Abstract
Background
Behaviourally HIV-infected adolescent females are at higher risk for abnormal cervical cytology and HPV infection compared to those who are uninfected, but data on perinatally HIV-infected adolescent females are lacking.
Methods
Cervical cytology, HPV infection and E6/E7 mRNA were assessed in sexually active 12–24-year-old adolescent females: perinatally HIV-infected (group 1, n = 40), behaviourally HIV-infected (group 2, n = 10), and HIV-uninfected (group 3, n = 10).
Results
Median age was lower in group 1 (18 years) than in groups 2 (24 years) and 3 (20.5 years) (P < 0.001), and median time since sexual debut was shorter: 2 vs 5 vs 4 years (P < 0.001). More trial participants in group 1 than group 2 were on antiretrovirals (90% vs 70%; P <0.001). Abnormal cervical cytology (atypical squamous cells of undetermined significance and higher) was observed in 30% (group 1), 40% (group 2) and 30% (group 3) (P = 0.92), whereas high-risk HPV infection was observed in 45%, 45% and 40%, respectively (P = 1.00). Positive E6/E7 mRNA was found in 28% of group 1, but not in other groups. High-risk HPV infection predicted abnormal cytology in all groups [OR 6.77, 95% confidence interval (CI) 1.99–23.0; P = 0.001). Additionally, plasma HIV RNA ≥50 copies/mL (OR 13.3, 95% CI 1.16–153.06; P = 0.04) predicted abnormal cytology in HIV-infected adolescent females.
Conclusions
Despite the younger age and shorter time since sexual debut, cervical cytological abnormalities and HPV infection were as common in perinatally HIV-infected as in behaviourally infected and uninfected adolescents. HPV vaccination, pre-cancer screening and antiretroviral treatment in HIV-infected female adolescents should be implemented to minimise the risk of cervical cancer.
Keywords: perinatal HIV, HPV, cervix, adolescents, Pap smear
Introduction
Worldwide, more women lose their lives to cervical cancer than any other cancer [1], and persistent infection with high-risk types of human papillomavirus (HPV), the most common sexually transmitted disease (STD), is the cause. HIV infection increases a woman’s risk for developing cervical cancer by up to 20-fold [2,3]. Prevalence and incidence of HPV infection among young adults who are sexually experienced is high [4,5], especially in resource-limited settings like Thailand, where HPV vaccination is not routinely available. Almost 25% of women younger than 20 years old worldwide are infected with HPV [4]. Over the past decade, those living with HIV are experiencing a rise in incidence and prevalence of genital cancers [6], probably due to highly active antiretroviral therapy (HAART) improving longevity. Studies in behaviourally HIV-infected female adolescents have noted higher prevalence of HPV infection and squamous intra-epithelial lesions compared to uninfected female adolescents [7]. High-risk HPV types in adolescents are associated with HIV seropositivity, and HPV persists longer in HIV-infected adolescents compared to those uninfected [8]. There is strong evidence for the association between HPV disease progression and degree and duration of immunosuppression among HIV-infected patients. Longer duration of HIV, high HIV RNA level and low CD4 cell count are risk factors for HPV progression to cancer, and the probability of HPV clearance in HIV-infected patients increases with rising CD4 cell counts [7,9,10]. Therefore, it is possible that adolescents with longer duration of HIV infection (i.e. perinatally infected) will be at higher risk for HPV infection and progression to cancer than those infected more recently via sexual transmission of HIV (i.e. behaviourally infected). However, previous research has concentrated on behaviourally HIV-infected adolescents and data in perinatally infected adolescents are sorely lacking [7,8,11–13]. In this study, we aimed to determine the prevalence of cervical cytological abnormalities, HPV infection and E6/E7 oncogenic mRNA in perinatally HIV-infected compared to behaviourally HIV-infected and HIV-uninfected female adolescents. We hypothesised that perinatally HIV-infected adolescents would have the highest prevalence of these abnormalities compared to the other two groups.
Methods
Study design and population
This was a feasibility study to explore the prevalence of HPV infection and abnormal cervical cytology amongst female adolescents who were perinatally HIV-infected, behaviourally HIV-infected and HIV-uninfected. A convenience sample of sexually active female adolescents was enrolled and assigned to one of three groups. Group 1 consisted of perinatally HIV-infected adolescents, who were enrolled at the HIV-Netherlands Australia Thailand (HIV-NAT) paediatric HIV research Clinic in Bangkok (n = 16) and the paediatric HIV clinic at Chiangrai Prachanukroh Hospital in Northern Thailand (n = 24). Group 2 consisted of behaviourally HIV-infected adolescents and group 3 included HIV-uninfected adolescents. Both groups 2 (n = 10) and 3 (n = 10) were recruited from the clients who came for HIV testing at the Thai Red Cross Anonymous clinic, one of the largest HIV voluntary counselling and testing clinics in Bangkok, Thailand.
Patients were eligible if they were female, aged 12–24 years old, had a history of vaginal intercourse with a male, and fulfilled the HIV status criteria as follows.
Group 1: documented positive HIV enzyme immunoassay (EIA) or nucleic acid testing (NAT) at any time and history of maternal HIV infection.
Group 2: documented positive EIA or NAT at any time after sexual debut without history of maternal HIV infection.
Group 3: documented negative HIV EIA and NAT.
Patients aged ≥18 years gave their consent. For patients aged 12–17 years consent was required from both themselves and their parents. The study was approved by the ethics committees of Chulalongkorn University in Bangkok and Chiangrai Prachanukroh Hospital in Chiang Rai.
At recruitment, patients were asked for their medical history and any information relevant to HIV infection, such as antiretroviral therapy (ART) use. CD4 cell count and HIV RNA data were obtained from medical records. All patients underwent physical examination and cervical sample collection in liquid-based cytology fluid (Liqui-PREP fluid, LGM International, Inc., Florida, USA) for cytology. Adolescents with abnormal cervical cytology results were invited back for colposcopy and biopsy. The cytology liquid was also used to determine HPV subtype and E6/E7 oncogenic messenger RNA (mRNA) quantification. An Audio Computer-assisted Self Interview (ACASI) was used to collect general demographic data, as well as sensitive information on sexual behaviour and behavioural risks. At the Bangkok site, patients were asked to return for a 12-month follow-up visit, at which the same procedures were performed.
Cervical cytology
Cervical sample collection was done by an experienced nurse or physician using a combined spatula and cytobrush technique. The cytology slides were read at the Cytology and Pathology Unit at the Department of Obstetrics and Gynecology, Chulalongkorn University by experienced cytotechnicians [10]. Results were classified according to the 2001 Bethesda system as: normal, atypical squamous cell of undetermined significance (ASC-US); atypical squamous cells – cannot exclude high-grade squamous intraepithelial lesion (ASC-H); low-grade squamous intra-epithelial lesion (LSIL); high-grade squamous intra-epithelial lesion (HSIL); or squamous cell carcinoma. All abnormal cytology slides and a randomly selected 10% of normal cytology slides were reviewed by a senior pathologist, who confirmed the final results.
HPV typing
Stored liquid-based cytology fluid was used for HPV typing by LINEAR ARRAY (LA HPV GT, Roche Molecular Systems, Inc., New Jersey, USA) to identify 37 HPV DNA genotypes: 13 high-risk genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), and 24 other HPV genotypes that are either possibly carcinogenic, non-carcinogenic or of unknown carcinogenicity (6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73 [MM9], 81, 82 [MM4], 83 [MM7], 84 [MM8], IS39 and CP6108).
Quantification of intracellular HPV E6/E7 mRNA
An aliquot from the liquid-based cytology fluid was used for intracellular HPV E6/E7 mRNA flow cytometric analysis using HPV OncoTect E6, E7 mRNA Kit (IncellDx, Menlo Park, CA, USA) [14]. The kit covers the detection of E6/E7 mRNA from HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. Flow cytometry was performed using 3-colour analysis on Beckman Coulter Cytomics FC500. A sample with ≥2% of cells exhibiting E6/E7 mRNA was considered positive. HPV E6/E7 oncoproteins mediate cancer development, and their overexpression can be measured by E6/E7 mRNA.
HPV vaccination
During the conduct of this study, HPV vaccination was offered to adolescents at the two clinics. Two products were used: Cervarix, that protects against HPV 16 and 18 (GlaxoSmithKline Biologicals, Rixensart, Belgium), and Gardasil, that protects against HPV 6, 11, 16 and 18 (Merck & Co, Inc, Whitehouse Station, NJ, USA). The information on uptake of vaccination and number of vaccines received was collected. All adolescents who elected to receive the vaccine had it after completing the baseline visit assessment.
Statistical methods
Demographic and relevant disease-related characteristics of the patient cohort were described and comparisons of categorical covariate across groups were made using a Chi-squared test, or Fisher’s exact test, as appropriate; continuous covariates across groups were analysed using a Kruskal–Wallis or Mann–Whitney U test, depending on whether comparisons were between all subject groups or among only HIV-infected subjects. Prevalence of abnormal cervical cytology, HPV infection, and E6/E7 mRNA positivity were calculated. The proportion with any abnormal cytology, or differing degrees of abnormal cytology between female adolescents in the three groups were compared using Fisher’s exact test. Changes in abnormal cytology and high-risk HPV genotypes over time in a subset with month 12 follow-up were described. Logistic regression models were used to assess predictors of abnormal cervical cytology at baseline in all adolescents; predictor covariates included HPV subtypes, E6/E7 mRNA positivity, sexual and social behaviour. Further models were developed for HIV-infected adolescents to assess the influence of HIV-related characteristics including HAART use, CD4 cell count, HIV RNA suppression, HIV acquisition method, HPV infection and E6/E7 mRNA positivity. Multivariate models were developed including covariates with P < 0.1 in univariate models, and P values in multivariate models were calculated using Wald tests.
Results
Demographic, behavioural and HIV-related characteristics
A total of 60 female adolescents was recruited for the study, 40 of whom were perinatally HIV-infected (group 1), 10 were behaviourally HIV-infected (group 2), and 10 were HIV-uninfected (group 3). The demographic characteristics for all adolescents and sexual behavioural characteristics for 58 adolescents who answered the ACASI questionnaire are shown in Table 1. The perinatally infected patients were younger and had a shorter time since sexual debut. They were more likely to use condoms and less likely to use emergency contraception (i.e. morning after pills). The HIV-uninfected adolescents were more likely to have sex in exchange for money or goods and to smoke cigarettes. More perinatally infected adolescents were on HAART and the duration on treatment was longer compared to those who were behaviourally infected. As a result, group 1 had higher HIV RNA suppression rates (P = 0.004) and tended to have higher CD4 cell counts (P = 0.07) than group 2.
Table 1.
Demographic, sexual and HIV-related characteristics
| Characteristics | Overall | Group 1 (perinatally HIV-infected females) | Group 2 (behaviourally HIV-infected females) | Group 3 (HIV-negative females) | P value |
|---|---|---|---|---|---|
| Number | 60 | 40 | 10 | 10 | |
|
| |||||
| Median age (IQR), yrs | 19 (17–21) | 18 (17–19) | 24 (20–24) | 20.5 (19–23) | <0.001 |
|
| |||||
| Median age (IQR) at sexual debut, yrs | 16 (15–17) | 16 (15–17) | 17 (16–18) | 15.5 (15–18) | 0.34 |
|
| |||||
| Median (IQR) time since sexual debut, yrs | 3 (1–4) | 2 (1–3) | 5 (4–7) | 4 (3–6) | <0.001 |
|
| |||||
| Lifetime sexual partners n (%) | 0.06 | ||||
| 1 | 14 (23.3) | 13 (32.5) | 0 (0) | 1 (10.0) | |
| 2–3 | 23 (38.3) | 16 (40.0) | 4 (40.0) | 3 (30.0) | |
| ≥ 4 | 19 (31.7) | 9 (22.5) | 4 (40.0) | 6 (60.0) | |
| Did not answer/missing | 4 (6.7) | 2 (5.0) | 2 (20.0) | 0 (0) | |
|
| |||||
| Number of partners in the last | 0.50 | ||||
| 3 months n (%) | |||||
| No partner | 6 (10.0) | 3 (7.5) | 2 (20.0) | 1 (10.0) | |
| 1 | 47 (78.3) | 33 (82.5) | 6 (60.0) | 8 (80.0) | |
| 2–3 | 4 (6.7) | 2 (5.0) | 1 (10.0) | 1 (10.0) | |
| ≥ 4 | 0 | 0 | 0 | 0 | |
| Did not answer/missing | 3 (5.0) | 2 (5.0) | 1 (10.0) | 0 | |
|
| |||||
| Frequency of sexual intercourse per month in previous 3 months | 0.20 | ||||
| 0–5 | 30 (50.0) | 19 (47.5) | 8 (80.0) | 3 (30.0) | |
| 5–10 | 17 (28.3) | 12 (30.0) | 1 (10.0) | 4 (40.0) | |
| ≥ 10 | 8 (13.3) | 5 (12.5) | 0 (0.0) | 3 (30.0) | |
| Did not answer/missing | 5 (8.3) | 4 (10.0) | 1 (10.0) | 0 (0.0) | |
|
| |||||
| Types of contraception n (%) | |||||
| Condoms | 33 (55.0) | 27 (67.5) | 4 (40.0) | 2 (20.0) | 0.02 |
| Hormonal contraception | 3 (5.0) | 2 (5.0) | 0 | 1 (10.0) | 0.70 |
| Morning-after pill | 3 (5.0) | 0 | 2 (20.0) | 1 (10.0) | 0.03 |
| Coitus interruptus | 13 (21.7) | 6 (15.0) | 3 (30.0) | 4 (40.0) | 0.15 |
| Contraceptive implant | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | 0.56 |
| Abstinence | 1 (1.7) | 0 | 0 | 1 (10.0) | 0.33 |
| Sterilisation | 6 (10.0) | 4 (10.0) | 1 (10.0) | 1 (10.0) | 1.0 |
| Injection contraception | 9 (15.0) | 7 (17.5) | 1 (10.0) | 1 (10.0) | 1.0 |
| No contraception | 9 (15.0) | 4 (10.0) | 2 (20.0) | 3 (30.0) | 0.23 |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | 0.56 |
|
| |||||
| Condom use n (%) | |||||
| Always | 15 (25.0) | 13 (32.5) | 2 (20.0) | 0 | 0.27 |
| Almost always | 9 (15.0) | 6 (15.0) | 2 (20.0) | 1 (10.0) | |
| Sometimes | 27 (45.0) | 15 (37.5) | 4 (40.0) | 8 (80.0) | |
| Never | 7 (11.7) | 5 (12.5) | 1 (10.0) | 0 | |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (2.5) | 0 | |
|
| |||||
| Ever had an STD n (%) | 5 (8.3) | 2 (5.0) | 2 (20.0) | 1 (10.0) | 0.20 |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | |
|
| |||||
| Sex for money/goods n (%) | 6 (10.0) | 2 (5.0) | 0 | 4 (40.0) | 0.02 |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | |
|
| |||||
| Smoking cigarettes n (%) | 49 (81.7) | 37 (92.5)7 | (70.0) | 5 (50.0) | 0.003 |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | |
|
| |||||
| Ever used alcohol n (%) | 33 (55.0) | 25 (62.5) | 3 (30.0) | 5 (50.0) | 0.25 |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | |
|
| |||||
| Ever used illicit drugs n (%) | 6 (10.0) | 3 (7.5) | 1 (10.0) | 2 (20.0) | 0.42 |
| Did not answer/missing | 2 (3.3) | 1 (2.5) | 1 (10.0) | 0 | |
|
| |||||
| HIV-related parameters | |||||
|
| |||||
| Number | 50 | 40 | 10 | ||
|
| |||||
| On HAART at month 0 n (%) | 43/50 (86.0) | 36/40 (90.0) | 7/10 (70.0) | n/a | 0.13 |
|
| |||||
| Median (IQR) duration on HAART before month 0, yrs (n = 43) | 6.9 (2.2–8.3) | 7.4 (3.3–8.5) | 0.6 (0.5–2.2) | n/a | <0.001 |
|
| |||||
| Median (IQR) CD4 on month 0 cells/μL (n = 50) | 542 (263–771) | 559 (359–832) | 343 (232–565) | n/a | 0.07 |
|
| |||||
| Median (IQR) HIV RNA on month 0, log10 copies/mL (n = 50) | 1.60 (1.60–1.74) | 1.60 (1.60–1.60) | 1.74 (1.60–5.45) | n/a | 0.02 |
| Missing n (%) | 0 | 0 | 3 (30.0) | ||
|
| |||||
| HIV RNA <50 copies/mL n (%) | 35 (70) | 32 (80) | 3 (30) | n/a | 0.001 |
| Missing n (%) | 0 | 0 | 3 (30.0) | ||
|
| |||||
| Disclosed HIV to last partner before engaging in sex n (%) | 0.17 | ||||
| ‘He knows’ (yes) | 26 (52.0) | 23 (57.5) | 3 (30.0) | n/a | |
| ‘He doesn’t know’ (no) | 17 (34.0) | 13 (32.5) | 4 (40.0) | ||
| ‘Not sure’ | 5 (10.0) | 3 (7.5) | 2 (20.0) | ||
| Did not answer/missing | 2 (4.0) | 1 (2.5) | 1 (10.0) | ||
Abbreviations: STD: sexually transmitted disease; HAART: highly active antiretroviral therapy
Cervical cytology, HPV infection and E6/E7 mRNA
Overall, 19 of 60 adolescents (31.7%) had abnormal cervical cytology (defined as ASC-US or higher, referred to hereafter as ASC-US+). Prevalence of ASC-US+ were similar among the three groups: 12 perinatally infected (30%), four behaviourally infected (40%) and three HIV-uninfected adolescents (30%), P = 0.92 (Table 2). LSIL was only observed in perinatally and behaviourally infected adolescents, and no one had HSIL or cancer. We offered colposcopy and biopsy to all adolescents with abnormal cytology with ASC-US+. However only four of 12 perinatally infected adolescents underwent this procedure (three with ASC-US and one with LSIL), while none in the other two groups did (0/4 in group 2 and 0/3 in group 3). Of the three with ASC-US, one had a biopsy result that could not exclude cervical intra-epithelial neoplasia (CIN) so she underwent a loop electrosurgical excision procedure, and the pathology of the tissue showed no CIN. For the other two adolescents with ASC-US, one had no CIN and one had CIN 1 on cervical biopsy. In the last adolescent with LSIL, the biopsy showed CIN 1.
Table 2.
Abnormal cytology, HPV infection and E6/E7 oncogenic mRNA positivity
| Outcome measures | Overall | Group 1 (perinatally HIV-infected females) | Group 2 (behaviourally HIV-infected females) | Group 3 (HIV-negative females) | P value |
|---|---|---|---|---|---|
| Number | 60 | 40 | 10 | 10 | |
|
| |||||
| Total abnormal Pap smear n (%) | 19 (31.7) | 12 (30.0) | 4 (40.0) | 3 (30.0) | 0.92 |
|
| |||||
| Abnormal Pap smear cytology n (%) | 0.27 | ||||
| ASC-US | 13 (21.7) | 9 (22.5) | 1 (10.0) | 3 (30.0) | |
| LSIL | 6 (10.0) | 3 (7.5) | 3 (30.0 | 0 | |
| HSIL | 0 | 0 | 0 | 0 | |
|
| |||||
| Any HPV infection n (%) | 36 (60.0) | 23 (57.5) | 8 (80.0) | 5 (50.0) | 0.32 |
|
| |||||
| High-risk HPV infection n (%) | 26 (43.3) | 18 (45.0) | 4 (40.0) | 4 (40.0) | 1.00 |
|
| |||||
| HPV infection group n (%) | 0.36 | ||||
| Non-high risk | 34 (56.7) | 22 (55.0) | 6 (60.0) | 6 (60.0) | |
| High-risk 16 and/or 18 | 15 (25.0) | 8 (20.0) | 3 (30.0) | 4 (40.0) | |
| High-risk HPV other than type 16 or 18 | 11 (18.3) | 10 (25.0) | 1 (10.0) | 0 | |
|
| |||||
| Infection with ≥2 high-risk HPV types n (%) | 9 (15.0) | 6 (15.0) | 1 (10.0) | 2 (20.0) | 0.87 |
|
| |||||
| E6/E7 positivity n (%)* | 11 (18.3) | 11 (27.5) | 0 | 0 | 0.02 |
Abbreviations: HPV: human papillomavirus; ASC-US: atypical squamous cell of undetermined significance; LSIL: low-grade squamous intra-epithelial lesion; HSIL: high-grade squamous intra-epithelial lesion.
Samples with ≥2% of cells exhibiting E6/E7 mRNA were considered positive.
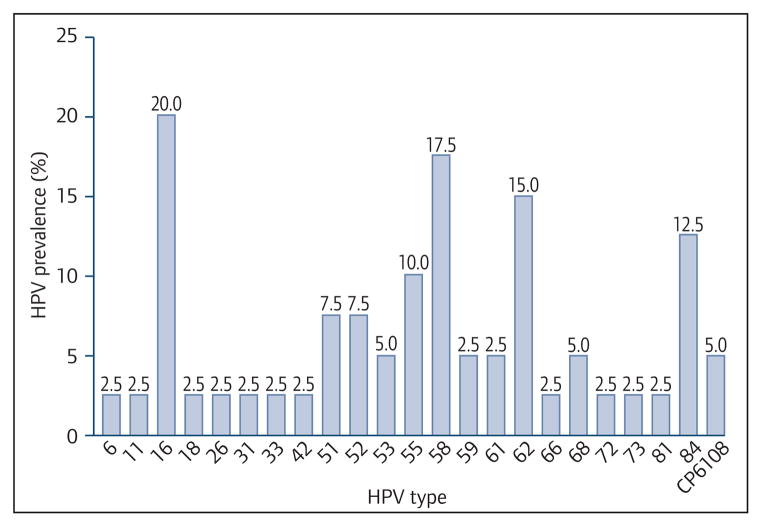
The overall prevalence rates of cervical infection with any HPV and high-risk HPV were 60% and 43%, respectively, and these were not statistically different between groups (Table 2). Of perinatally HIV-infected adolescents, 58% had any HPV infection, compared with 80% of behaviourally HIV-infected and 50% of HIV-uninfected adolescents (P = 0.32). Furthermore, 45% of perinatally HIV-infected adolescents had high-risk HPV infection, compared to 40% in the other two groups. The frequencies of HPV types did not differ between groups (data not shown), and high-risk HPV types other than those included in preventive vaccines (non-HPV 16, 18) featured prominently in all groups. Of the adolescents with abnormal cytology, 9/12 (75%), 3/4 (75%) and 2/3 (66%) of perinatally infected, behaviourally infected and HIV-negative females had infection with a high-risk HPV type. Infection with two or more high-risk HPV types was observed in about 15% of adolescents, without significant differences between groups. Figure 1 displays the prevalence of HPV types in 40 perinatally HIV-infected adolescents. HPV-16 was most common high-risk subtype (20%), followed by HPV-58 (17.5%) and HPV-51 and HPV-52 (both 7.5%). HPV-18 infection was observed in 2.5%. E6/E7 mRNA positivity was observed in 27.5% (95%CI 14.6–43.8%) perinatally HIV-infected, compared to 0% (95%CI 0–30.8%) in both behaviourally HIV-infected and HIV-uninfected adolescents (Table 2).
Figure 1.
HPV genotype prevalence among 40 perinatally HIV-infected adolescents
Predictors for abnormal cytology
Logistic regression analyses were performed to identify factors associated with abnormal cervical cytology (ASC-US+) at baseline among the 60 subjects in the study (Table 3). The only factor associated with abnormal cytology was cervical infection with high-risk HPV (OR 6.77, 95% CI 1.99–23.0; P = 0.001). Further models were developed for the 50 HIV-infected adolescents to assess the influence of HAART use, CD4 cell count, plasma HIV RNA ≥50 copies/mL, mode of HIV acquisition, HPV infection and E6/E7 mRNA positivity. In the univariate analyses, infection with a high-risk HPV type, HAART use, lower CD4 counts and plasma HIV-RNA ≥50 copies/mL were all associated with abnormal cytology. In a multivariate model, after adjusting for duration of HAART use and baseline CD4 cell count, cervical infection with high-risk HPV (OR 11.23, 95% CI 1.73–72.97; P = 0.01) and a HIV RNA of ≥50 copies/mL (OR 13.3, 95% CI 1.16–153.06; P = 0.04) were risk factors for abnormal cytology.
Table 3.
Logistic regression models for factors associated with abnormal cytology (ASC-US+) on Pap smear at baseline
| Model 1: All subjects | P value | Model 2: HIV-infected subjects only | P value | Adjusted model* | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | ||||
| HPV subtypes | 0.001 | 0.000 | 0.01 | |||
| No HPV or low-risk subtypes | 1 (ref) | 1 (ref) | 1 | |||
| High-risk subtypes | 6.7 (1.99–23.0) | 7.20 (1.86–27.8) | 11.23 (1.73–72.97) | |||
| E6/E7 mRNA positive | 0.75 (0.17–3.22) | 0.69 | 0.75 (0.17–3.31) | 0.70 | ||
|
| ||||||
| Demographics | ||||||
| Age at sexual debut <17 yrs | 1.06 (0.32–3.44) | 0.93 | 0.73 (0.19–2.55) | 0.59 | ||
|
| ||||||
| Smokes cigarettes | 0.80 (0.17–3.65) | 0.78 | 1.26 (0.12–13.2) | 0.85 | ||
|
| ||||||
| Ever used alcohol | 0.89 (0.28–2.81) | 0.85 | 0.70 (0.19–2.55) | 0.59 | ||
|
| ||||||
| Ever used illicit drugs | 0.81 (0.13–4.91) | 0.82 | 1.26 (0.12–13.24) | 085 | ||
|
| ||||||
| Subject group | 0.83 | 0.5 | ||||
| Perinatally HIV-infected | 1 (ref) | 1 (ref) | ||||
| Behaviourally HIV-infected | 1.56 (0.37–6.53) | 1.56 (0.37–6.53) | ||||
| HIV-uninfected | 1.00 (0.22–4.54) | |||||
|
| ||||||
| Sexual behaviour | ||||||
| Lifetime number of sexual partners | 0.63 | 0.58 | ||||
| 1 | 1 (ref) | 1 (ref) | ||||
| 2–3 | 1.83 (0.44–7.65) | 1.67 (0.39–7.32) | ||||
| 4 or higher | 3.67 (0.35–38.02) | 3.33 (0.33–34.83) | ||||
|
| ||||||
| Number of partners in last | 0.38 | 0.17 | ||||
| 3 months | ||||||
| None or one | 1 (ref) | 1 (ref) | ||||
| 2–3 | 2.53 (0.33–19.66) | 5.33 (0.44–64.36) | ||||
|
| ||||||
| Ever diagnosed with an STD | 0.24 (0.04–1.58) | 0.13 | 0.37 (0.05–2.97) | 0.35 | ||
|
| ||||||
| Condom use | 0.96 | 0.95 | ||||
| Always condom use | 1 (ref) | 1 (ref) | ||||
| Sometimes/almost always | 1.21 (0.31–4.65) | 1 16 (0.28–4.75) | ||||
| Never | 1.10 (0.15–8.13) | 1.38 (0.18–10.65) | ||||
|
| ||||||
| HIV-related characteristics | ||||||
| CD4 count at baseline (cells/μL) | 0.92 | 0.51 | ||||
| >350 | 1 (ref) | 1 (ref) | ||||
| 200–349 | 3.29 (0.80–20.10) | 3.14 (0.33–35.24) | ||||
| 0–199 | 9.37 (1.59–62.78) | 2.71 (0.22–33.02) | ||||
|
| ||||||
| Baseline HIV RNA ≥50 copies/mL | 14.5 (3.00–70.03) | 0.00 | 13.31 (1.16–153.06) | 0.04 | ||
|
| ||||||
| Duration used HAART before enrolment | 0.28 | 0.07 | 0.75 | |||
| No HAART before enrolement | 1 (ref) | 1 (ref) | ||||
| <3 yrs | 0.13 (0.02–0.98) | 0.58 (0.02–15.84) | ||||
| ≥ 3 yrs | 0.14 (0.02–0.89) | 1.30 (0.04–37.57) | ||||
Since apart from HPV grouping, all factors meeting criteria for entry into a multivariate model related to HIV, the adjusted model presented is relevant to the HI-infected subjects only.
Abbreviations: STD: sexually transmitted disease; HAART: highly active antiretroviral therapy.
Twelve-month follow-up
Forty adolescents at the Bangkok site were asked to return for a 12-month follow-up, and 13/20, 8/10 and 6/10 in groups 1, 2 and 3 did. Three of 27 adolescents had LSIL at baseline (one in group 1 and two in group 2), and by month 12, one had persistent LSIL, one regressed to ASC-US and the other to normal. Seven had ASC-US at baseline. At month 12, the ASC-US lesion had regressed to normal in five adolescents, whereas two, both perinatally infected, progressed to LSIL. Cytology remained normal in 14/17 with normal cytology at baseline. However, two perinatally infected adolescents had progression to either LSIL or ASC-US, and one who was HIV-uninfected had progression to ASC-US. All adolescents who had progression of lesions and the adolescent with persistent LSIL had at least one prior dose of vaccine prior to the 12-month follow-up visit; two of seven adolescents who had regression of lesions had no doses of vaccine, and the remaining five had at least one dose. Of the 27 adolescents with baseline and 12-month cytology results, 10 were infected with ≥1 high-risk HPV subtype at baseline, and of the majority (n = 7/10) had persistent infection of at least one high-risk subtype at month 12 (3/4 in group 1, 2/3 in group 2, 2/3 in group 3). Five acquired a new high-risk subtype at month 12 (three in group 1, and one each in groups 2 and 3). Among 17 with no HPV or no high-risk HPV infection at baseline, eight acquired a new high-risk HPV infection at month 12 (6/9 in group 1, 1/5 in group 2, 1/3 in group 3). In these adolescents, two of those in group 1, and one in group 3 acquired HPV 16 and/or 18 at month 12. Five of six from group 1 had received three doses of vaccine and one received two doses before month 12; the adolescents in groups 2 and 3 received one and two doses of vaccine before month 12, respectively.
Uptake of HPV vaccination
All 60 adolescents in the study were offered HPV vaccination after the first visit, and 52 (87%) received at least one dose of the vaccine. At time of study closure, 41 had completed all three doses and the remaining 11 are scheduled to complete their doses. No adolescent to date has failed to return for scheduled doses. Of the eight adolescents who did not agree to vaccination, two each were in groups 1 and 2, and four were in group 3.
Discussion
There is a paucity of data on cervical HPV infection and cytology among sexually active, perinatally HIV-infected female adolescents [15,16]. Although conducted in a small cohort, this report is, to our knowledge, among the first. In our adolescents, HPV infection of any type was observed in 58%, including 45% having high-risk HPV infection. This is higher than a US study of non-sexually active perinatally infected children and adolescents, where only 35% of females >12 years of age had any anogenital HPV infection (i.e. vulvar +/− perianal) [17]. We also found that 30% of our perinatally HIV-infected adolescents had abnormal cervical cytology, which was lower than the rate of 47% reported in sexually active perinatally infected adolescents enrolled in the Pediatric AIDS Clinical Trials Group protocol 219C [15]. HPV infection and abnormal cervical cytology were highly prevalent in behaviourally HIV-infected and in HIV-uninfected adolescents, at similar rates. The rate of E6/E7 oncogenic mRNA positivity was 27.5% amongst the perinatally infected adolescents; no adolescents in the other two groups exhibited E6/E7 mRNA positivity, although this should be interpreted with caution due to small sample sizes of these latter groups. Furthermore, infection with at least one high-risk HPV type and detectable plasma HIV-RNA were important predictors of cervical cytological abnormalities.
In our study, the younger and less sexually experienced perinatally infected group had similarly high rates of cervical cytological abnormalities and high-risk HPV infection as the behaviourally infected and uninfected adolescents. This is despite also having higher CD4 cell count and lower HIV RNA than the behaviourally infected adolescents. In addition, in those who returned for their 12-month follow-up, a fair number had persistence or progression of cytological abnormalities and had persistent high-risk HPV infection and/or acquired new HPV infection as observed by Brogly et al. [15]. It is possible that this is a result of prolonged immune suppression from HIV infection. Our perinatally infected adolescents were on average 18 years old and had been on HAART for about 7 years. We do not have information on their CD4 nadir, but they would most likely have initiated HAART according to the Thai National Guideline, which at that time, recommended treatment when the CD4 cell count was below 200 cells/μL or at diagnosis of an AIDS-defining illness. Nadir CD4 counts <200 cells/μL are known to be associated with a higher risk of abnormal cervical cytology in behaviourally infected adults [10]. In our current study, a CD4 cell count < 350 cells/μL was associated with a significantly increased risk of cytological abnormalities. A low CD4 cell count has been associated with cytological abnormalities in other studies [9,18]. The presence of immune suppression enables persistence of HPV, which in turn is necessary for the development of neoplastic lesions. It is also possible that hormonal surges around puberty can reactivate latent HPV infection, further contributing to the risk of HPV persistence [17].
ASC-US and LSIL were common among adolescents from all three groups in our study (30%, 40% and 30%, respectively), and none had HSIL or cervical cancer. The rate of ASC-US in our study was similar to that of the Reaching for Excellence in Adolescent Care and Health (REACH) cohort in the US (30%) of behaviourally HIV-infected and -uninfected adolescents. However, REACH had higher rates of LSIL (35%) and HSIL (5%) [7]. Strikingly, the behaviourally HIV-infected adolescents had a much higher rate of HSIL at 22% compared with 5% among HIV-uninfected adolescents (P<0.01) despite the two groups having similar rates of high-risk HPV infection and low-grade cytological abnormality [11]. These data suggest that HIV-infected adolescents are more likely to progress to high-grade lesions, and routine cytological screening in these adolescents should be a priority.
Persistent infection with high-risk HPV is a precursor to cervical cancer [7–9,18]. In our study, the rates of infection with high-risk HPV type were also similar among perinatally infected, behaviourally infected and uninfected adolescents, in spite of the HIV-uninfected group having somewhat greater sexual risk behaviours (e.g. number of life-time partners, frequency of sex, sex in exchange for goods or money). These HIV-uninfected adolescents were clients who walked in for HIV testing at the Thai Red Cross Anonymous Clinic, and probably represent a group at higher risk for HIV infection than the general Thai population. The rates of infection with any type of HPV (60%) or high-risk HPV type (43%) in our study are similar to published reports in behaviourally HIV-infected and HIV-uninfected adolescents, highlighting the importance of this infection across settings [7,8,11,19–22].
We evaluated the E6/E7 oncogenic mRNA, an early marker of high-risk HPV-mediated cell transformation, among the three groups of adolescents, and observed a positivity rate of about 30% in only the perinatally HIV-infected adolescents. This rate is higher than the 11% observed in HIV-uninfected adolescents of similar ages in a US study [22]. Studies have shown E6/E7 mRNA positivity to be superior to HPV testing in predicting abnormal cervical cytology [23], and a better predictor for abnormal pathology on biopsy than cytology screening [24]. However, in this study, we did not observe a significant association between E6/E7 mRNA positivity and abnormal cytology.
According to the American College of Obstetricians and Gynecologists, HIV-uninfected adolescents should begin screening for cervical cancer at age 21, regardless of the onset of sexual debut. It is advisable that behaviourally HIV-infected adolescents should have cervical cytology screening twice in the first year after HIV diagnosis and annually thereafter [25]. Although there are no specific guidelines for perinatally HIV-infected adolescents, our data suggest that abnormal cytology can occur within a few years after sexual debut; therefore, these adolescents should begin cervical cytology screening shortly after sexual debut. UK guidelines on the HYPNet website advise annual screening for perinatally infected young people from a year from coitarche with baseline colposcopy [26].
The best way to prevent HPV infection and subsequently abnormal cervical cytology is to immunise against HPV infection prior to sexual debut. Currently, HPV vaccination in the US is advised for females from the ages of 9 to 26 years old for the bivalent vaccine, and 11 to 26 years old for the quadrivalent vaccine [27–30]. The rates of uptake vary widely between settings. However, despite the low uptake in the US, a marked decrease in HPV prevalence in females aged 14–19 years has been seen [31]. In Thailand, HPV vaccine is not yet part of routine immunisation, and it costs approximately US$100 per course, which is unaffordable for most Thais. We were able to offer HPV vaccination to all participants in this study because of vaccine donations, and the uptake was high. As the age at sexual debut is becoming younger and HPV prevalence is the highest shortly after sexual debut [19], government healthcare costs associated with diagnosis and treatment of cases with abnormal cytology, could be significant and outweigh the costs of prevention. In HIV-infected adolescents at high risk for cervical cancer, routine HPV vaccination would be a prudent investment, even for developing countries such as Thailand [32,33]. Cervical pre-cancer screening programmes using cytology and possibly the addition of other biomarkers such as HPV DNA or E6/E7 mRNA remain essential for early diagnosis and management of cervical cancer.
There are several limitations of our study. First, the small sample size precluded robust statistical comparisons of the prevalence rates of abnormal cytology, E6/E7 oncoprotein and HPV prevalence rates between groups; however, recruitment for the study was difficult, particularly for subjects aged under 18 who may not have wanted their parents to know they were sexually active. Secondly, the longitudinal follow-up rates were low. Nevertheless, these data highlight the high prevalence of cervical cytological abnormalities in adolescent females, and show that high-risk HPV infection in perinatally HIV-infected adolescents can be similar to their behaviourally HIV-infected and uninfected peers, despite being younger and having a shorter time since sexual debut.
Acknowledgments
We are grateful to the patients and their families for participating in this study. We thank staff at HIV-NAT, the Thai Red Cross Anonymous Clinic and Chiangrai Prachanukroh Hospital for their assistance with the study conduct, as well as staff at Petchburi and Siriraj Hospitals for referring patients to our study. We thank members of the We Understand Group for being part of the community advisory committee for this study.
Funding support was provided through a grant to amfAR, The Foundation for AIDS Research, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), by the U.S. National Institutes of Health (NIH): National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD) and National Cancer Institute (NCI), with additional support from the AIDS Life Association.
GlaxoSmithKline donated Cervarix and the Ministry of Public Health donated Gardasil for our patients at the two participating sites. We thank Ms June Piraporn Ohata for her help in preparing the manuscript. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
HIV-NAT 139 Study group
HIV-NAT and The Thai Red Cross Anonymous Clinic: Torsak Bunupuradah, Thanyawee Puthanakit, Sunee Sirivichayakul, Jiranuwat Barisri, Amornrat Srimuan, Wanida Thiansanguankul, Chowalit Phadungphon, Naphassanant Laopraynak, Sudrak Lukhonpon, Chutima Saisaengjan.
Petchburi Hospital: Manee Yentang.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
This work has been presented in part at the 19th Conference on Retroviruses and Opportunistic Infections. March 2012, Seattle, WA, USA. Abstr. 896.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
References
- 1.Vaccarella S, Lortet-Tieulent J, Plummer M, et al. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Abraham AG, D’Souza G, Jing Y, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62:405–413. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny LA, Franceschi S, de Sanjose S, et al. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30 (Suppl 5):F168–174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Del Mistro A, Bertorelle R, Franzetti M, et al. Antiretroviral therapy and the clinical evolution of human papillomavirus-associated genital lesions in HIV-positive women. Clin Infect Dis. 2004;38:737–742. doi: 10.1086/381681. [DOI] [PubMed] [Google Scholar]
- 5.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 7.Moscicki AB, Ellenberg JH, Vermund SH, et al. Prevalence of and risks for cervical human papillomavirus infection and squamous intraepithelial lesions in adolescent girls: impact of infection with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2000;154:127–134. doi: 10.1001/archpedi.154.2.127. [DOI] [PubMed] [Google Scholar]
- 8.Vermund SH, Wilson CM, Rogers AS, et al. Sexually transmitted infections among HIV infected and HIV uninfected high-risk youth in the REACH study. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health. 2001;29:49–56. doi: 10.1016/s1054-139x(01)00296-8. [DOI] [PubMed] [Google Scholar]
- 9.Firnhaber C, Van Le H, Pettifor A, et al. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control. 2010;21:433–443. doi: 10.1007/s10552-009-9475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangclaviraj S, Kerr SJ, Chaithongwongwatthana S, et al. Nadir CD4 count and monthly income predict cervical squamous cell abnormalities in HIV-positive women in a resource-limited setting. Int J STD AIDS. 2008;19:529–532. doi: 10.1258/ijsa.2007.007222. [DOI] [PubMed] [Google Scholar]
- 11.Moscicki AB, Ellenberg JH, Crowley-Nowick P, et al. Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. J Infect Dis. 2004;190:1413–1421. doi: 10.1086/424466. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Li X, Zhang Z, et al. Association of human papillomavirus infection and abnormal anal cytology among HIV-infected MSM in Beijing, China. PLoS One. 2012;7:e35983. doi: 10.1371/journal.pone.0035983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YX, Xiong Y, Gui XE, et al. Epidemiologic risk profile of human papillomavirus infections in human immunodeficiency virus-positive Chinese women. Jpn J Infect Dis. 2011;64:411–416. [PubMed] [Google Scholar]
- 14.Narimatsu R, Patterson BK. High-throughput cervical cancer screening using intracellular human papillomavirus E6 and E7 mRNA quantification by flow cytometry. Am J Clin Pathol. 2005;123:716–723. [PubMed] [Google Scholar]
- 15.Brogly SB, Watts DH, Ylitalo N, et al. Reproductive health of adolescent girls perinatally infected with HIV. Am J Public Health. 2007;97:1047–1052. doi: 10.2105/AJPH.2005.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croucher AP, Jose S, McDonald S, et al. Sexual and reproductive health in a UK cohort of young adults perinatally infected with HIV. Sex Transm Infect. 2013;89:392–394. doi: 10.1136/sextrans-2012-050831. [DOI] [PubMed] [Google Scholar]
- 17.Moscicki AB, Puga A, Farhat S, Ma Y. Human papillomavirus infections in nonsexually active perinatally HIV infected children. AIDS Patient Care STDS. 2014;28:66–70. doi: 10.1089/apc.2013.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kravchenko J, Akushevich I, Sudenga SL, et al. Transitional probability-based model for HPV clearance in HIV-1-positive adolescent females. PLoS One. 2012;7:e30736. doi: 10.1371/journal.pone.0030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argyri E, Papaspyridakos S, Tsimplaki E, et al. A cross sectional study of HPV type prevalence according to age and cytology. BMC Infect Dis. 2013;13:53. doi: 10.1186/1471-2334-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciszek B, Heimrath J, Ciszek M. The application of human papilloma virus genotyping for the identification of neoplasm lesions in the cervix of women with abnormal cytology smears. Adv Clin Exp Med. 2012;21:759–766. [PubMed] [Google Scholar]
- 21.Kahn JA, Burk RD, Squires KE, et al. Prevalence and risk factors for HPV in HIV-positive young women receiving their first HPV vaccination. J Acquir Immune Defic Syndr. 2012;61:390–399. doi: 10.1097/QAI.0b013e3182676fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michala L, Argyri E, Tsimplaki E, et al. Human papilloma virus infection in sexually active adolescent girls. Gynecol Oncol. 2012;126:207–210. doi: 10.1016/j.ygyno.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Mockel J, Quaas J, Meisel H, et al. Human papillomavirus E6/E7 mRNA testing has higher specificity than liquid-based DNA testing in the evaluation of cervical intraepithelial neoplasia. Anal Quant Cytol Histol. 2011;33:311–315. [PubMed] [Google Scholar]
- 24.Pierry D, Weiss G, Lack B, et al. Intracellular human papillomavirus E6, E7 mRNA quantification predicts CIN 2+ in cervical biopsies better than Papanicolaou screening for women regardless of age. Arch Pathol Lab Med. 2012;136:956–960. doi: 10.5858/arpa.2011-0180-OA. [DOI] [PubMed] [Google Scholar]
- 25.ACOG Committee Opinion No. 463. Cervical cancer in adolescents: screening, evaluation, and management. Obstet Gynecol. 2010;116:469–472. doi: 10.1097/AOG.0b013e3181eeb30f. [DOI] [PubMed] [Google Scholar]
- 26.HYPNET/CHIVA. [accessed November 2014];Guidance on the management of sexual and reproductive health for adolescents living with HIV. 2011 Available at: http://www.hypnet.org.uk/pages/guidelines.php.
- 27.Centers for Disease Control and Prevention (CDC) FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 28.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent Human Papillomavirus Vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007 Mar 23;56(RR02):1–24. [PubMed] [Google Scholar]
- 29.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 30.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 31.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 32.Demarteau N, Van Kriekinge G, Simon P. Incremental cost-effectiveness evaluation of vaccinating girls against cervical cancer pre- and post-sexual debut in Belgium. Vaccine. 2013;31:3962–3971. doi: 10.1016/j.vaccine.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Termrungruanglert W, Havanond P, Khemapech N, et al. Cost and effectiveness evaluation of prophylactic HPV vaccine in developing countries. Value Health. 2012;15:S29–34. doi: 10.1016/j.jval.2011.11.007. [DOI] [PubMed] [Google Scholar]