Abstract
There is a growing interest in understanding how amyloid-β (Aβ) accumulation in preclinical Alzheimer’s disease relates to brain morphometric measures and cognition. Existing investigations in this area have been primarily conducted in older cognitively-normal (CN) individuals. Therefore, not much is known about the associations between Aβ burden, cortical thickness, and cognition in midlife. We examined this question in 109, CN, late-middle-aged adults (mean age=60.72±5.65 years) from the Wisconsin Registry for Alzheimer’s Prevention. They underwent Pittsburgh Compound B (PiB) and anatomical magnetic resonance (MR) imaging, and a comprehensive cognitive exam. Blinded visual rating of the PiB scans was used to classify the participants as Aβ+ or Aβ−. Cortical thickness measurements were derived from the MR images. The Aβ+ group exhibited significant thinning of the entorhinal cortex and accelerated age-associated thinning of the parahippocampal gyrus compared with the Aβ− group. The Aβ+ group also had numerically lower, but nonsignificant, test scores on all cognitive measures, and significantly faster age-associated cognitive decline on measures of Speed & Flexibility, Verbal Ability, and Visuospatial Ability. Our findings suggest that early Aβ aggregation is associated with deleterious changes in brain structure and cognitive function, even in midlife, and that the temporal lag between Aβ deposition and the inception of neurodegenerative/cognitive changes might be narrower than currently thought.
Keywords: Preclinical AD, amyloid, cortical thickness, cognition
1. Introduction
Recent research has indicated the existence of a preclinical stage of Alzheimer’s disease (AD) during which pathological changes gradually accumulate in the absence of detectable cognitive symptoms [1]. Converging evidence suggests that one of the earliest brain changes seen during this preclinical stage is an increase in brain amyloid-β (Aβ) deposition [1–3]. This Aβ deposition begins several years before the onset of symptoms and continues to increase as the disease progresses before it approaches a plateau approximately at the inception of clinical symptoms [1, 4–6].
Another observable feature of the preclinical stage of AD is an alteration in brain structure that, among other neurodegenerative effects, leads to reduced cortical thickness [7]. Automated measurement of cortical thickness from anatomical magnetic resonance imaging (MRI) scans is possible with the utilization of computer-aided techniques [8, 9]. Recently, studies have explored the connection between Aβ deposition and cortical thickness in preclinical AD using positron emission tomography (PET) tracers for Aβ, such as Pittsburg Compound B (PiB). For example, in a study of older cognitively-normal (CN) individuals, Becker and colleagues [10] found that Aβ deposition is associated with regional cortical thinning especially in posteromedial and lateral parietal structures. Similarly, Doré and colleagues [11] found that an increase in Aβ accumulation was associated with decreased cortical thickness in the posterior cingulate, precuneus, and hippocampus among CN individuals. Other studies using cerebrospinal fluid (CSF) biomarkers for Aβ have found similar associations between Aβ aggregation and structural brain changes in AD-susceptible regions [12]. Relatedly, although AD is characterized by a decline in cognition [1], the extent to which Aβ accumulation correlates with cognition among CN individuals is yet to be fully elucidated. Some investigations into the effect of Aβ on cognition among CN individuals find no associations [13–15] whereas others do reveal associations, largely in the domain of episodic memory [16, 17].
Most of the research on Aβ−related cortical thinning and cognitive dysfunction has focused on older CN individuals. Therefore, the manner and extent to which Aβ deposition affects brain structure and cognition in midlife remains relatively unexplored. This is an important knowledge gap because midlife is arguably when AD-related markers of Aβ, brain structure, and cognition are starting to be dynamic. Accordingly, in this study we sought to determine how Aβ accumulation relates to cognitive function and structural changes in AD-relevant brain regions among late-middle-aged adults at risk for AD. We investigated both the main effect of Aβ burden and its potential acceleration of normal age-associated alterations in our brain and cognitive outcome measures.
2. Materials and methods
2.1. Participants
Participants were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) cohort into this neuroimaging study. The WRAP is a longitudinal registry of approximately 1500 late-middle-aged adults who were cognitively healthy and between the ages of 40 and 65 at study entry [18]. One hundred nine consecutive participants were selected on the basis of having either an Aβ− or Aβ+ rating on their PiB-PET scan (see Qualitative PiB Rating section below) and also having a usable MRI scan. They constitute a subset of the individuals described in an earlier report from our group [19]. Mean age of the sample at time of brain scan was 60.72±5.65 and 62.4% were female. The sample was enriched with persons with a parental family history (FH) of AD (74.3%) as well as those positive for the apolipoprotein E ε4 allele (APOE4) (41.3%). The method for determining FH of AD has been described previously [18]. Study exclusion criteria included MRI contraindications, major neurological disorder (e.g., head trauma with loss of consciousness, neoplasms, and seizure disorders), current major psychiatric disease (e.g., schizophrenia), and abnormal MRI findings (e.g., ventriculomegaly). Table 1 summarizes participants’ background characteristics. The University of Wisconsin Institutional Review board approved all study procedures and each subject provided signed informed consent before participation.
Table 1.
Characteristics of study participantsa
| Variable | Aβ−, n=74 | Aβ+, n=35 | p-value |
|---|---|---|---|
| FH positive, % | 70.3 | 82.9 | .160 |
| APOE4 positive, % | 35.1 | 54.3 | .058 |
| Non-Hispanic White, % | 95.8 | 94.3 | .722 |
| Female, % | 55.4 | 77.1 | .029 |
| Age | 59.51 (5.82) | 63.27 (4.35) | <.001 |
| Education | 15.89 (2.37) | 16.57 (2.34) | .164 |
| MMSE | 29.15 (1.05) | 29.17 (1.34) | .945 |
| IQCODE | 46.59 (6.28) | 47.66 (5.86) | .405 |
| Interval between brain scan and cognitive assessment, months | 6.58 (6.03) | 6.64 (5.92) | .961 |
All values are mean (SD) except where otherwise indicated.
Aβ=amyloid-β; FH=family history of Alzheimer’s disease; APOE4=the varepsilon 4 allele of the apolipoprotein E gene; MMSE=Mini-Mental State Exam; IQCODE=Informant Questionnaire on Cognitive Decline in the Elderly.
2.2. Neuroimaging protocol
2.2.1. PET-PiB protocol
PiB data were acquired with a 70 minute dynamic acquisition followed by reconstruction using a filtered back-projection algorithm (DIFT). Data were corrected for random events, attenuation of annihilation radiation, deadtime, scanner normalization, and scatter radiation, then realigned and coregistered using SPM 8 (www.fil.ion.ucl.ac.uk/spm). Finally, the images were transformed into voxel-wise distribution volume ratio (DVR) maps of PiB binding using the time activity curve of cerebellar gray matter (GM) as the reference region. More detailed descriptions of PiB radiochemical synthesis, PiB-PET scanning, and DVR map generation may be found in a previous publication [19].
2.2.2. Qualitative PiB rating
To enhance potential clinical applicability and allow for the possibility of regional heterogeneity in Aβ deposition within this age range when Aβ burden may only be emerging, a qualitative approach was adopted for ascertaining cerebral amyloidosis. Specifically, after achieving high inter- and intra-reliability between two raters on a subset of PiB images (blinded to all pertinent subject characteristics such as age, sex, FH, APOE status, and cognitive function), a similarly-blinded single rater visually rated all DVR maps on the intensity and pattern of cortical amyloid binding as described previously [19]. Of the 109 subjects who provided data for our analyses, 74 were classified as amyloid negative (Aβ−) and 35 were classified as amyloid positive (Aβ+). An Aβ− rating was given when the scan demonstrates no cortical amyloid burden or only non-significant patchy/diffuse cortical GM binding not resembling an AD pattern. In contrast, an Aβ+ classification indicated that there was unambiguous GM amyloid binding in at least three cortical lobes resembling an AD disease pattern.
For descriptive purposes (and to perhaps provide some basis for comparison with studies that employ quantitative cut-points for Aβ positivity) we fitted a receiver operating characteristic curve with DVR data sampled bilaterally from the precuneus—a known inception site for Aβ aggregation [1, 20]—as the predictor and Aβ rating as the outcome. This analysis revealed an area under the ROC curve (95% CI) of .993 (.979, 1.00) for discriminating Aβ− and Aβ+ participants. A DVR cut-point of 1.10 yielded a sensitivity of .971 and a specificity of .973 for distinguishing both groups.
2.2.3. MRI protocol
The MRI scans were acquired in the axial plane on a GE x750 3.0T scanner with an eight channel phased array head coil (General Electric, Waukesha, WI). 3D T1-weighted inversion recovery-prepared SPGR scans were collected using the following parameters: TI/TE/TR=450ms/3.2ms/8.2ms, flip angle=12°, slice thickness=1mm no gap, FOV=256, matrix size=256×256×156.
2.2.4. Automated MRI image analysis
Thickness of cortical structures and volume of subcortical structures were obtained for select regions of interest (ROIs) using the FreeSurfer image analysis suite version 5.1.0 (http://surfer.nmr.mgh.harvard.edu/) [8, 9]. Briefly, the T1 MRI images were skull stripped and transformed into Talairach space. Then, using a standard atlas, the images are segmented, and volumes of subcortical regions, e.g., the hippocampus and amygdala, were obtained via this segmentation. Next, cortical surface meshes were created, defined as the gray/white matter boundary (the white matter surface) and the gray/CSF boundary (the pial surface). After topology correction and deformation of the surface meshes, the surfaces were parcellated based on a template atlas. Cortical thickness measurements were obtained by calculating the distance along a normal vector from each vertex in the white matter surface to the pial surface. The thickness values at each vertex within an ROI were averaged to obtain the ROI measurements. We focused on eight medial temporal and posteromedial cortex ROIs in this study. These ROIs were chosen because of their involvement in the AD cascade. Cortical thickness measurements were obtained from the entorhinal cortex, parahippocampal gyrus, fusiform gyrus, cingulate isthmus, posterior cingulate, and the precuneus whereas volumetric measurements were obtained from the hippocampus and amygdala. Measurements were averaged across hemispheres to obtain a single value for each ROI.
While the FreeSurfer automated procedure is 100% reproducible, user inspection and iterative control point editing is often required for maximal performance accuracy, and thus was implemented in this study in order to ensure proper cortical reconstruction. In our hands, intra- and inter-rater reliability is excellent (ICC > .99), based on a training sample of 10 brains of varying age and scan quality, rated by three technicians twice in blind fashion.
2.3. Neuropsychological assessment
All 109 participants completed a comprehensive neuropsychological battery [18] that included the Mini-Mental State Exam (MMSE) and other psychometric measures that span traditional cognitive domains of memory, attention, executive function, language, and visuospatial ability. Earlier factor analytic studies of the psychometric measures within the larger WRAP cohort [21, 22] showed that these tests map onto 6 cognitive factors: Immediate Memory, Verbal Learning & Memory, Working Memory, Speed & Flexibility, Visuospatial Ability, and Verbal Ability. The individual tests which loaded onto these factors were as follows: Immediate Memory: Rey Auditory Verbal Learning Test (RAVLT) Trials 1 and 2 [23]; Verbal Learning & Memory: RAVLT Trials 3–5 and Delayed Recall Trial [23]; Working Memory: Digit Span and Letter-Numbering Sequencing subtests from the Wechsler Adult Intelligence Scale–3rd edition [24]; Speed & Flexibility: Stroop Test interference trial [25], and Trail Making Test A and B [26]; Visuospatial Ability: Block Design and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI) [27] and Benton Judgment of Line Orientation [28]; Verbal Ability: Vocabulary and Similarities subtests from the WASI [27], Boston Naming Test [29], and Reading subtest from the Wide-Range Achievement Test, 3rd edition [30]. These factor scores were used in our evaluation of the association between Aβ retention and cognition in this study. The mean interval between brain scan and neuropsychological assessment was 6.60±5.97 months.
2.4. Statistical analyses
Group differences on background characteristics were analyzed using either t-tests or Chi-square tests as appropriate. We utilized age- and sex-adjusted ANCOVAs to investigate the main effect of amyloid burden (Aβ+ vs. Aβ−) on ROI measures of brain structure. For the hippocampus and amygdala, an additional adjustment was made for intracranial volume. To investigate whether Aβ induces an acceleration of age-associated structural brain changes, we fitted a series of multiple regression models that controlled for age and sex, while testing for interactions between age and Aβ rating. Where significant, these interactions would indicate a differential effect of aging on brain structure among Aβ+ subjects compared with Aβ− subjects.
Analyses of the relationship between amyloid burden and cognitive function were conducted in the same manner as those for brain structure. That is, age-, sex-, and education-adjusted ANCOVAs were utilized to investigate the main effect of amyloid burden (i.e., Aβ+ vs. Aβ−) on the cognitive factors whereas age-, sex-, and education-adjusted multiple regression models, that included age*Aβ rating interactions, were used to investigate whether age-related cognitive decline is more pronounced among Aβ+ individuals. All analyses were performed using SPSS 21.0 (IBM Corp., Armonk, NY), and only findings that met an alpha threshold of .05 (2-tailed) were deemed significant.
3. Results
3.1. Subject characteristics
The Aβ− and Aβ+ groups differed in age and sex, and these variables were included as covariates in our analyses. As expected, there was a tendency for APOE4+ persons to be disproportionally represented within the Aβ+ group, though this was nonsignificant. The groups did not differ in any other background characteristics, including the time interval between brain and cognitive assessment. These findings are shown in Table 1.
3.2. Aβ and brain structure
Table 2 summarizes our analyses of the main effect of Aβ burden on brain structure. The Aβ+ group exhibited significant cortical thinning in the entorhinal cortex compared with the Aβ− group. There were no group differences in any other ROIs examined.
Table 2.
| Region | Aβ−, n=74 | Aβ+, n=35 | p-value |
|---|---|---|---|
| Entorhinal | 3.47 (0.03) | 3.32 (0.05) | .017 |
| Parahippocampal | 2.65 (0.03) | 2.71 (0.04) | .266 |
| Fusiform | 2.62 (0.02) | 2.62 (0.02) | .988 |
| Cingulate isthmus | 2.49 (0.02) | 2.51 (0.03) | .615 |
| Posterior cingulate | 2.63 (0.02) | 2.58 (0.03) | .175 |
| Precuneus | 2.41 (0.01) | 2.37 (0.02) | .128 |
| Hippocampus | 3955.33 (44.74) | 3871.82 (66.03) | .315 |
| Amygdala | 1649.71 (25.20) | 1596.95 (37.19) | .260 |
All values are estimated mean (SE), adjusted for age and sex.
All values are thicknesses (in mm) except for hippocampus and amygdala, which are volumes in mm3.
Aβ=amyloid-β
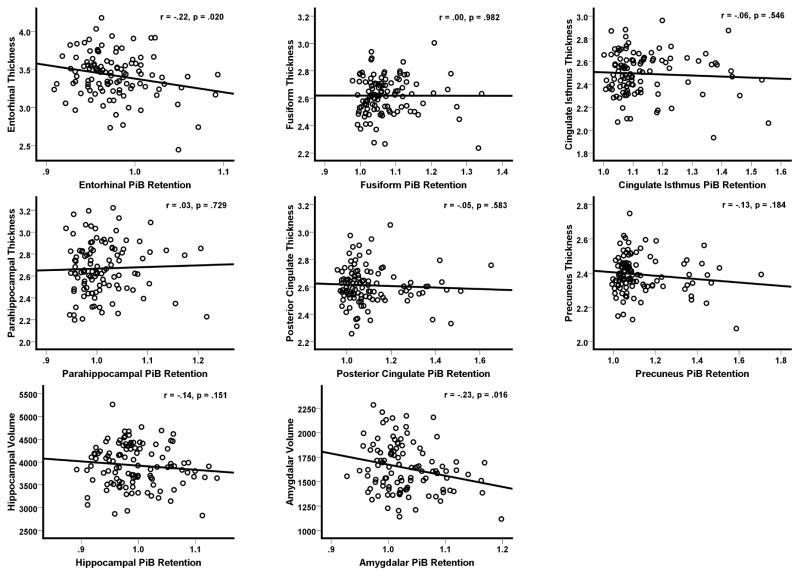
As a secondary analysis, we examined whether local amyloid burden is correlated with co-localized measures of brain structure using Pearson correlations. For this, FreeSurfer was used to extract mean PiB retention values within each of the eight ROIs by co-registering each participant’s DVR image to their FreeSurfer-rendered T1 volume, and then using FreeSurfer’s APARC+ASEG template as a mask for extracting mean PiB values in the ROIs. Correlations were conducted using bilateral measures obtained by averaging ROI values across hemispheres. Consistent with the group analyses shown in Table 2, we found that higher entorhinal cortex Aβ accumulation was correlated with decreased entorhinal cortical thickness (r (107)=−.22, p=.020). We also found a significant association between increased amygdala Aβ burden and decreased amygdala volume (r (107)=−.23, p=.016). Although the remaining correlations were all in the expected negative direction (i.e., higher Aβ being associated with reduced thickness/volume), they failed to meet our threshold for statistical significance (p’s > .1). These results are plotted in Figure 1.
Figure 1. Correlations between Aβ burden and co-localized MRI measures.
The plots depict correlations between regional cortical thickness and PiB binding extracted from the same brain region. PiB= Pittsburgh Compound B.
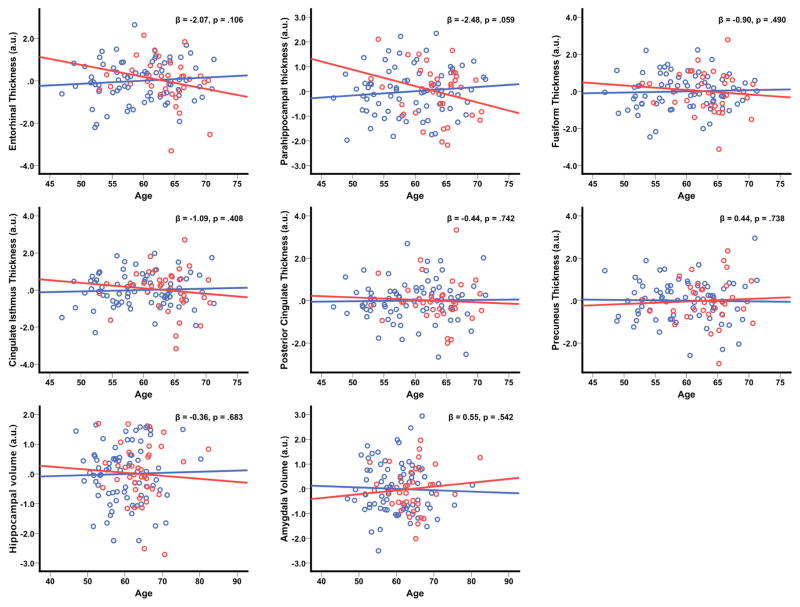
Our analyses of the impact of Aβ burden on age-related structural changes revealed a trend for the Aβ+ group to have more pronounced age-associated thinning of the parahippocampal gyrus compared with the Aβ− group (age*Aβ rating p=.059). Although none of the other ROIs were significant (age*Aβ rating p’s > .1), the beta coefficient for the age*Aβ rating term was negative in virtually all analyses, indicating a similar acceleration of age-associated thinning within the Aβ+ group. These findings are plotted in Figure 2.
Figure 2. Aβ burden accelerates age-related brain structural changes.
The plots depict predicted values derived from the regression equation (circles), with the line of best linear fit overlaid. Aβ=amyloid-β; red circles/line=Aβ+ group, blue circles/line=Aβ− group.
3.3. Aβ and cognition
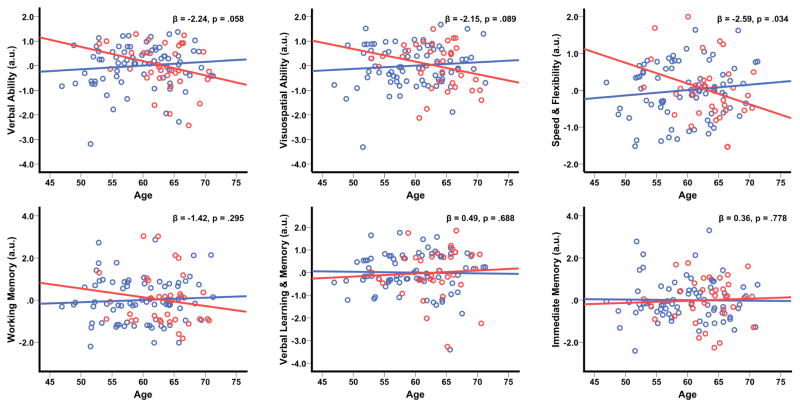
Results of the main effect of Aβ on cognition are summarized in Table 3. The Aβ+ group exhibited numerically lower scores on all six cognitive factors relative to the Aβ− group. However, these differences did not meet our criterion for statistical significance (p’s > .1). With respect to Aβ−induced acceleration of age-related cognitive decline, we found that Aβ+ participants demonstrated significantly greater age-associated cognitive decline on Speed & Flexibility (age*Aβ rating p=.034), and also showed trends for a faster rate of age-associated cognitive decline on Verbal Ability and Visuospatial Ability (age*Aβ rating p’s=.058 and .089 respectively). These results are shown in Figure 3.
Table 3.
Main effect of Aβ on Cognitiona
| Cognitive domain | Aβ−, n=74 | Aβ+, n=35 | p-value |
|---|---|---|---|
| Verbal Ability | 0.15 (0.11) | −0.05 (0.16) | .330 |
| Visuospatial Ability | 0.35 (0.11) | 0.11 (0.16) | .229 |
| Speed & Flexibility | 0.07 (0.09) | −0.16 (0.14) | .193 |
| Working Memory | 0.17 (0.14) | −0.11 (0.21) | .282 |
| Verbal Learning & Memory | −0.15 (.12) | −0.17 (0.18) | .938 |
| Immediate Memory | −0.14 (0.12) | −0.17 (0.19) | .905 |
All values are estimated mean (SE), adjusted for age, sex, and education.
Aβ=amyloid-β
Figure 3. Aβ burden accelerates age-related cognitive decline.
The plots depict predicted values derived from the regression equation (circles), with the line of best linear fit overlaid. Aβ=amyloid-β; red circles/line=Aβ+ group, blue circles/line=Aβ− group.
4. Discussion
In this study, we examined the relationship between Aβ burden, structural brain changes, and cognitive function among CN, late-middle-aged adults at risk for AD. We found that global Aβ burden was associated with cortical thinning in the entorhinal cortex. Thickness of the entorhinal cortex was also negatively correlated with co-localized PiB retention, further strengthening the evidence for the impact of Aβ burden on the structural integrity of the entorhinal cortex. In addition to this main effect of Aβ accumulation on brain structure, we also observed Aβ−induced acceleration of age-related structural change, especially within the parahippocampal gyrus. While Aβ accumulation did not exert a similar main effect on cognition as it did on brain structure within this overall cognitively-normal cohort, we found evidence that it was associated with faster age-related decline in cognitive performance, particularly in the domain of Speed & Flexibility.
The observed Aβ−induced alteration in the entorhinal cortex—detected in both global Aβ analyses and co-localized PiB/MRI analyses—is consistent with other studies revealing loss of cortical mass in the entorhinal region, as a result of amyloid aggregation in the elderly [11, 31]. The entorhinal cortex, which is a key afferent to the hippocampus, has been evidenced as a crucial center of AD pathology and one of the earliest areas to demonstrate degeneration [32]. Furthermore, volume of the entorhinal cortex is a reliable indicator of future decline from mild cognitive impairment to AD [33, 34] and, when tested in CN samples, is able to better predict future cognitive decline than structural changes in other medial temporal regions, including the hippocampus [34, 35].
Our correlational analyses of co-localized Aβ accumulation and brain structure also elicited an association between increased amyloid and decreased amygdala volume, which was not evident in our group analyses. The amygdala is a mesial temporal structure observed to decrease in mass early in disease progression, and further exhibits prominent volumetric decrease during the latter stages of AD [36]. Amygdala volume is a viable predictor of both future decline and the severity of decline in early and mild AD, and may be a better predictor than volume of the hippocampus [36–41]. In studies of AD individuals, the amygdala has been found to correlate with performance in the domains of memory, orientation, and recall [38, 39]. Similar results have been found in studies of CN individuals [42], with decreased amygdala volume consistently associated with more advanced cognitive decline. It is noteworthy that, although statistical significance in tests of the effect of co-localized Aβ on brain structure was only reached for the entorhinal cortex and amygdala, a general trend for increased Aβ to track with decreased brain structural integrity was observable across all of the ROIs examined. This suggests a rather pervasive effect of Aβ burden on brain morphometry, though this interpretation needs to be made with caution given the rather modest magnitude of the observed correlations.
Our findings also suggest that Aβ may accelerate the age-related loss of brain tissue in the parahippocampal gyrus. This is interesting because the parahippocampal gyrus encompasses the entorhinal cortex and, like the entorhinal cortex, is an early induction site for AD-related neurodegeneration [43]. Thus, our findings with respect to Aβ’s main effect on cortical thickness and its acceleration of age-related cortical thinning are quite convergent, jointly corroborating prior neuropathological studies that have shown the parahippocampal-entorhinal strip to be highly susceptible to the AD degenerative cascade [32, 43–46]. Similar to our co-localized PiB/MRI analyses, although only the parahippocampal gyrus neared statistical significance in our interrogation of potential Aβ−induced acceleration of age-related cortical thinning, there was a general tendency for increased Aβ to be associated with faster age-related cortical thinning across the other ROIs examined. This supports the notion that even ostensibly mild Aβ aggregation might not be innocuous, perhaps becoming more pernicious with the passage of time [47].
Understanding the linkage between Aβ and cognition is of great importance to the study of AD [48]. The evidence for an association between Aβ accumulation and cognition in CN cohorts is, however, mixed. Several cross-sectional studies in CN individuals have found no evidence of a relationship between Aβ accumulation and cognition [13–15], but some do find an association, primarily within the domain of episodic memory [16, 17]. A meta-analysis of studies investigating Aβ−cognition effects in CN individuals [49] suggested that while there is a modest association between amyloid accumulation and cognition, effect sizes in areas other than episodic memory and global cognition are small. Chetelat and colleagues [47] suggested that the variations in the observed effect of Aβ on cognition may be due to differences in the underlying characteristics of the study samples, especially in age and APOE4 status. In the present study, we found that, despite the apparent Aβ−induced degeneration of the entorhinal cortex and amygdala—two structures critical to episodic memory—there were no statistically significant main effect of Aβ on memory or other cognitive functions even though the Aβ+ group consistently exhibited lower cognitive test scores than the Aβ− group. Still, the effects of Aβ on cognition were not inconsequential, as we discovered an enhancing effect of Aβ on age-related cognitive decline, particularly in Speed & Flexibility, which is a cognitive function that is highly vulnerable to aging effects [50]. Figure 3 demonstrates that, in four of the six cognitive domains tested, Aβ+ individuals exhibit a marked decline in cognitive performance with increasing age, whereas Aβ− individuals do not exhibit such a decline. This acceleration of “normal” age-related changes might represent an important approach to the study of Aβ’s effect on cognition, especially in a relatively young cohort that is only starting to accumulate Aβ.
A key limitation of this study is its cross-sectional nature. Although we used statistical tests of interactions between age and Aβ rating to approximate the longitudinal effects of Aβ burden on brain structure and cognition, a truly prospective design with serial imaging and cognitive data would have enabled us to better track the progression of structural and cognitive changes among individuals with versus without Aβ burden. As the WRAP cohort is ongoing, and additional imaging and cognitive data are being collected, we will be uniquely positioned to investigate such questions in the future. Secondly, we determined cerebral amyloidosis via qualitative ratings. While this approach has potential clinical value (patients are likely to be more interested in whether their PiB scans are indicative of AD versus whether their PiB binding is 1.2) and has established precedence in the literature (for an excellent review, see Chetelat et al. [47]), there is a possibility that we might have made different observations had we used a quantitative approach such as by averaging PiB uptake in select ROIs. Similarly, although our use of an ROI approach to investigate the effect of Aβ burden on brain structure made our inquiry focused and hypothesis-driven, it may have resulted in a failure to detect findings that were outside of the examine ROIs. In addition, although FreeSurfer has been shown to yield segmentations that are reliable and concordant with manual measurements [51], we cannot entirely exclude the possibility that there might have been some variation in its performance across subjects within our sample. Finally, although our analyses overall showed that Aβ has a measurable, detrimental impact on brain structure and cognitive function, many of the tests did not attain statistical significance at the set threshold (i.e., p=.05). This might reflect inadequate power on our part to statistically detect these changes that are, arguably, only starting to occur and thus relatively subtle in magnitude. Future studies with larger sample sizes would be helpful in further testing this study’s objectives.
In summary, this study found that increased Aβ burden is associated with deleterious changes in brain morphometry and cognition in a late-middle-aged, CN cohort with risk factors for AD. These findings, and those from other groups [7, 10, 31, 34], provide evidence that the temporal window between Aβ deposition and the inception of neurodegenerative changes might be narrower than currently thought [1-3]. Further longitudinal analyses will help us better understand the prognostic value of Aβ deposition within this relatively young and risk-enriched cohort.
Acknowledgments
This research was supported by NIA grants K23 AG045957 (OCO), R01 AG021155 (SCJ), R01 AG027161 (MAS), P50 AG033514 (SA), and P50 AG033514-S1 (OCO); by a Veterans Administration Merit Review Grant I01CX000165 (SCJ); and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by Wisconsin Alumni Research Foundation, the Helen Bader Foundation, Northwestern Mutual Foundation, Extendicare Foundation, and from the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI. We gratefully acknowledge the assistance of Dustin Wooten, PhD, Ansel Hillmer, MSc, and Andrew Higgins with PET data production and processing; and Caitlin A. Cleary, BSc, Sandra Harding, MS, Jennifer Bond, BA, and the WRAP psychometrists with study data collection. In addition, we would like to acknowledge the support of researchers and staff at the Waisman Center, University of Wisconsin–Madison, where the brain scans took place. Finally, we thank participants in the Wisconsin Registry for Alzheimer’s Prevention for their continued dedication.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet neurology. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet neurology. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England journal of medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, et al. Brain beta-amyloid load approaches a plateau. Neurology. 2013;80:890–6. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 7.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 9.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 10.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Annals of neurology. 2011;69:1032–42. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, et al. Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA neurology. 2013;70:903–11. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 12.Fortea J, Sala-Llonch R, Bartres-Faz D, Llado A, Sole-Padulles C, Bosch B, et al. Cognitively preserved subjects with transitional cerebrospinal fluid ss-amyloid 1-42 values have thicker cortex in Alzheimer’s disease vulnerable areas. Biological psychiatry. 2011;70:183–90. doi: 10.1016/j.biopsych.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5553–63. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, et al. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA neurology. 2013;70:488–95. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain : a journal of neurology. 2007;130:2837–44. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 17.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain : a journal of neurology. 2009;132:1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Journal of geriatric psychiatry and neurology. 2005;18:245–9. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 19.Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiology of aging. 2014;35:576–84. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology. 2010;24:742–56. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koscik RL, La Rue A, Jonaitis E, Okonkwo OC, Johnson SC, Bendlin BB, et al. Emergence of mild cognitive impairment in late-middle-aged adults in the Wisconsin Registry for Alzheimer’s Prevention. Dement Geriatr Cogn Disord. 2014 doi: 10.1159/000355682. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M. Rey auditory verbal learning test: a handbook. Torrance, CA: Western Psychological Services; 1996. [Google Scholar]
- 24.Wechsler D. WAIS-III: Wechsler adult intelligence scale. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 25.Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop neuropsychological screening test manual. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- 26.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 27.Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 28.Benton AL. Neuropsychological assessment. Annual Review of Psychology. 1994;45:1–23. doi: 10.1146/annurev.ps.45.020194.000245. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EF, Goodglass H, Weintraub S. The Boston naming test. 2. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 30.Wilkinson GS. Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range Incorporated; 1993. [Google Scholar]
- 31.Whitwell JL, Tosakulwong N, Weigand SD, Senjem ML, Lowe VJ, Gunter JL, et al. Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? NeuroImage Clinical. 2013;2:249–57. doi: 10.1016/j.nicl.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Annals of neurology. 2000;47:430–9. [PubMed] [Google Scholar]
- 34.Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiology of aging. 2001;22:747–54. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 35.Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–96. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 36.Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC Alzheimer’s Disease Neuroimaging I. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry research. 2011;194:7–13. doi: 10.1016/j.pscychresns.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehericy S, Baulac M, Chiras J, Pierot L, Martin N, Pillon B, et al. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR American journal of neuroradiology. 1994;15:929–37. [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuno K, Wakai M, Takeda A, Sobue G. Medial temporal atrophy and memory impairment in early stage of Alzheimer’s disease: an MRI volumetric and memory assessment study. Journal of the neurological sciences. 2000;173:18–24. doi: 10.1016/s0022-510x(99)00289-0. [DOI] [PubMed] [Google Scholar]
- 39.Basso M, Yang J, Warren L, MacAvoy MG, Varma P, Bronen RA, et al. Volumetry of amygdala and hippocampus and memory performance in Alzheimer’s disease. Psychiatry research. 2006;146:251–61. doi: 10.1016/j.pscychresns.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Farrow TF, Thiyagesh SN, Wilkinson ID, Parks RW, Ingram L, Woodruff PW. Fronto-temporal-lobe atrophy in early-stage Alzheimer’s disease identified using an improved detection methodology. Psychiatry research. 2007;155:11–9. doi: 10.1016/j.pscychresns.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Cavedo E, Boccardi M, Ganzola R, Canu E, Beltramello A, Caltagirone C, et al. Local amygdala structural differences with 3T MRI in patients with Alzheimer disease. Neurology. 2011;76:727–33. doi: 10.1212/WNL.0b013e31820d62d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, et al. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dementia and geriatric cognitive disorders. 2010;29:75–81. doi: 10.1159/000264630. [DOI] [PubMed] [Google Scholar]
- 43.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiology of aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. discussion 78–84. [DOI] [PubMed] [Google Scholar]
- 44.Garcia Gil ML, Moran MA, Gomez-Ramos P. Ubiquitinated granular structures and initial neurofibrillary changes in the human brain. Journal of the neurological sciences. 2001;192:27–34. doi: 10.1016/s0022-510x(01)00587-1. [DOI] [PubMed] [Google Scholar]
- 45.Duyckaerts C, Colle MA, Dessi F, Piette F, Hauw JJ. Progression of Alzheimer histopathological changes. Acta neurologica Belgica. 1998;98:180–5. [PubMed] [Google Scholar]
- 46.Bancher C, Jellinger KA. Neurofibrillary tangle predominant form of senile dementia of Alzheimer type: a rare subtype in very old subjects. Acta neuropathologica. 1994;88:565–70. doi: 10.1007/BF00296494. [DOI] [PubMed] [Google Scholar]
- 47.Chetelat G, La Joie R, Villain N, Perrotin A, de La Sayette V, Eustache F, et al. Amyloid imaging in cognitively normal individuals, at–risk populations and preclinical Alzheimer’s disease. NeuroImage Clinical. 2013;2:356–65. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampel H. Amyloid-β and Cognition in Aging and Alzheimer’s Disease: Molecular and Neurophysiological Mechanisms. Journal of Alzheimer’s Disease. 2013;33:S79–S86. doi: 10.3233/JAD-2012-129003. [DOI] [PubMed] [Google Scholar]
- 49.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–8. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salthouse TA. Aging and measures of processing speed. Biological psychology. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 51.Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–66. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]