Abstract
Previously, we screened 38 inbred mouse strains for susceptibility to monkeypox virus (MPXV) and focused on wild-derived CAST mice because of their extreme vulnerability. Here, we provide further analysis of inbred mouse strains. NZW/Lac and C58 mice exhibited more weight loss than other classical inbred strains but all survived intranasal challenges with 104 to106 PFU of MPXV. Mice from three wild derived strains, in addition to CAST, exhibited severe weight loss and died or were euthanized. LD50 values for CASA, MOLF and PERA were 100, 6800 and >105 PFU, respectively. CASA was inbred independently from the same founders as CAST, whereas MOLF and PERA are genetically and geographically distinct. The MPXV susceptibility of the F1 progeny of CAST and either C57BL/6 or BALB/c indicated that resistance is dominant. Back-crossing the F1 progeny of C57BL/6 and CAST to CAST suggested more than one independent resistant locus.
Keywords: Poxvirus, Select agent, Virulence, Pathogenesis
Introduction
Monkeypox virus (MPXV) is the most severe poxvirus infection of humans, excluding variola virus, and has been designated as a Select Agent by the United States government because of the potential to exploit MPXV for bioterrorism. MPXV primarily infects rodents in Africa but can be transmitted to other animals as well as humans. Human monkeypox clinically resembles smallpox except for lower mortality and fewer human-to-human transmissions (McCollum and Damon, 2014; Parker et al., 2007). A virulent strain of MPXV is prevalent in the rain forests of central Africa, particularly in the Democratic Republic of the Congo, whereas a milder strain is present in West Africa. The latter was imported to the United States with infected dormice, rope squirrels and giant pouched rats in 2003 and spread to closely housed North American prairie dogs and ultimately to humans, resulting in 47 laboratory confirmed and additional clinically diagnosed human cases (Hutson et al., 2007; Reynolds and Damon, 2012). The ability to infect prairie dogs and other wild rodents and the occurrence of sporadic human MPXV infections in countries neighboring the Democratic Republic of the Congo, contribute to concerns that monkeypox may be an emerging disease.
Several small animal models including the American black-tailed prairie dog, the thirteen-lined ground squirrel, and the African dormouse have been used for studies of MPXV pathogenicity, antivirals and vaccines (Hutson and Damon, 2010; Parker and Buller, 2013). However, except for the African dormouse these animals are not readily raised in captivity and there are no commercial sources of the latter. Moreover, immunological reagents are not available for these rodents. Although the commonly used classical inbred mouse strains are relatively resistant to MPXV, a few wild-derived inbred strains are susceptible (Americo et al., 2010) and one of these, the CAST/EiJ mouse, has been further studied (Americo et al., 2014; Earl et al., 2012). The susceptibility to MPXV varied by age and route and was greater by the intraperitoneal route (LD50 = 14 PFU) compared to the intranasal route (LD50 = 680 PFU) for 6-week old female mice (Americo et al., 2010). Scarification and footpad inoculation only caused local lesions. The low interferon γ response of CAST mice to infection with MPXV and the protection afforded by exogenous interferon γ may be clues to the nature of their susceptibility (Earl et al., 2012). Moreover, the sensitivity of CAST mice extends to other orthopoxviruses including vaccinia virus and cowpox virus (Americo et al., 2014). The primary purpose of the present study was to analyze the susceptibility to MPXV of mouse strains that showed less severe symptoms than CAST mice in the initial screen and to gain insight into the genetics of resistance by cross breeding sensitive and resistant strains.
Results
Resistance of classical inbred mouse strains to MPXV
We previously screened 38 mouse strains, of which 32 were classically inbred, from the Jackson Laboratory Phenome Project for sensitivity to an intranasal (i.n.) dose of 2x104 PFU of the virulent MPXV-Z79-CB2 virus (Americo et al., 2010). NZW/LacJ and C58/J exhibited an average maximum 14% weight loss, which was greater than any of the other classical inbred strains. In that screen, C57BL/6J mice lost 4% of their weight and BALB/cJ mice lost no weight. The resistance of BALB/c mice was confirmed by the absence of mortality after infection with doses up to 107 PFU. We considered, however, that NZW/Lac and C58 mice might be more susceptible to MPXV at higher doses than the 2 X 104 PFU used in the screen. To further evaluate their susceptibility, NZW/Lac and C58 mice were infected with several doses of MPXV. The animals were monitored for signs of disease including hunched posture, ruffled fur, and lethargy for up to 18 days. Weight loss was recorded daily and is shown as percent of the pre-infection weight (Fig. 1A,B). Both strains infected with 106 PFU displayed signs of disease including maximal weight loss of 20 to 23%. Loss of weight was first observed between days 3–5 post-infection and continued until days 6–10, after which animals showed improved health, increased weight and recovery from disease. As reported in the original screen (Americo et al., 2010), both NZW/Lac and C58 mice (Fig. 1A, B) exhibited greater weight loss than C57BL/6 (Fig. 2A,B,C). With the 106 PFU dose, the difference in weight loss of NZW/Lac mice relative to C57BL/6 was highly significant (p<0.004) each day from 8 onwards; for C58 mice the difference from C57BL/6 was significant each day from 4 through 12 (p=0.001 to 0.04). With all three strains, weight loss was delayed and less severe at 105 PFU than at the higher dose and at 104 PFU there was only minor weight loss and minimal disease. In summary, no deaths were observed in any of the classical inbred strains even with an inoculum of 106 PFU, indicating a high degree of resistance to MPXV.
Fig. 1.
Weight loss and survival of classical and wild derived inbred mouse strains infected i.n. with MPXV. Weight loss of groups (n=5) of female NZW/lac (A) and C58 (B) classical inbred mice infected with 104–106 PFU of MPXV are shown. Weight loss and survival of groups (n=3–5) of female CASA (C, F), MOLF (D, G) and PERA (E, H) wild-derived inbred mice infected with 102–106 PFU of MPX are shown. Doses of MPXV are indicated by color.
Fig. 2.
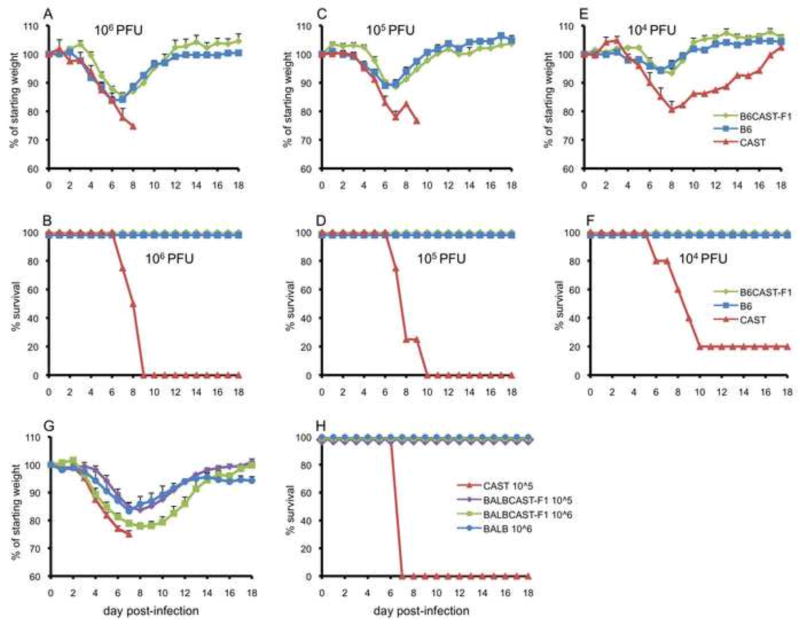
MPXV infection of the F1 generation of CAST mice crossed with C57BL/6 or BALB/c mice. Percent of starting weight (A, C, E) and survival (B, D, F) of parental CAST and C57BL/6 and F1 progeny infected i.n. with MPXV. BALB/c: 106 (n=5 male, 5 female), 105 (n=4 male, 4 female), 104 PFU (n=5 male, 5 female). CAST: 106 PFU (n=4 male), 105 PFU (n=4 male), 104 PFU (n=5 male). F1: 106 PFU (n=3 male, 3 female); 105 PFU (n=3 male, 3 female); 104 PFU (n=3 male, 3 female). Percent of starting weight (G) and survival (H) of parental CAST and BALB/c and F1 progeny infected i.n with MPXV. BALB/c: 106 PFU (n=5 female). CAST: 105 PFU (n=4 female). F1: 106 PFU (n=2 male, 4 female), 105 PFU (n=2 male, 3 female). Mouse strains are color-coded.
Susceptibility of wild-derived strains of mice to MPXV
In our initial multi-strain screen (Americo et al., 2010), three wild-derived strains showed signs of morbidity and mortality at the input dose of 2 × 104 PFU. CAST, MOLF/EiJ and PERA/EiJ mice exhibited greater than 20% weight loss and 100%, 75%, and 40% died or were euthanized, respectively. The sensitivity of CAST mice was further investigated and an LD50 of 680 PFU was determined (Americo et al., 2010). To more closely analyze the susceptibility of the MOLF, and PERA mice, we infected them i.n. with doses of MPXV ranging from 103 to 106 PFU. We also challenged CASA/RkJ mice, which were inbred independently from the same founder mice used to derive CAST mice, with 102 to 105 PFU of MPXV. The CASA mice lost substantial weight even at the lowest dose of 102 PFU (Fig. 1C), whereas MOLF (Fig. 1D) and PERA (Fig. 1E) mice lost substantial weight with 105 and 104 PFU but had only mild weight loss at 103 PFU. All CASA mice died or were euthanized after infection with 103 PFU or more (Fig. 1F) and a substantial number of MOLF mice succumbed at doses of 104 PFU (Fig. 1G) or more, whereas only 1 PERA mouse succumbed at 105 PFU (Fig. 1H). Based on these data, the LD50 values for CASA, MOLF and PERA were 100, 6,800 and >100,000 PFU, respectively. The LD50 of CASA was slightly lower than that previously determined for the closely related CAST strain (Americo et al., 2010). However, we continue to use CAST mice because of their greater availability than CASA.
Evidence for dominance of resistance over sensitivity to MPXV
In order to determine whether resistance of C57BL/6 mice to MPXV is a dominant or recessive trait, C57BL/6 female mice were crossed with CAST mice to produce F1 progeny. Groups of F1 mice (3 male and 3 female) as well as parental C57BL/6 and CAST mice were infected i.n. with 104, 105, or 106 PFU of MPXV. As shown in Fig. 2A-F, weight loss and survival of the F1 mice were indistinguishable from that of the resistant C57BL/6 parent regardless of sex. In the same experiment, parental CAST mice suffered severe weight loss and succumbed by day 10 post-infection with survival of only one mouse at the lowest dose (Fig. 2A–F). We also compared the F1 progeny with parental C57BL/6 and CAST mice infected with 103 PFU by the intraperitoneal route. Again, the F1 progeny were resistant to MPXV compared to CAST mice (data not shown). Thus, resistance to MPXV is a dominant characteristic of C57BL/6.
We also crossed female BALB/c mice with male CAST mice and infected the F1 progeny as well as the parental strains with 105 or 106 PFU of MPXV. The F1 progeny survived; whereas all CAST mice succumbed by day seven (Fig. 2G,H) indicating that resistance is dominant. However, at 106 PFU the F1 mice lost more weight than the parental BALB/c mice (p=0.03 to 0.004 from days 9 through 12) (Fig. 2G).
Evidence for multiple resistance loci to MPXV in C57BL/6 mice
To investigate whether dominance is due to more than a single genetic locus, the female F1 generation of parental C57BL/6 and CAST mice were backcrossed with male CAST mice. Nineteen progeny, 8 males and 11 females, were infected with 2 × 104 PFU of MPXV. Heterogeneity of weight loss was observed ranging from minimal C57BL/6-like to severe CAST-like for both male (Fig. 3A) and female (Fig. 3B) backcross mice. All 11 female backcross mice survived, whereas 3 of 8 male backcross mice (38%) did not (Fig. 3C). If resistance of C57BL/6 mice were determined by a single genetic locus, then 50% of the backcross progeny would be fully resistant and 50% fully sensitive. If resistance were determined by two independent loci, then 75% of the backcross progeny should be resistant and 25% sensitive. The survival of all female backcross mice strongly suggests multiple loci. A large number of backcross mice would be needed for single nucleotide polymorphism (SNP) analysis to identify resistant loci and evaluate the apparently greater sensitivity of male compared to female backcross mice.
Fig. 3.
MPXV infection of progeny from backcross of the F1 generation of parental CAST and C57BL/6 mice with CAST mice. Mice were infected i.n. with 2x104 PFU of MPXV. (A) Weight loss of male backcross progeny (n=8). (B) Weight loss of female backcross progeny (n=11). (C) Percent survival of mice. Mice are color coded according to key.
Discussion
Common classical inbred mice have mosaic genomes derived predominantly from the Western European Mus musculus domesticus with additional sequences mainly from the Japanese M. m. molossinus and exhibit limited diversity (Takada et al., 2013). Our previous (Americo et al., 2010) and present data demonstrating the relative resistance to MPXV infection displayed by more than 30 classical inbred strains, likely represent conserved genetic sequences. The two most sensitive classical inbred strains of 32 tested are NZW/Lac and C58, although all survived doses up to 106 PFU. In contrast, we found that genetically diverse wild-derived strains exhibit a broad range of susceptibilities to MPXV. CAST and CASA are the most sensitive with LD50 of less than 103, MOLF has intermediate sensitivity with a LD50 of less than 104 and PERA has a LD50 of greater than 105. CAST and CASA are species of M. m. castaneus that were derived from a small population of founder mice originally trapped in a grain storage facility in Thailand (JAX® NOTES Issue 456, Winter 1994). However, as CAST and CASA mice were inbred separately in different laboratories, the founder mice may also have been susceptible to MPXV. MOLF is an inbred species of M. m. molossinus that was derived from mice trapped in Japan and PERA is an inbred species of M. m. domesticus trapped in Peru. Not all wild-derived mice are highly susceptible to MPXV, however, since in our initial screen we found that SPRET/EiJ and CZECHII/EIJ lost no weight at all after infection with 2 X 104 PFU and PWK/PhJ lost less than 8%.
Crossbreeding of CAST with C57BL/6 and with BALB/c was carried out to investigate whether resistance or sensitivity to MPXV was dominant. In both cases the F1 progeny were relatively resistant to MPXV. Based on the number of survivors, a backcross of F1 females derived from CAST X C57BL/6 with male CAST mice indicated the likely presence of more than one resistance locus. However, there was a sex difference: 3 of 8 male backcross mice succumbed whereas the 11 female backcross mice survived. A much larger number of backcross mice than the 19 used here would be necessary to confidently map resistant loci by SNIP analysis. We also challenged the F1 generation between a cross of CAST and MOLF mice and found that they were more resistant to MPXV than the CAST parent (our unpublished data).
There is evidence from serial backcross experiments that multiple genes contribute to the resistance of C57BL/6 mice to ectromelia virus (Browstein et al., 1992). However, unlike MPXV, ectromelia is pathogenic in many classical inbred mouse strains. Increased severity of male compared to female mice has also been reported for infection of inbred mice with ectromelia virus (Browstein et al., 1992; Wallace et al., 1985). The Collaborative Cross panel (Threadgill and Churchill, 2012), which was derived by interbreeding eight different mouse strains, could provide an alternative method of mapping MPXV-resistance loci. Although the CAST mouse was included among the founders, the other seven strains in the panel all exhibit resistance to MPXV, possibly making it difficult to identify individual resistance genes.
For several reasons, we believe that the greater sensitivity of CAST mice compared to classical inbred strains is due to an inadequate immune response. Lung titers of MPXV in BALB/c mice following i.n. infection approach that of CAST mice but, in contrast to CAST mice, the virus is rapidly cleared. BALB/c mice make a more rapid and greater interferon γ response than CAST mice (Earl et al., 2014; Earl et al., 2012). In addition, interferon γ- and interferon γ receptor-knock-out C57BL/6 mice are less resistant to MPXV than parental C57BL/6 mice and exogenous interferon γ protects CAST mice against MPXV infection (Earl et al., 2012). The transcription factor STAT1 is involved in up regulating host response gene expression due to signaling by types I, II or III interferons and STAT1-deficient mice are even more susceptible to MPXV than interferon γ- and interferon γ receptor-deficient mice (Stabenow et al., 2010).
Materials and Methods
Cells and viruses
BS-C-1 cells were maintained at 37°C and 5% CO2 in modified Eagle minimal essential medium (EMEM; Quality Biologicals, Inc., Gaithersburg, MD) supplemented with 8% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 U of penicillin/ml, and 10 μg of streptomycin/ml. MPXV-Z79-CB2 (Americo, 2010), a clonal isolate derived from MPXV-Z79-005, was used in all experiments. Purified virus was prepared as described previously (Americo et al., 2010).
Mice
The following inbred mouse strains were obtained from Jackson Laboratories (Bar Harbor, ME): C57BL/6J, CAST/EiJ, MOLF/EiJ, C58/J, NZW/LacJ, CASA/RkJ, and F1 and back-cross progeny. BALB/c mice were obtained from Taconic Biotechnology, Germantown, NY). Mice were maintained in small, ventilated microisolator cages.
Inoculation of mice
Animal experiments were performed in an ABSL-3 facility with approval of the NIAID Animal Care and Use Committee and the Centers for Disease Control. On the day of infection, MPXV was thawed, sonicated, and diluted in phosphate buffered saline containing 0.05% bovine serum albumin. The titer of each dose was verified by plaque assay on BS-C-1 cells. Infections were performed by instillation of 10 μl of virus into one nostril. All wild-derived mice and progeny of wild-derived mice were lightly anaesthetized with isoflurane prior to infection. Mock-infected animals were inoculated with an equivalent volume of diluent. Animals were observed and weighed daily for up to 18 days. Animals that lost 30% of their starting weight were humanely euthanized in accordance with NIAID Animal Care and Use Guidelines
Highlights.
Classical inbred mice are resistant to monkeypox virus at intranasal doses of 106 infectious units
Wild-derived inbred mice have varied susceptibility with lethal doses of <100 infectious units
Resistance is dominant with evidence for multiple independent resistant loci
Acknowledgments
We thank Catherine Cotter for providing cells, the Comparative Medicine Branch, NIAID for care of animals and Thomas Kristie and Andrea Weisberg for assistance with the graphical abstract. The research was supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Americo JL, Moss B, Earl PL. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J Virol. 2010;84:8172–8180. doi: 10.1128/JVI.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Americo JL, Sood CL, Cotter CA, Vogel JL, Kristie TM, Moss B, Earl PL. Susceptibility of the wild-derived inbred CAST/Ei mouse to infection by orthopoxviruses analyzed by live bioluminescence imaging. Virology. 2014;449:120–132. doi: 10.1016/j.virol.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browstein DG, Bhatt PN, Gras L, Budris T. Serial backcross analysis of genetic resistance to mousepox, using marker loci for rmp-2 and rmp-3. J Virol. 1992;66:7073–7079. doi: 10.1128/jvi.66.12.7073-7079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Cotter CA, Moss B. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni) Virology. 2014;475C:150–158. doi: 10.1016/j.virol.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Moss B. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient interferon-gamma response. J Virol. 2012;86:9105–9112. doi: 10.1128/JVI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson CL, Damon IK. Monkeypox virus infections in small animal models for evaluation of anti-poxvirus agents. Viruses. 2010;2:2763–2776. doi: 10.3390/v2122763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson CL, Lee KN, Abel J, Carroll DS, Montgomery JM, Olson VA, Li Y, Davidson W, Hughes C, Dillon M, Spurlock P, Kazmierczak JJ, Austin C, Miser L, Sorhage FE, Howell J, Davis JP, Reynolds MG, Braden Z, Karem KL, Damon IK, Regnery RL. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. Am J Trop Med Hyg. 2007;76:757–768. [PubMed] [Google Scholar]
- McCollum AM, Damon IK. Human monkeypox. Clin Inf Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virology. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S, Nuara A, Buller RML, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- Reynolds MG, Damon IK. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends in Microbiology. 2012;20:80–87. doi: 10.1016/j.tim.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Stabenow J, Buller RM, Schriewer J, West C, Sagartz JE, Parker S. A mouse model of lethal infection for evaluating prophylactics and therapeutics against monkeypox virus. J Virol. 2010;84:3909–3920. doi: 10.1128/JVI.02012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada T, Ebata T, Noguchi H, Keane TM, Adams DJ, Narita T, Shin IT, Fujisawa H, Toyoda A, Abe K, Obata Y, Sakaki Y, Moriwaki K, Fujiyama A, Kohara Y, Shiroishi T. The ancestor of extant Japanese fancy mice contributed to the mosaic genomes of classical inbred strains. Genome Res. 2013;23:1329–1338. doi: 10.1101/gr.156497.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgill DW, Churchill GA. Ten years of the collaborative cross. Genetics. 2012;190:291–294. doi: 10.1534/genetics.111.138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GD, Buller RM, Morse HC., 3rd Genetic determinants of resistance to ectromelia (mousepox) virus-induced mortality. J Virol. 1985;55:890–891. doi: 10.1128/jvi.55.3.890-891.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]