Abstract
This preliminary study examined the extent to which regional brain activation during a reward cue antisaccade (AS) task was associated with 6-month treatment outcome in adolescent substance users. Antisaccade performance provides a sensitive measure of executive function and cognitive control, and generally improves with reward cues. We hypothesized that when preparing to execute an AS, greater activation in regions associated with cognitive and oculomotor control supporting AS, particularly during reward cue trials, would be associated with lower substance use severity at 6-month follow-up. Adolescents (n=14, ages 14-18) recruited from community-based outpatient treatment completed an fMRI reward cue AS task (reward and neutral conditions), and provided follow-up data. Results indicated that AS errors decreased in reward, compared to neutral, trials. AS behavioral performance, however, was not associated with treatment outcome. As hypothesized, activation in regions of interest (ROIs) associated with cognitive (e.g., ventrolateral prefrontal cortex) and oculomotor control (e.g., supplementary eye field) during reward trials were inversely correlated with marijuana problem severity at 6-months. ROI activation during neutral trials was not associated with outcomes. Results support the role of motivational (reward cue) factors to enhance cognitive control processes, and suggest a potential brain-based correlate of youth treatment outcome.
Keywords: adolescent, treatment outcome, marijuana, fMRI, antisaccade, reward
1. Introduction
Sensitivity to reward and inhibitory control play important roles in the development and maintenance of substance use behavior (Dawe et al., 2004; Koob and Volkow, 2010). Much less is known about brain-based indicators of reward sensitivity and inhibitory control in relation to substance use treatment outcome. Research with treatment-seeking adult substance users suggests that pre-treatment regional brain activity in response to fMRI tasks assessing cognitive control is associated with substance use treatment outcomes (Brewer et al., 2008; Kober et al., 2014). This pilot study of adolescents recruited from substance use treatment examined the extent to which regional brain activation associated with inhibitory control in a reward cue antisaccade (AS) fMRI task (Geier et al., 2010; Chung et al., 2011), administered during or shortly after treatment completion, was correlated with 6-month treatment outcome.
In research with substance dependent adults, two fMRI studies found that greater pre-treatment brain activation related to cognitive control, particularly in prefrontal regions, was associated with less substance use over follow-up (Brewer et al., 2008; Kober et al., 2014). Specifically, among cocaine dependent adults, greater pre-treatment regional brain activity during a Stroop color-word interference task in prefrontal regions, including the anterior cingulate and ventrolateral prefrontal cortex, was associated with less cocaine use during treatment (Brewer et al., 2008). Behavioral Stroop response, however, was not associated with cocaine use during treatment (Brewer et al., 2008). Further, among cannabis dependent males, greater pre-treatment Stroop-related activity in dorsal anterior cingulate cortex was associated with less cannabis use during treatment; and greater pre-treatment activation in prefrontal regions, such as ventrolateral prefrontal cortex, was associated with lower rates of cannabis use over 1-year follow-up (Kober et al., 2014). These studies of adult substance users suggest an inverse association between pre-treatment regional activation related to cognitive control and substance use outcomes over follow-up, and that task-related regional activation, relative to behavioral response, may be more strongly related to treatment outcome.
A sensitive marker of cognitive control of behavior and executive functioning is provided by antisaccade (AS) performance (Hutton and Ettinger, 2006). Performing an AS requires stopping a prepotent eye movement toward a salient stimulus in favor of a voluntary movement to the opposite spatial location (Hallett, 1978; Munoz and Everling, 2004). Advantages of using AS to assay cognitive control include its well-characterized neural circuitry, and the ability to isolate activity related to response preparation, which is critical to effective response inhibition (Everling et al., 1998; 2000; Luna et al., 2008). Processes supporting AS have a protracted development into adolescence, with the ability to consistently execute AS continuing to mature into young adulthood (Luna et al., 2008). In particular, when executing AS, adolescents, compared to adults, tend to rely more on less mature regions such as dorsolateral prefrontal cortex, relative to regions associated with inhibitory control such as the cortical eye fields (Luna et al., 2001). Research indicates that youth at high, relative to low, risk for substance involvement showed poorer AS performance (Habeych et al., 2006), and less activation of brain regions supporting AS (McNamee et al., 2008). These findings suggest possible deficits or delayed maturation in neural circuitry supporting AS among high risk youth, and the sensitivity of AS as a measure of cognitive control. A distributed network of fronto-subcortical-parietal regions subserves AS, including, for example, the frontal eye field (FEF), supplementary eye field (SEF), dorsolateral and ventrolateral prefrontal cortex (dlPFC and vlPFC), posterior parietal cortex, anterior cingulate cortex, basal ganglia, thalamus, and superior colliculus (Munoz & Everling, 2004; Luna et al., 2004; Jamadar et al., 2013).
The performance of tasks that involve strong cognitive control over behavior, such as AS, can be improved with the use of reward or incentive (Hardin et al., 2007; Geier et al., 2010). Adolescents, relative to adults, tend to be especially sensitive to the motivational effects of incentive on AS performance (Padmanabhan et al., 2011). In the context of reward cues, adolescents tend to show increased activity in striatal regions, whereas adults show greater activation in OFC (Padmanabhan et al., 2011), suggesting that adolescent behavior may be particularly influenced by bottom-up reward processing (striatum) in combination with a relatively immature OFC which supports executive processing of reward cues (Geier, 2013). Modulation of response inhibition by reward cues has been examined using an fMRI reward cue AS task in healthy adolescents (Geier and Luna, 2009; Geier et al., 2010; Padmanabhan et al., 2011), and youth with substance use disorder (SUD) (Chung et al., 2011). When SUD youth were compared with matched controls using the reward cue AS task, monetary incentive improved AS performance among SUD youth (Chung et al., 2011). Further, when preparing to execute AS (“preparation” phase) in reward trials, SUD youth, compared to controls, showed greater activation in prefrontal and oculomotor control areas associated with effective inhibitory control. The brain-based mechanisms by which incentives improve AS are thought to involve activation of regions involved in reward processing (e.g., ventral striatum [VS]), which in turn may enhance activity in regions associated with motor control (e.g., frontal eye fields) and executive function (e.g., prefrontal cortex) that support AS (Geier, 2013; Geier and Luna, 2009; Harsay et al., 2011).
As a measure of cognitive control, successful AS behavior and greater activation of cognitive control regions supporting AS would be expected to be associated with better substance use treatment outcome. Regional brain activation supporting AS, however, may be more strongly related to treatment outcome than AS behavioral performance, as found in a study using a Stroop task in treated adults (Brewer et al., 2008). Further, the use of an incentive (reward cue) to motivate cognitive control and to improve AS performance through greater regional activation supporting AS suggests that regional activation and AS performance during reward (versus neutral) trials will be more strongly associated with better treatment outcome. That is, an optimized or motivated level of inhibitory control may be an important indicator of lower substance use severity over follow-up among adolescents in substance use treatment.
This pilot study examined response to an fMRI reward cue AS task administered during or shortly after treatment completion as a correlate of 6-month outcomes in adolescent substance users. We predicted, based on the adult literature (e.g., Brewer et al., 2008), that regional brain activation associated with response inhibition would be more strongly associated with treatment outcome than AS behavioral performance. In addition, we hypothesized that when preparing to execute an AS (preparation phase), greater activation in regions involved in cognitive (e.g., dlPFC, vlPFC) and oculomotor (e.g., FEF, SEF) control which support AS would be associated with lower severity of substance involvement at 6-month follow-up. We also predicted that the association between regional activation and treatment outcome would be stronger for reward, relative to neutral, trials because reward cues may motivate and optimize effective cognitive control in adolescents. Study results could help to identify neurobiological mechanisms that are associated with substance use treatment outcomes in youth.
2. Material and Methods
2.1 Participants
Adolescents (ages 14-18) were drawn from a longitudinal, naturalistic study of youth recruited from community-based intensive outpatient (IOP) treatment for substance use (King et al., 2009; Chung and Maisto, 2009). Treatment involved group sessions three times per week, with each session lasting three hours. Recommended duration of IOP treatment was 6-8 weeks. Treatment focused on a goal of abstinence from alcohol and other non-prescribed drugs, with program content that included relapse prevention (e.g., exercising inhibitory control over substance use behavior, coping with urges) and facilitation of 12-step meeting attendance.
Adolescents included in these analyses (n=14, 71% male, mean age = 16.9 [SD=1.3], 93% Caucasian) had useable fMRI data and completed a follow-up assessment. Socioeconomic status was, on average, “middle-class” (Hollingshead, 1975; mean= 2.4, SD=1.3; range= 1-5, 1=highest and 5=lowest). Full scale IQ, determined by the Wechsler Abbreviated Scale of Intelligence (Psychological Corporation, 1999), was in the average range (mean=106.3, SD=10.6). Youth were primarily in intensive outpatient treatment for marijuana use, with 85.7% meeting criteria for a current (past 6-months) DSM-IV marijuana use disorder (Table 1). All youth reported at least 1 lifetime DSM-IV marijuana use disorder symptom over the course of the study. A majority (71.4%) of the sample had a current DSM-IV alcohol use disorder. Prior to treatment, average frequency of marijuana use was once per week, and average frequency of alcohol use was once per month (Table 1). The most common co-occurring lifetime psychiatric conditions included conduct disorder (43%), attention deficit hyperactivity disorder (29%), and major depression (21%).
Table 1.
Sample descriptive statistics (N=14)
| Baseline | ||
| Gender (% male) | 71.4 | |
| Age: Mean (SD) | 16.9 (1.3) | |
| Race/Ethnicity (% Caucasian) | 92.9 | |
| Multi-racial (%) | 7.1 | |
| Socio-economic status: Mean (SD) | 2.4 (1.3) | |
| Full scale intelligence quotient score: Mean (SD) | 106.3 (10.6) | |
| Current DSM-IV alcohol use disorder (%) | 71.4 | |
| Alcohol Abuse (%) | 64.3 | |
| Alcohol Dependence (%) | 7.1 | |
| Current DSM-IV marijuana use disorder (%) | 85.7 | |
| Marijuana Abuse (%) | 64.3 | |
| Marijuana Dependence (%) | 21.4 | |
| Current DSM-IV nicotine dependence (%) | 35.7 | |
| *Current DSM-IV “other drug” diagnosis (%) | 50.0 | |
| Other drug abuse (%) | 21.4 | |
| Other drug dependence (%) | 28.6 | |
| DSM-IV lifetime psychopathology | ||
| Major depression (%) | 21.4 | |
| Conduct disorder (%) | 42.9 | |
| Attention deficit hyperactivity disorder (%) | 28.6 | |
| Baseline | 6-month follow-up | |
| Frequency of substance use (past 6 months) | Mean (SD) | Mean (SD) |
| Alcohol use | 3.4 (1.6) | 3.9 (2.1) |
| Cannabis use | 4.9 (3.0) | 3.5 (3.0) |
| DSM-IV symptom count, past 6 months | Mean (SD) | Mean (SD) |
| Alcohol abuse and dependence symptoms | 1.9 (1.7) | 1.5 (1.8) |
| Marijuana abuse and dependence symptoms | 2.7 (2.1) | 1.4 (2.2) |
Notes: SD= standard deviation. Current= past 6-months. Frequency of substance use: 0=never tried, 1=no use in past 6 months, 2=less than once per month, 3=once per month, 4=2-3 times per month, 5=once per week, 6=2-3 times per week, 7=4-6 times per week, 8=daily. The total number of DSM-IV abuse and dependence symptoms met in the past 6-months was used for alcohol (maximum of 11 symptoms) and marijuana (maximum of 10 symptoms).
“Other drug” refers to substances other than alcohol, cannabis or nicotine. For “other drug” abuse (none in this category had other drug dependence): n=1 opiate abuse, n=1 opiate and cocaine abuse, n=1 cocaine and hallucinogen abuse. For “other drug” dependence: n=3 opiate dependence (1 also met criteria for cocaine abuse and sedative dependence), n=1 cocaine dependence (also had opiate, hallucinogen, and stimulant abuse).
2.2 Procedures
Adolescents in the parent longitudinal study were invited to participate in an add-on neuroimaging study of brain structure and functioning (Thatcher et al., 2010; Chung et al., 2011; Clark et al., 2012; Chung et al., 2013), and completed comprehensive substance use and psychiatric assessments at baseline, 6-months, and 1-year after baseline as part of the parent project. Baseline assessments were completed, on average, roughly 2 weeks after starting treatment (mean= 16.1 days, SD=8.4). Analyses focus on 6-month follow-up (i.e., on average, 6 months after the scan day), a period during which treatment gains are likely to be sustained (King et al., 2009). Two cases missed the 6-month follow-up, and reported on 6-month outcome data at the 12-month follow-up. Study protocols were approved by the University’s Institutional Review Board. Written informed consent (or assent from minor adolescents, and consent from the youth’s parent) was obtained prior to initiating study procedures. Youth were compensated for study participation.
Adolescents with a history of significant brain injury or other MRI contraindication (e.g., metal in the body) were excluded from the neuroimaging protocol. Youth were scanned, on average, 6 weeks (mean= 46.4 days, SD=14.1) after starting IOP. Most adolescents (8 out of 14) were still in IOP at the time of the scan; 3 had completed treatment (scans were completed within 2 weeks of completing treatment for these 3 cases), 2 adolescents were in aftercare at the time of the scan (1 was in aftercare for 1 week prior to the scan, the other was in aftercare for 4 weeks prior to the scan), and 1 dropped out after attending treatment for 1 month (and 3 days prior to the scan). Thus, scans were done after completion or dropout from the index treatment episode for 6 of the 14 cases (although 2 of these 6 cases continued in aftercare). Follow-up occurred, on average, 6 months after the scan day. The current analyses included a subset of cases (n=9 overlapping cases) from an earlier report that compared SUD youth with healthy controls (Chung et al., 2011). The prior report required cases to be matched to controls on sex and age, whereas no such matching was needed here. In preparing for the scan session, youth were instructed to abstain from alcohol and illicit substance use for at least 24 hours prior to scanning. No adolescent included in the analyses reported substance use <24 hours prior to the scan. Average number of days since last alcohol use prior to scan was 35.6 (SD=41.0), and for marijuana was 67.2 days (SD=83.1).
2.3 Measures of substance use and psychopathology
Data on substance use and psychopathology were obtained at each assessment by highly trained interviewers. Participants reported on frequency of alcohol, marijuana, tobacco, and other substance use in the past 6-months on a 9-point scale (0=never tried to 8=daily use). A Time Line Follow Back (Sobell and Sobell, 1995) was used to assess daily substance use at baseline (past 30 days) and on the scan day (covering the interval since the baseline assessment). The Structured Clinical Interview for DSM-IV SUDs (SCID; First et al., 2002), adapted for adolescents, with good reliability and concurrent validity (Martin et al., 2000), was used to assess the presence and age at onset and offset (to the nearest month) of SUD symptoms and diagnoses at each assessment. For cases that missed the 6-month assessment, report of SUD symptoms was collected since the baseline assessment at the 12-month follow-up, with ages of onset and offset coded to the nearest month, to determine symptom count over 6-month follow-up. DSM-IV Axis I psychopathology (e.g., conduct disorder) was evaluated using the adolescent version of the Schedule for Affective Disorders and Schizophrenia (K-SADS: Kaufman et al., 1997), which has demonstrated good inter-rater reliability (Clark et al., 1997).
2.4 fMRI reward cue antisaccade (AS) task
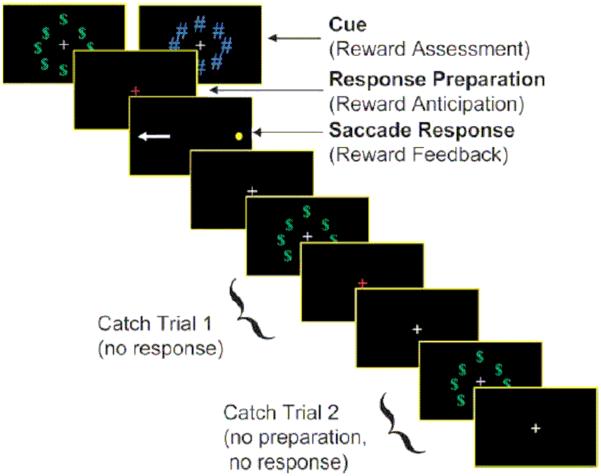
A fast-event related design was used to assess reward cue effects on activity unique to each epoch (i.e., cue, preparation, response execution) of the AS task (Geier et al., 2010; Chung et al., 2011). At the beginning of each AS trial, participants first viewed (1.5s) either (1) a ring of green dollar bill signs ($) around a central white fixation cross as a cue for reward trials, or (2) an equivalently sized, isoluminant ring of blue pound signs (#) around the fixation cross for neutral trials (see Figure). The ring then disappeared, and a red fixation cross was displayed for 1.5s signifying that an AS is to be performed. Finally, a peripheral target (yellow dot) appeared at an unpredictable horizontal location (± 3, 6, and 98 degrees visual angle). Participants were instructed to look at the mirror location (i.e., perform an antisaccade) during this time (1475 msec).
Figure.
fMRI antisaccade in the context of reward task: “incentive cue”, “preparation” and “response” epochs
Note: permission to reproduce the Figure depicting the fMRI task will need to be obtained from Cerebral Cortex
The AS task is a compound trial with an invariant sequence of components (i.e., motor response always follows response preparatory period). Thus, we included approximately 30% partial or “catch” trials, randomly inserted, in addition to jittered inter-trial intervals (Ollinger, Corbetta, et al., 2001; Ollinger, Shulman, et al., 2001) in order to deconvolve trial components. Two catch trial variants were presented and consisted of the trial terminating after either (1) the incentive cue (circles of “$” or “#”) (i.e. no response preparation or cue to perform an antisaccade) or (2) when the response preparation period ended (red fixation cross) (i.e. no peripheral target was presented). A jittered fixation period of 1.5, 3, or 4.5 seconds (randomly inserted), during which time subjects simply fixated on a central white cross presented on a black background, was included between all trials, compound and partial. Inclusion of partial trials and jittered inter-trial fixations allowed activity unique to each component of the task to be estimated independently (i.e., activity associated with response preparation can be estimated uniquely from reward image processing and motor processing).
The protocol included 14 complete reward trials, 6 partial reward trials (3 of each variant), 14 complete neutral trials, and 6 partial neutral trials (3 of each variant) in each run. Trials were pseudorandomized across runs. Each run lasted 5 min 9 sec and was presented 4 times for a total of 56 complete reward trials and 56 complete neutral trials. Participants were told that they could “win” (i.e., receive a “reward”) up to $10 for correct AS performance, with no monetary loss (i.e., no “punishment” for incorrect response), and were told to try to obtain the maximum amount. For the reward condition, the value of any single correct response was intentionally kept ambiguous to prevent participants from keeping a running total of earnings during the task. No feedback was provided to participants on AS performance. Participants who demonstrated compliance with the task received the full $10 “reward” at the end of the imaging session regardless of performance. All participants in the sample complied with the task.
2.5 Eye tracking
Participants were first oriented to the AS task outside of the scanner to ensure that they were able to perform the task. Eye movements were monitored in the scanner, and scored offline (Geier et al., 2010; Chung et al., 2011). In the scanner, eye movements were monitored using a long-range optics eye-tracking system (Model 504LRO, Applied Science Laboratories, Bedford, MA), which recorded eye position by pupil-corneal reflection obtained by a relay mirror mounted on the head coil with a resolution of 0.5° of visual angle. Simultaneous video monitoring provided data on task compliance. At the beginning of the session, a 9-point calibration procedure was performed. Stimuli were presented using E-prime (Psychology Software Tools, Inc., Pittsburgh, PA), projected onto a flat screen positioned behind the magnet. Subjects viewed the screen using a mirror mounted on a standard radiofrequency head coil. Eye movements were scored off-line using ILAB software (Gitelman, 2002) and in-house scoring programs written in MATLAB (Mathworks, Inc.) that calculated the direction, latency, and accuracy of responses.
Correct responses on the AS task were defined as those in which the first eye movement during the saccade epoch with velocity greater than or equal to 30°/s (Gitelman, 2002) was made toward the mirror location of the peripheral cue and extended beyond a 2.5°/visual angle from central fixation. Incorrect responses occurred when the first saccade during the saccade epoch was directed toward the peripheral stimulus and exceeded the 2.5°/visual angle central fixation zone. Trials in which no eye movements were generated, or in which the tracker lost fixation, were excluded from analyses. Prosaccade errors were consistently followed by movement to the appropriate location, indicating that instructions were understood, but the reflexive saccade was not effectively inhibited (cf. Velanova et al., 2008).
Behavioral variables included correct and incorrect AS latencies and errors in inhibitory response on rewarded and neutral trials. Sensitivity to reward on this task was indicated primarily by reduced inhibitory errors, and secondarily by shorter latency to correct AS, during reward compared to non-reward (neutral) trials (Hardin et al., 2007; Geier et al., 2010).
2.6 Image acquisition
Imaging data were acquired using a Siemens 3T Allegra Scanner. A gradient-echo echo-planar imaging sequence sensitive to blood oxygenation level dependent (BOLD) contrast (T2*) was used. Acquisition parameters were TR=1.5s TE=25ms, flip angle=70°, 64 × 64 acquisition matrix with field of view 20 × 20 cm. Twenty-nine 4mm-thick axial slices with no gap were collected, aligned to the anterior and posterior commissure (AC-PC line), generating 3.125 × 3.125 × 4mm voxels, which covered the entire cortex and most of the cerebellum. A series of magnetization-prepared rapid gradient echo (MPRAGE) images were acquired.
2.7 fMRI Data analysis
Preprocessing of the functional data followed standard techniques. Briefly, these included despiking using AFNI’s 3dDespike and slice timing correction. Mean relative motion per subject ranged between 0.05 mm and 0.25 mm. A few spikes in relative motion were found in three subjects exceeding 3 mm, but overall, less than 5% of the data contained motion greater than 1mm. MCFLIRT was used for motion correction (Jenkinson et al., 2002). Next, brain extraction, registration of functional to non-linearly registered anatomical data, spatial smoothing using SUSAN (Smith and Brady, 1997) with FWHM of 5 mm, high pass filtering of 0.008 Hz, and normalization were performed. Individual and group-level (mixed-effects) analyses were run using FSL to generate parameter estimates (PE) for Reward and Neutral conditions for each of the cue, preparation, and saccade phases. Analyses included only correct AS trials. Nuisance regressors included the six motion regressors used in motion correction, and the convolved hemodynamic response from trials that either resulted in an incorrect response or trials that could not be rated due to missing eye-tracking data (e.g., signal loss during tracking).
Analyses focused on a set of a priori regions of interest (ROIs) known to be involved in antisaccade performance (Luna et al., 2001; Velanova et al., 2008, 2009; Geier et al., 2010; Jamadar et al., 2013) and in reward processing (i.e., amygdala, orbitofrontal cortex, vmPFC, and striatum) (see Appendix for details on ROI location and volume). Antisaccade-related ROIs were drawn with a 10 mm or 7 mm (pre-supplementary motor area [preSMA], SEF) sphere surrounding the peak voxel of the associated cluster identified by neurosynth (www.Neurosynth.org) using the name of each ROI as a keyword. One exception to this was the ROI for posterior parietal cortex, which used the term “preparatory”, as this term provided a closer fit to activations from prior antisaccade studies. The resulting z-statistic images for these ROIs were then corrected for multiple comparisons using false discovery rate correction with a q-value of 0.05. We also ran whole-brain analyses, but since whole-brain analyses did not identify clusters of activation beyond the a priori ROIs, we report only the ROI results.
Parameter estimates, representing mean ROI activation, for reward and neutral conditions, for each of the three phases, were exported for analysis in SPSS. Results focus on the preparation phase, given prior research suggesting the importance of response preparation for effective response inhibition (Geier et al., 2010; Chung et al., 2011; Munoz and Everling, 2004). Substance use data (e.g., 6-month marijuana symptom count, number of days since last use of marijuana prior to the scan) were log transformed to normalize distributions. Only the bivariate correlation between vlPFC left sphere and 6-month marijuana symptom count was statistically significant (Reward: r= −.65, p<.01; Reward>Neutral: r= −.53, p<.05). Partial correlations of ROI activation and 6-month outcomes controlled for age and baseline level of the outcome variable, since they were associated with the marijuana outcome (age: r= −.58, p<.05; baseline marijuana symptom count: r= .69, p<.01). Gender was not associated with outcome (r= .05, p=.87), but was included as a covariate due to gender differences in adolescent brain development (Lenroot and Giedd, 2010). Number of days since last substance was not significantly associated with marijuana outcome (r= −.39, p=.17), but was included as a covariate due to the possible effect of number of days since last substance use on brain functioning.
We also ran the partial correlations excluding the 2 cases (one at a time) with the highest relative motion (note that these two cases were within the mean relative motion range of 0.05 mm and 0.25 mm per subject). Partial correlations with absolute value >.70 in the n=14 sample were robust to exclusion of these two cases (i.e., partial correlations with absolute value >.70 remained statistically significant at p<.05 when each case was excluded). Thus, we focus on reporting results for partial correlations with absolute value >.70 (although partial correlations with absolute value >.63 in the n=14 sample were significant at p<.05).
3. Results
3.1 Change in alcohol and marijuana involvement over 6-month follow-up
Table 1 presents descriptive statistics for alcohol and marijuana involvement at baseline and 6-month follow-up. Paired samples t-tests indicated no significant change over 6-month follow-up in frequency of alcohol use (t= −0.73, df=13, p=.48) or alcohol symptom count (t= 0.69, df=13, p=.50) in this primarily marijuana using sample. Marijuana use frequency declined, on average, but this reduction was not statistically significant (t= 1.32, df=13, p=.21). Marijuana symptom count, however, showed a significant decrease over follow-up (t=3.00, df=13, p=.01). Thus, analyses examining treatment outcome (i.e., change in substance involvement over follow-up) focused on marijuana symptom count.
3.2 Antisaccade behavioral performance
Table 2 reports descriptive statistics for behavioral AS performance. Across conditions, the average overall correct response rate was 74.9% (SD=17.9). Paired samples t-test indicated fewer errors in inhibitory response in the reward versus neutral condition (t=4.3, df=13, p=.001; see Table 2), indicating behavioral sensitivity to the reward condition. There was no significant effect of condition on latency to initiate a correct (t= 0.18, df=13, p=.86) or erroneous AS (t= 0.93, df=12, p=.37), but latency to correct AS was longer compared to AS errors in both neutral (t=4.3, df=12, p=.001) and reward conditions (t=3.5, df=13, p=.001). AS behavioral measures were not correlated with frequency of marijuana use or marijuana symptom count at 6-month follow-up (p>.2).
Table 2.
Behavioral results for reward and neutral antisaccade trials (N=14)
| Neutral Condition Mean (SD) |
Reward Condition Mean (SD) |
t-test | |
|---|---|---|---|
| Error rate (%) | 32.7 (22.6) | 18.0 (13.7) | 4.3, df=13, p=.001 |
| Latency of AS errors (ms) | 374.1 (51.6) 1 | 365.3 (70.7) | 0.9, df=12, p=.4 |
| Latency of correct ASs (ms) | 437.5 (63.5) | 434.9 (49.7) | 0.2, df=13, p=.9 |
Note:
n=13 (1 subject had division by 0)
AS=antisaccade, ms=millisecond, df= degrees of freedom,
3.3 Correlating fMRI preparatory phase ROI activation and 6-month marijuana symptom count
In the neutral condition, partial correlations (controlling for age, gender, number of days since last marijuana use prior to scan, and baseline marijuana symptom count) indicated no significant associations between ROI activation and 6-month marijuana symptom count (Table 3). By contrast, in the reward condition, activation in ROIs associated with reward (amygdala, nAcc), executive functioning (left vlPFC sphere), and oculomotor control (SEF sphere, putamen) were inversely correlated with marijuana symptoms at 6-months, controlling for the covariates. In the reward condition, those with fewer symptoms at 6-months tended to show greater activation in the ROI relative to baseline fixation, whereas those with more symptoms at follow-up tended to show deactivation in the ROI relative to baseline fixation. Likewise, when contrasting activation in reward versus neutral conditions, greater activation during reward versus neutral condition in ROIs associated with reward (left amygdala, left nAcc), executive function (right vlPFC sphere), and oculomotor control (putamen) were inversely correlated with 6-month marijuana symptom count, controlling for the covariates. Inspection of partial correlation plots did not indicate undue influence of outliers (see supplementary figures).
Table 3.
Partial correlations: fMRI rewarded antisaccade task ROI activation during preparation phase and marijuana symptom count at 6-month follow-up
| Region of Interest | Neutral | Reward | Reward>Neutral |
|---|---|---|---|
| Amygdala R | .10 | −.76* | −.57 |
| Amygdala L | .16 | −.79** | −.78** |
| Caudate R | −.03 | −.67 | −.62 |
| Caudate L | .00 | −.68 | −.70 |
| nAcc R | −.23 | −.92** | −.62 |
| nAcc L | −.10 | −.93** | −.74* |
| OFC R | .24 | −.67 | −.68 |
| OFC L | .19 | −.60 | −.67 |
| Putamen R | .05 | −.90** | −.75* |
| Putamen L | .09 | −.86** | −.75* |
| vmPFC | −.07 | −.54 | −.41 |
| dACC sphere | .36 | −.30 | −.51 |
| dlPFC L sphere | −.08 | −.63 | −.37 |
| dlPFC R sphere | .07 | −.55 | −.41 |
| FEF L sphere | .22 | −.52 | −.61 |
| FEF R sphere | .15 | −.68 | −.63 |
| PPC L sphere | −.16 | −.62 | −.47 |
| PPC R sphere | .07 | −.42 | −.38 |
| preSMA sphere | .06 | −.60 | −.64 |
| SEF sphere | −.16 | −.85** | −.55 |
| vlPFC L sphere | .11 | −.83** | −.66 |
| vlPFC R sphere | .46 | −.69 | −.75* |
Note: N=14
partial correlation >.70 and p<.05
p<.01
The partial correlations marked as statistically significant in the table also were identified as significant using false discovery rate correction with a q-value of 0.05.
6-month marijuana symptom count was log transformed to normalize the distribution.
Covariates: age, gender, number of days since last marijuana use prior to scan (square root transform), baseline marijuana symptom count.
R= right; L= Left; nACC= nucleus accumbens; OFC= orbitofrontal cortex; vmPFC= ventromedial prefrontal cortex; dACC= dorsal anterior cingulate; dlPFC= dorsolateral prefrontal cortex; FEF= frontal eye field; PPC= posterior parietal cortex; preSMA= presupplementary motor area; SEF= supplementary eye field; vlPFC= ventrolateral prefrontal cortex
4. Discussion
Results from this preliminary study support hypotheses that, when preparing to execute an AS, specifically during the reward cue condition, activation in regions associated with cognitive and oculomotor control was inversely correlated with marijuana symptom count at 6-month follow-up. Findings highlight the role of incentives (“reward cue”) in enhancing or motivating optimal levels of task-related activation to support effective inhibitory control, and indicate that this optimized level of activation during a cognitive control task was associated with fewer marijuana-related problems among treated adolescents over follow-up. Notably, youth with more marijuana symptoms over follow-up tended to show deactivation during the reward condition in ROIs that were associated with treatment outcome. The absence of an association between AS behavioral performance and treatment outcome is consistent with a study of treatment-seeking adults (Brewer et al., 2008), and suggests the potential utility of a brain-based, relative to behavioral, indicator in relation to treatment outcome.
The absence of an association between ROI activation and treatment outcome during neutral trials suggests the importance of motivating (by reward cue) optimal performance on a relatively challenging cognitive control task, such as antisaccade. Prior work with the reward cue AS task indicated that SUD youth do not generally lack inhibitory control, and that motivational cues (e.g., monetary incentive) can facilitate activation of regions involved in inhibitory control (Chung et al., 2011). For example, right vlPFC activation has been associated with inhibitory control (Aron and Poldrack, 2006), and specifically, inhibition of prepotent oculomotor responses (Massen, 2004). Results in this sample of treated youth indicating that greater activation of right vlPFC sphere during reward (relative to neutral) trials was associated with lower marijuana problem severity at follow-up suggest a potential role for reward in optimizing or motivating activation, particularly in this ROI, which in turn, was associated with treatment outcome.
This study’s finding that greater activation in regions associated with cognitive control (e.g., vlPFC) was associated with better marijuana outcomes in treated adolescents is similar to results obtained using an fMRI Stroop task in cocaine (Brewer et al., 2008) and cannabis dependent adults (Kober et al., 2014). The current study extends prior work with treatment seeking adults by using an AS task as a different assay of cognitive control, examining adolescents in community-based substance use treatment, and investigating the interplay of reward response and cognitive control in one task. An important difference between the prior work with adults and the current study of adolescents is that regional activation in cognitive control regions during reward, but not neutral, trials was associated with adolescent treatment outcomes. The finding in the current study that youth with more marijuana symptoms at follow-up tended to show deactivation in ROIs that were associated with outcome may reflect, for example, decreased sensitivity to reward or less efficient brain functioning during the reward condition. In addition, prior work with treatment seeking adults examined brain response prior to treatment, whereas in the current study of treated adolescents, brain response was assessed during or shortly after treatment completion. Nevertheless, research with both treated adults and this pilot study of treated adolescents suggests that regional activation associated with cognitive control is associated with subsequent substance use outcomes.
Notably, the reward cue antisaccade task leverages an individual’s sensitivity to reward to motivate or optimize cognitive control. Consistent with proposed brain-based mechanisms by which incentives might increase correct AS (Geier et al., 2010; Geier, 2013), greater activation during reward trials in regions involved in reward processing (e.g., amygdala, nAcc), oculomotor control (e.g., putamen, supplementary eye field sphere), and executive functioning (e.g., vlPFC) were associated with fewer marijuana symptoms at 6-month follow-up in treated adolescents. The association of activation during reward condition in nAcc, but not OFC, with marijuana outcome also is consistent with reliance on bottom-up processing of reward during adolescence, in the context of a relatively immature OFC (Geier, 2013). Adolescent sensitivity to reward cues, may bias action to immediate and salient rewards, such as substance use. However, the use of incentive to support cognitive control over behavior, for example, in contingency management interventions (Stanger and Budney, 2010), capitalizes on adolescent sensitivity to reward to promote positive behavior change and effortful control over behavior.
As a comparison to findings with treated adolescents in the current study, research on reward response with cocaine dependent adults in treatment correlated regional activation during reward processing using an fMRI monetary incentive delay task (MIDT) with treatment outcome, and found, in general, that reduced activity in regions involved in reward processing was associated with more positive response to behavioral therapy (Jia et al., 2011). In contrast, results from the current study suggest that youth with more marijuana symptoms at follow-up showed deactivation during the reward condition in ROIs associated with treatment outcome. Differences in results across these two studies may be due, in part, to differences in the role of reward in the two tasks (i.e., use of reward to enhance cognitive control versus response to monetary reward), differences in sample age (and brain development in adolescents versus adults), primary substance of abuse, and severity and duration of substance use. Importantly, with the rewarded antisaccade task, we were able to assess sensitivity to reward, inhibitory control, and their interplay (i.e., the use of reward to optimize inhibitory control) in a single task.
In the reward cue AS task, sensitivity to reward cue was used to increase top-down or executive cognitive control in inhibiting a prepotent response (saccade). The incentive used in this study was relatively small ($10 total upon task completion), and no feedback was provided to participants on whether an incentive was earned for correct antisaccade performance on a given trial, yet reward cue enhanced behavioral AS performance and influenced associated brain activation in predicted ways. Motivational factors that enhance inhibitory control may take different forms. For example, a study of adolescent marijuana users found that motivation in the form of hearing and reading “change talk” (statements supportive of positive behavior change) during exposure to marijuana cues activated brain areas related to response inhibition (e.g., inferior frontal gyrus), and that greater activation in these regions was associated with better 1-month outcomes (Feldstein Ewing et al., 2013). The potential effect of motivational factors in enhancing inhibitory control suggest, for example, that proximal reminders of incentive structure in a contingency management intervention (Stanger and Budney, 2010) or booster motivational interview sessions could help to prime inhibitory control among SUD youth.
Study limitations warrant comment. Generalizability of results is limited to youth recruited from community-based outpatient substance use treatment, most of whom were male and White, and primarily in treatment for marijuana use. This preliminary study analyzed a small sample of youth in substance use treatment. The small sample size and limited number of females precluded the ability to examine possible differences by sex. Although moderate to large effects were detected in hypothesized directions, and analyses included covariates, results need to be interpreted in the context of small sample size. Further, a relatively large number of ROIs was tested, and although false discovery rate was considered, replication is needed. Although both marijuana frequency and abuse/dependence symptom count declined over follow-up, only the reduction in marijuana symptom count was statistically significant, which may reflect limited sample size. However, similar results regarding alcohol and marijuana outcomes have been obtained in other research on adolescent treatment outcomes (Arias et al., 2014). Self-report of substance use and substance-related problems may be subject to bias, and biochemical verification of substance use was not conducted, although procedures to maximize validity (e.g., assurance of confidentiality) were used. fMRI data were not collected prior to initiating treatment, such that cognitive functioning at the time of the scan, which was associated with treatment outcome, could reflect, for example, effects of treatment or abstinence from substance use.
5. Conclusions
This preliminary study identified a potential neurobiological marker that was associated with adolescent substance use treatment outcome. As a next step, research in a larger adolescent sample that examines the temporal ordering of regional activation (e.g., reward-related ROIs influencing oculomotor control regions) is needed to test a mechanistic model by which incentives and other motivational factors (e.g., change talk) acutely enhance cognitive control, which could ultimately inform the development of neuroscience-informed interventions that aim to strengthen cognitive control. Further research is needed to probe the use of adolescent sensitivity to reward (bottom-up processing) to prime or enhance top-down cognitive control over behavior to improve youth treatment outcomes.
Supplementary Material
Highlights.
* Activation during rewarded antisaccade task correlated with later marijuana severity
* Antisaccade behavioral performance was not associated with treatment outcome
* Preliminary results suggest a brain-based correlate of treatment outcome in youth
Acknowledgements
Support for the conduct of the research and preparation of the manuscript was provided by funding from the National Institute on Alcohol Abuse and Alcoholism and National Institute on Drug Abuse (R01 AA014357, R21 AA016272, R21 AA017128, K02 AA018195).
Appendix
Regions of Interest for Rewarded Antisaccade task analyses
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Region of Interest |
Source | x | y | z | Radius (mm) |
Number of voxels |
| Amygdala R | Atlas | 434 | ||||
| Amygdala L | Atlas | 390 | ||||
| Caudate R | Atlas | 675 | ||||
| Caudate L | Atlas | 632 | ||||
| nAcc R | Atlas | 110 | ||||
| nAcc L | Atlas | 119 | ||||
| OFC R | Atlas | 1444 | ||||
| OFC L | Atlas | 1650 | ||||
| Putamen R | Atlas | 1011 | ||||
| Putamen L | Atlas | 979 | ||||
| vmPFC | Atlas | 1011 | ||||
| dACC sphere | Coordinate | 0 | 22 | 30 | 10 | 515 |
| dlPFC L sphere | Coordinate | −42 | 38 | 28 | 10 | 515 |
| dlPFC R sphere | Coordinate | 40 | 40 | 28 | 10 | 515 |
| FEF L sphere | Coordinate | −26 | −6 | 52 | 10 | 515 |
| FEF R sphere | Coordinate | 26 | −6 | 52 | 10 | 515 |
| PPC L sphere | Coordinate | −28 | −64 | 48 | 10 | 515 |
| PPC R sphere | Coordinate | 30 | −62 | 46 | 10 | 515 |
| preSMA sphere | Coordinate | 0 | 6 | 58 | 7 | 179 |
| SEF sphere | Coordinate | 0 | 0 | 68 | 7 | 179 |
| vlPFC L sphere | Coordinate | −48 | 36 | −4 | 10 | 515 |
| vlPFC R sphere | Coordinate | 48 | 36 | −6 | 10 | 515 |
Notes: Atlas= Harvard-Oxford Anatomical Atlas; R= right; L= Left; nACC= nucleus accumbens; OFC= orbitofrontal cortex; vmPFC= ventromedial prefrontal cortex; dACC= dorsal anterior cingulate; dlPFC= dorsolateral prefrontal cortex; FEF= frontal eye field; PPC= posterior parietal cortex; preSMA= presupplementary motor area; SEF= supplementary eye field; vlPFC= ventrolateral prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: permission to reproduce the Figure depicting the fMRI task will need to be obtained from Cerebral Cortex
The authors have no conflicts of interest to disclose.
References
- Arias A, Burleson J, Kaminer Y, Curry J, Dennis M. Examining the relationship between depression and alcohol use in youth being treated for a cannabis use disorder, poster presentation. Alcoholism: Clinical and Experimental Research. 2014;38:216A. Abstract 0861. [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, Thatcher D, Clark DB. Enhancing response inhibition by incentive: comparison of adolescents with and without substance use disorder. Drug and Alcohol Dependence. 2011;115:43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Maisto SA. "What I got from treatment": predictors of treatment content received and association of treatment content with 6-month outcomes in adolescents. Journal of Substance Abuse Treatment. 2009;37:171–181. doi: 10.1016/j.jsat.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Pajtek S, Clark DB. White matter integrity as a link in the association between motivation to abstain and treatment outcome in adolescent substance users. Psychology of Addictive Behaviors. 2013;27:533–542. doi: 10.1037/a0026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Chung T, Thatcher D, Pajtek S, Long E. Psychological dysregulation, white matter organization and substance use disorders in adolescence. Addiction. 2012;107:206–214. doi: 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Pollock N, Bukstein OG, Mezzich AC, Bromberger JT, Donovan JE. Gender and comorbid psychopathology in adolescents with alcohol dependence. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1195–1203. doi: 10.1097/00004583-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addictive Behaviors. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents' response to a cannabis intervention. Psychology of addictive behaviors. 2013;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research. New York State Psychiatric Institute; New York, NY: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. [Google Scholar]
- Geier C. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Hormones and behavior. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology, biochemistry, and behavior. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behavior Research Methods, Instruments, & Computers. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Folan MM, Luna B, Tarter RE. Impaired oculomotor response inhibition in children of alcoholics: The role of attention deficit hyperactivity disorder. Drug and Alcohol Dependence. 2006;82:11–17. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Cohen MX, Oosterhof NN, Forstmann BU, Mars RB, Ridderinkhof KR. Functional connectivity of the striatum links motivation to action control in humans. The Journal of Neuroscience. 2011;31:10701–10711. doi: 10.1523/JNEUROSCI.5415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Author; New Haven, CT: 1975. [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Jamadar SD, Fielding J, Egan GF. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Frontiers in psychology. 2013;4:749. doi: 10.3389/fpsyg.2013.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- King KM, Chung T, Maisto SA. Adolescents' thoughts about abstinence curb the return of marijuana use during and after treatment. Journal of Consulting and Clinical Psychology. 2009;77:554–565. doi: 10.1037/a0015391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, DeVito E, DeLeone C, Carroll K, Potenza M. Cannabis abstinence during treatment and one-year follow-up: Relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–2298. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug and Alcohol Dependence. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Massen C. Parallel programming of exogenous and endogenous components in the antisaccade task. The Quarterly journal of experimental psychology. A, Human experimental psychology. 2004;57:475–498. doi: 10.1080/02724980343000341. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature reviews: Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Developmental cognitive neuroscience. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation . Wechsler Abbreviated Scale of Intelligence (WASI) manual. Author; San Antonio, TX: 1999. [Google Scholar]
- Smith S, Brady JM. SUSAN—A New Approach to Low Level Image Processing. International Journal of Computer Vision. 1997;23:45–78. [Google Scholar]
- Sobell LC, Sobell MB. Assessment of drinking behavior. In: Allen JP, Columbus M, editors. Assessing Alcohol Problems. Humana Press; Bethesda, MD: 1995. pp. 55–74. [Google Scholar]
- Stanger C, Budney AJ. Contingency management approaches for adolescent substance use disorders. Child and adolescent psychiatric clinics of North America. 2010;19:547–562. doi: 10.1016/j.chc.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher DL, Pajtek S, Chung T, Terwilliger RA, Clark DB. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug and Alcohol Dependence. 2010;110:55–61. doi: 10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. The Journal of neuroscience. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.