Abstract
Children with prenatal alcohol exposure (PAE) may have cognitive, behavioral and brain abnormalities. Here, we compare rates of white matter and subcortical gray matter volume change in PAE and control children, and examine relationships between annual volume change and arithmetic ability, behavior, and executive function. Participants (n=75 PAE/64 control; age: 7.1–15.9 years) each received two structural magnetic resonance scans, ~2 years apart. Assessments included Wechsler Intelligence Scale for Children (WISC-IV), the Child Behavior Checklist (CBCL) and the Behavior Rating Inventory of Executive Function (BRIEF). Subcortical white and gray volumes were extracted for each hemisphere. Group volume differences were tested using false discovery rate (FDR, q<0.05). Analyses examined group-by-age interactions and group-score interactions for correlations between change in volume and raw behavioral scores. Results showed that subjects with PAE had smaller volumes than control subjects across the brain. Significant group-score interactions were found in temporal and parietal regions for WISC arithmetic scores and in frontal and parietal regions for behavioral measures. Poorer cognitive/ behavioral outcomes were associated with larger volume increases in PAE, while control subjects generally showed no significant correlations. In contrast with previous results demonstrating different trajectories of cortical volume change in PAE, our results show similar rates of subcortical volume growth in subjects with PAE and control subjects. We also demonstrate abnormal brain-behavior relationships in subjects with PAE, suggesting different use of brain resources. Our results are encouraging in that, due to the stable volume differences, there may be an extended window of opportunity for intervention in children with PAE.
Keywords: prenatal alcohol exposure, fetal alcohol spectrum disorders, magnetic resonance imaging, white matter, gray matter, brain development
1. Introduction
Children with heavy prenatal alcohol exposure (PAE) can be impaired in executive functions and overall intelligence (Astley and Clarren 2000; Mattson, Crocker et al. 2011), and may have structural brain abnormalities (Riley, Mattson et al. 1995). Diagnosis of fetal alcohol syndrome (FAS) is based on facial dysmorphology (such as short palpebral fissures, smooth philtrum, thin vermillion border); central nervous system abnormalities; growth deficiency; and documentation of alcohol exposure (Jones and Smith 1973; Hoyme, May et al. 2005). However, individuals with PAE may present across the full continuum of fetal alcohol effects, both with and without facial dysmorphology (Sampson, Streissguth et al. 1997).
Of the various cognitive domains affected in PAE, many studies have shown particularly poor arithmetic abilities (Streissguth, Barr et al. 1994; Goldschmidt, Richardson et al. 1996), and performance has been linked to a dose-response relationship where higher prenatal alcohol exposure relates to poorer arithmetic scores (Sampson, Streissguth et al. 2000). Additionally, those with PAE show many conduct and behavioral difficulties (Mattson, Crocker et al. 2011). 42% of children diagnosed with PAE also have attention-deficit hyperactivity disorders (ADHD) (Bhatara, Loudenberg et al. 2006). Similarly, deficiencies in working memory and conduct disorders are also quite prevalent in this population (Mattson, Calarco et al. 2006; Mattson, Crocker et al. 2011). The attention subscale of the Child Behavior Checklist (CBCL) has been found to discriminate between PAE and control subjects with 93% specificity (Lee, Mattson et al. 2004). The Behavior Rating Inventory of Executive Functioning (BRIEF) questionnaire has also been widely used to diagnose executive function deficits in PAE (Stevens, Nash et al. 2013).
The documented behavioral changes in PAE are accompanied by brain structural and functional alterations. Abnormalities of brain structures in children and adults with PAE have been reported frequently over the last few decades. Cross-sectional studies have shown smaller whole brain volume (Archibald, Fennema-Notestine et al. 2001; Sowell, Thompson et al. 2001; Lebel, Rasmussen et al. 2008; Roussotte, Sulik et al. 2012) and abnormalities in parietal, frontal, and temporal lobe white and gray matter structures in children with PAE compared to unexposed control subjects (Sowell, Thompson et al. 2002; Sowell, Mattson et al. 2008). Smaller white matter volumes and corpus callosum have also been widely reported (Sowell, Mattson et al. 2001; Wozniak and Muetzel 2011). Similarly, previous studies have shown smaller hippocampi, basal ganglia, as well as the global pallidus, caudate nucleus, putamen, and the thalamus in PAE (Archibald, Fennema-Notestine et al. 2001; Riikonen, Nokelainen et al. 2005; Nardelli, Lebel et al. 2011). While volume reductions associated with PAE are widespread, cross-sectional studies suggest that white matter and subcortical gray matter are disproportionately affected, with reductions of 9–13% relative to control subjects, compared to 7–8% for total brain volume and cortical gray matter (Astley, Aylward et al. 2009; Nardelli, Lebel et al. 2011).
Two cross-sectional studies examined age-related effects in subcortical gray matter volume in children with PAE. One report describes no significant volume-age correlations in the putamen, caudate, hippocampus or amygdala, but significantly age-related volume increases in the thalamus for control subjects and the globus pallidus for both groups (Nardelli, Lebel et al. 2011). No significant group-by-age interactions were noted for brain volumes in that study. Another study reported bigger hippocampi with age in control subjects but not in alcohol-exposed children aged 9–15 years (Willoughby, Sheard et al. 2008); again, interactions were not significant. Longitudinal studies are necessary to properly assess developmental trajectories. Longitudinal changes in cortical gray matter volume and white matter integrity have been recently described in this population where different trajectories of gray matter development (Lebel, Mattson et al. 2012) and white matter microstructure (Treit, Lebel et al. 2013) were observed in children with PAE, although similar rates of change have been observed in white matter volumes of frontal, parietal and callosal regions and subcortical regions (Treit, Lebel et al. 2013; Gautam, Nuñez et al. 2014).
In regards to directly assessing brain-behavior relationships, functional magnetic resonance studies (fMRI) show significantly lower activation during mental arithmetic tasks in the parietal and frontal regions in PAE (Santhanam, Li et al. 2009). Similarly, math performance has been related to white matter fractional anisotropy in those with PAE (Lebel, Rasmussen et al. 2010). In other clinical cohorts such as in those with anxiety and attention disorders, behavioral and conduct disorders have been linked with abnormal structure and activity of subcortical regions such as the caudate, striatum, amygdala and the hippocampus. Lower functional activity of the amygdala, hippocampus, and the striatum has been seen in youth with conduct disorders (Crowley, Dalwani et al. 2010; Fairchild, Hagan et al. 2013; Marsh, Finger et al. 2013), while smaller thalamus are found in children with ADHD (Xia, Li et al. 2012). In contrast, negative or non significant relationships in brain-behavior relationships have been reported previously both in typical children (Lebel, Rasmussen et al. 2010) and in typical older populations (Gautam, Cherbuin et al. 2011).
Recently, we reported on significantly different trajectories of brain activation in visuospatial attention and working memory tasks in those with PAE, where only decreases in brain activation over time were observed for PAE, and increases were observed for control subjects (Gautam, Nuñez et al. 2014). This study revealed that following subjects longitudinally can be informative on developmental changes that take place over time, and can be useful in understanding such processes in more detail. Hence, the objectives of the current study were to investigate whether children and adolescents with PAE have different rates of volume change with age in white matter and subcortical gray matter compared to control subjects through a longitudinal follow-up design. Secondly, since it is not known how longitudinal change in these white matter and subcortical volumes relate to behavioral symptoms and arithmetic ability in those with PAE, we aimed to determine brain-behavior relationships between age-related changes in subcortical volumes and arithmetic ability as well as parental reports of behavioral problems and executive function. Given previous reported relationships with change in white matter volumes increases in PAE and executive function (Gautam, Nuñez et al. 2014) and arithmetic ability (Lebel, Rasmussen et al. 2010), we hypothesized significant relationships between brain structure volume change and behavior in the PAE children.
2. Materials and Methods
2.1 Subjects
This study was part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) and included data from 139 subjects studied at three sites: Los Angeles, California (n=34: 18 male/16 female, 11 control/23 alcohol-exposed, 32 right-handed/2 left-handed), San Diego, California (n=20: 12 male/8 female, 11 control/9 alcohol-exposed, 17 right-handed/3 left-handed), and Cape Town, South Africa (n=85: 47 male/38 female, 42 control/43 alcohol-exposed, 81 right-handed/4 left-handed). Subjects were aged 7.1–15.9 years (12.3 ± 2.6 years) at the time of their first MRI scan, and were all scanned again approximately two years later at the same site with the same imaging protocol. Imaging data on subsets of these subjects have been previously published (Roussotte, Soderberg et al. 2010; Yang, Phillips et al. 2011; Yang, Roussotte et al. 2011), including longitudinal change in cortical volumes (Lebel, Mattson et al. 2012), longitudinal change in working memory and attention (Gautam, Nuñez et al. 2014) and relationships between longitudinal change in white matter and executive functions over time (Gautam, Nuñez et al. 2014).
Detailed alcohol exposure information (frequency of drinking, average drinks per occasion) was available for 38 of 75 alcohol-exposed subjects and 57 of 64 control subjects. Most control subjects had no prenatal alcohol exposure during any trimester of pregnancy; none had exposure in excess of 1 standard drink per week on average during pregnancy, or more than 2 drinks on any one occasion. Alcohol-exposed subjects had heavy PAE, defined as more than 4 standard drinks per occasion at least once per week, or more than 13 drinks per week at least once during pregnancy. Subjects without documentation of specific exposure amounts were also classified as PAE if there was reasonable suspicion of heavy prenatal alcohol exposure or documented maternal history of alcohol abuse, and evidence for neurobehavioral/neurocognitive deficits. Examples of reasonable reasons include (i) drinking reports obtained from a close relative who had close contact with the family (e.g. child’s grandmother), and (ii) orphanage documentation that the mother had registered as an alcoholic. For alcohol-exposed subjects, the average numbers of drinks per week were 12, 12, and 9 for the first, second and third trimesters, respectively. 30 alcohol-exposed subjects were classified as having FAS and 13 with partial FAS (PFAS) based on the Institute of Medicine guidelines as per the Hoyme criteria (Hoyme, May et al. 2005).
Detailed recruiting methods have been described elsewhere (Mattson, Foroud et al. 2010). In brief, control subjects were recruited from each site via advertisements, word of mouth, or national registers. Alcohol-exposed subjects were recruited through diagnostic clinics and/or previous studies. Alcohol exposure was confirmed through parent/guardian interviews, questionnaires, and social, medical and/or legal records, where available. Participants were excluded for: significant head injury with loss of consciousness for more than 30 minutes; significant physical or psychiatric disability that would prevent participation in the imaging and or cognitive assessments; if born younger than 34 weeks; or if presenting with any known causes of mental deficiency, such as chromosomal abnormalities.
After an explanation of procedures, all subjects provided assent and their parent/guardian provided written informed consent. The Institutional Review Boards at UCLA, SDSU, and the University of Cape Town approved all procedures.
2.2 Cognitive and Behavioral Assessments
Participants were assessed with the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV), including the arithmetic subtest. Higher scores in the arithmetic subset represent better performance. The Child Behavior Checklist (CBCL) and the Brief Rating Inventory of Executive Function (BRIEF) were also completed by parents/guardians. For this study, composite measures for conduct and attention problems from the CBCL were used, as well as the BRIEF global composite executive function measure. For these behavioral assessments, higher scores in all three subscales denote worse behavioral outcomes while lower scores represent more typical behaviors. The complete neurocognitive methods of the CIFASD project are described in detail elsewhere (Mattson, Foroud et al. 2010).
2.3 Image Acquisition
High resolution T1-weighted MRI were collected with the following parameters. In Los Angeles, California (1.5T Siemens Sonata): TR=1900, TE=4.38 ms, flip angle 15°, matrix 256 × 256 × 160, FOV 256 × 256 mm, total acquisition time 8:08; in Cape Town, South Africa (3T Siemens Allegra): TR=2200, TE=5.16 ms, flip angle 12°, matrix 256 × 256 × 160, field of view 256 × 256 mm, total time 7:04; in San Diego, California (3T GE Signa Excite): using TR=8, TE=3.0 ms, flip angle 12°, matrix 256 × 256 × 192, field of view 240 × 240 mm, total acquisition time 7:04. Final resolution was 1 × 1 × 1 mm3 for Los Angeles and San Diego, and 0.94 × 0.94 × 1 mm3 for Cape Town. Data demonstrating reliability of the T1-weighted scanning protocols across sites have been previously reported (Lebel, Mattson et al. 2012).
2.4 Image Processing
All data were processed through the longitudinal stream in FreeSurfer v5.1, using robust, inverse consistent registration (Reuter, Rosas et al. 2010) to create an unbiased within-subject template (Reuter and Fischl 2011). Information from each subject template was used to initialize longitudinal image processing, increasing repeatability and statistical power. Using these processing techniques, we obtained volumes for 34 white matter regions and 7 subcortical gray matter structures per hemisphere, as well as 5 callosal regions (for a total of 87 regions).
2.5 Statistical Analysis
Statistical analysis was conducted in SPSS v20 and R. We were ultimately interested in group differences in trajectories of volume change, rather than absolute differences at particular time points. However, as a first pass to help control for multiple comparisons, a preliminary analysis tested for group differences in subcortical volumes using mixed models with site and subject as random variables (including both time points for each subject). False discovery rate (FDR) correction was used to correct for multiple comparisons between groups; corrected values of q<0.05 were considered significant. Subsequently, we tested for group interactions for age trajectories only in regions that met the original FDR-corrected threshold for significant group volume differences. Mixed models were used, with site and subject as random variables, to examine group-age interactions. Significant results indicate differing developmental trajectories. Mixed models were also used, with site and subject as random variables and controlling for age, to test for correlations between volume changes and WISC-IV arithmetic scores (raw), CBCL attention and conduct problem scores, and BRIEF executive function composite scores, again only in regions with significant group volume differences. Significant brain-behavior interactions indicate different volume-score relationships in alcohol-exposed versus subjects. A p-value of 0.01 was considered significant for these follow-up tests; regions with 0.01<p<0.05 are reported as trends.
3. Results
3.1 Demographics
Group demographics are shown in Table 1. Age, sex, and handedness were not significantly different between groups. The average number of drinks per week was significantly higher in the alcohol-exposed group in all three trimesters (p<0.001). WISC-IV Arithmetic scores were significantly lower in the alcohol-exposed group than in control subjects (p=0.005). The CBCL scores for attention and conduct problems were significantly higher in the alcohol-exposed group (p<0.001), as was the BRIEF global executive composite score (p=0.049).
Table 1.
Demographic information for the alcohol-exposed and control groups. The alcohol-exposed group consisted of 75 subjects, and the control group was 64 subjects; as not all subjects had valid measurements for each variable, actual subject numbers are given in left column for each variable (nalcohol-exposed, ncontrol).
| Alcohol-exposed | Control | t-statistic for group differences | p-value | |
|---|---|---|---|---|
| Age at first scan (years; n=75, 64) | 12.3 ± 2.6 | 12.3 ± 2.5 | 0.25 | 0.98 |
| Gender (n=75, 64) | 46 male, 29 female | 31 male, 33 female | 2091* | 0.13 |
| Handedness (n=75, 64) | 69 right, 6 left | 61 right, 3 left | 2320* | 0.43 |
| Palpebral fissure length (PFL, cm; n=67, 58) | 2.42 ± 0.21 | 2.56± 0.13 | 4.4 | <0.001 |
| Lip Rank (n=65, 56) | 3.6 ± 0.7 | 3.2 ± 0.7 | −3.6 | <0.001 |
| Philtrum Rank (n=65, 55) | 3.6 ± 0.8 | 3.0 ± 0.9 | −3.9 | <0.001 |
| WISC Arithmetic, Scaled (n=73, 60) | 6.8 ± 2.5 | 8.3 ± 3.5 | 2.8 | 0.005 |
| CBCL Attention (n=71, 58) | 61 ± 9 | 54 ± 5 | −5.8 | <0.001 |
| CBCL Conduct (n=61, 55) | 60 ± 8 | 54 ± 5 | −5.3 | <0.001 |
| Alcohol exposure 1st trimester (Average # drinks/week; n=57, 38) | 12 ± 2 | 0 ± 0.0 | −8.2 | <0.001 |
| Prenatal alcohol exposure 2nd trimester (# drinks/week; n=57, 39) | 12 ± 2 | 0.1 ± 0.01 | −6.2 | <0.001 |
| Prenatal alcohol exposure 3rd trimester (# drinks/week; n=57, 39) | 9 ± 1 | 0 ± 0 | −6.1 | <0.001 |
For gender and handedness, the non-parametric Mann-Whitney U test statistic was used.
3.2 Group differences in white and subcortical gray matter volumes and development trajectories
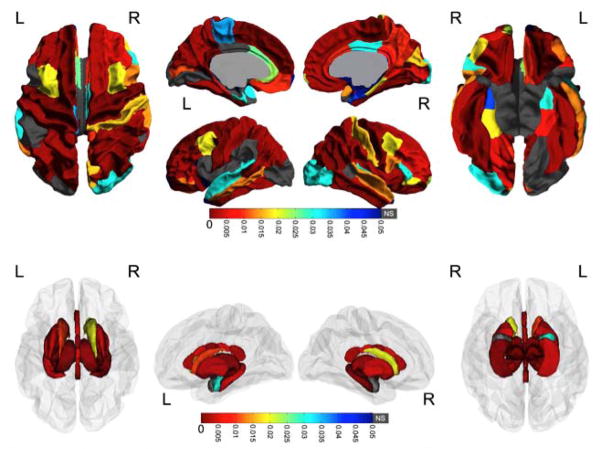
After FDR correction, most regions (79 of 87) had significant group differences; only the left pars opercularis, posterior cingulate and lateral occipital regions, the right amygdala, and the bilateral banks of the superior temporal sulcus and temporal poles did not have significant group differences of volume (Figure 1). Trajectories of volume change were examined only within regions that displayed significant group differences. Of these 79 regions, only the right and left transverse temporal regions had significant age-group interactions (p=0.041, 0.0071, respectively). In both cases, subjects with prenatal alcohol exposure displayed significant linear volume increases over time (p=0.0004, 0.04 in the left and right hemispheres, respectively), while age-related changes in control subjects were not significant (Figure 2). These significant group differences remained after controlling for variation in head size (Intracranial volume) at Time 1 (Supplementary Table 1).
Figure 1. Group differences in volume.
White matter and subcortical gray matter regions with significant volume differences between groups are colored according to their FDR-corrected significance values. Gray indicates no significant group differences. Note the widespread volume differences; in all cases subjects with prenatal alcohol exposure (PAE) had smaller volumes than control subjects.
Figure 2. Age-related changes in transverse temporal white matter regions.
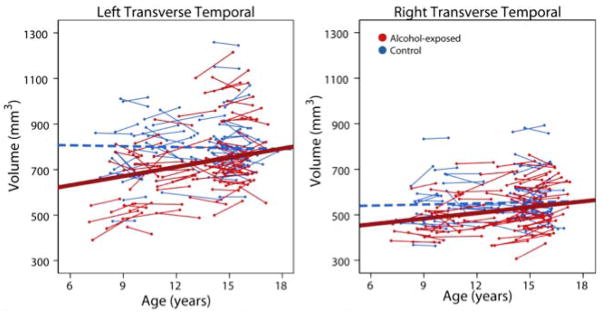
The only areas with significant age-group interactions were the bilateral transverse temporal regions, in which alcohol-exposed subjects (red) showed significant volume increases over time, but no significant changes were noted in control subjects (blue). The best fit line for control subjects (dotted blue line) was not significant and is just shown for reference.
For most brain regions, volume significantly increased over time, but trajectories were similar between groups. Only the anterior and mid-anterior corpus callosum, the left amygdala, banks of the superior temporal sulcus, isthmus cingulate, middle temporal, supramarginal, frontal pole, and insula; the right pars orbitalis, posterior cingulate, and rostral middle frontal; and bilateral caudal anterior cingulate, cuneus, entorhinal, lingual, medial orbital frontal, parahippocampal, temporal pole, rostral anterior cingulate areas showed no significant age-related changes over time.
3.3 Correlations between volume changes and cognition or behavior
Group interactions for correlations between volume changes and cognitive/behavioral scores were examined in the 79 regions with significant group differences of volume. These analyses were considered exploratory and thus a p-value of p<0.01 was used without further correction while those at p<0.05 were considered a trend. These secondary analyses would not survive a FDR correction.
Significant group interactions for WISC Arithmetic scores were observed in the right inferior temporal (p=0.003), right superior temporal (p=0.003) regions while others showed a trend (mid anterior corpus callosum (p=0.040), left middle temporal white matter (p=0.023), right supramarginal (p=0.03), and the right inferior parietal regions (p=0.029)), (Figure 3). Correlations between volume changes and WISC Arithmetic scores were more positive among participants with PAE subjects (positive for all regions except for middle corpus callosum) than among control subjects (negative for all except middle corpus callosum and middle temporal), indicating that higher arithmetic scores in subjects with PAE are often associated with larger volume increases over time.
Figure 3. Significant group-score interactions are shown for white matter and subcortical gray matter volumes for arithmetic scores.
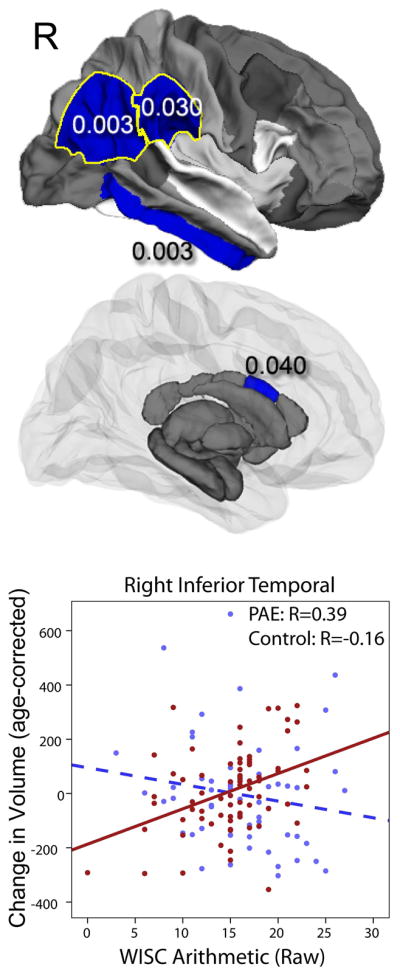
In several brain regions, baseline cognitive and/or behavioral scores were predictive of volume changes over time, and these relationships were different in subjects with prenatal alcohol exposure (PAE) than control subjects (interactions all p<0.05). PAE subjects with higher scores demonstrated larger volume increases over time (control subjects showed no significant relationships).
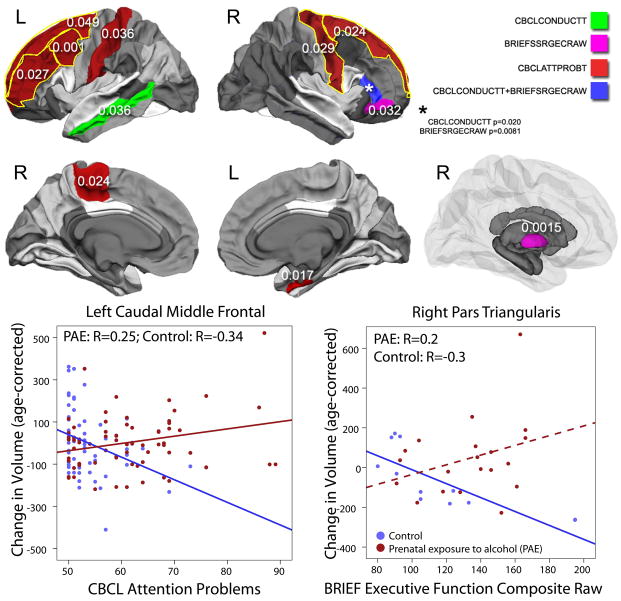
Significant group interactions were observed for CBCL attention problems in the left caudal middle frontal (p=0.001) region. Trends were also observed in the left rostral middle frontal (p=0.027), paracentral (p=0.024), and postcentral (p=0.036) regions, and in the right entorhinal (p=0.017) and precentral regions (p=0.029), and bilaterally in the superior frontal regions (L p=0.049, R p=0.024) (Figure 4). Relationships in participants with PAE were positive, indicating that worse attention problems were associated with larger white matter volume increases, while relationships in control subjects were negative, indicating that fewer attention problems were associated with larger volume increases.
Figure 4. Significant group-score interactions are shown for white matter and subcortical gray matter volumes.
In several brain regions, baseline cognitive and/or behavioral scores were predictive of volume changes over time, and these relationships were different in subjects with prenatal alcohol exposure (PAE) than control subjects (interactions all p<0.05). For behavioral scores in most cases, PAE subjects with worse behavioral problems demonstrated larger volume increases over time, while relationships in control subjects were opposite (worse behavior problems associated with smaller volume increases) or non-significant. Scatter plots are shown for select regions as examples; dotted lines indicate relationships which were not significant within that group.
Only trends were observed for interactions with CBCL conduct problems: in the left middle temporal (p=0.036) and right pars triangularis regions (p=0.020) (Figure 4). In the left middle temporal area, there was a non-significant negative correlation in PAE subjects and a non-significant positive correlation in control subjects. In the right pars triangularis, the opposite was true – correlations were positive in PAE and negative in control subjects, but non-significant (p=0.051, 0.1, respectively).
BRIEF executive function scores were differentially associated with volume changes in the right pallidum (p=0.001), pars triangularis (p=0.008) and a trend was observed in pars orbitalis (p=0.031) (Figure 4). In these regions, correlations were negative in control subject (more normal executive function associated with larger volume increases), and positive or not significant in participants with PAE.
4. Discussion
Using a large sample size and a longitudinal design, we show that children and adolescents with PAE have smaller subcortical volumes than unexposed control subjects, and that differences remain consistent across the age range (7–18 years). In most regions, group differences were stable over time. Hence, while those with PAE had smaller volumes than typically developing children across the age range, they nonetheless had similar rates of change in most brain regions. Brain-behavior relationships were also observed, showing that children and adolescents with PAE and worse behavior or executive function tended to have larger volume increases over time than those with fewer problems, particularly in frontal and temporal-parietal regions. Finally, brain-behavior correlations in controls were generally not significant.
The reduced white matter and subcortical gray matter volumes observed in children and adolescents with PAE is in good agreement with previous cross-sectional (Archibald, Fennema-Notestine et al. 2001; Astley, Aylward et al. 2009; Nardelli, Lebel et al. 2011; Roussotte, Sulik et al. 2011) and longitudinal studies (Treit, Lebel et al. 2013; Gautam, Nuñez et al. 2014). However, our findings of similar development trajectories contrast previous studies showing differential development of cortical gray matter volume (Lebel, Mattson et al. 2012) and white matter microstructure (Treit, Lebel et al. 2013) in children with PAE compared to typically developing control subjects. However, recent studies on white matter volume demonstrate similar trajectories in exposed and unexposed individuals (Gautam, Nuñez et al. 2014) and subcortical volume differences (Treit, Lebel et al. 2013). Given that development patterns are known to vary between white matter volume, gray matter volume, and white matter microstructure (anisotropy and diffusivity as measured by DTI), it is not surprising that children with PAE may demonstrate altered maturation in some areas and not others (Giedd, Blumenthal et al. 1999; Sowell, Thompson et al. 2004; Lebel and Beaulieu 2011). It is also possible that future studies with larger sample sizes and/or more imaging time points may reveal subtle developmental abnormalities in white matter volume. However, the current results suggest that these would at least be smaller in magnitude than the developmental abnormalities observed in cortical gray matter volume and white matter anisotropy and diffusivity.
The only region that did show differences in development trajectory was the transverse temporal area, a region involved in auditory processing. These differences were significant bilaterally, suggesting some consistency. In these regions, volume increases occurred at a slower rate in PAE subjects compared to controls, but both trajectories were linear (see Fig. 1). Children with prenatal alcohol exposure are more likely to have auditory processing difficulties than non-exposed children (Stephen, Kodituwakku et al. 2012), which may be related to abnormal white matter development in this area. However, children with PAE also have deficits in numerous other domains that affect brain areas that were not affected in this study, so more work needs to be done to understand these results.
Participants with PAE had poorer arithmetic scores at baseline than control subjects. This finding is well-documented in previous studies, and mathematical ability tends to be more impaired than other cognitive domains in individuals with PAE (Howell, Lynch et al. 2006). A previous study demonstrated correlations between white matter microstructure and mathematical ability in the parietal and cerebellar regions of children with PAE (Lebel, Rasmussen et al. 2010), though control subjects were not examined in this study. Here, we note that in temporal and parietal regions, arithmetic scores were positively associated with volume changes in subjects with PAE, but there were no significant associations in control subjects. Thus, while arithmetic ability may be related to brain structure in these areas at single time points, it appears that it is associated with developmental changes in temporal and parietal regions in children and youth with PAE.
Subjects with PAE also had more attention and conduct problems than control subjects, and we report a trend toward worse executive function (p=0.08) in this sample. Results showed that for behavioral measures, significant interactions were localized predominantly in the frontal and parietal areas. In subjects with PAE, higher rates of behavioral dysfunction predicted larger white matter volume increases over time, while control subjects generally showed the opposite, with higher rates of dysfunction related to smaller increases of white matter volume. These interactions in the areas important for planning, attention, and overall executive function abilities reveal that contributions of brain structure to cognition and behavior are unequal, and variable between groups.
Given the observed group differences in relation to brain-structure and behavior, it is possible that children and youth with PAE use brain resources differently and/or less efficiently than control subjects. Nonetheless, due to the increased behavioral and attentional problems as evidenced by the BRIEF and CBCL batteries, there are indications that despite increased volume in these regions over time, the cognitive function of these regions might still be impaired. This is because we cannot be certain about the underlying cellular differences that could be present in those with PAE. This theory is consistent with another study from our group on children with PAE, some of whom were also studied here (Gautam, Nuñez et al. 2014), showing group differences in white matter-executive function relationships between PAE and control subjects. In that study, we found that PAE children show differential relationships with age and executive function development compared with control subjects. Children with PAE showed significant cognitive improvements with age in relation to increased white matter volume, while control subjects showed no such relationships suggesting that cognitive functions may rely more heavily on white matter development in those with PAE than in control children.
Results correlating brain volume change with executive and cognitive function are also encouraging, at least from the view of neurodevelopment. Since exploratory in nature, the secondary analyses would not survive a FDR correction. Nonetheless, the positive relationships between arithmetic ability and white matter volume changes in PAE suggest that behavioral interventions or remediation in arithmetic skills to facilitate environmental exposures (which, in turn is likely to impact neurodevelopment) could lead to a general improvement of their arithmetic ability. Support for this view comes from previous interventions that have been conducted on children with PAE, showing that math interventions not only immediately improve standardized scores on CBCL and arithmetic tasks, but that these positive effects last at least 6–12 months after the intervention had stopped (Kable, Coles et al. 2007; Coles, Kable et al. 2009). These studies are particularly interesting, in that the intervention for a cognitive task also improved behavior. Since no neuroimaging data were collected on the participants during the trial, the brain structural changes accompanying the cognitive and behavioral improvements are unknown. However, given our current results, it is possible to speculate that some brain structural alterations could have occurred to lead to improved performances in the PAE participants in the study. Our findings of similar white matter volume change rates and recent findings of significant positive relationships with executive function change (Gautam, Nuñez et al. 2014) supports the view of these structures being crucial for improved performance over time. Given that these results would not survive multiple comparison correction, larger studies and longitudinal follow-up of cognitive function of PAE children will be needed to investigate this issue further.
Limitations
This study has several limitations. Firstly, as is typical of many studies of PAE, detailed alcohol consumption information was not available in PAE cases where biological relatives were not available for interviews. Nonetheless, all participants were identified according to the Institute of Medicine criteria, under which confirmed alcohol exposure is not required for FASD related diagnosis if dysmorphic facial features are identified. Secondly, although the group differences in brain volumes passed FDR corrections for multiple comparisons, the correlations between volume change in age and cognitive and behavioral change did not pass further multiple-comparison corrections. Another limitation of this study is the narrow range of behavioral scores in control subjects, as none had significant behavior or executive function problems. This limits our power to detect correlations and mandates caution in drawing conclusions within the control group. However, the wide range of scores in subjects with PAE makes those results more robust and more conclusive. Another limitation is the presence of confounding variables common to most studies of PAE. For instance, fathers of children with PAE often also drink more than fathers of control children (May, Gossage et al. 2005). Mothers of children with PAE are also more likely to have lower education and lower income (May, Gossage et al. 2008). Such indicators of poor socioeconomic status are independently correlated with poorer cognitive function in later life (Gale, O’Callaghan et al. 2004; Adler and Rehkopf 2008) and cannot be easily partialled out in our study. Finally, although we had neuroimaging data for two time points, behavioral data were only collected at Time 1 and therefore we could only assess how baseline neurobehavior scores correlated with change in brain structures. Future studies with behavioral data at multiple time points will be able to assess how structural brain changes might be related to behavioral change over time.
5. Conclusions
We demonstrate that the smaller volumes in children with PAE persist across childhood and adolescence, suggesting similar development trajectories and showing that these volume abnormalities do not worsen over time. This may be encouraging in that there may not be a critical period for white matter development in PAE, and interventions could be equally effective at any age. Finally, brain-behavior relationships indicated that PAE subjects with worse behavior or executive function tended to have larger volume increases over time in frontal and temporal-parietal regions than those with fewer problems, suggesting that children and youth with PAE may use brain resources differently than control subjects, even though volumetric developmental trajectories are similar.
Supplementary Material
Highlights.
Youth prenatally exposed to alcohol have smaller white matter volumes than control subjects
However, trajectories of volume changes over time are similar in both groups
Brain-behavior relationships are altered in subjects with prenatal alcohol exposure
Acknowledgments
This work was performed in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. This work was also supported by NIAAA grants U01 AA017122-01 to ERS and U24AA014811 to EPR, U01 AA014834 to SNM, and U01 AA11685 and R01 AA15134 to PAM; National Institute of Drug Abuse grant R01DA017831 to ERS, and National Institute of Child Health and Human Development R01 HD053893 to ERS.
Footnotes
Conflict of Interest
The authors have declared no conflicts of interest.
References
- Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, et al. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, et al. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33(10):1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: Introducing the 4-Digit diagnostic code. Alcohol and Alcoholism. 2000;35(4):400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Bhatara V, Loudenberg R, et al. Association of attention deficit hyperactivity disorder and gestational alcohol exposure: an exploratory study. J Atten Disord. 2006;9(3):515–522. doi: 10.1177/1087054705283880. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, et al. Math performance and behavior problems in children affected by prenatal alcohol exposure: intervention and follow-up. J Dev Behav Pediatr. 2009;30(1):7–15. doi: 10.1097/DBP.0b013e3181966780. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, et al. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PloS one. 2010;5(9):e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, et al. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013;54(1):86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, O’Callaghan FJ, et al. Critical periods of brain growth and cognitive function in children. Brain. 2004;127(2):321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- Gautam P, Cherbuin N, et al. Relationships between cognitive function and frontal grey matter volumes and thickness in middle aged and early old-aged adults: The PATH Through Life Study. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Gautam P, Nuñez SC, et al. Effects of prenatal alcohol exposure on the development of white matter volume and change in executive function. Neuroimage Clin. 2014;5:19–27. doi: 10.1016/j.nicl.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P, Nuñez SC, et al. Developmental Trajectories for Visuo-Spatial Attention are Altered by Prenatal Alcohol Exposure: A Longitudinal FMRI Study. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, et al. Prenatal alcohol exposure and academic achievement at age six: a nonlinear fit. Alcohol Clin Exp Res. 1996;20(4):763–770. doi: 10.1111/j.1530-0277.1996.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Howell KK, Lynch ME, et al. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31(1):116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kable JA, Coles CD, et al. Socio-cognitive habilitation using the math interactive learning experience program for alcohol-affected children. Alcohol Clin Exp Res. 2007;31(8):1425–1434. doi: 10.1111/j.1530-0277.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, et al. A Longitudinal Study of the Long-Term Consequences of Drinking during Pregnancy: Heavy In Utero Alcohol Exposure Disrupts the Normal Processes of Brain Development. The Journal of Neuroscience. 2012;32(44):15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. The Journal of neuroscience. 2012;32(44):15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, et al. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2010;34(2):354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, et al. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcoholism Clinical and Experimental Research. 2008;32(10):1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Lee KT, Mattson SN, et al. Classifying children with heavy prenatal alcohol exposure using measures of attention. Journal of the International Neuropsychological Society: JINS. 2004;10(2):271–277. doi: 10.1017/S1355617704102142. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, et al. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatry. 2013;54(8):900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, et al. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20(3):361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, et al. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, et al. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44(7–8):635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Gossage JP, et al. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health. 2005;95(7):1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Gossage JP, et al. Maternal Risk Factors for Fetal Alcohol Syndrome and Partial Fetal Alcohol Syndrome in South Africa: A Third Study. Alcoholism: Clinical and Experimental Research. 2008;32(5):738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, et al. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcoholism Clinical and Experimental Research. 2011;35(8):1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- Nardelli A, Lebel C, et al. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2011;35(8):1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, et al. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53(4):1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen RS, Nokelainen P, et al. Deep Serotonergic and Dopaminergic Structures in Fetal Alcoholic Syndrome: A Study with nor-Œ≤-CIT-Single-Photon Emission Computed Tomography and Magnetic Resonance Imaging Volumetry. Biological psychiatry. 2005;57(12):1565–1572. doi: 10.1016/j.biopsych.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, et al. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19(5):1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Roussotte F, Soderberg L, et al. Structural, metabolic, and functional brain abnormalities as a result of prenatal exposure to drugs of abuse: evidence from neuroimaging. Neuropsychol Rev. 2010;20(4):376–397. doi: 10.1007/s11065-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, et al. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping. 2011 doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, et al. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp. 2012;33(4):920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, et al. On categorizations in analyses of alcohol teratogenesis. Environ Health Perspect. 2000;108(Suppl 3):421–428. doi: 10.1289/ehp.00108s3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56(5):317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Li Z, et al. Effects of Prenatal Alcohol Exposure on Brain Activation During an Arithmetic Task: An fMRI Study. Alcoholism: Clinical and Experimental Research. 2009;33(11):1901–1908. doi: 10.1111/j.1530-0277.2009.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, et al. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18(1):136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, et al. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57(2):235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. NeuroReport. 2001;12(3):515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Kodituwakku PW, et al. Delays in auditory processing identified in preschool children with FASD. Alcohol Clin Exp Res. 2012;36(10):1720–1727. doi: 10.1111/j.1530-0277.2012.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SA, Nash K, et al. Towards identifying a characteristic neuropsychological profile for fetal alcohol spectrum disorders. 2. Specific caregiver-and teacher-rating. J Popul Ther Clin Pharmacol. 2013;20(1):e53–62. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, et al. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 1994;18(2):248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, et al. Longitudinal MRI Reveals Altered Trajectory of Brain Development during Childhood and Adolescence in Fetal Alcohol Spectrum Disorders. J Neurosci. 2013;33(24):10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S, Lebel C, et al. Longitudinal MRI Reveals Altered Trajectory of Brain Development during Childhood and Adolescence in Fetal Alcohol Spectrum Disorders. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(24):10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby A, Sheard ED, et al. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society: JINS. 2008;14(6):1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Muetzel R. What Does Diffusion Tensor Imaging Reveal About the Brain and Cognition in Fetal Alcohol Spectrum Disorders? Neuropsychology Review. 2011;21(2):133–147. doi: 10.1007/s11065-011-9162-1. [DOI] [PubMed] [Google Scholar]
- Xia S, Li X, et al. Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry research. 2012;204(2–3):161–167. doi: 10.1016/j.pscychresns.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Phillips OR, et al. Callosal Thickness Reductions Relate to Facial Dysmorphology in Fetal Alcohol Spectrum Disorders. Alcoholism, clinical and experimental research. 2011 doi: 10.1111/j.1530-0277.2011.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Roussotte F, et al. Abnormal Cortical Thickness Alterations in Fetal Alcohol Spectrum Disorders and Their Relationships with Facial Dysmorphology. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.