieval from Golgi | KDEL-R | KdelR/CG5183 | 1.303e-93 | −1.68 | n.d. | ||
| Rer1 | CG11857 | 8.289e-37 | −1.43 | n.d. | |||
| Cog1 | CG4848 | 5.633e-38 | −1.60 | Yes | |||
| Cog2 | ldlCp/CG6177 | 7.74e-101 | −1.27 | n.d. | |||
| Early Golgi retrograde traffic tethers –COG complex | Cog3 | Cog3/CG3248 | 3.817e-77 | −1.43 | n.d. | ||
| Cog4 | CG7456 | 7.34e-160 | −1.44 | n.d. | |||
| Cog5 | fws/CG6549 | 5.463e-60 | −1.22 | n.d. | |||
| Cog6 | CG1968 | 6.83e-153 | −1.26 | n.d. | |||
| Cog7 | Cog7/CG31040 | 2.033e-23 | −1.71 | n.d. | |||
| Cog8 | CG6488 | 1.510e-23 | −1.34 | n.d. | |||
Regulation by CrebA is based on microarray and/or in situ hybridization comparing CrebA null embryos to wild-type embryos. Note that fold change is from entire embryos, with many wild-type tissues not expressing CrebA.
indicates numbers with p values > 0.05. For all other numbers, p values were≤0.05. n.d. = not done. A comprehensive table of all secretory genes specifically examined for regulation by CrebA is included in the supplemental materials.
CrebA mutants have phenotypes consistent with CrebA’s role in secretion. The SG lumens are smaller and there are significantly fewer and smaller apical secretory vesicles than in wild type (Abrams and Andrew, 2005; Fox et al., 2010). The larval cuticle (secreted by epidermal cells) of CrebA mutants is weaker and less pigmented than that of wild type larvae (Abrams and Andrew, 2005). CrebA also functions in dendritic arborization in the sensory neurons during larval development (Iyer et al., 2013). Dendritic arborization is essential to form the neural circuits necessary for signaling. The homeodomain transcription factor Cut is required for dendritic elaboration as is elevated trafficking through the secretory pathway (Grueber et al., 2003; Cui-Wang et al., 2012). Iyer et al., recently showed that Cut regulation of COPII secretory components is indirect and requires CrebA, fully consistent with the previously described role for CrebA in upregulating secretory machinery in embryonic tissues (Iyer et al., 2013). As the phenotypic defects associated with the loss of the mammalian Creb3 proteins have emerged, it is clear that each family member also upregulates secretory capacity.
Creb3/Luman
Creb3/Luman/LZip was first identified in a yeast two-hybrid screen to find proteins that interact with the transcriptional co-activator host cell factor (HCF) protein (Lu et al., 1997). Early overexpression-based studies also identified Creb3 as an interacting protein with the Hepatitis C core protein (Jin et al., 2000), the CC chemokine receptor 1 (Ko et al., 2004), DC-STAMP (Eleveld-Trancikova et al., 2010), and Luman Recruitment Factor (Audas et al., 2008). Although Creb3 transcripts are detected quite broadly, the protein has been observed in only the trigeminal ganglion neurons, monocytes and bone marrow dendritic cells (Eleveld-Trancikova et al., 2010; Ko et al., 2004; Lu and Misra, 2000). One early study suggested a major role for Creb3 in the ERAD pathway since it binds the promoters of both the Herp and EDEM genes, two ERAD associated genes (Liang et al., 2006). More recent analysis of the gene expression changes associated with driving a constitutively-active form of Creb3 in the antigen-presenting dendritic cells of the bone marrow led to the identification of nearly 40 upregulated genes, including Creb3 itself (Sanecka et al., 2012). Importantly, several secretory pathway genes were highly upregulated, including Sec23a and Sec24d – COPII coat components and targets of other Creb3 family members – as well as Golga4, a Golgin protein, GBF1 (Golgi localized Arf GEF) and Arf4 (Golgi GTPase) (Sanecka et al., 2012).
Creb3-Arf4 signaling has recently been implicated in the Golgi stress response. Arf4 was identified in a screen designed to identify factors that confer resistance to Brefeldin A (BFA) induced apoptosis (Reiling et al., 2013). Arf4 knockdown prevented Golgi fragmentation and restored secretory function to BFA-treated cells (Reiling et al., 2013). Previous studies had indicated that Creb3 activated Arf4 (Jang et al., 2012), and, consistent with these findings, Creb3 transcriptionally upregulates Arf4 in response to BFA treatment. Several known pathogens, including Chlamydia and Shigella, utilize Golgi fragmentation both to acquire lipids from host cells and to interrupt secretory pathway function to prevent secretion of the cytokines that would trigger an immune response by the host (Heuer et al., 2009). Knocking down Arf4 prevents pathogen spread by preventing Golgi fragmentation, restoring function, and subsequently maintaining secretory pathway function (Reiling et al., 2013). Altogether these findings suggest that Creb3 proteins are not limited to stress in the ER but are also induced when other secretory organelles undergo stress. Clearly, bacterial pathogens have evolved to use this stress response to their advantage.
Creb3L1/OASIS
Creb3L1/OASIS was first identified as a gene enriched in astrocytes cultured long-term. Creb3L1 was subsequently revealed to be expressed at high levels in several secretory tissues, most notably in astrocytes, skeletal tissues, salivary glands, intestine, prostate gland and pancreas (Nikaido et al., 2001; Omori et al., 2002; Murakami et al., 2009). Importantly, recent reports analyzing the Creb3L1 mutant mouse have revealed a role for Creb3L1 in the differentiation, function and survival of many of the cell types in which it is expressed.
Creb3L1 is required for the differentiation of astrocytes and intestinal goblet cells. Differentiation of astrocytes requires demethylation of the Gfap promoter, a process regulated by the transcription factor GCM1 (Saito et al., 2012). Interestingly, Gcm1 transcription can be dramatically induced by co-expression of Creb3L1 and Creb3L4, suggesting that heterodimerization may synergistically activate certain downstream target genes (Saito et al., 2012). On the other hand, co-expression of Creb3L1 with Creb3 caused a downregulation in Gcm1 promoter activation, suggesting that Creb3 may act to inhibit the formation of the Creb3L1-Creb3L4 heterodimer necessary for the gene activation associated with astrocyte differentiation (Saito et al., 2012). This study suggests that co-expression of Creb3 proteins may not necessarily be for the purpose of redundancy, but may instead be to provide additional developmental control on gene transcription. Notably, there are fewer astrocytes in Creb3L1 mutants, suggesting a delay in differentiation.
In the intestine, Creb3L1−/− mice also display fewer mature goblet cells than their heterozygous littermates (Asada et al., 2012). Whereas early markers for intestinal cell specification are expressed normally or in some cases are increased (trefoil factor 3, tff3), markers for mature goblet cells, including mucin x2 (Muc2), Anterior gradient 2 (Agr2) and resistin-like β (Retnlb) are markedly reduced (Asada et al., 2012). Phenotypically, the goblet cells from the mutants have fewer, smaller secretory vesicles, and exhibit protein aggregation in the ER suggestive of secretory pathway dysfunction. The failure of the intestinal cells to fully mature suggests a potential link between terminal differentiation and upregulation of secretory capacity, mediated through Creb3L1.
Creb3L1 is also highly expressed in osteoblasts of the developing skeleton. Indeed, the most overt phenotype associated with loss of Creb3L1 is severe osteopenia, a disorder characterized by reduced bone density (Murakami et al., 2009). Microscopic analysis of the bone tissues revealed an accumulation of bone matrix proteins in the ER, suggesting a defect in protein transport through the secretory pathway. Gene expression analysis revealed that Creb3L1 does not regulate genes required for osteoblast specification but instead regulates the secreted components of the bone matrix, including col1a1 and col1a2 (Murakami et al., 2009). Additional targets of Creb3L1 were Xbp1 and the chaperone protein BiP, genes commonly upregulated during ER stress. Murakami et al. also showed that Creb3L1 mRNA is increased by the Runx2 transcription factor, which is activated downstream of BMP2 signaling. BMP2 signaling also induces mild ER stress in osteoblasts, thereby increasing the processing and activation of Creb3L1, and increasing bone matrix deposition (Murakami et al., 2009). A recent report indicates that the same pathways are activated during the healing of bone fractures (Funamoto et al., 2011).
In the pancreas, Creb3L1 is highly expressed during embryonic development with levels tapering off as differentiation occurs (Vellanki et al., 2010). It should be noted that Creb3L1 is detected in mature pancreatic islet cells, just at lower levels. Microarray studies in which Creb3L1 was overexpressed using the inducible β cell line, INS-1 832/13, resulted in the upregulation of genes associated with ECM production and trafficking, including the COPI vesicle transport protein, COPδ2, and the KDEL receptor, Kdelr3. Altogether, these findings are consistent with studies in Drosophila indicating that Creb3 proteins function in upregulating core components of the secretory pathway (Vellanki et al., 2010). Similarly, overexpression of activated Creb3L1 in HeLa cells induced expression of a large array of secretory pathway components, including proteins involved in cotranslational translocation, vesicle formation and trafficking (Fox et al., 2010).
Recent reports also suggest an unexpected role for Creb3L1 in the regulation of cell proliferation and survival following external cellular insults. Denard et al., showed that during virus infection, Creb3L1 is activated and functions to increase transcription of cell cycle inhibitors and to block expression of factors that promote cell proliferation (Denard et al., 2011). The same group has also shown that cancer cells expressing Creb3L1 are more sensitive to treatment with the chemotherapeutic agent doxorubicin (Denard et al., 2012). Cancer cell lines that express Creb3L1, when treated with doxorubicin, undergo RIP, activating Creb3L1 and increasing the expression of cell cycle inhibitor genes, again preventing cell proliferation. These studies suggest that tumors with high levels of Creb3L1 expression are more likely to respond to treatment with doxorubicin than those that do not express Creb3L1 (Denard et al., 2012). In mammary cancer lines, Creb3L1 is downregulated in highly metastatic cancers (Mellor et al., 2013). Mellor et al. recently found that mammary cancer cells that do express Creb3L1 are less invasive, less migratory and are more sensitive to hypoxia-induced apoptosis. Microarray analysis revealed a number of genes regulated by Creb3L1 that control cell proliferation and apoptosis (Mellor et al., 2013). Correspondingly, injection of cancer cells expressing Creb3L1 into mice resulted in smaller tumors and, in 70% of the mice, the tumor actually regressed in size (Mellor et al., 2013). Thus, expression of Creb3L1 correlates with inhibition of cell proliferation, perhaps coupling terminal differentiation with the cessation of cell division.
Creb3L2/BBF2H7
Creb3L2/BBF2H7 was first identified as a fusion protein with FUS that causes low-grade fibromyxoid sarcoma (Storlazzi et al., 2003). Recent studies in Xenopus, zebrafish and in mice point to Creb3L2 having an important role in regulating secretory function. In Xenopus, Creb3L2, as well as Xbp1, were identified by microarray analysis as transcription factors with preferentially increased notochord expression (Tanegashima et al., 2009). GO analysis of notochord enriched genes revealed that the 12 most over-represented GO terms refer to a single pathway, the secretory pathway (Tanegashima et al., 2009). Knockdown of Xbp1 by morpholino injection resulted in reduced secretory pathway gene expression, whereas Creb3L2 knockdown had little to no effect on secretory gene expression. Overexpression of either gene, nonetheless, was sufficient to increase secretory pathway expression, suggesting that Xbp1 and Creb3L2 may act coordinately to promote the high-level secretory pathway gene expression necessary for notochord development (Tanegashima et al., 2009).
In mammals, Creb3L2 is expressed in many tissues with very high expression in the chondrocytes of maturing cartilage (Saito et al., 2009). Creb3L2 functions at multiple levels during cartilage formation with a cell autonomous role in upregulating secretory pathway genes and a cell non-autonomous role in promoting the proliferation of undifferentiated chondrocytes. As such, mice and zebrafish deficient for Creb3L2 have shortened limbs or shortened cartilage structures, respectively (Saito et al., 2009; Melville et al., 2011). Microscopic analysis revealed defects in collagen secretion resulting in reduced cartilage associated ECM. Gene expression analysis in mice revealed that many secretory pathway genes are downregulated in Creb3L2−/− chondrocytes, with Sec23a, a COPII vesicle protein, showing the greatest downregulation (Saito et al., 2009). Correspondingly, the Sec23a or Sec24d knockdown phenotypes in zebrafish are quite similar to those of the Creb3L2 mouse mutant (Melville et al., 2011). Sox9 is considered the “master regulator” of chondrocyte differentiation since it is required for the secretion of ECM proteins, including two collagen genes, Col2a and Col11 (Lefebvre et al., 1997; Bridgewater et al., 1998). A recent study has shown that Creb3L2 is also directly regulated by Sox9, leading to a model wherein Sox9 has the dual role of upregulating Creb3L2 to increase the secretory machinery and upregulating the collagen cargo genes, thereby allowing for proper chondrocyte differentiation (Hino et al., 2014). In this case, Creb3L2 is facilitating collagen secretion by increasing the capacity of the secretory pathway machinery for increased cargo secretion.
The non-autonomous role for Creb3L2 in regulating chondrocyte proliferation maps to the C-terminal lumenal domain of the protein. In Creb3L2−/− mice, the number of proliferating chondrocytes is significantly reduced (Saito et al., 2009). Whereas the N-terminal domain failed to rescue the proliferation defect, expression of the C-terminal domain restored cell division in cultured fibroblasts (Saito et al., 2014). The secreted C terminus binds to Indian hedgehog (Ihh) and its receptor, Patched, to activate Hh signaling in neighboring cells, leading to an increase in parathyroid hormone related protein (PTHrP), which increases chondrocyte cell proliferation (Saito et al., 2014). Whether additional Creb3 family members can mediate cell signaling is unknown, but it is worth noting that, in an inducible cell culture system, ER stress induced secretion of the C-termini of both Creb3L1 and Creb3L4 (Saito et al., 2014).
Creb3L3/CrebH
Creb3L3/CrebH was first discovered to be highly expressed in hepatocytes and to be enriched in the small intestine and stomach. Subsequently, Creb3L3 has been shown to have important roles in the innate immune response, and in the regulation of iron, glucose and lipid homeostasis. In all cases, Creb3L3 upregulates genes encoding the liver or intestine secreted proteins required to maintain homeostasis.
During the inflammatory response, the liver increases production of the acute phase response (APR) proteins, which includes serum amyloid P (SAP) and C-reactive protein (CRP). This effect is largely regulated by the cleavage and activation of Creb3L3 by inflammatory cytokines, which activates transcription of both SAP and CRP (Zhang et al., 2006). Interestingly, enhanced expression of APR genes can be achieved through co-expression of Creb3L3 and ATF6, suggesting that heterodimerization of these two factors increase APR gene transcription (Zhang et al., 2006). Heterodimerization of Creb3L3 with other bZip factors seems to be a common theme as it has also been shown to interact with Xbp-1 to synergistically activate the hepcidin promoter (Vecchi et al., 2009). Hepcidin is a small peptide produced by the liver and required for iron homeostasis and it, too, is upregulated in response to proinflammatory cytokines (Vecchi et al., 2009).
Creb3L3 also has an important role in normal physiologic responses that require high-level secretory function, including gluconeogenesis and the production of liver- and intestine-specific secreted products. In response to fasting, the levels of nuclear Creb3L3 are increased to upregulate the expression of gluconeogenesis genes including PEPCK-C and G6Pase (Lee et al., 2010). Furthermore, in diabetic mice treated with Creb3L3 RNAi, the fasting blood glucose levels were significantly reduced, indicating that Creb3L3 is the major activator of the gluconeogenic program in mice under fasting conditions (Lee et al., 2010).
Apolipoproteins are secreted by the liver and intestine and are required for the transport of lipid molecules into the circulatory system. In cultured liver Hep-G2 cells, Creb3L3 expression was sufficient to induce the production and elevated secretion of cell-type specific cargoes including ApoA-IV and ApoA-1 (Barbosa et al., 2013). In vivo, expression analysis of the apolipoprotein genes revealed that Creb3L3 was not only required for ApoA-IV production but also for the production of ApoC2 (Xu et al., 2014). During disease states, such as liver steatosis, or fatty liver, the levels of Creb3L3 are significantly elevated and, correspondingly, the levels of ApoA-IV are increased (Xu et al., 2014). Because the liver plays such a crucial role in the regulation of circulating lipid levels, it is not surprising that Creb3L3 also has a role in the clearance of triglycerides from the plasma. Creb3L3−/− mice have higher circulating triglyceride levels than wild-type mice (Lee et al., 2011b), and microarray analyses revealed that in addition to the apolipoprotein genes being affected, there are also changes in additional triglyceride metabolism genes including Fgf21, a known regulator of plasma triglycerides (Lee et al., 2011b).
Similar to Creb3L1 and Creb3L2, Creb3L3 may also regulate cell proliferation. Creb3L3 transcript is significantly reduced in hepatocellular carcinoma cells (HCC) as compared to wild-type liver cells (Chin et al., 2005). Experiments in the HCC cell line HepG2 revealed that overexpression of Creb3L3 in these cells was sufficient to inhibit cell proliferation; the targets of Creb3L3 that affect cell proliferation, however, are currently unknown (Chin et al., 2005).
More is known about the mechanisms of Creb3L3 processing than for other family members. Creb3L3 is relatively unstable, with the nuclear form being degraded within an hour and the full-length form being completely degraded in 2–3 h (Bailey et al., 2007). Creb3L3 is N-glycosylated at three consensus glycosylation sites in the luminal domain (Chan et al., 2010). Mutating these sites leads to a significant reduction in Creb3L3-dependent transcriptional activation suggesting that N-glycosylation is critical for RIP cleavage and Creb3L3 activation (Chan et al., 2010).
Creb3L4/Creb4
Much of what is known about Creb3L4 function in humans was discovered through genome-wide expression studies to identify its downstream target genes (Ben Aicha et al., 2007). Consistent with the Creb3 factors in regulating secretory pathway genes, the major Creb3L4 targets include genes encoding the KDEL receptor (KDELR3), chaperone proteins, the O-glycosylating enzyme (GALNT3), and a Golgi assembly protein (Ben Aicha et al., 2007). Other major gene groups regulated by Creb3L4 include genes involved in transcription, sugar and lipid metabolism, channels and transporters, and genes involved in signal transduction, all classes of genes found to be regulated by the other members of this family. Creb3L4 is also expressed in the Paneth and Goblet cells of the intestine, and is regulated by the ETS-domain factor SPDEF (SAM-pointed domain containing ETS-like factor) (Gregorieff et al., 2009). SPDEF is required for Paneth and goblet cell maturation, with secretory progenitor cells accumulating in the SPDEF−/− mice (Gregorieff et al., 2009). Upregulation of Creb3L4 by SPDEF may be required in the Paneth and goblet cells to upregulate the secretory pathway components allowing them to differentiate and perform their secretory functions (Asada et al., 2011).
Creb3L4 may have an additional role in regulating cell survival; prostate cancer lines express Creb3L4 to higher levels than non-cancerous cells (Qi et al., 2002). In mice, loss-of-function mutations in Creb3L4 lead to viable, fertile animals despite there being a significant reduction in the number of sperm in the seminiferous tubules (Adham et al., 2005). This decrease in sperm count is due to increased apoptosis of the germ cells, suggesting that Creb3L4 promotes cell survival in both prostate cancer cells and in mouse sperm (Adham et al., 2005).
Concluding remarks
Studies of the UPR have both directly and indirectly implicated several transcription factors in adjusting secretory capacity in response to increased secretory load. Interestingly, all of the proteins are bZip transcription factors, including the proteins directly implicated in the UPR: ATF4, XBP1 and ATF6. These proteins function in yeast and in tissue culture cells to restore ER homeostasis by attenuating translation, by increasing the ERAD machinery to turnover unfolded proteins, and by increasing levels of many or most of the components of the secretory pathway. The Creb3 transcription factors were implicated in the UPR primarily because they are activated by the same mechanism (RIP) through which the UPR activates ATF6. This proteolytic processing liberates the N-terminal cytosolic transcription factor domain from the ER membrane, allowing these bZip proteins to enter the nucleus and regulate gene expression. Studies of the roles of the Creb3 family, in both tissue culture cells and in vivo, suggest a more physiologic role in secretion, as well as other functions. Indeed, in vivo studies of the canonical UPR proteins suggest that they also function in physiological secretion, often functioning either downstream or in parallel with the Creb3 family of proteins.
Studies of the Drosophila Creb3 protein, known as CrebA, have provided the most insight regarding the shared roles of all Creb3 proteins. CrebA is the only Creb3 family member in Drosophila and a combination of microarray studies, in situ analysis, as well as in vitro and in vivo DNA binding assays has revealed that CrebA regulates almost every known component of the early secretory pathway– acting directly in most cases (Table 1) (Abrams and Andrew, 2005; Fox et al., 2010). CrebA is not only necessary for the activation of secretory pathway component genes, it is also sufficient to activate every secretory pathway component gene that has been tested, as was nicely demonstrated using the engrailed enhancer to drive ectopic expression of CrebA in stripes in the embryonic ectoderm. Importantly, the same assay was used to drive expression of each of the five human Creb3 family members. The activated form of all five genes–Creb3, Creb3L1, Creb3L2, Creb3L4 and Creb3L4 – induced expression of every target gene that was tested, with each target gene encoding a component of a different complex in the secretory pathway (Fox et al., 2010; Barbosa et al., 2013). The ability to activate secretory gene expression in this heterologous system required the ATB domain that is unique to the Creb3 subfamily of bZip proteins (Barbosa et al., 2013). Moreover, CrebB, the Drosophila gene most closely related to CrebA–which does not have an ATB domain, did not have the same activity. Thus, coordinate transcriptional activation of secretory component machinery is an ancient role for the CrebA/Creb3 branch of bZip transcription factors, a role potentially masked in studies of mice mutants because of functional redundancy among family members.
Recent studies also suggest that Creb3 proteins may link upregulation of secretory pathway components to terminal differentiation, which typically involves cessation of cell division. In either virally infected or transformed cells, Creb3 members have been shown to either inhibit expression of cell cycle activators and/or activate expression of cell cycle inhibitors. Indeed, loss-of-function studies suggest that Creb3 activation is also linked to cell cycle regulation during the terminal differentiation of multiple secretory cell types. Determining if expression of the active forms of these molecules can both promote cell cycle exit and induce secretory programs in precursor cells is an important next test. If so, developing methods for expressing the active proteins in cancer cells could be a useful therapy.
Supplementary Material
Acknowledgments
We thank Caitlin Hanlon, Rajprasad Loganathan and Carolyn Machamer for critical reading of this manuscript. Our work on the transcriptional regulation of secretory capacity is supported by NIH K99 DE021461 (R.M.F.) and NIH RO1 DE013899 (D.J.A.).
Footnotes
Compliance with ethics guidelines
Rebecca M. Fox and Deborah J. Andrew declare that they have no conflict of interest.
References
- Abrams EW, Andrew DJ. Prolyl 4-hydroxylase alpha-related proteins in Drosophila melanogaster: tissue-specific embryonic expression of the 99F8-9 cluster. Mech Dev. 2002;112:1–2. 165–171. doi: 10.1016/s0925-4773(01)00636-0. [DOI] [PubMed] [Google Scholar]
- Abrams EW, Andrew DJ. CrebA regulates secretory activity in the Drosophila salivary gland and epidermis. Development. 2005;132(12):2743–2758. doi: 10.1242/dev.01863. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33(1):75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- Adham IM, Eck TJ, Mierau K, Müller N, Sallam MA, Paprotta I, Schubert S, Hoyer-Fender S, Engel W. Reduction of spermatogenesis but not fertility in Creb3l4-deficient mice. Mol Cell Biol. 2005;25(17):7657–7664. doi: 10.1128/MCB.25.17.7657-7664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833(11):2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15(22):6096–6110. [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Meyer HA, Hartmann E. Interactions between Spc2p and other components of the endoplasmic reticulum translocation sites of the yeast Saccharomyces cerevisiae. J Biol Chem. 2000;275(44):34068–34072. doi: 10.1074/jbc.M006126200. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119(Pt 11):2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- Aragón T, van Anken E, Pincus D, Serafimova IM, Korennykh AV, Rubio CA, Walter P. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2009;457(7230):736–740. doi: 10.1038/nature07641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi K. The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem. 2011;149(5):507–518. doi: 10.1093/jb/mvr041. [DOI] [PubMed] [Google Scholar]
- Asada R, Saito A, Kawasaki N, Kanemoto S, Iwamoto H, Oki M, Miyagi H, Izumi S, Imaizumi K. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J Biol Chem. 2012;287(11):8144–8153. doi: 10.1074/jbc.M111.332593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ast T, Cohen G, Schuldiner M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell. 2013;152(5):1134–1145. doi: 10.1016/j.cell.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Ast T, Schuldiner M. All roads lead to Rome (but some may be harder to travel): SRP-independent translocation into the endoplasmic reticulum. Crit Rev Biochem Mol Biol. 2013;48(3):273–288. doi: 10.3109/10409238.2013.782999. [DOI] [PubMed] [Google Scholar]
- Audas TE, Li Y, Liang G, Lu R. A novel protein, Luman/CREB3 recruitment factor, inhibits Luman activation of the unfolded protein response. Mol Cell Biol. 2008;28(12):3952–3966. doi: 10.1128/MCB.01439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Barreca C, O’Hare P. Trafficking of the bZIP transmembrane transcription factor CREB-H into alternate pathways of ERAD and stress-regulated intramembrane proteolysis. Traffic. 2007;8(12):1796–1814. doi: 10.1111/j.1600-0854.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- Bailey D, O’Hare P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid Redox Signal. 2007;9(12):2305–2321. doi: 10.1089/ars.2007.1796. [DOI] [PubMed] [Google Scholar]
- Barbosa S, Fasanella G, Carreira S, Llarena M, Fox R, Barreca C, Andrew D, O’Hare P. An orchestrated program regulating secretory pathway genes and cargos by the transmembrane transcription factor CREB-H. Traffic. 2013;14(4):382–398. doi: 10.1111/tra.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe CK, Miller EA. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics. 2013;193(2):383–410. doi: 10.1534/genetics.112.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont PJ, Chen WJ, San Pedro MN, Thuerauf DJ, Gellings Lowe N, Gude N, Hilton B, Wolkowicz R, Sussman MA, Glembotski CC. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart. Circ Res. 2010;106(2):307–316. doi: 10.1161/CIRCRESAHA.109.203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Aicha S, Lessard J, Pelletier M, Fournier A, Calvo E, Labrie C. Transcriptional profiling of genes that are regulated by the endoplasmic reticulum-bound transcription factor AIbZIP/CREB3L4 in prostate cells. Physiol Genomics. 2007;31(2):295–305. doi: 10.1152/physiolgenomics.00097.2007. [DOI] [PubMed] [Google Scholar]
- Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Beznoussenko GV, Parashuraman S, Rizzo R, Polishchuk R, Martella O, Di Giandomenico D, Fusella A, Spaar A, Sallese M, Capestrano MG, Pavelka M, Vos MR, Rikers YG, Helms V, Mironov AA, Luini A. Transport of soluble proteins through the Golgi occurs by diffusion via continuities across cisternae. Elife. 2014;3 doi: 10.7554/eLife.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122(Pt 10):1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AA, Jr, Martínez-Menárguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Jr, Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95(7):993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Borgese N, Fasana E. Targeting pathways of C-tail-anchored proteins. Biochim Biophys Acta. 2011;1808(3):937–946. doi: 10.1016/j.bbamem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80(1):71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol. 2013;14(6):382–392. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater LC, Lefebvre V, de Crombrugghe B. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J Biol Chem. 1998;273(24):14998–15006. doi: 10.1074/jbc.273.24.14998. [DOI] [PubMed] [Google Scholar]
- Bulleid NJ. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2012;4(11):4. doi: 10.1101/cshperspect.a013219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid NJ, Ellgaard L. Multiple ways to make disulfides. Trends Biochem Sci. 2011;36(9):485–492. doi: 10.1016/j.tibs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Capitani M, Sallese M. The KDEL receptor: new functions for an old protein. FEBS Lett. 2009;583(23):3863–3871. doi: 10.1016/j.febslet.2009.10.053. [DOI] [PubMed] [Google Scholar]
- Capoccia BJ, Jin RU, Kong YY, Peek RM, Jr, Fassan M, Rugge M, Mills JC. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J Clin Invest. 2013;123(4):1475–1491. doi: 10.1172/JCI65703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14(20):1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS, Munshi N, Horner J, Ivanova EV, Protopopov A, Anderson KC, Tonon G, DePinho RA. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11(4):349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CP, Mak TY, Chin KT, Ng IO, Jin DY. N-linked glycosylation is required for optimal proteolytic activation of membrane-bound transcription factor CREB-H. J Cell Sci. 2010;123(Pt 9):1438–1448. doi: 10.1242/jcs.067819. [DOI] [PubMed] [Google Scholar]
- Chapuy B, Tikkanen R, Mühlhausen C, Wenzel D, von Figura K, Höning S. AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic. 2008;9(7):1157–1172. doi: 10.1111/j.1600-0854.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Chartron JW, Gonzalez GM, Clemons WM., Jr A structural model of the Sgt2 protein and its interactions with chaperones and the Get4/Get5 complex. J Biol Chem. 2011;286(39):34325–34334. doi: 10.1074/jbc.M111.277798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Novick P, Ferro-Novick S. ER structure and function. Curr Opin Cell Biol. 2013;25(4):428–433. doi: 10.1016/j.ceb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin KT, Zhou HJ, Wong CM, Lee JM, Chan CP, Qiang BQ, Yuan JG, Ng IO, Jin DY. The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nucleic Acids Res. 2005;33(6):1859–1873. doi: 10.1093/nar/gki332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold C, Coventry J, Ponnambalam S, Monaco AP. Actin and microtubule regulation of trans-Golgi network architecture, and copper-dependent protein transport to the cell surface. Mol Membr Biol. 2004;21(1):59–66. doi: 10.1080/096870310001607350. [DOI] [PubMed] [Google Scholar]
- Cosson P, Lefkir Y, Démollière C, Letourneur F. New COP1-binding motifs involved in ER retrieval. EMBO J. 1998;17(23):6863–6870. doi: 10.1093/emboj/17.23.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Schröder-Köhne S, Sweet DS, Démollière C, Hennecke S, Frigerio G, Letourneur F. The Sec20/Tip20p complex is involved in ER retrieval of dilysine-tagged proteins. Eur J Cell Biol. 1997;73(2):93–97. [PubMed] [Google Scholar]
- Cottam NP, Ungar D. Retrograde vesicle transport in the Golgi. Protoplasma. 2012;249(4):943–955. doi: 10.1007/s00709-011-0361-7. [DOI] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87(3):391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, Harding HP. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci USA. 2012;109(15):E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross BC, Sinning I, Luirink J, High S. Delivering proteins for export from the cytosol. Nat Rev Mol Cell Biol. 2009;10(4):255–264. doi: 10.1038/nrm2657. [DOI] [PubMed] [Google Scholar]
- Csala M, Kereszturi É, Mandl J, Bánhegyi G. The endoplasmic reticulum as the extracellular space inside the cell: role in protein folding and glycosylation. Antioxid Redox Signal. 2012;16(10):1100–1108. doi: 10.1089/ars.2011.4227. [DOI] [PubMed] [Google Scholar]
- Cui-Wang T, Hanus C, Cui T, Helton T, Bourne J, Watson D, Harris KM, Ehlers MD. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell. 2012;148:1–2. 309–321. doi: 10.1016/j.cell.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio C, Caramelo JJ, Parodi AJ. UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin Cell Dev Biol. 2010;21(5):491–499. doi: 10.1016/j.semcdb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo JG, Stahmer KR, Miller EA. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim Biophys Acta. 2013;1833(11):2464–2472. doi: 10.1016/j.bbamcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79(1):777–802. doi: 10.1146/annurev-biochem-061608-091319. [DOI] [PubMed] [Google Scholar]
- Delic M, Rebnegger C, Wanka F, Puxbaum V, Haberhauer-Troyer C, Hann S, Köllensperger G, Mattanovich D, Gasser B. Oxidative protein folding and unfolded protein response elicit differing redox regulation in endoplasmic reticulum and cytosol of yeast. Free Radic Biol Med. 2012;52(9):2000–2012. doi: 10.1016/j.freeradbiomed.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Delic M, Valli M, Graf AB, Pfeffer M, Mattanovich D, Gasser B. The secretory pathway: exploring yeast diversity. FEMS Microbiol Rev. 2013;37(6):872–914. doi: 10.1111/1574-6976.12020. [DOI] [PubMed] [Google Scholar]
- Denard B, Lee C, Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife. 2012;1:e00090. doi: 10.7554/eLife.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denard B, Seemann J, Chen Q, Gay A, Huang H, Chen Y, Ye J. The membrane-bound transcription factor CREB3L1 is activated in response to virus infection to inhibit proliferation of virus-infected cells. Cell Host Microbe. 2011;10(1):65–74. doi: 10.1016/j.chom.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic V. A portrait of the GET pathway as a surprisingly complicated young man. Trends Biochem Sci. 2012;37(10):411–417. doi: 10.1016/j.tibs.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic V, Dötsch V, Sinning I. Endoplasmic reticulum targeting and insertion of tail-anchored membrane proteins by the GET pathway. Cold Spring Harb Perspect Biol. 2013;5(8):a013334. doi: 10.1101/cshperspect.a013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez P, Gautschi M, Helenius A. More than one glycan is needed for ER glucosidase II to allow entry of glycoproteins into the calnexin/calreticulin cycle. Mol Cell. 2005;19(2):183–195. doi: 10.1016/j.molcel.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Egea PF, Stroud RM, Walter P. Targeting proteins to membranes: structure of the signal recognition particle. Curr Opin Struct Biol. 2005;15(2):213–220. doi: 10.1016/j.sbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Eleveld-Trancikova D, Sanecka A, van Hout-Kuijer MA, Looman MW, Hendriks IA, Jansen BJ, Adema GJ. DC-STAMP interacts with ER-resident transcription factor LUMAN which becomes activated during DC maturation. Mol Immunol. 2010;47:11–12. 1963–1973. doi: 10.1016/j.molimm.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4(3):181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Fang H, Mullins C, Green N. In addition to SEC11, a newly identified gene, SPC3, is essential for signal peptidase activity in the yeast endoplasmic reticulum. J Biol Chem. 1997;272(20):13152–13158. doi: 10.1074/jbc.272.20.13152. [DOI] [PubMed] [Google Scholar]
- Fang H, Panzner S, Mullins C, Hartmann E, Green N. The homologue of mammalian SPC12 is important for efficient signal peptidase activity in Saccharomyces cerevisiae. J Biol Chem. 1996;271(28):16460–16465. doi: 10.1074/jbc.271.28.16460. [DOI] [PubMed] [Google Scholar]
- Feldheim D, Schekman R. Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J Cell Biol. 1994;126(4):935–943. doi: 10.1083/jcb.126.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol. 2001;152(3):595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RM, Hanlon CD, Andrew DJ. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J Cell Biol. 2010;191(3):479–492. doi: 10.1083/jcb.201004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RM, Vaishnavi A, Maruyama R, Andrew DJ. Organ-specific gene expression: the bHLH protein Sage provides tissue specificity to Drosophila FoxA. Development. 2013;140(10):2160–2171. doi: 10.1242/dev.092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000;10(5):203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Blobel G. Bovine opsin has more than one signal sequence. Nature. 1985;318(6044):338–343. doi: 10.1038/318338a0. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Watanabe Y, Uchida M, Ozaki M. Mammalian signal peptidase: partial purification and general characterization of the signal peptidase from microsomal membranes of porcine pancreas. J Biochem. 1984;96(4):1125–1131. doi: 10.1093/oxfordjournals.jbchem.a134930. [DOI] [PubMed] [Google Scholar]
- Funamoto T, Sekimoto T, Murakami T, Kurogi S, Imaizumi K, Chosa E. Roles of the endoplasmic reticulum stress transducer OASIS in fracture healing. Bone. 2011;49(4):724–732. doi: 10.1016/j.bone.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5(3):a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, te Heesen S, Graham TR, Aebi M, Emr SD. Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J Cell Biol. 1994;127(3):653–665. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz T, Stevens F, Argon Y. Orchestration of secretory protein folding by ER chaperones. Biochim Biophys Acta. 2013;1833(11):2410–2424. doi: 10.1016/j.bbamcr.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon AD, Latham CF, Miller EA. Vesicle-mediated ER export of proteins and lipids. Biochim Biophys Acta. 2012;1821(8):1040–1049. doi: 10.1016/j.bbalip.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R, Blobel G. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell. 1983;35(3 Pt 2):677–685. doi: 10.1016/0092-8674(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Gilmore R, Blobel G, Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson PA, Lock JG, Luke MR, Stow JL. Domains of the TGN: coats, tethers and G proteins. Traffic. 2004;5(5):315–326. doi: 10.1111/j.1398-9219.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol. 2011;3(11):a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45(2):106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg G, Shelness GS, Blobel G. A subunit of mammalian signal peptidase is homologous to yeast SEC11 protein. J Biol Chem. 1989;264(27):15762–15765. [PubMed] [Google Scholar]
- Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. e1331–1333. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Gristick HB, Rao M, Chartron JW, Rome ME, Shan SO, Clemons WM., Jr Crystal structure of ATP-bound Get3-Get4-Get5 complex reveals regulation of Get3 by Get4. Nat Struct Mol Biol. 2014;21(5):437–442. doi: 10.1038/nsmb.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112(6):805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Guo X, Mattera R, Ren X, Chen Y, Retamal C, González A, Bonifacino JS. The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev Cell. 2013a;27(3):353–366. doi: 10.1016/j.devcel.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Zanetti G, Schekman R. A novel GTP-binding protein-adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife. 2013b;2:e00160. doi: 10.7554/eLife.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman NA, Rojas FJ, Cutroneo KR. Collagen lysyl hydroxylation occurs within the cisternae of the rough endoplasmic reticulum. Arch Biochem Biophys. 1976;172(2):449–454. doi: 10.1016/0003-9861(76)90097-7. [DOI] [PubMed] [Google Scholar]
- Hamman BD, Hendershot LM, Johnson AE. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 1998;92(6):747–758. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Haze K, Okada T, Yoshida H, Yanagi H, Yura T, Negishi M, Mori K. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355(Pt 1):19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457(7230):731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Hino K, Saito A, Kido M, Kanemoto S, Asada R, Takai T, Cui M, Cui X, Imaizumi K. Master regulator for chondrogenesis, Sox9, regulates transcriptional activation of the ER stress transducer BBF2H7/CREB3L2 in chondrocytes. J Biol Chem. 2014 doi: 10.1074/jbc.M113.543322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67(1):49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- Hirst J, Irving C, Borner GH. Adaptor protein complexes AP-4 and AP-5: new players in endosomal trafficking and progressive spastic paraplegia. Traffic. 2013;14(2):153–164. doi: 10.1111/tra.12028. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Hu CC, Dougan SK, McGehee AM, Love JC, Ploegh HL. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. 2009;28(11):1624–1636. doi: 10.1038/emboj.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010;139(6):2038–2049. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes DJS. Vertebrate Collagens–Structures, Functions and Biomedical Applications. In: Scheibel T, editor. Fibrous Proteins. Landes Bioscience; Austin: 2008. pp. 12–29. [Google Scholar]
- Iyer SC, Ramachandran Iyer EP, Meduri R, Rubaharan M, Kuntimaddi A, Karamsetty M, Cox DN. Cut, via CrebA, transcriptionally regulates the COPII secretory pathway to direct dendrite development in Drosophila. J Cell Sci. 2013;126(Pt 20):4732–4745. doi: 10.1242/jcs.131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Naim HY. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol. 2001;11(18):1444–1450. doi: 10.1016/s0960-9822(01)00446-8. [DOI] [PubMed] [Google Scholar]
- Janda CY, Li J, Oubridge C, Hernández H, Robinson CV, Nagai K. Recognition of a signal peptide by the signal recognition particle. Nature. 2010;465(7297):507–510. doi: 10.1038/nature08870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SY, Jang SW, Ko J. Regulation of ADP-ribosylation factor 4 expression by small leucine zipper protein and involvement in breast cancer cell migration. Cancer Lett. 2012;314(2):185–197. doi: 10.1016/j.canlet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Cheng Z, Mandon EC, Gilmore R. An interaction between the SRP receptor and the translocon is critical during cotranslational protein translocation. J Cell Biol. 2008;180(6):1149–1161. doi: 10.1083/jcb.200707196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DY, Wang HL, Zhou Y, Chun AC, Kibler KV, Hou YD, Kung H, Jeang KT. Hepatitis C virus core protein-induced loss of LZIP function correlates with cellular transformation. EMBO J. 2000;19(4):729–740. doi: 10.1093/emboj/19.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, van Waes MA. The translocon: a dynamic gateway at the ER membrane. Annu Rev Cell Dev Biol. 1999;15(1):799–842. doi: 10.1146/annurev.cellbio.15.1.799. [DOI] [PubMed] [Google Scholar]
- Johnson N, Powis K, High S. Post-translational translocation into the endoplasmic reticulum. Biochim Biophys Acta. 2013;1833(11):2403–2409. doi: 10.1016/j.bbamcr.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Johnston HD, Foote C, Santeford A, Nothwehr SF. Golgi-to-late endosome trafficking of the yeast pheromone processing enzyme Ste13p is regulated by a phosphorylation site in its cytosolic domain. Mol Biol Cell. 2005;16(3):1456–1468. doi: 10.1091/mbc.E04-07-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalies KU, Hartmann E. Membrane topology of the 12- and the 25-kDa subunits of the mammalian signal peptidase complex. J Biol Chem. 1996;271(7):3925–3929. doi: 10.1074/jbc.271.7.3925. [DOI] [PubMed] [Google Scholar]
- Kaluza G, Repges S, McDowell W. The significance of carbohydrate trimming for the antigenicity of the Semliki Forest virus glycoprotein E2. Virology. 1990;176(2):369–378. doi: 10.1016/0042-6822(90)90007-e. [DOI] [PubMed] [Google Scholar]
- Kametaka S, Sawada N, Bonifacino JS, Waguri S. Functional characterization of protein-sorting machineries at the trans-Golgi network in Drosophila melanogaster. J Cell Sci. 2010;123(Pt 3):460–471. doi: 10.1242/jcs.055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan RJ, Freymann DM, Stroud RM, Walter P. The signal recognition particle. Annu Rev Biochem. 2001;70(1):755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- Kienzle C, von Blume J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014;24(10):584–593. doi: 10.1016/j.tcb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Kimata Y, Ishiwata-Kimata Y, Ito T, Hirata A, Suzuki T, Oikawa D, Takeuchi M, Kohno K. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol. 2007;179(1):75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko KI, Myllyla R, Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989;3:1609–1617. [PubMed] [Google Scholar]
- Ko J, Jang SW, Kim YS, Kim IS, Sung HJ, Kim HH, Park JY, Lee YH, Kim J, Na DS. Human LZIP binds to CCR1 and differentially affects the chemotactic activities of CCR1-dependent chemokines. FASEB J. 2004;18:890–892. doi: 10.1096/fj.03-0867fje. [DOI] [PubMed] [Google Scholar]
- Kode A, Mosialou I, Silva BC, Joshi S, Ferron M, Rached MT, Kousteni S. FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis. J Biol Chem. 2012;287(12):8757–8768. doi: 10.1074/jbc.M111.282897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Saito A, Hino S, Murakami T, Ogata M, Kanemoto S, Nara S, Yamashita A, Yoshinaga K, Hara H, Imaizumi K. BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol. 2007;27(5):1716–1729. doi: 10.1128/MCB.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457(7230):687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Hartmann E, Rapoport TA. A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 1993;3(3):72–75. doi: 10.1016/0962-8924(93)90066-a. [DOI] [PubMed] [Google Scholar]
- Lavieu G, Zheng H, Rothman JE. Stapled Golgi cisternae remain in place as cargo passes through the stack. Elife. 2013;2:e00558. doi: 10.7554/eLife.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci USA. 2011a;108(21):8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Giannikopoulos P, Duncan SA, Wang J, Johansen CT, Brown JD, Plutzky J, Hegele RA, Glimcher LH, Lee AH. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med. 2011b;17(7):812–815. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Chanda D, Yang J, Oh H, Kim SS, Yoon YS, Hong S, Park KG, Lee IK, Choi CS, Hanson RW, Choi HS, Koo SH. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 2010;11(4):331–339. doi: 10.1016/j.cmet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17(4):2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26(21):7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, VanValkenburgh C, Chen X, Mullins C, Van Kaer L, Green N, Fang H. Genetic complementation in yeast reveals functional similarities between the catalytic subunits of mammalian signal peptidase complex. J Biol Chem. 2003;278(51):50932–50939. doi: 10.1074/jbc.M307542200. [DOI] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441(7096):1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Lu R, Misra V. Potential role for luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency. J Virol. 2000;74(2):934–943. doi: 10.1128/jvi.74.2.934-943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Yang P, O’Hare P, Misra V. Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol. 1997;17(9):5117–5126. doi: 10.1128/mcb.17.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A. A brief history of the cisternal progression-maturation model. Cell Logist. 2011;1(1):6–11. doi: 10.4161/cl.1.1.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32(7):805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Määttänen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21(5):500–511. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Mandon EC, Trueman SF, Gilmore R. Translocation of proteins through the Sec61 and SecYEG channels. Curr Opin Cell Biol. 2009;21(4):501–507. doi: 10.1016/j.ceb.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandon EC, Trueman SF, Gilmore R. Protein translocation across the rough endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5(2):5. doi: 10.1101/cshperspect.a013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan M, Li X, Stefanovic S, Sharma A, Mateja A, Keenan RJ, Hegde RS. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 2010;466(7310):1120–1124. doi: 10.1038/nature09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Menárguez JA, Prekeris R, Oorschot VM, Scheller R, Slot JW, Geuze HJ, Klumperman J. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J Cell Biol. 2001;155(7):1213–1224. doi: 10.1083/jcb.200108029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack KE, Misselwitz B, Plath K, Rapoport TA. BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell. 1999;97(5):553–564. doi: 10.1016/s0092-8674(00)80767-9. [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441(7096):1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- McBride CE, Li J, Machamer CE. The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J Virol. 2007;81(5):2418–2428. doi: 10.1128/JVI.02146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee AM, Dougan SK, Klemm EJ, Shui G, Park B, Kim YM, Watson N, Wenk MR, Ploegh HL, Hu CC. XBP-1-deficient plasmablasts show normal protein folding but altered glycosylation and lipid synthesis. J Immunol. 2009;183(6):3690–3699. doi: 10.4049/jimmunol.0900953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor P, Deibert L, Calvert B, Bonham K, Carlsen SA, Anderson DH. CREB3L1 is a metastasis suppressor that represses expression of genes regulating metastasis, invasion, and angiogenesis. Mol Cell Biol. 2013;33(24):4985–4995. doi: 10.1128/MCB.00959-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville DB, Montero-Balaguer M, Levic DS, Bradley K, Smith JR, Hatzopoulos AK, Knapik EW. The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis. Dis Model Mech. 2011;4(6):763–776. doi: 10.1242/dmm.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon AK, Vidugiriene J. Topology of GPI biosynthesis in the endoplasmic reticulum. Braz J Med Biol Res. 1994;27(2):167–175. [PubMed] [Google Scholar]
- Merulla J, Fasana E, Soldà T, Molinari M. Specificity and regulation of the endoplasmic reticulum-associated degradation machinery. Traffic. 2013;14(7):767–777. doi: 10.1111/tra.12068. [DOI] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7(8):766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Meyer HA, Hartmann E. The yeast SPC22/23 homolog Spc3p is essential for signal peptidase activity. J Biol Chem. 1997;272(20):13159–13164. doi: 10.1074/jbc.272.20.13159. [DOI] [PubMed] [Google Scholar]
- Miller EA, Schekman R. COPII– a flexible vesicle formation system. Curr Opin Cell Biol. 2013;25(4):420–427. doi: 10.1016/j.ceb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SE, Collins BM, McCoy AJ, Robinson MS, Owen DJ. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature. 2007;450(7169):570–574. doi: 10.1038/nature06353. [DOI] [PubMed] [Google Scholar]
- Mironov AA, Beznoussenko GV, Nicoziani P, Martella O, Trucco A, Kweon HS, Di Giandomenico D, Polishchuk RS, Fusella A, Lupetti P, Berger EG, Geerts WJ, Koster AJ, Burger KN, Luini A. Small cargo proteins and large aggregates can traverse the Golgi by a common mechanism without leaving the lumen of cisternae. J Cell Biol. 2001;155(7):1225–1238. doi: 10.1083/jcb.200108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Hollien J. The unfolded protein response in secretory cell function. Annu Rev Genet. 2012;46(1):165–183. doi: 10.1146/annurev-genet-110711-155644. [DOI] [PubMed] [Google Scholar]
- Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem. 2009;146(6):743–750. doi: 10.1093/jb/mvp166. [DOI] [PubMed] [Google Scholar]
- Mullins C, Meyer HA, Hartmann E, Green N, Fang H. Structurally related Spc1p and Spc2p of yeast signal peptidase complex are functionally distinct. J Biol Chem. 1996;271(46):29094–29099. doi: 10.1074/jbc.271.46.29094. [DOI] [PubMed] [Google Scholar]
- Murakami T, Kondo S, Ogata M, Kanemoto S, Saito A, Wanaka A, Imaizumi K. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem. 2006;96(4):1090–1100. doi: 10.1111/j.1471-4159.2005.03596.x. [DOI] [PubMed] [Google Scholar]
- Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T, Kou I, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Wanaka A, Imaizumi K. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11(10):1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- Naim HY, Joberty G, Alfalah M, Jacob R. Temporal association of the N- and O-linked glycosylation events and their implication in the polarized sorting of intestinal brush border sucrase-isomaltase, aminopeptidase N, and dipeptidyl peptidase IV. J Biol Chem. 1999;274(25):17961–17967. doi: 10.1074/jbc.274.25.17961. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Wei JH, Seemann J. Modular organization of the mammalian Golgi apparatus. Curr Opin Cell Biol. 2012;24(4):467–474. doi: 10.1016/j.ceb.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT, Brown JD, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134(2):269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT, Walter P. Protein translocation across the endoplasmic reticulum. Curr Opin Cell Biol. 1994;6(4):510–516. doi: 10.1016/0955-0674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Nikaido T, Yokoya S, Mori T, Hagino S, Iseki K, Zhang Y, Takeuchi M, Takaki H, Kikuchi S, Wanaka A. Expression of the novel transcription factor OASIS, which belongs to the CREB/ATF family, in mouse embryo with special reference to bone development. Histochem Cell Biol. 2001;116(2):141–148. doi: 10.1007/s004180100279. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Nakano A. Identification of a gene required for membrane protein retention in the early secretory pathway. Proc Natl Acad Sci USA. 1993;90(17):8179–8183. doi: 10.1073/pnas.90.17.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyathi Y, Wilkinson BM, Pool MR. Co-translational targeting and translocation of proteins to the endoplasmic reticulum. Biochim Biophys Acta. 2013;1833(11):2392–2402. doi: 10.1016/j.bbamcr.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Oka OB, Bulleid NJ. Forming disulfides in the endoplasmic reticulum. Biochim Biophys Acta. 2013;1833(11):2425–2429. doi: 10.1016/j.bbamcr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366(Pt 2):585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb Perspect Biol. 2013;5(9):5. doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Imai J, Suzuki Y, Watanabe S, Tanigami A, Sugano S. OASIS is a transcriptional activator of CREB/ATF family with a transmembrane domain. Biochem Biophys Res Commun. 2002;293(1):470–477. doi: 10.1016/S0006-291X(02)00253-X. [DOI] [PubMed] [Google Scholar]
- Orlean P, Menon AK. Thematic review series: lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J Lipid Res. 2007;48(5):993–1011. doi: 10.1194/jlr.R700002-JLR200. [DOI] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B. Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol. 2005;21(1):529–550. doi: 10.1146/annurev.cellbio.21.012704.133214. [DOI] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81(4):561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett PA, Dietrich F, Bewersdorf J, Rothman JE, Lavieu G. Inter-Golgi transport mediated by COPI-containing vesicles carrying small cargoes. Elife. 2013;2:e01296. doi: 10.7554/eLife.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols MS, van Meel E, Oorschot V, ten Brink C, Fukuda M, Swetha MG, Mayor S, Klumperman J. hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat Commun. 2013;4:1361. doi: 10.1038/ncomms2360. [DOI] [PubMed] [Google Scholar]
- Qi H, Fillion C, Labrie Y, Grenier J, Fournier A, Berger L, El-Alfy M, Labrie C. AIbZIP, a novel bZIP gene located on chromosome 1q21.3 that is highly expressed in prostate tumors and of which the expression is up-regulated by androgens in LNCaP human prostate cancer cells. Cancer Res. 2002;62(3):721–733. [PubMed] [Google Scholar]
- Quinn RS, Krane SM. Abnormal properties of collagen lysyl hydroxylase from skin fibroblasts of siblings with hydroxylysine-deficient collagen. J Clin Invest. 1976;57(1):83–93. doi: 10.1172/JCI108273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raden D, Song W, Gilmore R. Role of the cytoplasmic segments of Sec61alpha in the ribosome-binding and translocation-promoting activities of the Sec61 complex. J Cell Biol. 2000;150(1):53–64. doi: 10.1083/jcb.150.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450(7170):663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- Reiling JH, Olive AJ, Sanyal S, Carette JE, Brummelkamp TR, Ploegh HL, Starnbach MN, Sabatini DM. A CREB3-ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nat Cell Biol. 2013;15(12):1473–1485. doi: 10.1038/ncb2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome ME, Rao M, Clemons WM, Shan SO. Precise timing of ATPase activation drives targeting of tail-anchored proteins. Proc Natl Acad Sci USA. 2013;110(19):7666–7671. doi: 10.1073/pnas.1222054110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, Wang Y, Eckhardt AE, Hill RL. Subcellular localization of the UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalacto-saminyltransferase-mediated O-glycosylation reaction in the sub-maxillary gland. Proc Natl Acad Sci USA. 1994;91(19):8935–8939. doi: 10.1073/pnas.91.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Domingos PM, Kang MJ, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 2007;26(1):242–252. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Li J, Kang MJ. Drosophila XBP1 expression reporter marks cells under endoplasmic reticulum stress and with high protein secretory load. PLoS ONE. 2013;8(9):e75774. doi: 10.1371/journal.pone.0075774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Imaizumi K. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009;11(10):1197–1204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- Saito A, Kanemoto S, Kawasaki N, Asada R, Iwamoto H, Oki M, Miyagi H, Izumi S, Sanosaka T, Nakashima K, Imaizumi K. Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat Commun. 2012;3:967. doi: 10.1038/ncomms1971. [DOI] [PubMed] [Google Scholar]
- Saito A, Kanemoto S, Zhang Y, Asada R, Hino K, Imaizumi K. Chondrocyte proliferation regulated by secreted luminal domain of ER stress transducer BBF2H7/CREB3L2. Mol Cell. 2014;53(1):127–139. doi: 10.1016/j.molcel.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Sanecka A, Ansems M, van Hout-Kuijer MA, Looman MW, Prosser AC, Welten S, Gilissen C, Sama IE, Huynen MA, Veltman JA, Jansen BJ, Eleveld-Trancikova D, Adema GJ. Analysis of genes regulated by the transcription factor LUMAN identifies ApoA4 as a target gene in dendritic cells. Mol Immunol. 2012;50(1–2):66–73. doi: 10.1016/j.molimm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Saraogi I, Shan SO. Molecular mechanism of co-translational protein targeting by the signal recognition particle. Traffic. 2011;12(5):535–542. doi: 10.1111/j.1600-0854.2011.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A. Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes. Mol Biol Cell. 2003;14(9):3605–3616. doi: 10.1091/mbc.E02-12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell DJ, Hebert DN. Protein translocons: multifunctional mediators of protein translocation across membranes. Cell. 2003;112(4):491–505. doi: 10.1016/s0092-8674(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Metz J, Schmid V, Denic V, Rakwalska M, Schmitt HD, Schwappach B, Weissman JS. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134(4):634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Stahl PD, Rohrer J. A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J Biol Chem. 2000;275(38):29694–29700. doi: 10.1074/jbc.M000571200. [DOI] [PubMed] [Google Scholar]
- Semenza JC, Hardwick KG, Dean N, Pelham HR. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990;61(7):1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21(1):81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Shao S, Hegde RS. A calmodulin-dependent translocation pathway for small secretory proteins. Cell. 2011a;147(7):1576–1588. doi: 10.1016/j.cell.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Hegde RS. Membrane protein insertion at the endoplasmic reticulum. Annu Rev Cell Dev Biol. 2011b;27(1):25–56. doi: 10.1146/annurev-cellbio-092910-154125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolik SM, Rose RE, Goodman RH. A cyclic AMP-responsive element-binding transcriptional activator in Drosophila melanoga-ster, dCREB-A, is a member of the leucine zipper family. Mol Cell Biol. 1992;12(9):4123–4131. doi: 10.1128/mcb.12.9.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Zeng X, Larese J, Ryoo HD. A modified UPR stress sensing system reveals a novel tissue distribution of IRE1/XBP1 activity during normal Drosophila development. Cell Stress Chaperones. 2013;18(3):307–319. doi: 10.1007/s12192-012-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Raden D, Mandon EC, Gilmore R. Role of Sec61alpha in the regulated transfer of the ribosome-nascent chain complex from the signal recognition particle to the translocation channel. Cell. 2000;100(3):333–343. doi: 10.1016/s0092-8674(00)80669-8. [DOI] [PubMed] [Google Scholar]
- Souid S, Lepesant JA, Yanicostas C. The xbp-1 gene is essential for development in Drosophila. Dev Genes Evol. 2007;217(2):159–167. doi: 10.1007/s00427-006-0124-1. [DOI] [PubMed] [Google Scholar]
- Sousa MC, Ferrero-Garcia MA, Parodi AJ. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992;31(1):97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167(1):35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128(6):1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Stefer S, Reitz S, Wang F, Wild K, Pang YY, Schwarz D, Bomke J, Hein C, Löhr F, Bernhard F, Denic V, Dötsch V, Sinning I. Structural basis for tail-anchored membrane protein biogenesis by the Get3-receptor complex. Science. 2011;333(6043):758–762. doi: 10.1126/science.1207125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling J, O’hare P. CREB4, a transmembrane bZip transcription factor and potential new substrate for regulation and cleavage by S1P. Mol Biol Cell. 2006;17(1):413–426. doi: 10.1091/mbc.E05-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlazzi CT, Mertens F, Nascimento A, Isaksson M, Wejde J, Brosjo O, Mandahl N, Panagopoulos I. Fusion of the FUS and BBF2H7 genes in low grade fibromyxoid sarcoma. Hum Mol Genet. 2003;12(18):2349–2358. doi: 10.1093/hmg/ddg237. [DOI] [PubMed] [Google Scholar]
- Strating JR, van Bakel NH, Leunissen JA, Martens GJ. A comprehensive overview of the vertebrate p24 family: identification of a novel tissue-specifically expressed member. Mol Biol Evol. 2009;26(8):1707–1714. doi: 10.1093/molbev/msp099. [DOI] [PubMed] [Google Scholar]
- Suh J, Hutter H. A survey of putative secreted and transmembrane proteins encoded in the C. elegans genome. BMC Genomics. 2012;13(1):333. doi: 10.1186/1471-2164-13-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanegashima K, Zhao H, Rebbert ML, Dawid IB. Coordinated activation of the secretory pathway during notochord formation in the Xenopus embryo. Development. 2009;136(21):3543–3548. doi: 10.1242/dev.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenheim N, Tarlinton DM, Crawford S, Corcoran LM, Hodgkin PD, Nutt SL. High rate of antibody secretion is not integral to plasma cell differentiation as revealed by XBP-1 deficiency. J Immunol. 2012;189(7):3328–3338. doi: 10.4049/jimmunol.1201042. [DOI] [PubMed] [Google Scholar]
- Thibault G, Ng DT. The endoplasmic reticulum-associated degradation pathways of budding yeast. Cold Spring Harb Perspect Biol. 2012;4(12):4. doi: 10.1101/cshperspect.a013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8(9):663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- Tohmonda T, Miyauchi Y, Ghosh R, Yoda M, Uchikawa S, Takito J, Morioka H, Nakamura M, Iwawaki T, Chiba K, Toyama Y, Urano F, Horiuchi K. The IRE1α-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep. 2011;12(5):451–457. doi: 10.1038/embor.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Pelham HR. The KKXX signal mediates retrieval of membrane proteins from the Golgi to the ER in yeast. Eur J Cell Biol. 1994;64(1):211–216. [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- Ungar D, Oka T, Brittle EE, Vasile E, Lupashin VV, Chatterton JE, Heuser JE, Krieger M, Waters MG. Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J Cell Biol. 2002;157(3):405–415. doi: 10.1083/jcb.200202016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, Martinez A, Galleguillos D, Armentano D, Schneider BL, Hetz C. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci USA. 2014;111(18):6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325(5942):877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellanki RN, Zhang L, Guney MA, Rocheleau JV, Gannon M, Volchuk A. OASIS/CREB3L1 induces expression of genes involved in extracellular matrix production but not classical endoplasmic reticulum stress response genes in pancreatic beta-cells. Endocrinology. 2010;151(9):4146–4157. doi: 10.1210/en.2010-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R, Wilson C, De Matteis MA. Exiting the ER: what we know and what we don’t. Trends Cell Biol. 2014;24(1):9–18. doi: 10.1016/j.tcb.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Vidugiriene J, Menon AK. The GPI anchor of cell-surface proteins is synthesized on the cytoplasmic face of the endoplasmic reticulum. J Cell Biol. 1994;127(2):333–341. doi: 10.1083/jcb.127.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann K, Lucas JL, Vuga D, Wang X, Brumm D, Stiles C, Kriebel D, Der-Sarkissian A, Krishnan K, Schweitzer C, Liu Z, Malyankar UM, Chiovitti D, Canny M, Durocher D, Sicheri F, Patterson JB. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J Biol Chem. 2011;286(14):12743–12755. doi: 10.1074/jbc.M110.199737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- Walter P, Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10(1):87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Wang F, Brown EC, Mak G, Zhuang J, Denic V. A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol Cell. 2010;40(1):159–171. doi: 10.1016/j.molcel.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wereszczynski J, McCammon JA. Nucleotide-dependent mechanism of Get3 as elucidated from free energy calculations. Proc Natl Acad Sci USA. 2012;109(20):7759–7764. doi: 10.1073/pnas.1117441109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett R, Kudlyk T, Pokrovskaya I, Schönherr R, Ungar D, Duden R, Lupashin V. COG complexes form spatial landmarks for distinct SNARE complexes. Nat Commun. 2013a;4:1553. doi: 10.1038/ncomms2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett R, Ungar D, Lupashin V. The Golgi puppet master: COG complex at center stage of membrane trafficking interactions. Histochem Cell Biol. 2013b;140(3):271–283. doi: 10.1007/s00418-013-1117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119(Pt 4):615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13(3):351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Xu X, Park JG, So JS, Hur KY, Lee AH. Transcriptional regulation of apolipoprotein A-IV by the transcription factor CREBH. J Lipid Res. 2014;55(5):850–859. doi: 10.1194/jlr.M045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YaDeau JT, Klein C, Blobel G. Yeast signal peptidase contains a glycoprotein and the Sec11 gene product. Proc Natl Acad Sci USA. 1991;88(2):517–521. doi: 10.1073/pnas.88.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273(50):33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119(9):2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BP, Craven RA, Reid PJ, Willer M, Stirling CJ. Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J. 2001;20(1–2):262–271. doi: 10.1093/emboj/20.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14(1):20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- Zanna PT, Sánchez-Laorden BL, Pérez-Oliva AB, Turpín MC, Herraiz C, Jiménez-Cervantes C, García-Borrón JC. Mechanism of dimerization of the human melanocortin 1 receptor. Biochem Biophys Res Commun. 2008;368(2):211–216. doi: 10.1016/j.bbrc.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bai N, Chang A, Zhang Z, Yin J, Shen W, Tian Y, Xiang R, Liu C. ATF4 is directly recruited by TLR4 signaling and positively regulates TLR4-trigged cytokine production in human monocytes. Cell Mol Immunol. 2013;10(1):84–94. doi: 10.1038/cmi.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


