Abstract
Although persons with dementia (PWD) and their family caregivers need in-home support for common neuropsychiatric symptoms (NPS), few if any assistive technologies are available to help manage NPS. This implementation study tested the feasibility and adoption of a touch screen technology, the Companion, that delivers psychosocial, nondrug interventions to PWD in their home to address individual NPS and needs. Interventions were personalized and delivered in-home for a minimum of 3 weeks. Post-intervention measures indicated the technology was easy to use, significantly facilitated meaningful and positive engagement, and simplified caregivers’ daily lives. Although intervention goals were met, caregivers had high expectations of their loved-one’s ability to regain independence. Care recipients used the system independently, but were limited by cognitive and physical impairments. We conclude the Companion can help manage NPS and offer caregiver respite at home. These data provide important guidance for design and deployment of care technology for the home.
Keywords: Dementia, nondrug Interventions, Assistive Technology, Aging in Place, caregiver burden, neuropsychiatric symptoms
INTRODUCTION
Dementia and Alzheimer’s disease, the most common form of dementia, are increasingly recognized as a public health crisis as they are difficult, costly diseases to manage and a cure is not imminent.1,2 The disappointing success of drug therapies3 has underscored the need for disease management strategies that include nonpharmacological interventions and support for patients and their caregivers in daily life, especially in the home. Over 70% of American seniors live in private homes,4 which is where many prefer to be and stay.5 Seniors fear loss of independence more than death and independence is a major factor in quality of life in persons with dementia (PWD).6,7
Managing the care of PWD while optimizing their quality of life is challenging, especially for family caregivers. These caregivers often have fulltime jobs yet spend many hours per week caring for a loved one at home.8–10 They provide help with instrumental activities of daily living (IADL) such as housekeeping and preparing meals, and with basic ADL such as bathing and toileting. Caregivers of PWD additionally have to cope with a host of neuropsychological symptoms such as apathy, anxiety, and agitation that are common across the dementia spectrum and stressful to handle.3,9,11–13 Assistive technologies could fill an important need in the support gap if they promoted independence, positive mood and behaviors, and quality of life without adding to caregiver burden.
PWD and their caregivers have indicated they need, but are lacking, support in four main areas: 1) symptoms of dementia such as loss of memory, 2) activities of daily living, 3) social contact and company, and 4) health monitoring and safety.14 Most information and communication technology (ICT) efforts have focused on monitoring and surveillance, which mainly enhance caregiver feelings of safety and security.15–17 ICT support for memory deficits and ADL comes primarily from electronic aids that facilitate the organization and execution of everyday tasks.16,17 These aids can improve prospective memory, i.e., remembering to do something in the future, including in PWD.16,18
Technology support for neuropsychiatric symptoms (NPS) is virtually disregarded as yet.16 NPS are not uncommonly treated with psychotropic drugs despite regulations to the contrary and questionable efficacy and safety of such drug interventions.12,19,20 Nonpharmacological approaches are based on theories that NPS stem from a combination of unmet needs (boredom, loneliness, sensory deprivation), maladaptive behavior reinforcement (tending to problem behavior only), and low stress thresholds that render PWD vulnerable.13 Common psychosocial treatments, therefore, include sensory interventions such as music, social contact such as real or simulated presence using photos and videos, and orientation to place and time using cues and reminders.13 Reminiscence, additionally, can reduce boredom, stimulate conversation, and preserve personal identity by remembering past events and experiences with the aid of artifacts such as personal photos or objects.21–23 Psychosocial interventions are associated with improved NPS and quality of life in PWD and caregivers,12,13,22–24 but traditionally depend on human interaction. This is a limiting and often unsustainable factor in an already burdened demographic.
The Companion (SimpleC, LLC, Atlanta, GA, USA) is a touch screen computer (no keyboard, no mouse) that was developed to deliver psychosocial interventions to PWD in their living environment using rich audiovisual programs (‘shows’) that combine images, music and messages from trusted individuals that are relevant and pleasing to the care recipient and thus engages him/her meaningfully and positively. Shows aim to address NPS that are barriers to independence and well-being. Reminiscence and simulated presence are provided through personal photos and videos, and by using the voices of loved-ones to make announcements or recount personal stories. The Companion cues and primes for important activities and routines through explicit verbal and timed reminders (e.g., “Time to drink some water”) followed by images that are congruent with the expected behavior (e.g., people drinking a glass of water). The reminders are an important source of orientation to time, which is also provided by a clock, date and day of the week displayed on the home screen that comes on when no show play. Preferred music accompanies each show unless requested otherwise. The Companion was developed in-field working with PWD and their formal and informal caregivers, predominantly in assisted living and memory care.
The goal of this study was to test the usability, feasibility and adoption of the Companion in a home- and community-based setting (i.e., private homes as opposed to formal care communities) in a small sample of dyads. A Companion was personalized for each of seven households and subsequently we determined barriers and facilitators to use and usefulness to PWD and their primary, family caregivers through structured observations, interviews and assessments. Participants used the system for at least three weeks.
METHODS
Sample
Given the focus on feasibility and usability, this study recruited a small sample (n= 7) that is considered adequate in usability and engineering psychology studies to capture the main barriers and facilitators to use of a system and its interface in order to properly inform redesign.25 Caregiver/care recipient dyads were recruited from a network of organizations and institutions, such as retirement communities with an independent living campus, the Georgia Chapter of the Alzheimer’s Association, adult day centers and a senior services center in one local county. They were identified from referrals and in response to recruitment flyers or face-to-face group presentations. All participating dyads received verbal and written communication about the details of the study, and written informed consent to participate was obtained from all participants. Ability to sign informed consent was assessed in care recipients26 and if unable to sign consent, care recipients signed an assent form.
Inclusion criteria were formally assessed during an in-home visit (next section) and were: living independently in a private home or independent living cottage or apartment containing at least 3 rooms separated by walls and doors; cohabitating couples or caregiving dyads; a dementia diagnosis or assistive need in care recipients; mild to moderate caregiver distress, and limited outside assistance from formal caregivers or otherwise. Care recipients had to be at least 50 years old; caregivers at least 21 yrs old. Exclusion criteria were: a care recipient Mini Mental State Examination (MMSE) score below 10; a dementia diagnosis in caregivers; severe caregiver distress; comorbid medical or psychiatric conditions in either the care recipient or caregiver that would prevent or impede completion of assessments or implementation of the trial; evidence or a suspicion of alcohol or physical abuse.
Screening & Baseline Assessments
Preliminary eligibility criteria were determined in a screening phone interview with caregivers that assessed candidates’ ages, living arrangements, support structures, comfort level with technology, medical diagnoses and conditions, the presence of neuropsychiatric symptoms and/or difficulties with activities of daily living in care recipients, and caregiver distress. Caregivers gave verbal informed consent to assess these variables. If participants seemed eligible, an in-home visit was scheduled to formally assess inclusion criteria. Baseline assessments were initiated after written informed consent documents were signed and included the following standardized measures: MMSE, standard version,27 to assess the presence and extent of cognitive impairments in care recipients; Barthel index28 to assess care recipient’s ability to complete ADL; Lawton scale29 to assess the presence and extent of difficulties with instrumental ADL (IADL); Cornell Scale for Depression in Dementia (CSDD)30 to assess the presence of major depressive disorder in care recipients; Neuropsychiatric Inventory (NPI), brief version,31,32 to assess the presence and severity of 12 neuropsychiatric symptoms and the associated caregiver distress; Caregiver Strain Index (CSI), modified,33 to evaluate objective aspects of caregiver burden; Zarit Caregiver Burden Scale34 to assess subjective aspects of caregiver burden. The MMSE was completed in private with the care recipient. All other assessments were completed based on caregiver report, typically in private or private conversation (NPI, CSDD) with the responsible investigator (R.K.).
Life Story and Care Needs Interview
In order to personalize the Companion for individual households and care recipients, a second home visit was scheduled to complete a life story interview preferably with both the caregiver and care recipient present, as well as a care needs interview with the caregiver, preferably in a private conversation with the investigator.
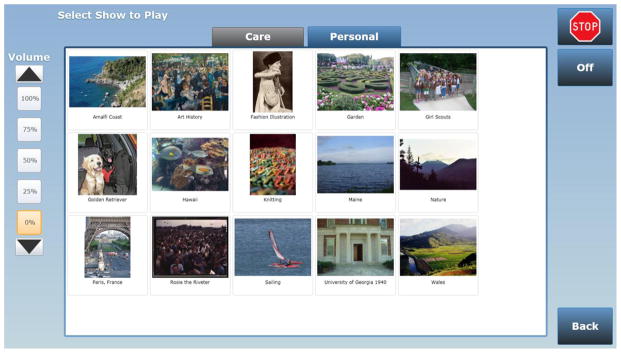
The life story interview aims to identify positive life events, memories, interests and circumstances that can support meaningful and positive individual engagement. It identifies topics and content conducive to reminiscing and personal wellness, including food preferences and important routines. This interview forms the basis for the Companion’s reminiscing feature or “Life Story menu” (Figure 1) consisting of shows on topics that speak to the individual and can be enjoyed by care recipients in isolation or together with others. Personal photos and music are used if available. The life story interview was developed by SimpleC based on reminiscence and dementia care best practices and our experience of working with older adults with dementia.
Figure 1.
Example (screenshot) of SimpleC Companion Life Story menu.
The care needs interview aims to identify the most pressing symptoms or needs in a care recipient or household and covers a wide range of challenges common in dementia and late life, including behavior and mood issues (20 symptoms), sleep, eating and hydration issues (10 symptoms), difficulties with communication and participation in social events and activities (12 symptoms), and difficulties with ADL (9 symptoms). The needs interview was developed by SimpleC and is partially based on formal assessments of neuropsychiatric symptoms in dementia (e.g., Neuropsychiatric Inventory31,32, Nursing Home Adjustment Checklist35) and part on years of experience working with older adults with dementia and their caregivers. In this study, baseline measures helped inform which symptoms were present and most pressing. During the Needs interview, caregivers selected 1 to 4 symptoms to become the target of intervention (“Goal”), and the current status for each target symptom was determined. This served as a reference (‘baseline’) for future status assessments and goal attainment scaling.36 Meaningful Engagement was made a goal for all participants to encourage Companion use and given the need for caregiver respite.
Creating Companion shows and menus
Based on the Life Story and Care Needs interviews, a menu of Life Story and Care shows was created and compiled by trained care specialist using proprietary methods and ICT systems. Depending on the available media, shows included personal photos and music, personal voice recordings and messages, or ‘generic’ photos and music from our media library that do not belong to individual users but nevertheless are relevant to their life story of care need. If requested, specific times were scheduled for the show(s) to come on automatically as opposed to starting them on-demand by touching the screen. Two investigators (R.K., C.K.) checked and approved the final offering after which the in-home install was scheduled.
Equipment Placement and Installation
While the Companion was personalized, an expert in assistive technology and environmental access visited participants in-home to evaluate the presence of environmental and other barriers in the home that could limit individual access to the technology. Based on this assessment, recommendations for Companion placement in the home were made. During the install, both the caregiver and care recipient were observed using a scripted protocol as they used and saw the Companion for the first time. Participants completed basic operations and tasks to inform the degree of technology usability or ‘user-friendliness’ and to get them acquainted with the system. Based on observations of individual performance, additional feedback and training were provided as necessary to make sure participants had a basic understanding and comfort level operating the Companion before investigators (A.A., R.K.) left the residence. In addition, caregivers were asked to fill out a questionnaire about their expectations of the technology’s usability and usefulness to themselves and care recipients. Question were adopted from Davis (1989) scales of Perceived Ease of use and Perceived Usefulness,37 which have repeatedly shown to predict technology acceptance.38 Caregivers were given a diary to fill out daily during the intervention period. The primary purpose of the diary was to facilitate use of the system, enhance caregiver’s comfort level with the system by having them complete a simple task every other day, and to document personal observations and experiences. Caregivers reported that maintaining the diary was easy and required little time each day to complete.
Follow-up Phone Calls & Diary
Caregivers received a phone call within a few days of installation to make sure the Companion was working properly and nothing prevented participants from using it. The purpose of specific shows was reviewed to remind caregivers of target symptoms and the interventions that had been created accordingly. Subsequently, caregivers were contacted once a week by phone to see how things were going, if they were using the technology, and if they had any questions or concerns.
Final Status, Goal Attainment & Exit Interview
At the end of the intervention period, a final home visit was scheduled to discuss participants’ experiences using a semi-structured interview. Questions were based on the diary and a technology adoption questionnaire that both the caregiver and care recipient filled out post-intervention. Caregiver questions were based on the pre-implementation questionnaire which was put in past tense to assess caregiver experiences and perceptions as they looked back on the intervention period. Answer categories (7 in total) ranged from Very True to Very Untrue. Care recipient questions were adapted with permission from Tiberio and colleagues who assessed attitudes and experiences with a robot in individuals with cognitive impairment.39 Care recipients answered with Yes or No.
To assess goal attainment, caregivers were asked to rate how care recipients were doing with respect to the target symptoms identified at the outset of the study (‘Better’ ‘Stable’, ‘Worse’, ‘Not Applicable’) and whether the current level of functioning, post-intervention, was as expected or not (“Much Less Than Expected”, “Somewhat Less Than Expected”, “As Expected”, “Somewhat More Than Expected”, and Much More Than Expected”).36
RESULTS
A. DESCRIPTIVE DATA
Sample
Twelve dyads were screened between September 2012 and August 2013 and completed baseline assessments. Five dyads (42%) were excluded over the course of the investigation because either the care recipient died (n=2, 17%), the care recipient deteriorated and was transferred to a nursing home (n=1, 8%), the caregiver deteriorated and was hospitalized repeatedly (n=1, 8%), or the spouses could not agree on the focus and goal of the intervention and how to proceed (n=1, 8%).
Included dyads (n=7) were all married couples and either lived in a house (57%) or apartment (43%). Four couples (57%) were living in a continuing care retirement community and three (43%) in private homes. Sample characteristics are shown in Table 1.
Table 1.
Sample Characteristics
| Care Recipients (n= 7) | Caregivers (n= 7) | |
|---|---|---|
| age, yr | 77 (60–88) | 79 (63–86) |
| gender, F/M | 3 (43%)/4 (57%) | 4 (57%), 3 (43%) |
| walking difficulties, Y/N | 5 (71%), 2 (29%) | 0 (0%), 7 (100%) |
| vision difficulties, Y/N | 1 (14%), 6 (86%) | 0 (0%), 7 (100%) |
| hearing difficulties, Y/N | 2 (29%), 5 (71%) | 2 (29%), 5 (71%) |
| fine motor problems, Y/N | 1 (14%), 6 (86%) | 1 (14%), 6 (86%) |
| Psychiatric history, Y/N | 2 (29%), 5 (71%) | 0 (0%), 7 (100%) |
| comorbid conditions, n | 1 (0–5) | 2 (0–6) |
| comorbid conditions | Hypertension (n=4,
57%) Diabetes (n=2, 29%) Congestive Heart Failure (n=1, 14%) High Cholesterol (n=1, 14%) Hydrocephalus (n=1, 14%) Hypothyroidism (n=1, 14%) Open Heart Surgery (n=1, 14%) Urinary Incontinence (n=1, 14%) Vascular Stent (n=1, 14%) |
Hypertension (n=4,
57%) Allergies (n=2, 29%) Urinary Incontinence (n=2, 29%) Diabetes (n=1, 14%) Diverticulitis (n=1, 14%) Enlarged Prostate (n=1, 14%) Gout (n=1, 14%) Heart Arrhythmia (n=1, 14%) High Cholesterol (n=1, 14%) Knee Problems (n=1, 14%) Macular Degeneration (n=1, 14%) |
Data are median (range) or frequency counts (percentage).
Physical Status
Care recipients had fewer reported comorbid conditions than caregivers (Table 1), but medical frailty varied substantially between individuals and couples, and was not formally assessed through medical records. Two care recipients (29%) used a walker inside the home and had limited mobility. Three others (43%) moved slowly and used a cane or walker outside the home. The vast majority of participants wore glasses, but despite this, one care recipient (14%) had blurred and double vision. In both subgroups, two individuals (28%) wore hearing aids. One care recipient (14%) had fine motor problems as reflected in difficulty holding a pen and with writing, and one caregiver (14%) had a numb left hand, but was right-handed. Two care recipients (14%) had a history of depression.
Cognitive Status
All care recipients had completed high school; six (86%) went to college and two (28%) completed advanced degrees. Four care recipients (57%) had received a diagnosis of dementia or mild cognitive impairment (MCI) in recent years, which their MMSE scores reflected (Table 2). Two care recipients (28%) had not received a diagnosis, but their MMSE scores suggested the presence of MCI or moderate dementia. One care recipient (14%) scored in the normal cognitive range but exhibited several NPS, which we considered an assistive need and eligibility for study inclusion. None of the participants had difficulty with the Reading item on the MMSE instructing them to close their eyes (Table 2).
Table 2.
Cognitive Profile of Care Recipients as measured by the Mini-Mental State Examination (MMSE).
| Care Recipient | Dementia or MCI diagnosis? | MMSE sum score | Registration | Orientation to Time | Orientation to Place | Recall | Attention Calculation | Naming | Repetition | Comprehension | Reading | Writing | Drawing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | 12 | |||||||||||
| 2 | No | 25 | |||||||||||
| 3 | No | 27 | |||||||||||
| 4 | Yes | 16 | |||||||||||
| 5 | Yes | 11 | |||||||||||
| 6 | Yes | 22 | |||||||||||
| 7 | No | 16 |
Cells highlighted in grey reflect impaired (imperfect) performance.
Functional Status & Dependence
The median score on the Barthel Index was 90 (range, 30 – 100), suggesting an overall high level of independence in completing ADL. Three care recipients (43%) were fully independent, three (43%) largely independent (sum scores, 70 – 90), whereas one care recipient (14%) was largely dependent on his wife to complete ADL. The most common ADL difficulties observed were getting dressed and getting up or down stairs (both n=3, 43%).
The median score on the Lawton IADL Scale was 1 (range, 0 – 8) for female care recipients and 2 (range, 1 – 5) for male care recipients. One male and one female care recipient were high-functioning and independent, but the majority of care recipients (71%) depended on their caregivers for IADL. The most common challenges, corrected for gender-bias, were shopping (n=5, 71%) and preparing and taking one’s medications (71%).
Neuropsychological Status
Table 3 lists the number and kinds of NPS observed in care recipients per caregiver self-report. The median NPI sum score was 9 (out of 96 maximum), the median number of symptoms reported 3 (out of 12 maximum), and the median distress sum score was 5 (out of 60 maximum). This confirmed that care recipients were experiencing NPS that gave rise to caregiver distress, but not extremely so. The most common symptoms were Agitation, Depression, and Sleep difficulties (n=3, 43%). None of the care recipients exhibited delusions, hallucinations, disinhibition or motor disturbances at the outset of the study.
Table 3.
Neuropsychological Symptoms as measured by the Neuropsychiatric Inventory (NPI) and Cornell Scale of Depression in Dementia (CSDD)
| Care Recipient | Tool | Number of Symptoms (sum score) | Severity sum score* | Distress sum score | Symptoms |
|---|---|---|---|---|---|
| 1 | NPI | 3 (10) | 4 | 6 | Agitation, Anxiety, Nighttime Behaviors |
| CSDD | 0 (0) | 0 | n/a | none | |
| 2 | NPI | 0 (0) | 0 | 0 | none |
| CSDD | 3 (4) | 1 | n/a | Difficulty Falling Asleep, Diurnal Variation of Mood, Multiple Physical Complaints | |
| 3 | NPI | 4 (10) | 4 | 6 | Appetite & Eating, Agitation, Elation or Euphoria, Irritability |
| CSDD | 1 (2) | 1 | n/a | Weight Loss | |
| 4 | NPI | 4 (7) | 5 | 2 | Depression, Anxiety, Irritability, Nighttime Behaviors |
| CSDD | 4 (4) | 0 | n/a | Multiple Awakenings During Sleep, Difficulty Falling Asleep, Multiple Physical Complaints, Irritability | |
| 5 | NPI | 0 (0) | 0 | 0 | none |
| CSDD | 2 (3) | 1 | n/a | Early Awakening, Lacks Energy | |
| 6 | NPI | 3 (9) | 4 | 5 | Depression, Apathy, Agitation |
| CSDD | 3 (5) | 2 | n/a | Irritability, Poor Self Esteem, Sad | |
| 7 | NPI | 4 (11) | 6 | 5 | Depression, Apathy, Nighttime Behaviors, Appetite and Eating |
| CSDD | 7 (7) | 0 | n/a | Anxiety, Sad, Irritability, Multiple Physical Complaints, Loss of Appetite, Diurnal Variation of Mood, Difficulty Falling Asleep |
The severity score reported for CSDD reflects the number of symptoms that were rated as severe, receiving the maximum score (2).
One care recipient (14%) showed evidence of significant depressive symptoms (CSDD sum score over 6, Table 3), but in none was major depressive disorder likely or obvious. The most common depressive symptoms (43%) were Irritability, Multiple Physical Complaints, and Difficulty Falling Asleep. None of the care recipients were suicidal per caregiver report.
Caregiver Distress
Table 4 details the level and source of caregiver distress. The median distress score on the mCSI was 5 (range, 0 – 9) out of 26 maximum. Most distress was generated by the confining nature of caregiving, restricting caregivers’ free time and free movement (n=5, 71%).
Table 4.
Caregiver distress as measured by the modified Caregiver Distress Scale (mCSI) and Zarit Caregver Burden Scale.
| Caregiver | Tool | Sum Score | Sources of Distress |
|---|---|---|---|
| 1 | mCSI | 3 | Caregiving is Confining, Changes Personal Plans, Upset by Changes in Person |
| Zarit | 10 | Angry around Loved-one, Afraid for Future, Strained Around Loved-one, Social Life Suffers, Uncomfortable to Have Friends Over | |
| 2 | mCSI | 0 | n/a |
| Zarit | 0 | n/a | |
| 3 | mCSI | 0 | n/a |
| Zarit | 11 | Loved-one Asks for More than Needs, Embarrassed over Behavior, Afraid for Future, Loved-one Dependent on Me, Should Be Doing More, Could Do a Better Job | |
| 4 | mCSI | 8 | Caregiving is Confining, Family Adjustments, Changes Personal Plans, Emotional Adjustments, Upsetting Behaviors, Upset by Changes in Person, Overwhelmed |
| Zarit | 34 | Loved-one Asks for More than Needs, No time for Self, Angry around Loved-one, Afraid for Future, Loved-one Dependent on Me, Strained around Loved-one, No Privacy, Social Life Suffers, Loved-one Expects me to Take Care of Him, Could do a Better Job | |
| 5 | mCSI | 9 | Sleep is Disturbed, Caregiving is Confining, Family Adjustments, Changes in Personal Plans, Upset by Changes in Person, Overwhelmed |
| Zarit | 21 | No time for Self, Afraid for Future, Loved-one Dependent on Me, Social Life Suffers, Loved-one Expects me to Take Care of Her, Lost Control Life | |
| 6 | mCSI | 5 | Physical Strain, Caregiving is Confining, Family Adjustments, Changes in Personal Plans, Time Demands |
| Zarit | 30 | Loved-one Asks for More than Needs, No time for Self, Afraid for the Future, Loved-one Dependent on Me, Strained Around Loved-one, No Privacy, Should be Doing More, Could do More | |
| 7 | mCSI | 6 | Sleep Disturbed, Physical Strain, Caregiving is Confining, Time Demands, Overwhelmed |
| Zarit | 19 | Stressed meeting other Responsibilities, Afraid Future, Loved-one Dependent on Me, Loved-one Expects me to Take Care of Him, Lost Control Life |
The maximum sum score on the mCSI is 26, suggesting regular distress. The maximum score on the Zarit Caregiver burden scale is 88, suggesting the caregiver nearly always feels burdened.
The median Zarit sum score was 19 (range, 0 – 34) out of 88 maximum, which suggested little to no caregiver burden overall. On an individual level, however, 4 caregivers (57%) showed evidence of mild to moderate burden. Caregivers most commonly felt Afraid about what the Future holds for their loved-one (n=6, 86%), that their loved-one is dependent on them (71%), that they lost control over their own lives (71%), and they feel uncertain about what to do about their loved-one (71%).
B. OUTCOME DATA
Technology Implementation & Feasibility
All couples (caregivers) had cell phones and the majority a computer (71%) and wireless Internet at home (86%). Few (14%) owned a smart phone, tablet or e-reader. The majority of caregivers (57%) identified themselves as “Tending to wait until a technology is widely adopted before you try it”.
Companions were installed in the home between February and October 2013. Although the intervention was scheduled to last 3 weeks, the median trial period was 31 days (range, 24 – 57 days). Longer trial periods accommodated unforeseen circumstances and interventions needing revisions per caregiver request. All participants accepted the intervention and no adverse events occurred.
Companions contained on average 8 Life Stories with personal media (range, 0 – 14 Stories), 29 Life Stories with personalized, but generic (non-personal) media, 2 Care Shows with personal media (range, 1 – 10 Shows) and 2 Care Shows with generic media.
Five out of 7 couples (71%) kept the Companion beyond the intervention for at least 45 days or more. Two couples are still using it after 33and 65 weeks, respectively.
Goals and Subjective Attainment
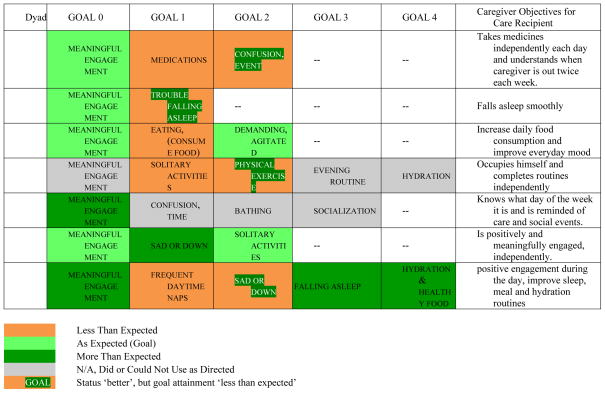
Table 5 shows the intervention goals caregivers selected based on symptoms and needs. Goal attainment is shown in different colors. Post-intervention, eleven out of 25 goals (44%) were as expected or better than expected whereas 8 goals (32%) were less than expected in the eyes of caregivers. Importantly, in four of these instances (50%) caregivers rated the care recipient’s status as ‘better’, but goal attainment as less than expected. One finding of note is that all 6 dyads who were able to use the Companion as intended reported that Meaningful Engagement was as expected or better than expected.
Table 5.
Intervention Goals and Goal Attainment Ratings at the End of the Intervention Period.
Positive experiences included many couples using and enjoying the reminiscing feature together, which some indicated they would not have done otherwise. All but 1 couple said the reminiscing was a positive experience and one caregiver stated after 8 months, long after the official trial ended: “The Companion is truly living up to its name these several months that [care recipient] has been in the nursing home”. Other positive experiences included the intervention helping caregivers be reminded of giving medications and feeling more secure and at ease when they were out, knowing the Companion was playing at home.
Barriers to use and usefulness included care recipients’ inability to use the Companion independently due to physical limitations (2); the Companion not offering a feature the caregiver had counted on (1), caregivers ignoring or muting all scheduled shows and not using interventions on demand (2); care recipients ignoring interventions even when they noticed them and perceived them as positive (2); care recipients’ unwillingness to share experiences with the caregiver (1); couples not having enough time or availability to experience the interventions due to circumstances (1). Three caregivers had hoped their loved-one would use the technology more even when (s)he did use it independently.
Technology Adoption
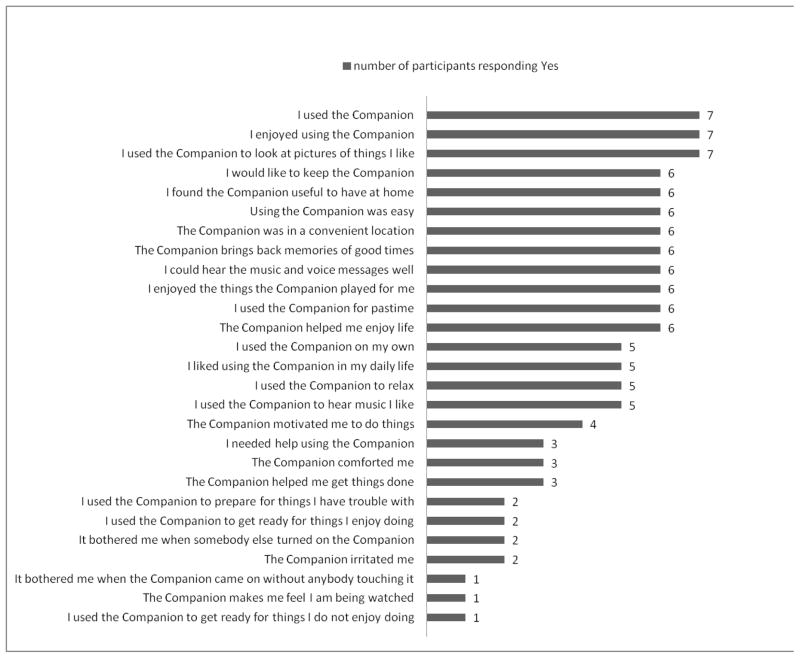
Figure 2 shows adoption metrics for care recipients. They perceived many aspects of the Companion and intervention positively, including ease of use and contents. The majority indicated it helped them relax, enjoy life and reminisce whereas it did not make them feel monitored or watched, nor did they find it to be irritating.
Figure 2.
Care Recipient (n=7) Technology Adoption
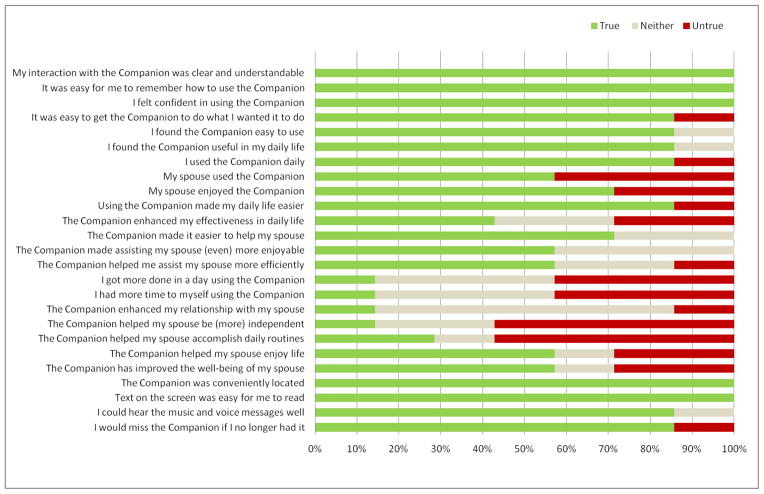
Caregivers were overwhelmingly positive in their assessment of the technology (Figure 3) and felt it made their daily lives easier and made helping their spouse easier. However, this did not necessarily make caregivers more efficient or effective in daily life nor helped them have more time to themselves. Respondents were more cautiously optimistic in these areas, suggesting that while assistive technologies such as the Companion can help mitigate symptoms and address needs in PWD, care burden and challenges remain. Perhaps most compelling is the ultimate question “I would miss the Companion if I no longer had it”, to which 86% of caregivers responded positively. This data point indicates the value of the system in the home.
Figure 3.
Caregiver (n=7) Technology Adoption
DISCUSSION
This study demonstrates that non-drug, psychosocial interventions can be delivered to seniors with dementia or assistive needs and their spouses at home using computer technology and help address symptoms and needs common in dementia and late life. Cohen-Mansfield and colleagues have argued and found that personalizing such interventions to meet individual needs and preferences is key to their success and efficacy by virtue of engagement, which is necessary for behavior change.13,40 As this study illustrates, advanced technology can be personalized in many ways by collecting and using personal facts and information and individually relevant audiovisual stimuli. In the future, if these items and data, including progress to wellness and intervention goals, are collected electronically and automatically, technology-based interventions can truly live up to their potential and make chronic conditions management available with ease. Chronic disease will never be easy but disease management can become easier with the help of smart technology.
We found that care recipients with mild cognitive impairment as well as moderate dementia were very interested in the possibilities of technology and could be tested using it for short as well as extended periods of time. Although the sample was small, technology adoption was excellent among both care recipients and caregivers as reflected in post-implementation questionnaire responses and a tendency to keep the Companion long after the official trial had ended. A third of care recipients (2/6) indicated they did not use the Companion independently. This did not necessarily relate to observed levels of cognitive impairment and suggests personal motivation and curiosity play a role. Physical limitations such as visual impairments and ambulation did affect independent technology use, which underscores the need for user screening and for systems that adapt to common user impairments, for instance by enlarging captions and graphics automatically if vision is impaired. Developing mobile applications and devices, in addition, will help accommodate ambulation difficulties and it is encouraging to see older adults adopting tablet technology in great numbers.41 It remains to be seen how seniors use mobile healthcare devices and applications in the home and daily life, and accordingly whether assistive technology can effectively address dementia symptoms and needs and relieve caregiver burden on a large scale.
A majority of care recipients in this study indicated they enjoyed the Companion shows, that they used it as a pastime and it brought back good memories, and that it helped them relax and enjoy life. This suggests the intervention facilitated meaningful and positive engagement, as intended, and supports quality of life, although none of these constructs were measured formally with standardized, dedicated questionnaires. Our findings are consistent with the work of other labs using technology to promote reminiscing and quality of life,42–45 and confirms that psychosocial interventions can be delivered using computer-based tools and digital media as opposed to a human being. The scope and depth of this positive engagement effect, for instance on symptoms such as depression and apathy, remains to be seen. There is some evidence to suggest that technology-assisted interventions can benefit mood and mood disorder symptoms, but most studies to date have included young rather than older adults.46 Technology studies with older adults tend to focus on security and monitoring, not disease and symptom management, or NPS are addressed using non-technological solutions such as environmental design.17,46 The current work did not focus on efficacy, and therefore, did not include post-implementation assessments of NPS, quality of life, cognitive function or caregiver distress. If efficacy were the objective, more dyads would need to be studied over longer periods of time, which we are in the process of doing. However, we question the usefulness of the repeat (pre-post) measurement of constructs such as cognitive function, ADL and NPS using standardized tools given that most symptoms, impairments and the underlying disease state are unlikely to change if not deteriorate in the face of a progressive neurodegenerative disease such as dementia. Even if a symptom or function were to improve, standardized tools are unlikely to reflect this as the tool’s overall score includes a number of items that will not have improved or are irrelevant and thus remain stable regardless of an intervention. Rather, it seems relevant to ask which specific symptoms or needs are an issue for a caregiver and patient and to focus on those symptoms and needs specifically and exclusively in terms of the intervention as well as outcome assessment. By definition, this requires an individualized metric of success or outcome, which we used and advocate here in the form of goal attainment scaling (discussed further below).
Questionnaire data did indicate that interventions helped care recipients prepare for things they have trouble with, or they enjoy doing or do not enjoy doing, which supports the notion that technology can prompt and motivate people to accomplish important health and wellness routines, such as daily oral hygiene, hydration and exercise. This is consistent with the small body of work on personal digital assistants and cognitive prosthetics.18,47,48 Our study makes an important contribution to the field as it tested in-field a technology solution that integrates various nondrug interventions such as cuing, priming, reminiscing, and simulated presence in one customizable system that meets an important, unaddressed need: managing common neuropsychological symptoms and care needs in the home.
These data also provide reason for cautious optimism. Whereas caregivers embraced the technology in terms of its ease of use and it facilitating and simplifying daily life, this did not necessarily translate into caregivers having more time to themselves or getting more done in a day. Caregivers were frequently instrumental in starting the Companion, and many would reminisce together with care recipients. While very positive, this cost caregivers time rather than freeing it up. Benefits to caregivers’ well being arguably also take time to manifest themselves, which longer and larger prospective trials should address.
It is further notable that the majority of caregivers did not feel the intervention made their loved-ones more independent, which is what many are looking for. Some expressed this quite clearly when we discussed and set intervention goals at the outset of studies and a loved-one’s dependence emerged as a prime psychological stressor from the Zarit caregiver burden scale in this sample. A key challenge for care technology and disease management systems, therefore, is to balance the requirement for personalization, which requires caregiver and care recipient input, with the requirement for caregiver respite. Caregivers are looking to simplify their lives, which is irreconcilable with the need to keep information and interventions up to date, relevant and personal. The disconnect between aspirations and actualization of independence and simplification may partially explain why goal attainment ratings by caregivers at the end of trials were surprisingly harsh in a number of cases and did not necessarily reflect the progress care recipients had made. This highlights the importance of setting realistic expectations (goals) on the one hand, and collecting objective, measurable outcomes on the other. In this trial we relied on caregiver’s subjective perception of goal attainment, but did not define a goal attainment scale (GAS) as some formal care providers have done in nursing home settings.36 Family caregivers have difficulty defining such a scale in our experience, and tend to set unrealistic goals, often unknowingly. Based on these experiences, we now define goals and help set an attainment scale in conversation with families, which works well and provides a more objective measure of progress and outcome. We also note that some goals (e.g., “I want Joe to be less agitated”) are harder to define and accomplish than others (“I want Joe to drink more water”). Goal setting and attainment scaling, in conclusion, takes time and consideration, but is a valuable method that lends itself well for measuring individual intervention effects in geriatric and long-term care environments,36 but also education 49, where needs vary greatly between individuals and within individuals over time. Focusing on standardized, group-based outcomes in these circumstances is frequently irrelevant and even unhelpful. Moreover, effective use of technology in older adults depends on whether or not a new technology satisfies an unmet need,50 which reinforces the need not only for personalization but also an individualized measure of outcome, which GAS provides. Recommendations to ensure methodological quality in applying this promising measurement approach can be found elsewhere.49
With respect to the home setting we note that these initial results suggest that a computer-based assistive technology can become part of the living environment and household routine. The size of the homes in this study was not an obvious barrier to hearing or seeing the interventions, but care was taken to make the Companion accessible to the care recipient by placing it in a convenient and relevant location. We also note that participants were considerably more frail than expected and study recruitment was a challenge given high attrition rates due to participant frailty and the sensitivity and complexity of the subject matter. Dementia is surrounded by myth, confusion and stigma, and something people need time to cope with if given a diagnosis. This hampers recruitment. In addition, many seniors, especially males in our experience, shy away from discussing difficulties they are experiencing and it is not always clear that something is wrong. This reluctance and uncertainty raises ethical dilemmas during recruitment and requires investigators to tread carefully. We also observed a general desire to focus on wellness and ability as opposed to disease and dysfunction. Consequently, the interventions in this demographic (independently living older adults) tend to focus on regaining independence and wellness rather than maintaining function and avoiding decline. The implication is that great care must be taken when implementing research and interventions that focus on assistive- and wellness needs in seniors.
In conclusion, this field-test indicated that a personalized computer-based tool for managing symptoms and needs common in dementia and late life was feasible, usable, and useful. The intervention facilitated meaningful and positive engagement in the home and helped care recipients and caregivers cope with symptoms and needs in daily life. Although a number of intervention goals were met, improving care recipient and caregiver independence is challenging and requires setting the right expectations as well as collecting objective measures of outcome.
Acknowledgments
Funding sources: This work was supported by a Fast-Track Small Business Innovation Research (SBIR) grant from the National Institute on Aging (1R44AG042206-01) awarded to Kerssens and SimpleC, LLC.
We thank all our community partners for their support of this project and invaluable help with recruitment. No partner is named specifically to protect the identity of study participants.
Footnotes
Part of this work was presented at the Gerontological Society of America 66th Annual Scientific Meeting, New Orleans, LA, 20 – 24 November 2013 (Abstract ID: 1692269)
Disclosures:
Chantal Kerssens, Renu Kumar, and Anne Adams are employees of SimpleC, LLC. Renu Kumar and Anne Adams were funded exclusively by grant 1R44AG042206-01.
Camilla Knott (Aptima, Inc) is a contractor on grant 1R44AG042206-01.
Jon Sanford and Wendy Rogers are consultants on grant 1R44AG042206-01.
Contributor Information
Chantal Kerssens, Vice President of Research and Clinical Innovation, SimpleC, LLC, Atlanta, GA.
Renu Kumar, Research Associate, SimpleC, LLC, Atlanta, GA
Anne E. Adams, Human Factors Specialist, SimpleC, LLC Atlanta, GA; Instructor, School of Psychology, Georgia Institute of Technology, Atlanta, GA.
Camilla C. Knott, Senior Scientist, Performance Assessment and Augmentation Division, Aptima, Inc, Washington, DC.
Laura Matalenas, Research Assistant, SimpleC, LLC; Undergraduate student, Engineering Psychology, Georgia Institute of Technology, Atlanta, GA.
Jon A. Sanford, Associate Professor, College of Architecture, Georgia Institute of Technology, Atlanta, GA; Director, Center for Assistive Technology & Environmental Access, Georgia Institute of Technology, Atlanta, GA; Research Architect, Rehabilitation R & D Center of Excellence, Department of Veterans Affairs, Atlanta, GA.
Wendy A. Rogers, Professor, School of Psychology, Georgia Institute of Technology, Atlanta, GA.
References
- 1.Alzheimer’s Association and Centers for Disease Control and Prevention; A.s. Association, editor. The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018. Chicago, IL: 2013. p. 64. [Google Scholar]
- 2.Alzheimer’s Association; Alzheimer’s Association, editor. Alzheimer’s & Dementia. Chicago, IL: 2012. 2012 Alzheimer’s Disease Facts and Figures. [Google Scholar]
- 3.Minati L, Edginton T, Bruzzone MG, Giaccone G. Current concepts in Alzheimer’s disease: a multidisciplinary review. Am J Alzheimers Dis Other Demen. 2009;24(2):95–121. doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doty P. National Academy on an Aging Society, editor. Public Policy & Aging Report. Washington, DC: 2010. The Evolving Balance of Formal and Informal, Institutional and Non-Institutional Long-Term Care for Older Americans: A Thirty-Year Perspective; pp. 3–9. [Google Scholar]
- 5.Prince Market Research. Attitudes of Seniors and Baby Boomers on Aging in Place. Nashville, TN: 2007. [Google Scholar]
- 6.Mathew Greenwald & Associates Inc. These four Walls… Americans 45+ Talk About Home and Community. Washington, DC: 2003. [Google Scholar]
- 7.Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual Life Outcomes. 2004;2:52. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzheimer’s Association; Alzheimer’s Association, editor. Alzheimer’s & Dementia. Chicago, IL: 2011. 2011 Alzheimer’s Disease Facts and Figures. [Google Scholar]
- 9.National Alliance for Caregiving. e-Connected Family Caregiver: Bringing Caregiving into the 21st Century. 2011. p. 64. [Google Scholar]
- 10.MetLife Mature Market Institute and Louis Tenenbaum. Aging in Place 2.0: Rethinking Solutions to the Home Care Challenge. Westport, CT: 2010. [Google Scholar]
- 11.Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis. 2009;18(1):11–30. doi: 10.3233/JAD-2009-1120. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg GRP, Lyketsos C. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22(3):346–72. doi: 10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry. 2001;9(4):361–81. [PubMed] [Google Scholar]
- 14.Lou CR, Giuliano A, Mulvenna MD. State of the Art in Electronic Asssitive Technologies for People with Dementia. In: Mulvenna MD, Nugent CD, editors. Supporting People with Dementia Using Pervasive Health Technologies. Springer; London: 2010. pp. 23–35. [Google Scholar]
- 15.Baruch J, Downs M, Baldwin C, Bruce E. A case study in the use of technology to reassure and support a person with dementia. Dementia. 2004;3(3):371–392. [Google Scholar]
- 16.Lauriks S, Reinersmann A, Van der Roest HG, Meiland FJ, Davies RJ, Moelaert F, Mulvenna MD, Nugent CD, Dröes RM. Review of ICT-based Services for Identified Unmet Needs in People with Dementia. In: Mulvenna MD, Nugent CD, editors. Supporting People with Dementia Using Pervasive Health Technologies. Springer; London: 2010. pp. 37–61. [DOI] [PubMed] [Google Scholar]
- 17.Lauriks S, Reinersmann A, Van der Roest HG, Meiland FJ, Davies RJ, Moelaert F, Mulvenna MD, Nugent CD, Dröes RM. Review of ICT-based services for identified unmet needs in people with dementia. Ageing Res Rev. 2007;6(3):223–46. doi: 10.1016/j.arr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson M, Cullen B, McGee-Lennon M, Brewster S, Evans JJ. The efficacy of cognitive prosthetic technology for people with memory impairments: A systematic review and meta-analysis. Neuropsychological Rehabilitation. 2013 doi: 10.1080/09602011.2013.825632. Epub ahead of print: p. http://dx.doi.org/10.1080/09602011.2013.825632. [DOI] [PubMed]
- 19.Ayalon L, Gum AM, Feliciano L, Areán PA. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med. 2006;166(20):2182–8. doi: 10.1001/archinte.166.20.2182. [DOI] [PubMed] [Google Scholar]
- 20.Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, Lyketsos CG. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5(5):245–55. doi: 10.1038/nrneurol.2009.39. [DOI] [PubMed] [Google Scholar]
- 21.Pittiglio L. Use of reminiscence therapy in patients with Alzheimer’s disease. Lippincotts Case Manag. 2000;5(6):216–20. doi: 10.1097/00129234-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Woods B, Spector A, Jones C, Orrell M, Davies S. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2005;(2):CD001120. doi: 10.1002/14651858.CD001120.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Webster JD, Bohlmeijer ET, Westerhof GJ. Mapping the future of reminiscence: A conceptual guide for research and practice. Research on Aging. 2010;32(4):527–64. [Google Scholar]
- 24.Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, Woods B, Beck C, Auer S, Lai C, Spector A, Fazio S, Bond J, Kivipelto M, Brodaty H, Rojo JM, Collins H, Teri L, Mittelman M, Orrell M, Feldman HH, Muñiz R. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–78. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen J. Usability Engineering. San Francisco: Morgan Kaufmann; 1993. [Google Scholar]
- 26.Resnick B, Gruber-Baldini AL, Pretzer-Aboff I, Galik E, Buie VC, Russ K, Zimmerman S. Reliability and Validity of the Evaluation to Sign Consent Measure. Gerontologist. 2007;47(1):69–77. doi: 10.1093/geront/47.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Md State Med J. 1965;14(2):61–65. [PubMed] [Google Scholar]
- 29.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 30.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–84. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 31.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 32.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 33.Thornton M, Travis SS. Analysis of the reliability of the Modified Caregiver Strain Index. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2003;58(2):S127–S132. doi: 10.1093/geronb/58.2.s127. [DOI] [PubMed] [Google Scholar]
- 34.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–55. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 35.Brandt J, Campodonico JR, Rich JB, Baker L, Steele C, Ruff T, Baker A, Lyketsos C. Adjustment to residential placement in Alzheimer disease patients: does premorbid personality matter? Int J Geriatr Psychiatry. 1998;13(8):509–15. doi: 10.1002/(sici)1099-1166(199808)13:8<509::aid-gps809>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 36.Gordon JE, Powell C, Rockwood K. Goal attainment scaling as a measure of clinically important change in nursing-home patients. Age Ageing. 1999;28(3):275–81. doi: 10.1093/ageing/28.3.275. [DOI] [PubMed] [Google Scholar]
- 37.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly. 1989;13(3):319–339. [Google Scholar]
- 38.Venkatesh V, Davis FD. A Theoretical Extension of the Technology Acceptance Model: Four Longitudinal Field Studies. Management Science. 2000;46(2):186–204. [Google Scholar]
- 39.Tiberio L, Cesto A, Cortelessa G, Padua L, Pellegrino AR. Assessing affective response of older users to a telepresence robot using a combination of psychophysiological measures. 2012 IEEE Ro-Man: The 21st IEEE International Symposium on Robot and Human Interactive Communication; 2012; Paris, France. pp. 833–838. [Google Scholar]
- 40.Cohen-Mansfield J, Dakheel-Ali M, Marx MS. Engagement in persons with dementia: the concept and its measurement. Am J Geriatr Psychiatry. 2009;17(4):299–307. doi: 10.1097/JGP.0b013e31818f3a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pew Internet & American Life Project; Pew Research Center’s Internet & American Life Project, editor. Pew Internet. Pew Research Center; Washington, D.C: 2012. Tablet and E-book reader Ownership Nearly Double Over the Holiday Gift-Giving Period; pp. 1–11. [Google Scholar]
- 42.Alm N, Dye R, Gowans G, Campbell J, Astell A, Ellis M. Designing an Interface Usable by People with Dementia. Conference on Universal Usability; 2003; Vancouver, British Columbia, Canada. pp. 156–157. [Google Scholar]
- 43.Yasuda K, Kuwabara K, Kuwahara N, Abe S, Tetsutani N. Effectiveness of personalised reminiscence photo videos for individuals with dementia. Neuropsychol Rehabil. 2009;19(4):603–19. doi: 10.1080/09602010802586216. [DOI] [PubMed] [Google Scholar]
- 44.Mihailidis A, Blunsden S, Boger JN, Richards B, Zutis K, Young L, Hoey J. Towards the development of a technology for art therapy and dementia: Definition of needs and design constraints. The Arts in Psychotherapy. 2010;37:293–300. [Google Scholar]
- 45.Astell AJ, Ellis MP, Bernardi L, Alm N, Dye R, Gowans G, Campbell J. Using a touch screen computer to support relationships between people with dementia and caregivers. Interacting with Computers. 2010;22:267–75. [Google Scholar]
- 46.Westphal A, Dingjan P, Attoe R. What can low and high technologies do for late-life mental disorders? Curr Opin Psychiatry. 2010;23(6):510–5. doi: 10.1097/YCO.0b013e32833d74d4. [DOI] [PubMed] [Google Scholar]
- 47.Alm N, Astell A, Ellis M, Dye R, Gowans G, Campbell J. A cognitive prothesis and communication support for people with dementia. Neuropsychol Rehabil. 2004;14(1/2):117–34. [Google Scholar]
- 48.Mihailidis A, Boger JN, Craig T, Hoey J. The COACH prompting system to assist older adults with dementia through handwashing: an efficacy study. BMC Geriatr. 2008;8(28) doi: 10.1186/1471-2318-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruble L, McGrew JH, Toland MD. Goal attainment scaling as an outcome measure in randomized controlled trials of psychosocial interventions in autism. J Autism Dev Disord. 2012;42(9):1974–83. doi: 10.1007/s10803-012-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin D, Hsieh C. The role of multimedia in training the elderly to acquire operational skills of a digital camera. Gerontechnology. 2006;(5):68–77. [Google Scholar]