Abstract
DNA damage has been implicated in aging, but direct evidence for a causal relationship is lacking, owing to the difficulty of inducing defined DNA lesions in cells and tissues without simultaneously damaging other biomolecules and cellular structures. Here we directly test whether highly toxic DNA double-strand breaks (DSBs) alone can drive an aging phenotype using an adenovirus-based system based on tetracycline-controlled expression of the SacI restriction enzyme. We deliver the adenovirus to mice and compare molecular and cellular end points in the liver with normally aged animals. Treated, 3-month old mice display many, but not all signs of normal liver aging as early as one month after treatment, including aging pathologies, markers of senescence, fused mitochondria, and alterations in gene expression profiles. These results, showing that DSBs alone can cause distinct aging phenotypes in mouse liver, provide new insights in the role of DNA damage as a driver of tissue aging.
DNA double-strand breaks (DSBs) are one of many types of DNA damage that occur spontaneously in all living organisms. DSBs can be induced by ionizing radiation, radio-mimetic chemicals or reactive oxygen species, but also during DNA replication when a polymerase encounters a single-strand lesion at a replication fork 1. DSBs pose problems for cells because their immediate and efficient repair by ligation is often constrained by their physical separation and/or the need to process damaged DNA termini 2,3. DSBs are repaired primarily by either homologous recombination (HR) or non-homologous end-joining (NHEJ). HR is an error-free pathway that utilizes sites of sequence homology, usually a sister chromatid, to repair breaks 4. NHEJ is error-prone, has no requirement for homology, and frequently causes deletions, insertions, and translocations 5. In the absence of repair, damaged cells can be eliminated by apoptosis. Alternatively, mitotically active cells can respond to DSBs by becoming senescent, the permanent cessation of cell division. DSBs can result in genome rearrangements, when multiple DSBs in the same cells are annealed erroneously 6. Thus, DSBs are highly toxic lesions that can promote cancer and, possibly, aging 7.
DSBs have been implicated in aging, through cell loss, the accumulation of senescent cells 8, or genome rearrangements 9. Interestingly, mammals show an age-related increase in foci of phosphorylated H2AX, a marker of DSBs, in various organs and tissues 10,11. Such foci may stem from the decreased propensity of a DSB to be repaired as a function of age 12, or may reflect an accumulation of senescent cells, which harbor persistent DNA damage foci 13. Additionally, DSBs have been indirectly linked to aging through the use of DSB repair-deficient mouse models, such as ERCC1- and Ku80-deficient mice. Mice harboring such DSB repair defects display multiple symptoms of premature aging and have a reduced lifespan 14,15. In humans, signs of premature aging have been observed in adult survivors of childhood cancer who had been treated with agents known to induce DSBs, such as ionizing radiation and a variety of chemotherapeutic drugs 16,17. However, it remains unclear whether these premature aging phenotypes are truly an effect of DSBs or collateral damage to other molecules such as lipids and proteins. To establish a definitive causal relationship between DSBs and aging, experimental animal models are needed in which DSBs can be specifically induced in cells and tissues.
Here, we use an adenoviral construct encoding the SacI restriction endonuclease inducible by doxycycline, previously described by us18, to directly test, for the first time, the possibility that DSBs alone can cause phenotypes associated with aging. The results indicate that one to two months after inducing DSBs in the liver, young mice show multiple symptoms of aging similar to those seen in untreated livers of normally aged control mice.
Results
Induction of DSBs by SacI adenovirus
We previously described a system for the quantitative introduction of DSBs in mammalian cells 18. The system consists of an adenoviral vector (AdV) containing a tetracycline-inducible, composite SacI restriction endonuclease, which is fused to a mutant estrogen receptor (ERT2), and a constitutively expressed reverse transactivator (rtTA) gene (Sac1 AdV; Fig. 1a). SacI recognizes a 6-bp palindromic sequence that occurs in the mammalian genome an estimated 1.3 million times, with ~130,000 sites expected to be available for cleavage in the context of chromatin 18. SacI binding and cleavage generates cohesive sticky ends, which should be easily re-ligated. However, a few of these ends can be eroded by endogenous exonuclease activities, thereby preventing re-ligation, creating a DSB, and activating the DNA damage response. In cultured cells, DNA restriction endonucleases have been shown to be mutagenic 19.
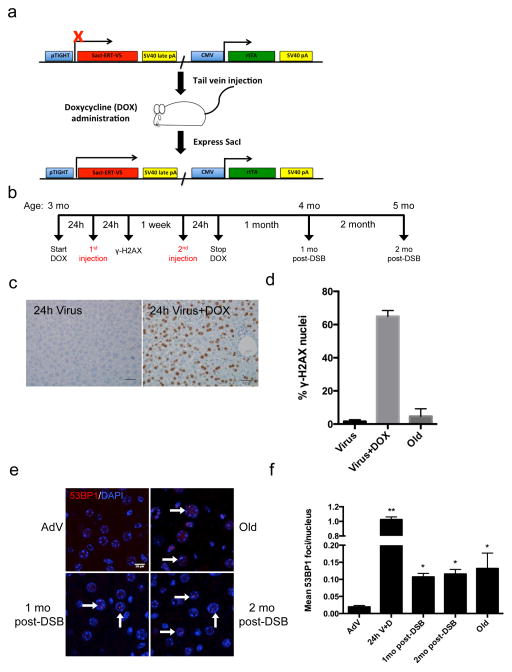
Figure 1. Double-strand break induction by SacI adenovirus.
(a) Schematic of the SacI adenoviral construct (SacI AdV) and its activation by doxycycline (DOX). (b) Schematic of the experimental timeline and mouse ages at which DSBs were induced. (c) Liver sections were immunostained for γ-H2AX 24 hours after SacI AdV tail-vein injection either without (left panel) or with (right panel) DOX administration, and old (28 month) mice as a control. Scale bar = 100μM. (d) Results were quantified and data shown as mean percentage of nuclei positively stained ± s.d,; Virus n=3, Virus+Dox n=2, Old n=3. (e) Representative images of liver sections stained with 53BP1 (red) and DAPI (blue), analyzed by confocal microscopy. White arrows indicate persistent 53BP1 foci. Scale bar = 10μM. (f) Mean number of 53BP1 foci per nucleus was quantified. Data shown are the mean ± s.e.m. of foci per nucleus, where AdV, 1mo, 2mo post-DSB n=4, 24h V+D n=2, and Old n=3; >300 nuclei were scored per animal. P values were calculated using student’s unpaired t-test to AdV samples. * P < 0.05, ** P < 0.01.
We injected the SacI AdV into the tail veins of young, 3-month old mice, 24 hours after administering DOX in their drinking water (Fig. 1b). The AdV treatment was repeated one week later, after which the mice were allowed to recover for a one- and two-month period prior to sacrifice. In parallel, young control animals received the SacI AdV without DOX (AdV control). As a positive control for aging phenotypes, we used naturally aged, 28-month-old mice, which were sacrificed at the same time. We specifically chose to analyze changes in liver, as intravenous administration of an adenovirus vector results mostly in hepatocyte transduction 20.
To confirm the induction of DSBs mostly in liver, we immunostained for γ-H2AX 24 hours after the first SacI AdV injection. Approximately 65% of hepatocytes in DOX-treated mice, compared to only 2% of hepatocytes in AdV control mice, contained nuclei that stained positively for γ-H2AX (Fig. 1c, d). Analysis of naturally aged mice showed a significant elevation of the frequency of γ-H2AX positive cells up to approximately 9%, corroborating data from others 10. For comparison, we also stained five other tissues for γ-H2AX after adenoviral injection and DOX treatment (Supplementary Fig. 1), showing that liver is by far the most robustly targeted tissue using our adenoviral vector, followed by a small portion of γ-H2AX positive cells in pancreas.
We also quantified DSBs in liver by staining for 53BP1 (Fig. 1e), which confirmed the γ-H2AX results at 24 hours after adenoviral injection and DOX, but also allowed counting the average number of DSBs as foci per nucleus. In our hands 53BP1 foci have a much better resolution for that purpose than γ-H2AX foci. The results indicated ~1 DSB on average per hepatocyte 24 hours after injection compared to ~0.02 foci in the untreated control mice (Fig. 1f). Most of these 53BP1 foci disappeared, presumably due to rapid repair and/or apoptosis, but some remained. Indeed, at one and two months after the AdV infections the levels were still significantly elevated in comparison with AdV control mice, i.e., 0.10 and 0.11 foci at one and two months, respectively, as compared to 0.02 in the controls. The levels at one and two months after treatment were about the same as what was observed in naturally aged, 28-month old mice. These results confirm that after an initially high level, DSB numbers in the liver of treated mice quickly decrease to physiological levels, comparable to what was observed in aged mice.
DSBs induce multiple normal liver ageing pathologies
Organ-specific patterns of multiple pathology are hallmarks of the aging process in many animal species. In the mouse, we and others have observed a set of pathological lesions that greatly increase in frequency with normal aging 15,21,22. To determine whether DSBs induce these same pathologies at early age, we performed hematoxylin and eosin staining on all liver sections from young treated with DOX and SacI adenovirus, SacI adenovirus alone, young, and old control mice (Fig. 2a), after which two independent pathologists, from different pathology centers, performed histopathological analysis in a blinded manner. Typical aging pathologies were scored based on a scale of 0–5, with zero being absent and five being most severe. The mean value ± standard error of the mean was calculated for all samples.
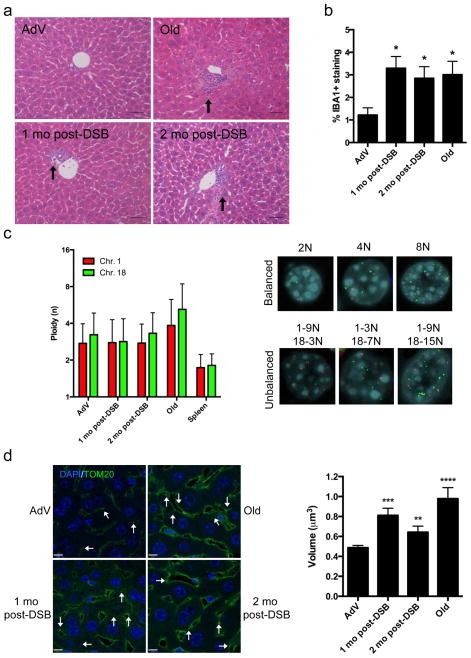
Figure 2. Phenotypic analysis of DSB-induced mouse liver.
(a) Representative H&E stained liver sections (portal vein orientation) assessed blinded for pathological characteristics of aging at 20X magnification. Black arrows indicate sites of lymphocytic infiltrates. (b) Quantification of activated macrophages determined by percentage of IBA1 staining. Data shown represents the mean ± s.e.m. from 3 images per n, where n=4 for all cohorts. (c) Dual-color interphase FISH was performed on liver sections. Ploidy for chromosome 1 (red) and chromosome 18 (green) was determined for 100 hepatocytes per n where n=3 for all cohorts. The ploidy of each chromosome was plotted as mean ± s.d. Representative images are shown for cells with balanced chromosome ploidy for 2N, 4N, and 8N and unbalanced cells. (d) Mitochondrial volume was quantified by immunofluorescent staining for TOM20 (green), a mitochondrial membrane bound protein, and nuclei (blue) and analyzed by confocal microscopy. White arrows indicate analyzable mitochondria after background noise subtraction from z-stack. Mean volume (in x-y-z planes) was calculated for 8 images per n (>2000 mitochondria), where AdV, 1mo, 2mo post-DSB n=4, and Old n=3. Data shown represents the mean ± s.e.m. Scale bar = 8 μM. P values were determined using the Kruskal-Wallis test to AdV samples followed by a post-hoc Dunn’s test. * P < 0.05, ** P < 0.01, *** P < 0.001.
When compared to both naturally aged (28-month-old) and young AdV control mice, DSB-induced young mice showed an elevated level of some, but not all, of the aging phenotypes observed in the naturally aged mice at one- and two-months after DSB induction (Table 1, Fig. 2a). Most notably, hepatocyte nuclei were enlarged (karyomegaly; Supplementary Fig 2a), inflammatory infiltrates and extramedullary hematopoiesis (EMH; Supplementary Fig 2c) were increased. Of note, intranuclear inclusions (Supplementary Fig 2b) trended towards significance for both one and two month post-DSB, but most likely was not significant due to sample size limitations. Another aging phenotype often found in liver of normally aged animals is lipofuscin, or pigment granules composed of oxidized lipid and protein residues of lysosomal digestion. However, while prominently present in normally aged mice, lipofuscin did not increase after DSB treatment (Table 1). Of note, young mice treated only with DOX but had not received an injection of SacI adenovirus did not display any changes in multiple pathology as compared to young, wild type controls (data not shown). To quantify the extent of karyomegaly, we scored hepatocyte size by volumetric analysis of DAPI-stained liver sections. The results (Supplementary Fig. 2d) confirmed a statistically significant increase in nuclear size in the DSB-induced mice. Hepatocytes have the ability to undergo changes in ploidy during development and aging 23, which could also contribute to karyomegaly. Thus, we utilized a quantitative dual-color interphase fluorescent in situ hybridization approach to score the ploidy of hepatocytes as compared to spleen as a control for normal diploid tissue 24. When analyzing two autosomes, Chr. 1 and 18, we did not observe a significant increase in average ploidy in hepatocytes, either one or two months after DSB-induction as compared to AdV controls (Fig. 2c, Supplementary Fig. 4). These results indicate that the observed increase in nuclear size in response to DSB treatment is not likely to be attributable to an increase in DNA content.
Table 1.
Histopathological analysis of DSB-induced mouse liver.
| Pathology | Young | AdV | 1mo post-DSB | 2mo post-DSB | Old |
|---|---|---|---|---|---|
| Karyomegaly | 1.62 ± 0.38 | 2.27 ± 0.14 | 3.00 ± 0* | 2.25 ± 0.25 | 4.00 ± 0** |
| Intranuclear inclusions | 0 ± 0 | 0.09 ± 0.10 | 0.38 ± 0.13# | 0.38 ± 0.13# | 3.00 ± 0** |
| Lobular infiltrates | 0.38 ± 0.24 | 0.81 ± 0.26 | 1.63 ± 0.13* | 1.38 ± 0.24 | 3.67 ± 0.33** |
| Portal infiltrates | 1.12 ± 0.12 | 0.90 ± 0.09 | 1.25 ± 0.15* | 1.25 ± 0.25 | 2.33 ± 0.66* |
| EMH | 0 ± 0 | 0 ± 0 | 0.75 ± 0.25* | 0.25 ± 0.25 | 2.33 ± 0.66* |
| Lipofuscin | 0.37 ± 0.12 | 0.20 ±0.13 | 0.25 ± 0.14 | 0 ± 0 | 2.0 ± 0.29* |
Mice were sacrificed and livers were harvested at one (1mo) or two months (2mo) post DSB induction. Livers were also harvested from untreated young mice (Young), young SacI AdV injected mice without DOX (AdV) one and two months after injection, and 28-month old untreated aged controls (Old). Liver sections were stained with hemotoxylin and eosin (H&E), and assessed blinded for pathological characteristics of aging. Pathology was scored on a scale of 0–5, with 0 having absent pathology and 5 having severe pathology. Young n=5, AdV n=6, 1mo post-DSB n=4, 2mo post-DSB n=4, Old n=3. Data shown are the mean values ± s.e.m. P values were calculated using the Kruskal-Wallis test to AdV samples followed by a post-hoc Dunn’s test.
P < 0.05,
P < 0.01.
P value = 0.06
As shown by pathology analysis, portal and lobular lymphocytic infiltrates are significantly increased after DSB-induction. To further test for infiltration of inflammatory cells, we immunostained liver sections with IBA1 (also known as AIF1), a common marker used for delineating activated macrophages. The results indicated a significant increase from 1.2% to approximately 3.3 and 2.8%, respectively, of infiltrating activated macrophages in one and two-month post-DSB livers as compared to young AdV control mice (Fig. 2b; Supplementary Fig. 3). An increase in activated macrophages to ~3% was also found in normally aged livers. These data suggest that white blood cells, either myeloid or lymphocytic, can infiltrate liver in response to DSBs, a putative cause of the age-related increase in inflammation.
Mitochondrial fusion is a well-documented aging phenotype, originally characterized in aging human hepatocytes 25. Given the potentially deleterious effects of DSBs and the well-documented role of mitochondrial defects in aging 26,27, we chose to analyze mitochondrial volume after DSB treatment, using immunofluorescent staining of TOM20, a component of the mitochondrial outer membrane complex responsible for shuttling in mitochondrial pre-proteins 28. Mitochondrial volume was found to be increased from 0.48 μm3 in AdV controls to 0.81 μm3 and 0.64 μm3 in one and two month post-DSB livers, respectively (Fig. 2d). In keeping with previous studies 25, we also found a drastic increase in volume in our aged cohort to 0.98 μm3. Thus, our data show that DSBs alone can significantly affect mitochondrial size, yet another, well-documented aging phenotype.
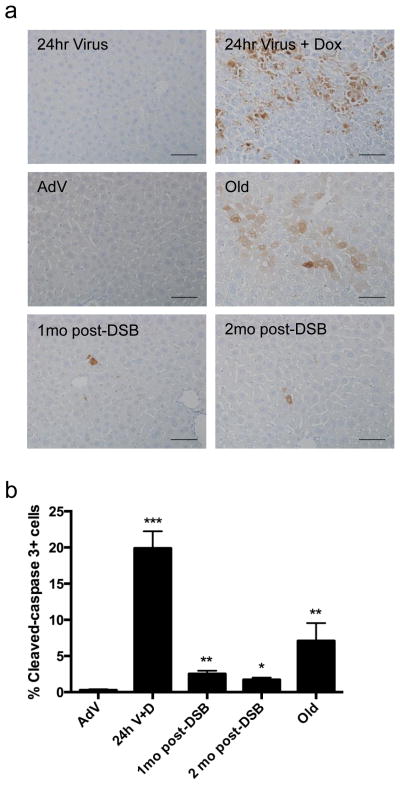
A major cellular hallmark of aging in liver as well as in other organs is an increase in apoptotic cells 29. Using immunostaining for cleaved caspase-3 as a measure of apoptosis, we observed a marked increase in apoptotic cells in 28-month-old, normally aged livers as compared to young, AdV control animals (Fig. 3), confirming findings by others 29,30. When we stained for cleaved caspase-3 at 24 hours after the first Sac1 AdV treatment, a large percent of apoptotic cells, ~20%, were present relative to 24-h AdV control mice (Fig. 3). Notably, we still observed an elevated percent of apoptotic cells in livers from mice one- and two-months after DSB induction, compared to livers from young adenoviral control mice (Fig. 3). These findings indicate that as late as two months after DSB induction, the number of apoptotic cells remains elevated. It is conceivable that this reflects delayed apoptosis, for example, due to further accumulation of damage.
Figure 3. Induction of apoptosis after DSB treatment.
(a) Liver sections were immunostained for cleaved caspase-3 after SacI adenoviral tail-vein injection as indicated. Magnification = 20X. Scale bar = 100μM. (b) Quantification of cleaved-caspase 3 from 3 images per n, where AdV, 1mo, 2mo post-DSB n=4, 24h V+D n=2, and Old n=3. P values were calculated using the Kruskal-Wallis test to AdV samples followed by a post-hoc Dunn’s test. * P < 0.05, ** P < 0.01, *** P < 0.001.
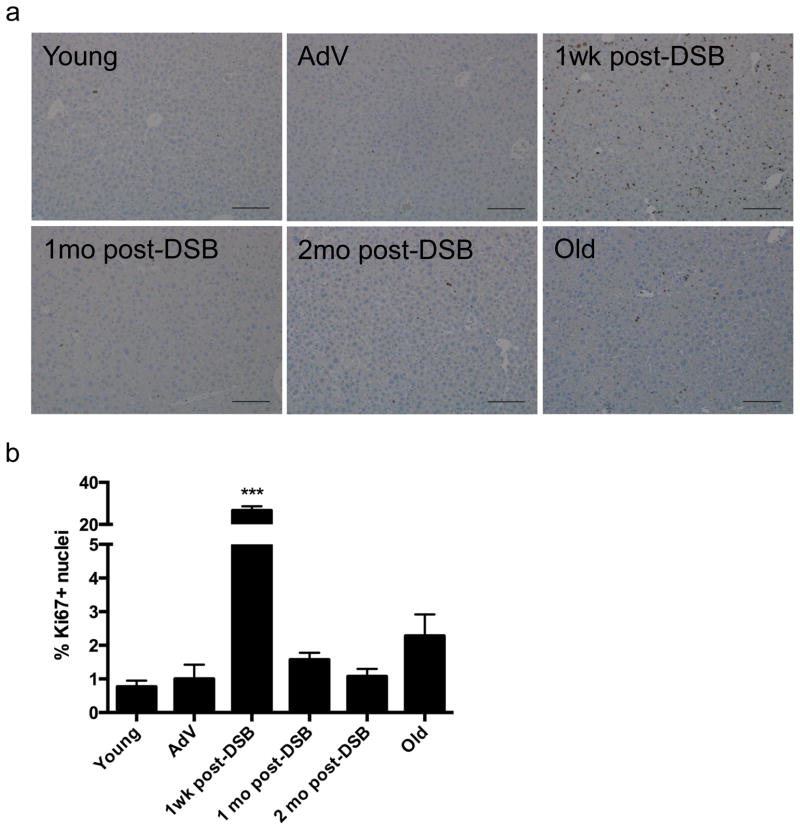
As suggested by the increase in apoptosis after the first wave of DSB induction, it is likely that somatic stem cell compartments are activated to stimulate liver regeneration. To confirm that cell proliferation occurred in these livers, we immunostained for a well-known nuclear proliferation marker, Ki67, shortly after DSB induction. We observed a wave of proliferation, ~26% Ki67+ nuclei, one week after the first DSB induction (Fig. 4). However, at one and two months after DSB induction, signs of significant proliferation had subsided (~1.6% and 1.1%, respectively) to levels almost similar to that of control mice, ~1%. These data indicate that the mouse liver undergoes robust regeneration after the first wave of DSBs, most likely to repopulate the great number of cells lost by apoptosis. We also observed a slight increase in Ki67 staining in 28-month-old normally aged mice, although this increase was not statistically significant. However, it is tempting to speculate that such a late increase in cellular proliferation reflects the regeneration of spontaneously damaged liver tissue that occurs even at old age.
Figure 4. Cell proliferation after DSB treatment.
(a) Liver sections were immunostained for Ki67 after SacI adenoviral tail-vein injection as indicated and images acquired at 10X magnification. Scale bar = 150 μM. (b) Quantification of the percent of Ki67+ nuclei of total nuclei from 3 images per n, where AdV, 1mo, 2mo post-DSB n=4, 24h V+D n=2, and Old n=3. Data represent the mean ± s.e.m. P values were determined using students unpaired t-test. *** P < 0.001
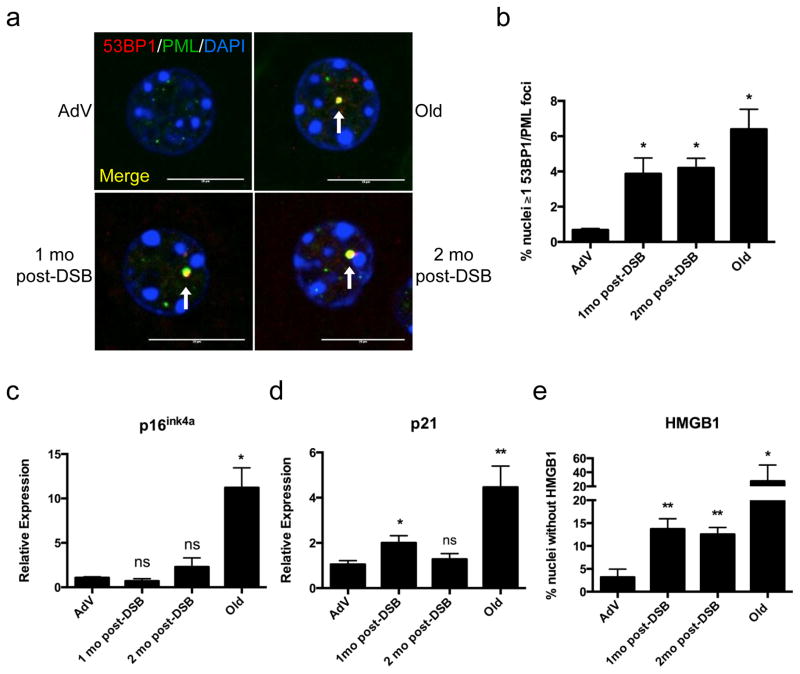
DSBs induce senescence-associated foci of persistent DNA damage
In addition to an increase in apoptotic cells, senescent cells have also been found at increased frequency in aged tissues 8,10. Indeed, cellular senescence is now considered a major hallmark of in vivo aging 31. Senescent cells are well characterized by persistent foci of γ-H2AX and 53BP1, markers of DSBs 10,32. The 53BP1 foci in senescent cells are termed DNA Segments with Chromatin Alterations that Reinforce Senescence (DNA-SCARS) 13, and are characterized by co-localization with PML nuclear bodies. Thus, we immunostained for 53BP1 and PML (Fig. 5a) one and two months after DSB induction, and compared 53BP1-PML co-localization to young AdV control and naturally aged mice. Mouse livers one and two months after DSB induction showed a significant increase in the number of nuclei with DNA-SCARS (4.2% and 3.9% of total hepatocytes, compared to 0.7% in AdV controls; Fig. 5b). The long-term presence of DNA-SCARS two months after DSB induction attests to the persistent nature of DSBs. In addition, we show for the first time that DNA-SCARS increase in the liver of normally aged mice (6.4% of total hepatocytes).
Figure 5. DSBs trigger senescence in mouse liver.
(a) Representative images of DNA-SCARS analyzed by confocal microscopy; 53BP1 (red), PML nuclear bodies (green), and DAPI (blue). White arrows indicate DNA-SCARS (co-localized PML and 53BP1). Scale bar = 10 μM. (b) Quantification of nuclei with ≥ 1 colocalized 53BP1 and PML focus. Data shown are the mean ± s.e.m. of all biological replicates for each cohort, where AdV, 1mo, 2mo post-DSB n=4, and Old n=3; >300 nuclei were scored per n. (c, d) Quantitative real time PCR (qPCR) analysis of (c) p16ink4a and (d) p21 expression; Relative expression was calculated using the ΔΔCT method and normalizing to GAPDH expression levels. (e) Liver sections were immunostained for HMGB1 and scored as the percent nuclei lacking HMGB1 staining. For (c, d, e) data shown represent the mean ± s.e.m. of all biological replicates for each cohort, where AdV, 1mo, 2mo post-DSB n=4, and Old n=6 for each cohort and for (e) >1000 nuclei were scored per n. P values were calculated using student’s unpaired t-test except for (b) where the Kruskal-Wallis test to AdV samples followed by a post-hoc Dunn’s test was performed. * P < 0.05, ** P < 0.01, *** P < 0.001, ns = not significant.
DSBs induce markers of cellular senescence
To confirm and further characterize DSB-induced cellular senescence, we examined three senescence markers: expression of p16ink4a, p21 33, and loss of nuclear high mobility group box 1 (HMGB1) 34. We found no statistically significant change in p16ink4a expression at one or two months after DSB induction (Fig. 5c). However, in concordance with previous studies 33, we did observe a significant increase in p16ink4a expression in livers of normally aged animals (Fig. 5c). By contrast, p21 expression increased ~2-fold one-month after DSB Induction, followed by a slight decline one month later (Fig. 5d). p21 expression was also increased, ~4.5-fold, in normally aged livers, as previously reported 35,36. Recently, loss of nuclear HMGB1, a chromatin associated protein, and its secretion was shown to be a hallmark of many senescent cells 34. HMGB1 is an alarmin, a dual function protein that can promote inflammation 37,38. Immunostaining for HMGB1 showed significant loss of nuclear HMGB1 in livers one and two months after DSB induction (~14% and ~13%, respectively; Fig. 5e). Normally aged livers also showed a significant loss of nuclear HMGB1 (~20% compared to ~3% for young controls), but as with many aging characteristics there was considerably high variation between aged animals. With the exception of p16, an increase of which could not be detected, these results point towards an accumulation of senescent cells in response to DSBs.
DSBs induce age-related changes in gene expression profiles
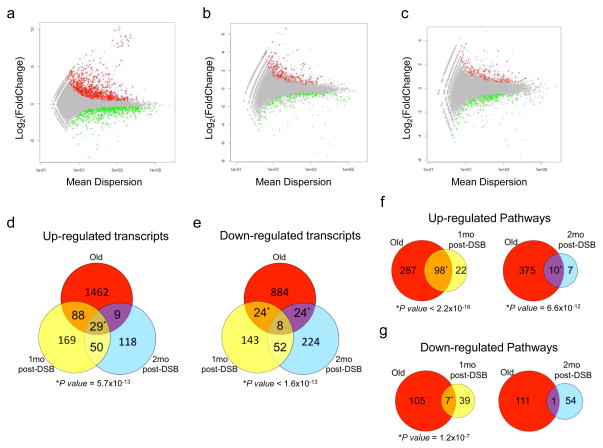
Because global gene expression profiles are excellent biomarkers of aging 30,39,40, we compared gene expression patterns in livers from normally aged mice and in mice at one and two months after DSB induction with those in livers from young AdV control mice. To date, most gene expression studies of aging have utilized microarrays. To more sensitively detect potential similarities between normal and DSB-induced liver aging, we used the more powerful method of RNA-seq. We sequenced directional libraries of liver from 3 young mice, 2 young AdV controls, 3 normally aged mice, and 3 mice at one and two months after DSB induction to an average depth of ~30 million paired-end reads. Reads were then aligned using GSNAP 41, with >80% mapping to the reference mouse genome (Supplementary Table 1). We assembled and counted transcripts using the Python-based program HTSeq, and called differentially expressed transcripts using DESeq, which utilizes a negative binomial test for calling differential expression between samples 42. Because expression differences during aging tend to be subtle 43, we refrained from setting an arbitrary cut-off for fold-changes, allowing us to detect all possible effects of DSBs. In addition, we performed two-dimensional principal component analysis after sample normalization and variance stabilization transformation to ensure no technical batch effects were present (Supplementary Fig. 5).
A total of 2,528 transcripts were differentially expressed in liver from normally aged mice, relative to young mouse liver, with 1,588 of these transcripts being upregulated and 940 downregulated (Fig. 6a). After comparing liver from young, AdV control mice to one-month post-DSB livers, 563 transcripts were found differentially expressed, with 336 being upregulated and 227 downregulated (Fig. 6b). The same comparison with two-month post-DSB mice showed 514 differentially expressed transcripts, with 206 being upregulated and 308 downregulated (Fig. 6c).
Figure 6. RNA-seq gene expression profiles of mouse liver after DSB induction.
Differential expression plots of the normalized mean count dispersion of transcripts versus normalized log2(Fold Change) for (a) Young versus Old, (b) AdV versus 1 mo post-DSB, and (c) AdV versus 2 mo post-DSB. P value cutoff is 0.05 for transcripts either significantly upregulated (red) or downregulated (green). (d–g) Venn diagrams. (d) upregulated or (e) or downregulated transcript overlaps of Old, 1 mo post-DSB, and 2 mo post-DSB as compared to their respective controls. (f, g) Significantly differentially expressed transcripts were subjected to gene ontology analysis for biological process using DAVID. (f) Overlap of 1 mo post-DSB or 2 mo post-DSB significantly upregulated or (g) downregulated pathways, compared to Old. P value of overlaps were determined using a binomial distribution test for 37,310 annotated transcripts and 13,301 GO terms for biological processes at time analysis was performed.
We analyzed transcript patterns from these three comparisons to assess the overlap between normal and DSB-induced aging. For upregulated transcripts, the one month post-DSB livers showed more overlap with normal old mouse liver than the two-month post-DSB livers, with a small, but highly significant overlap of 29 genes that were upregulated in all three groups (Fig. 6d). Interestingly, comparing the overlap of downregulated genes, both one- and two-months post-DSB mice independently showed significant overlap with old mice, but the combined transcriptional overlap was minimal and thus did not reach significance (Fig. 6e). Together, these results indicate that DSBs significantly contribute to but do not fully recapitulate the gene expression pattern of normally aged liver.
We performed gene ontology analysis on all differentially expressed transcripts from all three groups using DAVID (Database for Annotation, Visualization and Integrated Discovery) 44,45. For one- and two-month post-DSB livers this resulted in significant overlap for 98 and 10, respectively, biological processes with those upregulated by normal aging (Fig. 6f). Many of these up-regulated pathways are enriched for inflammatory and cell signaling processes (Table 2), including the immune response (Gene ontology (GO) term 0006955), leukocyte proliferation (GO: 0070661) and chemotaxis (GO: 0006935), which corroborates previous findings from meta-analyses of gene expression profiles of aged liver 43. Interestingly, regulation of apoptosis (GO: 0042981) in the one month post-DSB livers also significantly overlapped with normal aging, confirming our result of delayed apoptosis after DSB induction (Fig. 3). Other significantly upregulated pathways of interest, which overlap between DSB-induced and normal aging in liver, are cell adhesion (GO: 0007155) and regulation of actin filament polymerization (GO: 0030833), further providing evidence that DSBs induce a broad spectrum of normal age changes.
Table 2.
Top overlapping gene ontology pathways.
| GO term (biological process) | Old | 1mo post-DSB | 2mo post-DSB |
|---|---|---|---|
| Up Regulated | |||
| T cell activation (GO:0042110) | 9.4×10−13 | 5.2×10−11 | 2.0×10−3 |
| Immune response (GO:0006955) | 2.2×10−18 | 2.8×10−8 | NA |
| Cell activation (GO:0001775) | 5.5×10−18 | 4.6×10−8 | NA |
| Chemotaxis (GO:0006935) | 3.0×10−6 | 2.8×10−7 | 1.5×10−3 |
| T cell proliferation (GO:0042098) | 2.0×10−6 | 1.8×10−6 | 1.3×10−3 |
| Integrin-mediated signaling pathway (GO:0007229) | 3.4×10−4 | 9.0×10−6 | 2.8×10−3 |
| Lymphocyte proliferation (GO:0046651) | 3.0×10−5 | 2.1×10−5 | 4.1×10−3 |
| Leukocyte proliferation (GO:0070661) | 3.9×10−5 | 2.4×10−5 | 4.4×10−3 |
| Mononuclear cell proliferation (GO:0032943) | 3.9×10−5 | 2.4×10−5 | 4.4×10−3 |
| Cell cycle (GO:0007049) | 1.0×10−4 | 3.2×10−5 | 2.0×10−2 |
| Cell proliferation (GO:008283) | 5.1×10−5 | 1.5×10−4 | 1.2×10−2 |
| Regulation of apoptosis (GO:0042981) | 2.0×10−3 | 6.3 ×10−3 | NA |
| Cell adhesion (GO:0007155) | 1.8 ×10−5 | 1.8 ×10−3 | NA |
| Regulation of actin filament polymerization (GO:0030833) | 1.5 ×10−2 | 3.3 ×10−3 | NA |
| Down Regulated | |||
| Acute inflammatory response (GO:0002526) | 1.2×10−2 | 1.7×10−5 | NA |
| Steroid metabolic process (GO:0008202) | 3.7×10−3 | 2.2×10−4 | NA |
| Glucose metabolic process (GO:0006006) | 1.9×10−2 | 2.9×10−3 | NA |
| Monosaccharide metabolic process (GO:0005996) | 1.6×10−2 | 3.2×10−3 | 1.0×10−2 |
| Lipid biosynthetic process (GO:0008610) | 1.7×10−6 | 8.3×10−3 | NA |
The top 10 upregulated and top 5 downregulated overlapping gene ontology annotations for biological processes for 1 mo post-DSB or 2 mo post-DSB were compared to Old for upregulated and downregulated transcripts. P values were determined using Fisher’s exact test.
For pathways downregulated with normal aging we found distinctly less overlap, with 7 and 1 GO terms for one and two month post-DSB livers, respectively (Fig. 6f). Theses significantly downregulated pathways found to overlap between DSB-induced and normal liver aging were enriched for metabolic processes (Table 2), including monosaccharide (GO: 0005996), steroid metabolism (GO: 0008202), glucose metabolic process (GO: 0006006), and lipid biosynthetic process (GO: 0008610), also seen by others 30. This may point towards functional impairment of normal metabolism in both DSB-induced and normal aging.
These results are the first evidence that DSBs induce a broad spectrum of the same changes in transcript profile that also occurs during normal aging of the mouse liver.
Discussion
Taken together, our results indicate that DSBs alone are sufficient to cause part of the normal aging process in mouse liver. This finding is in keeping with the indirect evidence for DSBs as a potential driver of aging 7,9,46. Specifically, our data show that DSBs (1) induce most but not all pathological lesions in the livers of young animals that are normally only seen at old age; (2) result in increased apoptosis, cellular senescence and mitochondrial fusion in vivo, generally considered as part of the normal aging process; and (3) induce broad alterations in transcriptional expression profiles that overlap with many of the changes observed during normal aging of the liver, which confirm previously observed results obtained by others using microarrays. Of note, our observation of DNA-SCARS, never before reported in normally aged tissue, together with the evidence that DSBs induce cellular senescence 31,47, strongly suggest a primary role for DSBs in the normal aging process. We show that DSBs in vivo directly affect mitochondrial structure, which is in keeping with previous observations of mitochondrial defects in DNA repair deficient mice 48,49.
We conclude that DSBs can drive some aspects of the normal aging process, a conclusion that until now has been elusive due to the lack of models that allow studying “clean” DNA lesions, independent of possible side effects associated with genotoxic agents or progeroid human and mouse syndromes. The striking similarity between DSB-induced aging and its normal counterpart, at least in the liver, is remarkable given the great age difference between the treated young mice and the aged control group. Indeed, one might expect that many other aspects of normal aging, dependent on the extended time period of normal mouse life span and not necessarily due to DSBs, would confound the phenotypes found associated with DSB-induced aging so early in life.
Here, we analyzed only the liver, as the liver is known to be by far the main target after tail vein injection (Fig. 1c, Supplementary Fig. 1) 50. We also note that the side effects of SacI AdV treatment are minimal. As shown by the AdV controls, the DSB-inducing construct itself is not immunogenic and, in contrast to radiation or gene knockouts, side effects beyond the DSBs themselves are wholly absent.
Perhaps the strongest evidence that DSBs are capable of inducing a broad spectrum of normal age changes already at very early age is the significant overlap between gene expression profiles. This overlap in altered transcript profiles is not complete, but could be due, at least in part, to stochastic variation between animals, which in aged mice can be considerable 51. This may be responsible for some real overlap not reaching statistical significance. Indeed, the one and two month post-DSB patterns also differ from one another. However, it is conceivable that other, non-DSB, pro-aging factors play a role. For example, DSBs alone are apparently insufficient to accelerate lipofuscin accumulation. In this respect it is likely that the accumulation of protein aggregates and other age-related alterations in biological macromolecules drive aspects of the aging process independent of DNA damage 52. We also noted differences between normal and DSB-induced aging in cellular senescence markers. For example, p16ink4a expression was found by us and others 33, to increase significantly in normally aged liver, while no changes were observed in DSB-induced livers from young animals. It is plausible that the fraction of senescent cells in the DSB-induced livers was too low to detect a significant increase in p16ink4a. On the other hand, increased p21 as well as loss of nuclear HMGB1 were observed in both normal and DSB-induced, premature aging.
By what mechanism do DSBs induce distinct aging pathologies? One mechanism could be apoptosis. As we showed, an elevated rate of apoptosis persists at least two months after DSB induction, possibly affecting liver regenerative capacity. While cell loss alone is unlikely to explain all aspects of aging, widespread atrophy is certainly associated with normal aging. Second, DSBs can cause cellular senescence, which is emerging as a possible major cause of aging in vivo32,53. Senescence, despite being a safeguard against cancer, might cause age-related degeneration not only through a decrease in mitotic potential, thereby reducing regenerative capability, but also through the secretion of inflammatory cytokines 54, which can even promote hyperplastic growth in surrounding cells 47. Our observations of increased DNA-SCARS and loss of HMGB1 indicate an accumulation of senescent cells in response to DSBs in vivo. Furthermore, we provide evidence that clean DSBs can induce senescence, without persistent p16ink4a expression, and potentially dependent on a p53 response based on the observed increased in p21 and nuclear export of HMGB1. These markers, especially p21, may sustain the senescent state until further, irreversible phenotypes develop, such as changes in the epigenetic landscape 55. This may explain why we see a decrease in p21 two months after DSBs. While nuclear loss of HMGB1 may not fully cause a senescent state, its mechanism as an pro-inflammatory alarmin once exported out of the nucleus and ultimately out of the cell may partially cause the increase in activated macrophage infiltration due to its known interaction with IL-1β 38, thus reinforcing the SASP/inflammatory senescent phenotype regardless of p21 and p16ink4a expression. These results coupled with the increase in infiltrating lymphocytes and macrophages may explain the observed increase in inflammatory gene expression patterns. Moreover, previous studies have shown that unique respiratory bursts in macrophages can cause DNA damage in surrounding cells 56. Thus our evidence for persistent DNA damage and DNA-SCARS up to two months after the DSB treatment may well be sustained or even caused by the observed increase in infiltrating activated macrophages. Alternatively, the reduction in p21 two months after DSB induction may reflect the clearance of pre-malignant senescent hepatocytes by the immune system 57.
A third possible mechanism that could underlie the observed broad spectrum of gene expression profile changes is the demonstrated effect of DNA damage on metabolism. Indeed, similar alterations in lipid metabolism as we observed after DSB-induced aging have been reported for mouse models with defects in DNA repair 22,58.
Finally, when erroneously repaired, DSBs alter the genome or epigenome, which could explain some of the gene expression changes observed during both normal and DSB-induced aging 59.
In summary, our data on the molecular and cellular phenotypes triggered in mice upon the induction of clean DSBs, without side effects due to protein or lipid damage, enables — for the first time — the dissection of the component of aging that could be due to a defined DNA lesion alone. In keeping with indirect data on the likely pro-aging effect of DSBs, we show that DSBs can explain some of the chronic symptoms associated with physiological aging. Interestingly, our observation that aging symptoms are less severe at two months as compared to one month after treatment suggests that some of these phenotypes are reversible. To some extent this may be due to the short-term nature of the DSB induction. At such an early age, cell and tissue regenerative capacity is still high. However, similar phenotypes at old age may not be as reversible. Indeed, ageing is associated with a progressive decline in stem cell function, resulting in less effective tissue regeneration 60. While as yet we do not know the long-term effects of our DSB treatment, these could be similar to what is observed in adult survivors of pediatric cancer. Patients treated with harsh clastogens early in life see most adverse phenotypes disappear several months after the cessation of treatment only to return decades later, eerily mimicking premature aging 16,17. The experimental system described here should allow to systematically assess the long-term effects of DSBs in mice across tissues and test interventions to promote cell and tissue rejuvenation at different ages.
Methods
Adenoviral stock production
HEK 293A/tTS cells were cultured in DMEM supplemented with 10% Fetal Bovine Serum, 1% MEM non-essential amino acid, 4 mM L-glutamine, 1% penicillin-streptomycin, 1% sodium pyruvate. Adenoviral stocks, pAd/Tight-SEV5-CMV-rtTA (A/TSCR), were mass-produced in HEK 293A/tTS cells by infecting cells with crude A/TSCR virus at a MOI of 1. Cells were then incubated until a cytopathic effect of 90% was reached. Adenovirus was purified according to the Adeno-X™ Mega Purification Kit protocol (Clontech) and resuspended in 1X PBS.
Animals and tissue collection
All procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Albert Einstein College of Medicine. Three male Balb/C mice of age 27 months were obtained from the NIA (naturally aged mice), and allowed to recover one month after delivery before sacrificing. Male, 8-week old experimental and control mice were also obtained from the same NIA colony. When mice reached 12 weeks of age, doxycycline (DOX; 2mg/mL in 5% sucrose solution) was added to the drinking water 24 hours before receiving a tail-vein injection of .2mL of A/TSCR virus. Mice were removed from DOX 24 hours after the last tail-vein injection. Mice were sacrificed at the indicated intervals (24 hours, one week, one month, two months post-DSB) thereafter, liver was harvested and immediately fixed in 10% phosphate buffered formalin for up to 48 hours or flash frozen and kept at −80°C. Formalin-fixed tissues were paraffin embedded and 4.5μm sections were cut and used for immunohistochemistry and immunofluorescence.
Immunohistochemistry and immunofluorescence
Formalin-fixed paraffin embedded sections were deparaffinized, rehydrated, incubated in 0.01M citrate buffer (pH 6.0) at 95° C for 20 minutes and washed in 1X PBS according to standard protocols. Primary antibodies were anti-Histone H2A.X-S139ph at 1:1000 (Active Motif), anti-cleaved caspase-3 (Asp175) at 1:50 (Cell Signaling), anti-Ki67 at 1:400 (Dako), anti-IBA1 at 1:1500 (Wako). Secondary antibody was HRP conjugated goat anti-rabbit (Santa Cruz Biotechnology) at 1:1000. HRP activity was detected using a DAB substrate kit (Invitrogen). Liver sections were stained for pathological analysis according to established protocols using hematoxylin and eosin. For lipofuscin analysis, liver sections were deparaffinized, rehydrated, and mounted with mounting medium. Slides were then exposed to UV light and autofluorescent molecules (lipofuscin) assessed by a pathologist and given a score between 0–5 to correspond to aging pathologies. For immunofluorescence, liver sections were stained with anti-53BP1 (Bethyl Laboratories) at 1:500, anti-PML 36.1–104 (Millipore) at 1:50, anti-HMGB1 at 1:2000 (Abcam), and anti-TOM20 clone 2F8.1 (Millipore) at 1:500. Secondary antibodies were AlexaFluor 488 donkey anti-mouse and AlexaFluor 594 donkey anti-rabbit (Invitrogen) at 1:1000. Slides were counterstained with sudan black, mounted with ProLong Gold Antifade reagent with DAPI (Invitrogen) and allowed to cure overnight at room temperature.
Microscopy and analysis
Immunofluorescence images were acquired using a Leica SP5 laser scanning confocal microscope and a 40X oil objective with pinhole set to 1AU and line average set to 3, and analyzed using Volocity software; background thresholds were set based on young adenoviral control samples and antibody control samples. Immunohistochemistry images were acquired using a Nikon CoolScope at 10X or 20X magnification and analyzed using ImageJ software. HMGB1 immunofluorescence images were quantified using CellProfiler, an open-access image analysis program (www.cellprofiler.org).
Fluorescent in situ hybridization
Tissue FISH was performed according to a prior protocol and adapted for two color chromosome labeling 24,61. The BAC clones used were as follows: RP23-34K7 (Chr.1qA1) and RP23-16K15 (Chr.18qE1). Probes were labeled by nick translation using spectrum organge-dUTP and DY-415-dUTP. Liver sections were treated using Vysis Paraffin Pretreatment Reagent Kit (Abbott Molecular Inc., 02J02-032) with some modifications. Briefly, FFPE sections were baked at 56°C overnight on a slide warmer, deparaffinized by Citisolve (Thermo-Fisher) for 3× 10 minutes, dehydrated in 100% EtOH for 3× 10 minutes at room temperature, then air-dried. The slides were then treated with Pretreatment Buffer for 30 minutes at 80°C, washed with Wash Buffer for 5 minutes at room temperature, and then in purified water for 1 minute. Slides were immersed into Protease Solution for 18 minutes at 37°C, washed with purified water for 1 minute, then Wash Buffer for 5 minutes at room temperature. We dehydrated slides in a series of 70, 90, 100% ethanol. Co-denatured slides and probes at 76°C for 5 minutes; hybridized slides at 37°C overnight. Slides were placed into 2xSSC with 0.4% NP40 solution for 2 minutes at 72°C for detection, then air-dried. Slides were mounted with ProLong Gold Antifade reagent with DAPI (Invitrogen). FISH images were acquired using a Zeiss Axiovert 200 at 400X magnification.
qPCR
Total RNA was converted into cDNA using SuperScript III First-strand Synthesis Kit (Invitrogen) and 50 ng of random hexamers. qPCR was performed using 100 ng of cDNA and ABI StepOne Plus system for TaqMan® (ABI) assays. All calculations were performed using the ΔΔCT method, with TaqMan® assays normalized to GAPDH and biological replicate values representing the mean of technical triplicates. Primer and probes are as follows: p16Ink4a forward 5′-CCCAACGCCCCGAACT-3′, reverse 5′-GCAGAAGAGCTGCTATGTGAA-3′, probe 5′-TTCGGTCGTACCCCGATTCAGGTG-3′; p21 assay ID Mm04205640_g1; mouse GAPDH assay ID Mm99999915_g1.
Directional RNA-sequencing
Flash-frozen tissues were homogenized with 1.4 nm ceramic bead matrix and Trizol (Invitrogen) using the MP FastPrep-24 system. Total RNA quality was checked on an Agilent 2100 Bioanalyzer; only samples with a RNA Integrity Number greater than 8.5 were used for subsequent analysis. Total RNA was treated with DNaseI, column purified using the miRNeasy Mini Kit (Qiagen), and depleted of ribosomal RNA with Ribo-Zero Magnetic Gold Kit (Epicentre), followed by ethanol precipitation. Depleted RNA was converted to cDNA using SuperScript III First-Strand Synthesis Kit (Invitrogen) with 80 ng random hexamers and 50 μM oligo dT and subsequently ethanol precipitated. Single-stranded cDNA was converted to dsDNA by DNA polymerase I while incorporating dU/VTPs (10mM). Samples were fragmented in 1X TE to 200–300 bp using Covaris. After fragmentation, samples were purified using the MinElute PCR purification kit (Qiagen). Fragmented samples underwent standard end-repair, dA-tailing, and adapter ligation using Illumina TruSeq adaptors for multiplexing. Adaptor-ligated cDNA was treated with uracil-DNA glycosylase followed by enrichment PCR using Q5 polymerase (New England Biolabs) for 18 cycles. Libraries were size selected for 150–600 bp on a 2% low-melt ultra low range agarose gel stained with SYBR Gold (Invitrogen) to eliminate adaptor dimers. Purified libraries were clustered (7 samples per flow cell lane) and sequenced on an Illumina HiSeq2000 for 100bp paired-end reads.
Gene expression analysis
Pass filter sequences were aligned with GSNAP v2013-01-23 according to default settings with novel splicing, using the mm9_all reference genome 41. Counts were generated for each sample using HTSeq v0.5.3p9. Combined HTSeq counts were analyzed using DESeq v1.10.1 42 in RStudio v0.97.312. Significant differentially expressed genes were entered into DAVID v6.7 for pathway and gene ontology enrichment analysis using an EASE score of 0.05 with a gene count minimum of 3 for each GOTERM_BP_FAT 45. A Fisher’s exact test value below 0.05 was considered statistically significant for gene ontology pathway analysis. P value of overlap was determined using binomial distribution test.
Statistical methods
Statistical analyses for experiments not involving RNA-seq data were performed either in R v2.15.2 using RStudio v0.97.312 or in GraphPad Prism 6. For most analyses (unless otherwise stated) the mean value is shown with the standard error of the mean of biological replicates. P values were calculated using tests for parametric, student’s unpaired t-test, or non-parametric, Mann-Whitney test. Multiple group comparisons were performed using the Kruskal-Wallis test followed by a post-hoc Dunn’s test to correct for multiple testing. A significance value cut off of P < 0.05 was set for all tests.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant AG17242, the Ellison Medical Foundation, the Glenn Foundation, and the Sue Golding Graduate Division of the Albert Einstein College of Medicine. We thank Dr. Rani Sellers and the Histopathology Core, Dr. Shahina Maqbool and the Epigenomics Core, Dr. Cristina Montagna and the Molecular Cytogenetics Core, and the Analytical Imaging Facility of the Albert Einstein College of Medicine for their help and suggestions. We are also grateful to Dr. Sameh Youssef of the Dutch Molecular Pathology Center, Faculty of Veterinary Medicine, for performing the lipofuscin analysis. We also thank Brent Calder for his assistance with the analysis of the RNA-seq results and Dr. Tao Wang for assistance and recommendations in biostatistics.
Footnotes
AUTHOR CONTRIBUTIONS
RW, AM, and JV conceived and designed the experiments. RW carried out all experiments. SC, RL, and JC helped in senescence experiments. BM helped in performing statistical and RNA-seq data analysis. AdB performed pathology analysis and AdB and HvS assisted with the data analysis. RW and JV wrote the manuscript, which was read, edited and approved by all authors.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Accession codes
RNA-seq data have been deposited into the Sequence Read Archive under accession codes SRP053350 and SRP053429.
References
- 1.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 2.Mine-Hattab J, Rothstein R. Increased chromosome mobility facilitates homology search during recombination. Nature cell biology. 2012;14:510–517. doi: 10.1038/ncb2472. [DOI] [PubMed] [Google Scholar]
- 3.Soutoglou E, Misteli T. On the contribution of spatial genome organization to cancerous chromosome translocations. Journal of the National Cancer Institute. Monographs. 2008:16–19. doi: 10.1093/jncimonographs/lgn017. [DOI] [PubMed] [Google Scholar]
- 4.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annual review of biochemistry. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 5.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annual review of biochemistry. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697–700. doi: 10.1038/35015097. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Mitchell JR, Hasty P. DNA double-strand breaks: a potential causative factor for mammalian aging? Mechanisms of ageing and development. 2008;129:416–424. doi: 10.1016/j.mad.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedelnikova OA, et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nature cell biology. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 9.Dolle ME, et al. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nature genetics. 1997;17:431–434. doi: 10.1038/ng1297-431. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, et al. DNA damage response and cellular senescence in tissues of aging mice. Aging cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 11.Rube CE, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PloS one. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White RR, et al. Double-strand break repair by interchromosomal recombination: an in vivo repair mechanism utilized by multiple somatic tissues in mammals. PloS one. 2013;8:e84379. doi: 10.1371/journal.pone.0084379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodier F, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. Journal of cell science. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolle ME, et al. Broad segmental progeroid changes in short-lived Ercc1(−/Delta7) mice. Pathobiology of aging & age related diseases. 2011;1 doi: 10.3402/pba.v1i0.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oeffinger KC, et al. Chronic health conditions in adult survivors of childhood cancer. The New England journal of medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 17.Ness KK, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the st jude lifetime cohort study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maslov AY, Metrikin M, Vijg J. A dual-activation, adenoviral-based system for the controlled induction of DNA double-strand breaks by the restriction endonuclease SacI. BioTechniques. 2009;47:847–854. doi: 10.2144/000113237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeiffer P, Goedecke W, Obe G. Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis. 2000;15:289–302. doi: 10.1093/mutage/15.4.289. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Sato K, Hamada H. Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. Journal of virology. 2003;77:2512–2521. doi: 10.1128/JVI.77.4.2512-2521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijnhoven SW, et al. Accelerated aging pathology in ad libitum fed Xpd(TTD) mice is accompanied by features suggestive of caloric restriction. DNA Repair (Amst) 2005;4:1314–1324. doi: 10.1016/j.dnarep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Gregg SQ, et al. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55:609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan AW, et al. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faggioli F, Wang T, Vijg J, Montagna C. Chromosome-specific accumulation of aneuploidy in the aging mouse brain. Human molecular genetics. 2012;21:5246–5253. doi: 10.1093/hmg/dds375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Tauchi H. The formation of enlarged and giant mitochondria in the aging process of human hepatic cells. Acta pathologica japonica. 1975;25:403–412. doi: 10.1111/j.1440-1827.1975.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 26.Velarde MC, Flynn JM, Day NU, Melov S, Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging. 2012;4:3–12. doi: 10.18632/aging.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh Y, Lee KA, Kim WH, Han BG, Vijg J, Park SC. Aging alters the apoptotic response to genotoxic stress. Nature Medicine. 2001;8:3–4. doi: 10.1038/nm0102-3. [DOI] [PubMed] [Google Scholar]
- 30.Park JY, et al. Homeostatic imbalance between apoptosis and cell renewal in the liver of premature aging Xpd mice. PloS one. 2008;3:e2346. doi: 10.1371/journal.pone.0002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewitt G, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nature communications. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. The Journal of clinical investigation. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davalos AR, et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. The Journal of cell biology. 2013;201:613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards MG, et al. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding G, et al. Tubular cell senescence and expression of TGF-beta1 and p21(WAF1/CIP1) in tubulointerstitial fibrosis of aging rats. Experimental and molecular pathology. 2001;70:43–53. doi: 10.1006/exmp.2000.2346. [DOI] [PubMed] [Google Scholar]
- 37.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature reviews. Immunology. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 38.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 39.Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001;98:10630–10635. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonker MJ, et al. Life spanning murine gene expression profiles in relation to chronological and pathological aging in multiple organs. Aging cell. 2013;12:901–909. doi: 10.1111/acel.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26:873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennis G, Jr, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 45.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 46.Lans H, Hoeijmakers JH. Genome stability, progressive kidney failure and aging. Nature genetics. 2012;44:836–838. doi: 10.1038/ng.2363. [DOI] [PubMed] [Google Scholar]
- 47.Campisi J. Aging, cellular senescence, and cancer. Annual review of physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cogger VC, et al. Liver aging and pseudocapillarization in a Werner syndrome mouse model. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69:1076–1086. doi: 10.1093/gerona/glt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirschner K, Singh R, Prost S, Melton DW. Characterisation of Ercc1 deficiency in the liver and in conditional Ercc1-deficient primary hepatocytes in vitro. DNA repair. 2007;6:304–316. doi: 10.1016/j.dnarep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Smith TA, et al. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nature genetics. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 51.Bahar R, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 52.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature cell biology. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cruickshanks HA, et al. Senescent cells harbour features of the cancer epigenome. Nature cell biology. 2013;15:1495–1506. doi: 10.1038/ncb2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chong YC, Heppner GH, Paul LA, Fulton AM. Macrophage-mediated induction of DNA strand breaks in target tumor cells. Cancer research. 1989;49:6652–6657. [PubMed] [Google Scholar]
- 57.Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher B, et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS genetics. 2008;4:e1000161. doi: 10.1371/journal.pgen.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vijg J, Suh Y. Genome instability and aging. Annual review of physiology. 2013;75:645–668. doi: 10.1146/annurev-physiol-030212-183715. [DOI] [PubMed] [Google Scholar]
- 60.Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nature cell biology. 2011;13:506–512. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faggioli F, Vijg J, Montagna C. Four-color FISH for the detection of low-level aneuploidy in interphase cells. Methods Mol Biol. 2014;1136:291–305. doi: 10.1007/978-1-4939-0329-0_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.