Summary
Researchers dealing with the human leukocyte antigen (HLA) class I and killer immunoglobulin receptor (KIR) multi-gene families in humans are often wary of the complex and seemingly different situation that is encountered regarding these gene families in Old World monkeys. For the sake of comparison, the well-defined and thoroughly studied situation in humans has been taken as a reference. In macaques, both the major histocompatibility complex class I and KIR gene families are plastic entities that have experienced various rounds of expansion, contraction, and subsequent recombination processes. As a consequence, haplotypes in macaques display substantial diversity with regard to gene copy number variation. Additionally, for both multi-gene families, differential levels of polymorphism (allelic variation) and expression are observed as well. A comparative genetic approach has allowed us to answer questions related to ancestry, to shed light on unique adaptations of the species’ immune system, and to provide insights into the genetic events and selective pressures that have shaped the range of these gene families.
Keywords: MHC, KIR, rhesus macaque, co-evolution
An introduction to the MHC class I system
All jawed vertebrate species share the presence of a major histocompatibility complex (MHC) representing, in concert with the re-arranging families of T-cell receptors (Tcr) and the immunoglobulins (Ig), one of the hallmarks of the adaptive immune system. The MHC region is densely packed with several classes and/or clusters of genes, most of which play crucial roles in various immune responses. The MHC class I genes are divided into classical (class Ia) and non-classical (class Ib). The classical MHC class I genes are expressed in virtually all nucleated cells and are involved in generating adaptive immune responses to a plethora of pathogens that cause intracellular infections, such as viruses and mycobacteria. The non-classical MHC class I genes are often characterized by differential tissue expression, low levels of polymorphism, and the execution of specialized functions.
MHC class I molecules are essentially peptide receptors equipped with an antigen binding site and, under normal conditions, are transported to the cell surface, loaded with self-peptides. However, in the case of an infection, the MHC class I molecule may be loaded with a peptide that originates from a pathogen. It has been well established that T cells rearrange their receptors in the thymus, they are educated to become potentially reactive against self-MHC molecules loaded with non-self peptides. In particular, CD8+ cytotoxic T cells (CTLs) are able to lyse cells, indicating infection or a state of malignancy via the MHC class I pathway (1). Classical MHC class I genes display polymorphism, and each allotype has the property to bind its own spectrum of peptides. The biological relevance of allelic polymorphism is to minimize the possibility of one particular pathogen decimating an entire population (2). In accordance with this school of thought, many MHC class I allotypes may control either susceptibility or resistance to a large variety of chronic and infectious diseases.
Basic facts about macaques
Rhesus macaques (Macaca mulatta) are members of the Old World Monkey (OWM) family and have the widest geographical distribution of any nonhuman primate species. They occupy a wide range of biotopes in Asia and are native to countries such as Afghanistan, India, Pakistan, Nepal, Vietnam, Thailand, Burma, and Southern China. A closely related species is the cynomolgus macaque (Macaca fascicularis), which has a more southern geographical distribution, but introgression is documented to occur at the boundaries where both species meet (3).
Humans and macaques shared a common ancestor that lived approximately 25 million years ago (4, 5). This common ancestry is reflected by the fact that these species share many morphological and genetic features and are susceptible to infections by the same range of pathogens. Subsequently, both species may develop similar diseases. Macaques represent important model systems for the study of human biology and disease and have contributed to breakthrough discoveries in the fields of transplantation biology, infectious and chronic diseases, and vaccine development, as well as by the introduction of safe immunotherapy protocols into the clinic (6–13).
In the wild, macaques live in social groups (14), but in captivity, they are preferably kept in highly specialized centers or zoos that are able to provide all the necessary care in terms of the animals’ needs. Primate centers may house self-sustaining colonies of macaque species that have been pedigreed and characterized for many genetic traits over several decades. In the USA and Europe, biomedical studies have traditionally been conducted with rhesus macaques originating from the Indian continent. Due to the shortage of Indian macaques for biomedical studies, however, attention has shifted partly towards animals of Chinese origin. This has some relevance, as the rhesus macaque has its ancestral roots in the Indonesian archipelago, where the putative ancestral species is thought to have originated about 2 million years ago (15). The Indian and Chinese macaque populations were separated approximately 162 thousand years ago. Whereas the Chinese population expanded, the Indian population maintained its ancestral population size for a long time but at some point experienced a severe reduction (16). Cynomolgus monkeys colonized more southern territories during the ice age, but when water levels started to rise again due to climate changes, many populations became disconnected, for instance on the islands of the Indonesian archipelago. Cynomolgus macaque populations appear to display substantial levels of polymorphism in their genome, suggesting that the initial colonization of these territories was by a large group of individuals. At the present time, both species live in a wide geographic area and have populated many habitats and undoubtedly must have experienced different selective forces.
The Big Bang of MHC class I evolution in OWM
The human MHC or HLA system is located on the short arm of chromosome 6. This region comprises three classical class I genes designated HLA-A, HLA-B, and HLA-C. All of these genes display copious levels of allelic variation (17), and the corresponding gene products are transported to the cell surface of nucleated cells. MHC genes are encoded by the paternally and maternally inherited HLA-A, HLA-B, and HLA-C genes and are expressed in a co-dominant fashion that consequently allows heterozygous individuals to express two allotypes for each locus.
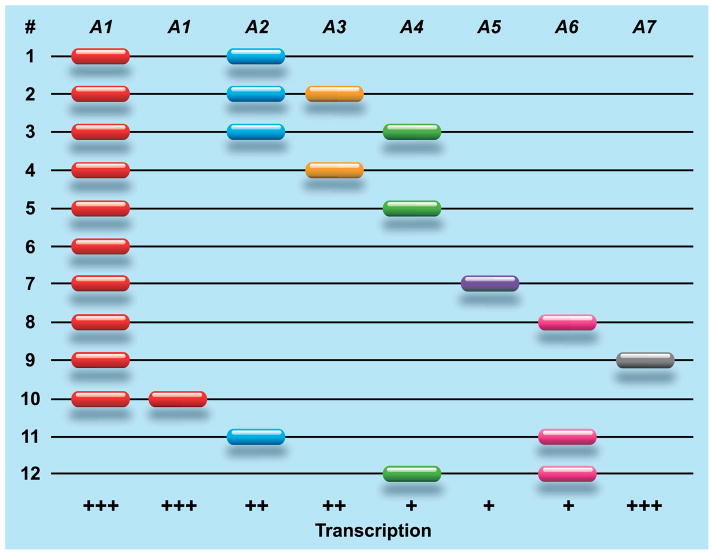
In macaques, the situation with regard to copy number variation of the MHC class I genes is radically different. Genomic mapping and segregation studies have illustrated that macaques experienced a substantial expansion of the MHC class I region (18–21), and this is apparently a common feature for all OWM species. In rhesus macaques (22, 23) and cynomolgus monkeys (23), a differential number of A gene region configurations have been encountered, each displaying another combination of different paralogous A genes. As can be seen, most region configurations in rhesus macaques possess – by convention – an A1 gene, whereas the A2–A7 genes show restricted haplotype distributions (Fig. 1). The high plasticity of the region is illustrated by the observation that two A1 genes may also be present on one configuration, but the contrasting situation where an A1 gene is absent is also documented (Fig. 1). Most likely the gene content–combination, number, and order of paralogous genes–of region configurations is altered in time by unequal crossing-over events. Identical or similar region configurations are encountered in other macaque species, demonstrating that they represent old entities that predated the speciation of macaques.
Fig. 1. Schematic representation of Mamu-A region configurations.
The Mamu-A1 gene is present on most region configurations but absent on # 11 and 12, and duplicated copies exist on configuration # 10. The A2 to A7 genes display a more restricted haplotype distribution, and most of them display differential transcription activity. Transcriptional activity is indicated as (+++) abundant, (++) moderate, and (+) low. The order and physical distance between the genes is only known for region configurations 1 and 5 (18, 20).
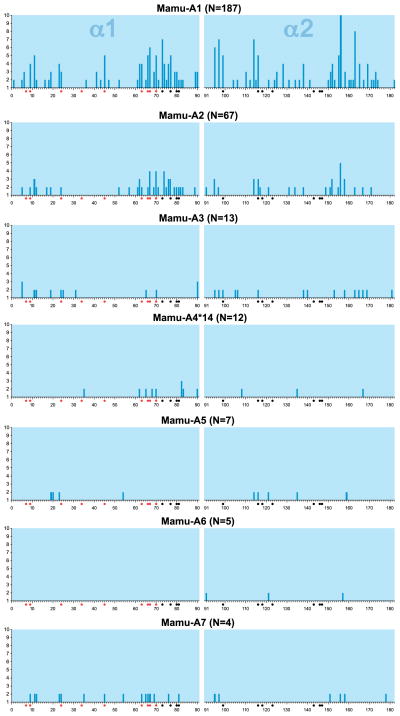
The Mamu-A1 gene seems to comprise a large number of allelic lineages, but within the lineages themselves only modest levels of polymorphism are encountered (24). The situation in humans is markedly different; lineages, such as HLA-A*02, are characterized by an extremely high number of alleles. As observed for HLA-A, the various Mamu-A1 lineages are highly diverse, and genetic distances between the lineages can be substantial. Most of the polymorphic residues encoded by exon 2 and 3 are contact residues mapping to the antigen-binding site, and as suchm, they define the specificity to bind particular peptides. A variability plot illustrates that the Mamu-A1 gene parades high levels of variation, which is mainly due to the existence of numerous distinct lineages (Fig. 2). The Mamu-A2 gene is polymorphic as well, and it encodes three lineages, with allelic variation mainly observed within Mamu-A2*05 (24). One of the other lineages, Mamu-A2*24, was generated by a recombination event (19, 25). The variability plot shows that the antigen-binding groove of the corresponding allotypes experienced purifying selection (Fig. 2), suggesting that this gene may control a more or less specialized function.
Fig. 2. Variability plot of different Mamu-A gene products.
Shown on the Y axis is the total number of different amino acids encountered at a given position. On the X axis are the alpha 1 and 2 domains, and the corresponding amino acids have been numbered sequentially. N represents the number of alleles that are encoded by the gene analyzed. The red and black dots indicate the contact residues in the B and F pocket, respectively.
The other paralogous A genes display modest levels of polymorphism, and again it seems that in these cases the antigen-binding groove has been subjected to purifying selection. Thus, the classical antigen presentation capacity of the A region is mainly controlled by the Mamu-A1 allotypes, whereas the other allotypes most likely execute more specialized functions.
The organization of the B region in macaques is more complex. For one haplotype, the presence and physical order of 19 B genes has been established by genomic sequencing of the entire region (18), and most of these genes seem to be intact. However, extended mRNA sequencing and segregation analyses have illustrated that haplotypes with different combinations of B genes are present in rhesus macaques. At this stage, at least 18 and 28 haplotypes have been determined in rhesus macaques (26, 27) and cynomolgus monkeys (26, 28), respectively. In contrast to the A region, it is not yet possible to discriminate between alleles and loci in the B region. The most parsimonious interpretation of the data is that all of these B entities represent distinct loci and that allelic variation for each of these paralogous genes is low. For a more definite answer, additional B haplotypes need to be sequenced at the genomic level to define the physical maps. For most B haplotypes, two to three genes appear to be transcribed at abundant levels. Referred to as majors, these genes seem to segregate with a certain number of minors that are in turn characterized by low transcription levels. Despite the fact that the recombination dynamics may differ significantly between macaque species (26), the haplotypes themselves seem to be stable, and several of them are shared between geographically distinct populations of macaque species. The mechanisms that govern the condition in which some of the intact genes are not or are poorly transcribed are unknown. An intriguing possibility may be that during recombination processes intact genes without functional promotors are positioned downstream of an active promotor. This would allow the reactivation of genes that had been silenced long ago and represents a sound strategy to avoid the adaption of pathogens to their host. Alternatively, the possibility that certain genes have been silenced due to epigenetic modifications cannot be excluded.
The conundrum of the missing C locus: what’s in a name?
In humans, the nomenclature of most MHC gene entities reflects their order of discovery. HLA-A and HLA-B were described first by serology, and this was followed by HLA-C. Thus, their naming does not reflect function or any evolutionary relationship. The HLA-B and HLA-C genes map tightly together, and this tandem is separated from HLA-A by a large genomic segment, which is consistent with the assumption that HLA-B and HLA-C genes shared a progenitor and arose from an ancient duplication event (29). Comparative studies on different primate species have illustrated that the A and B genes are old entities that were present in an ancestral primate species (30). The emergence of the C gene in fact arose from a duplication of the B gene, as was revealed by studies on the orangutan MHC (31). In humans, this gene was named C, but if nomenclature were to have been based on evolutionary knowledge, it could have been named HLA-B2. It has been claimed that the C locus is absent in OWM (32). One might wonder whether rhesus macaques indeed do lack the functional equivalent of the HLA-C gene, as they are known to possess multiple intact B genes per haplotype. Of course, we are dealing with independent duplication events. However, given the high levels of plasticity observed for the MHC, both at the gene and functional level, it is feasible that one of the duplicated B genes in rhesus macaques may execute, with regard to its preferential ligand structure, a function more or less similar to that of HLA-C in humans.
Allelic variation or gene copy number variation: choose your weapons
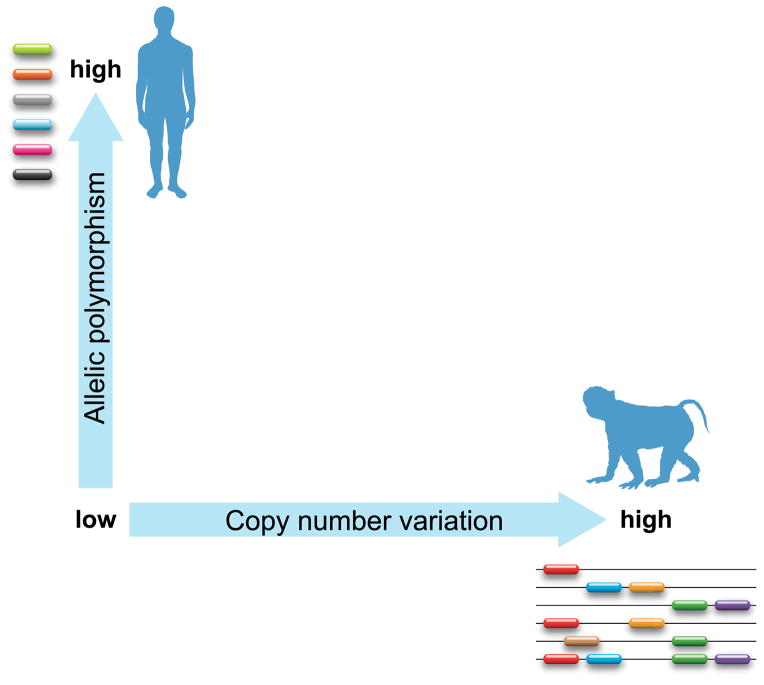
The Mamu-A1 gene controls a high number of lineages, most of them displaying limited levels of polymorphism. In humans, it is the other way around, with the HLA-A gene controlling a limited set of gene families/lineages, and each of them is represented by a huge number of alleles. To complicate matters, the A region in rhesus macaques displays gene copy number variation as well (Fig. 1). This contrasting situation is more prominent for the B genes. Nonetheless, one has to realize that human and macaque individuals seem to express approximately equal numbers of classical MHC class I allotypes. Therefore, the high number of duplicated genes present in macaques is not likely to result in a massive deletion of the T-cell repertoire in the thymus. Thus, the strategy to minimize the possibility of an entire population being decimated by one pathogen is in humans guarded by allelic polymorphism, whereas rhesus macaques seem to bank on combinations of immune response genes that are segregating in a Mendelian fashion (Fig. 3). Similar observations have been made for DR, located in the MHC class II region (33).
Fig. 3. The relationship between allelic heterogeneity and gene copy number variation for MHC class I.
Shown on the Y axis is an increasing scale depicting the number of alleles, whereas on the X axis an increasing scale in copy number variation per haplotype is shown. One extreme represents the situation in humans (one gene with a large amount of allelic variation), whereas rhesus macaques can be found on the other end of the spectrum (many region configurations but little allelic variation).
This makes one wonder which genetic modality was first: allelic variation or region configuration polymorphism. The situation observed in humans (one copy of the A, B, and C genes) is similar in chimpanzees and gorillas. The B region in orangutans shows a moderate expansion. Unfortunately, relatively little is known about the MHC of gibbons, but it seems to lack the C gene (34). In OWM, the A and B genes have expanded considerably. One model could be an expansion of the A and B genes during OWM radiation. However, there is an alternative explanation, since the orangutan genome also shows expansions of its MHC region. The common ancestor of humans, great apes, and OWM may have already possessed an expanded MHC class I gene region. Possibly during primate radiation, in the lineage leading toward great apes and humans, MHC class I genes were deleted. In contemporary humans, this ended in a situation involving three classical MHC class I genes, each displaying substantial levels of polymorphism. This model could explain why the genetic differences between the various lineages encoded by the HLA-A or -B genes are so markedly large. These days they are considered as alleles, but they may represent old relics of paralogous genes that are now localized on a similar position of the genome.
Majors and minors: differential expression profiles
In humans, the HLA-C allotypes are expressed at much lower levels than the HLA-A and HLA-B molecules (35). In rhesus macaques, next-generation sequencing protocols executed on pedigreed animals allowed monitoring of the transcription activity of multiple A and B genes. In our hands, only one of the A genes and two to three B genes per haplotype have been characterized by abundant transcription levels. It is likely that transcripts that are abundantly present are effectively translated and that the corresponding gene products are present at the cell surface. We have called these genes majors (19), and in many functional studies, these genes in particular control the classical adaptive immune responses (19, 36). For some MHC class I genes that are seemingly intact, no or low transcription (minors) has been documented. Hence, the question remains: what is their function or fate? In the case of extremely poor expression levels, which may be caused, for instance, by a leaky promotor system, transcriptional activity may be so low that translation is not effective, and the gene products do not reach the cell surface. A confounding factor that applies to most sequencing studies in macaques is that these have been conducted on virus-transformed B cells. This opens up the possibility that some of these genes may be differentially expressed in other tissues and cell types. One such an example has been recorded lately, as CD14+ monocytes express a unique subset of minors (37).
All that matters is function
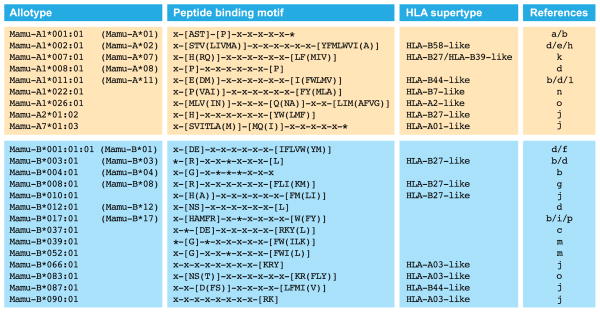
MHC class I molecules are receptors equipped with a groove that can accommodate small peptides, preferentially 9 amino acids in length. The groove contains six different pockets (designated A-F), and generally amino acid residue number 2 binds in the B pocket, whereas the carboxyl terminus will fit into the F pocket. Many variations on this theme may occur. Based on the amino acid composition of the groove, each allotype has its own capacity to bind a spectrum of peptides. The peptide-binding motifs have been determined for some of the major MHC class I molecules in rhesus macaques, and an overview has been provided (Fig. 4). The peptide-binding motifs defined for different HLA specificities have been clustered into supertypes (38). Some of the motifs found in humans and rhesus macaques are highly similar (Fig. 4); for instance, HLA-B27-like motifs are also present in rhesus macaques. The motifs defined for the Mamu-B*039:01 and -B*052:01 allotypes are shared with mice but seem to be absent in humans. Other motifs appear to be unique (39). The sharing of motifs is not due to common ancestry of the MHC class I allotypes but reflects convergent evolution. This phenomenon has been documented previously by comparing the MHC class I peptide-binding motifs between humans and chimpanzees (40).
Fig. 4. Overview of peptide-binding motifs in rhesus macaques.
Depicted are the Mamu-A and Mamu-B allotypes for which the peptide-binding motifs are known and their similarity to HLA supertypes. Names in brackets refer to the allotype designations that were used previously. In a peptide-binding motif, the conventional one-letter code identifies the preferred, or in brackets the tolerated amino acid residues at the corresponding position. An asterisk ‘*’ identifies a secondary anchor position. References: a. (122), b. (123), c. (2), d. (124), e. (125), f. (126) g. (127), h. (128), i. (129), j. (130), k. (131), l. (132) m. (133), n. (134), o. (135), p. (136).
Particular rhesus macaque MHC class I molecules are exported to the cell surface in a poor manner. Representatives of this phenomenon can be encoded by various loci, such as Mamu-A4*14:03, -B*012:02, and -I*01:02 (41). More so than humans, rhesus macaques are infected naturally with various types of retroviruses (42, 43). One possibility is that these retained gene products are actually highly specialized and monitor the activation of endogenous retroviruses. If such an event occurs, they may bind viral peptides and travel to the cell surface to signal infection. Another intriguing possibility is that these molecules have an intracellular ligand.
Non-classical MHC class I molecules in macaques
MHC-E: an old timer
In humans, the HLA-E gene executes a specialized function, as the pocket is hydrophobic and binds the leader segments of HLA-A, HLA-B, and HLA-C molecules (44). The complex of HLA-E and these leader segments are ligands for the NK cell receptors NKG2A and NKG2C, respectively (45). In essence, this system allows NK cells to scan whether expression of classical MHC class I antigens has been modified by viruses or malignancy. The peptide-binding pocket of the HLA-E gene product is highly conserved, and this level of conservation extends to macaque species (46, 47), suggesting that its function (48) is at least 30 million years old. One must realize that rhesus macaques display abundant transcription of multiple Mamu-A and -B-like gene products. This being the case, the supply of leader peptides to load HLA-E may then be substantial, and one might wonder whether this has an impact on the MHC class I recognition systems that work more specifically.
MHC-F: an enigma
HLA-F gene products are present on the cell surface of various sorts of activated lymphocytes (49). Recent work has suggested that HLA-F and MHC class I open conformers are ligands for particular sets of NK cell receptors of the KIR gene family (50). This function has been present over long evolutionary time spans, as evidenced by the sharing of similar sequences between humans and macaques (51).
The peculiar evolutionary history of HLA-G and Mamu-AG
In humans, the expression of HLA-G is mainly confined, together with HLA-C, to the placental trophoblast (52). In rhesus macaques and other OWM, the orthologue of HLA-G has been inactivated and represents a pseudogene (53). Its function and tissue distribution has been taken over by Mamu-AG, another duplicated A-related gene present in the MHC of the rhesus macaque (54, 55). The genome of OWM harbors multiple copies of this gene (18), and at this stage it is not clear how many of these are functional. The plasticity of the MHC system is illustrated in New World monkey species, where the ortholog of HLA-G controls a classical antigen presentation function, and in concordance, the gene is expressed in all nucleated cells (56).
Mamu-I: a nonclassical with an unknown function
All haplotypes in rhesus macaques seem to harbor a B-related gene, designated Mamu-I, which displays low variability with regard to its binding site (57). Orthologs of the gene are present in other OWM species as well (24), although at this stage, little is known about its function. It is transcribed in virally transformed B cells and seems to reside mainly intracellularly (41).
MIC genes: ancient indicators of stress
The HLA region encodes two functional genes that have been named MICA and MICB (17), though unlike the conventional MHC class I heavy chains, the MICA and MICB gene products do not associate with β2 microglobulin. MIC gene products are mainly expressed on epithelial cells of the gut and play an important role in signaling stress to the immune system. In macaque species, two copies are present per haplotype, but there is heterogeneity (58). Each haplotype comprises a MICB gene segregating either with an MICA gene or a MICA/B hybrid (59–61). As is found in humans, the genes are polymorphic.
The ligands of MHC class I gene products
T-cell receptors
Together with B cells, T lymphocytes represent the effector cells of the adaptive arm of the immune system. Whereas B cells may eventually secrete antibodies, T cells are equipped with a cell surface-fixed receptor. Functional TCRs are generated by gene rearrangement, and different gene segments that are being recruited from a large genomic arsenal are glued together. As a consequence, each individual expresses an enormous and unique repertoire of TCRs. The buildup of the repertoire and its eventual outcome are also dependent on the MHC phenotype of an individual. This huge TCR repertoire minimizes the possibility of escape from T-cell recognition. Most vertebrate species possess two classes of TCRs. The γδ-positive T cells are the first wave of cells leaving the thymus, and they play an important role in controlling homeostasis in the gut system. The γδT cells may utilize the MIC gene products, only expressed under conditions of cell-induced stress, or CD1 structures, MHC class I-like molecules not encoded within the MHC, as potential ligands (62, 63). The second wave of T cells leaving the thymus are CD4+ or CD8+, and these cells are characterized by an αβTCR. These receptors mainly interact with MHC class I and II molecules. The TCR and its repertoire are in essence similar in humans and rhesus macaques (64, 65) and are not discussed further here. As could be expected during evolution, various pathogens have evolved strategies aimed at avoiding immune recognition by modulating MHC class I cell surface expression (66, 67).
Natural killer cells and the missing-self hypothesis
A central theme in immunology is the capacity to discriminate between self and non-self structures, and various mechanisms have been employed to achieve this possibility. For instance, T cells are selected in the thymus, based on their capacity to stay non-reactive when they engage cells that have on their cell surface MHC molecules loaded with self-peptides. The situation changes dramatically when T cells encounter an MHC structure where a self-peptide has been changed for a peptide that originates from a pathogen.
There is a subset of large granular lymphocytes named natural killer (NK) cells. In the past, it was thought that they represented the innate arm of the immune response. Currently, however, it is widely considered that these cells bridge the gap between adaptive and innate immunity. Like T cells, NK cells protect the host from infection and control the unwanted growth of malignant cells. Unlike T and B cells, however, NK cells do not experience gene rearrangements, and as such, their recognition receptors are germ-line encoded. A crucial discovery was that cells that do lack MHC class I molecules are lysed by NK cells, and this was the basis for the postulation of the ‘missing-self’ hypothesis (68). Subsequently, it was assumed that NK cells must be equipped with inhibitory receptors that were able to interact with MHC molecules. Indeed, NK cells were found to possess receptors, such as CD94/NKG2A and -C dimers (indirect recognition via HLA-E) and members of the killer-cell immunoglobulin-like receptors (KIR) gene family that are interacting with MHC class I molecules (45, 69, 70). The family of NKG2 (KLRC) C-type lectin receptors and their invariant CD94 (KLRD1) partner are encoded by the natural killer complex (NKC), which in humans is located on chromosome 12. NK cells appear to possess an arsenal of both activating and inhibiting receptors. During a process called NK cell education, the interaction or lack thereof between NK receptors and self-MHC class I molecules determines the threshold level of activation (71, 72).
Introducing the KIR genes
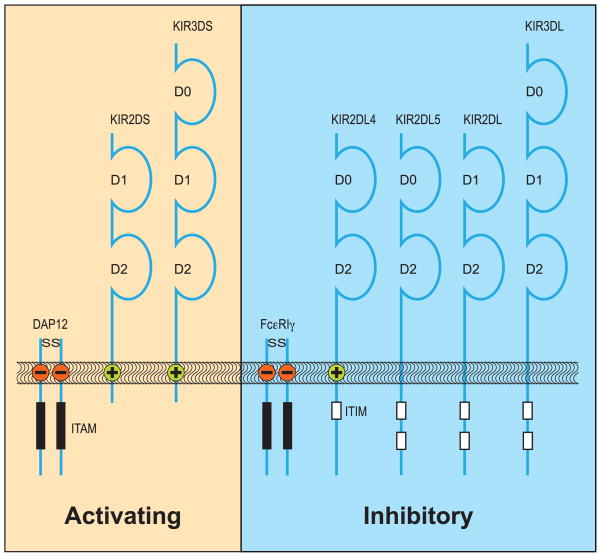
In both humans and macaques, the KIR genes map to the leukocyte receptor complex (LRC) located on chromosome 19 (73, 74). This family of NK cell receptors is highly polymorphic and displays extensive variability in copy number as well as a diverse and stochastic expression profile on individual NK cells. KIR nomenclature reflects the structure and function of these receptors. The first digit of KIR1D, KIR2D, or KIR3D indicates their number of Ig-like domains, whereas receptors with activating or inhibitory capacity have short (S) or long (L) tails, respectively (Fig. 5). Activating receptors share a positively charged amino acid residue in their transmembrane section that can associate with adaptor molecules such as DAP12 or the FcεRγ. These adapters have negatively charged amino acid residues in their transmembrane section and contain immune receptor tyrosine-based activation motifs (ITAM), and as such, they have a stimulatory signaling capacity (73, 74). Inhibitory receptors have immune receptor tyrosine-based inhibitory motifs (ITIM) in common, and the number of these motifs may vary from one to two. To denote KIR genes in non-human species, a prefix is used, for example ‘Mamu’ in rhesus macaques.
Fig. 5. KIR gene products in humans.
KIR molecules span the membrane. The inhibiting molecules (L) have ITIM structures in common, whereas the potential activating structures (S) need to transfer their signaling via adapter molecules that possess ITAM structures. Although KIR2DL4 pairs with an adaptor molecule, it is often portrayed as an inhibitory receptor due to its ITIM structure.
KIR in primates: evolution in the fast lane
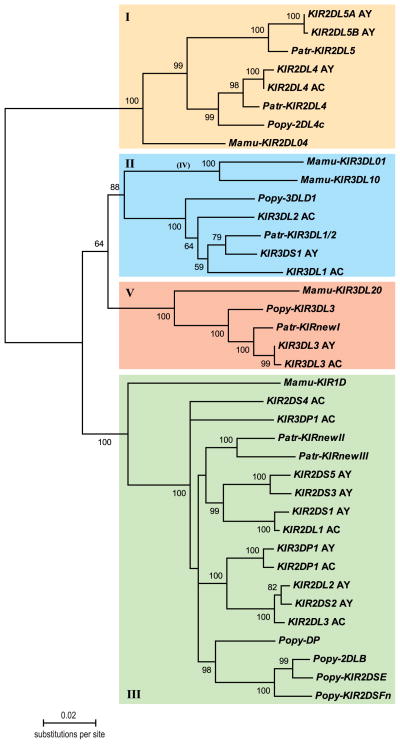
KIR genes have also been sequenced and annotated for various non-human primate species belonging to the great apes (34, 75, 76), OWM (48, 77, 78), and New World Monkeys (79, 80), suggesting that the ancestral structure was already present in a common ancestor (81). Based on phylogenetic analyses of KIR gene sequences, the various genes have been classified into four distinct lineages. For example, the human KIR2DL4 and KIR2DL5 genes (82, 83) cluster together into lineage I with their orthologous structures in chimpanzees (76), orangutans (75), and rhesus macaques (84) (Fig. 6). More or less similar observations have been made for the KIR genes clustering into lineages II, III, and V (Fig. 6). Although different primate species possess KIR genes with a similar architecture, all the KIR genes appear to have diverged in a species-specific way, which suggests rapid evolution.
Fig. 6. Neighbor-joining tree depicting different KIR gene lineages.
Intron 3 sequences were obtained from haplotypes that have been sequenced in humans (AC, AY), chimpanzees (Patr), orangutans (Popy), and rhesus macaques (Mamu), and were extracted from the EMBL database. Bootstrap values showing confidence levels above 50% have been indicated. The differently colored boxes represent KIR lineages I, II, III, and V.
KIR genes in rhesus macaques: a short overview
KIR2DL4 (lineage I)
KIR2DL4 is considered a framework gene in humans, as it is present on most haplotypes. The KIR2DL4 receptor has a hybrid character with regard to its signaling capacities, since it can deliver both adapter-mediated activating and ITIM-mediated inhibitory signals (Fig. 5). Macaques possess the orthologous Mamu-KIR2DL04 gene, which is present in most animals that have been analyzed. The detailed genomic structure has been reported, and the gene encodes two ITIM structures. In contrast to humans, where KIR2DL4 Ig-like domains are polymorphic, in rhesus and cynomolgus macaques KIR2DL04 has a peculiar distribution of polymorphic residues (84, 85). The ligand-binding domains are highly conserved, whereas the transmembrane and cytoplasmic sections have diverged considerably. Similar to humans, there are two major forms differing for their signaling potentials that also appear to have experienced balancing selection (84).
In humans, the natural ligand for KIR2DL4 may be soluble HLA-G, a splice variant of the non-classical MHC class I molecule, mainly expressed on cells of the trophoblast. It is generally accepted that because NK cells are the dominant lymphocyte present at the maternal-fetal interface during pregnancy, they assert a critical influence on its success. The Mamu-G gene has several deleterious mutations and is a pseudogene, but its function may have been taken over by Mamu-AG (53–55). At this stage, it is not known whether Mamu-AG serves as a ligand for Mamu-KIR2DL04. In both humans and macaques, KIR2DL4 alone is not essential for reproduction, as a substantial number of reported individuals with offspring lack the gene. This suggests that layers of redundancy exist.
KIR3DL/S (lineage II)
In humans, KIR possessing three domains, such as 3DL1/S1 and 3DL2, are grouped into lineage II, and map to the telomeric section of the KIR complex (86). Two of these are inhibitory receptors that recognize epitopes on HLA class I molecules. For instance, 3DL1 recognizes the HLA-Bw4 motif present on most HLA-B and some HLA-A molecules, whereas KIR3DL2 interacts with an epitope present on HLA-A3/A11 structures (Fig. 7). Through these inhibiting KIR receptors, NK cells scan for any malfunction with regard to their cognate MHC class I expression. The activating KIR3DS1 recognizes the Bw4 motif suboptimally and as a consequence is dependent on HLA-B-presented peptides from a restricted pool for its stabilization and functional binding (87). Orthologous genes have been discovered in rhesus macaques as well. One remarkable feature is that in OWM the KIR lineage II family has expanded dramatically (Fig. 7). As can be seen, in macaques at least 10 and 9 loci of inhibitory and activating receptors have been identified, respectively (78, 86, 88). As observed in humans, at the very least, several of these inhibitory and activating KIR receptors may recognize epitopes on MHC class I molecules (89–91). A unique feature of activating macaque lineage II genes is that the genomic organization of Mamu-KIR3DS appears to be conserved, and its putative RNA transcript would encode a transmembrane domain with charged activating residue and a long cytoplasmatic tail with two ITIMs. Analysis of the corresponding transcripts revealed a much shorter tail, and this deviation was explained by the presence of a splice site mutation in intron 8, causing the loss of both ITIMs, which converts this receptor to an activating one (77). One exception to this rule is Mamu-KIR3DS05, which has a premature stop codon introduced, suggesting that this activating KIR evolved independently.
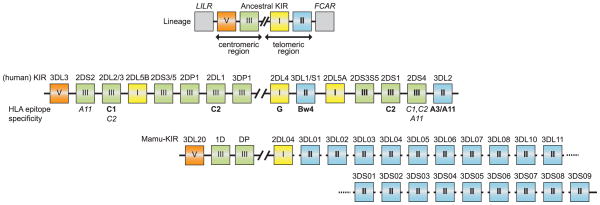
Fig. 7. Comparison of KIR gene organization in humans and rhesus macaques.
The top panel shows the predicted ancestral KIR gene organization depicting its boundary genes (LILR and FCAR), as well as the four KIR lineages. The human and rhesus macaque KIR gene clusters have been aligned at the position of the framework gene KIR2DL4 (lineage I: yellow box). The known ligands in humans are shown as well. The human KIR region shows expansion of lineage III at both the centromeric and telomeric region, whereas the rhesus macaques experienced expansion for lineage II at the telomeric region.
KIR2DL/S (lineage III)
In humans, lineage III KIR has been subject to expansion (Fig. 7). Nine loci have been classified, and their gene products have been found to bind dominant epitopes on HLA-C molecules designated C1 and C2 (74). KIR2DS2 and -2DS4 bind epitopes on HLA-A11 molecules, but this interaction is weak. During its evolution, lineage III KIR has been deposited on the telomeric section of the KIR complex by recombination processes as well (92). The situation is quite different in macaques, however, where the only transcribed lineage III KIR, Mamu-KIR1D, represents a truncated gene. This gene is highly conserved, but at present its function is not known (88, 93). The other linaege III member on the genome of macaques, Mamu-KIRDP, represents a pseudogene, orthologous to the pseudogene that is present in all hominids (94).
KIR3DL3 (lineage V)
There is only one locus of the KIR3DL3 framework gene in humans, and the ligand for its encoded receptor has not yet been identified (Fig. 7). In rhesus macaques, an orthologous structure is present on all haplotypes. This Mamu-KIR3DL20, thus designated as an acknowledgment of its being the 20th Mamu-KIR discovered, putatively encodes an inhibitory receptor with two ITIMs. In contrast to humans, in macaques (77) a truncated splice variant of Mamu-KIR3DL20 has been reported that lacks exon 4 and that could putatively encode a D0–D2 receptor similar to the structure of 2DL4 and 2DL5.
KIR gene-associated polymorphisms
Diversity: KIR haplotypes and copy number variation
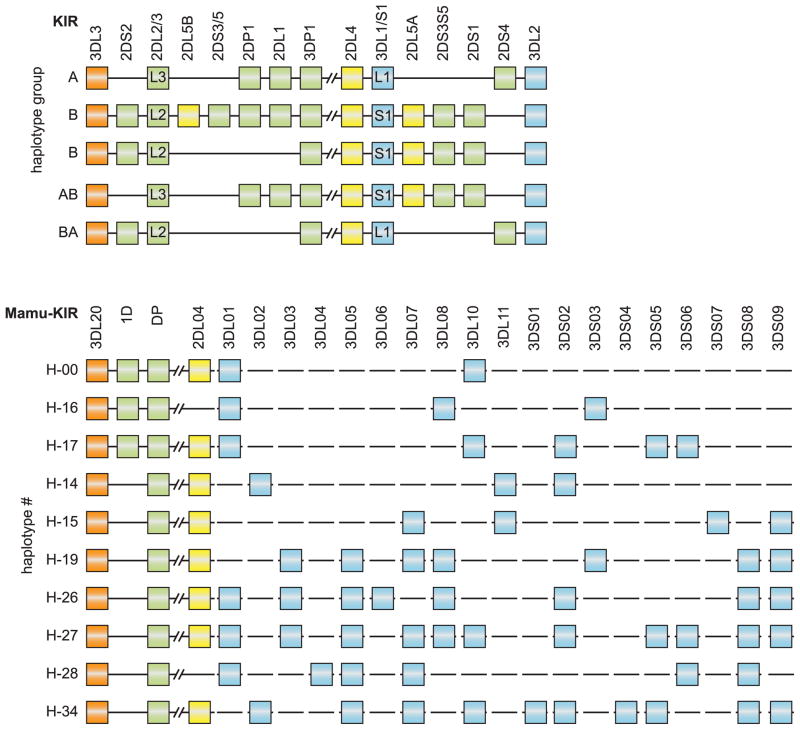
KIR haplotypes in humans display an impressive amount of copy number variation, as the presence and distribution has allowed the definition of group A and B haplotypes (95, 96). The B haplotypes are characterized by the presence of mostly activating genes such as the centromeric clusters KIR2DS2, KIR2DL5B, and KIR2DS3/5, and the telomeric clusters KIR3DS1, KIR2DL5A, KIR2DS3/S5, and KIR2DS1, whereas the A haplotypes lack these structures (Fig. 8). About 95% of the KIR haplotype diversity encountered in the human Caucasian population can be explained by recombination-like events that shuffled a particular centromeric region with a different telomeric region (92), and as a consequence, haplotypes with an intermediate A/B character have also been encountered. Gene content can be expanded and contracted by unequal crossing-over events, and the shuffling of domains by reciprocal recombination may generate novel hybrid entities (97). In rhesus macaques, a large number of haplotypes have been determined by segregation analysis, but in essence ‘A-ness or B-ness’ appears to be absent (88). With regard to the centromeric site, there seem to exist two fixed structures in macaques, with one carrying Mamu-KIR3DL20, -1D, and -KIRDP, whereas the other lacks Mamu-KIR1D. This is in contrast to the telomeric site, where lineage II KIR in rhesus macaques has significantly expanded. One can only conclude from this haplotype diversity that there must have been a substantial number of recombination events that affected these KIR loci (Fig. 8), as the number of lineage II KIR gene copies also differs significantly among haplotypes. This radical difference between species in both lineage expansion and haplotype architecture may be an indication that very different selective forces have been driving the evolution of the human and macaque KIR system, respectively.
Fig. 8. KIR haplotypes in humans and rhesus macaques.
The haplotypes in humans have been sequenced in detail, and the physical localization of the genes is known. For the rhesus macaque, this information is lacking, and therefore the rhesus macaque haplotypes have been inferred. For the human situation, the A and B, as well as their hybrid haplotypes (A/B and B/A), are shown. For the rhesus macaque, the haplotypes displaying gene copy number variation with regard to lineage II KIR genes are depicted.
Allelic variation and population dynamics
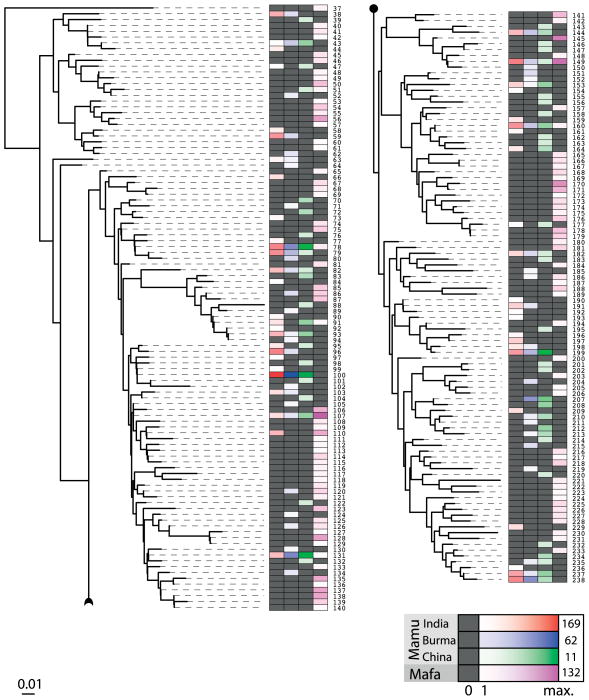
The KIR system in macaques is plastic, and any gene may be present or absent on a given haplotype (88). The extreme plasticity of KIR haplotypes in rhesus macaques is unrivalled in humans (93). As we have seen, several KIR genes in macaques display substantial levels of polymorphism. A next-generation sequencing study that was recently performed on a large panel of rhesus macaques originating from three different populations (Indian, Burmese, and Chinese) and a cohort of cynomolgus macaques has illustrated that almost every individual had a unique KIR repertoire. This was caused by a combination of the following factors: (i) macaques display substantially more haplotype variability than humans and (ii) the amount of allelic heterogeneity encountered was massive. In addition, we have observed a substantial amount of alternative splicing, indicating that different isoforms of the same gene occur. Taken together, this indicates that KIR polymorphisms in macaques dwarf those of the MHC (Blokhuis, manuscript in preparation). A comparison between the different individuals shows that some genes are shared between populations and species, whereas others may be unique for a certain population (Fig. 9). For example Mamu-KIR3DS5 is not found in animals of Chinese origin, while Mamu-KIR3DL04 is exclusively found in animals of Indian origin. This origin-specific evolution is not restricted to the lineage II genes, as exemplified by Mamu-KIR1D (III), which is present in 50% of the animals of Chinese origin but much less frequent in animals from Burmese or Chinese populations (93).
Fig. 9. KIR gene distributions in macaques.
Distribution of KIR loci in rhesus (Mamu) and cynomolgus (Mafa) macaques. For the rhesus macaque, individuals from India, Burma, and China have been analyzed. For the cynomolgus macaques (n=162), the origin of the animals is not precisely known. The color codes work as a heat-map; black denotes absent and darker shades of the color indicate that more individuals possess the KIR gene moiety.
KIR, disease, and reproductive success
Epistatic interactions between different MHC and KIR may have an impact on the health status of an individual. Epidemiological evidence for this phenomenon has been found in the human population: for example, in the case of infectious diseases (98–101). It has been long established that particular macaque MHC class I alleles are associated with differential outcomes in experimental disease settings (11, 102–104), but it is only in recent years that KIR has been studied in this context. On a modest scale compared with human studies in terms of numbers of subjects tested, similar sorts of KIR associations have been made in the simian immunodeficiency virus (SIV) model in rhesus macaques (105–108), which is studied in the context of AIDS vaccine research. In essence, these studies have demonstrated that NK cells play an important role in the containment of SIV infection but also that particular KIR-MHC combinations determine either a poor or a good prognosis, as is reflected by a high or low viral load, respectively. Peptide-dependent KIR-MHC interactions have been recorded both in humans and macaques (87, 109), and it has been suggested that HIV may actively evade T-cell-mediated immune responses by presenting peptides that together with class I bind strongly to inhibitory KIR (110). One could hypothesize that these combinations of activating KIR and MHC that actively engage viral peptides and prevent viral escape may be the ones providing SIV containment. Many studies have reported that the geographic origin of macaques used for this research is correlated to the specific evolution of MHC class I alleles. Similarly, it is now known that Mamu-KIR is rapidly evolving within populations, and most likely adapts to the local environmental pathogenic pressures (93). Experimental outcomes may differ greatly between animals of different origin, which would argue for revisiting the immunogenetics of these studies, and looking for pairs of MHC and KIR genes that have co-evolved in these populations.
As well as infectious diseases, reproductive success is proposed as one of the key selective forces to have shaped the human KIR system, because KIR-MHC combinations influence reproductive biology (111–114). The placentation system in macaques is very different from that of humans and great apes, though any evidence for the involvement of KIR is at present lacking. Whereas the interplay between reproductive success and protection from pathogens is proposed to have led to the balance of group A and B KIR haplotypes, this balance is distinctively absent in macaques.
Co-evolution of KIR and MHC
The expansion of KIR lineage III came with the rise of MHC-C as the dominant epitope for KIR in great apes and humans. In humans, however, HLA-C has a dual role: not only does it present peptides for immunity but most likely through interactions with KIR it allows NK cells to aid in vascular remodeling during placentation. It is these opposing selective forces that may be reflected by the balance in distribution of KIR A and B haplotypes. In contrast to humans, macaques do not experience the balance of these selective forces equally. Because pregnancy in rhesus macaques does not require extensive vascular remodeling, it is likely that most effort is put into monitoring/controlling infectious diseases. As a result, macaque KIR lineage II members may be skewed towards a role in the containment of infectious diseases.
It is an intriguing thought that the expanded Mamu-A and Mamu-B gene repertoire through co-evolution induced the expansion of potential KIR ligands or vice versa. Indirect data supporting such a claim indicate that several Mamu-KIR have specific Mamu-A and Mamu-B molecules as their ligands (41, 89, 91). Amino acid residues of Mamu-A and Mamu-B that make up the Mamu-B and Mamu-F pocket for peptide binding and presentation to TCRs encompass the same residues that define the Bw4 and Bw6 motifs that are being scanned by macaque lineage II KIR. It is also now well established that residues at the C-terminus of the class I-presented peptide directly interacts with KIR and can modulate NK cell function (115, 116). The combined polymorphism and diversity of the MHC class I system may therefore serve a dual purpose: to increase the capacity to present a diverse non-self peptide repertoire to TCRs and to facilitate the possibility that some of the residues might be compatible with either inhibitory or activating KIRs.
The delicate balance between immunity, pathogens, and malignancies
MHC class I molecules play a key role in signaling infection or a state of malignancy to the immune system. The effector functions are executed by CD8+ cytotoxic T cells or NK cells that may lyse the infected or malignant cells. Both cell types use different types of receptors. The T-cell repertoire is formed by rearrangement, and every individual expresses a potentially huge repertoire of different T cells with distinct receptors. As such, immune evasion by finding ‘a hole in the repertoire’ is not likely to happen. Therefore, pathogens as well as malignancies have evolved ways to manipulate their recognition, and many of these involve the MHC class I antigen presentation pathway. NK cells use different types of receptors to scan for the presence and absence of MHC class I antigens. Hence, the broad recognition structures involve LILRs (pan MHC class I) and NKG2A/CD94 in indirect recognition of MHC class I expression via HLA-E (44, 45). The more subtle types of MHC class I downregulation are monitored by means of the KIR receptors on NK cells. The other possibility is that pathogens have selected mechanisms in which MHC class I molecules are loaded preferentially with peptides that subtly resemble self-peptides. In such cases, an adequate T-cell response may not be activated.
In macaques, it has been seen that an inhibiting receptor was converted into an activating one due to a point mutation that altered a splice site. The KIR region is subject to frequent recombination. One possibility is that the heads (membrane distal segments) are placed every now and then in the context of a distinct tail (cytoplasmic section). If this were to be true, an inhibiting receptor could then switch to an activating one, or vice versa, which would be an excellent strategy to avoid adaptation or manipulation by pathogens. This would mean that an MHC complex that is loaded with a peptide from a pathogen that resembles ‘self’ would suddenly be confronted with an activating receptor instead of an inhibiting one. The changes could be picked up by the KIR receptors on NK cells. Of course, this might influence the rheostat status of the NK cells and possibly result in lysis of the infected cell. From an evolutionary point of view, pathogens that have evolved rapid immune evasion-like strategies are ones that are sooner or later most likely to produce those peptides that resemble self. Thus, the ability to switch between inhibiting and activating modalities is most likely an effective strategy to maintain control of rapidly evolving pathogens.
The next option the immune response has to deal with is that some pathogens like CMV have developed strategies to encode decoys (117, 118). A recent bioinformatics modeling study has illustrated that viral decoys are likely to diversify the repertoire of inhibitory KIRs (145). Therefore, it is possible that macaques are dealing with a number of infectious agents that are encoding decoys and/or pathogens that are banking on immune evasion strategies, leading to macaques having evolved such a complex KIR repertoire. Solid tumors are extremely rare in rhesus macaques and other OWM. One intriguing possibility is that this may be due to a highly efficient KIR repertoire on NK cells, which has been tailored to fight viral infections and has as a positive side-effect the efficient recognition of determinants of malignant cells. In the case of humans, there are several association studies involving KIR and the occurrence of cancer or therapy regarding blood-borne malignancies (119–121). For this reason, macaques may turn out to represent perfect models for studying and understanding cancer-related resistance and treatment.
One of the trade-offs for reproductive success in the human population, balance of the KIR lineage III repertoire, could be susceptibility to cancer and other malignancies (less effective recognition by KIR lineage II/III repertoire). Deep placentation most likely evolved late in evolution during the human-great ape lineage (114). Therefore, it may be that many other primate species use their NK cells and KIR repertoire to control infections and malignancies.
Acknowledgments
The authors wish to thank Donna Devine for editing the manuscript, and Henk van Westbroek for artwork. This work was in part supported by grants NIH/NIAID HHSN272201100013C, and 5R24OD011161-13.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Parham P, Ohta T. Population biology of antigen presentation by MHC class I molecules. Science. 1996;272:67–74. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 2.de Groot NG, Bontrop RE. The HIV-1 pandemic: does the selective sweep in chimpanzees mirror humankind’s future? Retrovirology. 2013;10:53. doi: 10.1186/1742-4690-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tosi AJ, Morales JC, Melnick DJ. Y-chromosome and mitochondrial markers in Macaca fascicularis indicate introgression with Indochinese M mulatta and a biogeogrphic barrier in the Isthmus of Kra. Int J Primatol. 2002;23:161–178. [Google Scholar]
- 4.Glazko GV, Nei M. Estimation of divergence times for major lineages of primate species. Mol Biol Evol. 2003;20:424–434. doi: 10.1093/molbev/msg050. [DOI] [PubMed] [Google Scholar]
- 5.Perelman P, et al. A molecular phylogeny of living primates. PLoS Genet. 2011;7:e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bontrop RE. Non-human primates: essential partners in biomedical research. Immunol Rev. 2001;183:5–9. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- 7.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217. doi: 10.1038/nature10841. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 9.Klein F, et al. Enhanced HIV-1 immunotherapy by commonly arising antibodies that target virus escape variants. J Exp Med. 2014;211:2361–2372. doi: 10.1084/jem.20141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara CW, et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature. 2013;504:248–253. doi: 10.1038/nature12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudd PA, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poliani PL, et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum Gene Ther. 2001;12:905–920. doi: 10.1089/104303401750195872. [DOI] [PubMed] [Google Scholar]
- 13.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehouse J, Micheletta J, Powell LE, Bordier C, Waller BM. The impact of cognitive testing on the welfare of group housed primates. PLoS One. 2013;8:e78308. doi: 10.1371/journal.pone.0078308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhesus Macaque Genome S, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez RD, et al. Demographic histories and patterns of linkage disequilibrium in Chinese and Indian rhesus macaques. Science. 2007;316:240–243. doi: 10.1126/science.1140462. [DOI] [PubMed] [Google Scholar]
- 17.Marsh SG, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291–455. doi: 10.1111/j.1399-0039.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daza-Vamenta R, Glusman G, Rowen L, Guthrie B, Geraghty DE. Genetic divergence of the rhesus macaque major histocompatibility complex. Genome Res. 2004;14:1501–1515. doi: 10.1101/gr.2134504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otting N, et al. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci U S A. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiina T, et al. Rapid evolution of major histocompatibility complex class I genes in primates generates new disease alleles in humans via hitchhiking diversity. Genetics. 2006;173:1555–1570. doi: 10.1534/genetics.106.057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins DI. The evolution of major histocompatibility class I genes in primates. Crit Rev Immunol. 1995;15:1–29. doi: 10.1615/critrevimmunol.v15.i1.10. [DOI] [PubMed] [Google Scholar]
- 22.Doxiadis GG, de Groot N, Otting N, Blokhuis JH, Bontrop RE. Genomic plasticity of the MHC class I A region in rhesus macaques: extensive haplotype diversity at the population level as revealed by microsatellites. Immunogenetics. 2011;63:73–83. doi: 10.1007/s00251-010-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otting N, de Vos-Rouweler AJ, Heijmans CM, de Groot NG, Doxiadis GG, Bontrop RE. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics. 2007;59:367–375. doi: 10.1007/s00251-007-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Groot NG, et al. Nomenclature report on the major histocompatibility complex genes and alleles of Great Ape, Old and New World monkey species. Immunogenetics. 2012;64:615–631. doi: 10.1007/s00251-012-0617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urvater JA, Steffen SR, Rehrauer W, Watkins DI. An unusual insertion in intron 2 of apparently functional MHC class I alleles in rhesus macaques. Tissue Antigens. 2000;55:153–156. doi: 10.1034/j.1399-0039.2000.550207.x. [DOI] [PubMed] [Google Scholar]
- 26.de Groot N, Doxiadis GG, Otting N, de Vos-Rouweler AJ, Bontrop RE. Differential recombination dynamics within the MHC of macaque species. Immunogenetics. 2014;66:535–544. doi: 10.1007/s00251-014-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otting N, Doxiadis GG, Bontrop RE. Definition of Mafa-A and -B haplotypes in pedigreed cynomolgus macaques (Macaca fascicularis) Immunogenetics. 2009;61:745–753. doi: 10.1007/s00251-009-0412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doxiadis GG, et al. Haplotype diversity generated by ancient recombination-like events in the MHC of Indian rhesus macaques. Immunogenetics. 2013;65:569–584. doi: 10.1007/s00251-013-0707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piontkivska H, Nei M. Birth-and-death evolution in primate MHC class I genes: divergence time estimates. Mol Biol Evol. 2003;20:601–609. doi: 10.1093/molbev/msg064. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZW, McAdam SN, Hughes AL, Dogon AL, Letvin NL, Watkins DI. Molecular cloning of orangutan and gibbon MHC class I cDNA. The HLA-A and -B loci diverged over 30 million years ago. J Immunol. 1992;148:2547–2554. [PubMed] [Google Scholar]
- 31.Adams EJ, Thomson G, Parham P. Evidence for an HLA-C-like locus in the orangutan Pongo pygmaeus. Immunogenetics. 1999;49:865–871. doi: 10.1007/s002510050566. [DOI] [PubMed] [Google Scholar]
- 32.Boyson JE, et al. The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J Immunol. 1996;156:4656–4665. [PubMed] [Google Scholar]
- 33.de Groot N, et al. Genetic makeup of the DR region in rhesus macaques: gene content, transcripts, and pseudogenes. J Immunol. 2004;172:6152–6157. doi: 10.4049/jimmunol.172.10.6152. [DOI] [PubMed] [Google Scholar]
- 34.Abi-Rached L, et al. A small, variable, and irregular killer cell Ig-like receptor locus accompanies the absence of MHC-C and MHC-G in gibbons. J Immunol. 2010;184:1379–1391. doi: 10.4049/jimmunol.0903016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apps R, et al. Influence of HLA-C expression level on HIV control. Science. 2013;340:87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budde ML, et al. Transcriptionally abundant major histocompatibility complex class I alleles are fundamental to nonhuman primate simian immunodeficiency virus-specific CD8+ T cell responses. J Virol. 2011;85:3250–3261. doi: 10.1128/JVI.02355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene JM, et al. Differential MHC class I expression in distinct leukocyte subsets. BMC Immunol. 2011;12:39. doi: 10.1186/1471-2172-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 2008;9:1. doi: 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Groot NG, et al. Unique peptide-binding motif for Mamu-B*037:01: an MHC class I allele common to Indian and Chinese rhesus macaques. Immunogenetics. 2013;65:897–900. doi: 10.1007/s00251-013-0734-5. [DOI] [PubMed] [Google Scholar]
- 40.de Groot NG, et al. AIDS-protective HLA-B*27/B*57 and chimpanzee MHC class I molecules target analogous conserved areas of HIV-1/SIVcpz. Proc Natl Acad Sci U S A. 2010;107:15175–15180. doi: 10.1073/pnas.1009136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosner C, Kruse PH, Lubke T, Walter L. Rhesus macaque MHC class I molecules show differential subcellular localizations. Immunogenetics. 2010;62:149–158. doi: 10.1007/s00251-010-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura M, et al. Characterization of simian T-cell leukemia virus type 1 in naturally infected Japanese macaques as a model of HTLV-1 infection. Retrovirology. 2013;10:118. doi: 10.1186/1742-4690-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montiel NA. An updated review of simian betaretrovirus (SRV) in macaque hosts. J Med Primatol. 2010;39:303–314. doi: 10.1111/j.1600-0684.2010.00412.x. [DOI] [PubMed] [Google Scholar]
- 44.O’Callaghan CA, et al. Structural features impose tight peptide binding specificity in the nonclassical MHC molecule HLA-E. Mol Cell. 1998;1:531–541. doi: 10.1016/s1097-2765(00)80053-2. [DOI] [PubMed] [Google Scholar]
- 45.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 46.Boyson JE, et al. The MHC E locus in macaques is polymorphic and is conserved between macaques and humans. Immunogenetics. 1995;41:59–68. doi: 10.1007/BF00182314. [DOI] [PubMed] [Google Scholar]
- 47.Lafont BA, Buckler-White A, Plishka R, Buckler C, Martin MA. Pig-tailed macaques (Macaca nemestrina) possess six MHC-E families that are conserved among macaque species: implication for their binding to natural killer receptor variants. Immunogenetics. 2004;56:142–154. doi: 10.1007/s00251-004-0663-4. [DOI] [PubMed] [Google Scholar]
- 48.LaBonte ML, Hershberger KL, Korber B, Letvin NL. The KIR and CD94/NKG2 families of molecules in the rhesus monkey. Immunol Rev. 2001;183:25–40. doi: 10.1034/j.1600-065x.2001.1830103.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee N, Ishitani A, Geraghty DE. HLA-F is a surface marker on activated lymphocytes. Eur J Immunol. 2010;40:2308–2318. doi: 10.1002/eji.201040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodridge JP, Burian A, Lee N, Geraghty DE. HLA-F complex without peptide binds to MHC class I protein in the open conformer form. J Immunol. 2010;184:6199–6208. doi: 10.4049/jimmunol.1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otting N, Bontrop RE. Characterization of the rhesus macaque (Macaca mulatta) equivalent of HLA-F. Immunogenetics. 1993;38:141–145. doi: 10.1007/BF00190901. [DOI] [PubMed] [Google Scholar]
- 52.Djurisic S, Hviid TV. HLA Class Ib Molecules and Immune Cells in Pregnancy and Preeclampsia. Front Immunol. 2014;5:652. doi: 10.3389/fimmu.2014.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of the rhesus monkey HLA-G ortholog. Mamu-G is a pseudogene J Immunol. 1996;157:5428–5437. [PubMed] [Google Scholar]
- 54.Bondarenko GI, et al. Characterization of cynomolgus and vervet monkey placental MHC class I expression: diversity of the nonhuman primate AG locus. Immunogenetics. 2009;61:431–442. doi: 10.1007/s00251-009-0376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of a novel MHC class I gene, Mamu-AG, expressed in the placenta of a primate with an inactivated G locus. J Immunol. 1997;159:3311–3321. [PubMed] [Google Scholar]
- 56.van der Wiel MK, Otting N, de Groot NG, Doxiadis GG, Bontrop RE. The repertoire of MHC class I genes in the common marmoset: evidence for functional plasticity. Immunogenetics. 2013;65:841–849. doi: 10.1007/s00251-013-0732-7. [DOI] [PubMed] [Google Scholar]
- 57.Urvater JA, et al. Mamu-I: a novel primate MHC class I B-related locus with unusually low variability. J Immunol. 2000;164:1386–1398. doi: 10.4049/jimmunol.164.3.1386. [DOI] [PubMed] [Google Scholar]
- 58.Seo JW, Walter L, Gunther E. Genomic analysis of MIC genes in rhesus macaques. Tissue Antigens. 2001;58:159–165. doi: 10.1034/j.1399-0039.2001.580303.x. [DOI] [PubMed] [Google Scholar]
- 59.Averdam A, et al. Genotyping and segregation analyses indicate the presence of only two functional MIC genes in rhesus macaques. Immunogenetics. 2007;59:247–251. doi: 10.1007/s00251-006-0187-1. [DOI] [PubMed] [Google Scholar]
- 60.Doxiadis GG, Heijmans CM, Otting N, Bontrop RE. MIC gene polymorphism and haplotype diversity in rhesus macaques. Tissue Antigens. 2007;69:212–219. doi: 10.1111/j.1399-0039.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 61.Meyer A, et al. High diversity of MIC genes in non-human primates. Immunogenetics. 2014;66:581–587. doi: 10.1007/s00251-014-0791-4. [DOI] [PubMed] [Google Scholar]
- 62.Luoma AM, et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity. 2013;39:1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu B, et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A. 2011;108:2414–2419. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bontrop RE, Otting N, Slierendregt BL, Lanchbury JS. Evolution of major histocompatibility complex polymorphisms and T-cell receptor diversity in primates. Immunol Rev. 1995;143:33–62. doi: 10.1111/j.1600-065x.1995.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 65.Olivieri DN, Gambon-Deza F. V genes in primates from whole genome sequencing data. Immunogenetics. 2015;67:211–228. doi: 10.1007/s00251-015-0830-9. [DOI] [PubMed] [Google Scholar]
- 66.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9:503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 67.Vossen MT, Westerhout EM, Soderberg-Naucler C, Wiertz EJ. Viral immune evasion: a masterpiece of evolution. Immunogenetics. 2002;54:527–542. doi: 10.1007/s00251-002-0493-1. [DOI] [PubMed] [Google Scholar]
- 68.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 69.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 70.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 71.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 72.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 73.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 74.Trowsdale J. Genetic and functional relationships between MHC and NK receptor genes. Immunity. 2001;15:363–374. doi: 10.1016/s1074-7613(01)00197-2. [DOI] [PubMed] [Google Scholar]
- 75.Guethlein LA, Flodin LR, Adams EJ, Parham P. NK cell receptors of the orangutan (Pongo pygmaeus): a pivotal species for tracking the coevolution of killer cell Ig-like receptors with MHC-C. J Immunol. 2002;169:220–229. doi: 10.4049/jimmunol.169.1.220. [DOI] [PubMed] [Google Scholar]
- 76.Khakoo SI, et al. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity. 2000;12:687–698. doi: 10.1016/s1074-7613(00)80219-8. [DOI] [PubMed] [Google Scholar]
- 77.Blokhuis JH, Doxiadis GG, Bontrop RE. A splice site mutation converts an inhibitory killer cell Ig-like receptor into an activating one. Mol Immunol. 2009;46:640–648. doi: 10.1016/j.molimm.2008.08.270. [DOI] [PubMed] [Google Scholar]
- 78.Kruse PH, Rosner C, Walter L. Characterization of rhesus macaque KIR genotypes and haplotypes. Immunogenetics. 2010;62:281–293. doi: 10.1007/s00251-010-0433-4. [DOI] [PubMed] [Google Scholar]
- 79.Cadavid LF, Lun CM. Lineage-specific diversification of killer cell Ig-like receptors in the owl monkey, a New World primate. Immunogenetics. 2009;61:27–41. doi: 10.1007/s00251-008-0342-y. [DOI] [PubMed] [Google Scholar]
- 80.Cadavid LF, Palacios C, Lugo JS. Bimodal evolution of the killer cell Ig-like receptor (KIR) family in New World primates. Immunogenetics. 2013;65:725–736. doi: 10.1007/s00251-013-0719-4. [DOI] [PubMed] [Google Scholar]
- 81.Sambrook JG, et al. Identification of the ancestral killer immunoglobulin-like receptor gene in primates. BMC Genomics. 2006;7:209. doi: 10.1186/1471-2164-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189:1093–1100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vilches C, Rajalingam R, Uhrberg M, Gardiner CM, Young NT, Parham P. KIR2DL5, a novel killer-cell receptor with a D0–D2 configuration of Ig-like domains. J Immunol. 2000;164:5797–5804. doi: 10.4049/jimmunol.164.11.5797. [DOI] [PubMed] [Google Scholar]
- 84.Blokhuis JH, van der Wiel MK, Doxiadis GG, Bontrop RE. Evidence for balancing selection acting on KIR2DL4 genotypes in rhesus macaques of Indian origin. Immunogenetics. 2009;61:503–512. doi: 10.1007/s00251-009-0379-6. [DOI] [PubMed] [Google Scholar]
- 85.Bimber BN, Moreland AJ, Wiseman RW, Hughes AL, O’Connor DH. Complete characterization of killer Ig-like receptor (KIR) haplotypes in Mauritian cynomolgus macaques: novel insights into nonhuman primate KIR gene content and organization. J Immunol. 2008;181:6301–6308. doi: 10.4049/jimmunol.181.9.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pyo CW, et al. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS One. 2010;5:e15115. doi: 10.1371/journal.pone.0015115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Connor GM, et al. Peptide-dependent recognition of HLA-B*57:01 by KIR3DS1. J Virol. 2015;89:5213–5221. doi: 10.1128/JVI.03586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blokhuis JH, van der Wiel MK, Doxiadis GG, Bontrop RE. The mosaic of KIR haplotypes in rhesus macaques. Immunogenetics. 2010;62:295–306. doi: 10.1007/s00251-010-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colantonio AD, et al. KIR polymorphisms modulate peptide-dependent binding to an MHC class I ligand with a Bw6 motif. PLoS Pathog. 2011;7:e1001316. doi: 10.1371/journal.ppat.1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosner C, Kruse PH, Hermes M, Otto N, Walter L. Rhesus macaque inhibitory and activating KIR3D interact with Mamu-A-encoded ligands. J Immunol. 2011;186:2156–2163. doi: 10.4049/jimmunol.1002634. [DOI] [PubMed] [Google Scholar]
- 91.Schafer JL, et al. KIR3DL01 recognition of Bw4 ligands in the rhesus macaque: maintenance of Bw4 specificity since the divergence of apes and Old World monkeys. J Immunol. 2014;192:1907–1917. doi: 10.4049/jimmunol.1302883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang W, et al. Copy number variation leads to considerable diversity for B but not A haplotypes of the human KIR genes encoding NK cell receptors. Genome Res. 2012;22:1845–1854. doi: 10.1101/gr.137976.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blokhuis JH, van der Wiel MK, Doxiadis GG, Bontrop RE. The extreme plasticity of killer cell Ig-like receptor (KIR) haplotypes differentiates rhesus macaques from humans. Eur J Immunol. 2011;41:2719–2728. doi: 10.1002/eji.201141621. [DOI] [PubMed] [Google Scholar]
- 94.Sambrook JG, et al. Single haplotype analysis demonstrates rapid evolution of the killer immunoglobulin-like receptor (KIR) loci in primates. Genome Res. 2005;15:25–35. doi: 10.1101/gr.2381205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uhrberg M, Parham P, Wernet P. Definition of gene content for nine common group B haplotypes of the Caucasoid population: KIR haplotypes contain between seven and eleven KIR genes. Immunogenetics. 2002;54:221–229. doi: 10.1007/s00251-002-0463-7. [DOI] [PubMed] [Google Scholar]
- 96.Uhrberg M, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 97.Wilson MJ, et al. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci U S A. 2000;97:4778–4783. doi: 10.1073/pnas.080588597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 99.Martin MP, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 100.Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pelak K, et al. Copy number variation of KIR genes influences HIV-1 control. PLoS Biol. 2011;9:e1001208. doi: 10.1371/journal.pbio.1001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bakker NP, et al. Resistance to collagen-induced arthritis in a nonhuman primate species maps to the major histocompatibility complex class I region. J Exp Med. 1992;175:933–937. doi: 10.1084/jem.175.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bontrop RE, Watkins DI. MHC polymorphism: AIDS susceptibility in non-human primates. Trends Immunol. 2005;26:227–233. doi: 10.1016/j.it.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 104.Evans DT, et al. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 105.Albrecht C, Malzahn D, Brameier M, Hermes M, Ansari AA, Walter L. Progression to AIDS in SIV-Infected Rhesus Macaques is Associated with Distinct KIR and MHC class I Polymorphisms and NK Cell Dysfunction. Front Immunol. 2014;5:600. doi: 10.3389/fimmu.2014.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaichompoo P, et al. Multiple KIR gene polymorphisms are associated with plasma viral loads in SIV-infected rhesus macaques. Cell Immunol. 2010;263:176–187. doi: 10.1016/j.cellimm.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hellmann I, Lim SY, Gelman RS, Letvin NL. Association of activating KIR copy number variation of NK cells with containment of SIV replication in rhesus monkeys. PLoS Pathog. 2011;7:e1002436. doi: 10.1371/journal.ppat.1002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hellmann I, Schmitz JE, Letvin NL. Activating KIR copy number variation is associated with granzyme B release by NK cells during primary simian immunodeficiency virus infection in rhesus monkeys. J Virol. 2012;86:13103–13107. doi: 10.1128/JVI.00325-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maloveste SM, et al. Degenerate recognition of MHC class I molecules with Bw4 and Bw6 motifs by a killer cell Ig-like receptor 3DL expressed by macaque NK cells. J Immunol. 2012;189:4338–4348. doi: 10.4049/jimmunol.1201360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alter G, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiby SE, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakimuli A, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A. 2015;112:845–850. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Norman PJ, et al. Co-evolution of human leukocyte antigen (HLA) class I ligands with killer-cell immunoglobulin-like receptors (KIR) in a genetically diverse population of sub-Saharan Africans. PLoS Genet. 2013;9:e1003938. doi: 10.1371/journal.pgen.1003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fadda L, et al. Peptide antagonism as a mechanism for NK cell activation. Proc Natl Acad Sci U S A. 2010;107:10160–10165. doi: 10.1073/pnas.0913745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vivian JP, et al. Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature. 2011;479:401–405. doi: 10.1038/nature10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beziat V, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilkinson GW, et al. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008;41:206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Re V, et al. Genetic diversity of the KIR/HLA system and outcome of patients with metastatic colorectal cancer treated with chemotherapy. PLoS One. 2014;9:e84940. doi: 10.1371/journal.pone.0084940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dutta A, Saikia N, Phookan J, Baruah MN, Baruah S. Association of killer cell immunoglobulin-like receptor gene 2DL1 and its HLA-C2 ligand with family history of cancer in oral squamous cell carcinoma. Immunogenetics. 2014;66:439–448. doi: 10.1007/s00251-014-0778-1. [DOI] [PubMed] [Google Scholar]
- 121.Verneris MR, Miller JS. KIR B or not to be? …that is the question for ALL. Blood. 2014;124:2623–2624. doi: 10.1182/blood-2014-09-596395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allen TM, et al. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 123.Dzuris JL, Sidney J, Appella E, Chesnut RW, Watkins DI, Sette A. Conserved MHC class I peptide binding motif between humans and rhesus macaques. J Immunol. 2000;164:283–291. doi: 10.4049/jimmunol.164.1.283. [DOI] [PubMed] [Google Scholar]
- 124.Hickman-Miller HD, et al. Rhesus macaque MHC class I molecules present HLA-B-like peptides. J Immunol. 2005;175:367–375. doi: 10.4049/jimmunol.175.1.367. [DOI] [PubMed] [Google Scholar]
- 125.Loffredo JT, et al. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J Immunol. 2004;173:5064–5076. doi: 10.4049/jimmunol.173.8.5064. [DOI] [PubMed] [Google Scholar]
- 126.Loffredo JT, et al. The high frequency Indian rhesus macaque MHC class I molecule, Mamu-B*01, does not appear to be involved in CD8+ T lymphocyte responses to SIVmac239. J Immunol. 2005;175:5986–5997. doi: 10.4049/jimmunol.175.9.5986. [DOI] [PubMed] [Google Scholar]
- 127.Loffredo JT, et al. Two MHC class I molecules associated with elite control of immunodeficiency virus replication, Mamu-B*08 and HLA-B*2705, bind peptides with sequence similarity. J Immunol. 2009;182:7763–7775. doi: 10.4049/jimmunol.0900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu J, et al. Diverse peptide presentation of rhesus macaque major histocompatibility complex class I Mamu-A 02 revealed by two peptide complex structures and insights into immune escape of simian immunodeficiency virus. J Virol. 2011;85:7372–7383. doi: 10.1128/JVI.00350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mothe BR, et al. Characterization of the peptide-binding specificity of Mamu-B*17 and identification of Mamu-B*17-restricted epitopes derived from simian immunodeficiency virus proteins. J Immunol. 2002;169:210–219. doi: 10.4049/jimmunol.169.1.210. [DOI] [PubMed] [Google Scholar]
- 130.Mothe BR, et al. Peptide-binding motifs associated with MHC molecules common in Chinese rhesus macaques are analogous to those of human HLA supertypes and include HLA-B27-like alleles. Immunogenetics. 2013;65:371–386. doi: 10.1007/s00251-013-0686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reed JS, et al. The role of MHC class I allele Mamu-A*07 during SIV(mac)239 infection. Immunogenetics. 2011;63:789–807. doi: 10.1007/s00251-011-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sette A, et al. Characterization of the peptide-binding specificity of Mamu-A*11 results in the identification of SIV-derived epitopes and interspecies cross-reactivity. Immunogenetics. 2005;57:53–68. doi: 10.1007/s00251-004-0749-z. [DOI] [PubMed] [Google Scholar]
- 133.Sette A, et al. A shared MHC supertype motif emerges by convergent evolution in macaques and mice, but is totally absent in human MHC molecules. Immunogenetics. 2012;64:421–434. doi: 10.1007/s00251-011-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Solomon C, et al. The most common Chinese rhesus macaque MHC class I molecule shares peptide binding repertoire with the HLA-B7 supertype. Immunogenetics. 2010;62:451–464. doi: 10.1007/s00251-010-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Southwood S, et al. Functional analysis of frequently expressed Chinese rhesus macaque MHC class I molecules Mamu-A1*02601 and Mamu-B*08301 reveals HLA-A2 and HLA-A3 supertypic specificities. Immunogenetics. 2011;63:275–290. doi: 10.1007/s00251-010-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu Y, et al. Structural basis of diverse peptide accommodation by the rhesus macaque MHC class I molecule Mamu-B*17: insights into immune protection from simian immunodeficiency virus. J Immunol. 2011;187:6382–6392. doi: 10.4049/jimmunol.1101726. [DOI] [PubMed] [Google Scholar]