Abstract
Background
The Brain-derived Neurotrophic Factor (BNDF) Val66Met variant and HMG-COA reductase inhibitors (statins) have been implicated in insulin resistance and diabetes risk. We sought to determine the effect of the BDNF Met variant and statin use on insulin resistance in schizophrenia and bipolar disorder using the homeostasis model assessment of insulin resistance (HOMA-IR).
Methods
A cross-sectional design was used and patients with diabetes or receiving medications affecting glucose regulation were excluded. Associations between insulin resistance and genotype were analyzed by ANOVA and regression analysis. Subjects were grouped by BDNF genotype as well as statin use.
Results
252 subjects with a mean age of 44 years were included. The group was 53% male and 59% had a schizophrenia diagnosis; 78% and 19% were receiving atypical antipsychotics(AAPs) and statin medications, respectively. Analysis showed schizophrenia subjects with the BDNF met allele as well as schizophrenia subjects with the BDNF met allele and statin combination had significantly higher HOMA-IR values compared to the other groups (p=0.046 and p=0.016, respectively).
Conclusions
These results suggest that in the metabolically high-risk population of schizophrenia the BDNF met allele alone and in combination with statin medications is associated with higher insulin resistance values. This was not seen in the bipolar population. Further validation of these associations remains necessary.
Introduction
Due to the overlapping nature of the psychopathology often seen with schizophrenia and bipolar disorder, antipsychotic use is a common therapeutic approach for patients with these serious mental illnesses. With the advent of the atypical antipsychotics (AAPs), the rates of undesirable side effects such as pseudoparkinsonism and tardive dyskinesia has significantly decreased, however metabolic complications such as weight gain and type II diabetes mellitus has increased within both of these patient populations1,2. In addition to the metabolic effects of the AAPs, poorer physical health, lifestyle habits and pharmacogenetics have been touted as having a role in the development of type II diabetes within these populations3–6. While many different pharmacogenetic variants have been implicated in the development of impaired glucose tolerance and other metabolic dysregulations seen with AAP use7–9, one that is currently emerging within the general population is the Brain-Derived Neurotrophic Factor (BDNF)10–12. The BDNF Val66Met (196 G/A) variant has been implicated in both schizophrenia and bipolar disorder due to its role in neuronal health, development and function. In general, the BDNF Val66Met variant has been shown to affect the predisposition, cognition, and brain structure in both illnesses13–15.
Outside of mental health, the BDNF protein and its Val66Met variant are involved in glucose regulation as BDNF levels have been shown to be low in the serum of animals and patients with insulin resistance, abnormal glucose regulation and diabetes10,16,17. Pharmacogenetically, a BDNF gene variant coding for its corresponding protein production has been identified at codon 66 resulting in a methionine (Met) being substituted for a valine (Val) amino acid. This substitution results in a drastic impairment of BDNF packaging and distribution18, and in animal and human models within the general population, the Met allele is associated with an increased risk of diabetes and insulin resistance through altered regulation of glucose metabolism and feeding habits19. Given the strong relationship between mental illness, AAP treatment, and glucose regulation, it is possible that BDNF genetic variability may mediate development of type 2 diabetes (T2DM) in a large percentage of patients treated with AAPs. However, the relationship between this variant and glucose dysregulation seen in schizophrenia and bipolar disorder has not been studied. While all metabolic complications carry a certain degree of risk, development of T2DM adds additional risks, associated with the micro and macrovascular complications, especially if other components of the metabolic syndrome are also present. Therefore, these patients require careful clinical monitoring.
It is standard of practice to treat the cholesterol abnormalities seen with AAP use with hydroxymethylglutaryl CoA reductase (HMG-CoA reductase) inhibitors also known as “statins”. These medications, while effective for lowering total cholesterol, have recently been implicated in increasing the risk of diabetes and insulin resistance and have also been found to have effects on circulating BDNF and the cleavage of pro-BDNF in certain populations20–23. Several meta-analyses have been published identifying this diabetes risk24–26 which culminated in additional warnings from the Food and Drug Administration (FDA) being added to the product labels of all medications within this class (i.e. atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin, lovastatin). Although this warning applies to any patient taking statin medications, the FDA advises their cautious use in populations at increased risk for diabetes like that of schizophrenia and bipolar disorder, however work delineating the true extent of this risk in those with a serious mental illness is unknown.
The primary aim of this investigation was to determine the relationship between the BDNF 66 Met allele and insulin resistance in schizophrenia and bipolar patients. Also, given the recent evidence of increased insulin resistance in at-risk populations using statin medications, we also sought to determine the relationship between statin medication use, BDNF 66Met allele carriers and insulin resistance in this same cohort.
Materials and Methods
Subjects and Data Collection
Participants were recruited from Southeastern Michigan mental health clinics and those meeting the following inclusion criteria were considered for the study: (i) Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, or bipolar disorder, (ii) 18–90 years old, (iii) no medication changes in the prior 6 months. Participants were excluded if they were unable or unwilling to give informed consent, had diabetes prior to starting mental health treatment, had any condition that would significantly affect glucose measures and insulin resistance analysis or had an active substance abuse diagnosis. The protocol was approved by the University of Michigan Institutional Review Boards.
Study visits were timed to take place within 2 hours of the subject’s usual wakening time, with most visits being completed by 11am. Height, weight, and hip and waist circumference were measured in all participants. Fasting plasma glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) measures were obtained, and the homeostasis model assessment of insulin resistance (HOMA-IR)27 was calculated for each patient using the following formula:
HOMA-IR = [fasting insulin (pIU/ml) × fasting blood glucose (mmol/L)]/22.5
Although various thresholds for insulin resistance have been identified28–31, work by Ascaso et. al. has identified a HOMA-IR above 2.6 as a threshold for insulin resistance32. This value was used since 1) HOMA-IR is more commonly used in research literature than other simple quantitative insulin resistance measurements, 2) the ease of which HOMA-IR can be applied to the clinical setting and 3) this value represents the 75th percentile of HOMA-IR values from a random sample of normal glucose metabolizing patients.
Subjects were excluded from this analysis if they had a documented diabetes diagnosis or were receiving treatment for diabetes since as can be seen from the calculation of HOMA-IR, treatment for diabetes would artificially lower insulin resistance values giving a false negative finding.
Each patient was assessed for metabolic syndrome using the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III Criteria)33 which include meeting at least 3 of the following criteria: (i) Waistline of >40 inches (men) or 35 inches (women), (ii) blood pressure ≥130/85 mmHg or currently being treated for hypertension with medication(s), (iii) triglycerides >150mg/dL, (iv) HDL < 50mg/dL (men) or 40mg/dL (women) or current lipid medication treatment and (v) fasting plasma glucose ≥100mg/dL or current diabetes medication treatment.
The medication histories of each participant were collected by medical record and patient interview. This included past or current antipsychotic, antidepressant or mood stabilizer use, current prescription drug use, and current non-prescription drug use including over-the-counter and herbal medications. Social histories were also completed for each participant and included past or present smoking habits and alcohol intake. Subjects were segregated in order to account for the differences in metabolic side effects for a given antipsychotic to include the following groupings: 1) olanzapine or clozapine, 2) quetiapine, risperidone, paliperidone or iloperidone and 3) all other antipsychotics or no antipsychotic.
Analytical Methods
Gene analysis was completed by first extracting DNA from whole blood using the salt precipitation method34. For genotyping the BDNF Val66Met variant (DBSNP rs6265) pyrosequencing primers were designed using Pyrosequencing Nucleotide Polymorphism (SNP) Primer Design Version 1.01 software (http://www.pyrosequencing.com) and genotypes were determined through the use of Pyrosequencing35. Conditions for the specific assay are available upon request.
Statistical Analysis
Due to the anticipated low genotype frequency of the BDNF 66MetMet genotype, the statistical analysis was performed by forming two groups: patient’s carrying the ValVal genotype and those carrying a Met allele (ValMet or MetMet genotypes). For our second aim, the same genotype groups were used, but they were stratified by presence or absence of statin use. The primary group of interest within this second aim was those subjects taking statins and carrying a 66 Met Allele compared to all others. Student t-tests and chi-square analyses were used to compare baseline demographics based on the two genotype groupings. ANOVA looked at the relationship of genotype, statins and HOMA-IR. Regression analysis was performed to analyze the relationship between BDNF genotype status, HOMA-IR, while controlling for variables we found to significantly effect HOMA-IR. HOMA-IR was used as the dependent variable while the genotype-based groups and the variables affecting HOMA-IR (detailed in results) were used as the independent variables. Finally, we first completed the analysis on the combined sample of our schizophrenia and bipolar cohort in order to assess an overall pharmacogenetic effect of the BDNF Val66Met genotype, statins and AAP use. We then analyzed based on each diagnosis given the differences in pathogenesis and medication use for these two diseases. All analyses were conducted using the JMP 9.0 statistical program. A p-value <0.05 with a 95% confidence interval was considered significant for this study.
Results
The initial screening population consisted of 314 males and females with schizophrenia or bipolar spectrum disorders. After excluding based on the criteria above (diagnosis of diabetes or current diabetes treatment), our final sample size for data analysis was 252 (148 with a schizophrenia spectrum diagnosis and 104 with a bipolar diagnosis). Of this sample of 252 subjects, 29% had schizophrenia, 24% had schizoaffective disorder, 6% had a diagnosis of schizophreniform disorder and 41% had bipolar I or II disorder. The average age of our population was 44.0 ± 11.9 years and 53% were male. Seventy-one percent were Caucasian, 22% were African-American and the rest (7%) were American Indian, Asian, Latino or Hispanic. The genotype frequencies for the BDNF Val66Met variant were 74% ValVal, 23% ValMet and 3% MetMet which was in Hardy Weinberg Equilibrium for the entire cohort as well as for each diagnosis spectrum (all p > 0.26). Eight participants were unable to be genotyped. Finally, approximately 19% of subjects in each diagnostic group was currently using statins at the time of this study. The most commonly used statin in our sample was simvastatin (50% of statin users) followed by atorvastatin (17% of statin users). There was no significant difference in statin type used between the schizophrenia and bipolar populations (p=0.2). The demographic data for the combined sample and for each diagnosis are in Table 1.
Table 1.
Analysis of Demographic Variables
| Variable | Schizophrenia Spectrum Disorders (n=148) |
Bipolar Disorder (n=104) |
Combined Sample (n=252) |
|---|---|---|---|
| Age (years ± s.d) | 44.8 ± 11.7 | 42.7 ± 12.0 | 43.9 ± 11.9 |
| % Male* | 66 | 35 | 53 |
| % Caucasian/%African American* | 61/30 | 84/11 | 71/22 |
| % On Atypical Antipsychotics* | 83 | 70 | 78 |
| % On Clozapine or Olanzapine/% On Risperidone, Quetiapine, Iloperidone or Paliperidone* | 28/37 | 15/56 | 23/43 |
| % On Statins | 19 | 18 | 19 |
| % Smoking* | 49 | 32 | 42 |
| % With ATP III/NCEP Metabolic Syndrome | 30 | 26 | 29 |
| BMI (kg/m2 ± s.d.) | 31.4 ± 7.15 | 32.1 ± 8.88 | 31.7 ± 7.9 |
| Blood pressure (mmHg ± s.d.) | 122/75 ± 16/12 | 124/73 ± 18/11 | 123/74 ± 17/11 |
| Total Cholesterol (mg/dL ± s.d.)* | 176 ± 39.2 | 192 ± 44.7 | 182 ± 42.2 |
| Triglycerides (mg/dL ± s.d.) | 131 ± 89.3 | 138 ± 104 | 134 ± 95.7 |
| High-Density Lipoprotein (mg/dL ± s.d)* | 52.7 ± 16.3 | 58 ± 14.9 | 54.9 ± 15.9 |
| Low-Density Lipoprotein (mg/dL ± s.d.)* | 108 ± 30.6 | 118 ± 38.2 | 112 ± 34.3 |
| Insulin (mU/L ± s.d.) | 22.9 ± 15.2 | 24.2 ± 20.8 | 23.5 ± 17.7 |
| Glucose (mg/dL ± s.d.) | 96.1 ± 12.5 | 96.0 ± 12.0 | 96.0 ± 12.3 |
| HbA1C% | 5.63 | 5.55 | 5.60 |
| HOMA-IR ± s.d. | 5.51 ± 3.92 | 5.91 ± 5.46 | 5.68 ± 4.62 |
| % BDNF Val66Met Genotype (%) | 73 ValVal/24 ValMet/3 MetMet | 77 ValVal/20 ValMet/3 MetMet | 74 ValVal/23 ValMet/3 MetMet |
| % With BDNF 66Met Allele | 28 | 23 | 26 |
indicates p<0.05 when comparing the Schizophrenia and Bipolar samples
Comparisons of the schizophrenia and bipolar cohorts (for the purposes of establishing a combined cohort) found a few differences that were significant. The schizophrenia cohort had a higher percentage of male and African-American subjects compared to the bipolar cohort (66% vs 35% and 30% vs 11%, respectively p < 0.05). There were also more schizophrenia subjects receiving atypical antipsychotics (83% vs. 70%, p=0.02) and differences in the percentage of subjects who were current smokers (49% vs. 32%, p=0.01) as well as differences in lipid levels between the two cohorts (See table 1 for percentage comparisons for following values. Total Cholesterol: p=0.003; HDL: p=0.01; LDL: p=0.02).
In preliminary analysis we found that in the combined sample, the Met allele carriers were older (46 versus 43 years old, p=0.043), less likely to be African-Americans (5% versus 26%, p=0.005), and had higher AAP use (89% versus 73%, p=0.010). There were no significant differences based on genotype when looking at the schizophrenia and bipolar sample individually.
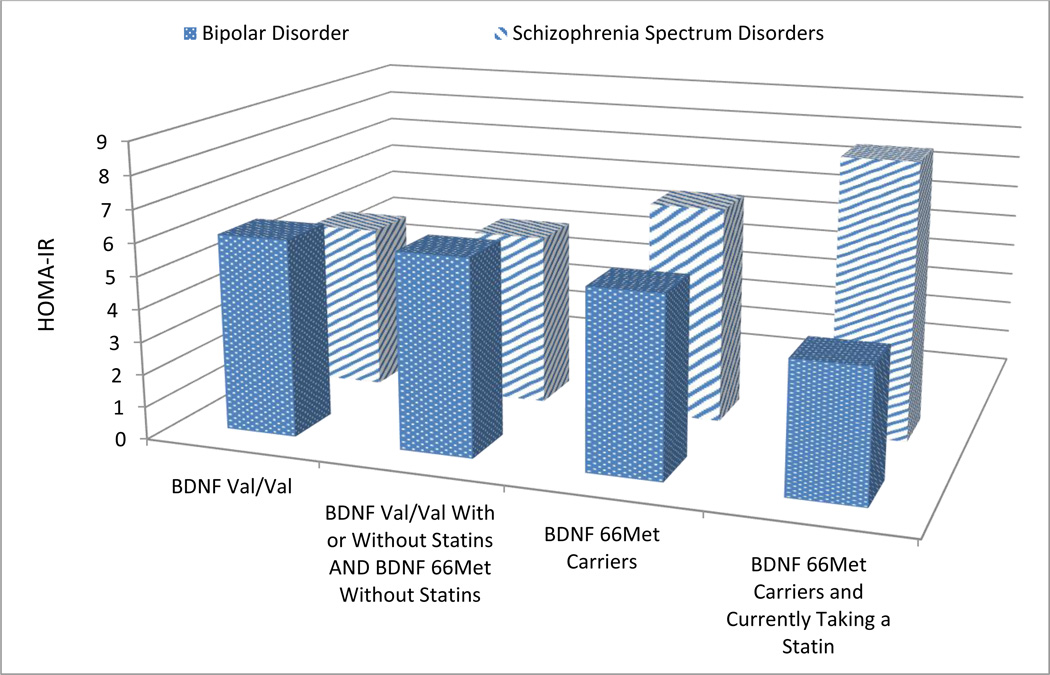
No significant relationship was found between the BDNF 66 Met allele and HOMA-IR for the group as a whole (Met allele HOMA-IR of 6.18 ± 0.594 vs. Val/Val genotype HOMA-IR of 5.52 ± 0.352, p=0.3). However when the group was segregated by primary psychiatric diagnosis, we found a significant effect of the BDNF 66Met allele on HOMA-IR in the schizophrenia sample where schizophrenia subjects carrying the Met allele had higher HOMA-IRs (p=0.046). No relationship was found for the bipolar group (analysis in Table 2, Figure 1).
Table 2.
Analysis of HOMA-IR based on BDNF Met carrier and Statin Status.
| Schizophrenia Spectrum Disorders |
Bipolar Disorder | Combined Sample | ||||
|---|---|---|---|---|---|---|
| Subject Groups |
HOMA-IR ± s.d. |
P-value | HOMA-IR ± s.d. |
P-value | HOMA-IR ± s.d. |
P-value |
| BDNF Val66 Carriers | 5.10 ± 0.400 | 0.046 | 6.04 ± 0.621 | 0.7 | 5.52 ± 0.352 | 0.3 |
| BDNF 66Met Carriers | 6.63 ± 0.642 | 5.47 ± 1.12 | 6.18 ± 0.594 | |||
| BDNF Val66 With or Without Statins AND BDNF 66Met Without Statins | 5.30 ± 0.350 | 0.016 | 5.99 ± 0.553 | 0.5 | 5.60 ± 0.312 | 0.2 |
| BDNF 66Met Carriers and Currently Taking a Statin | 8.45 ± 1.24 | 3.99 ± 2.74 | 7.15 ± 1.25 | |||
Figure 1. Graph of Analysis of HOMA-IR based on BDNF Met carrier and Statin Status.
This figure depicts the analysis detailed in Table 2. The groups “BDNF Val/Val” and “BDNF 66Met Carriers” are from the primary analysis of this study looking at the pharmacogenetic effect of BDNF Val66Met on insulin resistance. The groups “BDNF Val/Val With or Without statins AND BDNF 66Met Without Statins” and “BDNF 66Met Carriers and Currently Taking a Statin” are from the secondary analysis stratifying BDNF Val66Met status by current statin use. The figure depicts the differing effects seen in the bipolar and schizophrenia samples of adding these variables together on the insulin resistance measure of HOMA-IR.
There were no effects of statin medications on BDNF 66Met allele carriers in the entire cohort (Met allele plus statin HOMA IR =7.15 ± 1.25 vs. all other HOMA-IRs =5.6 ± 0.312, p=0.2). However, when subjects were segregated by diagnosis, schizophrenia subjects currently taking statin medications and carrying the Met allele had significantly higher HOMA-IRs compared to all other schizophrenia subjects (p=0.016). No relationship was found in the bipolar cohort (analysis in Table 2).
In addition to the previous analysis, a linear regression was performed for each dependent variable (e.g., BDNF and statin status) in which we controlled for age, race, AAP use, BMI and metabolic syndrome, due to the relationship between these variables, body habitus (primarily obesity) and insulin resistance as defined by HOMA-IR. This analysis again yielded no significant results for the bipolar sample. However, in the combined sample, there was a significant interaction between BMI and BDNF 66 Met carriers on HOMA-IR (f(1,1) = 3.90, p=0.049), where those with this allele, had higher levels of insulin resistance and similar BMIs compared to the Val/Val genotype groups. Given the number of analyses performed in this study we identify this result in the combined group as a trend that is driven by the findings within the schizophrenia subset. Within the schizophrenia subset, the effect of the BDNF 66 Met allele on HOMA-IR was significant by itself (f(11,135)=4.80, p=0.030) as well as 66 Met’s interaction with BMI on HOMA-IR (f(1,1) = 9.04, p=0.0032). Finally, only the interaction between BMI and genotype was seen in the schizophrenia sample when the regression analysis was stratified by statin use (f(1,1) = 6.26, p=0.037), where those with the 66 Met allele receiving a statin also had higher levels of insulin resistance at comparable BMIs.
Discussion
As part of this study we found a significant relationship between BMI, the BDNF 66 Met allele and insulin resistance in a schizophrenia population primarily treated with AAPs. Interestingly, our study showed that the pharmacogenetics of BDNF may not be the same across different populations that are prescribed AAPs or statins with the lack of a relationship seen in the combined and bipolar groups. This may suggest that glucose regulation is more dependent on genetic factors like BDNF within the schizophrenia population. Of note, is that within our schizophrenia and bipolar disorder samples, the HOMA-IR values are substantially higher than studies in the general population, which is not surprising given the rising incidence of diabetes and metabolic syndrome36–40. Studies including controls, subjects with new onset diabetes, those at high risk for diabetes, and HIV patients have HOMA-IR averages of 1.8–2.9 while our population average was much higher at 5.51 and 5.91 (as seen in Table 1) for the schizophrenia and bipolar samples, respectively. A large contributor to this elevated HOMA-IR seen in our populations may be the high percentage of AAP use (78% overall) with the majority being AAPs with greater metabolic side effect profiles. Although not statistically significant those schizophrenia subjects on clozapine, olanzapine, risperidone, paliperidone or quetiapine (68%) had a mean HOMA-IR of 5.8 versus those on ziprasidone, aripiprazole or 1st generation antipsychotics had a HOMA-IR of 5.0 (p=0.25) which is consistent with the literature41. Poor diet and a lack of activity could also be significant contributors to these high HOMA-IR values. Furthermore, for the combined sample, 80% (77% for schizophrenia and 82% for bipolar) had a HOMA-IR higher than a suggested threshold of 2.6 for insulin resistance. Again, this is not surprising given our populations’ overall high HOMA-IR values, AAP use, poor diet and lack of physical activity.
Our study contradicts that published by Krabbe and collegues which showed no association between the BDNF Val66Met polymorphism and HOMA-IR in the diabetic population10. Based on our data, we found a significant association between BMI, the BDNF 66 Met allele and insulin resistance within our schizophrenia sample only. This suggests that the BDNF Val66Met variant which has been studied for its psychiatric and cognitive effects14,42,43 may also have impact on the risk for insulin resistance within the schizophrenia population but perhaps not within any population that uses AAPs. BMI and HOMA-IR are easily measureable clinical variables that may represent potential risk factors in schizophrenia which could be targeted along with the BDNF Val66Met genotype to help predict those at greatest risk for insulin resistance. Based on this risk, subjects may then be targeted for specific interventions related to insulin resistance to prevent the development of diabetes and its detrimental comorbidities and cost. Using pharmacogenomics data along with subject specific clinical measures, personalized medicine interventions could be developed which guide specific medication use (e.g., AAPs with lower metabolic side effects profiles) or more intensive lifestyle modifications could be implemented as first line treatment for schizophrenia patients at greatest risk. Although the bipolar sample did not show a correlation with the BDNF 66Met variant, their high insulin resistance values suggest that they should be studied further in order to find more personalized pharmacogenomic approaches to help reduce their risk of diabetes as well.
Currently the use of statin medications within the general population is becoming more and more controversial. We hypothesized that use of a statin medication in subjects with the BDNF 66 Met allele may result in higher measures of insulin resistance. Based on our analysis, we found a significant relationship in schizophrenia patients taking statins and carrying the 66 Met allele resulting in a HOMA-IR measure about 1.5× higher than those patients either with the Val allele taking statins or those regardless of BDNF genotype not taking statins (Table 2). Also, the combination of the 66 Met allele and statin use resulted in a HOMA-IR value more than 1.2 times higher than those patients carrying the 66 Met allele alone. This relationship however was not seen in the combined sample and although not significant, an opposite effect was seen in the bipolar population suggesting that statins may not have the same detrimental effect it has on schizophrenia patients’ glucose regulation. After controlling for age, race, AAP use, BMI and metabolic syndrome, we found an interaction and these relationships remained (p=0.0032) suggesting that at any given BMI, schizophrenia patients with the BDNF 66 Met allele receiving a statin medication have significantly higher HOMA-IR values. The combined effect of statin use and the BDNF 66Met allele on HOMA-IR within the schizophrenia population could be a result of increased proteolytic cleavage (induced by statins) of the altered form of BDNF dictated by the Met variant whereas those schizophrenia patients with the Val/Val are unaffected by this increased cleavage since they are not producing the altered form of the BDNF protein18,22. Again, with further validation this could be used as a future personalized medicine approach in schizophrenia where the risks and benefits would need to be weighed when treating hyperlipidemia or perhaps alternate anti-hyperlipidemic medications would be used. Our results begin to suggest that statin medications can continue to be used in the bipolar population without the concern of increased insulin resistance.
Figure 2 depicts the differing trends in HOMA-IR seen between the schizophrenia and bipolar populations as more insulin resistance risk factors we investigated in this study (e.g., BDNF Val66Met polymorphism and statins) are added together. This result was interesting to the investigators as we hypothesized that the pharmacogenetic effects of BDNF and statins would be the same on both populations. The different trends could be a result of differing baseline characteristics between the groups (gender, AAP use, lipid levels, etc) that could not be fully controlled during analysis, pathophysiologic processes of the diseases or differences in baseline diabetes risk. Also, it may be possible that the BDNF protein may not be a major contributor to insulin resistance in the bipolar population and thus, the hypothesis of increased BDNF cleavage we proposed for the schizophrenia population would not have the same effect. Finally, although the mechanism for statin-induced insulin resistance has not been fully elucidated, differences in transporter expression between schizophrenia and bipolar patients could be a future line of investigation into the differences seen within our study44,45.
One major limitation to our study is that we did not analyze the BDNF levels of our participants. This would have allowed us to determine the mechanistic differences in BDNF levels based on the BDNF Val66Met variant while looking at insulin resistance measures. However, to date, the BDNF Val66Met has not been well correlated to BDNF levels46. Another limitation is that we used a relatively simple clinical tool to assess insulin resistance; however the HOMA-IR has been shown to be a good correlate to more invasive measures of insulin resistance such as the frequently sampled IV glucose tolerance test47,48. Limitations of our population make using a more validated insulin resistance tool like that of the hyperinsulinemic euglycemic glucose clamp very difficult but could be a future avenue of investigation in schizophrenia. The cross-sectional design of our study only gives us a snapshot of insulin resistance within our population of interest and would need to be repeated in order to confirm our results. Our study population was also not drug naïve and polypharmacy was fairly common in regards to overall number of medications. This makes it difficult to confirm the interaction of the BDNF gene, AAPs and statins within the mental illness. Our study was a candidate gene driven in nature and therefore we only looked at the BDNF Val66Met polymorphism because other BDNF polymorphisms have not been studied in insulin resistance in the literature. However, given the complexity of insulin resistance, possible associations of insulin resistance with other variants cannot be ruled out. Lastly, we did not control for multiple comparisons, but given the number of statistical tests that were carried out as part of this investigation, our most significant result in the schizophrenia population (interaction between BMI and 66Met allele on HOMA-IR) would remain this way even with the use of a more stringent significance level of 0.01.
Conclusion
Overall in our cross-sectional study of a schizophrenia and bipolar population receiving treatment with an antipsychotic we did not find a significant effect for the BDNF variant or statin use on insulin resistance for the group as a whole. However, when we stratified the sample by primary diagnosis, a significant association between the BDNF 66 Met allele, statin medications and BMI with increased insulin resistance measures in the schizophrenia sample was found. Although further validation may be needed, this may indicate that the BDNF Val66Met variant, thought to be promising in predicting insulin resistance and glucose dysregulation, may play a role in predicting the schizophrenia but not the bipolar population’s risk of developing insulin resistance and subsequently diabetes from AAP use. While this work is still preliminary these data provide more evidence regarding difference in the occurrence of diabetes between subjects with schizophrenia and bipolar disorder and give us more insight into the mechanisms behind these differences. This work will help to target genes of interest which can be followed up in future investigations focusing on personalized medicine for those with schizophrenia.
Acknowledgments
The following sources were utilized for this publication: The following funding sources were utilized for this publication: NIMH (R01 MH082784) and The National Alliance for Research in Schizophrenia and Depression (NARSAD)
References
- 1.Lambert BL, Cunningham FE, Miller DR, Dalack GW, Hur K. Diabetes risk associated with use of olanzapine, quetiapine, and risperidone in veterans health administration patients with schizophrenia. Am J Epidemiol. 2006;164(7):672–681. doi: 10.1093/aje/kwj289. [DOI] [PubMed] [Google Scholar]
- 2.Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry. 2002;159(4):561–566. doi: 10.1176/appi.ajp.159.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26(4):903–912. doi: 10.1093/oxfordjournals.schbul.a033504. [DOI] [PubMed] [Google Scholar]
- 4.Dynes JB. Diabetes in schizophrenia and diabetes in nonpsychotic medical patients. Dis Nerv Syst. 1969;30(5):341–344. [PubMed] [Google Scholar]
- 5.Felker B, Yazel JJ, Short D. Mortality and medical comorbidity among psychiatric patients: A review. Psychiatr Serv. 1996;47(12):1356–1363. doi: 10.1176/ps.47.12.1356. [DOI] [PubMed] [Google Scholar]
- 6.Suvisaari J, Perala J, Saarni SI, et al. Type 2 diabetes among persons with schizophrenia and other psychotic disorders in a general population survey. Eur Arch Psychiatry Clin Neurosci. 2008;258(3):129–136. doi: 10.1007/s00406-007-0762-y. [DOI] [PubMed] [Google Scholar]
- 7.De Luca V, Mueller DJ, de Bartolomeis A, Kennedy JL. Association of the HTR2C gene and antipsychotic induced weight gain: A meta-analysis. Int J Neuropsychopharmacol. 2007;10(5):697–704. doi: 10.1017/S1461145707007547. [DOI] [PubMed] [Google Scholar]
- 8.Ellingrod VL, Bishop JR, Moline J, Lin YC, Miller DD. Leptin and leptin receptor gene polymorphisms and increases in body mass index (BMI) from olanzapine treatment in persons with schizophrenia. Psychopharmacol Bull. 2007;40(1):57–62. [PubMed] [Google Scholar]
- 9.Ellingrod VL, Miller DD, Taylor SF, Moline J, Holman T, Kerr J. Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C variants. Schizophr Res. 2008;98(1–3):47–54. doi: 10.1016/j.schres.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 11.Golden E, Emiliano A, Maudsley S, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the baltimore longitudinal study of aging. PLoS One. 2010;5(4):e10099. doi: 10.1371/journal.pone.0010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hristova M, Aloe L. Metabolic syndrome--neurotrophic hypothesis. Med Hypotheses. 2006;66(3):545–549. doi: 10.1016/j.mehy.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 13.Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91(1–3):1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: Findings in schizophrenia. Curr Opin Psychiatry. 2011;24(2):122–127. doi: 10.1097/YCO.0b013e3283436eb7. [DOI] [PubMed] [Google Scholar]
- 15.Rybakowski JK. BDNF gene: Functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 2008;9(11):1589–1593. doi: 10.2217/14622416.9.11.1589. [DOI] [PubMed] [Google Scholar]
- 16.Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144(6):2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 17.Fujinami A, Ohta K, Obayashi H, et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008;41(10–11):812–817. doi: 10.1016/j.clinbiochem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 19.Lyons WE, Mamounas LA, Ricaurte GA, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X, Yang N, Xu Y, et al. Simvastatin treatment improves functional recovery after experimental spinal cord injury by upregulating the expression of BDNF and GDNF. Neurosci Lett. 2011;487(3):255–259. doi: 10.1016/j.neulet.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Tsai SJ. Statins may enhance the proteolytic cleavage of proBDNF: Implications for the treatment of depression. Med Hypotheses. 2007;68(6):1296–1299. doi: 10.1016/j.mehy.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Lu D, Jiang H, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25(2):130–139. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- 24.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: A meta-analysis. Diabetes Care. 2009;32(10):1924–1929. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 26.Sukhija R, Prayaga S, Marashdeh M, et al. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J Investig Med. 2009;57(3):495–499. doi: 10.2310/JIM.0b013e318197ec8b. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Esteghamati A, Ashraf H, Esteghamati AR, et al. Optimal threshold of homeostasis model assessment for insulin resistance in an iranian population: The implication of metabolic syndrome to detect insulin resistance. Diabetes Res Clin Pract. 2009;84(3):279–287. doi: 10.1016/j.diabres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Esteghamati A, Ashraf H, Khalilzadeh O, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in iran (SuRFNCD-2007) Nutr Metab (Lond) 2010;7:26. doi: 10.1186/1743-7075-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radikova Z, Koska J, Huckova M, et al. Insulin sensitivity indices: A proposal of cut-off points for simple identification of insulin-resistant subjects. Exp Clin Endocrinol Diabetes. 2006;114(5):249–256. doi: 10.1055/s-2006-924233. [DOI] [PubMed] [Google Scholar]
- 31.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54(2):333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 32.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26(12):3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 33.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 34.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19(19):5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh S, King CR, Garsa AA, McLeod HL. Pyrosequencing of clinically relevant polymorphisms. Methods Mol Biol. 2005;311:97–114. doi: 10.1385/1-59259-957-5:097. [DOI] [PubMed] [Google Scholar]
- 36.Chung JO, Cho DH, Chung DJ, Chung MY. Associations among body mass index, insulin resistance, and pancreatic beta-cell function in korean patients with new-onset type 2 diabetes. Korean J Intern Med. 2012;27(1):66–71. doi: 10.3904/kjim.2012.27.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Britton KA, Mukamal KJ, Ix JH, et al. Insulin resistance and incident peripheral artery disease in the cardiovascular health study. Vasc Med. 2012 doi: 10.1177/1358863X11436195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmslie JL, Porter RJ, Joyce PR, Hunt PJ, Shand BI, Scott RS. Comparison of insulin resistance, metabolic syndrome and adiponectin in overweight bipolar patients taking sodium valproate and controls. Aust N Z J Psychiatry. 2009;43(1):53–60. doi: 10.1080/00048670802534341. [DOI] [PubMed] [Google Scholar]
- 39.Serfaty L, Forns X, Goeser T, et al. Insulin resistance and response to telaprevir plus peginterferon alpha and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012 doi: 10.1136/gutjnl-2011-300749. [DOI] [PubMed] [Google Scholar]
- 40.Tsai A, Liou YJ, Hong CJ, Wu CL, Tsai SJ, Bai YM. Association study of brain-derived neurotrophic factor gene polymorphisms and body weight change in schizophrenic patients under long-term atypical antipsychotic treatment. Neuromolecular Med. 2011;13(4):328–333. doi: 10.1007/s12017-011-8159-5. [DOI] [PubMed] [Google Scholar]
- 41.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2010;123(2–3):225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung S, Chung HY, Jung J, Chang JK, Hong JP. Association among aggressiveness, neurocognitive function, and the Val66Met polymorphism of brain-derived neurotrophic factor gene in male schizophrenic patients. Compr Psychiatry. 2010;51(4):367–372. doi: 10.1016/j.comppsych.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Zhang XY, Chen DC, Xiu MH, et al. Cognitive and serum BDNF correlates of BDNF Val66Met gene polymorphism in patients with schizophrenia and normal controls. Hum Genet. 2012 doi: 10.1007/s00439-012-1150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki J, Iwashita M, Kono S. Statins: Beneficial or adverse for glucose metabolism. J Atheroscler Thromb. 2006;13(3):123–129. doi: 10.5551/jat.13.123. [DOI] [PubMed] [Google Scholar]
- 45.Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): Implications in glycaemic control. Diabetologia. 2006;49(8):1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 46.Terracciano A, Piras MG, Lobina M, et al. Genetics of serum BDNF: Meta-analysis of the Val66Met and genome-wide association study. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.616533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 48.Rossner SM, Neovius M, Mattsson A, Marcus C, Norgren S. HOMA-IR and QUICKI: Decide on a general standard instead of making further comparisons. Acta Paediatr. 2010;99(11):1735–1740. doi: 10.1111/j.1651-2227.2010.01911.x. [DOI] [PubMed] [Google Scholar]