Abstract
Working memory (WkM) is a fundamental cognitive process that serves as a building block for higher order cognitive functions. While studies have shown that children and adolescents utilize similar brain regions during verbal WkM, there have been few studies that evaluate the developmental differences in brain connectivity. Our goal was to study the development of brain connectivity related to verbal WkM in typically developing children and adolescents.
Thirty-five healthy children and adolescents, divided into three groups: 9–12 (children), 13–16 (young adolescents) and 17–19 (older adolescents) years, were included in this fMRI study. The verbal WkM task involved a modified Sternberg item recognition paradigm using three different loads. Brain connectivity analysis was performed using independent component analyses and regressing the components with the design matrix to determine task-related networks.
Connectivity analyses resulted in four components associated solely with encoding, four solely with recognition and two with both. Two networks demonstrated age-related differences with respect to load, 1) the left motor area and right cerebellum, and 2) the left prefrontal cortex, left parietal lobe, and right cerebellum. Post hoc analyses revealed that the first network showed significant effects of age between children and the two older groups. There was increasing connectivity with increasing load for adolescents. The second network demonstrated age-related differences between children and older adolescents. Children have higher task-related connectivity at lower loads, but they tend to equalize with the adolescents with higher loads. Finally, a non-load related network involving the orbital frontal and anterior cingulate cortices showed less connectivity in children.
Keywords: adolescents, brain connectivity, children, development, fMRI, verbal working memory
1. Introduction
Working memory (WkM) is considered to be one of the building blocks for higher cognitive functioning. It provides an essential interface between perception, attention, memory and action (Baddeley, 1996). WkM involves three primary processes: encoding information, actively maintaining this information on-line in memory, and finally, using the information to guide behavior. These processes are necessary for completing complex tasks (Baddeley, 1996). During encoding, the participant must attend to a task and construct an internal representation of the object. This mental representation must then be maintained in memory during a delay period, after which the mental representation may be used to decide upon a course of action.
There have been considerable efforts in non-human primates to understand the underlying neurobiology associated with WkM (Goldman-Rakic et al., 2004). Previous studies in humans have shown that WkM performance continues to improve from childhood, through adolescence, and into early adulthood (Luciana and Nelson, 2000; Luna et al., 2004). This development is associated with brain maturation, although the key elements involved have not yet been clearly delineated. There have been a number of functional imaging studies evaluating WkM in children and adolescents (Crone et al., 2006; Finn et al., 2010; Klingberg et al., 2002; O'Hare et al., 2008; Thomas et al., 1999). While children have been shown to activate similar brain regions as adults (Nelson et al., 2000; Olesen et al., 2003) there are several distinct developmental differences, although the findings are inconsistent.
(O'Hare et al., 2008) evaluated developmental differences in 12 children (7–10 years), 10 adolescents (11–15 years), and eight young adults (20–28 years) during an fMRI WkM task. They found increasing activation with increasing load in frontal, parietal and cerebellar regions in adolescents and adults, while children recruited only the left ventral prefrontal cortex with increasing WkM load. (Crone et al., 2006) also compared three age groups (8–12 years; n=14, 13–17 years; n=12, and 18–25 years old; n=18) and found that while children had poorer performance on a WkM task compared with adolescents and adults, they found no differences in the activation profile of the ventrolateral prefrontal cortex, a region associated with online maintenance. (Finn et al., 2010) followed ten female adolescents in their longitudinal fMRI study and found that younger adolescents have more activation in the hippocampus and older adolescents have a stronger relationship between behavioral performance and functional activity in the prefrontal cortex. (Klingberg et al., 2002) used functional MRI to measure brain activity during a WkM task in 13 participants between 9–18 years of age, and found a positive correlation between age-related increases in WkM capacity and brain activity in the superior frontal and intraparietal cortex. While a summary of these studies that utilized different age groups, methodologies, and regions of interest is challenging, nearly all studies show that there are age-related increases in specific areas associated with adolescent development.
WkM is disrupted in a number of psychiatric and neurological disorders, such as schizophrenia and Attention-Deficit/Hyperactivity Disorder (Diwadkar et al., 2011; Martinussen et al., 2005; White et al., 2011). Therefore, understanding the normal developmental trajectories of WkM is important to better understand when trajectories go awry. It is often unclear when during the course of development these abnormalities in WkM occur. Thus, having a good understanding of the normal development of WkM will help determine when in the course of development abnormal trajectories diverge from the normal trajectories.
Since brain function involves distributed neural networks, approaches that measure functional connectivity are well suited to study age-related network differences between childhood and late adolescence. Since the prefrontal cortex has a protracted development, our hypothesis was that connections between the prefrontal cortex and other outlying regions would strengthen from childhood through adolescence. Therefore our aim was to determine specific connections between the prefrontal cortex with other brain regions while performing a WkM task. The application of a data driven approach (Independent Component Analysis; ICA), allowed us to test other networks that are involved in verbal WkM. Therefore, our secondary aim was to assess alternative networks that show age-related differences in brain connectivity during verbal WkM tasks in typically developing children and adolescents. To our knowledge no other studies have examined developmental differences in functional connectivity associated with WkM performance in typically developing children and adolescents. However, there has been one recent study evaluating functional connectivity in adolescents (Finn et al., 2010).
2. Methods
2.1 Participants
Our participants consisted of typically developing children and adolescents between the ages of 9 and 19 years. To evaluate age-related differences, these participants were divided into three groups consisting of children (between the ages of 9 and 12 years; n=10), young adolescents (between the ages of 13 and 16 years; n=12), and older adolescents (between the ages of 17 and 19 years; n=13). Participants were recruited from advertisements in the local community, and via families who had participated in other MRI studies from our research group (Karatekin et al., 2010; White et al., 2011). Participants were excluded if they were pregnant, had a history of any psychiatric disorder, including a history of substance dependence or on-going substance abuse (within the past month), neurological disorders, head injuries, or a medical illness that involved the brain. Participants were also screened to assure that they had no contraindications for participation in an MRI study such as metal implants or claustrophobia. All participants underwent a thorough diagnostic assessment using the Kiddie-SADS-PL (Kaufman et al., 1997). Their socioeconomic status (SES) was measured by using the Hollingshead SES scale (Cirino et al., 2002). This study was performed at the University of Minnesota in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Institutional Review Board at the University of Minnesota. Informed consent and assent was obtained prior to participation.
2.2 Working Memory Paradigm
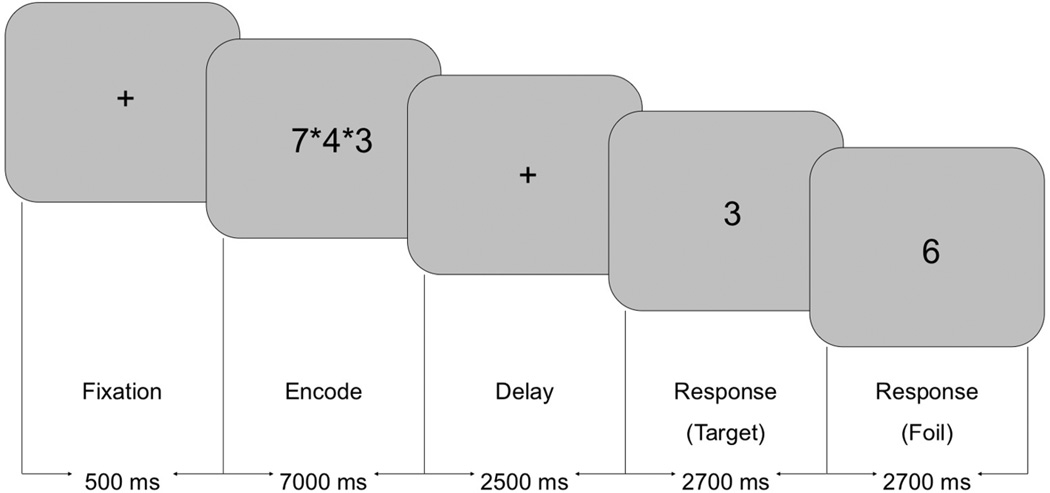
Verbal WkM was tested using a modified Sternberg Item Recognition Paradigm (SIRP) using three WkM loads (Sternberg, 1966) (Figure 1). The modified SIRP targeted encoding and retrieval of information and was easy enough to be performed well by children. The stimuli were designed as an integrated block and event-related paradigm and each run consisted of two blocks for each WkM Load (total = 6 blocks per run) (Roffman et al., 2008; White et al., 2011). During a WkM block, participants were initially presented with the word ‘Learn’. This was followed by the simultaneous presentation of one, three, or five digits for seven seconds (‘Encode’). After a short delay of 2.5 seconds, 16 single digits were presented sequentially at a rate of 2.7 seconds for each digit (‘Recognition’). The participants pushed their right thumb if the digit was a member of the memorized set (‘Target’), or their left thumb if the digit was not a member of the memorized set (‘Foil’). Accuracy and response time were measured for each response. All the participants who participated in this study had two practice sessions prior to the fMRI session. During the first practice session, participants were seated in a chair in front of a monitor and performed the WkM task with a team member describing the task. The second practice session was performed inside a mock scanner with stimuli identical to that used during the fMRI session. The participants practiced until they understood and were comfortable performing the task. Participants were told to respond as quickly as possible without making mistakes. During the fMRI session, a vacuum bag was placed around the back of the head to reduce head motion. The paradigm was programmed using E-Prime (Psychology Software Tools, Inc.) The participants wore a set of fMRI compatible gloves with buttons associated with each finger and thumb. There were three runs, each lasting five minutes and 58 seconds.
Figure 1.
Sternberg Item Recognition Paradigm
2.3 MRI Sequence
The MRI images were acquired with a 3T Siemens MR system (Erlangen, Germany) located at the Center for Magnetic Resonance Research at the University of Minnesota. After an initial localizer scan was obtained, a coronal scout image (12 slices; field of view (FoV) 224 mm, TR 2000 ms; TE 72 ms; resolution 2.3 × 1.8 × 2 mm) was obtained to locate the coronal midline. A second scout image was then attained using sagittal images acquired along the coronal midline (12 slices; FoV 224 mm; TR 2040 ms, TE 62 ms; resolution 1.2 × .9 × 2 mm). These sagittal slices were used to orient the volume along the anterior/posterior commissure (ACPC) plane. Functional images were obtained using a gradient echo sequence with 27 axial slices and an in-plane resolution of 3.4 × 3.4 mm, 4 mm slice thickness, and a 1 mm gap. Additional sequence parameters included: TE = 30 ms, TR = 2000 ms, flip angle = 90 degrees and FoV = 220 mm. A total of 177 volumes were obtained for each of the three runs (531 volumes in total).
2.4 Image Processing
All the functional images were preprocessed using a combination of Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/) (Cox, 1996) and FMRIB’s Software Library (FSL, FMRIB Software Library; FMRIB, Functional Magnetic Resonance Imaging of the Brain; http://www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2004). Following the conversion from DICOM to the Nifti format, slice timing correction and motion correction were performed using AFNI (Cox, 1996). Participants who were unable to complete three runs of the SIRP or participants who had greater than 2.5 mm of motion in the x, y, or z directions were excluded from the analyses. Images were oriented to standard Montreal Neurological Institute (MNI) space utilizing FSL in a 3-stage process. First, for each individual a mean echo planar imaging (EPI) image was generated from the fMRI time series. This mean EPI image was registered to an EPI template in standard space using a 12-parameter transformation (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Finally, the 12-parameter transformation was applied to the entire fMRI time series for each individual and each run. The data were spatially smoothed using an 8-mm full width at half-maximum Gaussian kernel (White et al., 2001).
2.5 Independent Component Analysis
Following the preprocessing steps, a group Independent Component Analysis (ICA) was performed on the preprocessed data (Calhoun et al., 2001a, b). The methods prescribed by this process were performed using GIFT (Matlab toolbox version 1.3c http://icatb.sourceforge.net). ICA allows a model free analyses of the data and thus was well suited as an initial step to derive specific brain networks. From this, we were able to test which of these networks were associated with our WkM task. We chose to use this approach, as it was our intent to initially extract network information and to use these networks to assess age-related differences in connectivity during WkM.
ICA is a statistical and computational data-driven technique that is designed to extract temporally related signals that are hidden within sets of random or unrelated variables. It assumes that the fMRI time series are linear mixtures of independent source signals that are buried within noise. The algorithm (infomax) was designed to extract maximally independent signals and their mixing coefficients. The principle behind ICA is that these maximally independent source signals represent temporally coherent groupings of BOLD signal change, often referred to as component maps. These components map the functional connectivity between different brain regions. Since ICA is a data-driven approach, the functional networks are generated without any assumptions about the shape of the hemodynamic time courses. The spatial maps generated by ICA were averaged together across the three scan sessions and the dimensionality was not constrained. This resulted in 26 independent component (IC) spatial maps for every participant. These IC spatial maps represent the regions of the brain related to a specific time course. Every voxel within a component spatial map contains a z score, with high z scores reflecting a greater contribution to the associated time course.
2.6 Component Selection
One of the strengths of ICA is its ability to detect noise-related components that represent signal artifacts such as head motion and eye movement. Thus, we first evaluated each of the spatial maps and eliminated those with motion or other artifacts. These were readily identified by symmetric activations on the opposite sides of the skull, activations within the ventricles, or activation within the eye itself. The second phase consisted of identifying and limiting the components to only those that were task-related. The SIRP has the advantage to be able to parse out the encoding, maintenance, and retrieval phases as separate time series. We did not calculate connectivity during the maintenance phase of the task, as the optimum method would be to parametrically alter the delay period to assess for effects of delay. Adding this additional measure would also have significantly increased the acquisition time, which would have been difficult especially for the younger children. The effect of load was determined via a mixed-model repeated measures ANCOVA using the beta weights that reflect task modulation at the different loads. The ICA component time courses were regressed against the design matrix for the working memory task in GIFT using a SPM5 general linear model (GLM) to obtain the beta weights for each load of the working memory task. The design matrix included columns for both encoding and recognition for each of the three WkM loads. The resulting beta weights from this regression analysis represent the degree to which each component was associated with the WkM task relative to the fixation baseline (i.e., a high beta weight represents a large task-related modulation of a component for a given regressor). The components that showed a statistically significant effect of load or age-related differences for either encoding, recognition or both were included in the study. These components were used to assess group differences using a mixed-model repeated-measures analysis of variance (ANOVA).
2.7 Statistical Analyses
The demographic data was assessed using chi-square for categorical data and ANOVA for normally distributed continuous data. We used the Kruskal-Wallis test for non-normally distributed continuous data. A 3 (age group) by 2 (encode/recognition) by 3 (load) by 3 (run) mixed-model repeated measures ANOVA was performed using age group, task, and load as the fixed effects, and subject as the random variable. We also used repeated measures ANOVA for post-hoc analysis comparing the three different age groups. The task-related beta-weights for each of the individual components were entered into a 3 (age group) by 3 (load) mixed-model repeated-measures ANOVA. To examine performance differences between the different age groups, a 3 (age group) by 3 (run) by 3 (load) mixed-model analysis of covariance (ANCOVA) was performed using response time (RT) and accuracy as covariates. We also analyzed age as a continuous variable using a mixed-model regression analysis. We examined differences in head motion during scanning using a 3 (age group) by 3 (run) repeated measures ANOVA. A Bonferroni correction was conducted to correct for multiple testing. The analyses were performed using SAS version 9.2 (Institute Inc., Cary, NC, USA).
3. Results
3.1 Study Population
From a total of 41 participants who completed scanning, six children were excluded due to significant motion. The 35 participants included in the study were between 9 and 19 years of age with a mean age ± S.D. of 15.0 ± 3.0. The total group included 16 girls and 19 boys. Age group subsamples included 10 children aged 9–12 (10.9 ± .9), 12 young adolescents aged 13–16 (15.2 ± 1.0) and 13 older adolescents aged 17–19 years old (18.1 ± .9). No significant differences in gender, socioeconomic status or handedness were found between these subgroups (Table 1). There were no significant differences in movement across age groups using both the maximum (F1,101 = 1.74, p = .190) and mean movement parameters derived from AFNI (F1,101 = .02, p = .903). All participants were debriefed after the task and were asked what strategy that they used to remember the numbers. All participants used the same strategy of repeating the numbers sequentially in their mind. They did this in the order that the numbers were presented, thus, without reordering and none of the subjects reported using a visual spatial strategy.
Table 1.
Demographic characteristics per age group
| Age group | |||||
|---|---|---|---|---|---|
| Children (9–12 years) |
Young adolescents (13–16 years) |
Older adolescents (17–19 years) |
p-value | ||
| Total (n=35) | 10 | 12 | 13 | NA | |
| Age (mean ±SD) | 10.9 ± .9 | 15.2 ± 1.0 | 18.1 ± .9 | NA | |
| Gender (male %) | 70.0 | 50.0 | 46.2 | NS | |
| Handedness (%) | Right | 80.0 | 66.7 | 84.6 | NS |
| Left | 0 | 0 | 7.7 | ||
| Both | 10.0 | 16.7 | 0 | ||
| No measurement | 10.0 | 16.7 | 7.7 | ||
| SES (mean ±SD) | 58.0 ± 7.6 | 54.0 ± 6.8 | 50.9 ± 6.6 | NS | |
Table note: NA = Not Applicable, NS = Not Significant
P-values were derived from ANOVAs for normally distributed continuous variables, Kruskal-Wallis test for non-normally distributed continuous variables and χ2-tests for categorical variables
3.2 Behavioral Results
Probe response time and probe accuracy
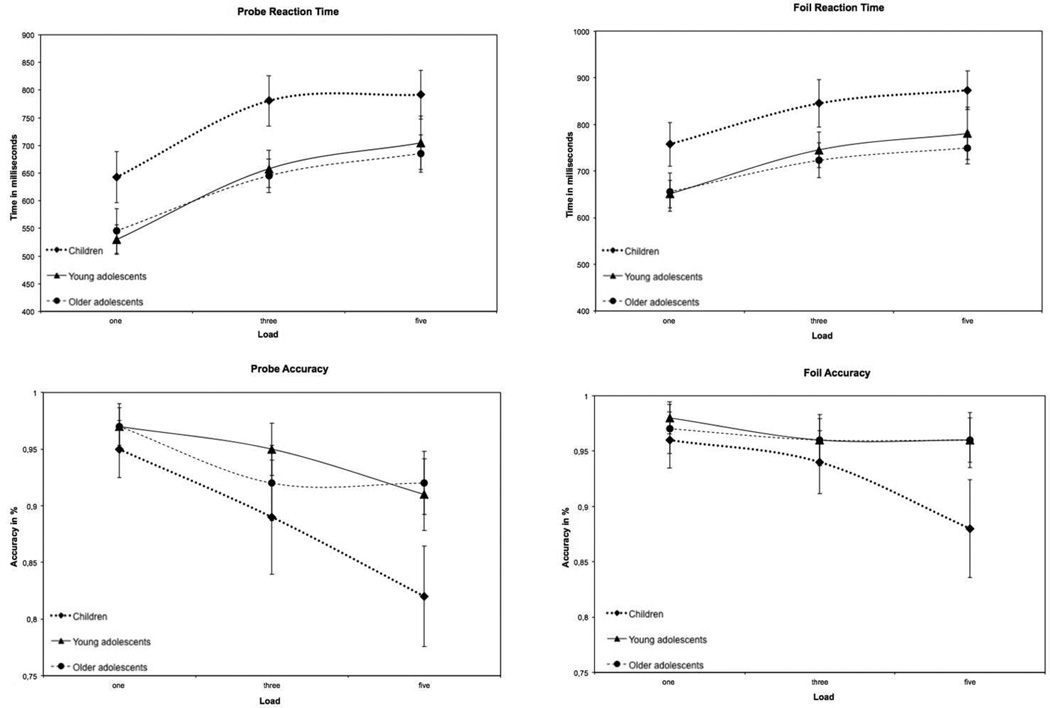
A mixed-model repeated-measures ANOVA found that both age group (F2,68 = 8.24, p < .001) and WkM load (F2,513 = 160.0, p < .0001) significantly affected probe response time (probe RT), and these factors did not interact. Children responded more slowly than older participants, and in all groups and the RT increased with increasing WkM load. For the probe accuracy there were significant main effects of run (F2,515 = 8.00, p < .001), age group (F2,63 = 5.0, p < .001), and load (F2,508 = 45.49, p < .0001). There was also an interaction between age group and load (F4, 508 = 5.42, p < .001) for probe accuracy. With increasing loads and successive runs, accuracy decreased. Thus, children between 9–12 years had longer response times and were less accurate for both probes and foils compared to the older participants (Figure 2).
Figure 2.
Behavioral results
Comparing the children and the younger adolescents in the post-hoc analysis showed that there were significant main effects of age group (F2, 46 = 12.47, p < .001), load (F2, 336 = 105.1, p < .0001), and run ((F2, 341 = 3.78, p = .02) for the probe RT using the mixed model repeated measures. In addition, there was an interaction effect of run by load (F4, 336 = 2.50, p = .04). There were significant main effects of age group (F1, 42 = 6.30, p < .02), load (F2, 331 = 40.9, p < .0001), and run ((F2, 338 = 7.29, p < .001) for the probe accuracy using the mixed model repeated measures analysis. In addition, there was also an interaction effect of age group by load (F2, 331 = 5.22, p = .006).
When comparing the children with the older adolescents, we found significant main effects of both age group (F2, 43 = 12.03, p = .001) and load (F2, 331 = 97.7, p < .0001) for the probe RT. No interaction effects were observed. There were significant main effects of age group (F1, 40 = 6.40, p = .02), load (F2, 327 = 35.2, p < .0001), and run ((F2, 331 = 5.09, p = .006) for the probe accuracy. In addition, there was also an interaction effect of age group by load (F2, 327 = 9.15, p < .001) and run by load (F4, 327 = 3.43, p = .009).
Finally, when comparing the younger adolescents with the older adolescents in the post-hoc analysis, the results showed a significant main effect of load (F2, 335 = 118.5, p < .0001) for the probe RT using the mixed model repeated measures. No interaction effects were observed. There were significant main effects of both load (F2, 358 = 17.2, p < .0001) and run ((F2, 358 = 4.17, p = .02) for the probe accuracy using the mixed model repeated measures. No interaction effects were observed.
Foil response time and foil accuracy
The mixed-model repeated-measures ANOVA showed that there were significant main effects for run (F2, 517 = 3.56, p < .05), age group (F2, 68 = 6.83, p < .001), and load (F2, 512 = 76.82, p < .0001) for foil response times. The response time for the foils (foil RT), with successive runs. There was also a run by load interaction (F4, 512 = 5.51, p < .001) with shorter response times associated with lower loads. The accuracy of the foil conditions showed main effects for both age group (F2, 64 = 3.49, p < .05), and load (F2, 508 = 14.49, p < .001). In addition, the accuracy of the foil condition also had an age group by load interaction (F4, 508 = 7.19, p < .001) (Figure 2).
In the post-hoc analysis we found significant main effects when comparing the children with the younger adolescents for age group (F2, 45 = 8.62, p = .005), and load (F2, 335 = 48.82, p < .0001) for the foil RT. In addition, there was an interaction effect of run by load (F4, 335 = 3.26, p = .01). There were significant main effects for both age group (F2, 42 = 4.87, p = .03) and load (F2, 331 = 16.73, p < .0001) for the foil accuracy. There was also an interaction effect of age group by load (F2, 331 = 8.44, p < .001).
In the comparison between the children and the older adolescents we found significant main effects for run (F1, 43 = 12.29, p = .001) and load (F2, 330 = 49.15, p < .0001) for the foil RT. In addition, there was an interaction effect of run by load (F4, 330 = 6.47, p < .0001). There were significant main effects for both age group (F2, 40 = 4.46, p = .04), and load (F2, 327 = 12.65, p < .0001) for the foil accuracy. There was also an interaction effect of age group by load (F2, 327 = 10.52, p < .001) and an interaction effect of run by age group (F2, 330 = 3.42, p = .03).
Finally, we compared the younger adolescents with the older adolescents and found a significant main effect for load (F2, 355 = 56.7, p < .0001) for the foil RT. In addition, we found an interaction effect of run by load (F4, 355 = 2.70, p = .03). There were no significant main effects for the foil accuracy using the mixed model repeated measures. No interaction effects were observed.
3.3 Imaging Results
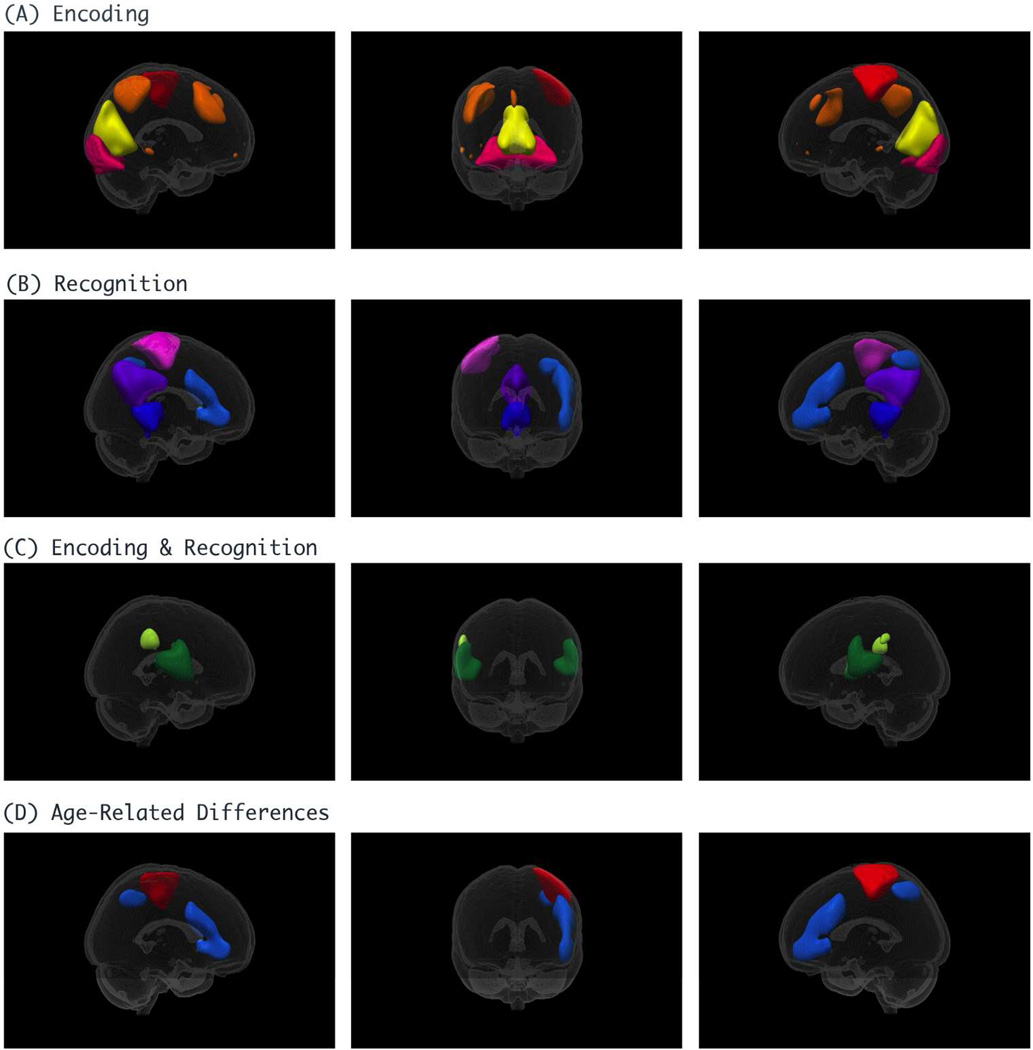
Out of a total of 26 components, 7 components were related to motion or other artifacts and were removed. We first evaluated networks that were related to load. Ten load-related components were grouped depending on whether they were significantly related to the encoding phase, recognition phase, or both using a mixed-model repeated-measures ANOVA; four ICs were associated solely with encoding, four solely with recognition, and two with both (Table 2 & Figure 3). Two IC networks demonstrated age-related differences with respect to load. A network involving the left motor area and the right cerebellum demonstrated age-related differences during encoding (F2,273 = 6.3, p = .002). This same network also showed an age group by run interaction (F2,269 = 4.8, p = .009). A network involving the left prefrontal cortex, the left parietal lobe, and the right cerebellum demonstrated age-related differences during recognition (F2,245 = 4.4, p = .013) (Table 2 & Figure 3).
Table 2.
Independent Components related to load
| Brain network | Effect of load | |
|---|---|---|
| Encoding | NumDF/DenDF/F/P | |
|
|
2/269/18.71/<.0001 | |
|
|
2/269/4.81/.0089 | |
|
|
2/301/24.41/<.0001 | |
|
|
2/269/12.91/<.0001 | |
| Recognition | NumDF/DenDF/F/P | |
|
|
2/269/7.54/.0006 | |
|
|
2/269/7.08/.0010 | |
|
|
2/269/3.07/.0479 | |
|
|
2/269/8.55/.0003 | |
| Encoding and Recognition | NumDF/DenDF/F/P Encoding | NumDF/DenDF/F/P Recognition |
|
|
2/305/14.40/<.0001 | 2/272/16.34/<.0001 |
|
|
2/301/7.72/.0005 | 2/269/16.98/<.0001 |
| Age-related Differences | NumDF/DenDF/F/P Encoding | NumDF/DenDF/F/P Recognition |
|
|
2/273/6.27/.0022 | - |
|
|
- | 2/245/4.40/.0133 |
NumDF = Numerator degrees of freedom, DenDF = Denominator degrees of freedom, F = F value
Figure 3.
Independent Components related to load
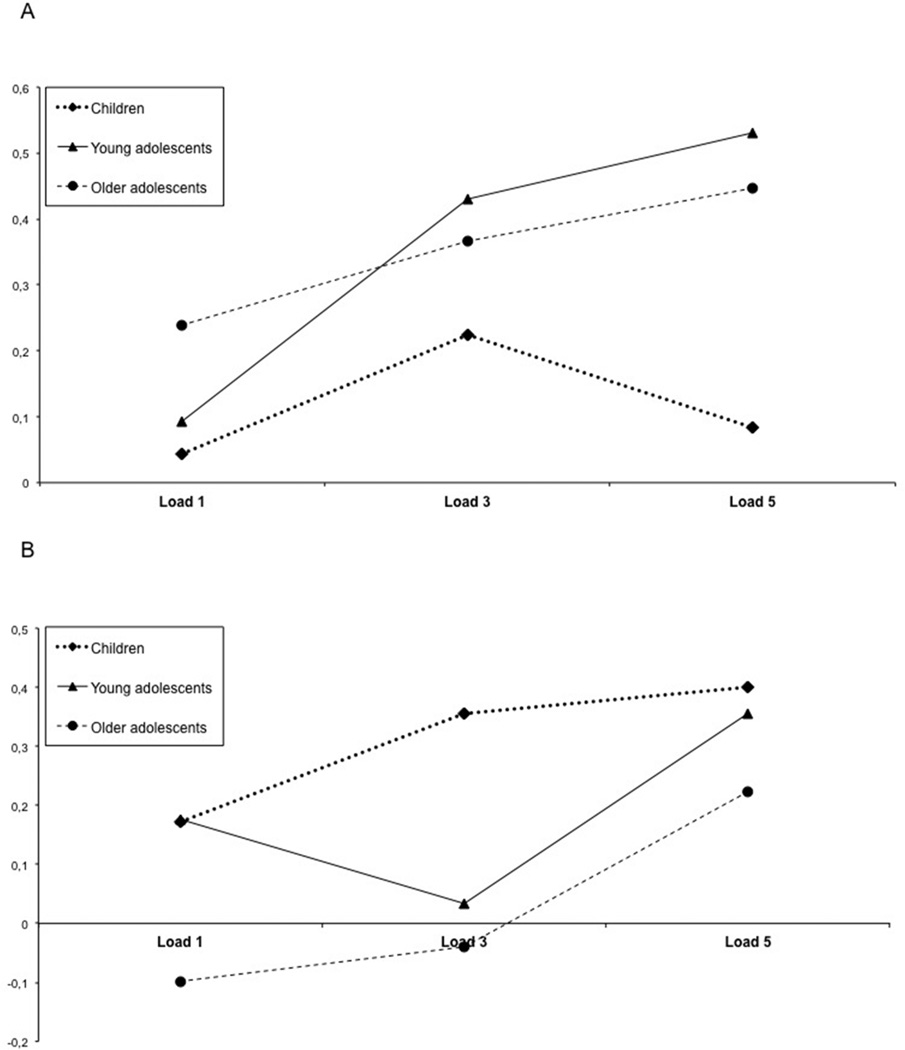
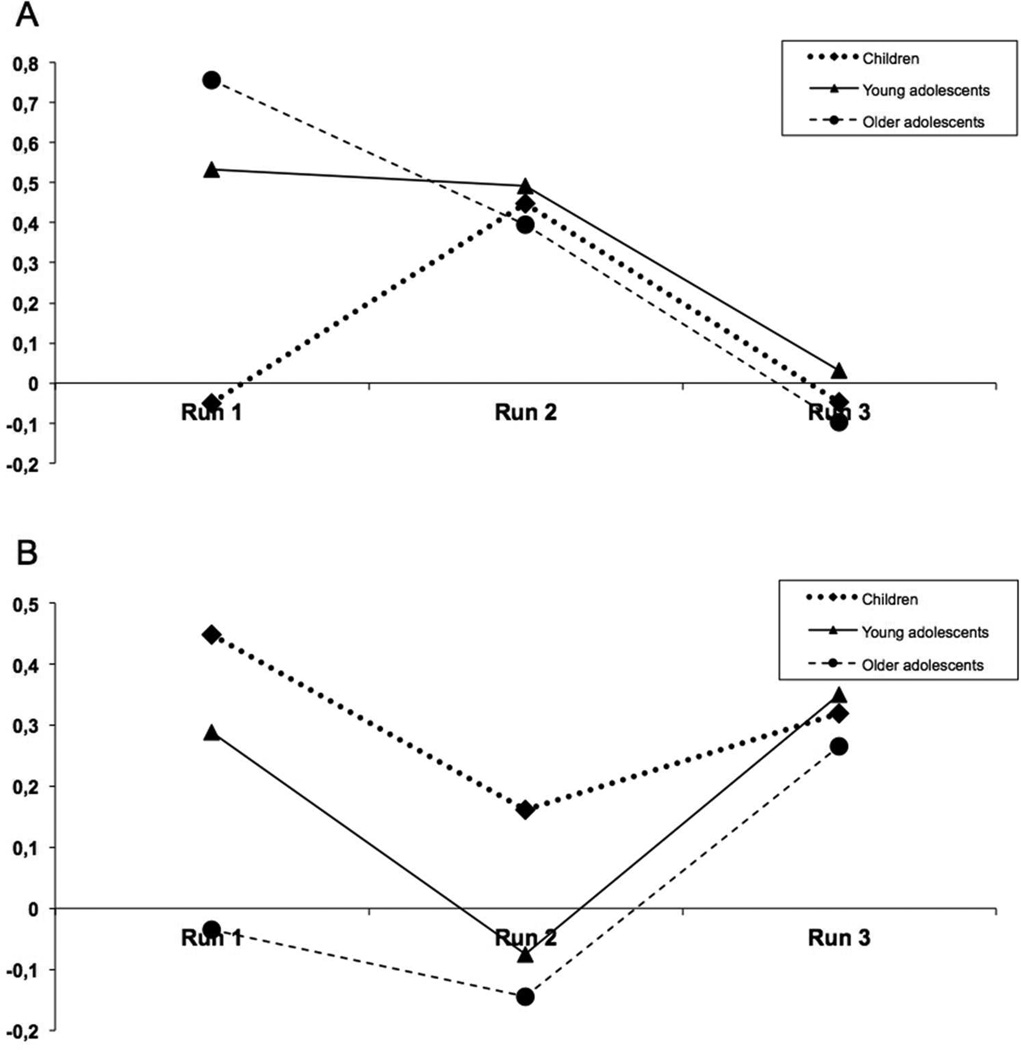
Post hoc analyses were performed to assess differences between each of the three different age groups. We found that the left motor/right cerebellar network showed a significant effect of age between the child group compared with both the younger adolescent group (F1,170 = 4.9, p = .029) and the older adolescent group (F1,188 = 11.0, p = .001). With greater load, adolescents showed greater functional connectivity within this network compared to the children (Figure 4a). There were no significant differences between the younger adolescent group and the older adolescent group. The interaction between age group and run showed a significant difference between the child group and the older adolescent group (F1,176 = 8.3, p = .005) (Figure 5a). These analyses were repeated using a mixed-model repeated measures ANCOVA with each of the behavioral measures (response time and accuracy) as covariates. None of the findings remained significant when performance was used as a covariate. When performing a separate analysis in which we compared the lowest load of the children with the highest load of the younger and older adolescents, we found significant differences during encoding (p = 0.024) for this network.
Figure 4.
Beta Weights for connectivity a) Left motor area, right cerebellum, b) Left parietal and pre-frontal cortex, right cerebellum
Figure 5.
Beta Weights for connectivity per run a) Left motor area, right cerebellum,
b) Left parietal and pre-frontal cortex, right cerebellum
The left prefrontal/left parietal/right cerebellar network showed age-related differences only between the child group and the older adolescent group (F1,185 = 9.2, p = .003). There were no significant differences between the child and young adolescent group, nor between the young adolescent and older adolescent groups (Figure 4b). There was also an age group by run interaction between the child group and the older adolescent group (F1,176 = 4.1, p = .043) (Figure 5b). None of the findings remained significant when the analyses were repeated using a mixed-model repeated measures ANCOVA with each of the behavioral measures (response time and accuracy) as covariates. The comparison of the lowest load of the children with the highest load of the younger and older adolescents, showed no significant differences during recognition (p = 0.476).
3.4 Age-related Differences Unrelated to Load
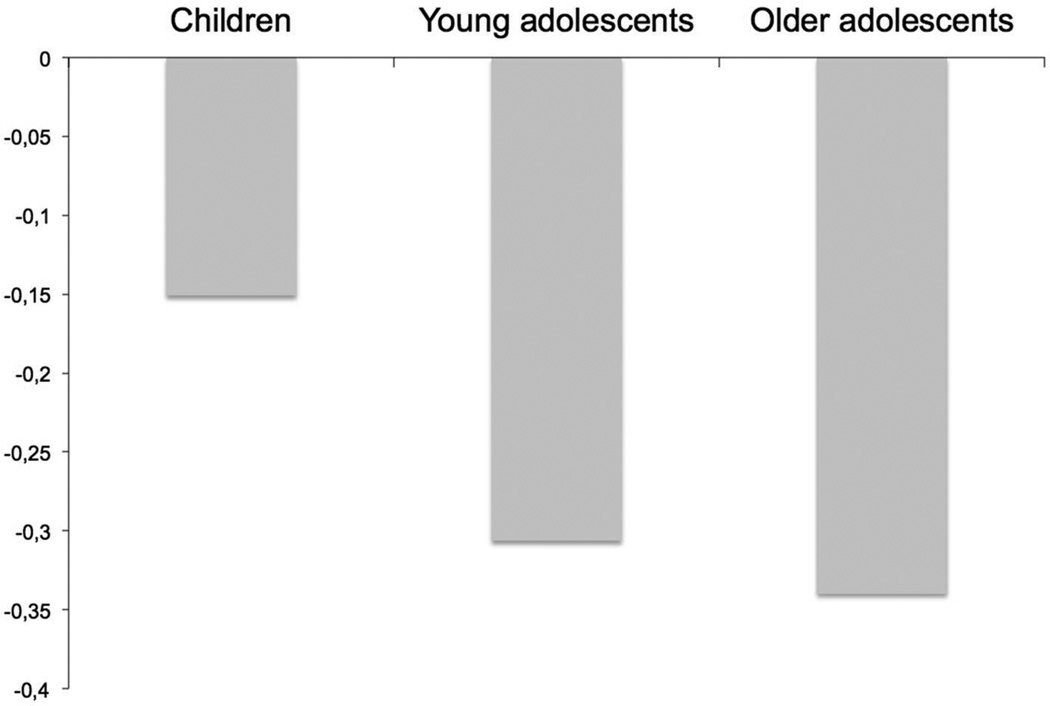
A network involving the anterior cingulate cortex and orbital frontal cortex demonstrated age-related differences during encoding (F2,301 = 3.1, p = .047). This network was related to the overall working memory task, but was not related to WkM load. Using post-hoc analysis we found that the anterior cingulate cortex and the orbital frontal cortex showed age-related differences only between the child group and the older adolescent group (F1,197 = 5.7, p = .018), although there was a trend between the younger and older adolescents (F1,215 = 3.0, p = .086, Figure 6). We also found an age group by run interaction between the child group and the older adolescent group (F1,197 = 3.9, p = .050). None of these findings remained significant when we used performance as a covariate. When comparing the lowest load of the children with the highest load of the younger and older adolescents, we found significant differences during encoding (p < 0.0001) in this network.
Figure 6.
Mean Beta Weights for connectivity of the anterior cingulate cortex and orbital frontal cortex
3.5 Age as a Continuous Variable
To confirm the age-related differences found in the three above described networks, we ran a mixed-model regression analysis with age as the random variable and load and run as fixed effects. The left motor area and right cerebellum network showed significant differences during encoding (F1,99 = 4.7, p = .032) and the left prefrontal, left parietal cortex, and the right cerebellum network showed significant differences during recognition (F1,99 = 5.1, p = .026). The third network involving the anterior cingulate cortex and the orbital frontal cortex, however, did not show significant differences during encoding using the mixed-model regression.
4. Discussion
In this fMRI study of typically developing children and adolescents, we demonstrated age-related differences between brain connectivity and verbal WkM in several distinct brain networks. These networks can be sub grouped into load-dependent and load independent networks. The age-related differences related to load were found in two specific brain networks involving 1) the left motor area and right cerebellum, and 2) the left prefrontal cortex, left parietal lobe, and right cerebellum. The first network is associated with motor functioning and the second network involves brain regions shown in prior studies to be involved in WkM performance (Nelson et al., 2000; Olesen et al., 2003; Thomas et al., 1999). Activations in the cerebellum have also been found in previous fMRI studies on WkM (Kirschen et al., 2010).
There have been several fMRI studies that evaluate developmental differences in working memory (Crone et al., 2006; Klingberg et al., 2002; Kwon et al., 2002; Thomas et al., 1999), although to our knowledge only one study has evaluated functional connectivity within working memory networks and found developmental differences in prefrontal and hippocampal connectivity (Finn et al., 2010). A major strength of this study was the longitudinal design and the homogeneous population of 10 females. However, they evaluated changes between mid- (mean age 15.1 years) to late adolescence (mean age 18.3 years), where we notice the major differences taking place between the children and mid- to late-adolescents. Thus, while there is clear overlap between our studies within the prefrontal cortex, the differences in motor networks could be attributed to the age of the sample or methodological differences between the two studies (data driven approach versus a region of interest approach).
Studies using traditional GLM analyses have shown age-related increases in activity in several brain regions: focal regions of the left and right dorsolateral prefrontal cortex, left ventrolateral prefrontal cortex, left premotor cortex and the left and right posterior parietal cortex. (Kwon et al., 2002) has shown that age was most predictive of brain activity. (Klingberg et al., 2002) found that older children showed higher activation in the superior frontal cortex and intraparietal cortex than younger children. We found age-related differences in functional connectivity in regions overlapping with these prior studies.
Several studies have compared resting state activity or baseline epochs with brain activation during a WkM task (Newton et al., 2011; Pyka et al., 2009; Pyka et al., 2012; Sala-Llonch et al., 2011; Zou et al., 2012). Zou et al. found that resting state activity can predict the behavioral performance and brain activation during WkM (Zou et al., 2012). Another study showed that connectivity during resting-state predicted the individual performance on a WkM task (Sala-Llonch et al., 2011). To our knowledge the relationship between resting state scans and brain activation during a WkM task has not been performed in children or adolescents. Since we did not collect resting-state fMRI scans as a part of this protocol, we are unable to test whether this relationship is also true during development. With the exponential rise in resting state studies, this is an important area for future research.
One network in which we found load- and age-related differences in functional connectivity between the child group and the older adolescent group was a left prefrontal, left parietal, and right cerebellar network. As this network has long been implicated in WkM function (Levy and Goldman-Rakic, 1999) it is not surprising that age-related differences would be identified within this network. Since performance suggests significant improvement with age, it is possible that the increased functional connectivity associated with age is tied to a better orchestration of brain function, translating to better performance. The fact that we found no differences between the child group and young adolescent group, or between the young adolescent and older adolescent group supports the idea of a developmental pathway in which young adolescents lie between children and older adolescents. The strength of the connectivity was stronger in children compared to the older adolescents, suggesting that children required greater coherence of neuronal activity with increasing WkM loads (Figure 4-b). This difference was no longer present when controlling for WkM performance, suggesting that performance differences were tied to the functional connectivity differences. This finding would be expected, given the strong relationship between task performance and age. This network does not survive stringent Bonferroni correction for multiple testing, thus it is possible that it is a Type II error. However, there is considerable evidence from prior studies as above described that would implicate that this network is associated with age-related differences in working memory.
In addition, we found age-related differences in a network associated with motor functioning (left motor area right cerebellum). In contrast with the above-mentioned network, this network showed differences between the child group compared with both the two older age groups. These differences in the motor network could possibly be a result of the prolonged developmental course of the cerebellum. It takes more time for the cerebellum to reach the peak volume in comparison with the cerebrum (Tiemeier et al., 2010). In this case there was greater functional connectivity in the adolescents compared to the children (Figure 4-a). Children had increasingly lower performance with increasing load compared to adolescents, and thus the differences could reflect less coherence with motor response networks in children. However, the age-related differences in this network were found during the encoding phase. Therefore, this age-related difference would be more difficult to explain by the manual motor response, as the participants did not press the button during the encoding phase.
The age-related differences that we found between children and adolescents performing a WkM task were not what we expected. In the cognitive network, involving the left prefrontal, left parietal, and right cerebellar network, the strength of the connectivity was stronger in children compared to the older adolescents, while in the motor network involving the left motor area and right cerebellum the functional connectivity was greater in adolescents in comparing to the children. We would have predicted that connectivity strengthens with age, especially in the cognitive domain. However, the measurement of task-related connectivity may be different than resting-state or structural connectivity. For example, increased effort on a task may translate to greater measured connectivity between regions. Alternatively, different brain regions could have different developmental trajectories, and this mismatch in regional development could influence network connectivity.
The network in which the connectivity is higher in adolescents is the network of the left motor area and the right cerebellum. This is the network that is specifically related to motor function. As mentioned above, this could be explained by the prolonged developmental course of the cerebellum, with the motor circuit in adolescents having more coherent connectivity due to better-developed cerebellar networks. The reason that the parietal/prefrontal/cerebellar network does not show the same pattern is perplexing. It may be that the children are exerting more effort for task completion, and thus there is greater connectivity within this network, including the cerebellar component. Another possibility is that adolescents are using alternate brain regions to complete the task, which results in more synchronous regions and greater noise in the system. This could have resulted in age-related differences in the strength of connections between the different regions.
The network including the left motor area and right cerebellum showed age-related differences during the encoding phase, while the more cognitive network including the left prefrontal, left parietal, and right cerebellar network showed significant differences during the recognition phase. Marvel and Desmond (Marvel and Desmond, 2010a) found that the dorsal cerebellar dentate co-activated with the SMA during encoding and that this likely represents the activation of an articulatory motor trajectory. During recognition they found that the ventral cerebellar dentate co-activated with prefrontal regions. These findings correspond very nicely with our results, as we found age-related motor differences during encoding and age-related cognitive differences during recognition. We can also distinguish between the motor and more cognitive pathways of the cerebellum during WkM (Marvel and Desmond, 2010a, b).
Interestingly, apart from the age-related differences, the cerebellum is involved in seven of the ten networks related to WkM in children (Table 2). This emphasizes the important role of the cerebellum in WkM tasks, which has been also documented from lesion (Kirschen et al., 2008) and transcranial magnetic stimulation (Desmond et al., 2005) studies.
A mixed-model regression analysis with age as the random variable and load and run as fixed effects was also performed on these two networks that were significantly related to load and age. We found that these two networks also showed age-related differences with age as a continuous variable in the model. These networks are strongly related with development along a linear trajectory.
A network involving the anterior cingulate cortex and orbital frontal cortex showed age-related differences that were not related to the load of the WkM task. Thus, this network showed age-related differences during encoding that was independent of the load. However, this network was not significant using age as a continuous variable, and thus it is possible that this network shows more non-linear effects, as evidenced in Figure 6.
Equally as interesting as the age-related differences in brain networks associated with WkM, is the fact that the majority of networks that we found were not different between the three age groups. This shows that the majority of functional brain networks associated with WkM show strong functional connectivity during the school age years and remain strong with development. We found four specific brain networks that were associated with encoding: 1) the right motor area and right cerebellum, 2) the right prefrontal and parietal cortex and left cerebellum and two networks involving both the occipital lobe (3 and 4). Four brain networks were associated with recognition: 1) the posterior cingulate cortex, 2) right motor area and left cerebellum, 3) left parietal and pre-frontal cortex and right cerebellum, and 4) a network involving the anterior and posterior cingulate cortex and medial cerebellum. We also demonstrated that the bilateral pre-frontal and parietal cortex and bilateral cerebellum and the right cerebellum and bilateral motor areas were associated with both encoding and recognition.
(Nelson et al., 2000) found comparable associations between working memory in children and activations in the prefrontal, posterior parietal, and anterior cingulate cortex. (Olesen et al., 2003) also found fronto-parietal activation associated with WkM in children. Thus, we provide evidence for mature functional connectivity patterns in children and adolescents within a number of WkM networks.
As expected, age-related differences were present in our behavioral data (Luciana and Nelson, 2000; White et al., 2010). Children had a significantly longer response time for both probes and foils compared to adolescents. The accuracy of the working memory task was also lower for all the three working memory loads in children.
A limitation of the study is the relatively small sample size per subgroup. Nevertheless, literature describing the development of brain connectivity associated with WkM is sparse and our findings mesh well with the sample sizes of the GLM and connectivity studies in the literature. To confirm our results, we also analyzed the age-related differences using a mixed-model regression analysis. Age as a continuous variable effectively increased the sample size and provided support for developmental differences in two load-dependent networks. Larger sample sizes may identify additional brain regions with smaller effect sizes that show age group-related differences in WkM performance. On the other hand, additional components could potentially be more prone to type II errors. Also, the test for age effects is certainly susceptible to type II error. However, when using Bonferroni correction, only the left motor area remains significant. Another limitation of our study is that considerable scanning time was spent during the retrieval phase of the task. Therefore the encoding phase has less power in comparison with the retrieval phase. An optimal design would have a balance between the encoding and retrieval time periods. However, we found significant age-related differences in connectivity in the left prefrontal cortex, left parietal lobe and right cerebellum during retrieval. Furthermore, there were as many load-related and age-related components during retrieval as during encoding. So the distribution of the networks during encoding and retrieval is the same, even with discrepancies in the duration of the encoding and retrieval phase. The question rises if the results would have been different if the study had been run with more even periods of encoding, maintaining and retrieval. Future studies could help to answer this question and possibly further optimize the design of the task. Another limitation is that we only used visually presented stimuli in this study. With auditory-presented stimuli, it is possible that we could have identified other age-related networks (Kirschen et al., 2010). As described by Kirschen et al. auditory presented stimuli during a WkM task are associated with greater medial cerebellar hemisphere activations while visual presented stimuli are associated with greater lateral hemisphere activations. Another limitation is that fatigue could have occurred during such long WkM trials. However, as presented in figure 5, the age-related networks look more alike during run 3 than the earlier runs, which may mean that fatigue tends to create a situation in which even older adolescents fall back to more basic network strategies.
In conclusion, it is important to better understand the developmental trajectories in functional connectivity as children progress through adolescence into early adulthood. It is an age period where the risk for specific psychiatric disorders increases dramatically. We found age-related differences in performance and brain connectivity during WkM tasks in 9–19 year old typically developing children and adolescents. An important finding in this study is evidence for a developmental trajectory in the left prefrontal, left parietal and right cerebellar network. This is an important network that has been shown to be associated with WkM performance. Future neuroimaging studies should evaluate brain connectivity in larger populations, beginning at a younger age, and using longitudinal designs. These studies may help inform when in the course of development the trajectories go awry in children with emerging psychopathology.
Acknowledgments
Funding
Mental Illness and Neuroscience Discovery Research Network (NIMH K08 MH068540), the NIH (2R01EB000840), ZonMw TOP (Project nummer: 91211021) the Mind Research Network, and through the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD) through the Essel and Blowitz-Ridgeway Foundations.
Abbreviations
- AFNI
Analysis of Functional NeuroImages
- BOLD
Blood Oxygen Level Dependent
- fMRI
functional Magnetic Resonance Imaging
- FSL
FMRIB’s Software Library
- ICA
independent component analyses
- SIRP
Sternberg Item Recognition Paradigm
- WkM
Working memory
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baddeley A. The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001a;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp. 2001b;13(1):43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9(2):145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Annals of neurology. 2005;58(4):553–560. doi: 10.1002/ana.20604. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, Axelson D, Rajarathinem R, Haddad L, Amirsadri A, Zajac-Benitez C, Rajan U, Keshavan MS. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: Comparing vulnerability markers. Progress in neuro-psychopharmacology & biological psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CL, Hinshaw S, D'Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(33):11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology. 2004;174(1):3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Karatekin C, White T, Bingham C. Shared and nonshared symptoms in youth-onset psychosis and ADHD. Journal of attention disorders. 2010;14(2):121–131. doi: 10.1177/1087054709347434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Desmond JE. Modality specific cerebro-cerebellar activations in verbal working memory: an fMRI study. Behavioural neurology. 2010;23(1–2):51–63. doi: 10.3233/BEN-2010-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Davis-Ratner MS, Milner MW, Chen SH, Schraedley-Desmond P, Fisher PG, Desmond JE. Verbal memory impairments in children after cerebellar tumor resection. Behavioural neurology. 2008;20(1–2):39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of cognitive neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(12):5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Neurodevelopmental assessment of cognitive function using CANTAB: Validation and future goals. In: Ernst R, editor. Functional Neuroimaging in Child Psychiatry. Cambridge: Cambridge University Press; 2000. pp. 379–397. [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex; a journal devoted to the study of the nervous system and behavior. 2010a;46(7):880–895. doi: 10.1016/j.cortex.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychology review. 2010b;20(3):271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Developmental psychology. 2000;36(1):109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Human brain mapping. 2011;32(10):1649–1659. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. NeuroImage. 2008;42(4):1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain research. Cognitive brain research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Pyka M, Beckmann CF, Schoning S, Hauke S, Heider D, Kugel H, Arolt V, Konrad C. Impact of working memory load on FMRI resting state pattern in subsequent resting phases. PloS one. 2009;4(9):e7198. doi: 10.1371/journal.pone.0007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyka M, Hahn T, Heider D, Krug A, Sommer J, Kircher T, Jansen A. Baseline activity predicts working memory load of preceding task condition. Human brain mapping. 2012 doi: 10.1002/hbm.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, White T, Clark VP, Fries J, Andreasen NC, Goff DC, Manoach DS. MTHFR 677C --> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val --> Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Pena-Gomez C, Arenaza-Urquijo EM, Vidal-Pineiro D, Bargallo N, Junque C, Bartres-Faz D. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex; a journal devoted to the study of the nervous system and behavior. 2011 doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10(3 Pt 1):327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. NeuroImage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, O'Leary D, Magnotta V, Arndt S, Flaum M, Andreasen NC. Anatomic and functional variability: the effects of filter size in group fMRI data analysis. NeuroImage. 2001;13(4):577–588. doi: 10.1006/nimg.2000.0716. [DOI] [PubMed] [Google Scholar]
- White T, Schmidt M, Karatekin C. Verbal and visuospatial working memory development and deficits in children and adolescents with schizophrenia. Early intervention in psychiatry. 2010;4(4):305–313. doi: 10.1111/j.1751-7893.2010.00204.x. [DOI] [PubMed] [Google Scholar]
- White T, Schmidt M, Kim DI, Calhoun VD. Disrupted functional brain connectivity during verbal working memory in children and adolescents with schizophrenia. Cerebral cortex. 2011;21(3):510–518. doi: 10.1093/cercor/bhq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo XN, Hong LE, Gao JH, Stein EA, Zang YF, Yang Y. Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Human brain mapping. 2012 doi: 10.1002/hbm.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]