Abstract
Cilia are highly conserved for their structure and also for their sensory functions. They serve as antennae for extracellular information. Whether the cilia are motile or not, they respond to environmental mechanical and chemical stimuli and signal to the cell body. The information from extracellular stimuli is commonly converted to electrical signals through the repertoire of ion-conducting channels in the ciliary membrane resulting in changes in concentrations of ions, especially Ca2+, in the cilia. These changes, in turn, affect motility and signaling pathways in the cilia and cell body to carry on the signal transduction. We review here the activities of ion channels in cilia from protists to vertebrates.
Keywords: animal physiology, invertebrate biology, vertebrate biology, cell biology, animal behavior
Introduction
Cilia are membrane-covered organelles that protrude from the surface of eukaryotic cells. The cytoskeleton of the cilium consists of nine doublet microtubules; motile cilia contain, in addition, two singlet microtubules in the center of the ring of doublets. In motile cilia, dynein arms induce sliding of adjacent doublet microtubules. We are perhaps most familiar with the function of motile cilia in moving sperm, cerebrospinal fluid, or respiratory mucus in mammals. However, there is a growing appreciation that cilia play critical signaling roles in eukaryotic cells (Bloodgood 2010, 2012). This revival of interest in cilia stems, in part, from the identification of ciliary gene defects in ciliopathies, which are human developmental syndromes that trace back to the failure of primary or motile cilia to either assemble or to perform signaling functions. (For more on ciliopathies, see the article “Cilia and Diseases”, by Brown and Witman, at the end of this special section). Almost all cells of the human body possess a single immotile primary cilium whose signaling functions are critical for normal development (Schneider et al. 2005, Fliegauf et al. 2006, Clement et al. 2009, McDermott et al. 2010, Amador-Arjona et al. 2011). If the cilia fail to form, or fail to possess the functional proteins necessary for signaling, critical cell functions will be missing. Since cilia are so highly conserved in their structure and function, it is possible to use model organisms including Chlamydomonas, ciliate protozoa, Caenorhabditis elegans, zebrafish, and mice for study.
The cilium is regarded as a cellular antenna that detects chemical, mechanical, osmotic, light, and gravitational stimuli (Pazour and Witman 2003, Singla and Reiter 2006, Berbari et al. 2009, Satir et al. 2010). The cilium has its own plasma membrane domain that receives and transduces chemical and electrical signals. The composition and functions of the ciliary membrane are distinct from those of the general plasma membrane on the cell. The cilium is also different in its extreme geometry, which may enhance its ability to detect weak or unusual stimuli, such as fluid flow through kidney tubules.
Proteomic studies (Mayer et al. 2009, Yano et al. 2013) have verified that the cilium is complex both in terms of structural proteins and signaling proteins. The latter can be grouped into three broad categories: receptor proteins; signal transduction components (e.g. G-proteins, cyclases, and kinases); and ion-conducting membrane channels that control the flow of current across the ciliary membrane. There is a wide variety of ciliary ion channels, including voltage-gated channels, some of which conduct calcium ions (Ca2+); channels that conduct potassium ions (K+); TRP channels, which non-selectively conduct cations; and others. Some, such as the cyclic-nucleotide-gated channels of olfactory cilia and the voltage-gated Ca2+ channels of ciliates, are found almost exclusively in the ciliary membrane. The activities of these ciliary ion channels can either induce or respond to signal transduction mechanisms localized to the cilia.
We focus in this review on electrical signaling associated with cilia. A variety of methods have been used to either directly measure or infer the mechanisms of electrical signaling in cilia. Many of these approaches are indirect. Demonstrating that a protein typically associated with electrical signaling is found on the cilium suggests that the cilium generates such a signal. Additional evidence in some cases has been provided by examining the cellular response after inhibiting or reducing the expression of a putative ciliary transduction molecule. It has also been possible to show that removing the cilium itself eliminates a normal electrical response. Fluorescent Ca2+ indicators can directly measure the concentration of free Ca2+ within the cilium. If this changes in a way that depends on extracellular Ca2+, it strongly suggests that a current is carried by Ca2+ across the ciliary membrane. More direct evidence comes from electrical measurements of ciliary transmembrane currents. This approach has been difficult because the small dimensions of cilia (generally 10–100 μm in length and 0.2–0.3 μm in diameter) pose a challenge for recording from cilia during signal transduction. Nonetheless, it has been possible to excise patches of plasma membrane from the sides of such cilia using very small glass micropipettes (Nakamura and Gold, 1987). It has also become possible to record from a single intact cilium (Kleene and Gesteland 1991, Kleene and Kleene 2012, DeCaen et al. 2013, Delling et al. 2013). Either procedure allows direct, high-fidelity recordings of the transmembrane current under various conditions.
Because of publication constraints, we have often needed to cite existing review articles instead of the many original works. These reviews are excellent sources for readers desiring more comprehensive presentations.
Cilia of invertebrates
Invertebrates use cilia (also called flagella in some species by some researchers) for movement and for sensation. Many of these functions are known to depend on electrical signaling.
Cilia of protists
Ciliates have been useful models for ciliary motility and sensory functions for over 100 years, even before direct electrical recording could be done to describe the membrane conductances associated with cilia. We will focus here on Paramecium as a primary example of ciliary electrical signaling because of the extensive literature on its cilium-powered swimming behavior, electrophysiology, and genetic dissection of behavior (Kung et al. 1975, Saimi and Kung 1987, Preston 1990).
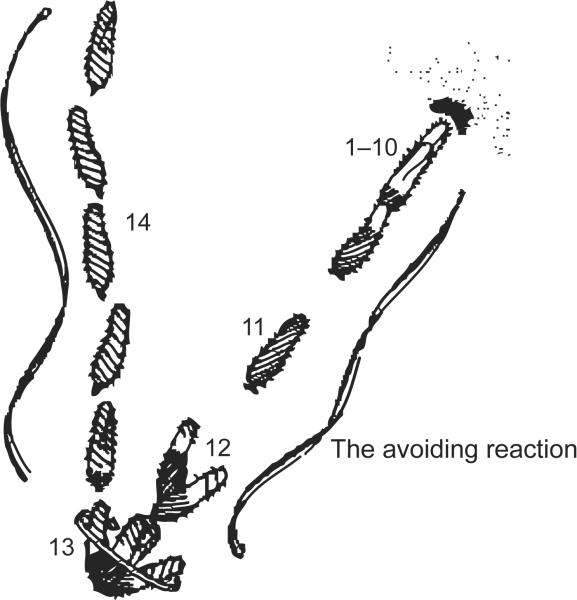
Paramecium cells are covered with 1000 or more cilia that beat toward the posterior of the cell. This coordinated beating propels the cell forward until it bumps into a solid object or there is a spontaneous reversal of the power stroke causing a temporary reversal of swimming direction (Figure 1). The reversal quickly ends and the cell swims off in a random new direction. At the turn of the last century, observant scientists noted that environmental stimuli affected swimming speed and turning frequency (Jennings 1906), which are dependent upon ciliary beat frequency and the reversal of the ciliary power stroke that causes the transient backward swimming (Machemer 1988a, 1988b).
Figure 1.
An image based on the sketch of the stages of an avoiding reaction drawn by Jennings (1906). Anterior mechanical stimulation by a cell swimming into an object leads to depolarization, opening of the Cav channels of the cilia, movement of the cell backward for a short time, twirling in place, and forward movement of the cell in a new direction. Depolarization by ionic stimuli causes the same avoiding reaction behavior. From Figure 5 of Eckert R (1972) “Bioelectric control of ciliary activity.” Science 176: 473–481, copyright 1972, with permission from Science.
Building upon these careful behavioral observations, important studies by Naitoh, Kaneko, Eckert, and Machemer (reviewed in Machemer, 1988a, 1988b) demonstrated that ciliary ion conductances and membrane potential control the frequency and direction of ciliary beating. Machemer and others elegantly demonstrated that the speed of swimming is dependent upon the membrane potential (Brehm and Eckert 1978, Machemer 1988a, 1988b, and Kutomi et al. 2012). Stimuli that hyperpolarize the cell slightly from rest increase ciliary beating toward the posterior of the cell and therefore swimming speed. Stimuli that depolarize slightly have the opposite effect. Depolarization above threshold initiates a graded Ca2+ action potential by opening the voltage-gated Ca2+ channels (Cav) that are located exclusively in the ciliary membrane (Dunlap 1977, Machemer and Ogura 1979) (Figure 2). The resulting increase in intraciliary Ca2+ reverses the direction of the power stroke of the cilia, resulting in the cell swimming backward. A rapidly activated voltage-gated K+ conductance (IKv) and slower Ca2+-activated K+ conductance (IKCa) return the membrane potential to the resting level. These K+ channels are also found in the cilia and, like the Cav channels, might be concentrated there and not in the soma (Brehm et al. 1978). The Ca2+ that activates the KCa channel has been shown to come from the Cav channels of the cilia (Satow and Kung 1980). Since there is no spillover of Ca2+ from the ciliary action potentials into the cell body, KCa channels are likely to reside in the ciliary membrane in order to be activated by Ca2+ influx into the cilium (Husser et al. 2004).
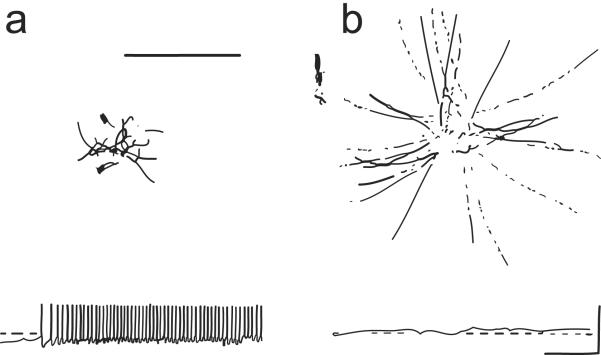
Figure 2.
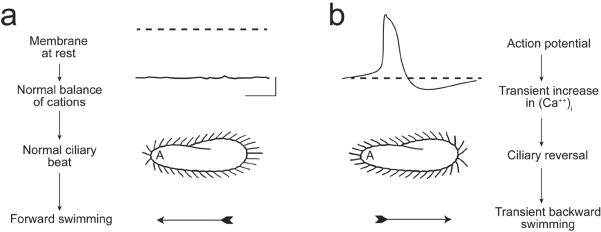
(a) These images illustrate that the resting membrane potential of Paramecium is negative (about −25 to −40 mV); the corresponding ciliary beat is toward the posterior of the cell and the cell swims forward. (b) In depolarizing solutions, such as high K+ or Ba2+, the cell's plasma membrane depolarizes and reaches threshold for the action potential. During the action potential, calcium ions (Ca2+) enter the cilia through voltage-gated channels; the high levels of Ca2+ change the power stroke of the cilia, which now beat most strongly toward the anterior and move the cell backward. The action potential is quickly terminated and the Ca2+ removed from or sequestered in the cilia, allowing ciliary beat and swimming to return to normal. From Kung et al. (1975) with permission from Science.
An advantage of ciliates like Paramecium for examining the role of cilia in signaling is that the cilia can be cleanly removed and the remaining cell body used for electrical recording (Dunlap 1977, Machemer and Ogura 1979). Functional elimination of one channel gene product at a time has also been achieved by mutation. The best known of these mutants are the Pawns, named for the chess piece because they cannot move backward for lack of a functional ciliary Ca2+ channel (Kung et al. 1975, Saimi and Kung 1987, Preston 1990). See more about these mutants below.
The duration of backward swimming is a function of the activity of Cav channels as well as the repolarizing K+ conductances. The cell swims backward for as long as it takes to sequester or export the extra Ca2+ from the cilium, which outlasts the duration of the action potential. In addition, other Ca2+-dependent channels that conduct sodium ions (Na+) or magnesium ions (Mg2+) can prolong the plateau of the action potential and increase the duration of backward swimming. Since Paramecium survives in a range of buffers, it is possible to assess the separate contributions of K+, Na+, and Mg2+ conductances to the backward swimming by ion substitution. While more than seven membrane currents have been identified by voltage clamp (Saimi and Kung 1987, Machemer 1988a), we will focus on those restricted to the cilia and electrical signaling of cilia.
As Preston observes (Preston 1990), Paramecium “broadcasts” the activity of ion channels through its swimming behavior; that is, the cells' swimming speed, turning frequency, and duration of backward swimming during the turn inform the observer about the cells' ciliary channel activity. This connection between behavior and channel activity opened up the possibilities for genetic dissection of swimming behavior (Kung et al. 1975). The seminal studies by Kung led to the identification of many useful mutants such as the Pawns, which cannot reverse their swimming direction and are devoid of the Cav conductance (Kung et al. 1975) (Figure 3), as is the case with deciliated cells (Dunlap 1977). These Pawn mutants have been useful for isolating and studying ion conductances in the absence of the ciliary Cav conductance. The K+ channels of Pawn mutants, for example, are normal (Satow and Kung 1980).
Figure 3.
Recordings and behavior of wildtype and pawn mutants of Paramecium in a depolarizing stimulus, barium chloride (BaCl2). (a) When wildtype cells are placed in a solution of BaCl2, they jerk about with rapid alternating backward and forward movements. These backward bouts correspond to the action potentials shown in the recording below. The dotted line is 0 mV. (b) Pawn mutants, when similarly placed in BaCl2, depolarize, as shown by the potential recording above 0 mV. However, the cells never reverse their swimming direction or ciliary beating. Instead they swim smoothly away from where they were placed. From Kung et al. (1975) with permission from Science.
A remarkable study of seemingly different mutants showed that their phenotypes were caused by alleles of the same gene. Paranoiacs and Pantophobiacs swim backward upon depolarizing stimulation and spontaneously for long periods of time, while Fast mutants swim quickly and smoothly without turns in the same depolarizing solution. The analysis of these mutants resulted in a dissection of the calmodulin molecule, with amino acid substitutions in the C-terminal lobe causing the long backward swimming phenotype and substitutions in the N-terminal domain causing the lack of backward swimming (Kung et al. 1992). In the first case, the mutant calmodulin did not activate the KCa channel that is critical for repolarizing the membrane, leading to prolonged depolarization and backward swimming. In the second case, the mutant calmodulin did not interact with the Ca2+-activated Na+ (NaCa) channel that, when open, normally prolongs the action potential and causes backward swimming.
Responses of Paramecium to mechanical stimuli rely upon the modulation of soma channels and ciliary Cav channels to drive the behavioral responses to stimuli. Mechanical stimulation of the posterior end of Paramecium leads to a hyperpolarization and fast swimming away from the stimulus, normally a predator, while mechanical stimulation of the anterior end of the cell induces depolarization and action potentials that cause the cell to back away. This latter response is the basis of the turn called the “avoiding reaction” that was demonstrated to open the voltage-gated Ca2+ channels of the cilia. The hyperpolarizing K+ conductance from posterior stimulation is unchanged by deciliation; similarly the depolarizing Ca2+ receptor current from anterior stimulation is unchanged by deciliation, but there are no Ca2+ action potentials without cilia (Machemer and Ogura 1979).
Attraction and repulsion responses of cells to chemical stimuli rely upon the modulation of the speed and frequency of turning (Van Houten 1998, Valentine et al. 2010). Attractants and repellents in general hyperpolarize and depolarize the cell, respectively, although the receptors are not necessarily located in the ciliary membrane. The hyperpolarization suppresses action potentials and also causes fast swimming that leads to accumulation of cells at the site of the stimulus; the depolarizations trigger action potentials and turns that cause the cells to disperse away from the site of the stimulus. Pawn mutants that cannot initiate turns through the voltage-gated Ca2+ channel activity do not normally respond to attractants or repellents. Thus the environmental stimuli control behavior through the modulation of ciliary ion channel activity.
The ion channel activities of the cilia result in membrane potential changes that, in turn, affect signal transduction pathways. The resulting second messengers have secondary effects on ciliary motility. For example, hyperpolarization stimulates the adenylyl cyclase activity of the cilia and also of the cell body (Schultz et al. 1992, Schultz et al. 1997). Cyclic AMP produced in the cilia modifies the ciliary beat direction and frequency making the cell swim fast forward (Hamasaki et al. 1991, Noguchi et al. 2004). Schultz and workers identified a linkage between a hyperpolarizing K+ conductance and cAMP production (Schultz et al. 1992). The connection then became more evident when the Schultz lab showed that the cyclase and the channel were domains of the same protein (Weber et al. 2004). This fusion protein was found expressed in cilia by green fluorescent protein tagging and by proteomic analysis (Yano et al. 2013). All genes for adenylyl cyclase in Paramecium code for these proteins that have both an N-terminal channel and C-terminal enzyme domain and a channel domain, but not all are found in the cilia (Yano et al. 2013). This intriguing K+ channel does not participate in the repolarization of the action potential, but may hold the explanation for the coupling of the hyperpolarization with rapid cAMP production when paramecia are stimulated with the attractant glutamate (Yang et al. 1997).
Paramecium shares ciliary ion channels with other organisms. For example, Chlamydomonas has in its flagellar membrane a voltage-gated Ca2+ channel that is necessary for the change in waveform in response to light or mechanical stimulation (Fujiu et al. 2009). The CatSper Ca2+ channels of the sperm flagellum are responsible for the change in waveform that occurs when the sperm is in the vicinity of the egg (Brenker et al. 2012). Cilia located on olfactory sensory neurons have no voltage-gated conductances, but they do have Ca2+-dependent K+ channels (Delgado et al. 2003). In contrast to these common ciliary channels, the channels that contribute to the sensory function of olfactory cilia (as discussed below) are missing from Paramecium. These include cyclic-nucleotide-gated channels that conduct cations with low selectivity, and Ca2+-gated channels that conduct chloride ions (Cl−). There is a report of a Cl− channel in cilia of Tetrahymena (Fujiwara-Hirashima et al. 1996).
Polycystic Kidney Disease (PKD) 2, a member of the transient receptor potential (TRP) family of non-selective cation channels, is found in primary cilia of some mammalian epithelial cells. As discussed below, PKD2 in such cells conducts Ca2+ among other ions and functions in mechanoreception (González-Perrett et al. 2001, Luo et al. 2003). Paramecium has a PKD2 ortholog in its ciliary membrane (Figure 4), although the evidence is not that it is a mechanoreceptor that conducts Ca2+ but rather a Mg2+ channel (Valentine et al. 2012). Direct recording from mammalian primary cilia (DeCaen et al. 2013, Delling et al. 2013) shows that, as in the case of Paramecium cilia, the primary cilium is a special Ca2+ signaling compartment isolated from the rest of the cell. Thus the Ca2+-permeable channels, such as the PKD2-dependent channels in primary cilia (Delmas et al. 2004) and the voltage-gated Ca2+ channels in Paramecium, function within a special Ca2+-signaling organelle (Jin et al. 2013). The roles of intraciliary Ca2+ in vertebrate phototransduction and olfactory transduction are also established and will be discussed below.
Figure 4.
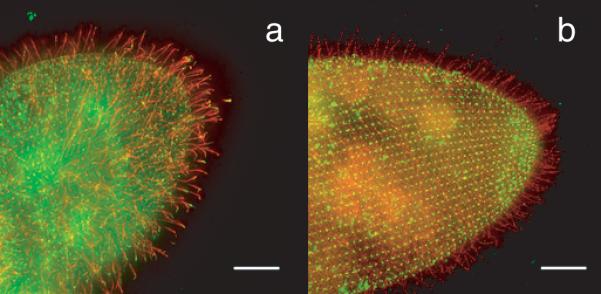
(a) Wildtype Paramecium cell with the FLAG-epitope-tagged small-conductance Ca2+-activated K+ channel SK1a. The FLAG-tagged channel is visualized with anti-FLAG primary and red fluorescent secondary antibody. The green fluorescence of the cell body and spots in the cilia are from an antibody against the folate chemoreceptor and secondary antibody. Note the red cilia, indicating that the SK1a channel localizes primarily to cilia. (b) Wildtype Paramecium cell with the PKD2 channel FLAG-epitope tagged. The red fluorescence indicates that this channel is present in cilia and also on the general cell surface. The green punctate fluorescence on the cell body is from the primary and secondary antibodies used to identify centrin, a protein known to be present in basal bodies at the base of the cilia. The scale bar indicates 10 micrometers. From Figure 6 in Valentine et al. (2012).
Paramecium shares with vertebrates not only orthologs of ciliary ion channels, but also some trafficking mechanisms that selectively allow membrane proteins to reach the ciliary membrane. For example, mammalian PKD2 depends upon the complex of Bardet-Biedl Syndrome (BBS) proteins to be properly situated in the ciliary membrane (Hoffmeister et al. 2011). Failure of the BBS proteins to form a complex and function in trafficking proteins to cilia leads to the many symptoms of the human Bardet-Biedl Syndrome, including developmental abnormalities and polycystic kidneys (Zaghloul and Katsanis 2009). In Paramecium, PKD2 and a small conductance calcium-activated K+ channel (SK1a) are not found in cilia of cells with reduced BBS complex proteins (Valentine et al. 2012). In contrast, the Paramecium ciliary voltage-gated Ca2+ channel is not dependent upon these BBS proteins or upon the accessory subunits that guide mammalian voltage-gated Ca2+ channels to their surface location (Dolphin 2012). It may turn out that the Pawn proteins are the accessory protein counterparts for Paramecium. Meckelin, another protein of the mammalian ciliary transition zone where proteins are selected to enter and leave the cilium, is also found in Paramecium and appears to function in ciliary development (Picariello et al. 2014).
Methods developed for recording from single cilia of vertebrates have not been successfully applied to Paramecium. However, there have been recordings from excised membrane patches that may have included ciliary membrane (Saimi and Ling 1990).
Cilia of other invertebrates
Many sensory functions in the soil nematode Caenorhabditis elegans depend on ciliated sensory neurons, and in these neurons several channels are specifically localized to the ciliary membrane. It is thus very likely that these channels underlie electrical signals generated within the cilia. Two such channels, TAX-2 and TAX-4, are gated (opened, activated) by cyclic GMP and modulate chemosensation, oxygen and CO2 sensation, photosensation, locomotion on surfaces, and swimming (reviewed by Johnson and Leroux, 2010). Several TRP channels are also enriched in neuronal sensory cilia of C. elegans (reviewed by Kahn-Kirby and Bargmann, 2006). OSM-9 and OCR-2, members of the TRPV class of channel, are required for olfaction, mechanosensation, and osmosensation. Normal male mating behavior requires the channel protein PKD-2 and another ciliary protein, LOV-1. These are homologues of the vertebrate proteins PKD2 and PKD1, respectively, which are discussed below. TRP-4, a channel of class TRPN, is found in the cilium of a mechanosensitive neuron. In this case, a membrane current in the neuron has been directly measured, and this current requires TRP-4 (Kang et al. 2010).
Ctenophores, also known as comb jellies, are the largest animals (ranging from a few millimeters to over one meter in size) to propel themselves with cilia. A recent review describes the history of studies of the structure and function of cilia in ctenophores and areas for future study of these cilia (Tamm 2014). Ctenophore cilia, arranged in groups, form the combs that characterize this phylum. As with the example of Paramecium above, the ciliary power stroke can reverse, underlying the escape behavior of the ctenophore. Modified stiff cilia in the ctenophore mouth help with ingestion. In the mouths of ctenophores of the genus Beroe, macrocilia beat in metachronal waves during feeding. These macrocilia, compound bundles of axonemes surrounded by a membrane, are activated to beat during feeding by neuronal depolarization of the macrociliary cells. The depolarization opens ciliary Ca2+ channels at the base of the macrocilium. In contrast, when the ciliary beat reverses, the channels along the entire macrocilium open. Cilia also play roles in sensory processes of the statocyst in balance and orientation of the ctenophore. Thus the cilia described above and more unusual ciliary structures underlie both sensation and motility in ctenophores.
In the fruit fly Drosophila, two channels of the TRPV class are present in the cilia of neurons in the chordotonal organ, an antennal organ required for sensing of sound. These channels, NAN and IAV, are homologous to the OCR-2 and OSM-9 channels, respectively, of C. elegans (Gong et al. 2004). NOMPC, a channel of the TRPN class that is homologous to the TRP-4 channel of C. elegans, is in the cilia of the chordotonal neurons as well as the cilia of the sensory neurons in the external tactile bristles (Lee et al. 2010). Mutations in these channel proteins compromise or eliminate the sensations that originate in the chordotonal organ and bristles.
Cilia of vertebrates
Most of our knowledge of electrical signaling in the cilia of vertebrates comes from indirect experimental approaches, with two prominent exceptions: the photoreceptor cells of the visual system and the sensory cilia of olfactory receptor neurons.
Photoreceptors of vertebrates
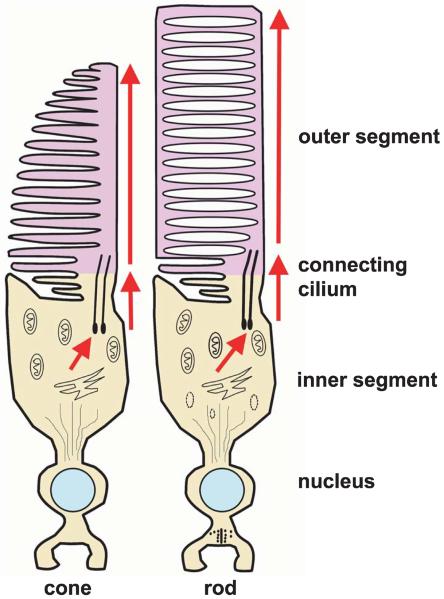
In the vertebrate retina, light is detected in the retina by photoreceptors called rods and cones. Each photoreceptor has an inner and an outer segment (Figure 5). The outer segment is a highly specialized cilium. In the dark, or when adapted to a constant level of illumination, the outer segment maintains a concentration of cyclic GMP (cGMP) sufficient to open channels in the plasma membrane. Ca2+ and Na+ enter the outer segment through these ligand-gated channels, while K+ exits the photoreceptor in the inner segment. The net effect of these two currents is to maintain the cellular membrane potential in a relatively depolarized state. When sufficient light strikes the outer segment, the influx of Ca2+ and Na+ is reduced, so the membrane potential becomes more negative (hyperpolarizing the cell). The mechanism of this is well understood (Figure 6). Some of the photons impinging on the retina are absorbed by the photopigment retinal; photon-induced isomerization of 11-cis retinal to all-trans-retinal results in a series of conformational changes in the protein portion (opsin) of rhodopsin located in the outer segment. One conformational state of the opsin protein activates a heterotrimeric G-protein (transducin), which in turn activates a cGMP phosphodiesterase. This phosphodiesterase enzymatically hydrolyzes some of the intracellular cGMP. With less cGMP, the influx of Ca2+ and Na+ into the outer segment is reduced. For additional details, see Pugh and Lamb (2000).
Figure 5.
Morphology of vertebrate cone and rod photoreceptors. Each includes an inner segment and an outer segment connected by a cilium. The inner segment contains the cell's biosynthetic machinery. Phototransduction takes place in the outer segment. In the rods, the outer segment contains free-floating membrane-bound disks. In cones, the disks remain continuous with the plasma membrane. Modified from Perkins BD, Fadool JM (2010) “Photoreceptor structure and development analyses using GFP transgenes.” Methods in Cell Biology 100: 205–218, copyright 2010, with permission from Elsevier.
Figure 6.
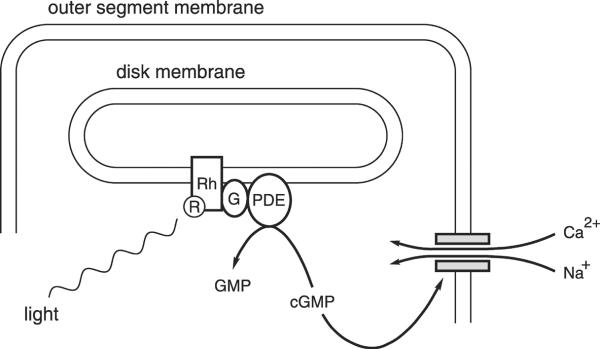
The mechanism of phototransduction in vertebrate rod photoreceptors. The schematic shows the end of a rod outer segment and just one of the many membranous disks inside. The parallel horizontal lines represent the inner and outer faces of the rod plasma membrane and the disk membrane as indicated. R, retinal; Rh, rhodopsin; G, the G-protein transducin; PDE, a cGMP phosphodiesterase; GMP, guanosine 5′-monophosphate; cGMP, cyclic GMP (guanosine 3′:5′-cyclic monophosphate); shaded rectangles, a cation-conducting channel activated by cGMP; Ca2+, calcium ions; Na+, sodium ions.
Olfactory cilia of vertebrates
Odors are detected by olfactory sensory neurons that line the nasal cavity. These neurons extend cilia (or in some species microvilli) into the nasal mucus. In amphibians, a neuron extends about six cilia, each 0.2 to 0.3 μm in diameter and on average 30 μm in length (Figure 7). These cilia are motile but do not beat synchronously or with a regular period. In mammals, each neuron projects on average 17 immotile olfactory cilia. The cilia increase the apical surface area of the neuron by a factor of about 40 (Menco 1997). This is expected to increase the probability that an odorant molecule will collide with a sensory membrane. Since 1956, it has been known that the neurons generate an electrical response to an appropriate odor (Ottoson 1956). For about 30 years, it has also been known that the neuronal cilia are required for this response (Getchell 1986). These clues motivated researchers to develop methods that allow direct electrical recording from the olfactory cilia. What follows is an outline of the principal odor transduction scheme that occurs within the cilium (Figure 8). Additional details and references are available elsewhere (Kaupp 2010).
Figure 7.
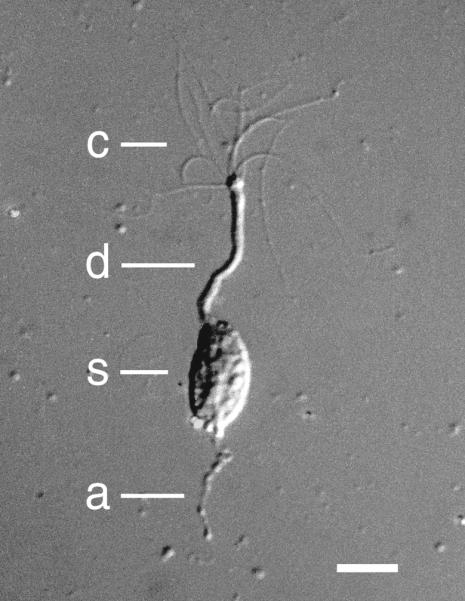
Photograph of an isolated frog olfactory receptor neuron under differential interference contrast optics. c, cilia; d, dendrite; s, soma; a, axonal segment. The calibration bar represents 10 micrometers. Modified from Kleene SJ, Gesteland RC (1981) “Dissociation of frog olfactory epithelium with N-ethylmaleimide.” Brain Research 229: 536–540, copyright 1981, with permission from Elsevier.
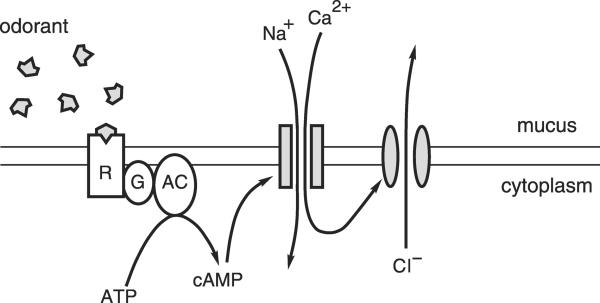
Figure 8.
The principal mechanism for transduction of odorous stimuli in vertebrate olfactory cilia. The parallel horizontal lines represent the inner and outer faces of the ciliary membrane of one cilium on an olfactory sensory neuron. R, odorant-receptor protein; G, Golf, a G-protein; AC, type III adenylyl cyclase; ATP, adenosine 5′-triphosphate; cAMP, cyclic AMP (adenosine 3′:5′-cyclic monophosphate); Na+, sodium ions; Ca2+, calcium ions; shaded rectangles, a cation-conducting channel activated by cAMP; shaded ellipses, a Ca2+-gated channel that conducts chloride ions; Cl−, chloride ions. Modified from Kleene SJ (2002) “The calcium-activated chloride conductance in olfactory receptor neurons.” Current Topics in Membranes 53: 119–134, copyright 2002, with permission from Elsevier.
The cilia of olfactory sensory neurons possess membrane proteins that bind odorants. Although there are several hundred such receptors across the population of neurons, the cilia of a given neuron have just one type of receptor. Typically this receptor binds several different odorants with fairly low affinity. If the odorant succeeds in activating the receptor, it in turn activates a heterotrimeric G-protein. This activates a type III adenylyl cyclase, causing cyclic AMP (cAMP) to be synthesized. cAMP gates the first of two types of transduction channel in the ciliary membrane. In the open state, these channels allow cations, principally Ca2+ and Na+, to flow from the mucus into the cilium. In the tiger salamander, it was possible to directly visualize the resulting increase in Ca2+ within the cilium using a Ca2+-sensitive fluorescent dye (Leinders-Zufall et al. 1997). The incoming positive ions depolarize the neuron. If the influx of Ca2+ is sufficient, the concentration of Ca2+ within the cilium becomes high enough to gate the second type of ciliary transduction channel. These channels then allow Cl− to exit the cilium. Negative ions leaving the cilium is electrically equivalent to positive ions entering, so this current also depolarizes the neuron, amplifying the initial depolarization. If the neuronal plasma membrane potential becomes positive enough, it can trigger an action potential that is transmitted to the olfactory bulb of the brain.
Electrical transduction of odors is a specialized property of the ciliary compartment. The two channel types are present elsewhere on the neuronal plasma membrane, but at much lower densities (about 300-fold lower in the case of the cAMP-gated channels (Kurahashi and Kaneko 1993)). An interesting and open question is how the extreme geometry of the cilium contributes to the efficiency of signal detection and amplification. In frog, for example, a cilium's length is on the order of 100 times its diameter. This geometry may be ideal for increasing binding of odor molecules to the surface, but it may also lead to unique challenges for optimizing signal transduction after an odor molecule binds. Where along the ciliary length would be the best location for the channels? An odor-binding event on the distal region of the cilium (i.e. farthest from the cell body) could be efficiently transduced by having the channels near the odorant receptor. However, currents from distally located channels will be attenuated as they are conducted along the length of the cilium toward the cell body. (This is called cable-conduction loss and also applies to passive conduction in nerve and muscle fibers.) Another design question involves the relative locations of the two types of transduction channel. Having the two channel types distributed in close proximity to one another should increase efficiency by allowing Ca2+ ions that enter through the cAMP-gated channels to rapidly activate nearby Cl− channels. By a combination of experiment and mathematical modeling, it has been inferred that both types of channel are concentrated in a common band a few micrometers from the base of the cilium (Flannery et al. 2006, French et al. 2010). Whether this is an optimal design is not yet understood, but other proteins associated with membrane signaling also show non-random distributions along the cilium (as summarized by Bloodgood (2012)). The first measurements of channel distributions along a cilium or flagellum may have been those of Beck and Uhl (1994) in Chlamydomonas.
The cilium's very large surface-to-volume ratio allows for very large fluctuations in ionic concentrations within the cilium. (Applying the estimates of Lindemann (2001) reveals that the surface-to-volume ratio for a rat olfactory cilium is about 190 times that of a spherical cell 10 μm in diameter.) During a large response, Ca2+ and Na+ entering through hundreds of ciliary channels can rapidly reach high concentrations within the small ciliary volume. In the absence of secondary mechanisms for expelling those ions, the Ca2+ within the cilium reaches millimolar levels in a modeled steady state (Lindemann 2001). Mechanisms for expelling ciliary Ca2+ do exist (reviewed by Kaupp (2010)). At the same time, intraciliary Ca2+ has been shown to reach at least 100 micromolar during the response to an odor (Delgado et al. 2004).
Other cilia of vertebrates
Most vertebrate cells in the body possess a single, non-motile primary cilium when the cell is not dividing. Among these primary cilia, renal primary cilia have received particular attention because defects in those cilia can lead to cystic kidney diseases. Mutations in ciliary proteins PKD1 (also known as polycystin-1 or PC1) and PKD2 (also known as polycystin-2, PC2, or TRPP2) cause autosomal dominant polycystic kidney disease (Bissler and Dixon 2005), and the mechanisms of this have been the subject of intense investigation (summarized by Ma et al. 2013).
In the kidney, the primary cilia extend into the lumens of the renal tubules. The renal filtrate (destined to become urine) flows through the tubules past these cilia and in the process deflects them. The evidence is strong that this mechanical stimulation initiates electrical signals in the renal primary cilium. Deflection of the cilium induces an increase in [Ca2+] in the cell body (Praetorius and Spring 2001). This response requires the cilium, external Ca2+, and three ciliary proteins – PKD1, PKD2, and TRPV4 (Praetorius and Spring 2003, Nauli et al. 2003, Köttgen et al. 2008). PKD2 and TRPV4 are members of the TRP family of channel proteins and are known to conduct Ca2+. The clear inference is that, on deflection of the cilium, channels in the cilium that include the PKD2 and/or TRPV4 subunits open, allowing a current of Ca2+ to enter the cilium. Only recently was it directly shown that Ca2+ levels increase within the cilium itself during deflection (Su et al. 2013, Jin et al. 2013). This advance was achieved by targeting Ca2+- sensitive fluorophores to the cilium. The evidence for electrical signaling in the renal primary cilium is strong. However, one direct measure, recording the electrical current across the ciliary membrane during signaling, has not yet been achieved. As noted above, such an approach has been successful with olfactory cilia and photoreceptors. Two methods for measuring the currents in primary cilia are now available. Raychowdhury et al. (2005) attached a recording pipette to the primary cilium after it was detached from the cell. More recently it has also become possible to record from a single, intact primary cilium while it is attached to the cell or following detachment from the cell (Figure 9) (Kleene and Kleene 2012, DeCaen et al. 2013, Delling et al. 2013).
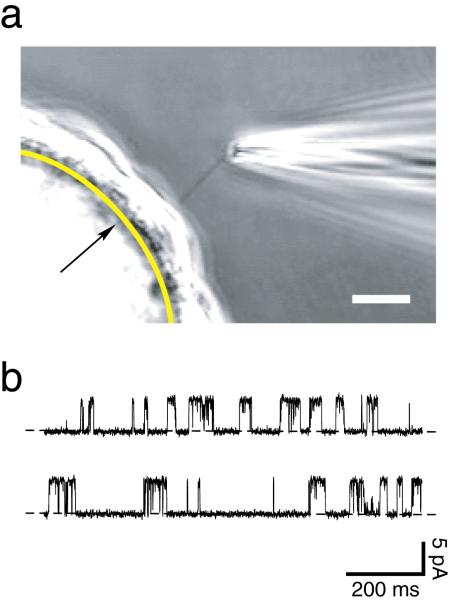
Figure 9.
(a) Photomicrograph of a method for electrical recording from an isolated primary cilium. A glass-coated bead is covered with a confluent layer of renal epithelial (IMCD-3) cells. A smooth arc (indicated by the arrow) has been drawn to represent the boundary between the bead and the cells. The primary cilium belonging to one cell is visible entering the tip of the recording micropipette. Cells are grown on beads to increase the visibility of the cilia. In a typical planar monolayer culture, the cilia cannot be resolved above the underlying cell layer. Another advantage of the procedure shown is that the bead is free-floating. This allows the cilium to enter the recording pipette as suction is applied to the pipette. Bar = 10 micrometers. From Figure 2 of Kleene and Kleene (2012). (b) A continuous 2-second recording of the current across the membrane of a renal primary cilium that was detached from the cell. The recording shows current fluctuations due to the opening and closing of a single membrane channel. The lower level of current (dashed lines) is seen when the channel is closed, and a current 5.2 picoamperes greater is seen when the channel is open. The membrane potential was held at 0 mV. This channel was activated by applying 4 μM Ca2+ to the cytoplasmic face of the membrane. pA, picoampere; ms, millisecond.
By recording directly from primary cilia, DeCaen et al. (2013) identified a spontaneously active non-selective cationic current in the ciliary membranes from two cell lines (derived from human retinal pigment epithelium (RPE) and from renal epithelium) and also the ciliary membranes of primary RPE cells and embryonic fibroblasts from mouse. In the human RPE cell line, the current was shown to require the TRP channel candidate subunits PKD1L1 and PKD2L1 (DeCaen et al. 2013). In a companion study of the human RPE cells (Delling et al. 2013), the cilium was found to be electrically distinct from the soma.. The resting membrane potential of the cilium was −18 mV, compared to −54 mV in the soma. Free [Ca2+] was 500–600 nM in the cilium but just 107 nM in the soma, even though movement of Ca2+ between the soma and the cilium was efficient. It is likely that ionic transport processes in the ciliary membrane maintain the distinct electrical state there.
Many details of the mechanosensitive response in renal primary cilia have not yet been determined. (See Hofherr and Köttgen (2011) for additional details and references.) PKD1, which is required for the response to flow-induced bending of the cilium, has structural characteristics of a receptor or adhesion molecule and colocalizes with PKD2 within the ciliary membrane. PKD1 is, however, an “orphan receptor”; the precise nature of the stimulus to which it responds is not known. It may be that PKD1 is mechanically sensitive, responding directly to ciliary bending induced by the flow of renal filtrate. It may also respond to a chemical ligand in the filtrate. One such candidate is exosome-like vesicles in the filtrate that bind to the ciliary surface and carry many signaling molecules. In the laboratory, many stimuli have been identified that activate PKD2 or channels that resemble PKD2: intracellular Ca2+, changes in pH or voltage, epidermal growth factor, fibrocystin, bradykinin and muscarinic agonists, swelling, external Ca2+, heat, and vasopressin. PKD2 is not the only channel protein found in the renal primary cilia. A functional TRP channel has four protein subunits, and the subunits are not always identical. In the renal cilium, PKD2 has been shown to combine with TRPV4 (Köttgen et al. 2008) and TRPC1 (Bai et al. 2008) subunits to form heteromeric channels. The α subunit of the epithelial Na+ channel (ENaC) is found in the cilium, and a ciliary channel similar to ENaC is activated by vasopressin (Raychowdhury et al. 2009). Finally, the voltage-dependent Ca2+ channel CaV1.2 and the type 5 dopamine receptor have been found in renal primary cilia; activation of the receptor causes an increase in ciliary Ca2+ mediated by the channel (Jin et al. 2013).
Electrical signals analogous to those in the non-motile renal primary cilia also appear to operate in the motile cilia of respiratory and oviductal epithelia (reviewed by Bloodgood 2010). In both of these motile cilia, mechanical stimulation leads to a change in ciliary beat frequency as well as an increase in Ca2+ in the cell body. As in the renal cells, these responses require extracellular Ca2+ and the TRPV4 channel protein. Since TRPV4 is found in these cilia, it is likely that they signal via a current of Ca2+ through TRPV4-dependent channels in the ciliary membrane. Respiratory cells give similar responses on exposure to bitter compounds. The cilia of respiratory cells contain three types of protein that contribute to transduction of bitter taste in the tongue: T2R receptor proteins, the G-protein α-gustducin, and the TRPM5 channel. Again, this strongly suggests electrical signaling by the cilium.
In many other vertebrate cilia, electrical signaling is suspected, but not confirmed, based on the presence on the cilia of receptors, channels, or other signaling proteins that might underlie such a response. (See Berbari et al. 2009 and Satir et al. 2010 for additional details and references.) The PKD2 channel subunit in particular has been identified on the cilia of embryonic node cells, ovarian granulosa cells, the cholangiocytes of bile ducts, vascular smooth muscle cells, and vascular endothelial cells. Other ciliary channels include TRPV4 in primary cilia of cholangiocytes (Gradilone et al. 2007) and the CatSper and Slo3 channels of sperm flagella (Zheng et al. 2013). In the brain, various primary cilia have been shown to have three G-protein-coupled receptors: serotonin 5-HT6 receptors, somatostatin receptor 3, and melanin-concentrating hormone receptor 1. Activation of each of these receptors mediates an electrical response in some cell type, but whether they initiate a current in any cilium is not yet known.
Future directions for research on ciliary ion channels and electrical signaling include the dissection of the trafficking of these channels into and retention within the ciliary membrane. The control of ciliary signaling begins with the proper delivery and maintenance of channels and accessory proteins. What is clear so far is that not all channels use the same trafficking processes from Golgi to ciliary membrane (Garcia-Gonzalo and Reiter 2012, Valentine et al. 2012).
Acknowledgments
This work was supported by National Institutes of Health grants R21 DK091917 and P30 DK090868 (Baltimore Polycystic Kidney Disease Research and Clinical Core Center) to SJK, R01 GM59988 to JVH, and P30 GM103498 for imaging. We thank Robert Bloodgood for review and critical reading of the manuscript.
References
- Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. Journal of Neuroscience. 2011;31:9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C-X, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Reports. 2008;9:472–479. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C, Uhl R. On the localization of voltage-sensitive calcium channels in the flagella of Chlamydomonas reinhardtii. Journal of Cell Biology. 1994;125:1119–1125. doi: 10.1083/jcb.125.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Current Biology. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissler JJ, Dixon BP. A mechanistic approach to inherited polycystic kidney disease. Pediatric Nephrology. 2005;20:558–566. doi: 10.1007/s00467-004-1665-z. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. Journal of Cell Science. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA. The future of ciliary and flagellar membrane research. Molecular Biology of the Cell. 2012;23:2407–2411. doi: 10.1091/mbc.E12-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P, Dunlap K, Eckert R. Calcium-dependent repolarization in Paramecium. Journal of Physiology. 1978;274:639–654. doi: 10.1113/jphysiol.1978.sp012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P, Eckert R. An electrophysiological study of the regulation of ciliary beating frequency in Paramecium. Journal of Physiology. 1978;283:557–568. doi: 10.1113/jphysiol.1978.sp012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krähling M, Müller A, Kaupp UB, Strünker T. The CatSper channel: a polymodal chemosensor in human sperm. EMBO Journal. 2012;31:1654–1665. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement CA, Kristensen SG, Møllgård K, Pazour GJ, Yoder BK, Larsen LA, Christensen ST. The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation. Journal of Cell Science. 2009;122:3070–3082. doi: 10.1242/jcs.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Bacigalupo J. Cilium-attached and excised patch-clamp recordings of odourant-activated Ca-dependent K channels from chemosensory cilia of olfactory receptor neurons. European Journal of Neuroscience. 2004;20:2975–2980. doi: 10.1111/j.1460-9568.2004.03778.x. [DOI] [PubMed] [Google Scholar]
- Delgado R, Saavedra MV, Schmachtenberg O, Sierralta J, Bacigalupo J. Presence of Ca2+-dependent K+ channels in chemosensory cilia support a role in odor transduction. Journal of Neurophysiology. 2003;90:2022–2028. doi: 10.1152/jn.01167.2002. [DOI] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Padilla F, Osorio N, Coste B, Raoux M, Crest M. Polycystins, calcium signaling, and human diseases. Biochemical and Biophysical Research Communications. 2004;322:1374–1383. doi: 10.1016/j.bbrc.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nature Reviews Neuroscience. 2012;13:542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. Journal of Physiology. 1977;271:119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery RJ, French DA, Kleene SJ. Clustering of cyclic-nucleotide-gated channels in olfactory cilia. Biophysical Journal. 2006;91:179–188. doi: 10.1529/biophysj.105.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Müller D, Thumfart J, Schermer B, Pazour GJ, Neumann HPH, Zentgraf H, Benzing T, Omran H. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. Journal of the American Society of Nephrology. 2006;17:2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- French DA, Badamdorj D, Kleene SJ. Spatial distribution of calcium-gated chloride channels in olfactory cilia. PLoS One. 2010;5:e15676. doi: 10.1371/journal.pone.0015676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiu K, Nakayama Y, Yanagisawa A, Sokabe M, Yoshimura K. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Current Biology. 2009;19:133–139. doi: 10.1016/j.cub.2008.11.068. [DOI] [PubMed] [Google Scholar]
- Fujiwara-Hirashima C, Anzai K, Takahashi M, Kirino Y. A voltage-dependent chloride channel from Tetrahymena ciliary membrane incorporated into planar lipid bilayers. Biochimica et Biophysica Acta. 1996;1280:207–216. doi: 10.1016/0005-2736(95)00292-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. Journal of Cell Biology. 2012;197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell TV. Functional properties of vertebrate olfactory receptor neurons. Physiological Reviews. 1986;66:772–818. doi: 10.1152/physrev.1986.66.3.772. [DOI] [PubMed] [Google Scholar]
- Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang D-J, Kaang B-K, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. Journal of Neuroscience. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proceedings of the National Academy of Sciences. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, LaRusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proceedings of the National Academy of Sciences. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Barkalow K, Richmond J, Satir P. cAMP-stimulated phosphorylation of an axonemal polypeptide that copurifies with the 22S dynein arm regulates microtubule translocation velocity and swimming speed in Paramecium. Proceedings of the National Academy of Sciences. 1991;88:7918–7922. doi: 10.1073/pnas.88.18.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister H, Babinger K, Gürster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. Journal of Cell Biology. 2011;192:631–645. doi: 10.1083/jcb.201007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofherr A, Köttgen M. TRPP channels and polycystins. Advances in Experimental Medicine and Biology. 2011;704:287–313. doi: 10.1007/978-94-007-0265-3_16. [DOI] [PubMed] [Google Scholar]
- Husser MR, Hardt M, Blanchard M-P, Hentschel J, Klauke N, Plattner H. One-way calcium spill-over during signal transduction in Paramecium cells: from the cell cortex into cilia, but not in the reverse direction. Cell Calcium. 2004;36:349–358. doi: 10.1016/j.ceca.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Jennings HS. Behavior of the Lower Organisms. Columbia University Press; 1906. [Google Scholar]
- Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cellular and Molecular Life Sciences. 2013;71:2165–2178. doi: 10.1007/s00018-013-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Leroux MR. cAMP and cGMP signaling: sensory systems with prokaryotic roots adopted by eukaryotic cilia. Trends in Cell Biology. 2010;20:435–444. doi: 10.1016/j.tcb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annual Review of Physiology. 2006;68:719–736. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nature Reviews Neuroscience. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Kleene SJ, Gesteland RC. Transmembrane currents in frog olfactory cilia. Journal of Membrane Biology. 1991;120:75–81. doi: 10.1007/BF01868593. [DOI] [PubMed] [Google Scholar]
- Kleene NK, Kleene SJ. A method for measuring electrical signals in a primary cilium. Cilia. 2012;1:17. doi: 10.1186/2046-2530-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. Journal of Cell Biology. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C, Chang S-Y, Satow Y, Van Houten JV, Hansma H. Genetic dissection of behavior in Paramecium. Science. 1975;188:898–904. [PubMed] [Google Scholar]
- Kung C, Preston RR, Maley ME, Ling K-Y, Kanabrocki JA, Seavey BR, Saimi Y. In vivo Paramecium mutants show that calmodulin orchestrates membrane responses to stimuli. Cell Calcium. 1992;13:413–425. doi: 10.1016/0143-4160(92)90054-v. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Kaneko A. Gating properties of the cAMP-gated channel in toad olfactory receptor cells. Journal of Physiology. 1993;466:287–302. [PMC free article] [PubMed] [Google Scholar]
- Kutomi O, Hori M, Ishida M, Tominaga T, Kamachi H, Koll F, Cohen J, Yamada N, Noguchi M. Outer dynein arm light chain 1 is essential for controlling the ciliary response to cyclic AMP in Paramecium tetraurelia. Eukaryotic Cell. 2012;11:645–653. doi: 10.1128/EC.05279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One. 2010;5:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. Journal of Neuroscience. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Predicted profiles of ion concentrations in olfactory cilia in the steady state. Biophysical Journal. 2001;80:1712–1721. doi: 10.1016/S0006-3495(01)76142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Molecular and Cellular Biology. 2003;23:2600–2607. doi: 10.1128/MCB.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nature Genetics. 2013;45:1004–1012. doi: 10.1038/ng.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machemer H. Electrophysiology. In: Gortz H-D, editor. Paramecium. Springer-Verlag; 1988a. pp. 186–215. [Google Scholar]
- Machemer H. Motor control of cilia. In: Gortz H-D, editor. Paramecium. Springer-Verlag; 1988b. pp. 216–235. [Google Scholar]
- Machemer H, Ogura A. Ionic conductances of membranes in ciliated and deciliated Paramecium. Journal of Physiology. 1979;296:49–60. doi: 10.1113/jphysiol.1979.sp012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Küller A, Daiber PC, Neudorf I, Warnken U, Schnölzer M, Frings S, Möhrlen F. The proteome of rat olfactory sensory cilia. Proteomics. 2009;9:322–334. doi: 10.1002/pmic.200800149. [DOI] [PubMed] [Google Scholar]
- McDermott KM, Liu BY, Tlsty TD, Pazour GJ. Primary cilia regulate branching morphogenesis during mammary gland development. Current Biology. 2010;20:731–737. doi: 10.1016/j.cub.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menco BP. Ultrastructural aspects of olfactory signaling. Chemical Senses. 1997;22:295–311. doi: 10.1093/chemse/22.3.295. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Kurahashi S, Kamachi H, Inoue H. Control of the ciliary beat by cyclic nucleotides in intact cortical sheets from Paramecium. Zoological Science. 2004;21:1167–1175. doi: 10.2108/zsj.21.1167. [DOI] [PubMed] [Google Scholar]
- Ottoson D. Analysis of the electrical activity of the olfactory epithelium. Acta Physiol Scand Suppl. 1956;35:1–83. [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Current Opinion in Cell Biology. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Picariello T, Valentine MS, Yano J, Van Houten J. Reduction of meckelin leads to general loss of cilia, ciliary microtubule misalignment and distorted cell surface organization. Cilia. 2014;3:2. doi: 10.1186/2046-2530-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. Journal of Membrane Biology. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. Journal of Membrane Biology. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Preston RR. Genetic dissection of Ca2+-dependent ion channel function in Paramecium. BioEssays. 1990;12:273–281. doi: 10.1002/bies.950120605. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Lamb TD. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga DG, de Grip WJ, Pugh EN, editors. Handbook of Biological Physics. Vol. 3. Amsterdam: 2000. pp. 183–254. [Google Scholar]
- Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. Journal of Biological Chemistry. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, Ramos AJ, Zhang P, McLaughin M, Dai X-Q, Chen X-Z, Montalbetti N, Del Rocío Cantero M, Ausiello DA, Cantiello HF. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. American Journal of Physiology Renal Physiology. 2009;296:F87–F97. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- Saimi Y, Kung C. Behavioral genetics of Paramecium. Annual Review of Genetics. 1987;21:47–65. doi: 10.1146/annurev.ge.21.120187.000403. [DOI] [PubMed] [Google Scholar]
- Saimi Y, Ling K-Y. Calmodulin activation of calcium-dependent sodium channels in excised membrane patches of Paramecium. Science. 1990;249:1441–1444. doi: 10.1126/science.2169650. [DOI] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. Journal of Cell Science. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y, Kung C. Ca-induced K+-outward current in Paramecium tetraurelia. Journal of Experimental Biology. 1980;88:293–303. doi: 10.1242/jeb.88.1.293. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Current Biology. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schultz JE, Guo Y, Kleefeld G, Völkel H. Hyperpolarization- and depolarization-activated Ca2+ currents in Paramecium trigger behavioral changes and cGMP formation independently. Journal of Membrane Biology. 1997;156:251–259. doi: 10.1007/s002329900205. [DOI] [PubMed] [Google Scholar]
- Schultz JE, Klumpp S, Benz R, Schürhoff-Goeters WJCh, Schmid A. Regulation of adenylyl cyclase from Paramecium by an intrinsic potassium conductance. Science. 1992;255:600–603. doi: 10.1126/science.1371017. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Su S, Phua SC, Derose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nature Methods. 2013;10:1105–1107. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm SL. Cilia and the life of ctenophores. Invertebrate Biology. 2014;133:1–46. [Google Scholar]
- Valentine M, Yano J, Van Houten J. Chemosensory transduction in Paramecium. Japanese Journal of Protozoology. 2010;41:1–8. [Google Scholar]
- Valentine MS, Rajendran A, Yano J, Weeraratne SD, Beisson J, Cohen J, Koll F, Van Houten J. Paramecium BBS genes are key to presence of channels in Cilia. Cilia. 2012;1:16. doi: 10.1186/2046-2530-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten J. Chemosensory transduction in Paramecium. European Journal of Protistology. 1998;34:301–307. [Google Scholar]
- Weber JH, Vishnyakov A, Hambach K, Schultz A, Schultz JE, Linder JU. Adenylyl cyclases from Plasmodium, Paramecium and Tetrahymena are novel ion channel/enzyme fusion proteins. Cellular Signalling. 2004;16:115–125. doi: 10.1016/s0898-6568(03)00129-3. [DOI] [PubMed] [Google Scholar]
- Yang WQ, Braun C, Plattner H, Purvee J, Van Houten JL. Cyclic nucleotides in glutamate chemosensory signal transduction of Paramecium. Journal of Cell Science. 1997;110:1567–1572. doi: 10.1242/jcs.110.20.2567. [DOI] [PubMed] [Google Scholar]
- Yano J, Rajendran A, Valentine MS, Saha M, Ballif BA, Van Houten JL. Proteomic analysis of the cilia membrane of Paramecium tetraurelia. Journal of Proteomics. 2013;78:113–122. doi: 10.1016/j.jprot.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. Journal of Clinical Investigation. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L-P, Wang H-F, Li B-M, Zeng X-H. Sperm-specific ion channels: targets holding the most potential for male contraceptives in development. Contraception. 2013;88:485–491. doi: 10.1016/j.contraception.2013.06.002. [DOI] [PubMed] [Google Scholar]