Abstract
Problem
Engaging communities in research increases its relevance and may speed the translation of discoveries into improved health outcomes. Many researchers lack training to effectively engage stakeholders, while academic institutions lack infrastructure to support community engagement.
Approach
In 2009, the Meharry-Vanderbilt Community Engaged Research Core began testing new approaches for community engagement, which led to the development of the Community Engagement Studio (CE Studio). This structured program facilitates project-specific input from community and patient stakeholders to enhance research design, implementation, and dissemination. Developers used a team approach to recruit and train stakeholders, prepare researchers to engage with stakeholders, and facilitate an in-person meeting with both.
Outcomes
The Core has implemented 28 CE Studios that engaged 152 community stakeholders. Participating researchers, representing a broad range of faculty ranks and disciplines, reported that input from stakeholders was valuable and that the CE Studio helped determine project feasibility and enhanced research design and implementation. Stakeholders found the CE Studio to be an acceptable method of engagement and reported a better understanding of research in general. A toolkit was developed to replicate this model and to disseminate this approach.
Next steps
The Core will collect data to better understand the impact of CE Studios on research proposal submissions, funding, research outcomes, patient and stakeholder engagement in projects, and dissemination of results. They will also collect data to determine if CE Studios increase patient-centered approaches in research and whether stakeholders who participate have more trust and willingness to participate in research.
Problem
To effectively translate scientific discoveries into improvements in individual and population health, community representatives should be involved in all stages of clinical and translational research.1,2 Community involvement can increase the quality and relevance of research,3 yet enhancing public participation in research is one of the central challenges facing clinical research enterprises.4 Engaging patients and consumers in research is complex, and current rigorous research training programs generally do not prepare researchers to identify, recruit, and convene stakeholders or prepare them for participation in research.5 Without appropriate training or experience, attempts to facilitate community and patient engagement are often ineffective, burdensome, and leave stakeholders feeling disenfranchised.6
Becoming proficient in community and stakeholder engagement requires training and hands-on experience, which may take years. Consequently, researchers without prior experience have limited options for engaging stakeholders in their research. The infrastructure and incentives at many academic health centers are not well-aligned to support community engagement. Significant gaps still exist in the methods used to engage communities in research and the process is often resource intensive and time consuming. 6,7
To address investigators' need for eliciting meaningful patient and community engagement, we developed the Community Engagement Studio (CE Studio), which provides a structured method for obtaining input from stakeholders to enhance the design, conduct, and dissemination of research.
Approach
With input from its Community Advisory Council, the Meharry-Vanderbilt Community-Engaged Research Core conceived the idea of the CE Studio in 2009. Over the next two years, two Clinical and Translational Science Award (CTSA) administrative supplements financially supported the development of the CE Studio model. A guided approach to patient and community engagement, this model allows researchers to obtain direct input from representative groups. Unlike most methods of community engagement, the CE Studio does not require individual researchers to recruit stakeholders and facilitate involvement. Instead, the CE Studio relies on a faculty/staff team with experience in patient and community engagement to identify stakeholders, prepare the investigator, and facilitate the interaction, minimizing investigator burden and maximizing efficiency.
The CE Studio is modeled after the Clinical and Translational Research Studio, an award-winning program that provides researchers with project-specific input from academic experts during an in-person meeting.8,9 In the community engagement model, the experts are patients or community stakeholders. A unique panel of stakeholders is constituted for each CE Studio and consists of individuals who represent the researcher's population of interest. These stakeholders possess firsthand knowledge, or lived experience, of a particular condition or a targeted community. The CE Studio stakeholders are consultants, not research subjects, and are compensated at a rate based on the local average value of volunteer time.
Investigators may request a CE Studio at any stage of their project, but are encouraged to do so in the early stages of idea generation or proposal development. Two to four weeks are needed to complete the planning and stakeholder recruitment process. The researcher meets with the CE Studio team to clarify the focus of the CE Studio, determine the characteristics of the stakeholder panel, and formulate the questions that will be posed to the stakeholders. The team also coaches the investigator on communicating effectively with non-researchers. The CE Studio staff recruits the study-specific stakeholder panel through existing relationships with community organizations and clinical practices and the diverse pool of individuals who have served in previous CE Studios. To prepare stakeholders, the CE Studio staff provides information about the process, research in general, and the specific project.
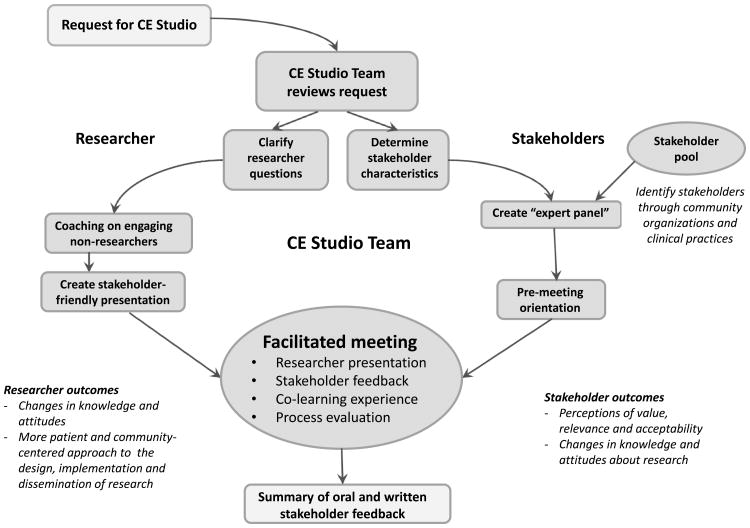
The two-hour face-to-face CE Studio is facilitated by an experienced, neutral moderator trained to ensure that the stakeholders are comfortable sharing their experiences and opinions and the researcher's questions are addressed. Each CE Studio begins with a brief presentation from the researcher. Two to three key questions are presented to the stakeholder panel, and the moderator facilitates the ensuing discussion. Notes are taken during the meeting and are later used to prepare a written summary for the investigator. At the conclusion, researchers and stakeholders complete a paper evaluation. The process for requesting and implementing a CE Studio is illustrated in Figure 1.
Figure 1.
The process for requesting and implementing a Community Engagement (CE) Studio. A CE Studio, developed by the Meharry-Vanderbilt Community Engaged Research Core in 2009, is a structured process facilitating project-specific input from community and patient stakeholders to enhance research design, implementation, and dissemination.
The estimated cost of a CE Studio is $2,000, which includes approximately 22 hours of staff time, four hours of faculty time, compensation for the moderator ($200), compensation for the stakeholders ($50 each), and food. There may be additional costs for advertising, stakeholder support (e.g., transportation, parking, child care), and interpretation.
Outcomes
To date, we have conducted 28 CE Studios for 23 researchers and engaged 152 patient and community stakeholders, with an average of eight stakeholders per session. The projects represent a broad range of health topics and types of research, including clinical trials and comparative effectiveness research. Nine of the 23 studies (39%) focused on minorities and underrepresented groups. Table 1 provides details on the researchers, topics, stakeholder characteristics, stakeholder recommendations and outcomes of a sample of completed CE Studios.
Table 1. Characteristics and Outcomes From a Sample of Community Engagement Studios, Meharry-Vanderbilt Community-Engaged Research Core, 2009–2014.
| Research topic | Investigator | Stakeholder | Focus | Stakeholder recommendations | Outcomes |
|---|---|---|---|---|---|
| Effect of sildenafil citrate on insulin resistance | Assistant professor in clinical pharmacology | African American women (n = 7) | Recruitment, retention |
|
|
| Biomarker for early lung cancer detection | Associate professor of medicine and cancer biology | African American men and women over 50; smokers for 25+ years (n = 10) | Recruitment, consent |
|
|
| Design for an ICU survivor clinic | Assistant professor in medicine in allergy/pulmonary and critical care | ICU survivors and their caregivers (n = 8) | Intervention design |
|
|
| Long-term effects of opioid analgesics | Assistant professor in health policy | Individuals with chronic pain who use opiates (n = 12) | Research design |
|
|
| Parent-based sleep education for children with insomnia | Professor in neurology | Parents of young children on the autism spectrum (n = 10) | Research design, dissemination |
|
|
| African Americans' attitudes on biospecimen donation | Assistant professor in internal medicine | African American adults (n = 9) | Survey development and implementation |
|
|
Abbreviations: ICU indicates intensive care unit; OT, occupational therapy; PT, physical therapy.
Researchers requesting CE Studios
The 23 researchers who have completed CE Studios hold faculty appointments across all ranks, including 12 junior investigators (52%) and 7 professors (30%), and represent a wide range of disciplines, including cancer epidemiology, biomedical informatics, pulmonary and critical care, neurology, human genomics, obstetrics, and clinical pharmacology. Four researchers sought multiple CE Studios to query different stakeholder groups (e.g., patients and community providers) or address distinct topics (e.g., survey design and participant recruitment). The most common reasons for requesting a CE Studio were to obtain input on research design, participant recruitment and retention, and dissemination of results.
Evaluations completed by researchers following each of the 28 CE Studios indicated that they felt that the stakeholder input improved the quality of their research (23 or 82% strongly agreed and 5 or 18% agreed). Overall, researchers were very satisfied with the CE Studio and agreed or strongly agreed that the right stakeholders were at the table and that the feedback was appropriate. Furthermore, 27 (96%) strongly agreed that the CE Studio was worth their time and 28 (100%) strongly agreed they would recommend the CE Studio to colleagues.
Patient and community stakeholders in CE Studios
Criteria for stakeholders include being a member of the investigator's target population (or having extensive knowledge of the population, e.g., as an advocate, caregiver, or provider), a willingness to share their knowledge, and an interest in improving research. The 152 stakeholders represent a broad range of health conditions and demographics, including populations often considered hard to reach. One hundred nine (72%) of the stakeholders are women and 71 (47%) are African American. Education ranges from high school diploma to terminal professional degrees. The 27 stakeholders who have participated in more than one CE Studio do not differ significantly from those who have participated only once.
Stakeholders reported an overwhelmingly positive experience with the CE Studio. One hundred and forty-seven (97%) strongly agreed or agreed that they received enough information from the investigator to give appropriate feedback and 150 (99%) believed their feedback would improve the project. Almost all stakeholders (150 or 99%) reported that the CE Studio was worth their time and 149 (98%) indicated they would be willing to participate again.
Feasibility and acceptability of CE Studios
To gain additional input regarding the feasibility and acceptability of the CE Studio, we conducted one focus group with stakeholders (n = 6) and one with researchers (n = 4). Several themes emerged. Stakeholders found the pre-meeting orientation to be very helpful in preparing them to provide feedback and noted that the CE Studio experience gave them a better understanding of the complexities and challenges of conducting research. Several stakeholders reported a sense of pride because their input was impacting the research, and many expressed an interest in receiving updates on the status of the research projects.
In the researcher group, investigators expressed appreciation for the stakeholders' input, and some reported that the experience led them to realize that they had previously overestimated their ability to effectively communicate their research to stakeholders. Researchers were pleased with the selection of stakeholders and found that the representativeness of the stakeholders added to the relevance and overall value of the feedback. Researchers thought that the CE Studio was appropriate for both well-established and early investigators who are conducting clinical research. The researchers encouraged continuing the CE Studios and advocated for marketing the program broadly within the academic institution.
Benefits of CE Studios
In the 28 post CE Studio evaluations, 22 researchers (79%) indicated that the CE Studio increased their understanding of and sensitivity to the study populations. Twenty researchers (71%) believed that the CE Studio input informed the feasibility of the project, and 17 (61%) stated that the input informed the strategies for recruitment and plans for dissemination.
Many of the stakeholder recommendations emphasized making research more patient-centered, culturally relevant, and accessible to potential research participants. The researchers reported that they used the stakeholder input to refine their proposals, revise recruitment materials, modify consent forms and add or increase participant compensation. Following their CE Studio, nearly half (13 or 46%) of the researchers made changes to an existing research project, 10 (36%) submitted grants, and 5 (18%) used the stakeholder input for quality improvement activities.
Next Steps
The early efforts support the acceptability and feasibility of the CE Studio and demonstrate that this approach can be used to efficiently and meaningfully engage patients and community stakeholders in different stages and across a range of research. To understand the long-term impact of CE Studios, we will prospectively collect data regarding the status of research proposal submissions and funding, recruitment and enrollment in studies, engagement of stakeholders in projects, and dissemination of research.
Although some researchers reported that the CE Studios enhanced the relevance and acceptability of research to the target population, we did not collect or examine changes in the research proposals, protocols, recruitment materials, or other documents. With funding from the Patient Centered Outcomes Research Institute, we have begun to examine whether the CE Studios lead to interventions, comparators, and outcomes that are more patient-centered.10
From the focus group, we learned that stakeholders developed a deeper appreciation for the complexity of clinical research and became invested in the project's success. To better understand the impact of CE Studios on stakeholders, we will collect data about stakeholders' trust of and willingness to participate in research. Additionally, we recognize that because our stakeholders are not selected randomly, their input may differ from the general population; therefore, we will seek to identify an appropriate comparison group.
A toolkit is available for programs interested in replicating this model (http://www.meharry-vanderbilt.org/ces-toolkit). Developed by a team of staff, faculty and community partners, the toolkit has evolved, reflecting refinements of the model and user feedback. For example, researchers recommended more time for preparation and coaching, more face-to-face time with the stakeholders, and more targeted recruitment of stakeholder panels. Stakeholders recommended that the CE Studio sessions be longer, that they be given more information about the project prior to the CE Studio, and that they receive follow-up communication about how their input impacted the study, as well as about the findings of the study.
Efforts to disseminate the model include invited demonstrations at four academic institutions and consultations with several others who learned about the model through the CTSA consortium. Three institutions (University of California, Davis; University of Arkansas for Medical Sciences; and Virginia Commonwealth University) have adopted the model. We plan to work with colleagues at these implementation sites to understand adaptations of the model to other institutions.
This approach to community engagement is not without its challenges. Chief among them is securing the resources necessary to create and sustain the model. Leveraging existing research support (including CTSA infrastructure) and requiring that researchers include the CE Studio in their research budgets are two strategies for covering costs. We believe that further study of this model will show that the benefits—including strengthening research proposals, increasing the relevance of the research, improving participant recruitment and retention of research participants, and building a cadre of research engaged stakeholders—far outweigh the costs to the researcher and the institution.
Acknowledgments
The authors wish to thank the Meharry-Vanderbilt Community Engaged Research Core Advisory Council, collaborators at the University of North Carolina at Chapel Hill, and the many community and patient stakeholders who have made the Community Engagement (CE) Studio program possible with their generous contributions of time and thoughtful input. The authors also thank Mr. Brett Poe and Dr. Alaina Boyer for assisting with the preparation of this manuscript.
Funding/Support: This work has been supported by Federal funds from the National Center for Research Resources and National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA) under the following award numbers: UL1RR024975-03S1, UL1-RR025747-02S1, U54-MD007593, and UL1-TR000445. The work was also supported by a research award from the Patient Centered Outcomes Research Institute: ME 1306-03342.
Contributor Information
Yvonne A. Joosten, Vanderbilt University School of Medicine; and Office for Community Engagement, Vanderbilt Institute for Medicine and Public Health, Nashville, Tennessee.
Tiffany L. Israel, Vanderbilt Institute for Medicine and Public Health, Vanderbilt University School of Medicine, Nashville, Tennessee.
Neely A. Williams, Community Partners Network, Nashville, Tennessee.
Leslie R. Boone, Vanderbilt Institute for Medicine and Public Health, Vanderbilt University School of Medicine, Nashville, Tennessee.
David G. Schlundt, Department of Psychology, Vanderbilt University, Nashville, Tennessee.
Charles P. Mouton, School of Medicine, Meharry Medical College, Nashville, Tennessee.
Robert S. Dittus, Associate vice-chancellor for public health and health care; senior associate dean for population health services, director, Institute for Medicine and Public Health, Department of Medicine, Vanderbilt University Medical Center; and director, Geriatric Research, Education and Clinical Center, VA Tennessee Valley Healthcare System, Nashville, Tennessee.
Gordon R. Bernard, Vanderbilt Institute for Clinical and Translational Research; and Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee.
Consuelo H. Wilkins, General Internal Medicine and Public Health, Vanderbilt University School of Medicine; School of Medicine, Meharry Medical College; and Meharry-Vanderbilt Alliance, Nashville, Tennessee.
References
- 1.Wilkins CH, Spofford M, Williams N, et al. Community representatives' involvement in Clinical and Translational Science Awardee activities. Clin Transl Sci. 2013;6:292–6. doi: 10.1111/cts.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leshner AI, Terry SF, Schultz AM, Liverman CT. The CTSA program at NIH: Opportunities for advancing clinical and translational research. National Academies Press; 2013. [PubMed] [Google Scholar]
- 3.Michener L, Cook J, Ahmed SM, Yonas MA, Coyne-Beasley T, Aguilar-Gaxiola S. Aligning the Goals of Community-Engaged Research: Why and How Academic Health Centers Can Successfully Engage With Communities to Improve Health. Acad Med. 2012;87:285–91. doi: 10.1097/ACM.0b013e3182441680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 5.McCloskey DJ, Aguilar-Gaxiola S, Michener JL. Principles of Community Engagement. 2nd. Bethesda: National Institutes of Health publication; 2011. pp. 11–7782. [Google Scholar]
- 6.Staley K. Exploring impact: Public involvement in NHS, public health and social care research. Eastleigh: INVOLVE; 2009. [Google Scholar]
- 7.McLaughlin H. Involving young service users as co-researchers: Possibilities, benefits and costs. British Journal of Social Work. 2006;36:1395–410. [Google Scholar]
- 8.Byrne DW, Biaggioni I, Bernard GR, et al. Clinical and translational research studios: a multidisciplinary internal support program. Acad Med. 2012;87:1052–9. doi: 10.1097/ACM.0b013e31825d29d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulley JM, Bernard GR. Proven processes: The Vanderbilt Institute for clinical and translational research. Clin Transl Sci. 2009;2:180–2. doi: 10.1111/j.1752-8062.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Improving Patient Engagement and Understanding Its Impact on Research through Community Review Boards. [Accessed April 10, 2014];2013 at http://pfaawards.pcori.org/node/20/datavizwiz/detail/3342.