Abstract
Alcohol dependence is frequently co-morbid with cognitive impairment. The relationship between these traits is complex as cognitive dysfunction may arise as a consequence of heavy drinking or exist prior to the onset of dependence. In the present study we tested the genetic overlap between cognitive abilities and alcohol dependence using polygenic risk scores (PGRS). We created two independent PGRS derived from two recent genome-wide association studies (GWAS) of alcohol dependence (SAGE GWAS: N=2750 & Yale-Penn GWAS: N=2377) in a population-based cohort, Generation Scotland: Scottish Family Health Study (GS:SFHS) (N=9863). Data on alcohol consumption and 4 tests of cognitive function: Mill Hill Vocabulary (MHV), Digit Symbol Coding, Phonemic Verbal Fluency (VF) and Logical Memory were available. PGRS for alcohol dependence were negatively associated with two measures of cognitive function: MHV (SAGE: p=0.009, β=−0.027& Yale-Penn: p=0.001,β=−0.034) and VF (SAGE: p=0.0008, β=−0.036 & Yale-Penn: p=0.00005, β=−0.044). VF remained robustly associated after adjustment for education and social deprivation however the association with MHV was substantially attenuated. Shared genetic variants may account for some of the phenotypic association between cognitive ability and alcohol dependence. A significant negative association between PGRS and social deprivation was found (SAGE: p=5.2 × 10−7, β=−0.054 & Yale-Penn: p=0.000012, β=−0.047). Individuals living in socially deprived regions were found to carry more alcohol dependence risk alleles which may contribute to the increased prevalence of problem drinking in regions of deprivation. Future work to identify genes which affect both cognitive impairment and alcohol dependence will help elucidate biological processes common to both disorders.
Keywords: Alcohol dependence, cognition, environment, genetics, polygenic, social deprivation
Introduction
Alcohol dependence is characterized by a maladaptive pattern of alcohol consumption that can lead to tolerance, withdrawal and a loss of control over intake that has negative psychological and physiological consequences. Cognitive impairment is a common feature of alcohol dependence (Hester et al., 2010) and persists after alcohol detoxification in 50–75% of cases (Parsons and Nixon, 1998; Smith and Atkinson, 1995). The relationship between alcohol dependence and cognitive impairment is complex. Cognitive impairment may increase the risk of alcohol dependence or arise as a consequence of prolonged heavy drinking. Family studies comparing non-alcoholic children of alcoholics to those with a negative family history of alcoholism find those with alcoholic parents perform worse on tests of executive function (Gierski et al., 2013; Ozkaragoz et al., 1997) suggesting that cognitive impairment may precede the onset of dependence.
Epidemiological studies have shown higher childhood IQ to be associated with less alcohol-induced hangovers in adulthood (Batty et al., 2006). However, higher childhood mental ability has also been associated with increased alcohol intake and alcohol related problems in adulthood (Batty et al., 2008). The relationship between alcohol dependence and cognitive ability is confounded by environmental exposures that obscure observational associations and causality. Social deprivation and the number of years spent in education correlate strongly with cognitive ability. Alcohol consumption is positively correlated with socio-economic status and education level (Corley et al., 2011; Grittner et al., 2012; Huerta and Borgonovi, 2010) however problem drinking is more prevalent in regions of social deprivation (Bromley et al., 2012). These factors interact as the effect of socioeconomic status negatively impacts cognitive function in individuals with a positive family history of alcoholism (Lovallo et al., 2013). Furthermore, social deprivation and education have a substantial genetic component that has been shown to overlap with the genetic basis of cognitive ability in this sample (Marioni et al., 2014).
A common genetic etiology may explain some of the overlap between alcohol dependence and cognitive impairment. The estimated heritabilities for alcohol dependence and adult general cognitive ability range from ~40–70% (Calvin et al., 2012; Enoch and Goldman, 2001; Haworth et al., 2010) however, few studies have examined their genetic overlap. One established approach to detect shared genetic effects between traits is to use polygenic risk profiling (Evans et al., 2013; Purcell et al., 2009). Summary data from a genome-wide association study (GWAS) of a disease of interest is used to determine the weighted number of risk alleles an individual in an independent sample carries. Polygenic risk profiles thus denote an individual’s genetic load for a particular disorder. By testing the association between a polygenic risk score for alcohol dependence and potential biological intermediates (cognitive ability) we are able to analyze the relationship between the two traits without having to measure alcohol dependence directly in the cohort being studied. Furthermore, associations between polygenic risk profiles and biological intermediates will not be confounded by environmental exposures and may highlight potentially causal pathways which warrant further study (Evans et al., 2013).
In the present study we calculated 2 independent polygenic risk profiles for alcohol dependence in 9863 members of Generation Scotland: the Scottish Family Health Study (GS:SFHS), a population based epidemiological cohort (Smith et al., 2006). Two polygenic risk scores (PGRS) were created using summary data from two independent European-American GWAS of alcohol dependence (SAGE, N=2750 & Yale-Penn, N=2377) (Gelernter et al., 2013). The GWAS summary data were meta-analysed and a third polygenic risk score created from the meta-analysis data. The Yale-Penn and SAGE datasets are the discovery samples from which GWAS summary statistics were derived and GS:SFHS is the sample in which polygenic risk scores were created and analysed. Alcohol dependence was not measured in GS:SFHS individuals and therefore polygenic risk profiles were used to explore the genetic relationship between alcohol dependence, cognitive ability, education and social deprivation.
Methods
Generation Scotland : the Scottish Family Health Study (GS:SFHS)
GS:SFHS is a family based epidemiological cohort recruited at random through general medical practices across Scotland. The protocol for recruitment is described in detail elsewhere (Smith et al., 2006). The cohort consists of 21,516 individuals over 18 years of age recruited if they had at least one other family member willing to participate. Genome-wide SNP data are available for 9863 individuals, and these are the individuals who are described and whose data are used in the present study (mean age = 52.2, SD=13.64) (5788 female, 4075 male).
Demographic information available included socio-economic deprivation measured using the Scottish Index of Multiple Deprivation (SIMD) 2009 matched to each participant’s postcode (Government, 2009). SIMD is not a direct measure of an individual’s socio-economic status, but is a ranking for their local area (6505 areas in total with an area population mean of ~800). It is derived from data on employment, income, health, education, housing, crime and access to services. SIMD is a rank number from 1 to 6505 and the lower the number the more socially deprived the geographical region. Each participant self-completed a pre-clinic questionnaire, which included information on their education by asking, ‘”how many years altogether did you attend school/study full-time?”’
Cognitive abilities were assessed using four tests. Verbal ability was assessed using the Mill Hill Vocabulary Scale, junior and senior synonyms (Raven, 1965). Immediate and delayed scores from the recall section of one story of the Wechsler Logical Memory test were summed to provide a measure of verbal declarative memory (Wechsler, 1998). The Wechsler Digit Symbol Coding test was used to measure processing speed (Wechsler, 1998). Executive function was measured using the letter-based phonemic verbal fluency test (letters C, F and L, for one minute each) (Lezak, 1995).
Alcohol consumption was assessed using a pre-clinical questionnaire. Participants were identified as current drinkers, former drinkers, or never drinkers. Consumption was measured in self-reported units of alcohol consumed in the previous week and converted into grams of alcohol/kg/week by multiplying units by 7.9 and dividing by the participant’s weight (measured in the research clinic) in kg. All components of GS:SFHS have received ethical approval from the NHS Tayside Committee on Medical Research Ethics (REC Reference Number: 05/S1401/89).
Genotyping and QC
Blood samples were collected using standard operating procedures and stored at the Wellcome Trust Clinical Research Facility Genetics Core, Edinburgh (www.wtcrf.ed.ac.uk) where they were genotyped using the IlluminaHumanOmniExpressExome -8v1.0 BeadChip and Infinum chemistry (Gunderson, 2009). The genotypes were then processed using the IlluminaGenomeStudio Analysis software v2011.1. The details of blood collection and DNA extraction are provided elsewhere (Smith et al., 2006).
Polygenic Profiling
Genotyping quality control was performed and SNPs with a minor allele frequency (MAF) <5%, significant deviation from Hardy-Weinberg equilibrium (p<0.001), or a call rate <98% were removed from further analyses. Individuals with genotyping call rates lower than 95% were also removed. Any strand-ambiguous SNPs were removed from the GS:SFHS dataset and genotypes were LD pruned using clump-based pruning (r2=0.25, 300kb window) to create a set of SNPs in linkage equilibrium. GS:SFHS genetic data included only raw-genotypes (unimputed data) and therefore only SNPs common to the Yale-Penn/SAGE samples and GS:SFHS were used to create polygenic scores.
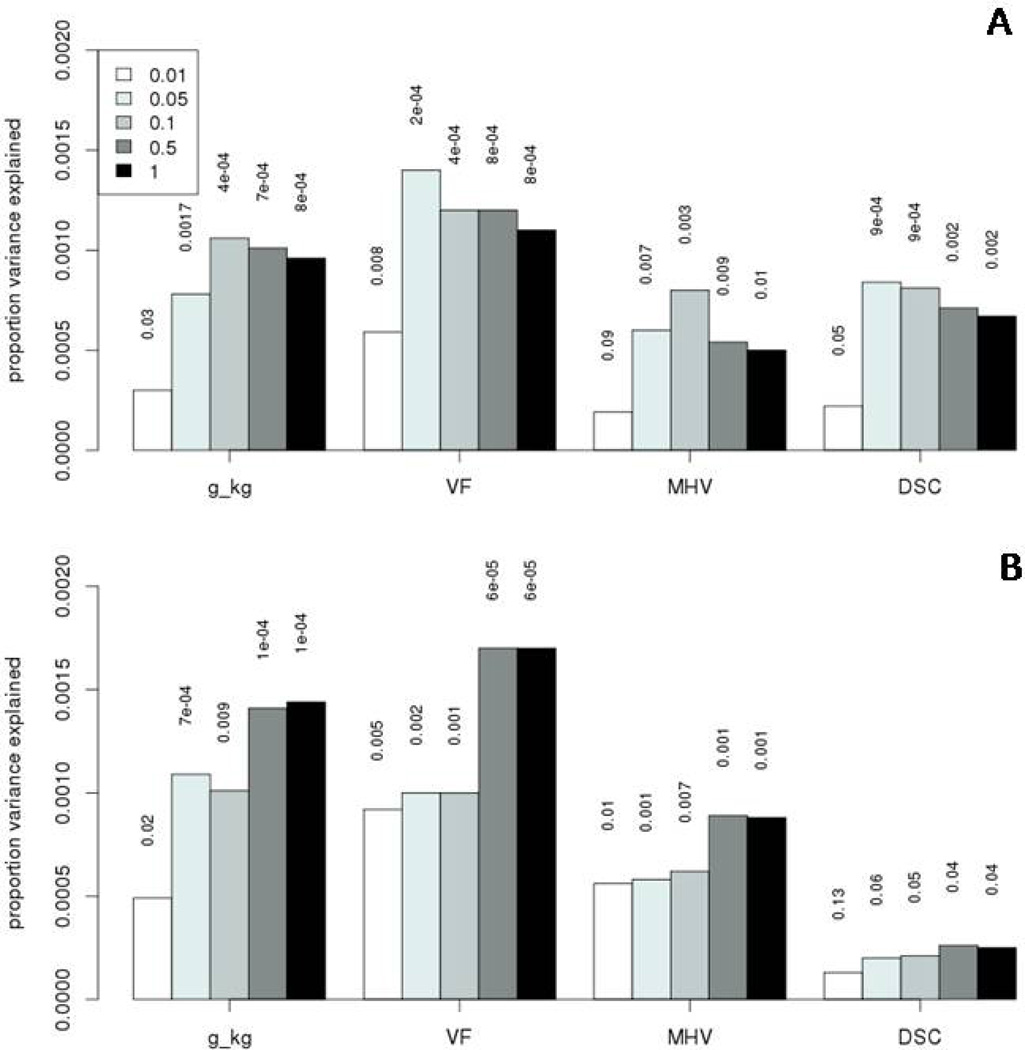
Polygenic risk scores for alcohol dependence were created in GS:SFHS using PLINK as previously described in detail (Purcell et al., 2009) using the summary GWAS data from the two independent (SAGE and Yale-Penn) GWAS of alcohol dependence (Gelernter et al., 2013). Both the Yale-Penn and SAGE alcohol dependence GWAS used an ordinal model to test for association. The imputed SNP allele dosage was the dependent variable and DSM-IV symptom counts for alcohol, cocaine and opioid dependence (adjusted for age, sex and ancestry principal components) were ordinal predictors. For 5,708,204 high quality SNPs with p-values in both the Yale-Penn and SAGE data sets, inverse variance meta-analysis was performed using METAL (Willer et al., 2010). These summary data were used to create a meta-analysis polygenic risk score for alcohol dependence (N=5127) in GS:SFHS. Polygenic risk scores were created using SNPs associated with alcohol dependence with p-value thresholds of 0.01, 0.05, 0.1, 0.5 and 1 in the Yale-Penn and SAGE GWAS; however, only data from the p-values for the ≤0.5 threshold are presented in the tables as these generally explained the largest amount of variance in the dependent variable (Figure 1 shows significance and variance explained at all p-value thresholds).
Figure 1.
A large majority (87%) of participants were born in Scotland and 95% in the United Kingdom. Roughly 82% of parents and 75% of grandparents were also born in Scotland. 99% of participants defined their ethnicity as ‘white’ (Smith et al., 2013). Multi-dimensional scaling (MDS) components were created according to the ENIGMA 1000 genomes protocol (ENIGMA, 2013) in the software package PLINK (Purcell et al., 2007). A plot of the first and second MDS components showing the genotyped individuals in GS:SFHS and 11 different ethnic HapMap populations is shown in eFigure 1. The GS:SFHS individuals cluster tightly with the HapMap CEU population and show little admixture with the other ethnic populations. Four MDS components were used to correct for population stratification.
The polygenic risk scores presented throughout the manuscript were created using all autosomal SNPs. However, as several known alcohol metabolism genes are located on chromosome 4q23 (ADH4, ADH5, ADH6, ADH1A, ADH1B, ADH1C), a separate polygenic risk score was calculated with chromosome 4 SNPs excluded to provide an estimate of polygenic risk for alcohol dependence that was independent of the SNPs within these loci.
Statistical Analysis
All variables were transformed towards normality where necessary using the Box-Cox transformation procedure implemented in the MASS package in R (Venables and Ripley, 2002). The ASReml-R (www.vsni.co.uk/software/asreml) software package was used to implement mixed linear model association analyses. Family structure was fitted as a random effect by creating an inverse of a relationship matrix using pedigree kinship information. All variables were scaled to have a mean of 0 and a standard deviation of 1, and the reported values of beta are standardized. Wald’s conditional F-test was used to calculate p-values for fixed effects. The proportion of variance explained by polygenic risk scores was calculated by taking the change in the sum of the residual variance and the additive genetic variance after removing the polygenic risk score from the model, then dividing this by the sum of residual variance and the additive genetic variance. Pearson’s correlations were used to determine the correlation between the two polygenic risk scores and alcohol consumption and cognitive/demographic variables, in unrelated individuals (N=6413) (eTable 1).
The validity of the alcohol dependence polygenic risk score was analysed by testing for association between polygenic risk for alcohol dependence and alcohol consumption. We then tested the association with cognitive ability test scores. Alcohol consumption was included as a fixed-effect covariate for all analyses except where alcohol consumption was the dependent variable. Any association between polygenic risk for alcohol dependence and cognitive ability would therefore reflect genetic overlap between the traits without the confounding effect of alcohol consumption. Two models were fit to test these associations, an unadjusted model which had age, sex, alcohol consumption, 4 MDS components and the polygenic risk score as fixed-effects. An adjusted model was also fitted which included social deprivation and education as additional fixed-effects.
To test the interaction of SIMD and polygenic risk score with cognitive measures as the dependent variable age, sex, alcohol consumption, polygenic risk score and SIMD were included as main effects and the interaction term ‘polygenic risk score*SIMD’. To control for confounders, each covariate was entered as an interaction with the genetic (polygenic risk score) and environmental (SIMD) effect as recommended elsewhere (Keller, 2013). The p-values presented are raw p-values uncorrected for multiple testing. False discovery rate (FDR) was implemented in the R package ‘fdrtools’ to estimate the local false discovery rate.
Results
Relationship of polygenic risk for alcohol dependence to alcohol consumption
The Pearson’s correlation between the 2 polygenic risk scores for alcohol dependence derived from the SAGE and Yale-Penn GWAS was 0.61. Polygenic risk for alcohol dependence in GS:SFHS was positively associated with alcohol consumption (SAGE:β=0.045, p=0.00003 & Yale-Penn: β=0.042, p=0.00009) (Table 1). The proportion of variance in alcohol consumption explained by risk scores was low: less than 0.2% (Figure 1). Using the polygenic risk score derived from the meta-analysis of the SAGE and Yale-Penn GWAS the effect size and variance explained increased modestly (β=0.049, p=2.8 × 10−6, r2=0.0022) (Table 1). The SAGE and Yale-Penn alcohol scores remained significantly associated with alcohol consumption when chromosome 4 SNPs were removed (Yale-Penn:β=0.034, p=0.001, r2=0.00102 & SAGE: β=0.036, p=0.0006, r2=0.0012) (eTable 1). The effect size (β) was reduced by 14–24% (Yale-Penn and SAGE scores respectively). Although chromosome 4 SNPs explain some of the variance in alcohol consumption, a significant polygenic risk signal is present on the remaining chromosomes.
Table 1.
Association of AD polygenic risk with alcohol consumption, education and SIMD. All associations are adjusted for age, sex and 4 MDS components to adjust for population stratification. Covariate adjusted models are presented with the inclusion of the other traits as covariates, e.g. alcohol consumption is adjusted for education and SIMD. P-values highlighted in bold remain significant after FDR correction for multiple comparisons.
| Trait | SAGE AD polygenic risk score |
SAGE AD polygenic risk score + covariates |
SAGE AD polygenic risk score |
SAGE AD polygenic risk score + covariates |
Meta-Analysis AD polygenic risk score |
Meta-Analysis AD polygenic risk score + covariates |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stats | P | Stats | P | Stats | P | Stats | P | Stats | P | Stats | P | |
| Alcohol Consumption | β =0.035, r2= 0.001 | 0.0007 | β = 0.045, r2=0.0019 | 0.00003 | β =0.04, r2=0.0014 | 0.00012 | β = 0.042, r2=0.0017 | 0.00009 | β =0.049, r2=0.002 | 2.8 × 10−6 | β =0.056, r2=0.003 | 2.1 × 10−7 |
| Education | β =−0.055, r2=0.0028 | 2.6 × 10−7 | β = −0.043, r2=0.0016 | 0.00006 | β =−0.033, r2=0.0009 | 0.0021 | β = −0.022, r2=0.0003 | 0.035 | β =−0.049, r2=0.0021 | 5.4 × 10−6 | β =0.035, r2=0.001 | 0.001 |
| SIMD | β =−0.054, r2=0.0028 | 5.2 × 10−7 | β = −0.044, r2=0.0018 | 0.00005 | β =−0.047, r2=0.002 | 0.000012 | β = −0.046, r2=0.0018 | 0.00003 | β =−0.063, r2=0.004 | 1.1 × 10−8 | β =0.056, r2=0.003 | 2.6 × 10−7 |
Polygenic risk scores derived from SNPs with a GWAS p-value ≤ 0.5 are presented.
Relationship of polygenic risk for alcohol dependence to socio-economic status and education
Social deprivation and the number of years in education were negatively associated with polygenic risk for alcohol dependence in GS:SFHS. Individuals living in more socially deprived regions (SAGE: β=−0.044, p=0.00005 & Yale-Penn: β=−0.046, p=0.00003) and who had spent fewer years in education (SAGE: β=−0.043, p=0.00006 & Yale-Penn: β=−0.022, p=0.04) had a significantly higher polygenic risk for alcohol dependence even after covariate adjustment (Table 1). As Mill Hill vocabulary test performance is correlated with social deprivation and education (r=0.24, p p=<2.2 × 10−16) (eTable 2) the association between alcohol dependence polygenic risk and deprivation was also tested in GS:SFHS after controlling for Mill Hill vocabulary. The negative association between polygenic risk and SIMD has a similar effect size after controlling for Mill Hill vocabulary (SAGE: p=0.000007, β =−0.047, Yale-Penn: p=0.0002, β=−0.039). However, education was not significantly associated with polygenic risk, and the effect sizes were markedly reduced, after the same adjustment (SAGE: p=0.44, β =−0.008 & Yale-Penn: p=0.41, β =0.008). The amount of variance in education and SIMD explained by polygenic risk for alcohol dependence was less than 0.36% across all models. The association between the meta-analysis PGRS and demographic and cognitive variables is presented in Table 1 and Table 2.
Table 2.
Relationship between SAGE, Yale-Penn and Meta-Analysis polygenic risk score and cognitive variables. Results are shown correcting for age + sex + 4 MDS components in the first instance, and with the addition of social deprivation (SIMD) education in the full model (score + covariates). P-values highlighted in bold remain significant after FDR correction for multiple comparisons.
| Polygenic Risk Score | Verbal Fluency | Mill Hill Vocabulary | Digit Symbol Coding | Logical Memory | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta (S.E.) |
r2 | p- value |
Beta (S.E.) |
r2 | p- value |
Beta (S.E.) |
r2 | p- value |
Beta (S.E.) |
r2 | p- value |
|
| SAGE score | −0.036 (0.01) | 0.001 | 0.0008 | −0.027 (0.01) | 0.0005 | 0.009 | −0.029 (0.009) | 0.0007 | 0.002 | −0.011 (0.01) | 4 × 10−6 | 0.28 |
| SAGE score + covariates | −0.024 (0.01) | 0.0004 | 0.027 | 0.001 (0.01) | 0 | 0.92 | −0.012 (0.009) | 3.8 × 10−5 | 0.24 | 0.0007 (0.01) | 0 | 0.94 |
| Yale-Penn score | −0.044 (0.01) | 0.002 | 5 × 10−5 | −0.034 (0.01) | 0.0009 | 0.001 | −0.019 (0.009) | 0.0003 | 0.04 | −0.015 (0.01) | 9.4 × 10−5 | 0.15 |
| Yale-Penn score + covariates | −0.048 (0.01) | 0.002 | 1 × 10−5 | −0.017 (0.01) | 0.0002 | 0.09 | −0.004 (0.009) | 0 | 0.66 | −0.012 (0.01) | 3 × 10−5 | 0.25 |
| Meta-Analysis score | −0.046 (0.01) | 0.002 | 3.6 × 10−5 | −0.035 (0.01) | 0.001 | 0.0009 | −0.028 (0.009) | 0.0009 | 0.003 | −0.015 (0.01) | 0.0001 | 0.152 |
| Meta-Analysis score + covariates | −0.034 (0.01) | 0.0009 | 0.002 | −0.009 (0.01) | 0 | 0.36 | −0.01 (0.009) | 7.2 × 10−6 | 0.31 | −0.005 (0.01) | 0 | 0.6 |
Polygenic risk scores derived from SNPs with a GWAS p-value ≤ 0.5 are presented.
Relationship of polygenic risk for alcohol dependence to cognitive function
A significant negative association between polygenic risk for alcohol dependence and performance on the verbal fluency test was observed (SAGE: β=−0.036, p=0.0008 & Yale-Penn: β=−0.044, p=0.00005) (Table 2). A significant negative association between Mill Hill vocabulary and alcohol dependence polygenic risk was also found (SAGE: β=−0.027, p=0.009 & Yale-Penn β=−0.034, p=0.001) although the SAGE p-value did not withstand correction for multiple testing. Digit symbol coding was negatively associated with polygenic risk for alcohol dependence (SAGE: β=−0.029, p=0.002, Yale-Penn: β=−0.019, p=0.04); however, the replication in the Yale-Penn dataset is not significant after correction for multiple testing. All associations between polygenic risk scores and cognitive measures have been corrected for self-reported alcohol consumption.
Education is strongly correlated with performance on the Mill Hill vocabulary test and the number of years spent in education (eTable 2) and SIMD, and intelligence are genetically correlated in this sample (Marioni et al., 2014). When education and SIMD were added as covariates to the model, the association between alcohol dependence risk score and cognitive function was substantially attenuated and no longer significant for Mill Hill vocabulary and digit symbol coding. Polygenic risk for alcohol dependence was significantly associated with verbal fluency and the effect sizes remained similar or reduced by ~33% after controlling for education and SIMD (SAGE: β=−0.024, p=0.027 & Yale-Penn: β=−0.048, p=0.00001) (Table 1), although the SAGE score association did not withstand correction for multiple testing.
Previous epidemiological studies have found a positive association between alcohol consumption and cognitive ability (Britton et al., 2004; Corley et al., 2011) and this was replicated in GS:SFHS (eTable 2). The proportion of variance in cognitive test scores explained by polygenic risk scores are shown in Figure 1 (less than 0.2% in all cases), where only measures of cognitive ability that are significantly associated with both SAGE and Yale-Penn polygenic risk scores are presented.
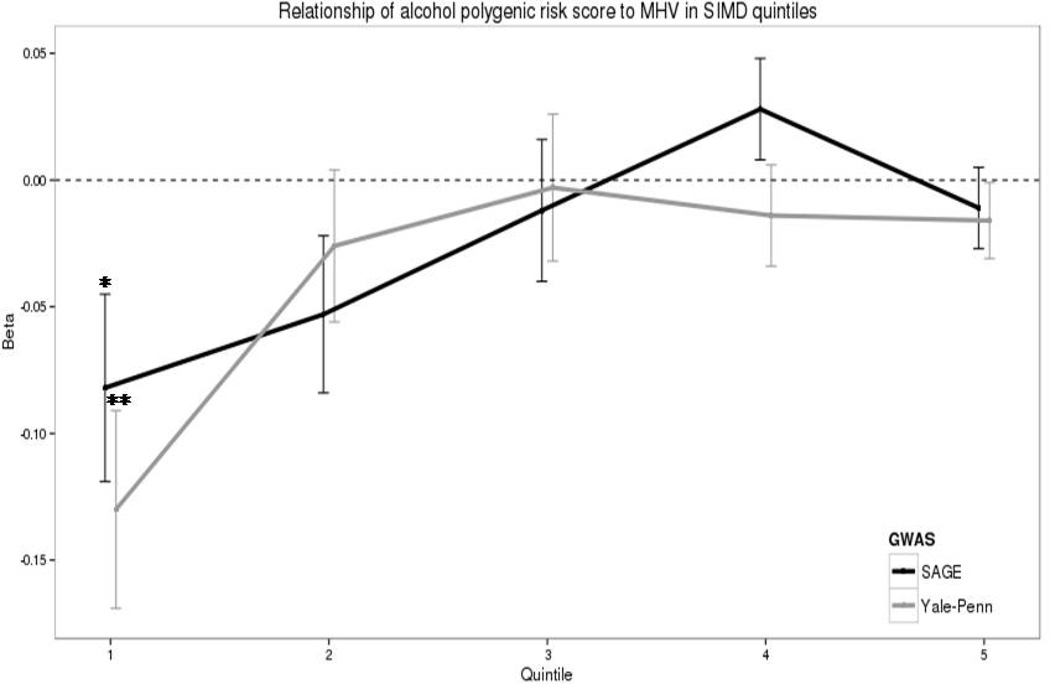
An interaction between SIMD and polygenic risk was observed when Mill Hill vocabulary was analysed (SAGE: β =0.026, p =0.01 & Yale-Penn: β =0.024, p=0.02) (Table 3) but this did not withstand correction for multiple testing. When the meta-analysed score was tested a significant interaction was also found (β =0.031, p=0.004) and this remained significant after correction for multiple testing. For illustrative purposes, figure 2 shows the association between polygenic risk scores and Mill Hill vocabulary in each quintile of SIMD rank. The impact of alcohol dependence polygenic risk on Mill Hill vocabulary scores appears most pronounced in individuals in the most socially deprived quintile (1), whereas in the least socially deprived quintile (5), polygenic risk has almost no effect on Mill Hill vocabulary performance (Figure 2).
Table 3.
Association of the interaction term SIMD*polygenic risk score with cognitive variables previously shown to be associated with polygenic risk for alcohol dependence. Results shown are corrected for age, sex, 4 MDS components and SIMD and polygenic risk score are included as main effects.
| Polygenic Risk Score | Verbal Fluency | Mill Hill Vocabulary |
Digit Symbol Coding |
|||
|---|---|---|---|---|---|---|
| Beta (S.E.) |
p-value | Beta (S.E.) |
p-value | Beta (S.E.) |
p-value | |
| SAGE score * SIMD | −0.0003 (0.01) | 0.98 | 0.026 (0.01) | 0.01 | 0.019 (0.009) | 0.04 |
| Yale-Penn * SIMD | −0.0035 (0.01) | 0.75 | 0.024 (0.01) | 0.02 | 0.016 (0.009) | 0.09 |
| Meta-Analysis * SIMD | −0.0018 (0.01) | 0.87 | 0.031 (0.01) | 0.004 | 0.023 (0.009) | 0.01 |
Polygenic risk scores derived from SNPs with a GWAS p-value ≤ 0.5 are presented. Bold highlighted p-values are significant after FDR correction.
Figure 2.
Discussion
We find that polygenic risk for alcohol dependence is positively correlated with alcohol consumption in this Scottish population-based sample, using scores derived from two independent GWAS of alcohol dependence. Individuals with a higher genetic load for alcohol dependence reported consuming significantly more alcohol. Despite alcohol consumption being positively correlated with cognitive ability in this sample, alcohol dependence polygenic risk is negatively associated with three measures of cognitive function: digit symbol coding, Mill Hill vocabulary and verbal fluency. These associations were independent of self-reported alcohol consumption. When education and social deprivation were added as covariates to the models, only the negative association with verbal fluency remained. These data suggest that lower cognitive functioning may precede alcohol dependence in individuals with a high genetic loading for the disorder, particularly in the domain of executive function (verbal fluency). Alcohol dependence polygenic risk is negatively correlated with SIMD and education. Individuals who carry more alcohol dependence risk alleles tend to live in regions of social deprivation and have spent fewer years in education. This may contribute to the increased prevalence of harmful drinking and alcohol misuse by individuals living in regions of social deprivation. The amount of variance explained by polygenic risk for alcohol dependence across all traits was less than 0.3%.
Other studies have found that alcohol consumption is positively correlated with socio-economic status (Corley et al., 2011; Grittner et al., 2012) and a study of young adults in Britain found a positive relationship between education level and alcohol consumption (Huerta and Borgonovi, 2010). However, despite higher socio-economic classes consuming more alcohol, individuals living in areas of social deprivation carry the burden of problem drinking and alcohol related disease (Bromley et al., 2012). The Scottish Health Survey 2012 found that men in low income households were most likely to engage in harmful drinking behaviour. Furthermore, a recent study found that men living in Scotland’s most deprived area (SIMD quintile 1) were significantly more likely to have an alcohol use disorder than males living in the least deprived area (SIMD quintile 5) (32% vs 21%) (Bromley et al., 2012). In the Welsh Health Survey (2003/2004–2007) respondents in the most socially deprived regions reported the most binge-drinking (Fone et al., 2013). In the present study we find that alcohol consumption is positively correlated with socio-economic status but that individuals living in areas of social deprivation in Scotland tend to carry more alcohol dependence risk alleles. This may be one reason that individuals in areas of social deprivation are more likely to develop alcohol use disorders despite consuming less alcohol overall than the rest of the population. A previous study of GS:SFHS found social deprivation to have a significant genetic component (Marioni et al., 2014) and we find that this overlaps with common genetic variation that increases risk for alcohol dependence. Whilst it is clear that social deprivation is a potent environmental risk factor for alcohol dependence future studies should consider that there may be a genetic component to these exposures that is relevant for alcohol dependence.
Cognitive deficits are common in alcohol dependent individuals, particularly in the domain of executive function (Fernandez-Serrano et al., 2010). However, a recent meta-analysis of cognitive deficits in alcoholism found that most cognitive impairments abate a year after alcohol detoxification (Stavro et al., 2013). We find that polygenic risk for alcohol dependence is associated with poorer cognitive ability in some domains, particularly on verbal fluency – a measure of executive function, independent of alcohol consumption. Our findings are supported by family studies showing that non-dependent offspring of alcoholics score lower on tests of executive function and language than those with a negative family history of alcoholism (Gierski et al., 2013; Nigg et al., 2004; Tapert and Brown, 2000). Furthermore, higher childhood IQ is associated with fewer alcohol-induced hangovers in adulthood (Batty et al., 2006). It is possible that lower cognitive ability is a risk factor for alcohol dependence in individuals with a high genetic risk for the disorder.
In our study, the strength of the negative relationship between alcohol dependence polygenic risk and digit symbol coding and Mill Hill vocabulary scores was attenuated when social deprivation and education were added as covariates. This may be because education and social deprivation are strongly correlated with Mill Hill vocabulary, and much of the variance in Mill Hill vocabulary is removed when adjusting for these variables. The causal direction is moot: higher verbal ability might be the result of more education and living in a more affluent area, or vice versa, or influences might flow dynamically in both directions (Deary and Johnson, 2010). It is important to recognize that when education is added as a control variable it is not just as an environmental factor, partly-genetically influenced cognitive ability also drives educational attainment to some degree.
We find no effect of social deprivation or education on the association between verbal fluency and polygenic risk for alcohol dependence. Verbal fluency is believed to be a frontal lobe process (Fuster, 2008) as patients with frontal lobe damage are significantly impaired on tests of phonemic word fluency (Robinson et al., 2012). Damage to the frontal lobes induces behaviour typically associated with addiction, such as the inability to defer immediate rewards for greater delayed rewards (Berlin et al., 2004). Indeed, chronic alcohol consumption is associated with metabolic and morphological changes in the frontal lobes, particularly the pre-frontal cortex (Adams et al., 1993; Pfefferbaum et al., 1997). Grey matter reductions in the prefrontal cortex of alcoholics are correlated with worse executive function (Chanraud et al., 2007). Here we show that polygenic risk for alcohol dependence is negatively associated with phonemic verbal fluency, suggesting that frontal lobe deficits may be pre-existing risk factors for the development of alcohol dependence. However, because the present study is correlational in nature, evaluation of this relationship requires a prospective design in which cognitive function is evaluated prior to the onset of alcohol dependence.
A recent study of healthy young adults found that exposure to social and personal adversity is a risk factor for executive function deficits in individuals with a positive family history of alcohol dependence (Lovallo et al., 2013). In support of this, we find the impact of polygenic risk for alcohol dependence on Mill Hill vocabulary to be greatest in individuals living in the most socially deprived areas. However, we also find that individuals living in social deprivation tend to carry more alcohol dependence risk alleles, which in turn is correlated with worse performance on Mill Hill vocabulary tests. Therefore a gene × environment interaction cannot be readily differentiated from a gene – environment correlation. Furthermore, the interaction between polygenic risk score and social deprivation only withstood correction for multiple testing when the meta-analysed risk score was used and therefore these results need to be interpreted cautiously until replicated in an independent sample.
There are some limitations to this study. Information on alcohol dependence was not available in the GS:SFHS dataset and therefore we do not know to what extent subjects with alcohol dependence are influencing our analyses. In an independent study, the Scottish Health Survey 2012 found that 21% of men and 11% of women were classed as hazardous drinkers. However, as we are able to control for alcohol consumption it is unlikely to be a significant confounder, although our measure of alcohol consumption is based on self-report and potentially underestimated. Another limitation is that number of individuals in the original GWAS for alcohol dependence was relatively low (SAGE N=2377, Yale-Penn N=2750). Previous studies using polygenic risk scores have used GWAS datasets comprising 7000–22,000 individuals (McIntosh et al., 2013; Purcell et al., 2009). However, we were able to able to find a robust association with alcohol consumption using our risk scores suggesting they are valid tools to investigate the genetic overlap between disorders. The variation in weekly alcohol consumption explained by the alcohol dependence PGRS is low, less than 0.1%. We created a meta-analysed polygenic risk score that utilized summary statistics from the Yale-Penn and SAGE GWAS combined (N=5127) to try to increase the amount of variance explained in our traits of interest. This score explained 0.3% of the variance in alcohol consumption. A large GWAS of schizophrenia (N~150,000) was used to create polygenic risk scores in an independent schizophrenia cohort. These scores explained 7% of the variance in schizophrenia (Consortium, 2014) and demonstrate that as the size of a discovery GWAS increases the amount of variance explained by polygenic risk score increases. Considering that the genetic overlap between alcohol consumption and abuse is not perfect (rG=0.61) (Dick et al., 2011) and the original GWAS for alcohol dependence had fewer individuals it is understandable that the variance explained by polygenic risk scores is low. Finally, not all of the associations we report withstand correction for multiple testing. The associations between digit symbol coding, education and alcohol dependence polygenic risk scores were modest and do not survive correction for multiple testing across both datasets. However, it is notable that Mill Hill vocabulary, digit symbol coding and education are nominally associated with alcohol dependence polygenic risk with the same direction of effect observed for each score.
The data presented in this study provide evidence that polygenic risk for alcohol dependence associates with alcohol consumption, social deprivation and some domains of cognitive ability in a large population based sample. These findings allow us to understand better the biological mechanisms underlying these traits and their associations. Cognitive ability may not only be a result of chronic alcohol consumption, but a pre-disposing risk factor for the development of alcohol dependence, although longitudinal data are required to test this hypothesis. By understanding the relationship among alcohol dependence, social deprivation and cognitive ability we may identify individuals at high risk to develop alcohol dependence and inform health interventions to reduce the burden of alcohol misuse on society. Thus, prospective evaluation of the findings reported here may create a basis for focused prevention efforts.
Supplementary Material
Acknowledgements
We are grateful to the families who took part in GS:SFHS, the GPs and Scottish School of Primary Care for their help in recruiting them, and the whole GS team, which includes academic researchers, clinic staff, laboratory technicians, clerical workers, IT staff, statisticians and research managers.
Funding
The Chief Scientist Office of the Scottish Government and the Scottish Funding Council provided core support for Generation Scotland. GS:SFHS was funded by a grant from the Scottish Government Health Department, Chief Scientist Office, number CZD/16/6. Genotyping services for a part of the Yale GWAS study were provided by the Center for Inherited Disease Research (CIDR) and Yale University (Center for Genome Analysis). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University (contract number N01-HG-65403). The publicly available datasets used for the analyses described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000092.v1.p1 through dbGaP accession number phs000092.v1.p. Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through the NIH Genes, Environment and Health Initiative [GEI] (U01 HG004422). SAGE is one of the genome-wide association studies funded as part of the Gene Environment Association Studies (GENEVA) under GEI. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01 HG004446). Assistance with data cleaning was provided by the National Center for Biotechnology Information. Support for collection of datasets and samples was provided by the Collaborative Study on the Genetics of Alcoholism (COGA; U10 AA008401), the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392), and the Family Study of Cocaine Dependence (FSCD; R01 DA013423). Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NIH contract "High throughput genotyping for studying the genetic contributions to human disease" (HHSN268200782096C). The authors TKC and AMMcI acknowledge with gratitude the financial support received for this work from the Dr Mortimer and Theresa Sackler Foundation. PAT, DJP, IJD and AMMcI are members of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged. Supported in part by National Institutes of Health grants RC2 DA028909, R01 DA12690, R01 DA12849, R01 DA18432, R01 AA11330, R01 AA017535, P50 AA12870, MSTP T32GM07205, and CTSA 8UL1TR000142.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Macintyre S. Childhood IQ and life course socioeconomic position in relation to alcohol induced hangovers in adulthood: the Aberdeen children of the 1950s study. J Epidemiol Community Health. 2006;60:872–874. doi: 10.1136/jech.2005.045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Schoon I, Emslie C, Hunt K, Gale CR. Childhood mental ability and adult alcohol intake and alcohol problems: the 1970 British cohort study. Am J Public Health. 2008;98:2237–2243. doi: 10.2105/AJPH.2007.109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II Study. Am J Epidemiol. 2004;160:240–247. doi: 10.1093/aje/kwh206. [DOI] [PubMed] [Google Scholar]
- Bromley C, Corbett J, Day J, Doig M, Gharib W, Given L, Gray L, Leyland A, MacGregor A, Marryat L, Maw T, McConnville S, McManus S, Mindell J, Pickering K, Roth M, Sharp C. National Statistics :The Scottish Health Survey - The main report. A national statistics publication for Scotland. 2012 [Google Scholar]
- Calvin CM, Deary IJ, Webbink D, Smith P, Fernandes C, Lee SH, Luciano M, Visscher PM. Multivariate genetic analyses of cognition and academic achievement from two population samples of 174,000 and 166,000 school children. Behav Genet. 2012;42:699–710. doi: 10.1007/s10519-012-9549-7. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Consortium SWGotPG. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley J, Jia X, Brett CE, Gow AJ, Starr JM, Kyle JA, McNeill G, Deary IJ. Alcohol intake and cognitive abilities in old age: the Lothian Birth Cohort 1936 study. Neuropsychology. 2011;25:166–175. doi: 10.1037/a0021571. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Johnson W. Intelligence and education: causal perceptions drive analytic processes and therefore conclusions. Int J Epidemiol. 2010;39:1362–1369. doi: 10.1093/ije/dyq072. [DOI] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS. Measures of current alcohol consumption and problems: two independent twin studies suggest a complex genetic architecture. Alcohol Clin Exp Res. 2011;35:2152–2161. doi: 10.1111/j.1530-0277.2011.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENIGMA. The Enhancing Neuroimaging Genetics through Meta Analysis (ENIGMA) Consortium : ENIGMA2 Genetics Support Team. ENIGMA2 1KGP Cookbook (v3) 2013 [Online]. [Google Scholar]
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep. 2001;3:144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- Evans DM, Brion MJ, Paternoster L, Kemp JP, McMahon G, Munafo M, Whitfield JB, Medland SE, Montgomery GW, Timpson NJ, St Pourcain B, Lawlor DA, Martin NG, Dehghan A, Hirschhorn J, Davey Smith G. Mining the human phenome using allelic scores that index biological intermediates. PLoS Genet. 2013;9:e1003919. doi: 10.1371/journal.pgen.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Fone DL, Farewell DM, White J, Lyons RA, Dunstan FD. Socioeconomic patterning of excess alcohol consumption and binge drinking: a cross-sectional study of multilevel associations with neighbourhood deprivation. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. 4th Edition. London: 2008. [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, Anton R, Preuss UW, Ridinger M, Rujescu D, Wodarz N, Zill P, Zhao H, Farrer LA. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2013;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, Cohen R, Kahn JP, Limosin F. Executive functions in adult offspring of alcohol-dependent probands: toward a cognitive endophenotype? Alcohol Clin Exp Res. 2013;37(Suppl 1):E356–E363. doi: 10.1111/j.1530-0277.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- Government TS. Scottish Index of Multiple Deprivation. 2009 http://www.scotland.gov.uk/topics/statistics/simd/.
- Grittner U, Kuntsche S, Gmel G, Bloomfield K. Alcohol consumption and social inequality at the individual and country levels--results from an international study. Eur J Public Health. 2012;23:332–339. doi: 10.1093/eurpub/cks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson KL. Whole-genome genotyping on bead arrays. Methods Mol Biol. 2009;529:197–213. doi: 10.1007/978-1-59745-538-1_13. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2010;15:1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Lubman DI, Yucel M. The role of executive control in human drug addiction. Curr Top Behav Neurosci. 2010;3:301–318. doi: 10.1007/7854_2009_28. [DOI] [PubMed] [Google Scholar]
- Huerta MC, Borgonovi F. Education, alcohol use and abuse among young adults in Britain. Soc Sci Med. 2010;71:143–151. doi: 10.1016/j.socscimed.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry. 2013;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. New York Oxford University Press; 1995. [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2013;37:616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Davies G, Hayward C, Liewald DC, Kerr SM, Campbell AC, Luciano M, Smith BH, Padmanabhan S, Hocking LJ, Hastie ND, Wright AF, Porteous DJ, Visscher PM, Deary IJ. Molecular genetic contributions to socioeconomic status and intelligence. Intelligence. 2014;44:7. doi: 10.1016/j.intell.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Gow A, Luciano M, Davies G, Liewald DC, Harris SE, Corley J, Hall J, Starr JM, Porteous DJ, Tenesa A, Visscher PM, Deary IJ. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;73:938–943. doi: 10.1016/j.biopsych.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Ozkaragoz T, Satz P, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14:31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. J Stud Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. The Mill Hill vocabulary scale. London H.K.Lewis; 1965. [Google Scholar]
- Robinson G, Shallice T, Bozzali M, Cipolotti L. The differing roles of the frontal cortex in fluency tests. Brain. 2012;135:2202–2214. doi: 10.1093/brain/aws142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BH, Campbell A, Linksted P, Fitzpatrick B, Jackson C, Kerr SM, Deary IJ, Macintyre DJ, Campbell H, McGilchrist M, Hocking LJ, Wisely L, Ford I, Lindsay RS, Morton R, Palmer CN, Dominiczak AF, Porteous DJ, Morris AD. Cohort Profile: Generation Scotland: Scottish Family Health Study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J Epidemiol. 2013;42:689–700. doi: 10.1093/ije/dys084. [DOI] [PubMed] [Google Scholar]
- Smith BH, Campbell H, Blackwood D, Connell J, Connor M, Deary IJ, Dominiczak AF, Fitzpatrick B, Ford I, Jackson C, Haddow G, Kerr S, Lindsay R, McGilchrist M, Morton R, Murray G, Palmer CN, Pell JP, Ralston SH, St Clair D, Sullivan F, Watt G, Wolf R, Wright A, Porteous D, Morris AD. Generation Scotland: the Scottish Family Health Study; a new resource for researching genes and heritability. BMC Med Genet. 2006;7:74. doi: 10.1186/1471-2350-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Atkinson RM. Alcoholism and dementia. Int J Addict. 1995;30:1843–1869. doi: 10.3109/10826089509071058. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth Edition. New York: Springer; 2002. [Google Scholar]
- Wechsler D. WAIS-III UK Administration and Scoring Manual. London: Psychological Corporation; 1998. [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.