Abstract
Alcohol use is prevalent during adolescence, yet little is known about possible long-lasting consequences.. Recent evidence suggests that adolescents are less sensitive than adults to ethanol’s aversive effects, an insensitivity that may be retained into adulthood after repeated adolescent ethanol exposure. This study assessed whether intermittent ethanol exposure during early or late adolescence (early-AIE or late-AIE, respectively) would affect ethanol conditioned taste aversions 2 days (CTA1) and >3 weeks (CTA2) post-exposure using supersaccharin and saline as conditioning stimuli (CS), respectively. Pair-housed male Sprague-Dawley rats received 4 g/kg i.g. ethanol (25%) or water every 48 hours from postnatal day (P) 25–45 (early AIE) or P45–65 (late AIE), or were left non-manipulated (NM). During conditioning, 30 min home cage access to the CS was followed by 0, 1, 1.5, 2 or 2.5 g/kg ethanol i.p., with testing 2 days later. Attenuated CTA relative to controls was seen among early and late AIE animals at both CTA1 and CTA2, an effect particularly pronounced at CTA1 after late AIE. Thus, adolescent exposure to ethanol was found to induce an insensitivity to ethanol CTA seen soon after exposure and lasting into adulthood, and evident with ethanol exposures not only early but also later in adolescence.
Introduction
Adolescence is a developmental stage characterized by age-specific alterations that remain highly conserved across species. In addition to neural, hormonal, and physical transformations, changes in behavior are also evident, including adolescent-associated increases in novelty seeking, social activity and risk taking (See Spear, 2000 and 2010 for a review). Initiation of alcohol use also occurs largely in adolescence (Faden, 2006), with some of this use reaching high levels. Recent statistics within the United States have determined that approximately 5.1% of 8th graders, 15.6% of 10th graders, and 23.7% of 12th graders reported binge drinking (5+ drinks) within the previous 2 weeks (Johnston, O’Malley, Bachman, & Schulenberg, 2013), often on multiple occasions (Patrick & Schulenberg, 2013). Beginning alcohol use early in adolescence may be particularly problematic, with the age of initiation of alcohol use (Dawson, Li, & Grant, 2008) joining levels of binge drinking during adolescence (Windle & Zucker, 2010) as strong predictors of subsequent alcohol dependence. Recent work has raised the possibility that consequences of binge drinking in early versus late adolescence may differ (See Spear, 2014, in revision, for review). For instance, two studies in humans highlighted differences in parietal lobe activation when comparing current early (Tapert et al., 2004) and late (Tapert et al., 2001) adolescent binge drinkers during a spatial working memory task. Likewise, in simple rodent models of adolescent ethanol exposure, administration of ethanol early in adolescence resulted in context retention deficits, whereas exposure beginning just a week later did not, although this exposure later in adolescence resulted in a context extinction deficit similar to that seen in adults (Broadwater & Spear, 2013). Such findings suggest that there may be distinct consequences of binge-drinking depending on whether the exposure begins in early or late adolescence.
The more than two-fold greater per occasion use of alcohol during adolescence than seen in adulthood is not only evident in humans (Hughes, 2010; Masten, Faden, Zucker, & Spear, 2009), but also in other mammalian species such as rodents (Doremus, Brunell, Rajendran, & Spear, 2005; Vetter, Doremus-Fitzwater, & Spear, 2007). Consequently, rodent models have been employed to explore factors contributing to the enhanced ethanol intake during this developmental transition. Such factors have shown that adolescents are relatively insensitive to many ethanol effects when compared to adults, especially to properties of the drug which likely serve as cues to limit intake (for review, see Doremus-Fitzwater et al., 2010; Spear and Varlinskaya, 2005). However, while adolescents display attenuated sensitivity to ethanol-induced sedative (Silveri & Spear, 1998), motor impairing (White et al., 2002), social disrupting (Varlinskaya & Spear, 2002) and aversive (Anderson, Varlinskaya, & Spear, 2010) effects, they also conversely exhibit enhanced sensitivity to other consequences of ethanol, including ethanol-induced social facilitation (Varlinskaya & Spear, 2006), memory impairments (Markwiese, Acheson, Levin, Wilson, & Swartzwelder, 1998), and possibly the rewarding properties of the drug (Pautassi, Myers, Spear, Molina, & Spear, 2008; Ristuccia & Spear, 2008).
Conditioned taste aversion (CTA) is a method often used to determine the dysphoric effects of ethanol. In this procedure, ingestion of a novel flavor (CS) is paired with the effects of a specific drug (unconditioned stimulus, US). When the animals are later given an opportunity to consume the CS, the degree to which the animal avoids the solution is an indicator of the relative dysphoria experienced in the initial pairing. Previous studies from our laboratory have demonstrated a relative insensitivity to ethanol induced CTA in adolescents, with a higher dose (Anderson et al., 2010; Vetter-O'Hagen, Varlinskaya, & Spear, 2009) and more CS-US pairings (Anderson et al., 2010) needed to produce attenuated intake of the CS in adolescents than adults. Moreover, prior work by Green and Grahame (2008) reported a negative correlation between CTA and ethanol intake, suggesting that a lower sensitivity to the aversive properties of a drug may be an important contributor to greater levels of intake. It has been suggested that the overall hedonic value of a drug is a function of the balance between its rewarding and aversive effects (Riley, 2011). Thus, the relative insensitivity of adolescents to the aversive effects of ethanol may facilitate increased drinking during development.
To explore lasting consequences of alcohol exposure during adolescence, a variety of models of adolescent intermittent ethanol (AIE) exposure have been used in rodents in recent years. Under some, but not all, circumstances, adolescent-typical phenotypes are retained into adulthood after AIE, including studies examining behavior and cognition, in addition to electrophysical and neural characteristics (see Spear and Swartzwelder, 2014, for review). For instance, adolescent-typical elevations in ethanol consumption (Alaux-Cantin et al., 2013; Broadwater & Spear, 2013), attenuations in ethanol-induced CTA (Alaux-Cantin et al., 2013; Diaz-Granados & Graham, 2007), greater ethanol-induced impairment in working memory (Risher et al., 2013) and increased impulsivity (Gilpin, Karanikas, & Richardson, 2012) have all been reported in adult rats weeks following AIE exposure.
To assess whether ethanol exposure during early versus late adolescence differentially influences later aversive properties of ethanol, the current study assessed the impact of AIE during early (P25–45) and late (P45–65) adolescence on ethanol CTA. Two separate post-exposure to test periods were examined, with CTA assessed immediately following the last exposure (CTA-1; withdrawal) and 3-weeks post exposure (CTA-2). All animals were given both tests, with separate tastants used for the two CTA sessions, with the first session (CTA-1) using a sweet (supersaccharin (SS)) and the second (CTA-2) using a salty (sodium chloride (NaCl)) solution. Studies have shown rodents to readily consume both tastants (SS: Morales et al., 2014; NaCl: Li, Hsiao, and Li, 2013; Rowland, Morian, Nicholson, and Salisbury, 1995). Utilizing two distinct tastants rules out issues of memory retention across tests, and helped ensure the animals consumed enough of the CS during the CTA-2 conditioning session. Blood ethanol levels following ethanol challenge were assessed after both CTA tests to determine possible contributions of chronic tolerance to the CTA findings that were obtained.
Methods
Subjects
A total of 288 Sprague-Dawley male rats bred and reared in our colony at Binghamton University were used in this study. The day after birth, all litters were culled to 8–10 pups and housed with their dams until weaning on postnatal day (P) 21, at which time animals were pair-housed with same-sex littermates. Animals were maintained in a temperature controlled (20–22° C) vivarium on a 12-/12-h light/dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and tap water. All procedures were conducted in accord with guidelines established by the National Institutes of Health using protocols approved by the Binghamton University Institutional Animal Care and use Committee.
Experimental design and animal assignment
The design of this experiment was a 2 exposure age (early: P25–P45; late: P45–65) × 3 exposure condition (EtOH, H20, or NM) × 5 CTA training dose (0.0, 1.0, 1.5, 2.0, and 2.5 g/kg EtOH) factorial. Initial assignment sample size was 8–10 animals per group. Animals were assigned to groups randomly, with the constraints that: (a) no more than one animal from any given litter was placed in a particular test group to avoid confounding litter with treatment effects (Zorrilla, 1997); and (b) animals were assigned to different training doses in CTA-1 and CTA-2 (using a counterbalanced design).
Procedure
Chronic exposure
Animals were chronically exposed to either water or ethanol from P25–45 (early) or P45–65 (late). During this regimen, subjects were weighed and given 4 g/kg ethanol or water intragastrically (i.g.) every 48 hours for a total of 11 exposures. Ethanol was intubated as a 25% (v/v) solution in tap water, while subjects in the water condition were intubated with an equivalent volume of water alone. Animals assigned to the NM group were weighed on the first (P25/45) and last (P45/65) exposure days. Following the last exposure, there was a 48-hour period prior to the onset of the CTA-1 procedure to allow animals to recover from any potential acute withdrawal effects.
CTA-1 training/test procedure
Beginning on P48 or 68, each pair of animals was 50% water restricted. To calculate water restriction, water intake for each pair of animals over the previous 24-hour period was measured and half of this amount was provided for the following 24-hours. Twenty-four hours after the onset of the water restriction period, the conditioning session occurred. At the onset of each session (conditioning and test), animals were weighed and each housing pair was separated in their home-cage with a wire-mesh divider 15 minutes prior to a 30-minute conditioning/test session. Separating the animals in this way allows for measurement of individual consumption in homecage testing without the stress of isolate housing, and has been used previously in our laboratory (e.g. Anderson, Varlinskaya and Spear, 2010). At the onset of conditioning, each animal was provided with one bottle containing a supersaccharin (SS) solution (3% sucrose, 0.125% saccharin in water; modified from Ji et al., 2008; see Morales et al., 2014). Immediately following the 30-minute access to the tastant serving as the conditioned stimulus (CS), the bottle was removed and the animal was injected with the designated training dose via interperitoneal (i.p.) administration of a 20% (v/v) solution in physiological saline; 0 dose controls were injected with 0.9% saline isovolumetric to the highest dose of ethanol administered. Each housing pair together received the same drug challenge, and remained separated with the wire-mesh divider for an additional 15 minutes. Upon removal of the mesh divider, each pair of animals was provided with ad libitum access to a fresh bottle of water. The following day (P50 or 70), animals again underwent 24 hours of 50% water restriction. On P51 or 71, animals were given a test session consisting of 30 minute access to SS. Fifteen minutes after the end of the access period, the mesh divider was removed and animals were again given ad libitum water access. The following day (P52 or 72), animals were again injected i.p. with their assigned training dose and remained unseparated in their homecage for 30 minutes, at which point tail bloods were collected for assessment of blood ethanol concentrations (BECs).
CTA-2 training/test procedure
Following blood collection after CTA-1 testing, animals sat undisturbed in their homecages until P70 (early AIE animals) or 90 (late AIE animals), when the above procedure was again repeated, but with two modifications. Animals received a different training dose during the second CTA procedure than was used during CTA-1 (with dose conditions counterbalanced across animals). Further, a different CS, sodium chloride (NaCl) (0.9%) was used to avoid the potential confound of memory retention of the prior CS aversion across CTA-1 dose conditions.
Ethanol analyses
On P73 or 93, animals were again injected i.p. with their assigned training dose, and left undisturbed in their homecage until they were euthanized via decapitation for assessment of BECs 30 minutes post-injection. Trunk blood and brains collected and maintained at −80°C until analysis. Blood ethanol concentrations (BEC) were assessed via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (GC) (Wilmington, DE) and procedures in standard use in our laboratory (e.g. see Willey et al., 2012).
Data Analysis
Early and late AIE animals were analyzed separately for intake of the CS in mls on each conditioning and test day. Data from 10 animals were only included in CTA-1 analysis due to experimenter error during CTA2 training. Animals that consumed less than 1 ml of the CS on either conditioning day were excluded from analysis only for that CTA training/test session (i.e., CTA-1 or CTA-2), as were animals whose measured consumption was ≥ 2 standard deviations from the mean (and hence likely to reflect bottle leakage). In total, data from 23 animals from CTA-1 and 20 from CTA-2 were excluded, with no more than 2 animals excluded per group, resulting in a final n of 7–10 per group.
CTA-1 and CTA-2 data were analyzed separately. Baseline intake at each of the two test intervals was analyzed via a 3 (Exposure condition: ethanol, water, NM) × 2 (Exposure age: early, late) × 5 (Dose) ANOVA to determine if there were differences in pre-conditioning consumption of the tastant across the groups. Given the presence of such differences in CTA-1 (see results), test day intakes were transformed to percent baseline for each animal prior to analysis of the test day data. Additionally, CTA-1 test day consumption of late animals violated Levene’s homogeneity of variance assumption, with these data being successfully transformed via square root transformation.
To explore main effects of Dose and Exposure (and/or their interaction) emerging in each of these analyses, doses effective for producing CTA in each exposure condition were determined using the Dunnett’s test, using the saline control group as the comparison group. Although this focus on dose-response analysis within each group was made a priori, due to the nonorthogonal nature of these contrasts, they cannot be strictly considered planned comparisons (Ruxton and Beauchamp, 2008). All findings were considered significant at p < 0.05.
Results
CTA-1 Data (training/testing beginning 48 hours post- AIE exposure
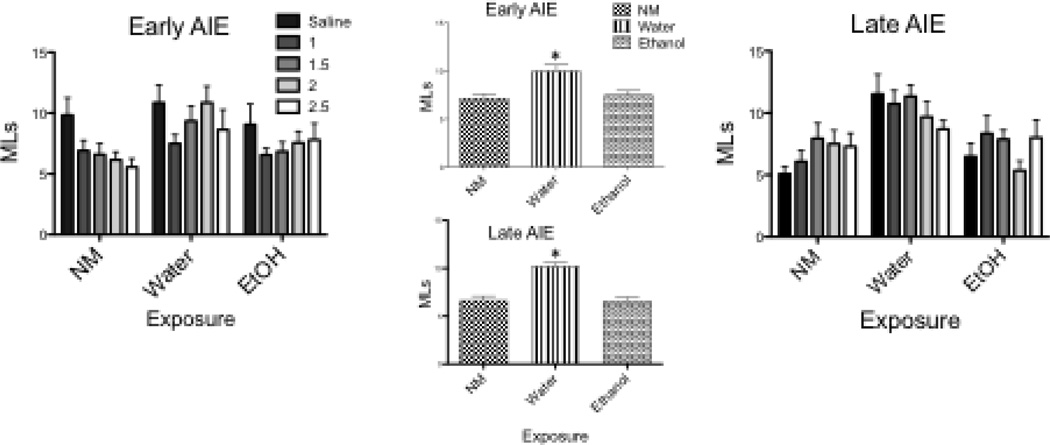
Baseline Intake (Figure 1)
Figure 1.
A main effect of Exposure in baseline intake during CTA conditioning was seen in both early (F[2,111] = 6.27, p = 0.003) and late (F[2,125] = 20.85, p < 0.001) AIE animals, with animals in the water exposure group drinking significantly more SS than other groups (see insert). A main effect of Dose was seen in the early AIE group (F[4,111] = 3.01, p = 0.02), with animals assigned to receive saline consuming more than all other doses. * Denotes significant difference from other doses; # Indicates significant difference from other exposure groups
The ANOVA of baseline intake during CTA-1 revealed a main effect of Exposure in both early (F[2,111] = 6.27, p = 0.003) and late (F[2,125] = 20.85, p < 0.001) AIE animals, with animals exposed to water i.g. at either age drinking significantly more SS compared to the other groups (see inserts to Figure 1). Analysis also revealed a main effect of Dose (F[4,111] = 3.01, p = 0.02) in the early AIE group, with animals assigned to receive saline immediately after the baseline intake period consuming more of the tastant compared to the other dose assignments. Due to this inadvertent sampling bias and the baseline elevation in SS intake in the water groups, CTA-1 test day data from both exposure ages were analyzed as percent baseline.
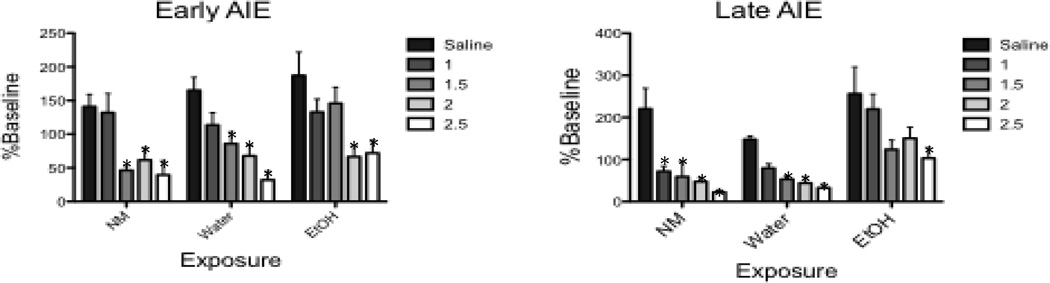
Test day intake (Figure 2)
Figure 2.
Dunnett’s post hoc tests of test day intake during CTA-1 revealed an attenuated sensitivity to ethanol CTA in both ethanol-exposed groups * Denotes significant difference relative to saline-injected controls within each adolescent exposure condition
The ANOVA of % baseline intake during the CTA-1 test session in the early AIE group revealed main effects of Exposure (F[2,111] = 4.69, p = 0.011), and Dose (F[4,111] = 15.51, p < 0.001). Dunnett’s planned comparisons revealed that both water exposed and NM control animals showed less % intake on test day than did saline control animals following the three highest doses of ethanol (1.5, 2 and 2.5 g/kg), whereas % intakes of animals chronically exposed to ethanol were significantly lower than saline control animals only after the 2 highest doses of ethanol (2 and 2.5 g/kg). Analysis of the square root transformed data of the late AIE animals also revealed main effects of Exposure (F[2,125] = 17.47, p < 0.001), and Dose (F[4,125] = 12.15, p < 0.001). Dunnett’s planned comparisons determined that animals in the NM group had significantly lower % baseline intakes than saline control animals following all doses of ethanol, with significant attenuations in intake evident in water exposed animals after the 3 highest doses (1.5, 2, and 2.5 g/kg). In contrast, animals in the ethanol group exhibited significantly lower % baseline intakes than saline control animals only after the highest dose (2.5 g/kg).
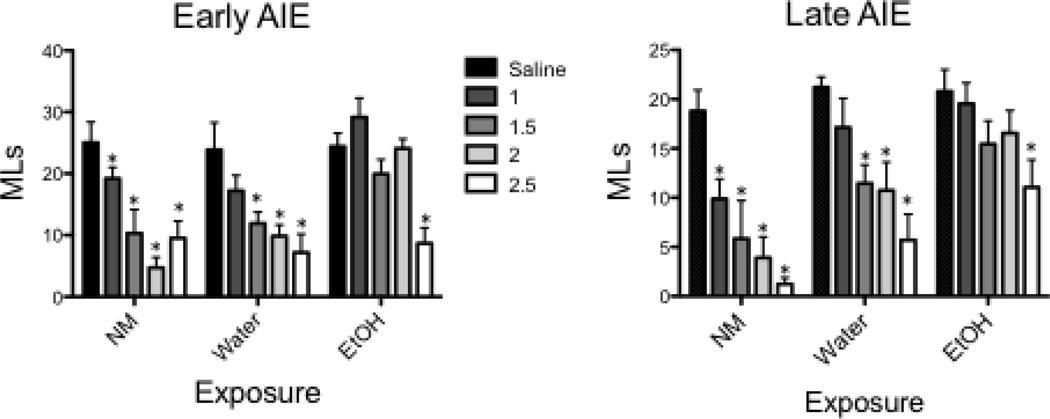
CTA-2 data (3- weeks post-exposure)(Figure 3)
Figure 3.
Dunnett’s post hoc tests of test day intake during CTA-2 revealed an attenuated sensitivity to ethanol CTA in both ethanol-exposed groups. * Denotes significant difference relative to saline-injected controls within each adolescent exposure condition
Baseline intake
The ANOVA of baseline intake during CTA-2 revealed no main effects in either early or late AIE groups (data not shown).
Test day intake
The ANOVA of CS tastant intake in mls during the CTA-2 test session in early AIE animals showed significant effects of Dose (F[4,110] = 15.86, p = 0.0001) and Exposure group (F[2,110] = 12.3, p < 0.001) and their interaction, (F[8,110] = 2.24, p < 0.03), with the ANOVA of test day intake of Late AIE animals revealing main effects of Dose (F[4,107] = 15.23, p < 0.001) and Exposure group (F[2,107] = 17.87, p < 0.001). Dunnett’s test revealed that among early and late exposure groups, NM control animals significantly decreased test day intake relative to their saline controls following all doses of ethanol (1, 1.5, 2, 2.5 g/kg), whereas intake was significantly suppressed among animals in the water exposed groups after 1.5, 2 and 2.5 g/kg of ethanol. In contrast, in both the early and late exposure age group, ethanol animals significantly decreased their intake relative to saline controls only following the highest dose of ethanol (2.5 g/kg).
BECs
The ANOVAs examining tailblood BECs following CTA-1 revealed only expected dose effects on BECs in both early (F[4,110] = 234.02, p < 0.001) and late (F[4, 121] = 2.62, p < 0.04) exposure age groups (Table 1). There were no main effects or interactions involving exposure age or condition. The ANOVA examining trunk blood BECs following CTA-2 in the early AIE animals revealed both a main effect of Dose (F[4,110]= 195.43, p < 0.001), and Exposure (F[2,110]=7.39, P<0.01), with early AIE animals exposed to water having slightly but significantly higher BECs than the other exposure groups (Table 1). The ANOVAs examining BECs in late AIE animals revealed only the expected dose effect (F(4, 105) = 203.28, p < 0.001).
Table 1.
BECs following CTA-1 and CTA-2
| Early exposure | Late exposure | |||||||
|---|---|---|---|---|---|---|---|---|
| Dose | Dose | |||||||
| 1.0 | 1.5 | 2 | 2.5 | 1 | 1.5 | 2 | 2.5 | |
| Post-CTA-1 BEC (tailblood) | ||||||||
| NM | 77.68 ± 4.22 | 131.9 ± 9.57 | 176.46 ± 14.24 | 248.34 ± 21.75 | 82.38 ± 5.39 | 136.84 ± 11.11 | 196.2 ± 18.72 | 212.91 ± 26.57 |
| Water | 98.7 ± 5.17 | 136.52 ± 6.96 | 514.55 ± 8.16 | 212.23 ± 11.8 | 84.41 ± 7.51 | 122.83 ± 11.8 | 181.73 ± 15.18 | 246.01 ± 17.14 |
| EtOH | 82.11 ± 4.03 | 140.16 ± 13.29 | 184.36 ± 8.86 | 230.98 ± 9.23 | 82.5 ± 6.23 | 127.14 ± 5.89 | 192.24 ± 13.77 | 189.38 ± 16.78 |
| Post-CTA-2 BEC (trunk blood) | ||||||||
| NM | 89.73 ± 5.63 | 140.28 ± 6.73 | 211.73 ± 10.93 | 206.08 ± 24.28 | 99.67 ± 3.82 | 144.41 ± 10.77 | 214.48 ± 13.11 | 283.44 ± 14.7 |
| Water | 103.26 ± 2.68 | 178.31 ± 9.26 | 227.16 ± 8.08 | 265.33 ± 17.26 | 105.16 ± 5.48 | 166.45 ± 9.59 | 228.8 ± 14.02 | 316.5 ± 15.2 |
| EtOH | 78.9 ± 7.31 | 160.9 ± 17.36 | 191.66 ± 10.71 | 222.6 ± 19.91 | 128.89 ± 19.94 | 174.44 ± 17.06 | 219.69 ± 19.33 | 257.36 ± 23.13 |
Bolded values denote a significant difference from Early AIE CTA-2 NM and ethanol animals
Discussion
The results of the current study demonstrate that regardless of whether adolescents were exposed to ethanol in early or late adolescence, animals that received binge-like exposure to alcohol during adolescence exhibited notable long-lasting attenuations in the aversive effects of ethanol relative to their counterparts that did not receive ethanol during adolescence. During testing shortly after the end of the exposure periods, this effect was more pronounced in late AIE animals, with these animals requiring a higher dose (2.5 g/kg) than early AIE animals (2.0 g/kg) to express ethanol CTA. This slight difference of exposure age failed to hold through to CTA-2, with both early and late AIE animals tested in adulthood not expressing CTA until the highest (2.5 g/kg) dose – a dose notably higher than necessary to produce CTA in their control counterparts. Given that adolescent animals are normally less sensitive than adults to ethanol’s aversive effects, as indexed via ethanol CTA (Anderson et al., 2010), these findings are reminiscent of other work showing that ethanol exposure during adolescence sometimes results in the retention of an adolescent-typical phenotype into adulthood (see Spear and Swartzwelder, 2014, for a review).
The differences in baseline intake prior to CTA-1 across the to-be-administered CTA dose conditions must reflect incidental variations associated with random assignment, given that treatment of these groups was identical prior to the baseline test. During the baseline intake sessions for CTA-1, however, the chronic water animals from both exposure ages were also found to consume more of the “supersac” solution used as the tastant than the other exposure groups. This effect of prior exposure condition could potentially reflect an effect of the presumably mild stress associated with repeated gavage, with ethanol-exposed animals perhaps not expressing similar increases due to anxiolytic effects of ethanol. Many studies examining the effects of stress during adolescence have investigated long-term differences, with mixed results often depending on stressor severity, rat strain, and sex (e.g. see McCormick and Green, 2013 for a review). In the few studies examining immediate effects of stress during adolescence, however, males were found to be relatively resistant to decreases in sucrose consumption (Bourke & Neigh, 2011; Ducci et al., 2009; Hong et al., 2012), with females sometimes even reported to show increased sucrose consumption following repeated mild stress (Bourke & Neigh, 2011; Pohl, Olmstead, Wynne-Edwards, Harkness, & Menard, 2007). Regardless of the factors contributing to this gavage effect seen with CTA-1, it did not persist until CTA-2. It is possible that by this test, sufficient time had elapsed for recovery from the potentially mild stress of gavage. Alternatively (and perhaps at least as likely), it is possible that the accentuated intake after repeated gavage may be restricted to sweet stimuli such as “supersac” and may not emerge with a non-sweet taste stimulus such as the salt solution used as the tastant in CTA-2.
Test day intake during CTA-1 revealed that animals that received intermittent exposure to ethanol during adolescence exhibited attenuated sensitivity to the aversive properties of ethanol, with this insensitivity slightly more pronounced in late AIE animals. At testing in adulthood (CTA-2), however, comparable insensitivities were seen in AIE animals exposed at either of the two ages. These findings are consistent with previous studies also showing attenuated ethanol CTA after ethanol exposure in adolescence, despite notable differences in exposure mode [i.g. here vs. i.p. (Alaux-Cantin et al., 2013) and vapor exposure (Diaz-Granados & Graham, 2007)] in other studies.
While the current data are in accordance with previous findings that early AIE results in attenuated CTA in adulthood, the similarity in finding with late AIE contrasts with that reported by Alaux-Cantin et al., (2013) using i.p. exposure. The age at which early and late exposures were administered differed only slightly between studies, with Alaux-Cantin and colleagues using P30–43 and P45–58 for early and late adolescence, respectively, and whereas these groups were represented by P25–45 and P45–65 in the current study. Hence, it is likely that route of administration or other differences across labs may contribute to this and other differences reported between i.p. and i.g. routes with AIE data (e.g., compare results of i.p. (Alaux-Cantin et al., 2013) and i.g. (Broadwater et al., 2011) on later ethanol consumption). For a number of response measures, studies comparing early and late adolescent AIE exposure have observed that ethanol exposure beginning pre-pubertally and extending into puberty produce different consequences that those beginning later in adolescence (see Spear, 2014, under revision), with early exposure often resulting in an extension of an adolescent-typical phenotype, and late exposure resulting in more adult-typical consequences. From these CTA data, it would appear that sensitivity to ethanol’s aversive effects might be an exception, with exposure to ethanol throughout a broad range of adolescence sufficient to attenuate later sensitivity to ethanol aversion in a way that could serve to promote later intake.
One possible contributor to the considerable resistance to ethanol CTA seen following both early or late AIE is a general degradation of the US during conditioning, a phenomenon known as the US pre-exposure effect. The US pre-exposure effect suggests that previous exposure to the US, in the present case, repeated prior exposure to ethanol, will lead to a retardation in the acquisition of a conditioned response using that US (Randich & LoLordo, 1979). However, the current study, along with previous studies (Diaz-Granados & Graham, 2007), provides evidence to suggest that the US pre-exposure effect is not responsible for the attenuation in CTA after AIE. First, the interstimulus interval between exposures is either 4 days or 3 weeks, with previous results showing a diminished US pre-exposure effect by 4 days post-exposure (Misanin, Hoefel, Riedy, & Hinderliter, 1997). Additionally, the route of administration differed between pre-exposure (i.g.) and CTA conditioning (i.p.), which contrasts with consistency in route used in prior studies showing US pre-exposure effects. Together, the combined effect of the long interstimulus intervals and different routes of administration would seemingly make it unlikely that the attenuation in CTA seen after AIE is merely a result of a diminished association between the US and CS.
Blood ethanol concentrations following an acute ethanol injection did not differ between exposure groups following CTA-1. This finding is in accordance with prior data showing no differences in BECs across groups following adolescent intermittent ethanol exposure (Diaz-Granados & Graham, 2007; Przybycien-Szymanska, Mott, Paul, Gillespie, & Pak, 2011). At CTA-2, animals in the water pre-exposed group displayed higher BECs at challenge when compared to both ethanol exposed and NM animals, whereas attenuated CTA was seen in AIE animals relative to both water and NM animals. Hence, the attenuated ethanol CTA seen after AIE exposure at either age does not appear to be related simply to metabolic tolerance, but rather reflects a pharmacodynamic effect, complementing prior studies.
The persisting attenuations in ethanol CTA after AIE may reflect an effect of the ethanol on ongoing neuronal development. The adolescent brain is undergoing rapid and substantial reorganization, including modifications in areas related to motivation and reward (see Doremus-Fitzwater, Varlinskaya and Spear, 2010 for a review). The neurocircuitry connecting the prefrontal cortex (PFC) and subcortical reward regions continues to develop throughout adolescence, with, for instance, overall excitatory drive and synaptic density to the PFC exhibiting notable declines during adolescence (Gourley et al., 2012; Huttenlocher, 1984; Salimi et al., 2008; Zecevic, Bourgeois, & Rakic, 1989; See Selemon, 2013 for review), while glutamatergic projections from the basolateral amygdala to the PFC continue to emerge during adolescence (Cunningham, Bhattacharyya, & Benes, 2008). DA receptor densities within the dorsal striatum peak during this stage of development, followed by a substantial pruning of these receptors during the adolescent-to-adult transition period, a finding mirrored in both human (Seeman et al., 1987) and rodent (Tarazi & Baldessarini, 2000; Teicher, Krenzel, Thompson, & Andersen, 2003) studies. Extended exposure to ethanol during adolescence could exact lasting alterations within circuitry underlying rewards and aversions (see Lammel, 2012 for review of circuitry), possibly delaying or abating the normal ontogenetic progression in these and other neural systems (see Spear, 2013 for review). There has been little investigation to date of the effects of AIE on such circuitry.
Results have reliably demonstrated that adolescent rodents are less susceptible to the aversive effects of many drugs of abuse, including ethanol (Anderson et al., 2010; Philpot, Badanich, & Kirstein, 2003) and cocaine (Schramm-Sapyta, Morris, & Kuhn, 2006). Likewise, CTA for non-addictive substances such as lithium chloride is also reduced in adolescents (Schramm-Sapyta et al., 2006), suggestive perhaps of a general insensitivity to aversive effects at this time. Since current and prior results have demonstrated an extension of adolescent-typical behaviors into adulthood following exposure to large amounts of alcohol in adolescence, it would be interesting to examine the response to LiCl in adulthood following AIE to determine whether the attenuated CTA is general or drug specific after AIE. A limitation of the current study is the exclusion of an adult exposure group, thus it is unknown if the results seen following early and late AIE are adolescent-specific, or if comparable results would be seen in adults. Diaz-Granados and Graham (2007), however, found effects of attenuated CTA to ethanol following repeated ethanol exposures to be evident after adolescent but not adult ethanol exposure, suggesting that the notable insensitivity to aversive effects of ethanol seen after AIE may be specific to adolescent exposure. Assessment of females would also be helpful, with prior research reporting both attenuated (Chambers, Sengstake, Yoder, & Thornton, 1981; Sherrill, Berthold, Koss, Juraska, & Gulley, 2011) and enhanced (Morales et al., 2014; Morales & Spear, 2013) sensitivity to aversive effects of drugs of abuse in adolescent and adult females when compared to males. Although little investigated, when studied females have been observed to be more resistant to other effects of AIE than males (e.g., Varlinskaya, Truxell, & Spear, 2014).
The current data provide evidence that repeated exposure to binge levels of ethanol intake in either early or late adolescence leads to an insensitivity to the aversive effects of ethanol in adulthood. Drug abuse is often thought to be related to the relative balance between the rewarding and aversive properties of the drug, with attenuated sensitivity to aversive effects often associated with increased intake of ethanol (see Riley, 2011 for a review), perhaps more strongly so than an enhanced sensitivity to ethanol’s rewarding effects per se (Green & Grahame, 2008 among others). Hence, the prolonged insensitivity to aversive properties of ethanol following AIE may promote increased ethanol intake in adulthood, a hypothesis supported by recent findings (Alaux-Cantin et al., 2013). Overall, it appears that repeated exposure to binge-levels of alcohol during either early or late adolescence results in a retention of an adolescent-typical insensitivity to ethanol aversion into adulthood, possibly permitting relatively high levels of ethanol consumption and contributing to later alcohol-related problems.
Highlights.
Adolescent Intermittent Ethanol (AIE) resulted in attenuated CTA in adulthood
AIE in late adolescence resulted in greater CTA attenuation 2 days post-exposure
All AIE animals were equally insensitive when tested 3 weeks post-exposure
No metabolic tolerance was noted following AIE
AIE results in a decreased sensitivity to aversive effects of ethanol in adulthood
Acknowledgements
This work was supported by funds from RO1-AA018026 and UO1-AA019972-01 to LPS from NIAAA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60(1):112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res. 2013;256:10–19. doi: 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011;35(8):1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB, Yoder RL, Thornton JE. Sexually dimorphic acquisition of a conditioned taste aversion in rats: effects of gonadectomy, testosterone replacement and water deprivation. Physiol Behav. 1981;27(1):83–88. doi: 10.1016/0031-9384(81)90303-6. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18(7):1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95(1–2):62–72. doi: 10.1016/j.drugalcdep.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31(12):2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Roy A, Shen PH, Yuan Q, Yuan NP, Hodgkinson CA, Goldman D. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166(9):1031–1040. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcohol Clin Exp Res. 2006;30(6):1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7(2):e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32(7):2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42(1):1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Flashner B, Chiu M, ver Hoeve E, Luz S, Bhatnagar S. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol Behav. 2012;105(2):269–275. doi: 10.1016/j.physbeh.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. State Estimates of Substance Abuse from the 2006–2007 National Surveys on Drug Use and Health. DIANE Publishing; 2010. [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88(5):488–496. [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19(1):1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on drug use: 2012 overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KC, Hsiao S, Li JS. Conditioned taste aversion as instrumental punishment. J Exp Psychol Anim Behav Process. 2013;39(3):294–297. doi: 10.1037/a0031822. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22(2):416–421. [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. A developmental perspective on underage alcohol use. Alcohol Res Health. 2009;32(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- Misanin JR, Hoefel TD, Riedy CA, Hinderliter CF. Remote and proximal US preexposure and aging effects in taste aversion learning in rats. Physiol Behav. 1997;61(2):221–224. doi: 10.1016/s0031-9384(96)00371-x. [DOI] [PubMed] [Google Scholar]
- Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: impact of age and sex. Behav Brain Res. 2014;261:323–327. doi: 10.1016/j.bbr.2013.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Spear LP. Differences in sensitivity to ethanol-induced conditioned taste aversions emerge after pre- or post-pubertal gonadectomy in male and female rats. Behav Brain Res. 2013;240:69–75. doi: 10.1016/j.bbr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE. Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Res. 2013;35(2):193–200. [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32(11):2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27(4):593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121(3):462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6(4):e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, LoLordo VM. Associative and nonassociative theories of the UCS preexposure phenomenon: implications for Pavlovian conditioning. Psychol Bull. 1979;86(3):523–548. [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103(1):69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, Acheson SK. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS One. 2013;8(5):e62940. doi: 10.1371/journal.pone.0062940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Autonomic responses to ethanol in adolescent and adult rats: a dose-response analysis. Alcohol. 2008;42(8):623–629. doi: 10.1016/j.alcohol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Morian KR, Nicholson TM, Salisbury JJ. Preference for NaCl solutions in sham drinking Sprague-Dawley rats: water deprivation, sodium depletion, and angiotensin II. Physiol Behav. 1995;57(4):753–757. doi: 10.1016/0031-9384(94)00321-1. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behavioral Ecology. 2008;19(3):690–693. [Google Scholar]
- Salimi K, Glantz LA, Hamer RM, German TT, Gilmore JH, Jarskog LF. Regulation of complexin 1 and complexin 2 in the developing human prefrontal cortex. Synapse. 2008;62(4):273–282. doi: 10.1002/syn.20492. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84(2):344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1(5):399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225(1):104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. New York: Norton; 2010. [Google Scholar]
- Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52(2) Suppl 2:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2014 doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18(1):29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Krenzel E, Thompson AP, Andersen SL. Dopamine receptor pruning during the peripubertal period is not attenuated by NMDA receptor antagonism in rat. Neurosci Lett. 2003;339(2):169–171. doi: 10.1016/s0304-3940(02)01475-1. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48(2):146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 2014;48(5):433–444. doi: 10.1016/j.alcohol.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73(3):673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Windle M, Zucker RA. Reducing underage and young adult drinking: how to address critical drinking problems during this developmental period. Alcohol Res Health. 2010;33(1–2):29–44. [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50(1):11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]