Abstract
Reading is a critical life skill in the modern world. The neural basis of reading incorporates a distributed network of cortical areas and their white matter connections. The cerebellum has also been implicated in reading and reading disabilities. However, little is known about the contribution of cerebellar white matter pathways to major component skills of reading. We used diffusion magnetic resonance imaging (dMRI) with tractography to identify the cerebellar peduncles in a group of 9–17 year old children and adolescents born full term (n=19) or preterm (n=26). In this cohort, no significant differences were found between fractional anisotropy (FA) measures of the peduncles in the preterm and full term groups. FA of the cerebellar peduncles correlated significantly with measures of decoding and reading comprehension in the combined sample of full term and preterm subjects. Correlations were negative in the superior and inferior cerebellar peduncles and positive in the middle cerebellar peduncle. Additional analyses revealed that full term and preterm groups demonstrated similar patterns of reading associations within the left SCP, MCP and left ICP. Partial correlation analyses showed that distinct sub-skills of reading were associated with FA in segments of different cerebellar peduncles. Overall, the present findings are the first to document associations of microstructure of the cerebellar peduncles and the component skills of reading.
Keywords: Cerebellum, White Matter, Reading, Diffusion Tensor Imaging, Tractography, preterm birth, prematurity
Introduction
Reading is an essential skill for academic and occupational success in the modern world. It is comprised of two related components: the ability to decode single words, a process of mapping orthographic information into phonological information, and the ability to comprehend, a process of extracting meaning from written words and sentences (Hoover and Gough 1990). Skilled reading is supported by a distributed network of cortical brain areas and the white matter fiber pathways that connect them (Deheane 2009; Price 2012; Vandermosten, et al. 2012; Wandell and Yeatman 2013). Structures in the cerebellar cortex are also implicated in reading and its components (Desmond and Fiez 1998; Stoodley and Schmahmann 2009). Cerebellar regions link primarily to contralateral cerebral association cortices with an orderly topographic mapping (Buckner, et al. 2011). Evidence from anatomical mapping and functional connectivity studies suggests that regions within the anterior and posterior lobes of the cerebellum contribute to reading via connections to associative cortices of the frontal, parietal, and temporal lobes (Buckner, et al. 2011, D'Angelo and Casali 2012; Koziol, et al. 2014; Price 2012; Ramnani 2006; Stoodley 2012). However, studies have yet to establish whether the structural properties of the cerebellar peduncles are associated with the core skills of reading. Attaining this knowledge would deepen current understanding of the neural bases of reading and the role of cerebellar white matter connections in specific aspects of human cognition.
The first imaging studies to implicate the cerebellum in cognitive processes were early positron emission tomography (PET) studies involving single word reading (Peterson, et al. 1988; Peterson, et al. 1989). Since these initial studies, numerous other functional imaging studies have identified several regions within the left and right hemispheres of the cerebellum, particularly lobules VI and VII of the posterior lobes, that are associated with processing orthographic, phonological, and semantic information during reading tasks (Booth, et al. 2007; Price 2012; Stoodley 2012; Turkeltaub, et al. 2002). Neuroimaging studies have also identified important differences in cerebellar activation and structural abnormalities in cerebellar gray and white matter volumes that are associated with impaired reading abilities, including those observed in children and adults with dyslexia (Eckert, et al. 2003; Eckert, et al. 2005; Fernandez, et al. 2013; Laycock, et al. 2008; Leonard, et al. 2001; Stoodley and Stein 2011). For example, adult dyslexics demonstrate diminished rightward asymmetries in cerebellar gray matter volume as compared to typical readers (Rae, et al. 2002). Based on these and related findings, the cerebellar theory of dyslexia posits that mild cerebellar dysfunction is at the biological root of dyslexia (Nicholson, et al 2001; Fawcett 2011). The theory attempts to explain dyslexia as failure of skill automatization, drawing upon differences in muscle tone and postural instability as well as differences in blood flow to the cerebellar cortex and vermis in typical versus dyslexic readers. However, the role of the cerebellum in reading and its disorders is still debated (Barth, et al. 2010), and its contributions to reading may involve processes such as articulatory monitoring process during overt reading (Ben-Yehudah and Fiez 2008), amplification and refinement of phonological representations (Booth, Wood et al., 2007), or word retrieval (Frings, et al. 2006; Jansen, et al. 2005).
The cerebellar cortices, including regions activated during reading tasks (Booth, et al. 2007; Price 2012; Stoodley 2012; Turkeltaub, et al. 2002), communicate with subcortical structures, thalamic nuclei, motor and associative cortices of the cerebrum via afferent and efferent white matter tracts that course through the three major pathways of the cerebellum; the superior, middle, and inferior cerebellar peduncles (SCP, MCP, ICP, respectively; Naidich, et al. 2009). The superior cerebellar peduncles (SCP) contain primarily efferent pathways that emerge from the deep cerebellar nuclei (i.e., dentate, emboliform, fastigial and globase), pass through the ipsilateral superior cerebellar peduncle into the dorsal pons, decussate at the level of the inferior colliculus, and travel to the thalamus and then to the contralateral cerebral cortex. The middle cerebellar peduncle (MCP) is a major afferent pathway that carries input fibers from the contralateral cerebral cortex via pontine nuclei across the midline of the cerebellum to the contralateral cerebellar cortex (Naidich, et al. 2009). The inferior cerebellar peduncles (ICP) contain mostly afferent pathways, feeding signals from the spine and the olivary nucleus into the cerebellum, as well as efferent pathways from the cerebellum to the vestibular nuclei (Naidich, et al. 2009). The ICP has a distinctive branching pattern in which each branch terminates on a single Purkinje neuron (Fujita and Sugihara 2013).
A common method for assessing the microstructural properties of white matter is diffusion magnetic resonance imaging (dMRI). The predominant measure derived from dMRI measurements to index white matter microstructure is fractional anisotropy (FA), which represents the level of directional preference in the pattern of water diffusion at any voxel. FA is known to be affected by several tissue properties, including myelin composition, axonal density, directional coherence and axonal thickness (Alexander, et al. 2007). Reading abilities in both children and adults have been shown to be associated with FA of the left arcuate fasciculus, temporal callosal pathways, fronto-occipital, uncinate, and inferior longitudinal fasciculi (Beaulieu, et al. 2005; Cummine, et al. 2013; Deutsch, et al. 2005; Dougherty, et al. 2007; Klingberg, et al. 2000; Niogi and McCandliss 2006; Odegard, et al. 2009; Richards, et al. 2008; Steinbrink, et al. 2008; Yeatman, et al. 2011). The direction of association of FA and reading skill is positive in many studies (Beaulieu, et al. 2005; Cummine, et al. 2013; Deutsch, et al. 2005; Klingberg, et al. 2000; Niogi and McCandliss 2006; Richards, et al. 2008; Steinbrink, et al. 2008) but also negative in several studies (Dougherty, et al. 2007; Odegard, et al. 2009; Yeatman, et al. 2011).
To date, white matter properties of the cerebellar peduncles have not been examined in association with reading skills in children. This oversight has occurred, in part, because certain dMRI and volumetric methods are poorly suited to reliably identify, co-register, and characterize the small and intersecting pathways of the cerebellum (Bookstein 2001; Davatzikos 2004). It is possible to overcome these barriers and identify the cerebellar peduncles within individual subjects using diffusion-based tractography algorithms (Stieltjes, et al. 2001). Recent computational methods provide the ability to go beyond a single mean FA value for each white matter tract, and instead, generate tract profiles that characterize FA as it varies along the trajectory of the tract, thus achieving enhanced sensitivity and accuracy (Gong, et al. 2005; Yeatman, et al. 2012; Yeatman, et al. 2011).
In the present study, we sought to determine whether structural properties of the cerebellar peduncles would demonstrate associations with reading skills. To explore these associations, we used the largest sample we had available, a sample of healthy children and adolescents born full term or preterm in whom we had previously acquired both behavioral measures of reading and dMRI scans. We know that preterm children in this particular sample scored in the normal range in various tests of cognition, language and reading (Lee, et al. 2011), differed significantly in certain cerebral white matter properties compared to full term peers (Feldman, et al. 2012a), and showed positive correlations between FA and reading abilities in several cerebral white matter regions, including the corpus callosum, right forceps major and forceps minor, right and left inferior frontal-occipital, inferior longitudinal, superior longitudinal, and uncinate fasciculi (Feldman, et al., 2012b). Neither the microstructural characteristics of the cerebellar peduncles nor their structure-function associations have been explored in the current sample.
Children born preterm represent a potentially interesting group to contrast with children born full term because they are at-risk for deficits in reading (Aarnoudse-Moens, et al. 2009), injury to cerebral white matter structures (Anderson, et al. 2003; Back 2006; Volpe 2009) and injury to the cerebellum (Limperopoulos, et al. 2010; Limperopoulos, et al. 2005a; Limperopoulos, et al. 2005b). Prematurity-related cerebellar injury has also been linked to secondary impairments in remote cerebral cortical growth and functional disabilities (Johnson, et al. 2005; Limperopoulos, et al. 2005a,b). However, the extent that prematurity may affect the development of white matter tracts of the cerebellar peduncles is not currently well understood.
The overall goals of the present study were to determine whether structural properties of the cerebellar peduncles differ between children born preterm and full term, and whether the same structural properties correlate with two core components of reading: decoding and comprehension. To achieve these goals, we applied diffusion-based tractography and isolated the left and right SCP, MCP, and left and right ICP in each of the participants. Using this approach, we obtained a summary measure, mean tract-FA, and a FA profile, depicting FA variations along the trajectory of each tract. Structural lateralization of the peduncles was evaluated as well, by comparing FA of the left and right tracts for each of the peduncles. Mean tract-FA, FA profiles, and tract-lateralization were compared for each of the cerebellar peduncles between the full term and preterm groups. We anticipated group differences in the structural properties of the cerebellar peduncles, based on prior dMRI evidence for group-based variations in several cerebral white matter tracts (Feldman, et al. 2012b), and based on the results of studies that found volumetric differences documented in the cerebellum of prematurely born children (Limperopoulos, et al. 2010; Limperopoulos, et al. 2005a; Limperopoulos, et al. 2005b; Srinivasan, et al. 2006).
Our next goal was to assess the relation between FA of the cerebellar peduncles and performance on tasks probing decoding and reading comprehension. Associations between FA of the cerebellar peduncles and reading measures were first examined in the combined sample of full term and preterm children, in order to identify the tracts and tract regions demonstrating significant correlations. We then considered whether the associations between FA and reading are consistently found in each of the groups. Based on their similar performance in reading (Feldman, et al., 2012b), we anticipated similar patterns of association in the two groups. We followed up with several analyses that examined the selectivity of these associations. This was achieved by (1) calculating the association of FA with a general measure of non-verbal IQ and (2) calculating partial correlations with one component of reading while controlling for the other, and vice versa. Finally, to interpret the direction (positive/negative) of the correlations between FA and reading, we examined whether reading was associated with axial diffusivity or radial diffusivity, the two parameters that contribute to FA.
We hypothesized that FA of the SCP and the MCP would demonstrate significant associations with both decoding and comprehension. The rationale for this hypothesis was that these pathways likely convey afferent and efferent signals between the cerebellum and association cortices of the frontal, parietal, and temporal lobes, (D'Angelo and Casali 2012; Koziol, et al. 2014; Ramnani 2006) implicated in reading (Booth, et al. 2007; Price 2012; Turkeltaub, et al. 2002). We considered the left and right cerebellar tracts separately because many functional imaging studies observe lateralized activity in the right cerebellum during reading (Marien, et al. 2001; Price 2012) and because dyslexia has been associated with abnormal lateralization of the cerebellar gray matter volume (Rae, et al. 2002). We did not anticipate substantial associations between FA within the ICP and reading measures, given anatomical and physiological evidence that the ICP conveys mostly sensorimotor information to the cerebellum via spinocerebellar and olivocerebellar connections (Error! Hyperlink reference not valid.; Naidich et al., 2009).
Experimental Procedures
Subjects
Participants included children and adolescents between the ages of 9–17 years old born either full term (n= 19; 9 males, mean age =12.9 ±2.2) or preterm (n=26, 13 males, mean age = 12.8 ± 2.3) who had enrolled in the Palo Alto, CA site of a larger multi-site study of prematurity outcomes (Feldman, et al. 2012a; Feldman, et al. 2012b; Lee, et al. 2011). Preterm birth was defined as gestational age < 36 weeks and birth weight < 2500 grams. Exclusion criteria included active seizures, complications of a ventriculoperitoneal shunt, congenital malformations, meningitis or encephalitis, receptive vocabulary standard score < 70, sensory impairments or a native language other than English. Preterm subjects in the present sample were high-functioning, scoring within the normal range for their age on measures of intelligence and reading (see Table 1). We excluded from analysis one child who had no arcuate fasciculus bilaterally, had very abnormal values of FA throughout the brain, and who was described in a case study (Yeatman and Feldman 2013). Four subjects born preterm had ventricular dilation on MRI. In three of them, on whom we had neonatal records, Grade III or IV intraventricular hemorrhages were documented on neonatal ultrasounds. However, review by a neuroradiologist found no evidence of cerebellar injury in any of these subjects. We therefore included these participants in the preterm sample.
Table 1.
Subject Demographics and Mean Tract-FA Measures
| Behavioral Measures | Preterm (n = 26) | Full Term (n=19) | t or X2 | ||
|---|---|---|---|---|---|
| Boys -- n (%) | 13 | (50) | 9 | (47.3) | 0.03 |
| Low maternal education -- n (%) | 3 | (11.5) | 6 | (31.5) | 2.76 |
| Mean age in years (stdev) | 12.80 | (2.3) | 12.9 | (2.2) | .149 |
| Mean gestational age in weeks (stdev) | 28.2 | (2.2) | 39.2 | (1.1) | 21.6* |
| Mean birth weight in grams (stdev) | 1159.1 | (427.4) | 3153.9 | (407.0) | 15.9* |
| WJ-III Basic Reading Skills Cluster, SS (stdev) | 105.3 | (13.4) | 106.7 | (10.0) | .402 |
| WJ-III Passage Comprehension, SS (stdev) | 102.5 | (12.7) | 108.1 | (14.0) | 1.4 |
| Performance IQ, WASI, SS | 106.2 | (14.5) | 107.3 | (12.9) | .261 |
| Mean Tract-FA | F | ||||
| SCP-L mean FA (stdev) | 0.34 | (0.03) | 0.33 | (0.05) | 1.92 |
| SCP-R mean FA (stdev) | 0.34 | (0.04) | 0.32 | (0.04) | 1.92 |
| MCP mean FA (stdev) | 0.50 | (0.06) | 0.48 | (0.04) | 1.92 |
| ICP-L mean FA (stdev) | 0.46 | (0.04) | 0.44 | (0.04) | 1.92 |
| ICP-R mean FA (stdev) | 0.45 | (0.05) | 0.45 | (0.04) | 1.92 |
P < 0.05
stdev = standard deviation
SS = standard score
WJ-III = Woodcock-Johnson Tests of Achievement – 3rd Edition
WASI = Wechsler Abbreviated Scale of Intelligence
SCP = Superior Cerebellar Peduncle
MCP = Middle Cerebellar Peduncle
ICP = Inferior Cerebellar Peduncle
FA = fractional anisotropy (mean calculated across the tract)
L = left
R = right
The Stanford University institutional review board approved this study and consent procedures. A parent or legal guardian provided informed written consent and children provided written assent. Participants were compensated for participation.
Behavioral Measures
Reading abilities were assessed with the Woodcock-Johnson III Tests of Achievement (WJ-III; (Woodcock, et al. 2001)). The Basic Reading Skills Cluster of the WJ-III assesses decoding skills as a composite of two subtests: word identification (untimed overt reading of a list of real words) and word attack (untimed overt reading of a list of pseudo-words). The Passage Comprehension subtest of the WJ-III (Woodcock, et al. 2001) was used to assess reading comprehension skills. In this subtest, participants are asked to read covertly 1–2 sentences and fill in the blank(s) (a cloze procedure). Standard scores were calculated based on birth date. Subjects were determined to be outliers if scores on behavioral measures deviated by 3 standard deviations or more from the mean. Based on this criterion, one preterm subject had to be excluded from the analyses of decoding scores.
Intellectual ability was examined using the Wechsler Abbreviated Scale of Intelligence (WASI), a widely used, nationally standardized test of general intellectual ability that measures both verbal and nonverbal cognitive ability (Kaufman 1994; Wechsler 1999). Performance IQ is composed of Block Design and Matrix Reasoning subtests and assesses nonverbal cognitive abilities.
MRI Acquisition and Processing
MR images were acquired on a 3T Signa Excite (GE Medical Systems, Milwaukee, WI) at Stanford University. T1 images included one IR-prep 3D FSPGR scan in the axial plane (field of view (FOV) = 24 ×15.6 cm, matrix size = 256 × 192, 1.2mm slices, TI = 300 ms, flip angle = 15 degrees, NEX=1) and two high resolution inversion recovery (IR)-prep 3D fast spoiled gradient (FSPGR) scans also collected in the axial plane (FOV = 24 × 18 cm, matrix size = 260 × 192, 0.9 mm slices, TI = 300 ms, flip angle = 15 degrees, number of excitations (NEX) = 1). The second set of T1 images were co-registered to the first FSPGR using a mutual information maximization algorithm (SPM5, http://www.fil.ion.ucl.ac.uk/spm/) and subsequently averaged for improved contrast. A trained research assistant manually identified the anterior and posterior commissures on the midsagittal plane, and these points were used to align the averaged anatomical image to a canonical ac-pc orientation, using a rigid body transformation (no warping was applied).
For dMRI and tractography, a diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence (TE = 80 ms, TR = 6500 ms, FOV = 24 × 24 cm, matrix size = 128 × 128) was used to acquire 60 slices, 2 mm thick, along 30 diffusion directions (b = 900). The sequence was repeated 4 times, and 10 non-diffusion weighted (b = 0) volumes were collected.
Diffusion MR images were pre-processed with open-source software, mrDiffusion (http://white.stanford.edu/software/). Eddy current distortions and subject motion in the diffusion weighted images were removed by a 14-parameter constrained non-linear co-registration algorithm based on the expected pattern of eddy-current distortions, given the phase-encoding direction of the acquired data (Rohde, et al. 2004). Diffusion data were aligned to the T1 anatomical scans that had been averaged and rotated to align with the ac-pc plane. Alignment was achieved by registering the b0 images to the resampled T1 image using the same mutual information maximization algorithm as above. Diffusion tensors were fit using a robust least-squares algorithm designed to remove outliers at the tensor estimation step (Chang, et al. 2005). We computed the eigenvalue decomposition of the diffusion tensor and the resulting eigenvalues were used to compute fractional anisotropy (FA) (Basser and Pierpaoli 1996). No scans were lost due to excessive head motion.
Fiber Tracking
Fiber tractography and data analysis were performed using mrDiffusion (http://white.stanford.edu/software/) and Matlab (Mathworks, Natick, MA). Fiber tracking was performed using a deterministic streamlines tracking algorithm (STT) (Chang, et al. 2005; Conturo, et al. 1999; Mori, et al. 1999), with a fourth-order Runge-Kutta path integration method (Press, et al. 2002). The fiber tracking algorithm was seeded with a white matter mask defined as all the voxels with FA value greater than 0.2. For tracking purposes, a continuous tensor field was estimated using trilinear interpolation of the tensor elements. Tracking proceeded in all directions and stopped when FA dropped below a minimum threshold of 0.15 or when the angle between two adjacent steps was greater than 30°.
Fiber Tract Identification
Each fiber group was identified in individual brains by constraining the results of fiber tracking with two ROIs, manually placed on the principal diffusion direction map (red-green-blue (RGB) map) by a child-neurologist (YL) who was blind to group assignment. ROI placement on the RGB map was guided by annotated RGB maps in a MRI atlas (Mori, et al. 2005) (see subsequent section for detailed description of ROI placement procedures). A child neuroradiologist confirmed ROI placement.
Tract editing was performed manually and individually using Quench, a 3D visualization tool (http://white.stanford.edu/newlm/index.php/QUENCH) where tracts were presented against the background of the averaged T1 image. Tract editing was performed based on known tract anatomy (Naidich, et al. 2009) and was guided by published cerebellar tracking results in adults (Mori, et al. 2005; Stieltjes, et al. 2001). Two independent raters (YL and KET) visually inspected each fiber group to assure that it was consistent with anatomical guidelines.
ROI Placement
Superior Cerebellar Peduncle (SCP), Left and Right
One spherical ROI (5mm) was placed on the dentate nucleus in the axial plane of the RGB map at the level of the medial pons. The second spherical ROI (5mm) was placed on the ipsilateral SCP (green voxels on the RGB map) at the level of the ponto-mesencephalic junction. At this level, the SCP appears both green (fibers project anteriorly toward the thalamus) and red (fibers that begin to decussate across the midline) (Mori, et al., 2005). Robust tracts were detected bilaterally, similar in anatomic configuration to previous descriptions of these tracts (Granziera, et al. 2009; Stieltjes, et al. 2001). Using deterministic tracking methods, we could not follow this tract dorsally through the decussation towards the contralateral cortex, but rather identified fibers continuing on the ipsilateral side to the cortex (Figure 1a), as described by (Mori, et al. 2005). We therefore clipped the tract at the level of the decussation, and analyzed its diffusivity properties below that point (Figure 1b). This approach identified the right SCP in all subjects. The left SCP could not be successfully identified in one subject, likely due to limitations of deterministic tractography (Wahl, et al. 2010; Yeatman, et al. 2011). This subject was excluded from analysis of the left SCP only.
Figure 1. FA profiles of cerebellar peduncles.
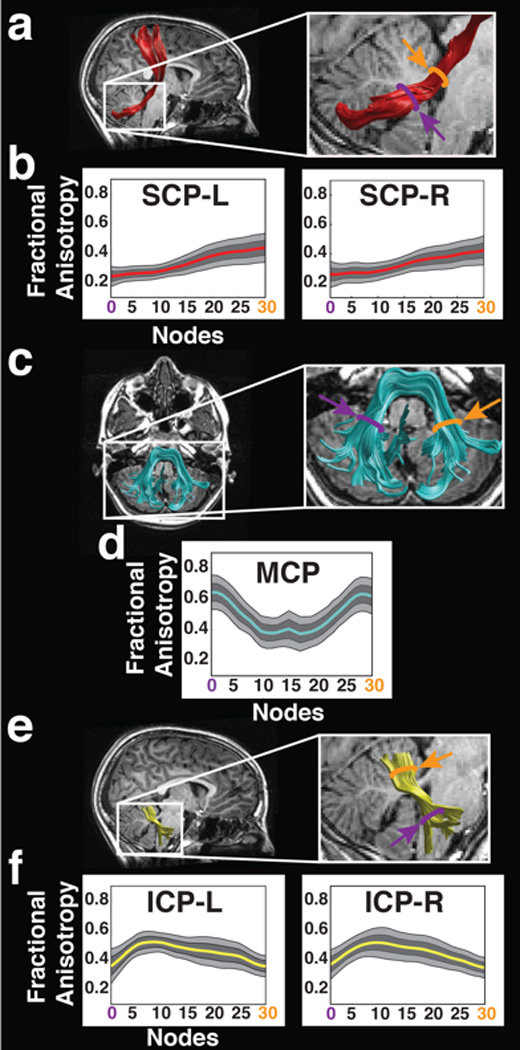
Renderings of 3 individual cerebellar tracts are displayed on sagittal (a,e) or axial (c) T1 images of a representative single subject. Purple and orange lines in expanded images (a, c, e - right panels) indicate location of the two ROIs used to identify the SCP (a-red), MCP (c-cyan) and ICP (e-yellow). Only right hemisphere ROIs are displayed for the SCP and ICP. Tract mean group FA profiles (b, d, f) are color-coded to match tract renderings. FA values are plotted for 30 equidistant nodes between two defining ROIs. Location of ROIs correspond to purple (location 0 – ROI1) and orange (location 30 – ROI2) arrows in FA profiles (b,d,f) and T1 images (a,c,e). Boundaries of the 25th and 75th percentiles are indicated by dark gray shading. Boundaries of the 10th and 90th percentiles are indicated by light gray shading. (FA = fractional anisotropy; SCP = superior cerebellar peduncle; MCP = middle cerebellar peduncle; ICP = inferior cerebellar peduncle; L = left; R = right).
Middle Cerebellar Peduncle (MCP)
The MCP was consistently identified on the axial RGB map at the level of the medial pons, in the most caudal slice in which the dentate nucleus was visible (Figure 1 c, d). At this level, the MCP appears green within the cerebellum and red within the pons (Mori, et al. 2005). Pontine fibers of the MCP first exit the pontine nuclei and decussate across the midline (red) before entering the cerebellum, where fibers are predominantly oriented in the anterior-posterior direction (green). We captured the MCP by placing two spherical ROIs (5mm) within the cerebellum at this axial slice: one placed on the central portion of the right MCP (green voxels on the RGB map) and the other placed on the central portion of the left MCP (same color). This approach produces streamlines that bifurcate into two separate branches as described by (Mori, et al. 2005), reflecting the passage of ponto-cerebellar fibers around the pontine nuclei (Naidich, et al. 2009). Since calculations of tract profiles assume non-branching tracts, streamlines passing posterior to the pontine nuclei as the pontine crossing tract were removed manually using Quench. One full term subject was excluded from analyses of the MCP because only the pontine crossing tract could be identified for this subject.
Inferior Cerebellar Peduncle (ICP), Right and Left
The first spherical ROI (5mm) was placed on the ICP (blue voxels on the RGB map) defined on the axial plane at the level of the medulla, inferior to the dentate nucleus. At this level, the ICP appears blue as fibers that originate from within the spinal cord and inferior olive travel dorsally into the cerebellum (Mori, et al., 2005; Naidich, et al. 2009). The second spherical ROI (5mm) was placed on the ipsilateral ICP (green voxels on the RGB map) defined on the axial plane at the level of the ponto-mesencephalic junction. At this level, the ICP appears green as fibers begin to project posteriorly and enter the cerebellum. The resulting fiber group consisted of only streamlines passing through both ROIs (Figure 1e,f). The trajectory of the ICP matched the configuration described in previous adult studies (Granziera, et al. 2009; Mori, et al. 2005; Stieltjes, et al. 2001). This approach identified the left and right ICP in all subjects.
Fiber Tract Quantification
The same ROIs (Fig. 1a–f) were used to isolate the central trajectory of each of the fascicles. Tract extremities beyond these ROIs to either side were clipped. The central portion of the tract is generally consistent across individuals, whereas the extreme ends of a fascicle typically vary dramatically across subjects. Therefore, diffusion properties of each cerebellar peduncle were quantified only along the central portion of the fascicle (Figure 1). This procedure generates for every tract and for every individual, a FA profile: a description of FA variations along the central portion of the tract. Each FA profile was resampled into 30 equidistant nodes between the two ROIs. FA was calculated at each node by taking a weighted average of the FA values at that node across all fibers belonging to this tract. Each fiber’s contribution to the average was weighted by the probability that a fiber was a member of the fascicle, computed as the Mahalanobis distance from the fiber tract core (Yeatman, et al. 2012). This procedure minimizes the contribution of fibers located further from the fiber tract core that are more likely to reflect a mixture of gray and white matter or of different tracts, and so minimizes the effect of partial voluming on diffusion property estimates. Mean tract-FA was calculated by averaging the FA values of all the 30 nodes belonging to the same tract.
Laterality Index
Laterality indices for the SCP and ICP were determined by calculating the difference in the mean tract-FA of the left and right tracts divided by their sum (L-R/L+R). The laterality index of the MCP was calculated by splitting the MCP to nodes 1–15 (left) and 16–30 (right). Laterality profiles for the SCP and ICP were next generated by calculating a laterality index at each of the 30 nodes along the FA profiles. A laterality profile for the MCP was generated by calculating a laterality index at each of the 15 nodes along the FA profiles of the left and right halves of the MCP.
Statistical Analyses: Group Comparisons
Chi-square tests and two-tailed t-tests for independent samples were used to examine whether preterm (PT) and full term (FT) children differed on demographic variables and behavioral outcome measures.
The following statistical analyses were used to assess the structural characteristics of the cerebellar peduncles and compare FA and lateralization of the cerebellar peduncles between the PT and FT groups. A 2 by 2 by 3 mixed analysis of variance (ANOVA) was conducted with tract-FA as the dependent measure, Group (PT/FT) as a between subject variable, and Hemisphere (Left or Right) and Tract (SCP, MCP, ICP) as the within-subject variables. For completeness, we conducted a second analysis replacing Group with Gender as the between subject variable (all other variables remain the same).
To achieve greater sensitivity for local group differences restricted to specific tract segments, two-tailed t-tests for independent samples were next calculated between the FA values of PT and FT subjects at each node along the FA profile, separately for each tract. The same procedure was taken to test group differences in the laterality profiles of each tract. A family-wise error corrected cluster size was computed using a nonparametric permutation method to control for multiple comparisons (Nichols and Holmes 2002), within a tract. A minimum cluster size of 6 adjacent nodes was required for significance.
Statistical Analyses: Associations with Reading
Evidence for associations between reading skills and FA of the cerebellar peduncles was first assessed by calculating Spearman correlation coefficients between reading skills (decoding, comprehension) and mean tract-FA in the combined sample of full term and preterm children. Spearman rank correlations were calculated due to evidence for non-normal distribution in FA measures in a preliminary exploration of the data. To increase sensitivity for local associations, we next calculated Spearman correlation coefficients between reading skills (decoding, comprehension) of the combined sample and their local FA values at 30 nodes along the trajectory of each tract (see (Yeatman, et al. 2012) for a similar approach, calculating correlations along the FA profiles of the major cerebral tracts). A family-wise error corrected cluster size of 6 adjacent nodes was found sufficient for corrected significance for analyses performed within each tract separately (Nichols and Holmes 2002). To control for multiple comparisons across the five tracts and two reading measures, the significance of the Spearman correlations between behavioral measures and mean FA of significant clusters was determined using a false discovery rate (FDR) of 5% (Benjamini and Hochberg 1995). Post-hoc Spearman partial correlations were performed to confirm that associations were not affected by the age of participants.
To assess whether the associations observed in the combined group were present in both full term and preterm groups, we extracted the mean FA of the significant clusters and calculated Spearman correlations in each group separately, between these FA values and children’s reading scores.
Post-Hoc Statistical Analyses
To examine the domain specificity of the associations between reading and FA of the cerebellar peduncles, Spearman correlations were first calculated between a general measure of non-verbal intelligence (performance IQ; PIQ) and the FA profile of each cerebellar tract. In tract nodes showing significant correlations with both PIQ and reading measures, we calculated partial correlations between mean FA from significant clusters and reading measures, while controlling for PIQ.
Specificity of the reading-FA correlations to one reading component was tested by calculating partial Spearman correlations between mean FA from significant clusters of the relevant peduncles (left and right SCP, MCP and left ICP, all showing significant correlations with either of the reading measures) and one of the reading measures, while controlling for the other reading measure (decoding or comprehension). Finally, to interpret the direction of associations (positive/negative) observed between FA and reading measures, we examined possible associations between reading measures and both mean axial diffusivity (AD) and mean radial diffusivity (RD). Specifically, we calculated Spearman correlations between each of the reading measures and mean AD or mean RD from clusters demonstrating significant correlations between reading skills and FA.
Results
Structural Characteristics of the Cerebellar Peduncles in the entire sample
The five cerebellar peduncles were identified in all participants (Figure 1) except for the left SCP, which was missed in one preterm child (see Methods above). Visual inspection of FA profiles revealed that FA varied along each tract in a consistent pattern across individual participants (Figure 1b, d, f). The laterality indices of the three peduncles approached zero (mean LIs=0.003, −0.011 and 0.005 in the SCP, MCP and ICP, respectively). A similar pattern was documented in both the full term and preterm groups, separately (FT: mean LIs=−0.007, −0.013 and 0.003; PT: mean LIs= −0.001, −0.010 and 0.010 for the SCP, MCP and ICP respectively). All nodes along the laterality profiles were close to 0 in the combined sample and in the PT and FT groups, separately.
Group Comparisons: preterm versus full term
Group comparison of mean tract-FA
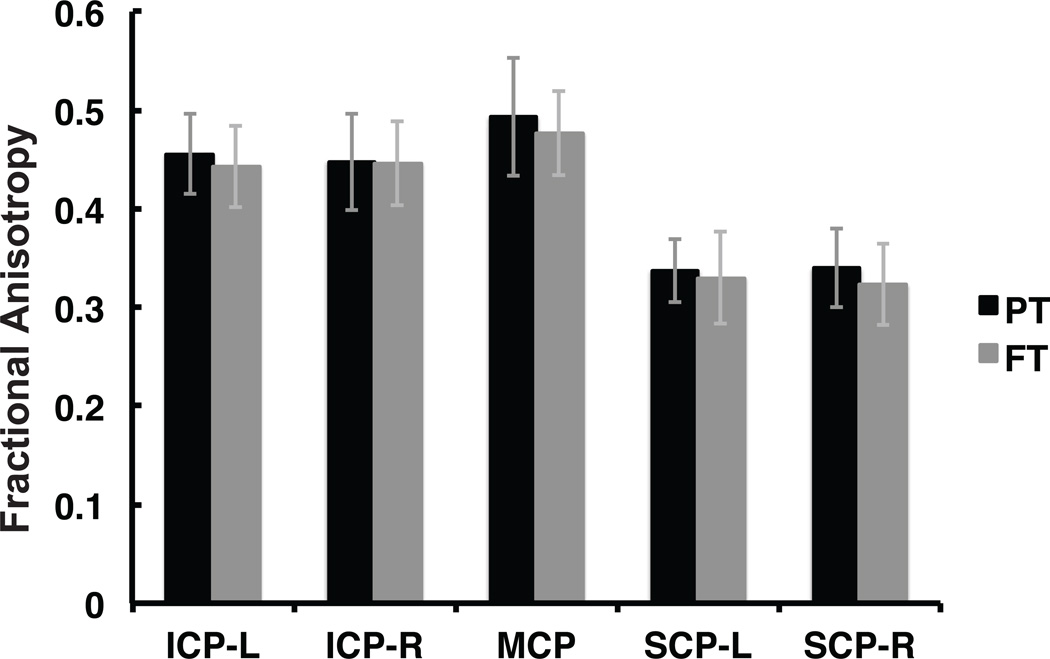
Mean tract-FA did not differ significantly between the FT and PT groups in any of the five cerebellar peduncles (Table 1, Figure 2). Specifically, there was no significant main effect of Group (PT vs. FT), nor any significant interaction with Group, in a Group x Hemisphere x Tract mixed ANOVA over mean tract-FA measures (Group main effect: F(1,41) = 1.92, P >0.1; Group x Tract: F(2,82) = 1.38, P >0.1; Group x Hemisphere F(1,41) = 0.025, P >0.1). The same ANOVA indicated no significant effect of Hemisphere either (Hemisphere main effect: F(1,41) = 0.004, P >0.1), supporting the impression that the cerebellar peduncles were not lateralized. There was a highly significant main effect of Tract (F(2,82) = 919.98, P <0.001), reflecting differences between the cerebellar peduncles in overall mean tract-FA. Mean tract-FA did not differ significantly between males and females in any of the cerebellar peduncles (P >0.1).
Figure 2.
Mean tract-FA is similar between FT and PT children. No group differences are observed between FT and PT groups for mean FA of the left and right ICP, MCP, left and right SCP. Mean FA for FT group is plotted in gray. Mean FA for PT group is plotted in black. Error bars represent ± 1 standard deviation. (FA = fractional anisotropy; FT = full term; PT = preterm; ICP = inferior cerebellar peduncle; MCP = middle cerebellar peduncle; SCP = superior cerebellar peduncle; L=left; R=right).
Group comparison of FA profiles
Independent t-tests in nodes along the FA profile of each peduncle revealed that there were no significant group differences found in any specific region along any of the five cerebellar peduncles (no clusters sized ≥ 6, family-wise error corrected (Nichols and Holmes, 2002).
Group comparison of tract lateralization
Independent t-tests in nodes along the laterality profiles of the three peduncles revealed that there were no significant group differences in any specific region of the SCP, MCP and ICP (no clusters sized ≥ 6, family-wise error corrected (Nichols and Holmes, 2002)).
Associations between Reading and FA of the Cerebellar Peduncles
Given that we did not record any effect of prematurity on the structural properties of the cerebellar peduncles in this particular cohort, we conducted associative analyses in the combined sample of full term and preterm children to increase power for identifying potentially significant associations between reading and structural properties of the cerebellar peduncles. We then considered whether the associations between FA and reading were consistently found in each of the full term and preterm groups separately.
Associations between Decoding and FA of the Cerebellar Peduncles
Combined Group Analyses
We analyzed the association between mean tract-FA and decoding in the five cerebellar peduncles, across all participants. The degree of association was statistically significant in the ICP-L (rs = −0.32, P =0.032), trended towards significance in the SCP-L (rs = −0.29, P =0.064) and in the MCP (rs = 0.29, P =0.064), and was not significant in the right ICP and right SCP.
After establishing evidence for associations between FA and decoding, we then assessed the same associations along the trajectory of the cerebellar peduncles. In this more detailed analysis, we observed that the association between decoding and FA was significant in segments of the left SCP, MCP and left ICP (Figure 3; Table 2). Specifically, significant negative associations were observed between decoding and FA within dorsal segments of the left SCP (Figure 3a–b) and within dorsal segments of the left ICP (Figure 3e–f). A significant positive association was observed between decoding and FA within medial segments of the MCP (Figure 3c–d). Correlations between mean FA within the significant clusters and decoding remained statistically significant after controlling for multiple comparisons across the five tracts at a 5% criterion for false discovery rate. Spearman partial correlations confirmed that all associations between decoding and mean cluster FA remained significant after controlling for age (left SCP: rs = −0.38, P = 0.01; MCP: rs = 0.50, P =0.0006; left ICP: rs= −0.50, P =0.006). Decoding was not significantly associated with FA in any clusters of either the right SCP or the right ICP.
Figure 3. FA of the left SCP, MCP and left ICP is associated with decoding skills.
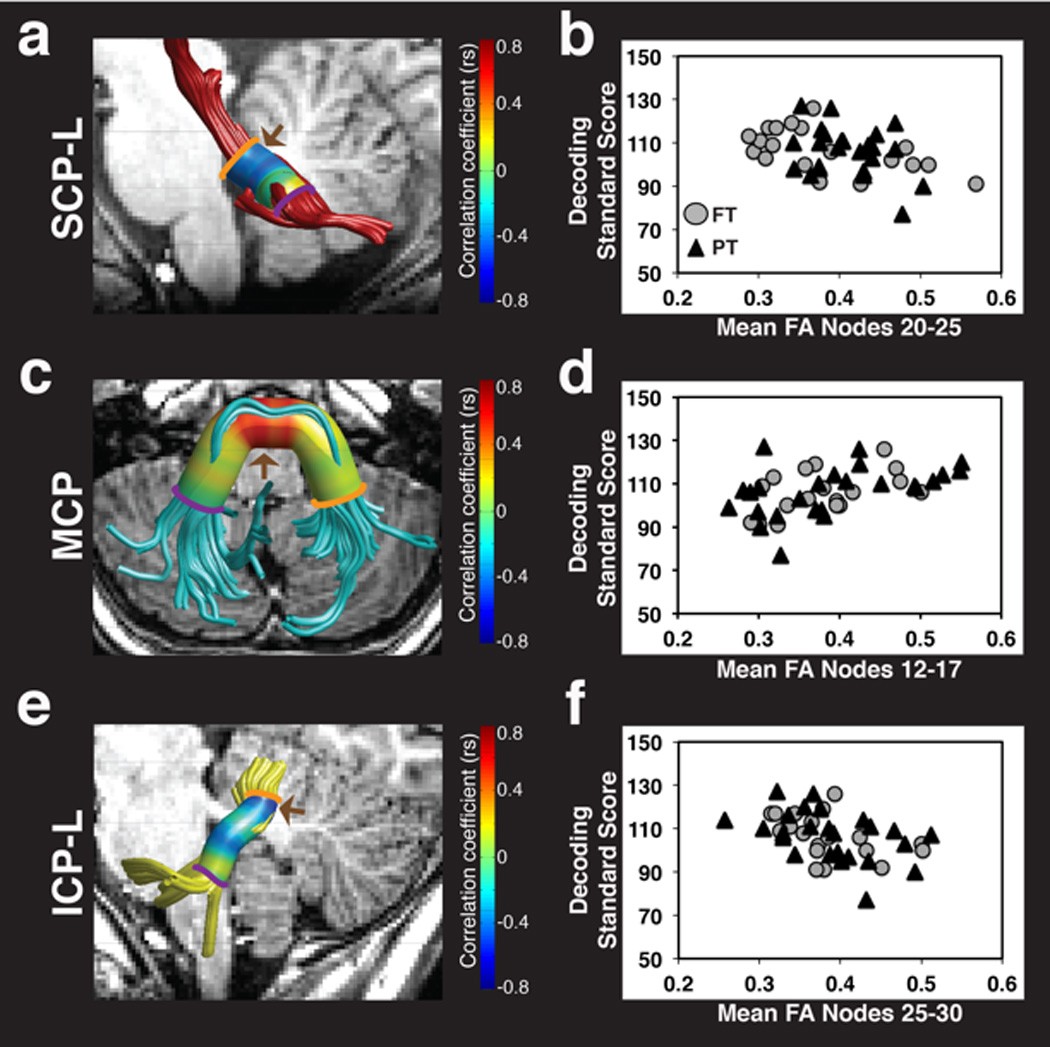
Strength of correlations between decoding standard scores and FA at 30 equidistant nodes are displayed on a colored cylinder surrounding tract renderings for the left SCP (a), MCP (c) and left ICP (e). Negative associations are observed between decoding skills and FA of the left SCP (a) and left ICP (e) as indicated in blue. Positive associations are observed between decoding skills and FA of the MCP (c) as indicated in red. Brown arrows (a,c,e) indicate cluster location where significant associations between FA and decoding skills survived within tract multiple comparisons corrections. Scatter plots represent the association between decoding standard scores and significant cluster mean FA for the left SCP (b), MCP (d) and left ICP (f). Color bar represents Spearman correlation coefficients. (FA = fractional anisotropy; SCP = superior cerebellar peduncle; MCP = middle cerebellar peduncle; ICP = inferior cerebellar peduncle; L = left; R = right; FT = full term; PT = preterm; rs = Spearman correlation coefficient).
Table 2.
Spearman correlations between mean FA of significant clusters and reading scores in the combined sample
| Decoding (SS) Combined Group |
Comprehension (SS) Combined Group |
|||
|---|---|---|---|---|
| White matter tract | rs | P | rs | P |
| SCP-L (nodes‡ 20–25) | −0.38* | P = 0.012 | −0.34* | P = 0.025 |
| SCP-R | ns | ns | −0.36* | P = 0.016 |
| MCP (nodes 12–17) | 0.51* | P < 0.001 | 0.51* | P < 0.001 |
| ICP-L (nodes 25–30) | −0.48* | P < 0.001 | −0.40* | P = 0.007 |
| ICP-R | ns | ns | ns | ns |
P< 0.05
Significant at 5% False Discovery Rate (FDR) criterion
FA extracted from mean FA across the nodes within the significant cluster
Nodes for a significant cluster (6-adjacent nodes)
SS = Standard score
rs = Spearman correlation coefficient
SCP = Superior Cerebellar Peduncle
MCP = Middle Cerebellar Peduncle
ICP = Inferior Cerebellar Peduncle
ns = non-significant
L = Left
R = Right
Separate Analyses in PT and FT
We repeated the association analyses in each group (PT, FT) separately, and confirmed that the direction of association was the same in both groups. Associations of FA and decoding were negative in the SCP-L and ICP-L in both groups, and positive in the MCP in both groups. We found significant negative associations in the full term group between mean cluster FA and decoding within the SCP-L (rs = −0.56, P =0.012) and ICP-L (rs = −0.49, P =0.035) (Table 3). In the preterm group, we found a significant positive association between mean cluster FA and decoding in the MCP (rs = 0.57, P =0.002) and a significant negative association between mean cluster FA and decoding in the ICP-L (rs = −0.47, P =0.016; Table 3).
Table 3.
Spearman correlations between mean FA of significant clusters and reading measures for Full Term and Preterm Groups
| Decoding (SS) Full Term |
Decoding (SS) Preterm |
Comprehension (SS) Full Term |
Comprehension (SS) Preterm |
|||||
|---|---|---|---|---|---|---|---|---|
| White matter tract§ | rs | P | rs | P | rs | P | rs | P |
| SCP-L (nodes‡ 20–25) | −0.56* | P= 0.012 | −0.25 | P= 0.222 | −0.45 | P= 0.055 | −0.18 | P= 0.393 |
| SCP-R | ns | ns | ns | ns | −0.74* | P < 0.001 | −0.10 | P = 0.646 |
| MCP (nodes 12–17) | 0.40 | P = 0.103 | 0.57* | P = 0.002 | 0.58* | P = 0.012 | 0.53* | P = 0.005 |
| ICP-L (nodes 25–30) | −0.49* | P = 0.035 | −0.49* | P = 0.016 | −0.37 | P = 0.121 | −0.41* | P= 0.038 |
| ICP-R | ns | ns | ns | ns | ns | ns | ns | ns |
P< 0.05
FA extracted from mean FA across the nodes within the significant cluster
Nodes for a significant cluster (6-adjacent nodes)
SS = Standard score
rs = Spearman correlation coefficient
SCP = Superior Cerebellar Peduncle
MCP = Middle Cerebellar Peduncle
ICP = Inferior Cerebellar Peduncle
ns = non-significant
L = Left
R = Right
Associations between Comprehension and FA of the Cerebellar Peduncles
Combined Group Analyses
The association between comprehension and mean tract-FA of the five cerebellar peduncles was assessed in the combined sample. The degree of association trended towards statistical significance in the ICP-L (rs = −0.28, P =0.060), and was not significant in any of the other peduncles. To interrogate the association in further detail, we then examined the associations of FA and comprehension along the trajectory of the tracts. We found significant associations between comprehension and FA within clusters of the left and right SCP, MCP and left ICP (Figure 4; Table 2). Significant negative associations were apparent between FA and passage comprehension within dorsal segments of the left SCP (Figure 4a–b), the right SCP (Figure 4c–d) and the left ICP (Figure 4g–h). Significant positive associations between passage comprehension and FA were observed within medial segments of the MCP (Figure 4e–f). Correlations between mean FA of the significant clusters and comprehension remained statistically significant after controlling for multiple comparisons across the five tracts at a 5% criterion for false discovery rate. Spearman partial correlations confirmed that all associations between passage comprehension and mean cluster FA remained significant after controlling for age (left SCP: rs = −0.34, P =0.03, right SCP: rs = −0.36, P =0.02, MCP: rs = 0.53, P <0.001, and left ICP: rs = −0.40, P =0.007). Associations between passage comprehension and FA along the right ICP were still not significant even in this more sensitive analysis.
Figure 4. FA of the left and right SCP, MCP and left ICP is associated with passage comprehension skills.
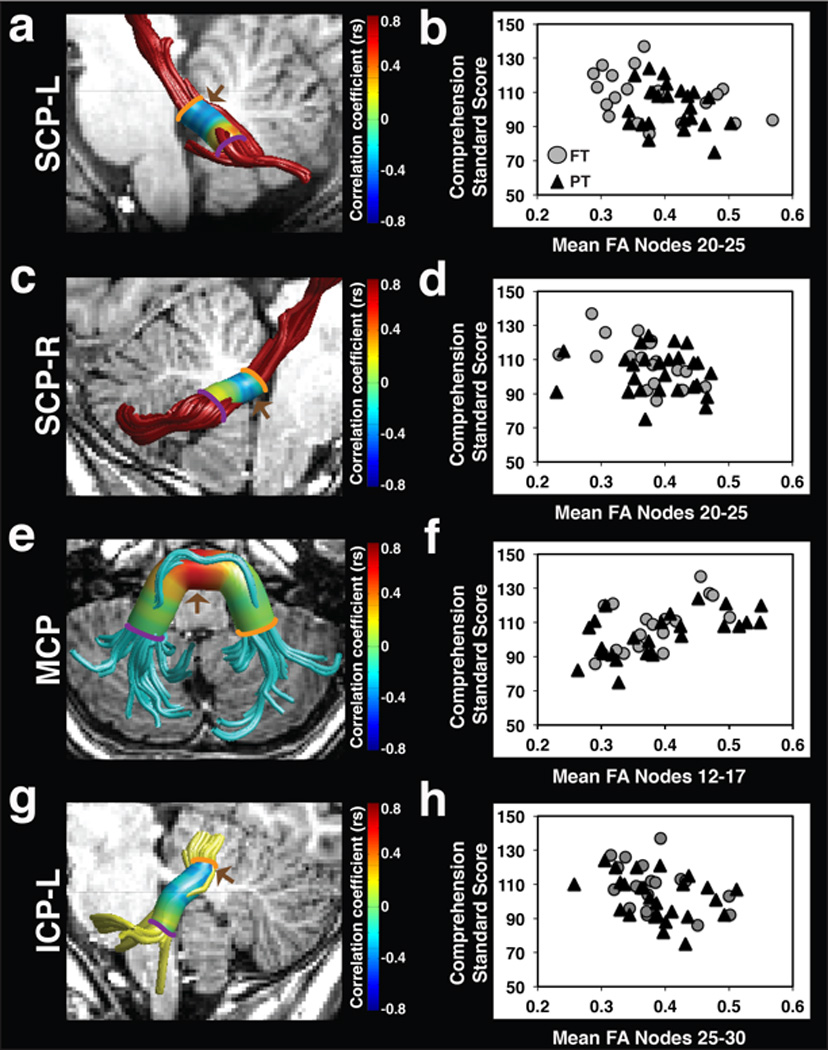
Strength of correlations between passage comprehension standard scores and FA at 30 equidistant nodes are displayed on the cylinder surrounding tract renderings for the left SCP (a), right SCP (c), MCP (e) and left ICP (g). Passage comprehension standard scores are negatively associated with FA of the left and right SCP (a,c), and left ICP (g) as indicated in blue. Passage comprehension standard scores are positively associated with FA of the MCP (e) as indicated in red. Brown arrows (a,c,e,g) indicate cluster location where significant associations between FA and passage comprehension skills survived within-tract multiple comparisons corrections. Scatter plots represent the association between passage comprehension standard scores and significant cluster mean FA for the left SCP (b), right SCP (d), MCP (f) and left ICP (h). Color bar represents Spearman correlation coefficients. (FA = fractional anisotropy; SCP = superior cerebellar peduncle; MCP = middle cerebellar peduncle; ICP = inferior cerebellar peduncle; L = left; R = right; FT = full term; PT = preterm; rs = Spearman correlation coefficient).
Separate Analyses in PT and FT
We repeated the association analyses in each group (PT, FT) separately, and found that the direction of association was the same in both groups; associations of FA and comprehension were negative in the SCP-L, SCP-R and ICP-L, and positive in the MCP. We found significant associations in the full term group between mean cluster FA and passage comprehension in the SCP-R (rs = −0.74, P <0.001) and MCP (rs = 0.58, P =0.012), with a trend in the SCP-L (rs = −0.45, P =0.055) (Table 3). We found significant associations in the preterm group between mean cluster FA and passage comprehension within the MCP (rs = 0.53, P =0.005) and ICP-L (rs = −0.41, P =0.038) (Table 3).
Specificity of Associations with Reading
Correlations with Performance IQ
To explore the domain specificity of associations between cerebellar tracts and reading skills, we calculated the correlations between FA profiles and performance IQ, a non-verbal cognitive measure that, in the present sample, was correlated with reading ability (PIQ and decoding association: rs = 0.44, P =0.003; PIQ and comprehension association: rs = 0.47, P =0.001). The results revealed significant positive associations between PIQ and FA only in the MCP, in regions similar to the regions associated with reading, nodes 12–17 (rs = 0.51, P <0.001). No significant associations between FA and PIQ were observed in any segment along the bilateral ICP and bilateral SCP. Spearman partial correlations confirmed that reading associations in the MCP remained significant after controlling PIQ for both decoding (rs = 0.37, P =0.017) and passage comprehension (rs = 0.35, P =0.020).
Separating the contribution of each reading component
To explore whether the observed associations could be explained by similar or distinct underlying processes indexed by decoding and comprehension, we extracted mean FA from the significant clusters of the left and right SCP, MCP and left ICP and calculated additional Spearman partial correlations between these FA values and one of the reading measures, while controlling for the other reading measure. After controlling for performance on passage comprehension, FA of the left ICP (but not MCP or L-SCP) continued to be significantly correlated with decoding (rs = −0.33, P =0.032; Table S1). Conversely, after controlling for decoding, FA of the right SCP (but not L-ICP, MCP, L-SCP) remained significantly correlated with passage comprehension (rs = −0.41,P =0.006; Table S2).
Associations with AD and RD
Finally, we performed analyses within the clusters that showed significant associations of FA and reading to determine if axial diffusivity or radial diffusivity were associated with reading. These analyses revealed significant negative correlations between radial diffusivity of the MCP and both decoding (rs = −0.39, P =0.009) and comprehension (rs = −0.39, P =0.010). Axial and radial diffusivity were not significantly associated with reading measures within the SCP-L, SCP-R, or ICP-L (P >0.1).
Discussion
Summary of results
The present study is the first to our knowledge to relate the structural properties of the cerebellar peduncles with reading abilities. We applied diffusion MRI and tractography algorithms to identify and characterize the cerebellar peduncles in this sample of 9–17 year old children and adolescents born either full or preterm. Variations in FA profiles appeared similar across individuals. In contrast to our initial predictions, we were unable to detect any statistically significant differences in the mean tract-FA values or FA profiles of the cerebellar pathways comparing the full term and preterm groups. We did not find evidence of lateralization in FA values of the cerebellar peduncles in either the combined sample or in the full term or preterm groups separately, nor did we find a significant difference between the lateralization patterns of the two groups. No significant gender differences were observed in mean tract-FA of the SCP, MCP or ICP.
As hypothesized, fractional anisotropy measures extracted from the left and right SCP and MCP were significantly associated with measures of decoding and comprehension skills in this sample of full term and preterm participants. Contrary to initial hypotheses, fractional anisotropy extracted from the left ICP was also found to be significantly correlated with decoding and comprehension skills. These associations were revealed in correlation analyses performed along the trajectories of the tracts as opposed to associations with mean tract-FA values. The pattern and direction of associations were similar in the full term and preterm groups, though the clusters that reached statistical significance were not identical in the two groups.
We performed additional analyses to assess the specificity of our results to the domain of reading, using performance IQ as a measure of domain-general cognitive skills. These analyses revealed that, unlike the pattern of associations for reading, the associations of FA and performance IQ were found only in the MCP and the correlations with reading remained significant after controlling for performance IQ in this cluster. Further, after controlling for comprehension, significant associations remained in the left ICP for decoding. Similarly, after controlling for decoding, significant associations remained in the right SCP for comprehension. Taken together, these findings show that white matter connections of the cerebellar peduncles demonstrate selective associations with the core skills of reading.
Structural Properties of Cerebellar Peduncles
Similar to cerebral tracts, FA was observed to vary along the trajectory of the SCP, MCP and ICP (Yeatman, Dougherty et al. 2011). The observed variations appear consistent with established patterns in adults (Stieltjes, et al. 2001) and are most likely related to anatomical features, such as the narrowing of the tracts and the density of crossing fibers (Yeatman, Dougherty, et al. 2011). Similar to a previous report in healthy adults (Thiebaut de Schotten, et al. 2011), we did not find evidence of laterality in FA values of the cerebellar peduncles. We also did not observe significant gender differences in FA of the cerebellar peduncles. The latter finding contrasts with other dMRI studies involving large samples of healthy adults in which FA of the left cerebellum was found to be higher in males, even after controlling for height (Kanaan, et al., 2012). Further studies will tell whether the lack of significant group differences in the present study stems from the difference in analysis methods, namely the tractography approach used here compared to voxel-based methods used elsewhere to extract FA measures of the peduncles (Kanaan, et al., 2012, 2014). In addition, more studies employing larger sample sizes than the present study are needed in order to understand how potential gender differences in the microstructure of the cerebellar peduncles may contribute to cognitive skills, including those important for reading.
The effect of prematurity on microstructural properties of the cerebellar peduncles
In the present study, we were unable to detect significant group differences in the microstructure of the SCP, MCP, or ICP in preterm compared to full term children. These results contrast with our initial predictions and with several studies of cerebral tracts that find FA to be lower in children born preterm than in controls, particularly within the corpus callosum, superior longitudinal fasciculus and inferior frontal-occipital fasciculus (Mullen, et al. 2011; Nagy, et al. 2003; Vangberg, et al. 2006). However, it is worth noting that not all studies find significantly lower FA in preterm groups. Some find no group differences (Feldman, et al. 2012b; Frye, et al. 2010) and others find regions of higher FA in the preterm than full term group (Allin, et al. 2011; Eikenes, et al. 2011). We recognize that the present sample size may not be adequate for detecting subtle group differences in cerebellar tract FA in either direction. Still, the findings suggest that cerebellar pathways in this relatively high functioning preterm sample, who were free of macroscopic cerebellar injury, were not severely altered. In separate on-going longitudinal studies of younger preterm children, we plan to examine whether cerebellar FA differences exist at earlier stages of development and whether early group differences in FA, if they exist, may resolve either as children get older or as they acquire specific skills for reading.
Cerebellar peduncles and reading
As hypothesized, we observed significant associations between FA of the MCP and SCP with decoding and passage comprehension skills. Similar to cerebral pathways, the MCP and SCP likely contribute to reading abilities by integrating and relaying information processed across distributed neural systems (Ben-Shachar, et al. 2007; Deheane 2009; Price 2012). Based on evidence from functional neuroimaging and anatomical mapping studies, it is plausible that these pathways convey information important for controlling both motor and cognitive aspects of reading (D'Angelo and Casali 2012; Koziol, et al. 2014; Price 2012; Ramnani 2006; Strick, et al. 2009). Indeed, anatomical mapping studies performed in monkeys have revealed that left inferior prefrontal regions and left premotor areas of the frontal lobes are reciprocally connected with the right hemisphere of the cerebellum via multi-synaptic connections conveyed by the SCP and MCP, respectively (Ramnani 2006; Strick, et al. 2009). In humans, regions of the left inferior prefrontal regions are commonly activated during language and reading tasks (Deheane 2009; Price 2012; Turkeltaub, et al. 2002). There is also sufficient evidence demonstrating that the right hemisphere of the cerebellum may be selectively involved in linguistic processes (Mariën, et al. 2013; Marien, et al. 2001). Indeed, the right lateral posterior cerebellum is consistently activated during tasks of semantic and phonological word retrieval (Frings, et al. 2006; Jansen, et al. 2005) and has also been implicated in working memory processes subserving language processes (Desmond and Fiez 1998). In addition, gray matter volume of the right cerebellar declive in lobule VI has been demonstrated to accurately distinguish dyslexic readers from control readers (Pernet, et al., 2009). The association of comprehension with FA of the right SCP may thus reflect the importance of this pathway in conveying neural information relevant for higher-order linguistic processes involved in understanding written text. However, we cannot readily explain why we found associations of decoding and FA in the SCP-L and not SCP-R, since studies find reading-related skills associated with left inferior prefrontal regions that connect to the cerebellum via the SCP-R (Jansen, et al. 2005; Stoodley, et al. 2012). Further studies are needed to confirm whether the cerebellar peduncles contribute selectively to specific linguistic, motor and other domain-general cognitive processes that are critically involved in reading but were not isolated here. Such studies can establish with more specificity the sub-skills of comprehension that rely on projections of the right SCP and the sub-skills shared between decoding and comprehension that may rely on the left SCP and MCP.
Contrary to initial hypotheses we also observed evidence for associations of FA within the left ICP with decoding and comprehension. Moreover, after controlling for performance on passage comprehension, FA of the left ICP remained significantly correlated with decoding, suggesting a specific role in the reading process. This finding may reflect the importance of olivocerebellar pathways for oculomotor functions (Voogd and Barmack 2006) required for controlling eye-movements during word reading, though it is not clear why the region would be selectively associated with decoding and not comprehension, because both tasks require oculomotor control. Alternatively, the ICP-L, like the other peduncles, may be responsible for conveying information relevant for broader cognitive and motor functions (D'Angelo and Casali 2012; Koziol, et al. 2014; Ramnani 2006). Again, further studies employing tasks more specific than those employed here should be performed to assess the selectivity of the cerebellar peduncles for specific linguistic, sensorimotor, and/or cognitive processes.
Associations with reading in full term and preterm participants
In this sample, the pattern of associations between FA and reading skills was extremely similar in the two groups of participants. In both preterm and full term participants we found negative associations of FA and reading in the SCP-L and ICP-L and positive associations in the MCP. These similarities may indicate that the present sample of preterm participants did not sustain any direct or indirect damage to the cerebellar peduncles. White matter development within the cerebellum is earlier than white matter development in areas of the cortex (Saksena, et al. 2008). Even if the children born preterm had sustained any microscopic injury to the cerebellum at birth, it may have resolved by school age. We note that, even though the direction of correlation was similar in the two groups, the degree of association did not always reach statistical significance in both subgroups. For example, the association between FA in the SCP-R and comprehension in the full term group was strong and significant, while in the preterm group it did not reach significance. We did not directly assess whether the degree of correlation was statistically different in the two groups because such analyses require larger samples, beyond the sample size of this study. However, it is worth noting that children born preterm frequently show behavioral characteristics suggestive of weak frontal lobe functions, including poor executive function skills and attention control (Aarnoudse-Moens, et al. 2009). It is thus plausible that cerebellar-frontal lobe connections may be less effective in achieving these functions in the preterm group. Future studies involving larger samples are required to explore whether there are functional differences in the contributions of cerebellar-frontal efferent pathways in reading abilities of children born preterm.
MCP and non-verbal IQ
We interpret findings from exploratory analyses with PIQ as evidence that the present pattern of associations observed with reading measures was unlikely to reflect associations with general cognitive skills that are not specific to reading. In performing these analyses, we observed a novel association between the microstructural properties of the MCP and non-verbal IQ. These findings suggest that the MCP may be associated with a range of cognitive skills, including those important for reading. Given the unique sample of full and preterm children in the present study, further research is necessary to test the generality of these associations in other populations, including adults and persons with specific reading impairments, or in neurodevelopmental conditions associated with cerebellar disturbances, such as autism (Allen and Courchesne 2003; Amaral, et al. 2008).
Interpreting the direction of FA associations with reading
Unexpectedly, we found negative associations between FA and reading in the SCP and ICP, but positive associations in the MCP. Fractional anisotropy served as the primary index of tract microstructure in the present study. FA is a scalar value that reflects the normalized standard deviation of the 3 eigenvalues of the diffusion tensor (Beaulieu 2002). FA increases when the eigenvalues differentiate, that is, when AD increases, RD decreases, or both. It has been argued that AD reflects axonal status, while RD reflects myelin (Kumar, et al. 2008; Kumar, et al. 2010; Kumar, et al. 2012; Song, et al. 2002; Song, et al. 2005). Within the clusters that showed significant associations between FA and reading, we found significant negative associations between RD and reading only in the MCP. These findings may be taken as supporting the interpretation that the degree of myelination in the MCP may contribute positively to reading skill; the more myelinated the MCP is the more restricted the diffusion along the direction perpendicular to the preferred diffusion direction is, yielding faster information transfer and better reading skill. However, we recognize that RD is an inadequate measure for assessing myelin (Mezer, et al. 2013; Wheeler-Kingshott and Cercignani 2009; Yeatman, et al. 2014). Better understanding of the relation between tissue properties of the cerebellar peduncles and reading could be achieved using imaging methods that tap into myelin content and axonal diameter more directly than FA (e.g., Assaf, et al. 2008; Mezer, et al. 2013).
Negative associations of FA and reading have been found in other studies. There is increasing evidence from studies performed in both healthy children (Dougherty, et al. 2007; Odegard, et al. 2009; Yeatman, et al. 2011) and preterm participants (Frye, et al. 2008) for negative associations with reading skills within several cerebral pathways, including the corpus callosum and arcuate fasciculus. Both positive and negative correlations with FA should be interpreted with caution; they can stem from covariation of any of a number of tissues factors (or of a combination of factors) with reading performance (Jones and Cercignani 2010). Indeed, some of these factors are expected to affect information transmission efficacy in opposite ways. For example, increased axonal diameter could enhance information transmission but reduce FA, yielding negative association of FA with behavior. In addition, within a voxel, variation in the proportion of axons oriented in different directions could give rise to both negative and positive correlations with behavior (Jones 2010). Directional (in)coherence is expected to play an important role, particularly in explaining FA variation in cerebellar pathways, given the underlying architecture of multiple intersecting thin fiber tracts (Granziera, et al. 2009). For these reasons, it is difficult to offer straightforward interpretations for positive and negative associations, such as the ones documented here in terms of tissue properties. Quantitative methods combined with high angular resolution diffusion imaging techniques will likely be important for disambiguating amongst the multiple tissue properties known to contribute to FA (Tournier, et al. 2008; Tuch, et al. 2002; Wedeen, et al. 2008), including axonal density, distribution of axonal diameter within the voxel, degree of myelination, and directional coherence of the population of axons within the voxel (Basser and Pierpaoli 1996; Beaulieu 2002; Pierpaoli, et al. 1996). Knowledge of these tissue properties is likely to inform interpretations of the present positive and negative associations with reading measures that cannot be disambiguated with dMRI alone.
Limitations and Future Directions
The current sample included children and adolescents born either full term or preterm and a large range of ages at scanning. These factors raise the concern that developmental changes in FA and factors related to preterm birth could explain the present associations with reading measures. Nevertheless, several analyses suggest that significant associations observed in the cerebellar pathways were unlikely to be explained by factors related to prematurity or age at scanning. Specifically, associations between FA and reading measures remained significant after controlling for age at scanning. This possibility is further minimized by the fact the we used age-standardized reading measures, a tool typically used to allow joint analysis of reading scores acquired in children of varying ages. Further, we observed no group differences in either mean tract-FA or FA profiles for any of the cerebellar peduncles.
Another set of limitations arises from the imaging parameters and diffusion tensor algorithms we used. Diffusion MRI is not sufficiently sensitive to disambiguate among many factors that influence transmission efficiency. As suggested above, future studies should use multiple methods to evaluate myelin content, axonal caliber, proportion of crossing fibers, and other characteristics that can influence FA values (Assaf, et al. 2008; Mezer, et al. 2013; Tournier, et al. 2008; Tuch, et al. 2002; Yeatman, et al. 2014). Higher-resolution methods beyond the diffusion tensor model employed here are required for distinguishing tracts that interdigitate within the left and right SCP, such as dento-thalamic pathways (Granziera, et al. 2009; Tournier, et al. 2008). To properly assess the functional specificity of these pathways for distinct cognitive, linguistic and motor processes involved in reading, future studies would have to use more specific tasks than the composite measures employed here. In sum, fine tuning both the segmentation of cerebellar tracts and the assessment of reading components will assist in establishing the functional specificity of distinct cerebellar pathways for reading sub-skills.
As acknowledged above, we observed similar FA profiles and similar patterns of correlations in the cerebellar pathways of prematurely born children compared to full term children. This contrasts with previous findings from our lab in the cerebral white matter of full term and preterm children (Feldman, et al. 2012b). It is possible that these findings may reflect differences in the pathophysiology of cerebral versus cerebellar white matter development following preterm birth (Back and Rosenberg 2014). This possibility should be explored in future studies involving animal models for prematurity.
It is important to note that in this study we evaluated children across a wide range of reading abilities; we did not focus on dyslexia. To date, there are no published studies that use diffusion imaging to assess the structural characteristics of the cerebellar pathways in dyslexia, nor to relate those properties to specific language and reading components, even though there are strong predictions made in this regard (Jednorog, et al. 2013; Stoodley and Stein 2011). Such studies can inform clinicians and educators with respect to the neuropathological and compensatory mechanisms underlying language and reading impairments (Limperopoulos 2005; Vandermosten, et al. 2012).
Conclusions
The findings presented here are the first to implicate the microstructural properties of the cerebellar peduncles in explaining individual differences in the core abilities of reading. These results have significant implications for neurocognitive models of human behavior and disease that must now begin to consider the functional contributions of cerebellar pathways. Ongoing studies of younger children will help to determine whether similar associations exist at earlier reading stages to support learning or rather emerge later to support mature and automatic reading. Further research in clinical populations is needed to understand how cerebellar white matter tracts contribute to good and poor reading abilities. Overall, the present findings demonstrate the potential use of diffusion MRI for measuring the contribution of cerebellar white matter tracts to a broad range of human behaviors over the course of cognitive development.
Supplementary Material
Acknowledgements
This work has been supported in part by the National Institutes of Health, NICHD grants (RO1-HD69162, RO1-HD46500) and the Clinical and Translational Science Award 1UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources, National Institutes of Health. We thank the children and families who participated in our study; Jason D. Yeatman, Robert Dougherty, and Brian Wandell for helpful suggestions regarding the analyses; and the developmental-behavioral pediatrics research group for discussions of the results.
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-Analysis of Neurobehavioral Outcomes in Very Preterm and/or Very Low Birth Weight Children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Courchesne E. Differential Effects of Developmental Cerebellar Abnormality on Cognitive and Motor Functions in the Cerebellum: An fMRI Study of Autism. A J Psychiat. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Allin MPG, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RAA, McGuire P, Rifkin L, Murray RM, Nosarti C. White Matter and Cognition in Adults Who Were Born Preterm. PloS one. 2011;6:e24525. doi: 10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of Autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Anderson P, Doyle L, Study VIC. Neurobehavioral Outcomes of School-Aged Children Born Extremely Low Birth Weight or Very Preterm in the 1990s. JAMA. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: A Method for Measuring Axon Diameter Distribution from Diffusion MRI. Magn Reson Med. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA. Perinatal White Matter Injury: The Changing Spectrum of Pathology and Emerging Insights into Pathogenetic Mechanisms. Ment Retard Dev D R. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- Back SA, Rosenberg PA. Pathophysiology of Glia in Perinatal White Matter Injury. Glia. 2014;62:1790–1815. doi: 10.1002/glia.22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AE, Denton CA, Stuebing KK, Fletcher JM, Cirino PT, Francis DJ, Vaughn S. A Test of the Cerebellar Hypothesis of Dyslexia in Adequate and Inadequate Responders to Reading Intervention. J Int Neuropsychol Soc. 2010;16:526–536. doi: 10.1017/S1355617710000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and Physiological Features of Tissues Elucidated by Quantitative-Diffusion-Tensor MRI. J Magn Reson Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The Basis of Anisotropic Water Diffusion in the Nervous System - a Technical Review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging Brain Connectivity in Children with Diverse Reading Ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White Matter Pathways in Reading. Curr Opin Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Fiez JA. Impact of Cerebellar Lesions on Reading and Phonological Processing. Ann N Y Acad Sci. 2008;1145:260–274. doi: 10.1196/annals.1416.015. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- Bookstein FL. "Voxel-Based Morphometry" Should Not Be Used with Imperfectly Registered Images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T. The Role of the Basal Ganglia and Cerebellum in Language Processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The Organization of the Human Cerebellum Estimated by Intrinsic Functional Connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. Restore: Robust Estimation of Tensors by Outlier Rejection. Magn Reson Med. 2005;53:1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME. Tracking Neuronal Fiber Pathways in the Living Human Brain. Proc Natl Acad Sci USA. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummine J, Dai W, Borowsky R, Gould L, Rollans C, Boliek C. Investigating the Ventral-Lexical, Dorsal-Sublexical Model of Basic Reading Processes Using Diffusion Tensor Imaging. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0666-8. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Casali S. Seeking a Unified Framework for Cerebellar Function and Dysfunction: From Circuit Operations to Cognition. Front Neural Circuits. 2012;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C. Why Voxel-Based Morphometric Analysis Should Be Used with Great Caution When Characterizing Group Differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Deheane S. Reading in the Brain: The Science and Evolution of Human Invention. New York: Viking Penguin; 2009. [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging Studies of the Cerebellum: Language, Learning and Memory. Trends Cogn Sci. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Dougherty R, Bammer R, Siok W, Gabrieli J, Wandell B. Children's Reading Performance Is Correlated with White Matter Structure Measured by Diffusion Tensor Imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-Callosal Pathway Diffusivity Predicts Phonological Skills in Children. Proc Natl Acad Sci USA. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical Correlates of Dyslexia: Frontal and Cerebellar Findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical Signatures of Dyslexia in Children: Unique Information from Manual and Voxel Based Morphometry Brain Measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eikenes L, Løhaugen GC, Brubakk A-M, Skranes J, Håberg AK. Young Adults Born Preterm with Very Low Birth Weight Demonstrate Widespread White Matter Alterations on Brain DTI. Neuroimage. 2011;54:1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Fawcett AJ. Balance and Reading Are Separate Symptoms of Dyslexia. Dev Med Child Neurol. 2011;53:294–295. doi: 10.1111/j.1469-8749.2010.03886.x. [DOI] [PubMed] [Google Scholar]
- Feldman H, Lee E, Loe I, Yeom K, Grill-Spector K, Luna B. White Matter Microstructure on Diffusion Tensor Imaging Is Associated with Conventional Magnetic Resonance Imaging Findings and Cognitive Function in Adolescents Born Preterm. Dev Med Child Neurol. 2012a;54:809–814. doi: 10.1111/j.1469-8749.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and Reading Skills in School-Aged Children and Adolescents Born Preterm Are Associated with White Matter Properties on Diffusion Tensor Imaging. Neuropsychologia. 2012b;50:3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez VG, Stuebing K, Juranek J, Fletcher JM. Volumetric Analysis of Regional Variability in the Cerebellum of Children with Dyslexia. Cerebellum. 2013;12:906–915. doi: 10.1007/s12311-013-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles HG, Hein-Kropp C, Gizewski ER, Diener HC, Timmann D. Cerebellar Involvement in Verb Generation: An fMRI Study. Neurosci Lett. 2006;409:19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Frye R, Hasan K, Malmberg B, Desouza L, Smith K, Landry S. Superior Longitudinal Fasciculus and Cognitive Dysfunction in Adolescents Born Preterm and at Term. Dev Med Child Neurol. 2010;52:760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Liederman J, Papanicolaou A. Splenium Microstructure Is Related to Two Dimensions of Reading Skill. Neuroreport. 2008;19:1627–1631. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Sugihara I. Branching Patterns of Olivocerebellar Axons in Relation to the Compartmental Organization of the Cerebellum. Front Neural Circuits. 2013 doi: 10.3389/fncir.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S, Xiao J, Guo X. Asymmetry Analysis of Cingulum Based on Scale-Invariant Parameterization by Diffusion Tensor Imaging. Hum Brain Mapp. 2005;24:92–98. doi: 10.1002/hbm.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granziera C, Schmahmann JD, Hadjikhani N, Meyer H, Meuli R, Wedeen V, Krueger G. Diffusion Spectrum Imaging Shows the Structural Basis of Functional Cerebellar Circuits in the Human Cerebellum in Vivo. PLoS ONE. 2009;4:e5101. doi: 10.1371/journal.pone.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover W, Gough P. The Simple View of Reading. Reading and Writing. 1990;2:127–160. [Google Scholar]
- Jansen A, Floel A, Van Randenborgh J, Konrad C, Rotte M, Forster AF, Deppe M, Knecht S. Crossed Cerebro-Cerebellar Language Dominance. Hum. Brain Mapp. 2005;24:165–172. doi: 10.1002/hbm.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednorog K, Gawron N, Marchewka A, Heim S, Grabowska A. Cognitive Subtypes of Dyslexia Are Characterized by Distinct Patterns of Grey Matter Volume. Brain Struct Funct. 2013;219:1697–1707. doi: 10.1007/s00429-013-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Bodensteiner JB, Lotze TE. Frequency and Nature of Cerebellar Injury in the Extremely Premature Survivor with Cerebral Palsy. J Child Neurol. 2005;20:60–64. doi: 10.1177/08830738050200011001. [DOI] [PubMed] [Google Scholar]
- Jones D. Challenges and Limitations of Quantifying Brain Connectivity in Vivo with Diffusion MRI. Imaging Medicine. 2010;2:341–355. [Google Scholar]
- Jones DK, Cercignani M. Twenty-Five Pitfalls in the Analysis of Diffusion MRI Data. NMR Biomed. 2010;23:803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Allin M, Picchioni MM, Barker GJ, Daly E, Shergill SS, Woolley J, McGuire PK. Gender differences in white matter microstructure. PLoS One. 2012:e38272. doi: 10.1371/journal.pone.0038272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan RA, Chaddock C, Allin M, Picchioni MM, Daley E, Shergill SS, McGuire PK. Gender influence on white matter microstructure: a tract-based spatial statistics analysis. PLoS One. 2014:e0091109. doi: 10.1371/journal.pone.0091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A. Intelligent Testing with the WISC-III. New York, NY: Wiley; 1994. [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli J, Moseley M, Poldrak R. Microstructure of Temporoparietal White Matter as a Basis for Reading Ability: Evidence from Diffusion Tensor Magnetic Resonance Imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K and others. Consensus Paper: The Cerebellum's Role in Movement and Cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion Tensor Imaging Demonstrates Brainstem and Cerebellar Abnormalities in Congenital Central Hypoventilation Syndrome. Pediatr Res. 2008;4:275–280. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Harper RM. Rostral Brain Axonal Injury in Congenital Central Hypoventilation Syndrome. J Neurosci Res. 2010;88:2146–2154. doi: 10.1002/jnr.22385. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Regional Brain Axial and Radial Diffusivity Changes During Development. J Neurosci Res. 2012;90:346–355. doi: 10.1002/jnr.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock SK, Wilkinson ID, Wallis LI, Darwent G, Wonders SH, Fawcett AJ, Griffiths PD, Nicolson RI. Cerebellar Volume and Cerebellar Metabolic Characteristics in Adults with Dyslexia. Ann N Y Acad Sci. 2008;1145:222–236. doi: 10.1196/annals.1416.002. [DOI] [PubMed] [Google Scholar]
- Lee ES, Yeatman JD, Luna B, Feldman HM. Specific Language and Reading Skills in School-Aged Children and Adolescents Are Associated with Prematurity after Controlling for IQ. Neuropsychologia. 2011;49:906–913. doi: 10.1016/j.neuropsychologia.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, King WM, Freeman A. Anatomical Risk Factors for Phonological Dyslexia. Cereb Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ. Cerebellar Injury in the Premature Infant Is Associated with Impaired Growth of Specific Cerebral Regions. Pediatr Res. 2010;68:145–150. doi: 10.1203/PDR.0b013e3181e1d032. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. Late Gestation Cerebellar Growth Is Rapid and Impeded by Premature Birth. Pediatrics. 2005a;115:688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, Robertson RL, Moore M, Akins P, Volpe JJ and others. Impaired Trophic Interactions between the Cerebellum and the Cerebrum among Preterm Infants. Pediatrics. 2005b;116:844–850. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Küper M and others. Consensus Paper: Language and the Cerebellum: An Ongoing Enigma. Cerebellum. 2013;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP. The Lateralized Linguistic Cerebellum: A Review and a New Hypothesis. Brain Lang. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Mezer A, Yeatman JD, Stikov N, Kay KN, Cho N-J, Dougherty RF, Perry ML, Parvizi J, Hua LH, Butts-Pauly K and others. Quantifying the Local Tissue Volume and Composition in Individual Brains with Magnetic Resonance Imaging. Nat Med. 2013;19:1667–1672. doi: 10.1038/nm.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-Dimensional Tracking of Axonal Projections in the Brain by Magnetic Resonance Imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PC, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. Preterm Birth Results in Alterations in Neural Connectivity at Age 16 Years. Neuroimage. 2011;54:2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson J, LIlja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H and others. Preterm Children Have Disturbances of White Matter at 11 Years of Age as Shown by Diffusion Tensor Imaging. Pediatr Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Naidich T, Duvernoy H, Delman B, Sorenson A, Kollias S, Haacke E. Duvernoy's Atlas of the Human Brain Stem and Cerebellum: High-Field MRI, Surface Anatomy, Internal Structure, Vascularization and 3 D Sectional Anatomy. Vienna: Springer; 2009. [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric Permutation Tests for Functional Neuroimaging: A Primer with Examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends Neurosci. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left Lateralized White Matter Microstructure Accounts for Individual Differences in Reading Ability and Disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain Connectivity in Non-Reading Impaired Children and Children Diagnosed with Developmental Dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci. 2009;10:67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S, Fox P, Posner M, Mintun M, Raichle ME. Positron Emission Tomographic Stuides of the Cortical Anatomy of Single-Word Processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Peterson S, Fox P, Posner M, Mintun M, Raichle ME. Positron Emission Tomographic Studies of the Processing of Single Words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion Tensor Mr Imaging of the Human Brain. Radiol. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Press W, Teukolsky S, Vetterling W, Flannery B. Numerical Recipes in C++:The Art of Scientific Computing. Cambridge, UK: Cambridge Univ Press; 2002. [Google Scholar]
- Price CJ. A Review and Synthesis of the First 20 Years of Pet and Fmri Studies of Heard Speech, Spoken Language and Reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, Dixon RM, Lee MA, Thompson CH, Styles P, Richardson AF, Stein JF. Cerebellar Morphology in Developmental Dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The Primate Cortico-Cerebellar System: Anatomy and Function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Richards T, Stevenson J, Crouch J, Johnson LC, Maravilla K, Stock P, Abbott R, Berninger V. Tract-Based Spatial Statistics of Diffusion Tensor Imaging in Adults with Dyslexia. AJNR. 2008;29:1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive Approach for Correction of Motion and Distortion in Diffusion-Weighted Mri. Magn Reson Med. 2004;51:103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Saksena S, Husain N, Malik GK, Trivedi R, Sarma M, Rathore RS, Pandey CM, Gupta RK. Comparative Evaluation of the Cerebral and Cerebellar White Matter Development in Pediatric Age Group Using Quantitative Diffusion Tensor Imaging. Cerebellum. 2008;7:392–400. doi: 10.1007/s12311-008-0041-0. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination Revealed through Mri as Increased Radial (but Unchanged Axial) Diffusion of Water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH, Armstrong RC. Demyelination Increases Radial Diffusivity in Corpus Callosum of Mouse Brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Srinivasan L, Allsop J, Counsell SJ, Boardman JP, Edwards AD, Rutherford M. Smaller Cerebellar Volumes in Very Preterm Infants at Term-Equivalent Age are Associated with the Presence of Supratentorial Lesions. AJNR. 2006;27:573–579. [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller H, Juengling F, Kassubek J, Riecker A. The Contribution of White and Gray Matter Differences to Developmental Dyslexia: Insights from DTI and VBM at 3.0T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S. Diffusion Tensor Imaging and Axonal Tracking in the Human Brainstem. Neuroimage. 2001;14:723–735. doi: 10.1006/nimg.2001.0861. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Stein JF. The Cerebellum and Dyslexia. Cortex. 2011;47:101–116. doi: 10.1016/j.cortex.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The Cerebellum and Cognitiion: Evidence from Functional Imaging Studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional Topography of the Cerebellum for Motor and Cognitive Tasks: An Fmri Study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P, Dum R, Fiez J. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DG, Catani M. Atlasing Location, Asymmetry and Inter-Subject Variability of White Matter Tracts in the Human Brain with Mr Diffusion Tractography. Neuroimage. 2011;54:49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P. Resolving Crossing Fibres Using Constrained Spherical Deconvolution: Validation Using Diffusion-Weighted Imaging Phantom Data. Neuroimage. 2008;42:617–625. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High Angular Resolution Diffusion Imaging Reveals Intravoxel White Matter Fiber Heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquière P. A Qualitative and Quantitative Review of Diffusion Tensor Imaging Studies in Reading and Dyslexia. Neurosci Biobehav Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in White Matter Diffusion Anisotropy in Adolescents Born Prematurely. Neuroimage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain Injury in Premature Infants: A Complex Amalgam of Destructive and Developmental Disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Barmack NH. Oculomotor Cerebellum. Prog Brain Res. 2006;151:231–268. doi: 10.1016/S0079-6123(05)51008-2. [DOI] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P. Microstructural Correlations of White Matter Tracts in the Human Brain. Neuroimage. 2010;51:531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Yeatman JD. Biological Development of Reading Circuits. Curr. Opin. Neurobiol. 2013;23:261–268. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Weschler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation/ A brand of Harcourt Assessment, Inc; 1999. [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. Diffusion Spectrum Magnetic Resonance Imaging (Dsi) Tractography of Crossing Fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. About “Axial” and “Radial” Diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Woodcock LJ, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca: Riverside Publishing; 2001. [Google Scholar]
- Yeatman J, Wandell B, Mezer A. Lifespan Maturation and Degeneration of Human Brain White Matter. Nature Comm In press. 2014 doi: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Feldman HM. Neural Plasticity after Pre-Linguistic Injury to the Arcuate and Superior Longitudinal Fasciculi. Cortex. 2013;49:301–311. doi: 10.1016/j.cortex.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PloS One. 2012;7(11):e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, Ben-Shachar M. Anatomical Properties of the Arcuate Fasciculus Predict Phonological and Reading Skills in Children. J Cogn Neurosci. 2011;23:3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.