Abstract
Neural activity is tightly coupled to energy consumption, particularly sugars such as glucose. Yet we find that, unlike mature neurons and astrocytes, neural stem/progenitor cells (NSPCs) do not require glucose to sustain aerobic respiration. NSPCs within the adult subventricular zone express enzymes required for fatty acid oxidation and show sustained increases in oxygen consumption upon treatment with a polyunsaturated fatty acid. NSPCs also demonstrate sustained decreases in oxygen consumption upon treatment with etomoxir, an inhibitor of fatty acid oxidation. In addition, etomoxir decreases the proliferation of subventricular zone NSPCs without affecting cellular survival. Finally, higher levels of neurogenesis can be achieved in aged mice by ectopically expressing PGC1a, a factor that increases cellular aerobic capacity by promoting mitochondrial biogenesis and metabolic gene transcription. Regulation of metabolic fuel availability could prove a powerful tool in promoting or limiting cellular proliferation in the central nervous system.
Keywords: neural stem cell, progenitor, neurogenesis, fatty acid oxidation, proliferation, differentiation
Introduction
Neural stem/progenitor cells (NSPCs), which retain the capacity to produce new neurons and glia in the adult mammalian brain, reside in the subventricular zone of the lateral ventricle (SVZ) and the subgranular zone of hippocampal dentate gyrus (DG) 1, 2. While newly-born cells in the inner molecular cell layer of DG migrate locally, cells from SVZ migrate long distances along the rostral migratory stream to populate the olfactory bulb with new GABAergic interneurons including dopaminergic neurons 3. The control of neurogenesis in the adult brain is notoriously complex, and depends upon factors in both the extracellular environment and limitations within the cellular machinery 4. Since NSPCs reside in a "neurogenic niche" fortified by blood vessels and astrocytes 5, these cells have direct access to contents in the bloodstream, including substrates to fuel energy metabolism. In this study we investigated the substrates required by adult mammalian neural stem cells to maintain their metabolic and neurogenic activity.
Catabolism, the production of energy in the form of ATP, is necessary to sustain cellular activity. The adult brain primarily depends upon the oxidation of carbohydrates such as glucose to fuel energy production. Although the brain comprises about 2% of body weight, this organ accounts for approximately 25% of organismal glucose consumption and 20% of oxygen consumption. Neuronal activity is indeed tightly coupled to glucose uptake 6. Yet intriguingly, neurons and astrocytes have recently been shown to compartmentalize metabolic processes 7, 8. Glutamate released by neurons during synaptic transmission triggers astrocytic uptake of blood-borne glucose. Astrocytes then undergo glycolysis, metabolizing glucose into pyruvate then releasing excess end products in the form of lactate into the extracellular space via the monocarboxylate transporter MCT4 9, 10. Neurons take up this extracellular lactate via the monocarboxylate transporter MCT2, and convert it back to pyruvate in a reaction catalyzed by lactate dehydrogenase 8, 11. Both neurons and astrocytes then use pyruvate intracellularly to fuel oxidative metabolism. Physiological studies over recent years have shown that neurons prefer lactate as an bioenergetic substrate 11, 12, although both glucose and lactate can strongly stimulate oxidative metabolism in mature neurons 12, 13. Yet the metabolic fuel requirements of adult neural stem cells are not known.
In this report, we demonstrate that neural stem cells derived from the mouse subventricular zone do not require glucose to sustain oxygen consumption, unlike other cell types that have been characterized in the adult brain. Instead, adult NSPCs are capable of utilizing multiple fuel sources to maintain the high levels of aerobic respiration necessary for cellular division. In particular, fatty acids stimulate respiratory activity in these cells.
Beta-oxidation of fatty acids is performed by numerous cell types, most notably cardiac myocytes and skeletal muscle tissue 14. Although mature cells within the adult brain depend on carbohydrates such as glucose for energy production, the fetal brain oxidizes primarily free fatty acids and other related substrates derived from the mother’s milk 15, 16. Metabolic dependence upon fatty acids appears to be retained in adult neural stem cells. In this study, we performed gain-of-function and loss-of-function studies to test whether beta-oxidation contributes to energy production and acts as a limiting factor in cellular proliferation. Here we report that adult neural stem cells in the subventricular zone rely upon fatty acid oxidation to support aerobic respiration and proliferative activity, although other fuels can also be oxidized.
Materials and Methods
Immunohistochemistry
All experiments were performed as approved by the University of Washington Institutional Animal Care and Use Committee (IACUC) and United Kingdom Home Office Project Licence Number 60/4386. Full details on experimental techniques are available in Supplemental Materials and Methods. For immunohistochemical studies, wild-type adult C57BL/6 mice were euthanized and brain tissues were processed for cryosectioning. 10µm-thick slices were subjected to 0.01M sodium citrate at 100°C for ten minutes for antigen retrieval. Sections were then rinsed with PBS containing 0.1% Triton X-100 (PBST). Non-specific staining was blocked for 2 hours in PBS with 0.1% Triton X-100 and 5% donkey serum (blocking solution). Sections were incubated overnight at 4°C with appropriate antibodies diluted in blocking solution (details in Supplemental Table 1A). Sections were then rinsed with PBST. The appropriate secondary antibodies were diluted by 1:250 in blocking solution and placed on sections for 2 hours at room temperature (details in Supplemental Table 1B). Sections were rinsed and co-stained with Hoechst diluted to 1µg/mL in PBST. Fluorescence microscopy was performed using a Zeiss Apoptome Microscope with attached camera and Axiovision software.
Laser-Capture Microdissection
Cells within subventricular zone (SVZ), olfactory bulb (OB), or cortex were microdissected using the Zeiss PALM MicroBeam Laser-Capture Microdissection Microscope. Cells were collected using the RNAqueous MicroKit (Ambion AM1931) and stored at −20°C until ready for real-time PCR analysis.
Real-Time PCR
Real-time PCR was used to quantify metabolic transcripts in laser-captured tissue samples and serum-exposed dissociated cells. The real-time PCR was based on the procedure described previously 17 which quantifies fluorescence emitted by dyes VIC and FAM which are conjugated to probes complementary to cDNA reverse-transcribed from mRNA transcripts. The assay was performed on the StepOnePlus™ Real-Time PCR System (Applied Biosystems). Samples from each neuroanatomical area or serum-exposure timepoint were each run in triplicate to quantify total copy number. Each sample was normalized to the standard curve, using the equation VALUE = (CT – INTERCEPT)/SLOPE. These values were normalized to B-actin. Total copy number of cDNA representing ACADL, CPT1, and MCT2 transcripts were compared between neuroanatomical areas (in laser-captured samples) or between serum-exposure timepoints (in cell cultures) using a two-tailed t-test in Excel. Error bars are SEM.
Serum-free primary culture of mouse neural stem/progenitor cells
Adult NSPCs were isolated as previously described 18. Briefly, wild-type C57BL/6 mice, 3 months of age, were transcardially perfused with ice-cold saline. Brain tissue from the subventricular zone was mechanically and enzymatically dissociated with collagenase-DNase solution. To remove debris, myelin and red blood cells, the cell suspension was mixed with a percoll solution and centrifuged. The isolated progenitor cells were grown in proliferation media, consisting of Dulbecco’s modified Eagle’s medium/F12 (Omega Scientific DM-25, Tarzana, CA) supplemented with 2 mM glutamine, 1% N2 (Gibco, Carlsbad, CA), 50 lg/ml heparin (Sigma, St Louis, MO), 20 ng/ml epidermal growth factor (Peprotech, Rocky Hill, NJ), and 20 ng/ml fibroblast growth factor-2 (Peprotech, Rocky Hill, NJ). Cultures were passaged by mechanical dissociation in the presence of trypsin-EDTA, and used for in vitro experimentation between passages 3 and 12. Cultures contain a relatively homogenous, stable population of NSPCs (90% Nestin+; 80% CD133+) 18. In Supp Figure 4, serum exposure involved the use of normal DM25 medium supplemented with 10% fetal bovine serum. In Figure 6, neuronal differentiation was achieved with 2% B27 supplement in Neurobasal Medium. All in vitro experiments were performed three times with separate biological replicates (each with three technical replicates), except Seahorse Analyzer experiments shown in Figure 3, which were performed three times with separate biological replicates (each with five technical replicates). Error bars are SEM.
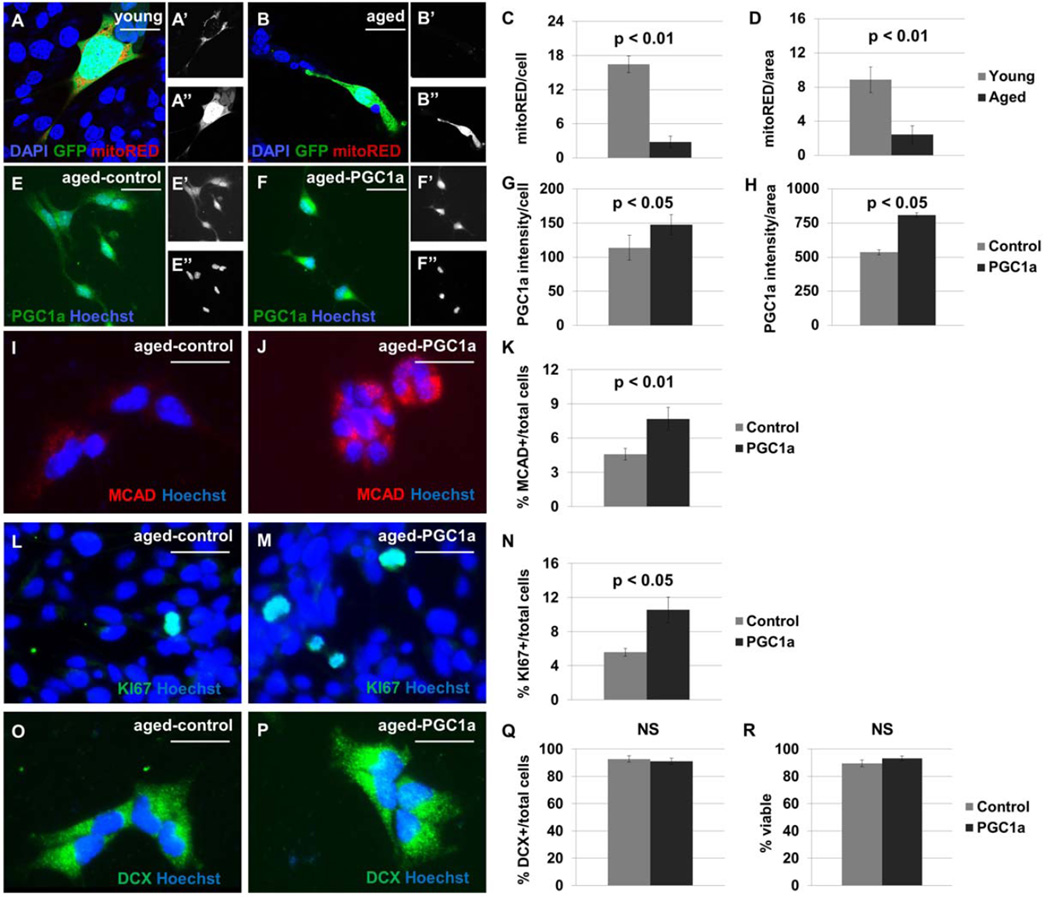
Figure 6. Ectopic PGC1α expression increases MCAD+ mitochondrial content and KI67+ mitotic index in cultured NSPCs.
NSPCs from the young adult (A) and aged adult (B) mouse brain were labeled with MitoTracker Red; separate channels are shown to display this mitochondrial marker (’), and GFP (”). Fewer mitoRED+ mitochondria are observed in cultures from aged adult brain than from young adult brain (p<0.01), when normalized per cell (C) or per unit area (D). NSPC cultures derived from 18-month-old mouse brain were transduced with lentivirus to express GFP alone (E) or PGC1α-GFP (F). Separate channels are shown to display PGC1α expression (’), and the pan-nuclear marker Hoechst (”). A 150% increase in nuclear PGC1α expression, compared with control, was observed by immunocytochemistry (p<0.05, G–H). Cells expressing PGC1α demonstrate higher MCAD expression than control cells (p<0.01, I–K). Cultures transduced with PGC1α-GFP have a higher KI67+ index under growth conditions (p<0.05, L–N). The cultures have no difference in DCX+ index (p>0.05, O–Q) or trypan blue-viability (p>0.05, R) after a four-day differentiation protocol. Scale bars represent 20 µm.
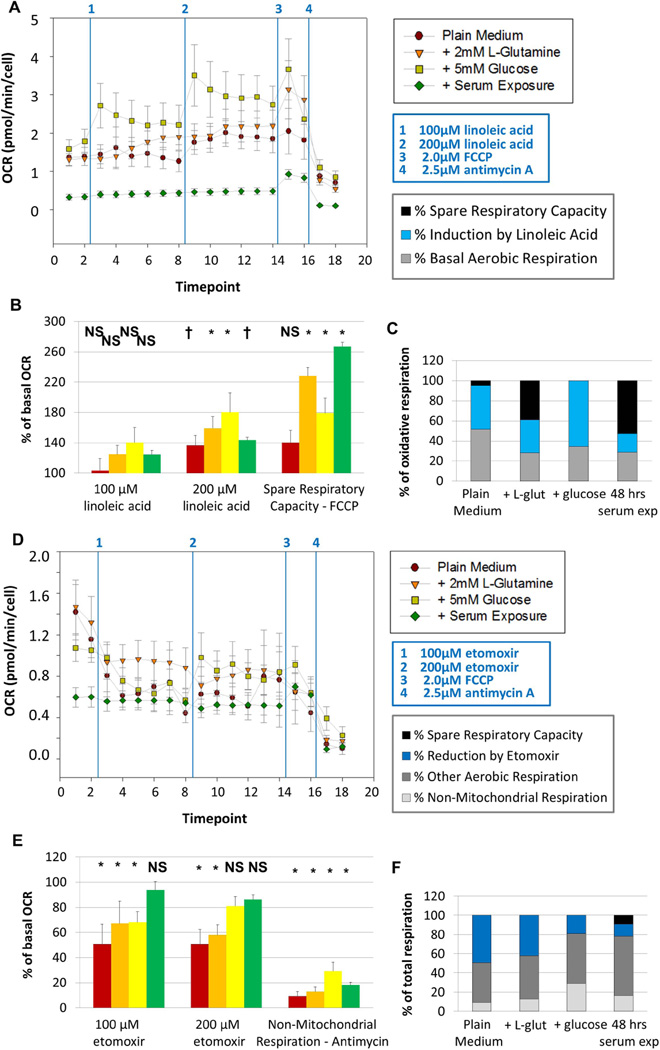
Figure 3. Fatty acid oxidation is a primary contributor to aerobic respiration in primary-cultured NSPCs.
The oxygen consumption rate (OCR, A and E) and extracellular acidification rate (ECAR, B and F) of primary-cultured NSPCs were assessed using the Seahorse Analyzer. Baseline measurements were taken for cells in plain medium (- glutamine – glucose, red circles), for cells in medium + L-glutamine (orange triangles), for cells in medium + glucose (yellow squares), and for cells exposed to 10% fetal bovine serum (FBS) for 48 hours prior to the experiment (green diamonds). Cells were then treated with 100µM linoleic acid, a polyunsaturated fatty acid. The concentration was then increased to 200µM of linoleic acid. Cells were then treated with 2.0µM FCCP, which induces maximal respiration, and 2.5µM Antimycin A, which completely inhibits aerobic respiration. Cells do not show significant changes in OCR upon treatment with 100µM linoleic acid, but cells do significantly increase OCR upon further addition of linoleic acid (B). The fraction of maximal oxidative respiratory capacity that is utilized under basal conditions and after linoleic acid treatment is shown (C). In a separate experiment, cells were treated with 100µM etomoxir, an inhibitor of CPT1, the rate-limiting enzyme in fatty acid oxidation; the concentration was then increased to 200µM etomoxir (D). All cells show significant decreases in OCR upon treatment with 100µM etomoxir, except cells that had been exposed to serum (E). Cells in plain medium or in medium containing L-glutamine maintain decreased OCR upon further addition of etomoxir, while cells with glucose show a partial recovery (E). The fraction of total respiratory capacity attributed to non-mitochondrial respiration, fatty acid oxidation and other aerobic metabolism is shown (F). Measurements were taken every nine minutes; data from three experiments were averaged together. NS, not significant. †, p < 0.05. *, p < 0.01.
Extracellular flux analysis in live cells
For Figure 2, oxygen consumption rate (OCR) was measured using a flow culture system adapted for extracellular flux analysis as described 19. This system utilizes an ultrastable oxygen sensor based on the detection of the decay of the phosphorescent emission from an oxygen-sensitive dye. For Figure 3, OCR was measured using the Seahorse XF24 Extracellular Flux Analyzer as previously described 20. OCR measurements were normalised to cell count per well and were averaged across three independent experiments each with multiple replicates. Results were statistically compared using two-tailed t-tests in Excel and plotted in Prism.
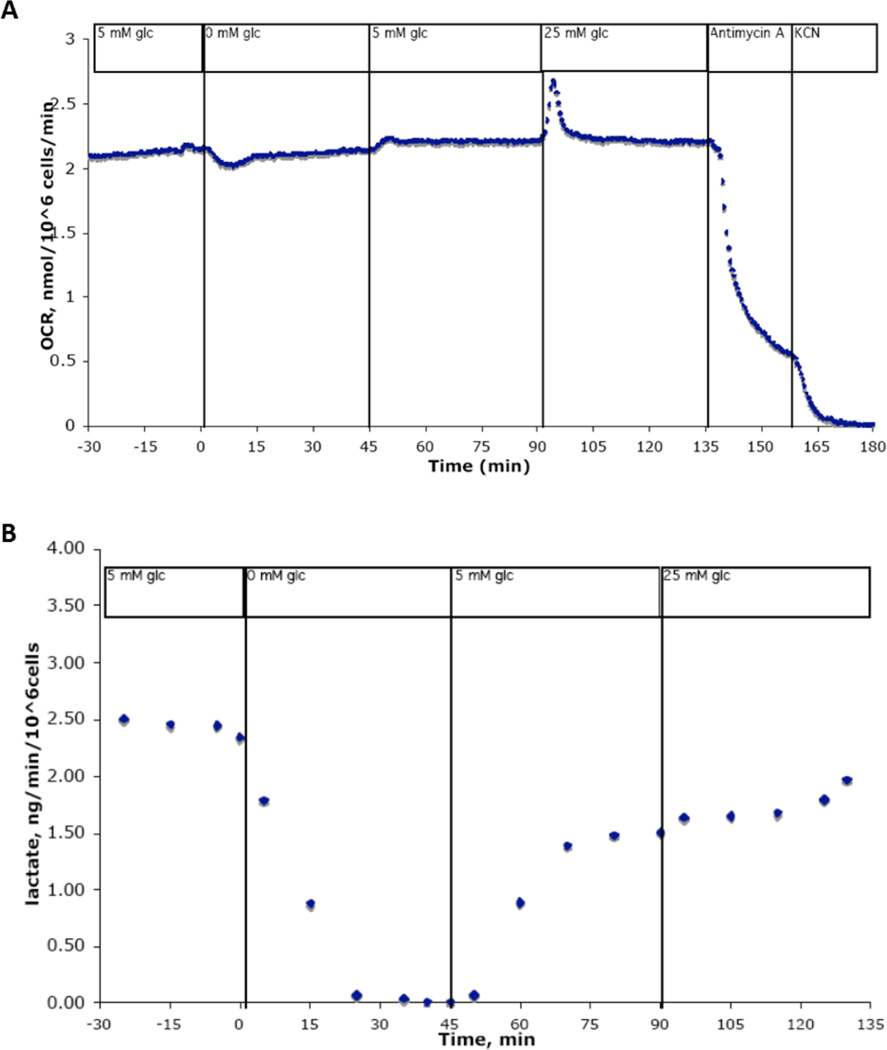
Figure 2. Neural stem cells do not require glucose to sustain aerobic respiration.
Shown is a representative trace of cultured NSPCs in a flow culture system outfitted for continuous metabolic analysis. Cells were exposed to differing concentrations of glucose over 210 minutes. Undifferentiated NSPCs do not sustain changes in oxygen consumption rate when glucose concentration is decreased to 0mM, then returned to 5mM, then increased to 25mM for extended periods of time (A), although simultaneous measures of lactate production drop to zero within 25 minutes of glucose removal (B).
Assessment of cellular proliferation and viability
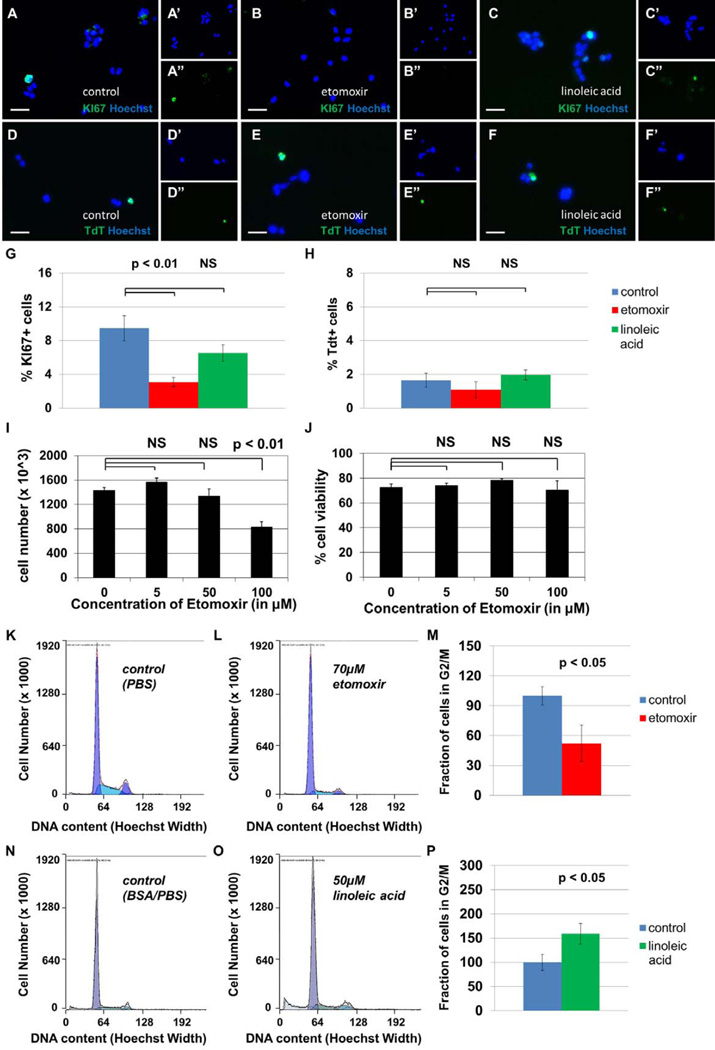
To quantify the fractions of actively cycling cells in the population, we employed two methods: immunocytochemical labelling of KI67 and FACS-based mitotic profiling. NSPCs were plated at a density of ~10,000 cells per well on coated glass coverslips for 24 hours in growth medium. Cells were then treated with 10µL of dPBS, 5mM etomoxir or 5mM linoleic acid, for a final concentration of 100µM etomoxir and 100µM linoleic acid. Immunohistochemistry was performed as above. TUNEL+ apoptotic cells were quantified as directed using a TdT Reagent Kit (Chemicon S7160). All cells were co-stained with Hoechst diluted to 1µg/mL in PBS containing 0.1% Triton X-100. Coverslips were mounted onto glass slides using Citifluor anti-fade glycerol reagent. Fluorescence microscopy was performed using a Zeiss Axioskop with attached camera and Axiovision software. Treatment groups were compared using two-tailed t-tests in Excel.
To make additional measurements of cellular proliferation, we performed FACS-based mitotic profiling. Cells were plated at a density of 10^6 cells per plate and incubated at 37°C for 24 hours in growth medium. Cells were then treated with dPBS, etomoxir at a final concentration of 70µM or linoleic acid at a final concentration of 50µM. 24 hours after treatment, cells were labelled with 2µg/mL Hoechst in permeabilization solution for one hour, then subjected to fluorescence-associated cell sorting using LSRII. Mitotic profile analysis was performed using ModFit software. 10,000 readings were taken for each replicate. Treatment groups were compared using two-tailed t-tests in Excel.
To quantify the fraction of viable cells using an alternative method, we employed the ViCell automated cell counter to measure total number of cells and the number of dead cells labelled positively with Trypan Blue dye. Cells were plated at 500,000 per plate and counted 48 hours later. Fractions of viable cells in each treatment group were compared using two-tailed t-tests in Excel.
Pharmacological inhibition of fatty acid and lactate transport in vivo
Female C57B/6 mice between 5–7 weeks of age were injected intraperitoneally with 40mg/kg etomoxir in PBS, 40mg/kg alpha-cyano-4-hydroxycinnamate (4-CIN) in 1% methanol-PBS, or with similar volumes of PBS. Animals were injected once per day for three days and sacrificed on the fourth day, approximately 24 hours after the final injection. Perfusion and immunohistochemistry were performed as described above. Fluorescence microscopy was performed using a Zeiss Apoptome with attached camera and Axiovision software. Cell counts were compared using two-tailed t-tests in Excel.
Lentiviral-mediated gene delivery of PGC1a and GFP in vitro and in vivo
To restore the relevant enzymatic machinery for fatty acid oxidation in aged NSPCs, we targeted neural stem/progenitor cells within SVZ for genetic modification by intracerebral injection of high-titer lentivirus encoding either GFP alone or peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1α) tagged with GFP. Two groups of mice, aged 21 months, were injected in subventricular zone with either a control virus expressing GFP or a virus expressing PGC1α tagged with GFP (n = 4 per group). Additional animals injected in a different location were used as controls to assess the extent of lentiviral infection and target gene expression. Animals were caged socially and euthanized by transcardial perfusion two months after surgery. Tissues were fixed, sectioned, stained, and visualized as described above. Cell counts were compared using two-tailed t-tests in Excel.
Results
Expression of fatty acid oxidation enzymes in adult subventricular zone and hippocampus
Brain energy metabolism has been shown to shift from beta-oxidation of fatty acids to glucose-based metabolism during weaning 15, 16. As a result, oxidation of fatty acids and ketone bodies in the CNS decreases drastically during maturation. We hypothesized that NSPCs however maintain a metabolic phenotype similar to less-differentiated cells in the fetal brain. To test this question, we assessed whether enzymes required for fatty acid oxidation were present in NSPCs located within neurogenic areas of the adult brain.
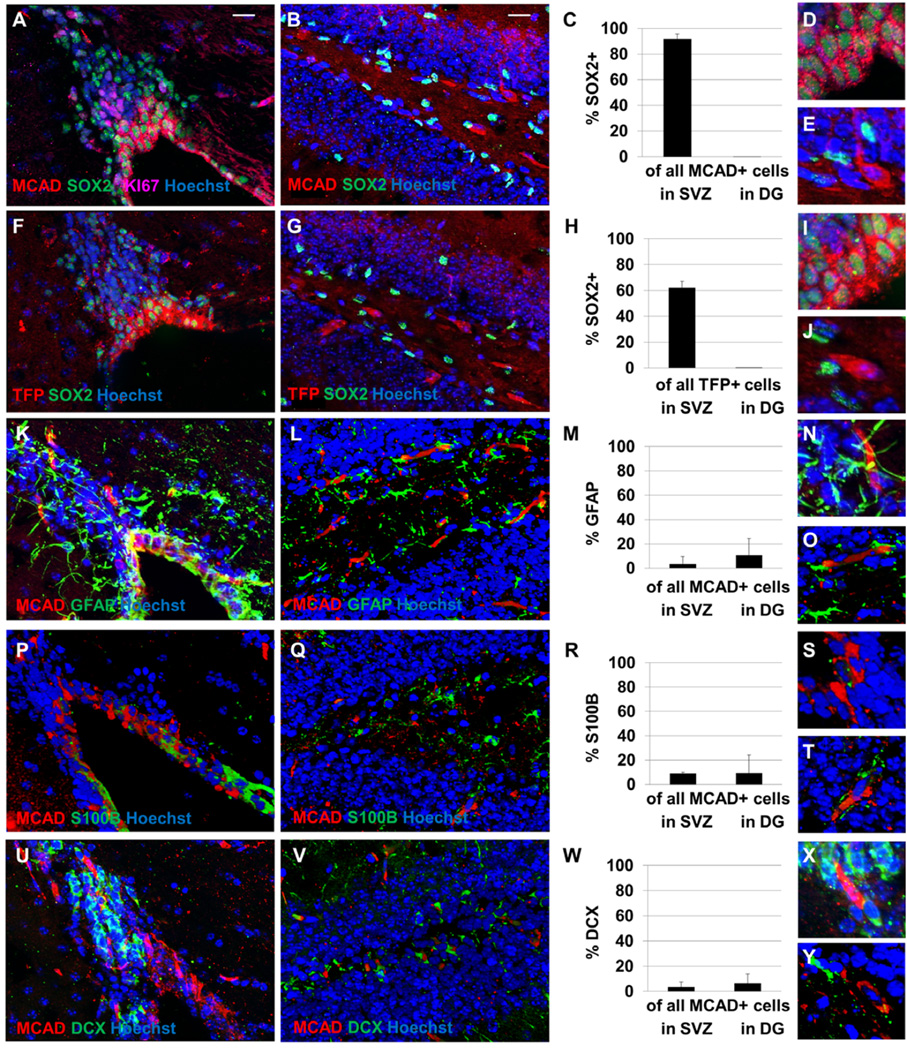
Cells expressing Medium Chain Acyl CoA Dehydrogenase (MCAD) and TriFunctional Protein (TFP) are observed in the subventricular zone and hippocampal dentate gyrus (Figure 1). Positively-labeled cells are also observed in the hypothalamic nuclei (not shown), as has been previously observed 21, 22. Cells in hippocampal dentate gyrus that labelled positively for markers of fatty acid oxidation did not co-label with SOX2, a marker of progenitor cells. The cells expressing fatty acid oxidation enzymes in this area therefore do not appear to be a progenitor population. However, a majority of cells in SVZ containing these markers demonstrate co-labelling with SOX2, suggesting that SOX2+ cells there are capable of fatty acid oxidation. However very few MCAD+ cells in SVZ co-label with KI67, an S-phase marker, suggesting that actively-proliferating cells are not undergoing fatty acid oxidation. MCAD+ cells observed in SVZ also do not co-label with GFAP+ astrocytes, S100B ependymal cells, or DCX+ neuroblasts (Figure 1). A negative control with no primary antibody is shown in Supplemental Figure 1 and individual channel images are shown in Supplemental Figures 2 and 3.
Figure 1. NSPCs in adult mouse subventricular zone express enzymes required for fatty acid oxidation.
Cells expressing Medium Chain Acyl CoA Dehydrogenase (MCAD, A–E) and TriFunctional Protein (TFP, F–J) are observed in the subventricular zone (A, F) and hippocampal dentate gyrus (B, G). A majority of cells expressing these enzymes in SVZ are observed to be co-labelled with SOX2, while none of the cells expressing fatty acid oxidation enzymes in dentate gyrus are labelled with SOX2 (C, H). Higher-magnification images of MCAD+ cells (D–E) and TFP+ cells (I–J) in SVZ (D, I) and DG (E, J) are shown. In SVZ, fewer than 5% of MCAD+ cells co-label with KI67 and fewer than 5% of KI67+ cells co-label with MCAD. Most MCAD+ cells (in red) do not co-label with GFAP+ astrocytes (K–O), S100B+ ependymal cells (P–T), or DCX+ neuroblasts (U–Y). All tissues were stained with the pan-nuclear marker Hoechst (in blue) to demonstrate total cell number. Scale bars represent 20 µm.
Because MCAD is a critical enzyme in the beta-oxidation of medium-length fatty acids, all long-chain and medium-chain fatty acids will be processed by this enzyme. Therefore, if a cell undergoes fatty acid oxidation it will contain this particular enzyme. Therefore we have co-labelled tissues with a mouse monoclonal antibody against MCAD in combination with polyclonal antibodies against GFAP, S100B, and doublecortin to investigate the identity of cells capable of fatty acid oxidation.
Changes in metabolic gene expression during cellular maturation
Differentiation into a mature neuron or astrocyte may involve modulating the metabolic machinery by up-regulating gene products necessary for cell-specific metabolic activity. Recent studies have shown that differentiation of embryonic day 14 (E14) neural stem cells into astrocytes, by addition of fetal bovine serum (FBS) to the culture medium, induces astrocyte-specific metabolic characteristics such as glycogenolysis during the same timeframe necessary to develop typical morphological and immunohistochemical characteristics of astrocytes 23, 24. We hypothesized that differentiating cells in the adult brain may recapitulate developmental processes by becoming less dependent on beta-oxidation of fatty acids and becoming more dependent upon carbohydrates and monocarboxylates.
To test whether metabolic machinery is remodelled in neurogenic areas of the adult brain, we performed laser-capture micro-dissection and real-time PCR to quantify metabolic transcripts in NSPCs from adult mouse subventricular zone, adult-born neurons from the olfactory bulb, and embryonically-born neurons from cortex (Supplemental Figure 4A–E). Cells taken from olfactory bulb do not have significantly lower levels of transcripts for the fatty acid oxidation enzymes Long-Chain Acyl CoA Dehydrogenase (ACADL) and Carnitine Palmitoyl Transferase I (CPT1), although cortical cells do have significantly lower levels of these transcripts compared with SVZ cells. Like cortical cells, cells taken from olfactory bulb demonstrate significantly higher levels of transcripts for Monocarboxylate Transporter 2 (MCT2) compared with SVZ cells.
Next, we aimed to establish a timeline of metabolic gene expression during in vitro differentiation of adult NSPCs over 24 hours of serum exposure (Supplemental Figure 4D–L). We observed no changes in the quantity of transcripts for ACADL or CPT1 during this time period. However, we did observe significant increases in MCT2 transcripts. Together, these findings suggest that adult-born neurons and glia acquire lactate transport machinery during differentiation but do not lose expression of enzymes required for fatty acid oxidation. This change in gene expression manifests early, as cells are only starting to show morphological and immunohistochemical signatures of differentiation.
Substrates employed by NSPCs to support aerobic respiration
Previous studies have shown that changes in glucose concentration cause strong, sustainable changes in the oxygen consumption rate (OCR) of both neurons and astrocytes 12, 13. Here we tested the response of NSPCs to changes in glucose concentration using a flow culture system to measure OCR and the extracellular acidification rate caused by continuous lactate production (an indirect measure of glycolytic activity). We observed that OCR remains steady through changes in glucose concentration, although lactate production drops to zero within 25 minutes of glucose removal (Figure 2). Upon addition of glucose, a slight rise in OCR is observed, with the opposite effect observed upon removal of glucose. However, OCR returns to baseline levels shortly after any change in glucose concentration. These data suggest that neural stem cells do metabolize glucose, but are not dependent upon glucose to sustain aerobic respiration.
Since primary-cultured NSPCs do not require glucose to sustain aerobic respiration, they must have alternative metabolic fuel requirements than other cells within the adult brain. We hypothesized that fatty acids are instead used by NSPCs as substrates to power aerobic metabolism. To test this hypothesis, we compared the responses of undifferentiated and serum-differentiated NSPCs to the polyunsaturated fatty acid linoleic acid and the drug etomoxir, which inhibits the rate-limiting enzyme in fatty acid oxidation (carnitine palmitoyl transferase, CPT1). For these experiments, OCR was measured in live cells in real time using a Seahorse Analyzer. The addition of polyunsaturated fatty acids increases OCR significantly, even in the presence of alternative metabolic substrates such as L-glutamine or glucose (Figure 3A–C). Pharmacological inhibition of fatty acid oxidation significantly decreases OCR in all groups (Figure 3D–F); however cells with access to glucose can recover OCR upon increased concentrations of etomoxir (Figure 3D–E). Cells that had been exposed to serum for 48 hours prior to experiments demonstrate an abrogated response to etomoxir, only slightly decreasing OCR in response to this inhibitor of fatty acid oxidation (Figure 3D–F). However, these cells are still capable of increasing respiratory rate in response to linoleic acid (Figure 3A–C).
Effects of pharmacological inhibition of fatty acid oxidation in vitro
To test whether fatty acid oxidation plays a role in cellular survival and proliferation, we treated primary-cultured mouse NSPCs with 100µM etomoxir, 100µM linoleic acid, or a vehicle control, then stained with either KI67, a cell cycle marker, or TdT, a marker of apoptosis (Figure 4A–H). The fraction of KI67+ proliferating cells decreases with etomoxir treatment, while the fraction of TdT+ apoptotic cells does not change significantly in either treatment group. In a separate assay, we found that doses of etomoxir approaching 100 µM decrease cell number significantly without affecting cell viability (Figure 4I–J). We then subjected cells to FACS-based mitotic profiling as an additional measure of proliferative activity (Figure 4K–P). We again observed significant decreases in the fraction of actively cycling cells upon etomoxir treatment. Since etomoxir decreases KI67+ index, the total cell count, and the fraction of cells in S+G2/M phase of the cell cycle, without affecting the TdT+ or Trypan Blue+ cell population, the function of this catabolic pathway appears to be important for the proliferation of neural stem cells. These data suggest that inhibition of fatty acid oxidation blocks proliferation of NSPCs without affecting rates of apoptosis.
Figure 4. Inhibition of fatty acid oxidation decreases proliferation in cultured NSPCs, but does not affect cellular survival.
Sample photomicrographs of cells treated with PBS (A,D), 100 µM etomoxir (B,E), or 100 µM linoleic acid (C,F) are shown, stained with either KI67, an S-phase cell cycle marker (A–C), or TdT, a marker of apoptosis (D–F). Separate channels are shown to display Hoechst, a pan-nuclear marker (’), and TdT or KI67 (”). The fraction of KI67+ proliferating cells decreases after 24 hours treatment with etomoxir (p<0.05, G) and does not change after 24 hours of treatment with linoleic acid (p>0.05, not significant, G). The fraction of TdT+ apoptotic cells does not change significantly in either treatment group (p>0.05, not significant, H). The total cell count after 48 hours is significantly decreased in the presence of etomoxir at 100 µM concentrations (p<0.01, I) while cell viability remains unaffected (p>0.05, not significant, J). Proliferation was also assessed by FACS-based mitotic profiling of primary-cultured NSPCs. In one experiment, cells were treated with vehicle control (PBS, K) or 70 µM etomoxir (L). The fraction of cells in G2/M phase of the cell cycle decreased from control levels after 24 hours treatment with etomoxir (p<0.05, M). In a separate experiment, cells were treated with vehicle control (N) or 50 µM linoleic acid (O). The fraction of cells in G2/M phase of the cell cycle increased upon treatment with linoleic acid (p<0.05, P). Scale bars represent 20 µm.
Effects of pharmacological inhibition of fatty acid oxidation in vivo
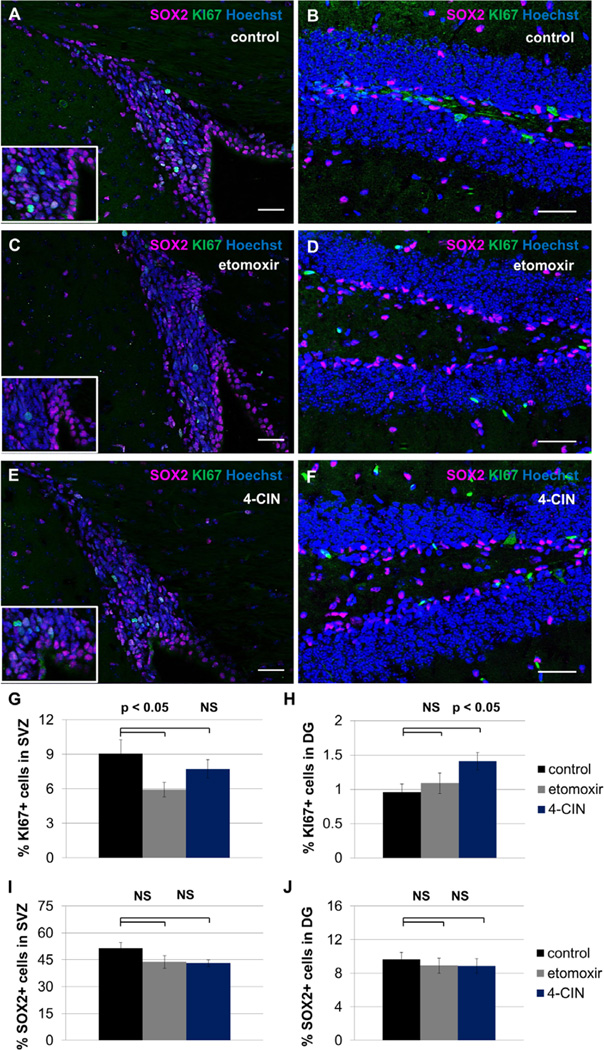
To test whether inhibition of fatty acid oxidation blocks proliferation of NSPCs in vivo, female mice between 5–7 weeks of age were injected intraperitoneally on three consecutive days with 40mg/kg etomoxir, 40mg/kg alpha-cyano-4-hydroxycinnamate (4-CIN), or similar volumes of saline. The fraction of KI67+ cells and the fraction of SOX2+ cells were assessed immunohistochemically (Figure 5). We observed a significant decline in KI67+ cells in the subventricular zone in etomoxir-treated animals but no significant decline in KI67+ cells in this area in 4-CIN-treated animals. The SOX2+ population in SVZ is unaffected in both treatment groups. We observed no decline in KI67+ cells in the hippocampal dentate gyrus; indeed, 4-CIN treatment was associated with a significant increase in mitotic index in this area. SOX2+ cells in DG are unaffected by treatment with either etomoxir or 4-CIN. These data suggest that SVZ and hippocampal DG have different responses to metabolic inhibitors which might be predicted by enzyme expression patterns observed in Figure 1
Figure 5. Inhibition of fatty acid oxidation decreases proliferation in SVZ but not hippocampal dentate gyrus.
Adult mice received three injections of saline (A, B), 40mg/kg etomoxir (C, D) or 40mg/kg alpha-cyano-4-hydroxycinnamate (4-CIN) (E, F). The fraction of KI67+ cells and the fraction of SOX2+ cells was assessed within SVZ (A, C, E) and hippocampal dentate gyrus (B, D, F). Etomoxir treatment is associated with a significant decrease in KI67+ cells in SVZ (p<0.05, G), while the effect of 4-CIN on this population was not significant (p>0.05, G). KI67+ cell number in the hippocampal dentate gyrus was unchanged upon etomoxir treatment (p>0.05, H), but significantly increased upon 4-CIN treatment (p<0.05, H). The SOX2+ progenitor cell population was unaffected in both areas in both treatment groups (p>0.05, not significant, J). Scale bars represent 40 µm.
Rescue of neurogenic activity in the aged brain by increasing aerobic metabolic capacity
Few neurons are produced in the aged brain, and the majority of neurogenic decline occurs by middle age. Aged NSPCs exhibit decreases in both mitochondrial content and aerobic respiratory activity 25. We hypothesized that expanding the aerobic capacity of the remaining population of NSPCs in the aged brain may boost neurogenesis and rescue regenerative activity. To do so, we performed lentiviral-mediated rescue of a mitochondrial biogenesis factor within aged SVZ NSPCs. The target gene, Peroxisome proliferator-activated receptor Gamma Co-activator 1 alpha (PGC1α), induces mitochondrial biogenesis, coordinating an increase in mitochondrial number and metabolic gene expression, which has been shown to rescue oxidative respiration in cardiac myocytes 26, 27.
To test whether PGC1α affects metabolic gene expression in NSPCs, we overexpressed PGC1α-GFP or GFP alone (as a control) in cultured NSPCs from the aged mouse brain. Lentiviral-mediated transduction increases PGC1α expression and MCAD+ mitochondrial content (Figure 6A–K). Grown in proliferation medium, cultures transduced with PGC1α have a higher KI67+ index compared with controls (Figure 6L–N). Sustained in differentiation medium (2% B27 in Neurobasal Medium), cultures transduced with PGC1α have no difference in DCX+ index or viability (Figure 6O–R). These results indicate that PGC1α increases respiratory capacity and proliferative activity, but do not affect the propensity of cells to survive or undergo neuronal differentiation.
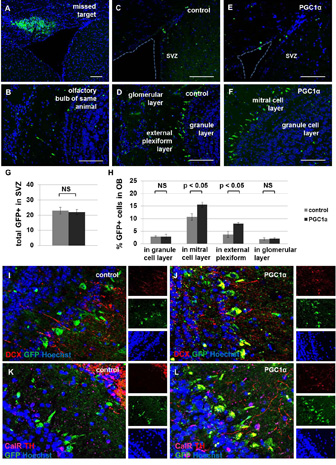
We targeted NSPCs within SVZ for genetic modification by intracerebral injection of high-titer lentivirus tagged with green fluorescent protein (GFP). Two groups of mice, aged 21 months, were injected with either the control virus expressing GFP or the virus expressing PGC1α tagged with GFP under the same constitutive promoter. The animals were sacrificed two months after surgery. We observed similar numbers of GFP+ cells remaining in the subventricular zone of control and experimental animals; however, ectopic expression of PGC1α was associated with an increased number of GFP+ cells in some areas of the olfactory bulb, specifically the mitral cell layer and the external plexiform layer (Figure 7A–H). While GFP+ cells in the olfactory bulb of control animals did not co-label with neuronal markers, PGC1α-overexpressing GFP+ cells co-labelled with doublecortin and tyrosine hydroxylase (Figure 7I–L).These data suggest that increased metabolic capacity can promote the regenerative potential of aged NSPCs to a limited extent.
Figure 7. Ectopic PGC1α expression increases SVZ-based neurogenesis in aged mice.
Injection of lentivirus into a target location with non-migratory cells demonstrates the extent of infection in the mouse brain two months post-injection (A). The olfactory bulb (OB) in this same animal is observed to contain few GFP+ cells, as expected (B). The remainder of mice were injected into the target location containing proliferative cells along the lateral ventricle. These animals have very few GFP+ cells in the SVZ when analyzed two months after inducing ectopic expression of GFP alone (C) or PGC1α + GFP (E). Most GFP+ cells are observed in the olfactory bulb two months after infection with virus encoding GFP alone (D) or PGC1α + GFP (F). The total number of GFP+ cells in SVZ was not significantly different between groups (p>0.05, G). The fraction of total cells expressing GFP was significantly higher in the PGC1α group compared with the control group in some areas of olfactory bulb, specifically in the mitral cell layer and the external plexiform layer (p<0.05, H) but not in the granule cell layer or the glomerular layer (p>0.05, H). GFP+ cells in OB overexpressing PGC1α co-labelled with doublecortin (DCX, I–J) and tyrosine hydroxylase (TH, K–L), but not calretinin (CalR, K–L). Note: NS, not significant. Scale bars represent 100 µm.
Discussion
Molecular mechanisms shown to regulate the rate of adult neurogenesis include both cell-intrinsic factors, such as tumor suppressor proteins 28–30, and factors within the microenvironment, such as growth factors 31. In addition, some behaviors affect neurogenic activity. For example, voluntary exercise has been shown to increase cellular proliferation in the adult hippocampus 2 while diets high in monounsaturated fatty acids decrease the BrdU+ proliferative cell index in the adult hippocampus 32. Although molecular pathways within the cell and factors within the microenvironment have been proposed to regulate the rate of adult neurogenesis 33, a blood-borne factor directly linking behavioral activity with neurogenic activity has not been identified. Metabolic fuel availability may provide such a link. Manipulating the fuel availability of neural stem cells may indeed uncover mechanisms by which organismal behavior, energy consumption, and cellular activity are coupled in the adult mammalian brain.
In this study, we identify the metabolic fuel requirements of NSPCs and the underlying enzymatic machinery in these cells and we describe how both the enzymatic machinery and the physiological response to catabolic substrates changes during the process of differentiation. In addition, we tested whether the availability of metabolic substrates, particularly fatty acids, can limit proliferative activity in vivo using pharmacological and genetic methods.
Fatty acids are present in the bloodstream of adult mammals, commonly bound to serum albumin. The concentration of free fatty acids in human blood has been estimated at 444 µM, or 74% of albumin concentration estimated at 600 µM 34. The total concentration of fatty acids in human serum has been estimated at 3290 µg/mL, including saturated fats, monounsaturated fatty acids and polyunsaturated fatty acids 35. A full 30% of these fatty acids (1009 µg/mL) in human blood plasma are in the form of linoleic acid, which is present in quantities approaching 3.6mM. Linoleic acid is therefore a readily available metabolic substrate.
Fatty acids can enter a cell directly or be produced within the cell itself. Indeed fatty acids have been shown to be produced endogenously in adult NSPCs 36. These fatty acids can be used as raw materials to build cell membranes or be metabolized into cannabinoids, eicosenoids, and other bioactive agents. Alternatively, they can be used to produce energy within the mitochondrion by a process called beta-oxidation. To enter the mitochondrion, a fatty acid must be esterified to acyl CoA by Acyl CoA Synthetase. Within the inter-membrane space, the fatty acyl-CoA is then converted to an acyl-carnitine by Carnitine Palmitoyl Transferase I (CPT1) and is transported across the inner mitochondrial membrane by Carnitine Translocase. Once inside the mitochondrial matrix, the carnitine is coverted back into a fatty acyl-CoA. This molecule is first oxidized by a chain-length-specific Acyl CoA Dehydrogenase (i.e. MCAD, LCAD), in the process reducing FAD to FADH2. The resulting molecule is hydrated, oxidized again, and then cleaved into a fatty acyl-CoA and acetyl-CoA in a final step called ketothiolysis. These three steps are performed by the same enzyme, TriFunctional Protein (TFP). The acetyl-CoA molecule is used in other bioenergetic pathways such as the Kreb’s Cycle, while the remaining fatty acyl-CoA (now two carbons shorter than the original molecule) restarts the four-step process of beta-oxidation. Critically, beta-oxidation can be inhibited pharmacologically with etomoxir, which blocks the activity of the rate-limiting transport enzyme CPT1.
The various experiments performed in this study yield a consistent image of the metabolic substrates preferred by NSPCs and their newly-differentiated progeny. SOX2+ progenitor cells in SVZ but not hippocampal dentate gyrus express enzymes required for fatty acid oxidation, and inhibition of this pathway in vivo decreases proliferative activity within SVZ but not DG. As expected, cellular differentiation, both in vivo and in vitro, leads to increased expression of the monocarboxylate transporter. However, the same cells do not lose expression of fatty acid oxidation enzymes. Correspondingly, serum-exposed NSPCs retain responsiveness to linoleic acid but are unaffected by inhibition of fatty acid oxidation. The differentiated cells therefore seem able to metabolize either glucose or fatty acids, depending on substrate availability, better than the undifferentiated cells. Undifferentiated NSPCs rely greatly upon fatty acid oxidation for their proliferative activity, since etomoxir treatment leads to significant decreases in aerobic respiratory activity, KI67+ index, and FACS mitotic profile. We observed significant increases after linoleic acid treatment in FACS-based assays but not in KI67+ labelling index. This discrepancy may be due to the difference in a suspension assay versus a monolayer assay. A detergent effect of the fatty acid could have caused mitotic cells loosely adherent to the coverslip to be washed away during staining; since all cells were spun down and collected for FACS-based mitotic profiling, such an occurrence would not have led to a loss of cells. The necessity of fatty acid oxidation for neurogenic activity was borne out by further experiments in vivo. The relative importance of fatty acids over lactate was demonstrated in a pharmacological study using etomoxir and 4-CIN, respectively. The results in the SVZ were consistent with other findings in this study, supporting the requirement of fatty acid oxidation for proliferative activity. However, the results in the hippocampus were intriguing. It appears that inhibiting lactate metabolism with 4-CIN actually boosts stem cell identity and activity in DG. Perhaps new neurons are forced to remain in cell cycle when rendered unable to utilize the preferred substrate of neurons. Further experiments are needed to understand the metabolic fuel requirements of NSPCs in DG.
The remaining population of stem-like cells in the aged brain demonstrate decreased aerobic capacity. Specifically, aged NSPCs have decreased mitochondrial mass and lower baseline oxygen consumption rates compared with young NSPCs 25. In this report we show that expanding the aerobic capacity of the remaining population of NSPCs in the aged brain, by ectopically expressing PGC1α within the SVZ, increases mitotic activity in vitro and boosts new neuron number in some areas of the olfactory bulb. PGC1α is a broad regulator of aerobic metabolism, increasing mitochondrial content, mitochondrial DNA replication, and metabolic gene expression 26, 27, 37. Yet this factor can have other effects on a cell, particularly on neuronal gene expression. Interestingly, one recent study demonstrated reduced parvalbumin (PV) expression and a decreased number of PV interneurons in animals lacking PGC1α 38 while another study demonstrated reduced tyrosine hydroxylase (TH) expression upon ectopic PGC1α expression in dopaminergic neurons 39. These studies suggest that PGC1α may play a critical role in establishing cellular identity in the central nervous system. The mechanisms by which catabolic strategies and mitochondrial remodelling link to neuronal physiological function remains an active area of study.
Since catabolic activity is a fundamental characteristic of a cell, exquisitely optimized to meet energetic needs and constraints, the identification of metabolic substrates required by the adult neural stem cell is imperative to understanding the process of regeneration. It is possible that different cell types rely upon different bioenergetic fuels. Different neuronal and glial sub-types in the brain may have distinctive “batteries” which confer cell-specific properties, like glycolytic fast-twitch and fat-burning slow-twitch fibers in skeletal muscle tissue. In addition, molecular sensors that coordinate catabolic and anabolic activity within brain cells may contribute to sensing the local availability of bioenergetic substrates and adjusting physiological activity accordingly. Characterizing cell-specific metabolic machinery may therefore inform on cellular identity and physiological function.
In light of this study, an improved understanding of metabolic limitations on cellular proliferation may aid strategies to boost regenerative potential in the injured or aged central nervous system. However, further testing is needed before an expansion of metabolic capacity can be considered as an approach to counter age-related cognitive decline. In particular, it is important to assess whether such interventions can enhance cognition in animal models and whether any side effects are observed from augmenting proliferation in the endogenous neural stem/progenitor population.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Mr Behnum Habibi, Ms Claire Boothman, Mr Don Maris, Ms Jennifer Webb, Dr Casey Wilson, Dr John Yarham, Dr Stuart Williamson, and Dr Francesco Bruni for technical assistance. This project was funded by Newcastle University Centre for Brain Ageing and Vitality (supported by the Biotechnology and Biological Sciences Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council and Medical Research Council [G0700718]), the UK NIHR Biomedical Research Centre in Age and Age Related Diseases award to the Newcastle upon Tyne Hospitals NHS Foundation Trust, MRC Centre for Neuromuscular Disease [G000608-1], and The Wellcome Trust Centre for Mitochondrial Research [096919/Z/11/Z]. This work was also supported in part by and the DRC Cell Functional Analysis Core (NIH Grant DK17047, to I.R.S.). This work was not supported by (NIH Grant AG038305, awarded to P.J.H.)
Footnotes
Author Contributions:
E.A.S designed, performed, and analysed experiments. R.M. performed cell culture, tissue cryosectioning, immunohistochemistry, and microscopy for experiments shown in Figures 4 and 5. I.R.S. contributed intellectually and designed experiments shown in Figures 2 and 3. A.T. contributed intellectually and performed surgeries for experiments shown in Figure 7. S.M. assisted with Seahorse experiments shown in Figure 3. Initial experiments (metabolic physiology shown in Figure 2 and ViCell/FACS analysis shown in Figure 4) were performed in the lab of P.J.H. All other experiments were performed in the lab of D.M.T. who provided funding, resources, and intellectual support. E.A.S. wrote the manuscript, and all other authors contributed edits.
The authors declare no competing financial interests and no potential conflicts of interest.
References
- 1.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;15:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla ALD. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 4.Stoll EA. Advances toward regenerative medicine in the central nervous system: challenges in making stem cell therapy a viable clinical strategy. Molecular and cellular therapies. 2014;2 doi: 10.1186/2052-8426-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 8.Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Itoh Y, Esaki T, Shimoji K, et al. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc Natl Acad Sci U S A. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 11.Bouzier-Sore AK, Voisin P, Canioni P, et al. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab. 2003;23:1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- 12.Wyss MT, Jolivet R, Buck A, et al. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna MC, Hopkins IB, Carey A. Alpha-cyano-4-hydroxycinnamate decreases both glucose and lactate metabolism in neurons and astrocytes: implications for lactate as an energy substrate for neurons. J Neurosci Res. 2001;66:747–754. doi: 10.1002/jnr.10084. [DOI] [PubMed] [Google Scholar]
- 14.Sack MN, Rader TA, Park S, et al. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan KJ, Bender A, Taylor RW, et al. A multiplex real-time PCR method to detect and quantify mitochondrial DNA deletions in individual cells. Analytical biochemistry. 2007;370:127–129. doi: 10.1016/j.ab.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Stoll EA, Habibi BA, Mikheev AM, et al. Increased re-entry into cell cycle mitigates age-related neurogenic decline in the murine subventricular zone. Stem Cells. 2011;29:2005–2017. doi: 10.1002/stem.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet IR, Khalil G, Wallen AR, et al. Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Technol Ther. 2002;4:661–672. doi: 10.1089/152091502320798303. [DOI] [PubMed] [Google Scholar]
- 20.Wu M, Neilson A, Swift AL, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lam TKT, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 22.Lopez M, Lelliott CJ, Vidal-Puig A. Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. Bioessays. 2007;29:248–261. doi: 10.1002/bies.20539. [DOI] [PubMed] [Google Scholar]
- 23.Brunet JF, Grollimund L, Chatton JY, et al. Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia. 2004;46:8–17. doi: 10.1002/glia.10348. [DOI] [PubMed] [Google Scholar]
- 24.Brunet JF, Allaman I, Magistretti PJ, et al. Glycogen metabolism as a marker of astrocyte differentiation. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoll EA, Cheung W, Mikheev AM, et al. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J Biol Chem. 2011;286:38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 27.Lehman JJ, Barger PM, Kovacs A, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jablonska BAA, Vandenbosch R, Belachew S, Berthet C, Kaldis P, Gallo V. Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone. J Cell Biol. 2007;179:1231–1245. doi: 10.1083/jcb.200702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medrano S B-CM, Atienza MB, Rahimi D, Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikheev AM, Stoll EA, Mikheeva SA, et al. A syngeneic glioma model to assess the impact of neural progenitor target cell age on tumor malignancy. Aging Cell. 2009;8:499–501. doi: 10.1111/j.1474-9726.2009.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 32.Lindqvist A, Mohapel P, Bouter B, et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 33.Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10:59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- 34.Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–240. [PubMed] [Google Scholar]
- 35.Harris JI, Hibbeln JR, Mackey RH, et al. Statin treatment alters serum n-3 and n-6 fatty acids in hypercholesterolemic patients. Prostaglandins Leukot Essent Fatty Acids. 2004;71:263–269. doi: 10.1016/j.plefa.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Knobloch M, Braun SMG, Zurkirchen L, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 38.Lucas EK, Markwardt SJ, Gupta S, et al. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark J, Silvaggi JM, Kiselak T, et al. Pgc-1alpha overexpression downregulates Pitx3 and increases susceptibility to MPTP toxicity associated with decreased Bdnf. PLoS One. 2012;7:e48925. doi: 10.1371/journal.pone.0048925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.