Abstract
Cytomegalovirus (CMV) reactivation in non-immune suppressed critically ill patients is an area of increasing interest. CMV has long been appreciated as a pathogen in immunocompromised hosts. CMV reactivates in approximately one-third of latently infected non-immune suppressed hosts during critical illness, however, its role as a pathogen in these patients remains unclear. CMV reactivation has been linked to bacterial sepsis, and likely results from inflammation, transient immune compromise, and viral epigenetic changes. While CMV may improve immune response to some bacterial infections, other data suggest that CMV induces exaggerated responses to severe infections that may be harmful to latently infected hosts. These results also suggest that previous infection history may explain significant differences seen between human septic responses and murine models of sepsis. While critically ill human hosts clearly have worse outcomes associated with CMV reactivation, determining causality remains an area of investigation, with randomized control trials currently being performed. Here we review the current literature, and highlight areas for future investigation.
Keywords: Cytomegalovirus, sepsis, reactivation
Introduction
Since its original description and then isolation by Margaret Gladys Smith [1,2], cytomegalovirus (CMV) has become well recognized as a pathogen in hosts with impaired immunity. This is most noticeable in those with impaired immunity, such as seen in congenital infection, in those whom the immune system is intentionally suppressed, such as occurs in transplantation, and in disease imposed impairment of immunity, such as in human immunodeficiency virus and acquired immune deficiency syndrome.
With advances in monitoring and detection methods, CMV has been recognized to reactivate in numerous settings in individuals that are not chronically immune suppressed (reviewed in [3]). As will be discussed, there are growing data suggesting that such reactivation events could be pathogenic in previously immune competent patients during critical illness [4]. In the current review immune competent patients that have CMV reactivation will be referred to as non-immune suppressed, acknowledging that these previously immune competent patients might have transient immune compromise as a consequence of their illness.
Incidence of Reactivation
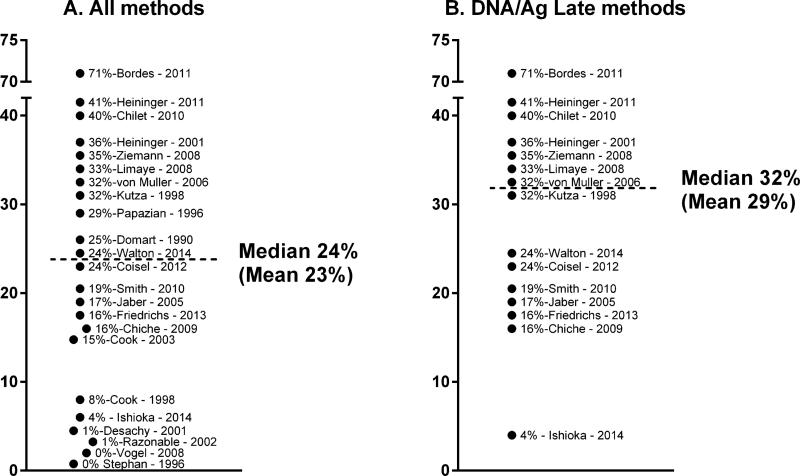
Since the earliest descriptions of CMV reactivation in immune competent hosts, there have now been more than 20 studies demonstrating reactivation in non-immune suppressed patients during critical illness [5-27]. There is significant variability in estimated reactivation rates, most of which may be explained by methodology or kinetics. When these results are summarized, the median/mean rates of reactivation respectively are 24% and 23% (Figure 1A). It is becoming increasingly apparent that certain patient populations have different risks, with burn patients having much higher rates of reactivation for example than cardiac patients [27,10,5]. We now know that most reactivation events occur 7-28 days after onset of critical illness [10], likely explaining why studies utilizing very early monitoring (<8 days) showed a 0-1% reactivation rate [23,24,26,25]. In addition, several published studies used viral culture or shell vial methods [22,21,14,13], and while these studies were fundamental to confirm true reactivation by recovering live virus, these methods have lower sensitivity than current PCR or antigenemia based methodologies. If these early detection and low sensitivity studies are excluded, it seems that roughly 1 in 3 patients with critical illness will have CMV reactivation (Figure 1B).
Figure 1.
Cytomegalovirus reactivation rates from previously published studies.1A all studies included.
1B. Results from all studies using DNA or antigen based testing and monitoring >8 days.
Sepsis and CMV
Bacterial sepsis is an associated trigger of CMV reactivation that was first recognized in the 1990's. Domart et al first showed that a significant number of non-immune suppressed patients with mediastinitis following cardiac surgery had CMV reactivation [14]. Subsequent work by Prosch and Volk and the Berlin group showed in a trio of manuscripts that CMV reactivation occurs at a high rate in septic patients, and suggested that this reactivation might be a consequence of TNF and nuclear factor-κβ stimulation of the major immediate early promoter [28-30]. This clinical association was later experimentally confirmed by combining murine models of CMV latency and polymicrobial sepsis [31], and then subsequently with direct administration of inflammatory mediators in the murine latency model [32]. It has been recently proposed that reactivation events associated with sepsis are a consequence of inflammatory stimulation of the major immediate early promoter, transient immune compromise, and likely some component of epigenetic regulation of viral DNA (reviewed in [33]).
Once the connection between bacterial sepsis and viral reactivation was solidified, focus was next turned to the consequences of such reactivation events. It has been shown that pulmonary inflammatory responses induced by polymicrobial sepsis are exaggerated in mice with latent CMV [34]. This exaggerated inflammatory response, something that we have termed CMV-ALI (CMV-associated lung injury) [4], is associated with enhanced pulmonary fibrosis in latently infected mice after sepsis [34]. Virgin et al demonstrated subsequently that previous infection with CMV or the Epstein Barr Virus homolog γ-herpes virus-68 can confer protection against subsequent bacterial challenges [35]. The mechanism for this resistance appeared to be macrophage activation [35], and more recent work suggests that for CMV infection this may be a consequence of enhanced Toll like receptor (TLR) expression and responsiveness on infected macrophages [36]. This enhanced TLR responsiveness is accompanied by enhanced CD14 expression, thereby increasing macrophage responsiveness to TLR-2, TLR-4, and TLR-5 ligands.
Interestingly, this enhanced TLR responsiveness might actually provide a survival advantage for the virus, by ensuring that the major immediate early promoter is tickled repeatedly (if not continuously) by endogenous bacteria throughout an infected hosts lifetime, perhaps causing shedding of virus and thus transmission opportunities. If this hypothesis is true, then one would expect to see some differences in germ free hosts after CMV infection. It is known that CMV infection can induce dramatic CMV-specific T-cell responses, a phenomenon that is popularly referred to as ‘memory inflation’. Curiously germ free mice do not develop memory inflation after CMV infection, but do develop memory inflation after bacterial reconstitution [37]. If CMV-specific T-cells are inflating in response to viral transcriptional activity, then one could speculate that host bacteria facilitate such activity. This makes the enhanced TLR/CD14 expression after CMV infection even more interesting, leaving hosts even more susceptible to bacterial stimulation and inflammation. Such stimulation might explain the perpetual low level viral transcriptional activity during “latency” shown by the Reddehase group [38,39], and in moments of immune weakness during relative health allow intermittent shedding, giving survival advantage to the virus. Conversely, such enhancements in toll like receptors might also contribute to the exaggerated immune responses seen during sepsis, becoming detrimental when those same hosts encounter severe bacterial infections.
Whether such viral preconditioning by CMV has a beneficial or detrimental impact on humans during bacterial septic challenges is unknown. On the one hand, it is logical that a viral infection that enhances immunity to bacterial infections should benefit the septic host. If this is true, then latently infected mice should show enhanced survival following bacterial sepsis, which is not consistent with our experience (unpublished data). Likewise, IgG positive patients should also show improved outcomes during sepsis, but the single study to date evaluating this shows no such benefit [40]. In fact, this study shows no association with improved or worsened outcomes in CMV-IgG positive patients [40]. One shortcoming of this trial however is that IgG titers were not studied, and all studies to date that have correlated CMV-serostatus with detrimental outcomes have found such associations only in those with the highest IgG titers [41-43].
On the other hand, the septic response is considered by many to be a deranged host response, and it is equally logical that CMV preconditioning might contribute to such exaggerated inflammation. Hosts with coincident CMV reactivation and bacterial infections have more inflammation and immune system activation that is accompanied by an increased risk of septic shock, supporting the detrimental hypothesis [44,35,34]. By looking at the CMV-specific immune responses to infection in human hosts, it seems clear that not all naturally occurring infections are equal and show a broad mix of infectious titers [41-43,45]. This mix of high and low titer infections may in part explain why the work by De Vlieger et al did not show an advantage or disadvantage of previous CMV infection in outcome after sepsis or critical illness.
Differences between human and murine sepsis responses
It is quite interesting that the study of sepsis has been plagued by significant differences between human and murine septic responses. History is now littered with many therapies for bacterial sepsis that have looked extremely promising in murine models, only to fail in subsequent human studies [46]. In fact, recent genomic work has suggested that septic responses in mice are mostly disparate from humans [46].
One major difference between humans and murine models that has gone overlooked is the immune experience of subjects in sepsis studies. Welsh, Selin et al first popularized the idea that previous immune responses to infectious challenges might shape and influence subsequent responses to new antigens, a concept that they termed “heterologous immunity” (reviewed in [47]). Most adult humans have been exposed to multiple previous virus infections, not to mention a standard battery of immunizations that may have significant and long lasting impact on their immune responses to sepsis. For example, 60% of patients have been infected by CMV prior to onset of critical illness, and the prevalence of other herpes family viruses are also very high during adulthood (reviewed in [3]). As previously discussed, precedent herpesvirus infection has the potential to significantly alter host responses to sepsis [48,34]. Thus, comparing “immune experienced” human immune responses that have been manipulated by innumerable previous infectious encounters to relatively “immune naïve” immune responses in mice is likely a comparison between the figurative apple and orange. Given the myriad combinations of precedent antigen experience, including the number, type, sequence, organism load and of course timing of exposure (recent versus remote), it may be required for murine models of sepsis to include such immune preconditioning to adequately recapitulate human responses to sepsis.
CMV reactivation: pathogen or bystander
It is clear that patients that suffer CMV reactivation during their critical illness have an associated mortality of roughly double that of those without reactivation [49]. It is interesting that this mortality rate is similar to that seen in HIV patients with DNAemia (2-4X more likely to die) - independent of HIV load and CD4 counts and despite HAART [50]. Recent clinical data have suggested that it isn't merely CMV, but the magnitude of the immune response to it that influences CMV-related mortality [42].
The association between CMV reactivation and mortality naturally prompts the question of pathogen or bystander. Several authors have suggested that CMV reactivation events are merely an indicator of host immune compromise, which has been associated by itself with worsened outcomes [51,15]. Because most investigators agree that CMV is never fully quiescent, requiring constant immune surveillance to maintain functional latency, it makes sense that transient immune compromise during critical illness could allow reactivation [39,38]. It is now clear that sepsis can induce contraction of CMV-specific T-memory in mice, thereby facilitating transcriptional reactivation [52]. Likewise sepsis has been shown to cause contraction of CMV-specific IgG in humans [26]. Limited data on CMV-specific T-immunity in non-immune suppressed humans during critical illness show persistence of CMV-specific T-cells [8]. Given the lack of pre-illness baseline data, however, it is impossible to know if the presence of such T-cells is indicative of “intact” CMV-specific immune function or some fraction thereof. Also consistent with the immune compromise hypothesis is the observation that other human herpes viruses reactivate during sepsis [15].
For CMV to cause harm, one would expect that similar to immune suppressed patients, there would be end organ injury from virus activity [53]. Among other organs, CMV is known to develop latent infections in both lungs and liver [54-56]. Patients with pulmonary reactivation have significantly prolonged durations of mechanical ventilation (Reviewed in [3], [13,6,7,21,18,11,9,20,5,16], and there are murine data to suggest worse pulmonary inflammation and lung injury in mice with latent CMV during sepsis [34]. There are also data confirming worse hepatitis in patients with reactivation [21]. As previously discussed, patients latently infected with CMV that have subsequent bacterial infections have increased risk of septic shock [44]. Altogether these associations are intriguing but nonetheless circumstantial evidence that CMV reactivation is harmful in non-immune suppressed patients.
Treatment trials ongoing
Probably the most effective way to answer the question of whether CMV reactivation is pathogenic or merely a bystander in human disease will be properly controlled randomized trials with antiviral therapy [57]. In the case of immune suppressed transplant patients, CMV was perceived as a definite pathogen, and this led to widespread antiviral use when such agents became available. It was not until decades later that properly controlled trials in immune suppressed patients proved a benefit [58]. Fortunately, there has been a more circumspect and deliberate approach to antiviral treatment in critically ill patients with CMV reactivation.
There has been considerable debate about the antiviral strategy that should be used in these patients because only 1 in 3 are expected to have reactivation. Available animal data suggest that the most effective reactivation prevention strategy will be early prophylaxis [59], but this approach would see 2/3 of critically ill patients without reactivation receiving potentially toxic medications. Alternatively, using preemptive therapy will limit the number of people receiving antivirals to those suffering reactivation, but it is unclear whether delayed treatment will have benefit [59].
There are currently three such clinical trials to address this important question in non-immune suppressed ICU patients. The first reactivation prevention trial is still ongoing (Boeckh and Limaye, NCT01335932) and compares ganciclovir to placebo in patients with ARDS. Of the two others, one has been recently completed and evaluated antiviral prophylaxis using high dose acyclovir versus low dose ganciclovir (Bion, Cowley, and Moss NCT01503918). The third trial is also still underway evaluating preemptive therapy with ganciclovir or acyclovir respectively for CMV or HSV (Papazian NCT02152358). There are some encouraging new data that suggest improved survival for treatment of herpes simplex virus in critically ill non-immune suppressed patients [60]. Nonetheless, given the risks associated with available agents, it seems that the most prudent role for now for treating CMV reactivation will be to await results of ongoing trials.
Conclusions
There is a strong and long standing association between CMV reactivation and sepsis. It is currently unclear whether CMV and sepsis are friend or foe. On the one hand, there is evidence that previous CMV infection can protect against subsequent bacterial infection by enhanced macrophage activation. On the other hand this same immune enhancement may contribute to exaggerated inflammatory responses during sepsis leading to septic shock and patient mortality. Determining how preconditioned immune responses to persistent herpes viruses impact subsequent immune system activation and inflammatory responses may provide significant insight into septic responses. Given the enormous number of patients that harbor latent CMV that become critically ill, and the attendant mortality associated with CMV reactivation, developing better understanding of CMV reactivation and possible new strategies to prevent it may significantly contribute to patient outcome.
Acknowledgements
This work was funded in part by NIH GM 066115
Footnotes
Disclosure of conflict of interest statement:
The authors have no conflicts of interest to declare
Research involving human participants and/or animals:
Not applicable to this review
Informed Consent:
Not Applicable to this review
Contributor Information
Sara Mansfield, Department of Surgery, The Ohio State University Medical Center, Columbus, OH 43210.
Marion Griessl, Department of Surgery, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215.
Michael Gutknecht, Department of Surgery, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215.
Charles H. Cook, Department of Surgery, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215
References
- 1.Smith MG. Propagation of salivary gland virus of the mouse in tissue cultures. Proc Soc Exp Biol Med. 1954;86(3):435–440. doi: 10.3181/00379727-86-21123. [DOI] [PubMed] [Google Scholar]
- 2.Smith MG. Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc Soc Exp Biol Med. 1956;92(2):424–430. doi: 10.3181/00379727-92-22498. [DOI] [PubMed] [Google Scholar]
- 3.Guidry CA, Mansfield SA, Sawyer RG, Cook CH. Resistant Pathogens, Fungi, and Viruses. Surg Clin North Am. 2014;94(6):1195–1218. doi: 10.1016/j.suc.2014.08.010. doi: http://dx.doi.org/10.1016/j.suc.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook CH, Trgovcich J. Cytomegalovirus reactivation in critically ill immunocompetent hosts: A decade of progress and remaining challenges. Antiviral Res. 2011;90(3):151–159. doi: 10.1016/j.antiviral.2011.03.179. doi:10.1016/j.antiviral.2011.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordes J, Maslin J, Prunet B, d'Aranda E, Lacroix G, Goutorbe P, Dantzer E, Meaudre E. Cytomegalovirus infection in severe burn patients monitoring by real-time polymerase chain reaction: A prospective study. Burns. 2011;37(3):434–439. doi: 10.1016/j.burns.2010.11.006. doi:10.1016/j.burns.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F, Meisner C, Jahn G, Koenigsrainer A, Unertl K, Hamprecht K. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Critical care. 2011;15(2):R77. doi: 10.1186/cc10069. doi:10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29(3):541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Chilet M, Aguilar G, Benet I, Belda J, Tormo N, Carbonell JA, Clari MA, Costa E, Navarro D. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. J Med Virol. 2010;82(8):1384–1391. doi: 10.1002/jmv.21825. doi:10.1002/jmv.21825. [DOI] [PubMed] [Google Scholar]
- 9.Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. 2008;36(12):3145–3150. doi: 10.1097/CCM.0b013e31818f3fc4. [DOI] [PubMed] [Google Scholar]
- 10.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang M-L, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Patients. JAMA. 2008;300(4):413–422. doi: 10.1001/jama.300.4.413. doi:10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Muller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, Durmus N, Hampl W, Mertens T. Active cytomegalovirus infection in patients with septic shock. Emerging Infectious Diseases. 2006;12(10):1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26(5):1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 13.Papazian L, Fraisse A, Garbe L, Zandotti C, Thomas P, Saux P, Pierrin G, Gouin F. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84(2):280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery [see comments]. Chest. 1990;97(1):18–22. doi: 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, Green JM, Storch GA, Hotchkiss RS. Reactivation of Multiple Viruses in Patients with Sepsis. PLoS ONE. 2014;9(6):e98819. doi: 10.1371/journal.pone.0098819. doi:10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coisel Y, Bousbia S, Forel J-M, Hraiech S, Lascola B, Roch A, Zandotti C, Million M, Jaber S, Raoult D, Papazian L. Cytomegalovirus and Herpes Simplex Virus Effect on the Prognosis of Mechanically Ventilated Patients Suspected to Have Ventilator-Associated Pneumonia. PLoS ONE. 2012;7(12):e51340. doi: 10.1371/journal.pone.0051340. doi:10.1371/journal.pone.0051340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CA, Conroy LT, Pollock M, Ruddy J, Binning A, McCruden EA. Detection of herpes viruses in respiratory secretions of patients undergoing artificial ventilation. J Med Virol. 2010;82(8):1406–1409. doi: 10.1002/jmv.21794. doi:10.1002/jmv.21794. [DOI] [PubMed] [Google Scholar]
- 18.Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault P-F, Eledjam J-J. Cytomegalovirus Infection in Critically Ill Patients: Associated Factors and Consequences. Chest. 2005;127(1):233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 19.Friedrichs I, Bingold T, Keppler OT, Pullmann B, Reinheimer C, Berger A. Detection of herpesvirus EBV DNA in the lower respiratory tract of ICU patients: a marker of infection of the lower respiratory tract? Med Microbiol Immunol. 2013;202(6):431–436. doi: 10.1007/s00430-013-0306-1. doi:10.1007/s00430-013-0306-1. [DOI] [PubMed] [Google Scholar]
- 20.Chiche L, Forel JM, Roch A, Guervilly C, Pauly V, Allardet-Servent J, Gainnier M, Zandotti C, Papazian L. Active Cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37(6):1850–1857. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 21.Cook CH, Martin LC, Yenchar JK, Lahm MC, McGuinness B, Davies EA, Ferguson RM. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003;31(7):1923–1929. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- 22.Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. 1998;176(4):357–360. doi: 10.1016/s0002-9610(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 23.Desachy A, Ranger-Rogez S, Francois B, Venot C, Traccard I, Gastinne H, Denis F, Vignon P. Reactivation of human herpesvirus type 6 in multiple organ failure syndrome. Clin Infect Dis. 2001;32(2):197–203. doi: 10.1086/318474. [DOI] [PubMed] [Google Scholar]
- 24.Razonable RR, Fanning C, Brown RA, Espy MJ, Rivero A, Wilson J, Kremers W, Smith TF, Paya CV. Selective reactivation of human herpesvirus 6 variant a occurs in critically ill immunocompetent hosts.[see comment]. J Infect Dis. 2002;185(1):110–113. doi: 10.1086/324772. [DOI] [PubMed] [Google Scholar]
- 25.Stephan F, Meharzi D, Ricci S, Fajac A, Clergue F, Bernaudin JF. Evaluation by polymerase chain reaction of cytomegalovirus reactivation in intensive care patients under mechanical ventilation. Intensive Care Medicine. 1996;22(11):1244–1249. doi: 10.1007/BF01709343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel T, Vadonis R, Kuehn J, Eing BR, Senninger N, Haier J. Viral reactivation is not related to septic complications after major surgical resections. APMIS. 2008;116(4):292–301. doi: 10.1111/j.1600-0463.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 27.Ishioka H, Sanui M, Tsutsumi Y, Yanase F, Shiotsuka J. Low prevalence of active cytomegalovirus infection in a cardiovascular intensive care unit. Journal of intensive care. 2014;2(1):12. doi: 10.1186/2052-0492-2-12. doi:10.1186/2052-0492-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Docke WD, Prosch S, Fietze E, Kimel V, Zuckermann H, Klug C, Syrbe U, Kruger DH, von Baehr R, Volk HD. Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343(8892):268–269. doi: 10.1016/s0140-6736(94)91116-9. [DOI] [PubMed] [Google Scholar]
- 29.Stein J, Volk HD, Liebenthal C, Kruger DH, Prosch S. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. J Gen Virol. 1993;74(11):2333–2338. doi: 10.1099/0022-1317-74-11-2333. [DOI] [PubMed] [Google Scholar]
- 30.Prosch S, Staak K, Stein J, Liebenthal C, Stamminger T, Volk HD, Kruger DH. Stimulation of the Human Cytomegalovrus IE Enhancer/Promoter in HL-60 Cells by TNFalpha Is Mediated via Induction of NF-kappaB. Virology. 1995;208(1):197–206. doi: 10.1006/viro.1995.1143. [DOI] [PubMed] [Google Scholar]
- 31.Cook C, Zhang X, McGuinness B, Lahm M, Sedmak D, Ferguson R. Intra-abdominal Bacterial Infection Reactivates Latent Pulmonary Cytomegalovirus in Immunocompetent Mice. J Infect Dis. 2002;185:1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 32.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, Tumor Necrosis Factor Alpha, or Interleukin-1{beta} Triggers Reactivation of Latent Cytomegalovirus in Immunocompetent Mice. J Virol. 2006;80(18):9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seckert CK, Griessl M, Buttner JK, Freitag K, Lemmermann N, Hummel M, Liu XF, Abecassis M, Angulo A, Messerle M, Cook CH, Reddehase M. Immune Surveillance of Cytomegalovirus Latency and Reactivation in Murine Models: Link to Memory Inflation. In: Reddehase MJ, editor. Cytomegaloviruses. Vol. 1. Caister Academic Press; Norfolk, UK: 2013. pp. 374–416. [Google Scholar]
- 34.Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006;34(3):842–849. doi: 10.1097/01.ccm.0000201876.11059.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447(7142):326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 36.Smith PD, Shimamura M, Musgrove LC, Dennis EA, Bimczok D, Novak L, Ballestas M, Fenton A, Dandekar S, Britt WJ, Smythies LE. Cytomegalovirus Enhances Macrophage TLR Expression and MyD88-Mediated Signal Transduction To Potentiate Inducible Inflammatory Responses. The Journal of Immunology. 2014;193:5604–5612. doi: 10.4049/jimmunol.1302608. doi:10.4049/jimmunol.1302608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka K, Sawamura S, Satoh T, Kobayashi K, Noda S. Role of the Indigenous Microbiota in Maintaining the Virus-Specific CD8 Memory T Cells in the Lung of Mice Infected with Murine Cytomegalovirus. J Immunol. 2007;178(8):5209–5216. doi: 10.4049/jimmunol.178.8.5209. [DOI] [PubMed] [Google Scholar]
- 38.Seckert CK, Griessl M, Buttner JK, Scheller S, Simon CO, Kropp KA, Renzaho A, Kuhnapfel B, Grzimek NK, Reddehase MJ. Viral latency drives 'memory inflation': a unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med Microbiol Immunol. 2012 doi: 10.1007/s00430-012-0273-y. doi:10.1007/s00430-012-0273-y. [DOI] [PubMed] [Google Scholar]
- 39.Kurz SK, Reddehase MJ. Patchwork Pattern of Transcriptional Reactivation in the Lungs Indicates Sequential Checkpoints in the Transition from Murine Cytomegalovirus Latency to Recurrence. J Virol. 1999;73(10):8612–8622. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vlieger G, Meersseman W, Lagrou K, Wouters P, Wilmer A, Peetermans W, Van den Berghe G, Van Wijngaerden E. Cytomegalovirus serostatus and outcome in nonimmunocompromised critically ill patients. Crit Care Med. 2012;40(1):36–42. doi: 10.1097/CCM.0b013e31822b50ae. [DOI] [PubMed] [Google Scholar]
- 41.Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to Cytomegalovirus, Inflammation, All-Cause and Cardiovascular Disease-Related Mortality in the United States. PLoS ONE. 2011;6(2):e16103. doi: 10.1371/journal.pone.0016103. doi:10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strandberg TE, Pitkala KH, Tilvis RS. Cytomegalovirus antibody level and mortality among community-dwelling older adults with stable cardiovascular disease. JAMA. 2009;301(4):380–382. doi: 10.1001/jama.2009.4. [DOI] [PubMed] [Google Scholar]
- 43.Wang GC, Kao WHL, Murakami P, Xue Q- L, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus Infection and the Risk of Mortality and Frailty in Older Women: A Prospective Observational Cohort Study. Am J Epidemiol. 2010;171(10):1144–1152. doi: 10.1093/aje/kwq062. doi:10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miggins M, Hasan A, Hohmann S, Southwick F, Casella G, Schain D, Liu H, Bihorac A, Moldawer L, Efron P, Ang D. The Potential Influence of Common Viral Infections Diagnosed during Hospitalization among Critically Ill Patients in the United States. PLoS ONE. 2011;6(4):e18890. doi: 10.1371/journal.pone.0018890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. doi:10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1222878110. doi:10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsh RM, Selin LK. No one is naive: The significance of heterologous T-cell immunity. Nature Reviews Immunology. 2002;2(6):417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 48.Barton ES, White DW, Virgin HW. Herpesvirus Latency and Symbiotic Protection from Bacterial Infection. Viral Immunology. 2009;22(1):3–4. doi: 10.1089/vim.2008.0100. doi:doi:10.1089/vim.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infections in non-immunosuppressed ICU patients. Crit Care Med. 2009;37(8):2350–2358. doi: 10.1097/CCM.0b013e3181a3aa43. [DOI] [PubMed] [Google Scholar]
- 50.Wohl DA, Zeng D, Stewart P, Glomb N, Alcorn T, Jones S, Handy J, Fiscus S, Weinberg A, Gowda D, van der Horst C. Cytomegalovirus Viremia, Mortality, and End-Organ Disease Among Patients With AIDS Receiving Potent Antiretroviral Therapies. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;38(5):538–544. doi: 10.1097/01.qai.0000155204.96973.c3. [DOI] [PubMed] [Google Scholar]
- 51.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15(5):496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell J, Trgovcich J, Kincaid M, Zimmerman PD, Klenerman P, Sims S, Cook CH. Transient CD8-memory contraction: a potential contributor to latent cytomegalovirus reactivation. J Leukoc Biol. 2012;92(5):933–937. doi: 10.1189/jlb.1211635. doi:10.1189/jlb.1211635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Muller L, Mertens T. Human cytomegalovirus infection and antiviral immunity in septic patients without canonical immunosuppression. Medical Microbiology and Immunology. 2008;197(2):75–82. doi: 10.1007/s00430-008-0087-0. [DOI] [PubMed] [Google Scholar]
- 54.Seckert CK, Renzaho A, Tervo H- M, Krause C, Deegen P, Kuhnapfel B, Reddehase MJ, Grzimek NKA. Liver Sinusoidal Endothelial Cells Are a Site of Murine Cytomegalovirus Latency and Reactivation. J Virol. 2009;83(17):8869–8884. doi: 10.1128/JVI.00870-09. doi:10.1128/jvi.00870-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67(9):5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toorkey CB, Carrigan DR. Immunohistochemical detection of an immediate early antigen of human cytomegalovirus in normal tissues. J Infect Dis. 1989;160(5):741–751. doi: 10.1093/infdis/160.5.741. [DOI] [PubMed] [Google Scholar]
- 57.Cook CH. Cytomegalovirus Reactivation in “Immunocompetent” Patients: A Call for Scientific Prophylaxis. J Infect Dis. 2007;196(9):1273–1275. doi: 10.1086/522433. [DOI] [PubMed] [Google Scholar]
- 58.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-Analysis: The Efficacy of Strategies To Prevent Organ Disease by Cytomegalovirus in Solid Organ Transplant Recipients. Ann Intern Med. 2005;143(12):870–880. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 59.Forster MR, Trgovcich J, Zimmerman P, Chang A, Miller C, Klenerman P, Cook CH. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res. 2010;85(3):496–503. doi: 10.1016/j.antiviral.2009.12.004. doi:DOI: 10.1016/j.antiviral.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traen S, Bochanen N, Ieven M, Schepens T, Bruynseels P, Verbrugghe W, Jorens PG. Is acyclovir effective among critically ill patients with herpes simplex in the respiratory tract? J Clin Virol. 2014;60(3):215–221. doi: 10.1016/j.jcv.2014.04.010. doi:10.1016/j.jcv.2014.04.010. [DOI] [PubMed] [Google Scholar]