Abstract
Elevated levels of protein kinase CK2 (formerly casein kinase 2 or II) have long been associated with increased cell growth and proliferation both in normal and cancer cells. The ability of CK2 to also act as a potent suppressor of apoptosis offers an important link to its involvement in cancer since deregulation of both cell proliferation and apoptosis are among the key features of cancer cell biology. Dysregulated CK2 may impact both of these processes in cancer cells. All cancers that have been examined show increased CK2 expression, which may also relate to prognosis. The extensive involvement of CK2 in cancer derives from its impact on diverse molecular pathways controlling cell proliferation and cell death. Downregulation of CK2 by various approaches results in induction of apoptosis in cultured cell and xenograft cancer models suggesting its potential as a therapeutic target.
Keywords: CK2, casein kinase 2, CKII, cancer, CK2 dysregulation, CK2 upregulation, prognostic marker, therapeutic target, nanoparticle, nanocapsule
Introduction
Investigations from several laboratories have resulted in detailed description of the characteristics of protein kinase CK2 as documented in a number of recent reviews [see e.g., 1–8]. CK2 is a ubiquitous serine/threonine protein kinase that is among the most highly conserved proteins in nature. Its heterotetrameric structure consists of two catalytic subunits (~ 42 kDa α and 38 kDa α′) and two regulatory subunits (~ 28 kDa β) in α2β2, αα′β2, or α′2β2 configurations. The two catalytic subunits are linked through the β subunits, which are stimulatory rather than inhibitory to the kinase activity [9]. The β subunits may form a linkage with the nuclear matrix [10]. CK2 phosphorylates a large number of substrates, many of which are involved in gene expression and cell growth [2, 3, 5, 6, 11, 12]. Studies on the biological functions of CK2 have followed a circuitous route, and to further complicate the field, a considerable amount of earlier work did not use the name casein kinase 2 or CK2. For example, pioneering studies on phosphorylation of non-histone proteins in the nucleus in response to altered cell growth suggested the involvement of protein kinase activity towards these acidic proteins that later could be attributed to CK2 [see e.g., 12], and in fact many substrates of CK2 are nuclear-associated [e.g., 13]. An important feature of CK2 biology is the recognition that CK2 dynamically localizes to various cellular compartments under diverse conditions [14–16]. Indeed, nuclear structures such as chromatin and nuclear matrix acutely demonstrate modulations in CK2 in response to various growth and cell death stimuli [2, 4, 11, 12, 14–18]. As discussed subsequently, the nuclear matrix appears to be a key locus for CK2 signaling in the nucleus with regard to its function in cell proliferation and cell death. While CK2 is present in all cells (both normal and cancer), the focus of this review is to highlight its functionality in the cancer cell phenotype as compared with that in the normal, and to document aspects of CK2 function in cancer cell biology that promote consideration of this protein as a target for cancer therapy.
CK2 status in normal vs. cancer cells
Early studies of protein kinase signaling in the prostate [19] led to identification of CK2 as an important signal in the androgen- and growth factor-mediated regulation of growth in a normal cell growth model [20]. A key feature of CK2 regulation of cell growth and death relates to nuclear signaling, as evidenced by its shuttling to and from nuclear structures such as chromatin and the nuclear matrix in response to altered growth stimuli; these features of CK2 were originally documented by us [e.g., 2, 4, 11, 12, 14, 21–28]. Although the mechanisms involved in the in vivo regulation of CK2 are not fully understood, our work has suggested that CK2 targeting to specific loci in the cell represents an important aspect of its intracellular dynamics [e.g., 2, 14, 15, 17, 23, 29]. CK2 dynamics in the nuclear matrix appear to be the most remarkable indicator of cell signaling response leading to growth or death. Much evidence suggests that the nuclear matrix is intimately involved in cell growth, proliferation, and apoptosis [30–33]. For example, in androgen-responsive prostate cells, removal of survival signals causes loss of CK2 from the nuclear structures and is associated with inhibition of cell growth and induction of apoptosis. Restoration of the growth stimulus (androgen or growth factors) promotes survival and proliferation and is associated with an early shuttling of CK2 to the nucleus. Indeed, in the rat prostate model, loss of CK2 from the nuclear matrix at 24 h after androgen ablation is a marked 80%, with a concomitant 3-fold change in the number of cells in apoptosis. At 48 h, more than 92% of the CK2 is lost from the nuclear matrix with a concomitant 21-fold change in the number of apoptotic cells [14, 20, 25, 28]. That the nuclear matrix is the locale of several CK2 substrates further emphasizes the importance of these observations linking CK2 shuttling to and from the nuclear structures in response to various stimuli [2, 11, 12]. Recent studies have provided further evidence that downregulation of CK2 expression promotes an early loss of CK2 from the nuclear matrix and is associated with induction of apoptosis in prostate tumor in vivo [34,35]. These observations suggest the potential involvement of a subcellular compartment of CK2 (i.e., that in the nuclear matrix) in the regulation of cell growth and apoptosis.
CK2 has been found to be upregulated in all cancers that have been examined. This increase in CK2, however, does not manifest itself at the level of message expression but rather at the level of protein, as determined in various studies by measurement of enzyme activity, immunohistochemistry or immunoblot analysis [see e.g., 3, 11]. However, it seems that elevated levels of CK2 alone are not indicative of dysregulation since high levels of CK2 are intrinsically present in certain organs such as brain and testes. CK2 in various cell types remains at a distinct and stable level under normal conditions. During cell proliferation, CK2 protein levels increase, then return subsequently to the original basal level characteristic of the particular cell type. As discussed later, stable levels of CK2 appear to be critical to homeostasis in the cell. Several features of CK2 status in cancer cells are noteworthy. First, tumor cells have a high nuclear concentration of CK2, whereas in normal cells, the protein is diffused in various compartments. Even more important, CK2 elevation in neoplasia reflects a complex state of dysplasia, not simply the proliferative state of the tumor cells [36]. Second, dysregulation of CK2 expression may relate to the severity of disease and even serve as a prognostic indicator [37, 38], an idea reinforced by recent observations in other laboratories [39, 40]. Third, as discussed subsequently, the recent discovery that CK2, besides being involved in cell growth and proliferation, is a potent suppressor of apoptosis supports its role in cell survival and firmly ties CK2 upregulation and function to the cancer cell phenotype [4, 11, 21, 41, 42].
Oncogenic potential of CK2
By itself, CK2 does not appear to be an oncogene. The proposal for the potential involvement of CK2 in oncogenesis has been based on observations that CK2 expression was upregulated in all cancers examined [e.g., 3, 8, 11]. Although it had long been appreciated that CK2 is elevated during normal and cancer cell proliferation, its role in the cancer cell phenotype remained unclear, leading to the assumption that elevated amounts of CK2 in cancer cells were simply a reflection of their proliferative status [3]. However, we originally demonstrated that increased CK2 expression in cancer cells reflected not only proliferation but also the state of dysplasia [36].
A significant aspect of CK2 biology is that it is essential for cell survival [e.g., 43, 44]. Accordingly, attempts to produce CK2α and CK2β-knockout mice have been unsuccessful (44,45). Moreover, modest downregulation of CK2 in the nuclear compartment (chromatin and matrix) leads to induction of widespread cell death by apoptosis [11, 12, 22, 34, 35]. In this context, it must be emphasized that cellular CK2 expression tends to be stable, and relatively small changes in the balance of CK2 expression have a large impact on cellular homeostasis. The apparent dysregulation of CK2 in various cancers suggests that at some stage in the process of transformation it undergoes significant change in its status, imparting an oncogenic potential to cells. Although CK2 by itself is not an oncogene, experimental animal studies have corroborated the remarkable contributory oncogenic potential imparted by dysregulation of CK2. For example, overexpressed CK2α in combined transgenic expression with c-myc or Tal-1 resulted in a significant increase in the incidence of leukemia and lymphoma in mice [46–49]. Likewise, expression of CK2α in mouse mammary gland under control of the MMTV-LTR resulted in a transgenic mouse model of breast cancer with several features resembling the human disease [48]. In each case, modest overexpression of the CK2α transgene was sufficient to evoke enhanced oncogenic potential in the mice. These studies suggest that CK2 involvement in oncogenesis may be coupled to its modulation of the activity of other oncogenic signals in the cells. Noteworthy are the observations that CK2 can influence the stability of c-myc [49], and serve as a positive regulator of Wnt signaling in relation to tumorigenesis [50].
CK2: A key suppressor of apoptosis
Programmed cell death or apoptosis is a key component of cell physiology, and its dysregulation is recognized as a critical factor in oncogenesis. The latter aspect is especially pertinent to prostate cancer [41, 42, 51]. The initial observations that suggested a role for CK2 in the suppression of apoptosis were twofold. First, growth stimulus deprivation (androgen or growth factors) resulted in rapid loss of CK2 from the nuclear compartment (e.g., chromatin and matrix), which preceded the appearance of apoptosis [22]. Second, chemical and physical agents that induce apoptosis (e.g., etoposide, diethylstilbestrol, heat shock) result in rapid shuttling of CK2 to the nuclear matrix in PC-3 and ALVA-41 cells as an initial protective response [21, 52]. Similar observations have been made with respect to CK2 response to radiation [53]. Proof of the protective role of nuclear CK2 was obtained when ectopic overexpression of CK2α prior to treatment with apoptosis-inducing agents was found to protect these cells strongly from apoptosis [21]. The specificity of the protective effect resided in the α but not the β subunit [21]. Further investigation has demonstrated that death receptor-mediated apoptosis in ALVA-41 and PC-3 cells also is suppressed by prior overexpression of CK2α [54,55]. Thus, our work has established that CK2 can afford protection against apoptosis mediated via different pathways (intrinsic and extrinsic). Recent studies of other cancer models have provided additional supportive data for the role of CK2 in receptor-mediated apoptosis [53, 56–58]. It is noteworthy that several components of the apoptosis machinery are candidate substrates for phosphorylation by CK2 [e.g., 17]. Other pertinent observations are that CK2 facilitates repair of DNA single-strand breaks [59] and is a key regulator of the tumor suppressor PML through ubiquitin mediated degradation on CK2 mediated phosphorylation [60]. Interestingly, the c-terminal truncated form of the tumor suppressor adenomatous polyposis coli (APC) protein, which is an early event in most colon adenomas and carcinomas [61], has been shown to associate with CK2 without affecting its activity, whereas the full-length APC protein interaction with CK2 results in reducing its activity thereby exerting a growth inhibitory effect [62]. Finally, IGFBP-3 has been found to be specifically phosphorylated by CK2, which influences its apoptotic activity, an observation that is particularly significant since the IGF axis is believed to play an important role in the pathogenesis of many cancers [63].
CK2 interaction with other signaling molecules
In the foregoing, we discussed characteristics of CK2 in normal versus cancer cells and how CK2 upregulation contributes to suppression of apoptosis and oncogenesis. Overall, the data would suggest that CK2 affects a variety of molecules involved in diverse processes and, in fact, impacts on a wide range of cellular biochemical activities. This is not surprising considering that a rather large number of potential CK2 substrates have been identified by various investigators, and many of these substrates are members of complex signaling networks. [for details see e.g., 8, 11, 13, 17, 18]. Since CK2 is a ubiquitous enzyme present in both normal and cancer cells, it is possible that its activity towards a subset of substrates may depend on the state of oncogenesis, or that its activity towards certain substrates may have a more profound effect in supporting the biological activities of cancer cells. An elegant schematic account of potential links of CK2 to other pathways was given recently [8]. Here, we explore some of the signaling targets of CK2 pertaining to cancer.
Importantly, it appears that CK2 has a global role in transcription-related chromatin remodeling [27, 64, 65]. CK2 interactions and activity are intertwined with RNA polymerase I, II and III protein complex functions in general, and are specifically associated with various pre-mRNA transcription and splicing factors [66–73]. CK2 phosphorylation of numerous transcription and splicing factors likely changes their activity [see e.g., 11], and thus profoundly affects gene expression and proliferation. An effect of CK2 in androgen receptor-mediated transcriptional activity by androgen in prostate cells has been demonstrated [74], and it is conceivable that similar activity may take place in other cells in which steroid hormone receptors are involved. The role of CK2 in the regulation of Wnt signaling through phosphorylation of β-catenin in breast cancer has been well documented [48, 50]. Likewise, considerable evidence has been provided linking the function of CK2 in aberrant activation of NFκB through activation of various IKK in breast cancer models [75–79]. CK2 phosphorylation of the splicing factor RNPS1 increases both the splicing and translation of a reporter pre-mRNA [67]. Molecules such as PTEN and Akt play important roles in cancer [e.g., 80, 81]. PTEN is phosphorylated by CK2, which influences its stability (82–85). Interestingly, Akt is activated by phosphorylation at various sites by Akt kinases, and was demonstrated to undergo phosphorylation by CK2 at a specific site (Ser 129), which results in its hyperactivation [86]. CK2 plays an important role in the functions of the RNA-processing protein nucleophosmin (protein B23), most notably through an impact on its nuclear localization [87–90]. The involvement of p53 and MDM2 in cancer is also well known [91,92], and the role of CK2-mediated phosphorylation of these proteins has been investigated extensively [e.g., 47, 93–98].
CK2 also plays a global role in the regulation of apoptotic pathways, and several downstream targets in the apoptotic machinery are impacted by CK2 activity [17]. These include, e.g., Bid [99, 100], Bad [101], Max [102], Faf1 [103], Bcl2 and Bcl-xL [104, 105], caspase 2 [106], caspase-inhibiting protein ARC [107], and inhibitor of apoptosis proteins (IAPs), including survivin [104, 108]. These studies have shown that survivin activity is regulated by CK2 at the level of transcription. Investigation of cIAP1, cIAP2, XIAP, and survivin in prostate cancer cells demonstrated their differential distribution in the cytoplasmic and nuclear compartments and also their expression levels were responsive to altered CK2. Interestingly, decreased IAP levels in response to the apoptosis-inducing agents TNFα and TRAIL was potently blocked on forced overexpression of CK2 [104]. Of note are the observations that fibronectin protection of TNFα-induced apoptosis is via the AKT/survivin pathway [109].
How does CK2 function in cancer cells?
To examine the role of CK2 in cancer cells as compared with the normal, it may be appropriate to briefly mention some key distinguishing aspects of cancer cell biology. Cancer cells can commandeer diverse molecular pathways to maintain themselves, and several distinct features of cancer cells can be identified, as discussed in several thoughtful reviews [see e.g., 80, 81, 110]. A number of hallmarks of cancer cells have been described–deregulated growth (self-sufficiency of growth signals, and insensitivity to antigrowth signals), evading cell death (apoptosis), limitless replicative potential, sustained angiogenesis, tissue invasion and metastasis, and genome instability [80, 81]. These features are present to varying degrees in all cancers; however, it seems that the particularly pervasive characteristics of cancer cells are deregulated proliferation and deregulated apoptosis [110]. These considerations raise the question as to how CK2 functions link with these hallmark features of cancer? Here, we attempt to discuss these issues.
As stated earlier, CK2 has been found to be upregulated in all cancers examined [see e.g., 3, 11], and we propose that increased CK2 expression is a contributing and/or sustaining factor in the deregulated growth of cancer cells. While CK2 is also upregulated in normal cells during proliferation, it subsequently returns to basal levels characteristic of the particular cell type. On the other hand, cancer cells appear to acquire a ‘stable’ new elevated level of CK2 expression higher than the level in counterpart normal cells [36, 38, 39, 111]. Further, the upregulation of CK2 in cancer cells reflects not only proliferation but the pathological status of various cells in a given field [36]. Following an example of prostate cancer cells which demonstrate androgen sensitivity (such as LNCaP) and androgen independence (such as PC-3), it was apparent that CK2 was equally functional in these cases and did not depend on the nature of growth factor requirement by the cells [20]. Finally, elevated levels of CK2 could contribute to oncogenicity in a given cell type by combining with another oncogene, as has been well illustrated in several aforementioned studies along these lines [45–49].
The second important feature of cancer is deregulated apoptotic activity. It appears that in some cancers the decrease in apoptotic activity is an even more important factor in cancer progression than increases in proliferation [e.g., 41, 42, 110]. As discussed above, CK2 is a potent suppressor of apoptosis [17, 21, 22]. Upregulation of CK2 can impact both the intrinsic and extrinsic apoptotic pathways mediated by diverse signals [8, 17]. Therefore, increased CK2 expression represents a mechanism by which cancer cells suppress normal apoptotic activity and cellular response to apoptosis-inducing agents.
Sustained angiogenesis is another important acquired capability of cancer [80, 81]. Clearly, the ability of cancers to generate an appropriate blood supply network in the tumor is critical to its survival. While the function of CK2 in the process of angiogenesis has not been extensively investigated, there are indications that it plays a role in this process. For example, in experimental models of angiogenesis and retinal neovascularization, it was demonstrated that CK2 was involved in these processes. The data further suggested that inhibition of CK2 caused a suppression of angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites [112–114]. We have also observed that when CK2 was downregulated by systemic administration of antisense CK2α ODN to mice bearing a xenograft model of human prostate cancer, there was a marked reduction in microvasculature as indicated by the endothelial marker Cd31/Pecam-1 [35]. Of further note are the observations that CK2 may play a role in the regulation of HIF-1 activity, a hypoxia-responsive factor involved in tumor angiogenesis [115]. Together, these observations hint that CK2 may be an important player in the maintenance of angiogenesis in the tumor microenvironment.
With respect to the question of a role for CK2 in tissue invasion and metastasis, at present there are no systematic studies on this subject. Based on observations that CK2 may serve as a prognostic marker [37–40, 111], it may be surmised that cancer that exhibits a higher level of CK2 dysregulation is likely to be more aggressive and hence invasive. It is conceivable also that CK2 influences cancer cell invasion and metastasis by affecting the activity of molecules that are known to play a role in these processes. For example, green tea polyphenol epigallocatechin-3-gallate (EGCG) induced estrogen receptor alpha expression, reversing the invasive phenotype of breast cancer through activation of FOXO3a. Further, EGCG was found to inhibit the invasive phenotype of mouse mammary tumor cells driven by nuclear factor-kappaB, c-Rel and protein kinase CK2 [116]. Similarly, mice transgenic for MMTV-c-Rel and CK2α produce mammary tumors and cell lines which demonstrate a highly invasive phenotype [78]. It may be noted that EGCG and resveratrol caused a moderate inhibition of CK2 in prostate cancer cells, and this may also represent a mode of their activity as chemopreventive agents [117]. CK2-mediated phosphorylation of E-cadherin appears to influence its interaction with beta-catenin [118]. CK2 appears to also regulate adherens junction dynamics. For example, it was observed that disruption of adherens junctions is associated with a decrease in E-cadherin phosphorylation by CK2 [119]. Profiling of protein kinases in transformation of human ovarian epithelium in cell culture models has identified various kinases including CK2 to be associated with phenotypic manifestations of cancer progression [120]. Likewise, a few observations relating to the effect on matrix metalloproteases may be noted. It has been documented that UVB irradiation of human dermal fibroblasts causes CK2 mediated upregulation of MMP-1 and MMP-3 translation, whereas their major tissue inhibitor of matrix metalloproteinase-1 is not affected by CK2 [121]. Moreover, a recent study on the invasion-promoting MT1-MMP, which is directly linked to tumorigenesis and metastasis transactivation, show that its expression is strongly correlated with that of several genes including CK2α [122]. In studies of breast cancer, observations on HDAC2 phosphorylation by CK2 may be important for tumor progression [123]. To sum, while the data are limited, it would appear that CK2 may also significantly influence the processes of invasion and metastasis.
CK2: A potentially important target for cancer therapy
An eventual goal of the investigations on CK2 is to translate basic observations to utility in patients. Induction of apoptosis is an attractive approach that has gained much attention as a cancer therapeutic. We illustrate the potentiality of this approach by using prostate cancer therapy as an example. Prostate cancer is initially androgen sensitive and responds to androgen ablation. However, after the initial remission, the disease relapses in a generally fatal form because of the emergence of an androgen-refractory phenotype whose growth depends on a variety of autocrine/paracrine factors [e.g., 124]. Although docetaxel-based chemotherapy can improve survival in this situation, most patients die from chemoresistant disease. A number of molecular therapeutic approaches that have employed various gene targets have been investigated, but challenges such as design of delivery vectors and the effectiveness of the target gene remain [see, e.g., 125–127]. The IAPs and survivin have been proposed as targets for cancer therapy [128, 129]. However, as discussed above, their activity is strongly influenced by CK2, and thus it is likely that strategies to affect these molecules may be hampered by CK2 which is upregulated in cancer cells. A number of attempts to target protein kinases also have been undertaken [e.g., 125, 130]. However, we are the first to have proposed that downregulation of CK2 may be a strategic approach to inducing apoptosis in cancer cells, and thus merits consideration as a target in prostate cancer cells that may lead to novel cancer therapies [22]. Indeed, as discussed below, a compelling case can be made in support of our hypothesis.
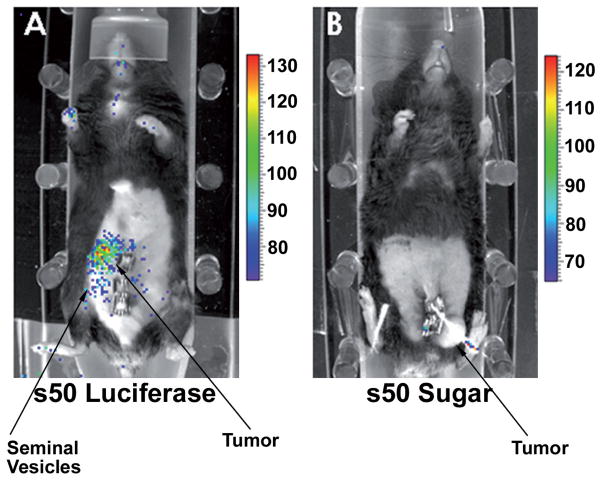
We believe that to eradicate all tumor cells, it is important to consider targeting gene(s) that are uniquely indispensible for cell survival, as otherwise, tumor cells will escape death by recruiting an alternate pathway. This might be the situation, for example, with investigations directed toward a target such as Bcl-2 [127]. Molecular targeting of protein kinases to downregulate their activity has excellent potential as a therapeutic approach, and indeed has been attempted in a few cases [125, 127, 129]. Of note, the kinase targets in these reports were not critical for cell survival, which might limit their utility for clinical translation. This is not the case for CK2 because it is essential for cell survival. We initiated this work by employing antisense CK2α ODN to downregulate CK2 in cell culture and xenograft models (22, 34). However, different types of therapeutic agents that cause molecular or chemical downregulation of CK2 may be considered for such approaches. The importance of CK2 as a potential target for cancer therapy is supported by several considerations. First, compared with several other protein kinases, CK2 appears to be profoundly responsive to modulations of mitogenic signals in prostate cells [2, 11, 12, 18, 28]. Second, dysregulated elevation of CK2 in cancer cells reflects the pathologic status of the tumor [2, 11, 36–40]. Third, CK2 downregulation impacts not only cell growth and proliferation but also apoptotic activity in cancer cells, making its targeting a two-edged sword [4, 11, 12, 17]. Fourth, CK2 is indispensable for cell survival, and there appear to be no redundant pathways to compensate for its downregulation [43, 44]. In the case of prostate cancer, downregulation of CK2 would target both the androgen-sensitive and -refractory cells, producing an equally effective response regardless of the prostate cancer cell phenotype in vivo. Support for these considerations is derived from other recent studies. For example, targeting CK2 phosphorylation sites has been employed as means of inducing cell death which is presumably mediated by blocking of phosphorylation of CK2 substrates in cancer cells [131]. Likewise, chemical inhibition of CK2 appears to be a potential approach to achieve a therapeutic effect in experimental models [132]. Induction of apoptosis in cancer cells upon downregulation of CK2 may also rely on production of intracellular H2O2 which is known to have several downstream targets in the apoptotic machinery [133]. While the above views provide a strong rationale for considering CK2 as a target for cancer therapy, it is important to note that CK2 is a ubiquitous enzyme and hence a key consideration would be to employ strategies that would spare normal cells while targeting CK2 in cancer cells. Clearly, the ubiquitous and indispensible nature of CK2 would raise concerns about host toxicity if it were downregulated in normal cells. Specific molecular downregulation of the targeted signal in tumors should be a key goal, and to that end, we have developed a novel nanocapsule approach to deliver the CK2 targeting agent specifically to primary and metastatic tumors in vivo. The use of s50 (sub-50 nm) nanocapsules composed of tenfibgen (a subdomain of the endogenous human protein tenascin) to deliver the various therapeutic agents to tumors while sparing normal cells should be a key to utilizing CK2 as the target for therapy [35, 55]. Further, such a strategy has a strong potential of targeting tumor metastases. Figure 1 illustrates that the tenfibgen nanocapsule carrying a luciferase cargo specifically targets the prostate cancer in vivo while sparing normal cells. Thus, delivery of a therapeutic agent that would downregulate CK2 through this approach should have a strong potential of eradicating the tumor while sparing the normal tissues in vivo.
Figure 1.
Strategy for tumor cell-specific delivery of CK2-targeting agents through s50 tenfibgen nanocapsules. Orthotopic implantation of TrampC2 tumor cells (2 × 106) was carried out and the tumor was allowed to develop for 4 weeks. To image the tumor position by luciferase Xenogen method, mouse A was injected intraperitoneally with 12 μg of s50 nm tenfibgen nanocapsules bearing luciferase plasmid, and mouse B with s50 nm tenfibgen capsules bearing sugar (trehalose). After 7 days, the mice were injected intraperitoneally with 120 mg/kg of luciferin, and imaged after 15 min in a Xenogen system. Mouse B (treated with s50 nm tenfibgen nanocapsules bearing sugar) has a tumor, but no signal is apparent (see arrow). However, mouse A (treated with s50 nm tenfibgen nanocapsules bearing the luciferase plasmid) shows signal in the tumor and tumor cell-invaded seminal vesicles which were proximal to the tumor (the primary tumor was located behind the tubules).
Concluding remarks
The above discussion highlights the significance of CK2 in cancer biology. While much work is needed to elaborate on CK2 functions in cancer, a considerable amount of information supports the notion that it is a key player in the pathogenesis of cancer. Further, CK2 is a remarkably nodal molecule with its consistent upregulation in cancer, impact on a large number of cellular processes, and its essential for survival nature. Thus, CK2 merits serious consideration as a highly significant therapeutic target [17, 18, 35, 55]. Finally, we wish to emphasize that this report is not a comprehensive review of CK2 in relation to cancer but rather a brief overview of aspects of this subject, and we acknowledge that some of the views expressed here are likely to be speculative as considerable additional studies are needed to continue to define molecular links of CK2 involvement in cancer.
Acknowledgments
This work is supported in part by a U.S.P.H.S. Research Grant CA-15062 awarded by the National Cancer Institute, Department of Health and Human Services, and in part by the Medical Research Funds of the Department of Veterans Affairs.
References
- 1.Allende JE, Allende CC. Protein kinase CK2 –an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed K. Nuclear matrix and protein kinase CK2 signaling. Crit Rev Eukaryot Gene Expr. 1999;9:329–336. doi: 10.1615/critreveukargeneexpr.v9.i3-4.170. [DOI] [PubMed] [Google Scholar]
- 3.Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20:391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: An emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 5.Pinna LA. Protein kinase CK2: A challenge to canons. J Cell Sci. 2002;115:3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 6.Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyerin W, Ackermann K. The genes encoding human protein kinase CK2 and their functional links. Progr Nucl Acid Res Mol Biol. 2003;74:239–273. doi: 10.1016/s0079-6603(03)01015-8. [DOI] [PubMed] [Google Scholar]
- 8.Guerra B, Issinger OG. Protein kinase CK2 in human disease. Curr Med Chem. 2008;15:1870–1886. doi: 10.2174/092986708785132933. [DOI] [PubMed] [Google Scholar]
- 9.Graham KC, Litchfield DW. The regulatory β subunit of protein kinase CK2 mediates formation of tetrameric CK2 complexes. J Biol Chem. 2000;275:5003–5010. doi: 10.1074/jbc.275.7.5003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Davis AT, Ahmed K. Mechanism of protein kinase CK2 association with nuclear matrix: Role of disulfide bond formation. J Cell Biochem. 1998;69:211–220. [PubMed] [Google Scholar]
- 11.Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16:573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed K, Davis AT, Wang H, Faust RA, Yu S, Tawfic S. Significance of protein kinase CK2 nuclear signaling in neoplasia. J Cell Biochem Suppl. 2000;35:130–135. doi: 10.1002/1097-4644(2000)79:35+<130::aid-jcb1136>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 14.Tawfic S, Faust RA, Gapany M, Ahmed K. Nuclear matrix as an anchor for protein kinase CK2 nuclear signalling. J Cell Biochem. 1996;62:165–171. doi: 10.1002/(sici)1097-4644(199608)62:2<165::aid-jcb4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Faust M, Montenarh M. Subcellular localization of protein kinase CK2 – A key to its function? Cell Tissue Res. 2000;301:329–340. doi: 10.1007/s004410000256. [DOI] [PubMed] [Google Scholar]
- 16.Filhol O, Martiel JL, Cochet C. Protein kinase CK2: a new view of an old molecular complex. EMBO Rep. 2004;5:351–355. doi: 10.1038/sj.embor.7400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 – A key suppressor of apoptosis. Adv Enzyme Regul. 2008;48:179–187. doi: 10.1016/j.advenzreg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Ahmad KA, Unger G, Slaton JW, Ahmed K. CK2 signaling in androgen-dependent and -independent prostate cancer. J Cell Biochem. 2006;99:382–391. doi: 10.1002/jcb.20847. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed K, Ishida H. Effect of testosterone on nuclear phosphoproteins of rat ventral prostate. Mol Pharmacol. 1971;7:323–327. [PubMed] [Google Scholar]
- 20.Guo C, Yu S, Davis AT, Ahmed K. Nuclear matrix targeting of the protein kinase CK2 signal as common downstream response to androgen or growth factor stimulation of prostate cancer cells. Cancer Res. 1999;59:1146–1151. [PubMed] [Google Scholar]
- 21.Guo C, Yu S, Davis AT, Green JE, Ahmed K. A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem. 2001;276:5992–5999. doi: 10.1074/jbc.M004862200. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Davis A, Yu S, Ahmed K. Response of cancer cells to molecular interruption of the CK2 signal. Mol Cell Biochem. 2001;227:167–174. [PubMed] [Google Scholar]
- 23.Ahmed K, Yenice S, Davis A, Goueli SA. Association of casein kinase 2 (CK-2) with nuclear chromatin in relation to androgenic regulation of rat prostate. Proc Natl Acad Sci USA. 1993;90:4426–4430. doi: 10.1073/pnas.90.10.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawfic S, Ahmed K. Association of casein kinase 2 (CK-2) with nuclear matrix: Possible role in nuclear matrix protein phosphorylation. J Biol Chem. 1994;269:7489–7493. [PubMed] [Google Scholar]
- 25.Tawfic S, Ahmed K. Growth stimulus-mediated differential translocation of casein kinase 2 to the nuclear matrix: Evidence based on androgen action in the prostate. J Biol Chem. 1994;269:24615–24620. [PubMed] [Google Scholar]
- 26.Yu S, Davis AT, Guo C, Green JE, Ahmed K. Differential targeting of protein kinase CK2 to the nuclear matrix upon transient overexpression of its subunits. J Cell Biochem. 1999;74:127–134. [PubMed] [Google Scholar]
- 27.Guo C, Davis AT, Ahmed K. Dynamics of protein kinase CK2 association with nucleosomes in relation to transcriptional activity. J Biol Chem. 1998;273:13675–13680. doi: 10.1074/jbc.273.22.13675. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Wang H, Davis A, Ahmed K. Consequences of CK2 signaling to the nuclear matrix. Mol Cell Biochem. 2001;227:67–71. [PubMed] [Google Scholar]
- 29.Guerra B, Siemer S, Boldyreff B, Issinger OG. Protein kinase CK2: Evidence for a protein kinase CK2β subunit fraction, devoid of the catalytic CK2α subunit, in mouse brain and testicles. FEBS Lett. 1999;462:353–357. doi: 10.1016/s0014-5793(99)01553-7. [DOI] [PubMed] [Google Scholar]
- 30.Getzenberg RM, Pienta KJ, Ward WS, Coffey DS. Nuclear structure and the three dimensional organization of DNA. J Cell Biochem. 1991;47:289–299. doi: 10.1002/jcb.240470402. [DOI] [PubMed] [Google Scholar]
- 31.Berezney R. The nuclear matrix: A heuristic model for investigating genomic organization and function in the cell nucleus. J Cell Biochem. 1991;47:109–123. doi: 10.1002/jcb.240470204. [DOI] [PubMed] [Google Scholar]
- 32.Stein GS, Montecino M, van Wijnen AJ, Stein JL, Lian JB. Nuclear structure-gene expression interrelationships: Implications for aberrant gene expression in cancer. Cancer Res. 2000;60:2067–2076. [PubMed] [Google Scholar]
- 33.Barrett TJ, Spelsberg TC. Nuclear matrix and steroid hormone action. Vitam Horm. 1999;55:127–163. doi: 10.1016/s0083-6729(08)60935-8. [DOI] [PubMed] [Google Scholar]
- 34.Slaton JW, Sloper DT, Unger G, Davis A, Ahmed K. Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol Cancer Res. 2004;2:712–721. [PubMed] [Google Scholar]
- 35.Ahmad KA, Wang G, Slaton JW, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anti-cancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Faust RA, Niehans GA, Gapany M, Hoistad D, Knapp D, Cherwitz D, Davis A, Adams GL, Ahmed K. Subcellular immunolocalization of protein kinase CK2 in normal and carcinoma cells. Int J Biochem Cell Biol. 1999;31:941–949. doi: 10.1016/s1357-2725(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 37.Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: Association with malignant transformation. Cancer Lett. 1996;101:31–35. doi: 10.1016/0304-3835(96)04110-9. [DOI] [PubMed] [Google Scholar]
- 38.Gapany M, Faust RA, Tawfic S, Davis A, Adams GL, Ahmed K. Association of elevated CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med. 1995;1:659–666. [PMC free article] [PubMed] [Google Scholar]
- 39.Laramas M, Pasquier D, Filhol O, Ringeisen F, Desxotes JL, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2α) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer. 2007;43:928–934. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 40.O-Charoenrat P, Rusch V, Talbot SG, Sarkaria I, Viale A, Socci N, Ngai I, Rap P, Singh B. Casein kinase II alpha subunit and C1-inhibitor are independent predictors of outcome in patients with squamous cell carcinoma of the lung. Clin Cancer Res. 2004;10:5792–5803. doi: 10.1158/1078-0432.CCR-03-0317. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyprianou N, Bruckheimer EM, Guo Y. Cell proliferation and apoptosis in prostate cancer: significance in disease progression and therapy. Histol Histopathol. 2000;15:1211–1223. doi: 10.14670/HH-15.1211. [DOI] [PubMed] [Google Scholar]
- 43.Padmanabha R, Chen-Wu JLP, Hanna DE, Glover CVC. Isolation, sequencing, and disruption of the yeast CKA2 gene: Casein kinase II is essential for viability in S. cerevisiae. Mol Cell Biol. 1990;10:4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchou T, Nernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B. Disruption of the regulatory β subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seldin DC, Lou DY, Toselli P, Landesman-Bollag E, Dominguez I. Gene targeting of CK2 catalytic subunits. Mol Cell Biochem. 2008;316:141–147. doi: 10.1007/s11010-008-9811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIα. EMBO J. 1996;15:5160–5166. [PMC free article] [PubMed] [Google Scholar]
- 47.Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2 α collaborate in the development of thymic lymphomas in mice. Oncogene. 1998;16:2965–2974. doi: 10.1038/sj.onc.1201854. [DOI] [PubMed] [Google Scholar]
- 48.Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, Seldin DC. Protein kinase CK2: Signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001;227:153–165. [PubMed] [Google Scholar]
- 49.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21:5280–5288. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 50.Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signaling and tumorigenesis. Mol Cell Biochem. 2005;274:63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- 51.Zörnig M, Hueber AO, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochim Biophys Acta. 2001;1551:F1–F37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 52.Davis AT, Wang H, Zhang P, Ahmed K. Heat shock mediated modulation of protein kinase CK2 in the nuclear matrix. J Cell Biochem. 2002;85:583–591. doi: 10.1002/jcb.10158. [DOI] [PubMed] [Google Scholar]
- 53.Yamane K, Kinsella TJ. CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. Cancer Res. 2005;65:4362–4367. doi: 10.1158/0008-5472.CAN-04-3941. [DOI] [PubMed] [Google Scholar]
- 54.Wang G, Ahmad KA, Ahmed K. Modulation of death receptor-mediated apoptosis by CK2. Mol Cell Biochem. 2005;274:201–205. doi: 10.1007/s11010-005-2952-0. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Unger GM, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells: A potential approach to cancer therapy. Mol Cell Biochem. 2005;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]
- 56.Izeradjene K, Douglas L, Delaney A, Houghton JA. Casein kinase II (CK2) enhances death-inducing signaling complex (DISC) activity in TRAIL-induced apoptosis in human colon carcinoma cell lines. Oncogene. 2005;24:2050–2058. doi: 10.1038/sj.onc.1208397. [DOI] [PubMed] [Google Scholar]
- 57.Izeradjene K, Douglas L, Delaney A, Houghton JA. Influence of casein kinase II in tumor necrosis factor-mediated apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res. 2004;10:6650–6660. doi: 10.1158/1078-0432.CCR-04-0576. [DOI] [PubMed] [Google Scholar]
- 58.Ravi R, Bedi A. Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res. 2002;62:4180–4185. [PubMed] [Google Scholar]
- 59.Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW. The protein kinase CK2 facilitates repair of chromosomal DNA single strand breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 60.Scaglioni PP, Yung TM, Cai LF, Erdjument-Bromage H, Kaufman AJ, Singh B, Teruya-Feldstein J, Tempst P, Pandolfi PP. A CK2-dependent mechanism of degradation of the PML tumor suppressor. Cell. 2006;126:269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 61.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 62.Homma MK, Li D, Krebs EG, Yuasa Y, Homma Y. Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein. Proc Natl Acad Sci USA. 2002;99:5959–5964. doi: 10.1073/pnas.092143199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cobb L, Koyama S, Cohen P. Site-specific phosphorylation by intracellular kinases determines the apoptotic activity of IGFBP-3 in prostate cancer. AACR Annual Meeting Proceedings; Los Angeles, CA. 2007. p. Abstract #4393. [Google Scholar]
- 64.Guo C, Davis AT, Yu S, Tawfic S, Ahmed K. Role of protein kinase CK2 in phosphorylation of nucleosomal proteins in relation to transcriptional activity. Mol Cell Biochem. 1999;191:135–142. [PubMed] [Google Scholar]
- 65.Barz T, Ackermann K, Dubois G, Eils R, Pyerin W. Genome-wide expression screens indicate a global role for protein kinase CK2 in chromatin remodeling. J Cell Sci. 2003;116:1563–1577. doi: 10.1242/jcs.00352. [DOI] [PubMed] [Google Scholar]
- 66.Cardenas ME, Gasser SM. Regulation of topoisomerase II by phosphorylation: a role for casein kinase II. J Cell Sci. 1993;104:219–225. doi: 10.1242/jcs.104.2.219. [DOI] [PubMed] [Google Scholar]
- 67.Trembley JH, Tatsumi S, Sakashita E, Loyer P, Slaughter CA, Suzuki H, Endo H, Kidd VJ, Mayeda A. Activation of pre-mRNA splicing by human RNPS1 is regulated by CK2 phosphorylation. Mol Cell Biol. 2005;25:1446–1457. doi: 10.1128/MCB.25.4.1446-1457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghavidel A, Schultz MC. TATA binding protein-associated CK2 transduces DNA damage signals to the RNA polymerase III transcriptional machinery. Cell. 2001;106:575–584. doi: 10.1016/s0092-8674(01)00473-1. [DOI] [PubMed] [Google Scholar]
- 69.Cabrejos ME, Allende CC, Maldonado E. Effects of phosphorylation by protein kinase CK2 on the human basal components of the RNA polymerase II transcription machinery. J Cell Biochem. 2004;93:2–10. doi: 10.1002/jcb.20209. [DOI] [PubMed] [Google Scholar]
- 70.Lewis BA, Sims RJ, 3rd, Lane WS, Reinberg D. Functional characterization of core promoter elements: DPE-specific transcription requires the protein kinase CK2 and the PC4 promoter. Mol Cell. 2005;18:471–481. doi: 10.1016/j.molcel.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Bierhoff H, Dundr M, Michels AA, Grummt I. Phosphorylation by casein kinase 2 facilitates rRNA gene transcription by promoting dissociation of TIF-IA from elongating RNA polymerase I. Mol Cell Biol. 2008;28:4988–4998. doi: 10.1128/MCB.00492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panova TB, Panov KI, Russell J, Zomerdijk JC. Casein kinase 2 associates with initiation-competent RNA polymerase I and has multiple roles in ribosomal DNA transcription. Mol Cell Biol. 2006;26:5957–5968. doi: 10.1128/MCB.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnston IM, Allison SJ, Morton JP, Schramm L, Scott PH, White RJ. CK2 forms a stable complex with TFIIIB and activates RNA polymerase III transcription in human cells. Mol Cell Biol. 2002;22:3757–3768. doi: 10.1128/MCB.22.11.3757-3768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Götz C, Bachmann C, Montenarh M. Inhibition of protein kinase CK2 leads to a modulation of androgen-receptor dependent transcription in prostate cancer cells. Prostate. 2007;67:125–134. doi: 10.1002/pros.20471. [DOI] [PubMed] [Google Scholar]
- 75.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Traish AM, Mercurio F, Sonenshein GE. Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor kappaB in breast cancer. Cancer Res. 2001;61:3810–3818. [PubMed] [Google Scholar]
- 76.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor kappaB, transformed phenotypes, and survival of breast cancer cells. Cancer Res. 2002;62:6770–6778. [PubMed] [Google Scholar]
- 77.Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Seldin DC, Sonenshein GE. Inducible IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65:11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
- 78.Belguise K, Guo S, Yang S, Rogers AE, Seldin DC, Sherr DH, Sonenshein GE. Green tea poly-phenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007;67:11742–11750. doi: 10.1158/0008-5472.CAN-07-2730. [DOI] [PubMed] [Google Scholar]
- 79.Pando MP, Verma IM. Signal dependent and independent degradation of free and NF-κB bound IκB-α. J Biol Chem. 2000;14:21278–21286. doi: 10.1074/jbc.M002532200. [DOI] [PubMed] [Google Scholar]
- 80.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 81.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 82.Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- 83.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 84.Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:1–3. doi: 10.1016/s0014-5793(02)03274-x. [DOI] [PubMed] [Google Scholar]
- 85.Torres J, Rodriguez J, Myers MP, Valiente M, Graves JD, Tonks NK, Pulido R. Phosphorylation-regulated cleavage of the tumor suppressor PTEN by caspase-3: implications for the control of protein stability and PTEN-protein interactions. J Biol Chem. 2003;278:30652–30660. doi: 10.1074/jbc.M212610200. [DOI] [PubMed] [Google Scholar]
- 86.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 87.Tawfic S, Olson MOJ, Ahmed K. Role of protein phosphorylation in post-translational regulation of protein B23 during programmed cell death in the prostate gland. J Biol Chem. 1995;270:21009–21015. doi: 10.1074/jbc.270.36.21009. [DOI] [PubMed] [Google Scholar]
- 88.Szebeni A, Hingorani K, Negi S, Olson MOJ. Role of protein kinase CK2 phosphorylation in the molecular chaperone activity of nucleolar protein B23. J Biol Chem. 2003;278:9107–9115. doi: 10.1074/jbc.M204411200. [DOI] [PubMed] [Google Scholar]
- 89.Lawson K, Larentowicz L, Laury-Kleintop L, Gilmour SK. B23 is a downstream target of polyamine-modulated CK2. Mol Cell Biochem. 2005;274:103–114. doi: 10.1007/s11010-005-3066-4. [DOI] [PubMed] [Google Scholar]
- 90.Louvet E, Junéra HR, Berthuy I, Hernandez-Verdun D. Compartmentation of the nucleolar processing proteins in the granular component is a CK2-driven process. Mol Biol Cell. 2006;17:2537–2546. doi: 10.1091/mbc.E05-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheikh MS, Fornace AJ., Jr Role of p53 family members in apoptosis. J Cell Physiol. 2000;182:171–181. doi: 10.1002/(SICI)1097-4652(200002)182:2<171::AID-JCP5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 92.Yap DBS, Hsieh JK, Chan FSG, Lu X. mdm2: a bridge over the two tumour suppressors, p53 and Rb. Oncogene. 1999;18:7681–7689. doi: 10.1038/sj.onc.1202954. [DOI] [PubMed] [Google Scholar]
- 93.Allende-Vega N, McKenzie L, Meek D. Transcription factor TAFII250 phosphorylates the acidic domain of Mdm2 through recruitment of protein kinase CK2. Mol Cell Biochem. 2008;316:99–106. doi: 10.1007/s11010-008-9816-3. [DOI] [PubMed] [Google Scholar]
- 94.Hjerrild M, Milne D, Dumaz N, Hay T, Issinger OG, Meek D. Phosphorylation of murine double minute clone 2 (MDM2) protein at serine-267 by protein kinase CK2 in vitro and in cultured cells. Biochem J. 2001;355:347–356. doi: 10.1042/0264-6021:3550347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McKendrick L, Milne D, Meek D. Protein kinase CK2-dependent regulation of p53 function: evidence that the phosphorylation status of the serine 386 (CK2) site of p53 is constitutive and stable. Mol Cell Biochem. 1999;191:187–199. [PubMed] [Google Scholar]
- 96.Götz C, Kartarius S, Schwar G, Montenarh M. Phosphorylation of mdm2 at serine 269 impairs its interaction with the retinoblastoma protein. Int J Oncol. 2005;26:801–808. [PubMed] [Google Scholar]
- 97.Prowald A, Schuster N, Montenarh M. Regulation of the DNA binding of p53 by its interaction with protein kinase CK2. FEBS Lett. 1997;408:99–104. doi: 10.1016/s0014-5793(97)00399-2. [DOI] [PubMed] [Google Scholar]
- 98.Schuster N, Götz C, Faust M, Schneider E, Prowald A, Jungbluth A, Montenarh M. Wild-type p53 inhibits protein kinase CK2 activity. J Cell Biochem. 2001;81:172–183. doi: 10.1002/1097-4644(20010401)81:1<172::aid-jcb1033>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 99.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 100.Olsen BB, Petersen J, Issinger OG. BID, an interaction partner of protein kinase CK2α. Biol Chem. 2006;387:441–449. doi: 10.1515/BC.2006.059. [DOI] [PubMed] [Google Scholar]
- 101.Klumpp S, Maurer A, Zhu Y, Aichele D, Pinna LA, Krieglstein J. Protein kinase CK2 phosphorylates BAD at threonine-117. Neurochem Intl. 2004;45:747–752. doi: 10.1016/j.neuint.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Krippner-Heidenreich A, Talanian RV, Sekul R, Kraft R, Thole H, Ottleben H, Luscher B. Targeting of the transcription factor Max during apoptosis: phosphorylation-regulated cleavage by caspase-5 at an unusual glutamic acid residue in position P1. Biochem J. 2001;358:705–715. doi: 10.1042/0264-6021:3580705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olsen BB, Jessen V, Højrup P, Issinger OG, Boldyreff B. Protein kinase CK2 phosphorylates the Fas-associated factor FAF1 in vivo and influences its transport into the nucleus. FEBS Lett. 2003;546:218–222. doi: 10.1016/s0014-5793(03)00575-1. [DOI] [PubMed] [Google Scholar]
- 104.Wang G, Ahmad KA, Ahmed K. Impact of protein kinase CK2 on inhibitor of apoptosis proteins (IAPs) in prostate cancer cells. Mol Cell Biochem. 2008;316:91–97. doi: 10.1007/s11010-008-9810-9. [DOI] [PubMed] [Google Scholar]
- 105.Wang G, Ahmad KA, Ahmed K. Role of CK2 in regulation of TRAIL induced apoptosis in prostate cancer cells. Cancer Res. 2006;66:2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- 106.Shin S, Lee Y, Kim W, Ko H, Choi H, Kim K. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J. 2005;24:3532–3542. doi: 10.1038/sj.emboj.7600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li PF, Li J, Muller EC, Otto A, Dietz R, von Harsdorf R. Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Mol Cell. 2002;10:247–258. doi: 10.1016/s1097-2765(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 108.Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci USA. 2006;103:15079–15084. doi: 10.1073/pnas.0606845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fornaro M, Plescia J, Chheang S, Tallini G, Zhou YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor-α-induced apoptosis via the Akt/survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 110.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 111.Yenice S, Davis AT, Goueli SA, Akdas A, Limas E, Ahmed K. Nuclear Casein kinase 2 (CK-2) activity in human normal, benign hyperplastic, and cancerous prostate. Prostate. 1994;24:11–16. doi: 10.1002/pros.2990240105. [DOI] [PubMed] [Google Scholar]
- 112.Ljubimov AV, Caballero S, Aoki A, Grant MB, Castellon R. Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:4583–4591. doi: 10.1167/iovs.04-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kramerov AA, Saghizadeh M, Pan H, Kabosova A, Montenarh M, Ahmed K, Penn JS, Chan CK, Hinton DR, Grant MB, et al. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Amer J Path. 2006;168:1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kramerov AA, Saghizadeh M, Caballero S, Shaw LC, Li Calzi S, Bretner M, Montenarh M, Pinna LA, Grant MB, Ljubimov AV. Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites. Mol Cell Biochem. 2008;316:177–186. doi: 10.1007/s11010-008-9831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mottet D, Ruys SP, Demazy C, Raes M, Michiels C. Role of casein kinase 2 in the regulation of HIF-1 activity. Int J Cancer. 2005;117:764–774. doi: 10.1002/ijc.21268. [DOI] [PubMed] [Google Scholar]
- 116.Belguise K, Guo S, Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res. 2007;67:5763–5670. doi: 10.1158/0008-5472.CAN-06-4327. [DOI] [PubMed] [Google Scholar]
- 117.Ahmad KA, Harris NH, Johnson AD, Lindvall HCN, Wang G, Ahmed K. Protein kinase CK2 modulates apoptosis induced by resveratrol and EGCG in prostate cancer cells. Mol Cancer Therap. 2007;6:1006–1012. doi: 10.1158/1535-7163.MCT-06-0491. [DOI] [PubMed] [Google Scholar]
- 118.Catimel B, Layton M, Church N, Ross J, Condron M, Faux M, Simpson RJ, Burgess AW, Nice EC. In situ phosphorylation of immobilized receptors on biosensor surfaces: application to E-cadherin/beta-catenin interactions. Anal Biochem. 2006;350:277–288. doi: 10.1016/j.ab.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 119.Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, Vincent C, Schmitt D. The disruption of adherens junctions is associated with decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257:255–264. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- 120.Wong AS, Kim SO, Leung PC, Auersperg N, Pelech SL. Profiling of protein kinase in the neoplastic transformation of human ovarian surface epithelium. Gynecol Oncol. 2001;82:305–311. doi: 10.1006/gyno.2001.6280. [DOI] [PubMed] [Google Scholar]
- 121.Brenneisen P, Wlaschek M, Schwamborn E, Schneider LA, Ma W, Sies H, Scharffetter-Kochanek K. Activation of protein kinase CK2 is an early step in the ultraviolet B-mediated increase in interstitial collagenase (matrix metalloproteinase-1, MMP-1) and stromelysin-1 (MMP-3) protein levels in human dermal fibroblasts. Biochem J. 2002;365:31–40. doi: 10.1042/BJ20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rozanov DV, Savinov AY, Williams R, Liu K, Golubkov VS, Krajewski S, Strongin AY. Molecular signature of MT1-MMP: transactivation of the downstream universal gene network in cancer. J Cancer Res. 2008;68:4086–4096. doi: 10.1158/0008-5472.CAN-07-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun JM, Chen HY, Moniwa M, Litchfield DW, Seto E, Davie JR. The transcriptional repressor Sp3 is associated with CK2-phorphorylated histone deacetylase 2. J Biol Chem. 2002;277:35783–35786. doi: 10.1074/jbc.C200378200. [DOI] [PubMed] [Google Scholar]
- 124.Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate. 2000;42:150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 125.Cho-Chung YS. Antisense and therapeutic oligonucleotides: Toward a gene-targeting cancer clinic. Exp Opin Ther Patents. 2000;10:1711–1724. [Google Scholar]
- 126.Olie RA, Zangemeister-Wittke U. Targeting tumor cell resistance to apoptosis induction with antisense oligonucleotides: Progress and the therapeutic potential. Drug Resist Updates. 2001;4:9–15. doi: 10.1054/drup.2001.0181. [DOI] [PubMed] [Google Scholar]
- 127.Gleave ME, Monia B. Antisense therapy for cancer. Nat Rev Cancer. 2005;5:468–479. doi: 10.1038/nrc1631. [DOI] [PubMed] [Google Scholar]
- 128.Reed JC. Apoptosis-based therapies. Nat Rev Drug Disc. 2002;2:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 129.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nature Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 130.Cho YS, Kim MK, Tan L, Srivastava R, Agrawal S, Cho-Chung YS. Protein kinase A RIα antisense inhibition of PC3M prostate cancer cell growth: Bcl-2 hyper-phosphorylation, Bax up-regulation, and Bad-hyperphosphorylation. Clin Cancer Res. 2002;8:607–614. [PubMed] [Google Scholar]
- 131.Perea SE, Reyes O, Baladron I, Perera Y, Farina H, Gil J, Rodriguez A, Bacardi D, Marcelo JL, Cosme K, Cruz M, Valenzuela C, López-Saura PA, et al. CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Mol Cell Biochem. 2008;316:163–167. doi: 10.1007/s11010-008-9814-5. [DOI] [PubMed] [Google Scholar]
- 132.Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J, Kazimierczuk Z, Pinna LA, Heisterkamp N. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007;21:178–180. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- 133.Ahmad KA, Wang G, Ahmed K. Intracellular hydrogen peroxide production is an upstream event in apoptosis induced by downregulation of CK2 in prostate cancer cells. Mol Cancer Res. 2006;4:331–338. doi: 10.1158/1541-7786.MCR-06-0073. [DOI] [PubMed] [Google Scholar]