Abstract
Testosterone and inflammation have been linked to the development of common age-associated diseases affecting the prostate gland including prostate cancer, prostatitis, and benign prostatic hypertrophy. We hypothesized that testosterone regulates components of prostate tight junctions which serve as a barrier to inflammation, thus providing a connection between age- and treatment-associated testosterone declines and prostatic pathology. We examined the expression and distribution of tight junction proteins in prostate biospecimens from mouse models and a clinical study of chemical castration, using transcript profiling, immunohistochemistry and electron microscopy. We determined that low serum testosterone is associated with reduced transcript and protein levels of Claudin 4 and Claudin 8, resulting in defective tight junction ultrastructure in benign prostate glands. Expression of Claudin 4 and Claudin 8 was negatively correlated with the mononuclear inflammatory infiltrate caused by testosterone deprivation. Testosterone suppression also induced an auto-immune humoral response directed toward prostatic proteins. Testosterone supplementation in castrate mice resulted in re-expression of tight junction components in prostate epithelium and significantly reduced prostate inflammatory cell numbers. These data demonstrate that tight junction architecture in the prostate is related to changes in serum testosterone levels, and identify an androgen-regulated mechanism that potentially contributes to the development of prostate inflammation and consequent pathology.
Keywords: testosterone supplementation, inflammation, tight junction, claudin
INTRODUCTION
The androgen receptor (AR), and androgenic hormones including testosterone (T) and its primary intraprostatic metabolite, dihydrotestosterone (DHT), direct the normal development and function of the prostate gland (1). Ligand-AR complexes regulate a broad range of physiological events in the prostate that include cellular differentiation, proliferation, metabolism and secretory function (1, 2). These processes are regulated through a complicated cross-talk of cellular interactions that involve reciprocal paracrine signals between distinct epithelial and mesenchymal cell types (3). Androgens and the AR also modulate pathological processes that affect the prostate gland including the aging-related diseases of prostate carcinoma and benign prostatic hypertrophy (BPH)(4). Pharmacological interventions designed to treat these diseases have focused on suppressing the prostatic AR axis (5-7). Paradoxically, as men age from the 4th to 8th decades, the time period associated with increased incidence rates of BPH and prostate cancer, serum testosterone levels gradually decrease at a relatively constant rate (8, 9). The relationships between declining androgens and prostate pathology remains poorly understood.
Increasingly, androgens are recognized to influence other diseases, such as the metabolic syndrome, a condition associated with low serum testosterone and a generalized elevation in systemic markers of inflammation (10-13). Some degree of inflammation has been identified in the majority of prostate biopsies acquired from hypogonadal men, and infiltrates are frequently detected in and around foci of atrophy that are characterized by an increased epithelial proliferation index (14, 15). Inflammation disrupts the integrity of prostate epithelium leading to elevated serum prostate specific antigen (PSA) levels (14, 16) and is hypothesized to provide a favorable environment for cancer development, and induce a precursor lesion long before the formation of a tumor (17). Inflammation has also been associated with lower urinary tract symptoms (LUTS) that include pelvic pain and voiding symptoms (18).
To date, the etiology and pathogenesis of inflammation in the relatively sterile environment of the prostate remains unclear. Previous studies point to a role for androgens as modulators of systemic immune and inflammatory responses (19-21). Androgens are critical for maintaining the immune privilege status of organs such as the testes where they regulate the permeability of the blood-testis barrier through the tight junction protein Claudin 3 (Cldn3) (22). Claudins, occludins and junction adhesion molecules (JAMs) are the three major constituents of tight junctions. These transmembrane proteins form continuous strands, tightly sealing two adjacent cells and forming barriers to the passage of cells, molecules, and ions. Similar to the blood-testis barrier that influences immune system access, tight junctions are also present between basal cells residing adjacent to the prostatic acinar basement membrane, and between columnar epithelial cells (23, 24). The possibility that the prostate is normally an immune privileged organ is supported by the fact that allogeneic tissue grafts can survive in the gland for extended time periods without immunologic eradication (25). The blood-prostate barrier restricts molecules from passing from the interstitial compartments into the tubular compartments, in a fashion similar to that of the blood-testis barrier (26). Although it has been reported that several claudin proteins are expressed in the prostate(27), little is known concerning the regulation of prostatic tight junctions and their potential role(s) in association with prostatic inflammation and pathology.
In this study, we sought to provide evidence supporting the hypothesis that tight junctions in the prostate gland are maintained by androgens, and this blood-prostate-barrier serves to indirectly suppress inflammatory responses by restricting access to prostatic epithelial antigens. We evaluated the expression of tight junction proteins and quantitated inflammatory infiltrates in the prostates of castrated mice and humans. Low androgen concentrations correlated with decreased expression of tight junction components and with elevated numbers of intraprostatic inflammatory cells. A systemic immune response to prostatic proteins in conjunction with testosterone suppression also occurred. Repletion of testosterone restored tight junction protein expression and was associated with a dramatic reduction in the numbers of intraprostatic inflammatory cells. These findings have implications for studies of aging-associated pathology, testosterone replacement in the aging population, and immune-based therapeutics targeting prostate cancer.
METHODS
Animal Studies
Animal studies were approved by the Fred Hutchinson Cancer Research Center’s IACUC. Male C57BL6J mice at 12 weeks of age were purchased from The Jackson Laboratory. Mice were castrated using standard methods and sacrificed for serum and prostate tissue examination at 2, 4 and 8 weeks. At 2 weeks post castration, a group of animals was implanted with either testosterone (T) (5.0 mg/pellet; 21-day release) or placebo pellets subcutaneously, and then sacrificed at 2 or 3 weeks after T treatment. At least 3 animals were used for each time point of the experiment.
Clinical Specimens
Clinical studies were approved by the University of Washington Institutional Review Board and all patients signed written informed consent. Prostate tissue was obtained at prostatectomy from patients with clinically localized prostate cancer enrolled in a neoadjuvant study evaluating the effects of lupron or estradiol on prostate gene expression. All patients underwent a prostatectomy after 3 weeks of treatment and tissues were snap frozen for subsequent gene expression and immunohistochemical studies. Human prostates from age-matched eugonadal patients with low-grade prostate cancers without hormone manipulation were used for the control group.
Laser Capture microdissection and RNA Amplification
Prostate epithelial cells were microdissected from OCT embedded mouse and human prostate tissues using an Arcturus Veritas Laser Capture Microdissection System (Mountain View, California). Approximately 3000 cells were captured from each sample, and RNA was isolated using PicoPure RNA isolation Kit (Arcturus Inc). RNA was amplified with message Amp™ II aRNA kit from Applied Biosystems, and the amplified RNA was quantified using a NanoDrop 1000 UV-Vis Spectrophotometer (NanoDrop Technologies).
Quantitative RT-PCR Analysis
1.5ug amplified RNA of each sample was used to generate cDNA by oligodT primed reverse transcription reactions. Quantitative PCR reactions were performed in triplicates using an Applied Biosystems 7700 sequence detector (Foster City, California) with master mix containing 5ng of cDNA from each sample, 1uM of each primer pair, and SYBR Green PCR from Applied Biosystems. The sequences of primers for mouse cDNA were as follows: Cldn4, 5-gccttctctgtggtttcttgttt-3′ and 5-gcaggtgccatttattgtagaaa-3′; Cldn8, 5′-aggtccaatgaaatgtgtttgtt-3 and 5′-tcatttcagcactgcttttagttc-3′. The sequences of primers for human cDNA were as follows: CLDN4, 5′-ccccttccaaggacactaatga-3′ and gaagaggaaaaaccccaggact; CLDN8, 5′-tcctcttctcccagaggctttt-3′ and ccctggaaaagcagtttgaatg-3′.
Immunoblotting
Sera from mice were screened for the presence of autoantibodies by Western blotting. Prostate proteins were separated by SDS-PAGE, transferred to membranes and incubated with 1:200 diluted sera from castrated mice or normal mice overnight at 4°C. Primary antibody was detected with a HRP-conjugated goat anti-mouse secondary antibody (Bio-Rad) and the ECL Western blotting detection system (Amersham Pharmacia).
Immunoprecipitation
Whole mouse prostate tissues were homogenized in RIPA buffer. Protein lysate was precleared by adding 75ul Protein A/G Agarose beads (Thermo Scientific), and incubated at 4′C for 2 hours. After centrifugation, beads were discarded, and supernatants were added to mouse sera from either castrated mice or intact mice, and incubated at room temperature for 2 hours. Immune complexes were precipitated with 50 μl of protein A/G-Agarose beads 4′C for 1 hour. After three washes in cold RIPA buffer, beads were boiled in 60μl 4× sample buffer for 5 minutes. After centrifugation, the supernatant was subjected to SDS-PAGE.
Mass Spectrometry
The protein bands from Coomassie stained gels were excised and cut into 1mm cubes. After washing with water, the samples were dehydrated by vacuum centrifugation followed by incubation for 1 hour at 56°C in 10 mM DTT in 100 mM ammonium bicarbonate. After removing the DTT solution, 55mM iodoacetamide in 100mM ammonium bicarbonate was added and incubated for 45 minutes in the dark at room temperature. The samples were dried by vacuum centrifugation, followed by digesting the gel pieces in buffer containing trypsin and 50mM ammonium bicarbonate, at 37′C overnight. After centrifugation, the supernatants were collected, dried by vacuum centrifugation, and analyzed by mass spectrometry, Peptide spectra were identified from the X!Tandem search against the IPI mouse database.
Immunohistochemistry
Mouse prostates from castrated and control mice were prepared as described previously (28). For Cldn4 and Cldn8 staining, paraffin sections were incubated overnight with 1:200 diluted rabbit anti-Cldn4 antibody and rabbit anti-Cldn8 (Zymed Laboratories). A goat anti-rabbit antibody conjugated to Alexa Fluor 568 (Molecular Probes) was used at dilution of 1:1000. For CLDN4, cytokeratin and DNA triple staining, human prostate sections were incubated with mouse anti-CLDN4 antibody (Zymed Laboratories) and rabbit anti-cytokeratin antibody (DAKO Cytomation), overnight at 4°C. Goat anti-mouse antibody conjugated to Alexa Fluor 568 (Molecular Probes) and goat anti-rabbit antibody conjugated to Alexa Fluor 488 (Molecular Probes) were used at 1:1000 dilution for 30 min at 25°C, washed with PBS, then mounted in Vectashield mounting medium with DAPI.
CD3 antigen retrieval was performed in a Black and Decker steamer for 20 minutes in preheated Trilogy buffer (Cell Marque, Hot Springs AZ) and cooled for 20 minutes. F4/80 and CD68 slides were steamed for 20 minutes in preheated Target Retrieval solution (pH6, Dako) and cooled for 20 minutes. Slides were rinsed 3 times in wash buffer and all subsequent staining steps were performed at room temperature using the Dako Autostainer. Endogenous peroxide activity was blocked using 3% H2O2 for 8 minutes followed by protein blocking. Slides were blocked in 15% goat serum and 5% mouse serum in Tris-buffered saline (TBS) containing 1% BSA for 10 minutes. All antibodies were incubated on the tissue for 30 minutes and then washed. CD3 (MCA1477, Serotec) was used at 10 μg/ml, F4/80 (MCA497, Serotec) was used at 20 μg/ml and CD68 PG-M1 (M0876, Dako) was used at 1.9 μg/ml. Antibodies were detected using biotinylated goat anti-rat antibody (112-065-167, Jackson ImmunoResearch) or biotinylated goat anti-mouse antibody (115-066-062, Jackson ImmunoResearch) at 1:200 for 30 minutes followed by chromogenic detection using Vector Elite ABC reagents. The staining for all slides was visualized with 3,3′-diaminobenzidine (DAB, Dako) for 7 minutes, and the sections were counter-stained with hematoxylin (Dako) for 2 minutes. Concentration-matched isotype control slides were run for each tissue sample (Jackson ImmunoResearch Laboratories).
Radioimmunoassay (RIA)
Serum testosterone was assayed by double antibody RIA using an assay from Diagnostic Systems Laboratories, Inc (Catalog number DSL-4100, Webster, TX). All samples were assayed at one time. Minimum limit of detection for this assay is 0.05 ng/ml and intra-assay coefficient of variation is 5.7%.
Quantification of inflammatory cells and claudin expression
After immunohistochemical staining, 10 random microscopic fields from the peripheral zone (human) or lobe (mouse) from each sample were photographed, and the inflammatory cells were independently counted by two researchers (F.V.L and J.M). The mean number of inflammatory cells in each treated prostate sample was compared to that of control groups.
Images from the claudin-stained human prostates were also scored by two independent researchers (J.M and F.V.L) based on the following scale: 4= strong membranous staining in epithelial cells; 3= strong membranous staining in majority of epithelial cells, but not in every cell; 2= faint membranous staining, but visible; 1= membranous staining barely detectable; 0= membranous staining barely detectable, with irregular cytoplasmic clumps. Average scores were compared to control groups.
Statistical Analysis
All data are expressed as mean ± SE (standard error). Statistical comparisons between groups were performed using GraphPad Prism 4.0b software. Unpaired t-tests were used for comparing treatment and control groups. The association between claudin expression and inflammatory cells were analyzed with Pearson’s correlation. P values <0.05 were considered significant for all the analysis.
RESULTS
Serum testosterone suppression results in the loss of tight junction protein expression in the mouse prostate
To examine the role of androgens on the regulation of cellular tight junctions in the prostate, we castrated a group of adult mice and quantitated transcripts encoding tight junction proteins at time-points of 2, 4, and 8 weeks. We specifically acquired prostate epithelial cells by laser capture microdissection, amplified the RNA, and performed quantitative RT-PCR for occludin, Tjp1, and 23 members of the Claudin gene family (Supplemental figure 1). We determined that Cldn4, Cldn7, and Cldn8 were the most abundantly expressed Claudin transcripts in prostate epithelium, and found that Cldn4 (mean difference of the cycle threshold numbers is 3.266 ± 0.6151; p<0.01) and Cldn8 (mean difference of the Ct numbers is 0.9158 ± 0.3793; p<0.033) transcripts were significantly decreased following castration (Figure 1).
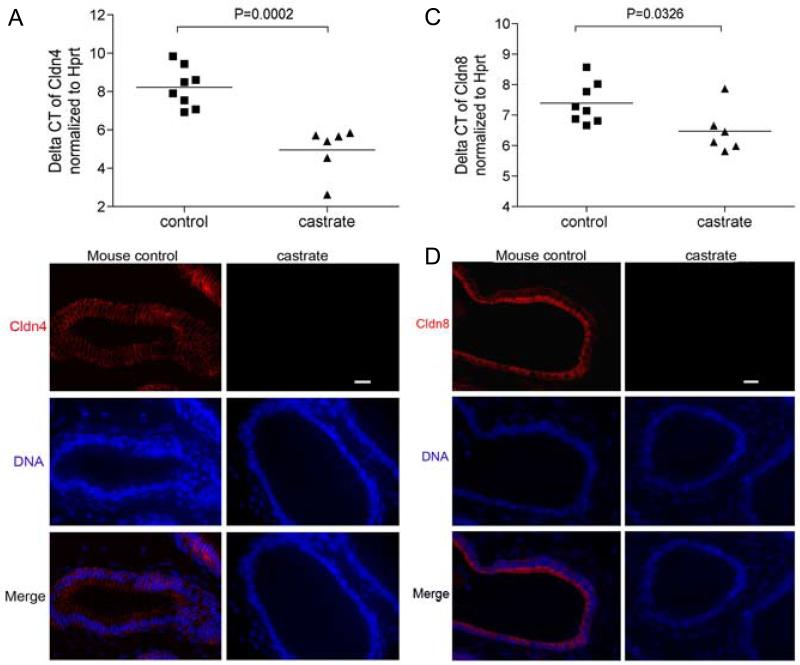
Figure 1. Testosterone suppression is associated with the loss of Claudin 4 and Claudin 8 expression in prostate epithelium.
Quantitative RT-PCR analysis of Cldn4 expression (A) and Cldn8 expression (C) in the prostate epithelium of castrated and control mice. The relative abundance of Cldn4 and Cldn8 normalized to Hprt, was significantly reduced compared to normal controls, representing as mean ± SE (mean difference of the cycle threshold numbers is 3.266 ± 0.6151; p=0.0002 for Cldn4) and mean difference of the Ct numbers is 0.9158 ± 0.3793; p=0.0326 for Cldn8) (B) Immunofluorescence detection of Cldn4 on prostate sections from control and castrated mice. Cldn4 proteins were distributed within the epithelium of the mouse prostates, and expression was substantially decreased in the prostates of castrated mice; (D) Immunofluorescence detection of Cldn8 on prostate sections from control and castrated mice, Cldn8 proteins were localized at the intercellular sites in the epithelium of control mouse prostates, some of the proteins concentrated at the edge of the luminal compartment. Cldn8 became undetectable in the prostates of 4 week post castrated mice. Scale bar =20 μm, applied to all images.
To confirm and extend these results, we determined the distribution of Cldn4 and Cldn8 proteins in the prostates of eugonadal and castrated mice using immunofluorescence microscopy (Figure 1). In eugonadal mice, both Cldn4 and Cldn8 proteins were localized to intercellular tight junction sites within the epithelium, although some Cldn8 protein was localized at the apical-most regions of luminal cell plasma membranes. In castrated mice, the expression of both Cldn4 and Cldn8 proteins became progressively uneven and eventually undetectable by 4 weeks after castration (Figure 1).
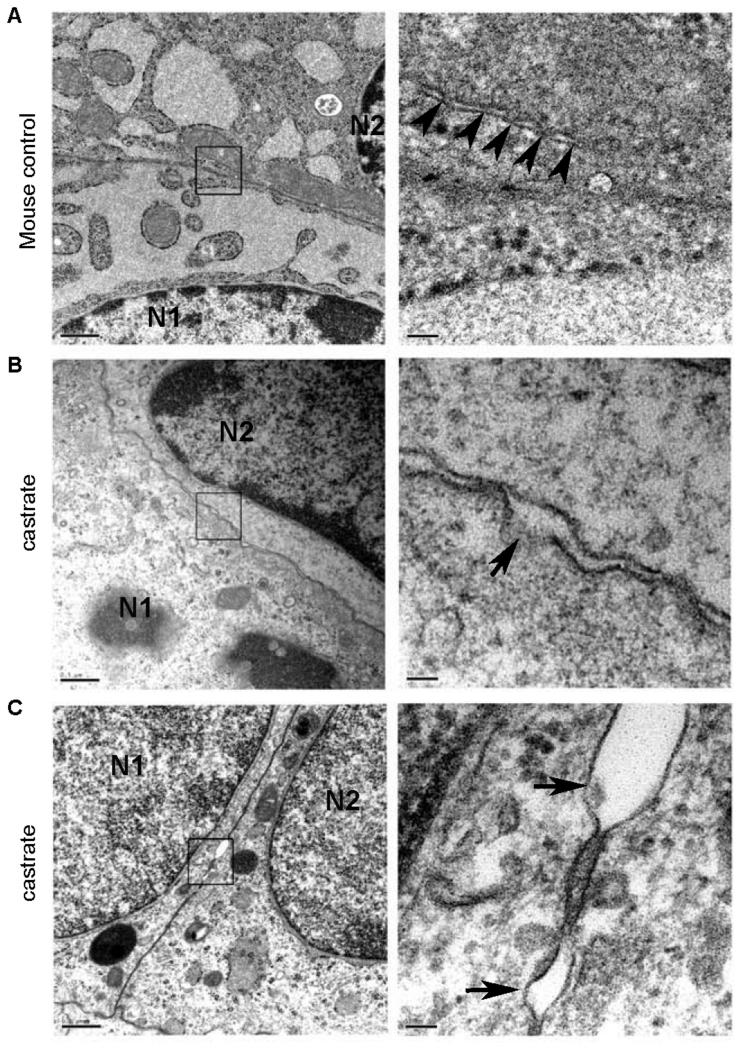
To determine the impact of the androgen suppression and loss of Cldn4 and Cldn8 expression on tight junction architecture, we examined the ultrastructure of the prostate epithelium from normal and castrated mice using electron microscopy (Figure 2). Cellular tight junctions contain a series of kissing points (arrows) that bring the lipid bilayers on opposing epithelial cells into intimate contact (Figure 2). In castrated mice, the tight junctions in the prostate epithelium lack obvious kissing points, and intercellular membranous contacts are diminished.
Figure 2. Ultrastructure of prostate epithelium in normal and castrated mice.
Normal epithelial tight junctions comprise a series of kissing points (A) (arrows) that juxtapose the lipid bilayers on opposing epithelial cells into intimate contact. In castrated mice, the tight junctions in the prostate epithelium lack obvious contact points (B,C), and the intercellular membranous contact regions are separated (arrows).
To determine if the administration of testosterone would promote the re-expression of tight junction proteins in the prostate, we performed immunofluorescence microscopy to evaluate the expression of Cldn4 and Cldn8. We found that Cldn4 expression was restored at sites of tight junctions in previously castrated mice after 2 weeks of testosterone treatment (Supplemental Figure 2). Cldn8 became detectable at tight junction regions of basal epithelium and was strongly expressed at the apical surface of luminal epithelial cells (Supplemental Figure 2). In contrast, Cldn4 and Cldn8 remained undetectable in the prostates of castrated mice that received placebo or no treatment (Supplemental Figure 2). These data indicate that testosterone replacement can re-establish the expression of tight junction components that were diminished by androgen suppression.
Testosterone suppression reduces the expression of tight junction proteins CLDN4 and CLDN8 in human prostate epithelium
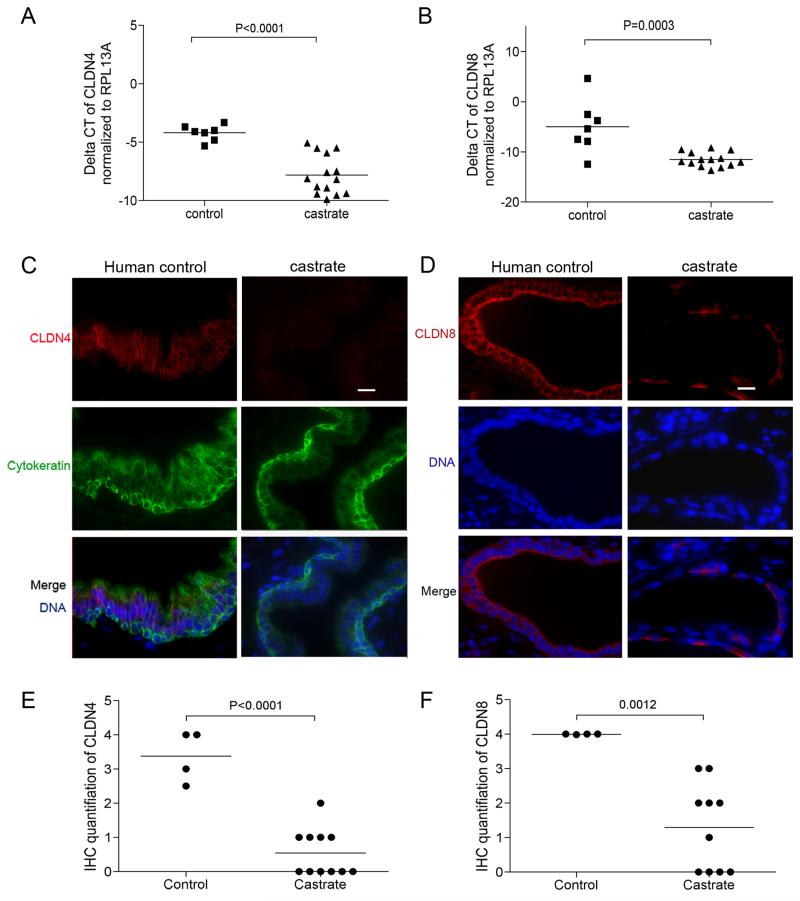
To determine the role of androgens on tight junction regulation in the human prostate, we examined prostate tissues acquired from patients who received 3 weeks of treatment that produced castrate levels of serum testosterone, and age-matched eugonadal control patients. We specifically acquired benign prostate epithelial cells by laser capture microdissection, amplified the RNA, and performed quantitative RT-PCR for Claudin gene family members (Supplemental figure 3). We determined that CLDN1, CLDN3, CLDN4, CLDN7, and CLDN8 were abundantly expressed in normal prostate epithelium, and determined that CLDN4 (mean difference of Ct numbers is 3.603 ± 0.6654; p<0.001) and CLDN8 (mean difference of Ct number is 6.532 ± 1.492; p<0.001) were significantly decreased following castration (Figure 3A,B).
Figure 3. Androgen suppression decreases the expression of CLDN4 and CLDN8 in the human prostate.
Transcript levels of CLDN4 (mean difference of Ct numbers is 3.603 ± 0.6654; p<0.0001) (A) and CLDN8 (mean difference of Ct number is 6.532 ± 1.492; p=0.0003) (B) were significantly decreased in prostate epithelium following testosterone suppression. (C) Immunofluorescence detection of CLDN4 on prostate sections from normal control and castrated men, CLDN4 proteins are localized at the intercelluar tight junction sites within the epithelium of eugonadal prostates, but only detectable at very low levels in the prostates from castrated men. Cytokeratin was stained as a marker for epithelial cells; (D) immunofluorescence detection of CLDN8 on prostate sections from normal control and castrated men. Within the epithelium of eugonadal prostates, CLDN8 proteins are localized at the intercellular tight junction sites of the basal compartment, and strong signals of CLDN8 staining was detected along the edge of luminal compartment of the epithelium. CLDN8 staining was decreased in the prostates from castrated men. Scale bar =20 μm, applied to all images. The mean scores of claudin protein expression levels from prostates following androgen suppression were significantly lower than the control group (the difference of mean scores for CLDN4 is 2.830 ± 0.4101, (P<0.0001) (E); the difference of mean scores for CLDN8 is 2.698 ± 0.6413 (p= 0.0012)(F))
Similar to the findings in mouse prostate, CLDN4 and CLDN8 proteins are generally evenly distributed at intercellular tight junction sites, with relatively more concentrated CLDN8 expression located toward the apical regions of the luminal cell plasma membranes (Figure 3C,D). Both CLDN4 and CLDN8 protein levels were detectable at substantially lower levels in the prostates of men treated with either lupron or estradiol (Figure 3C,D): the difference of mean IHC scores for CLDN4 expression between the controls versus treated patients is 2.830 ± 0.4101, (P<0.001) and for CLDN8 is 2.698 ± 0.6413 (p<0.01) (Figure 3E,F).
Testosterone suppression is associated with prostate inflammation
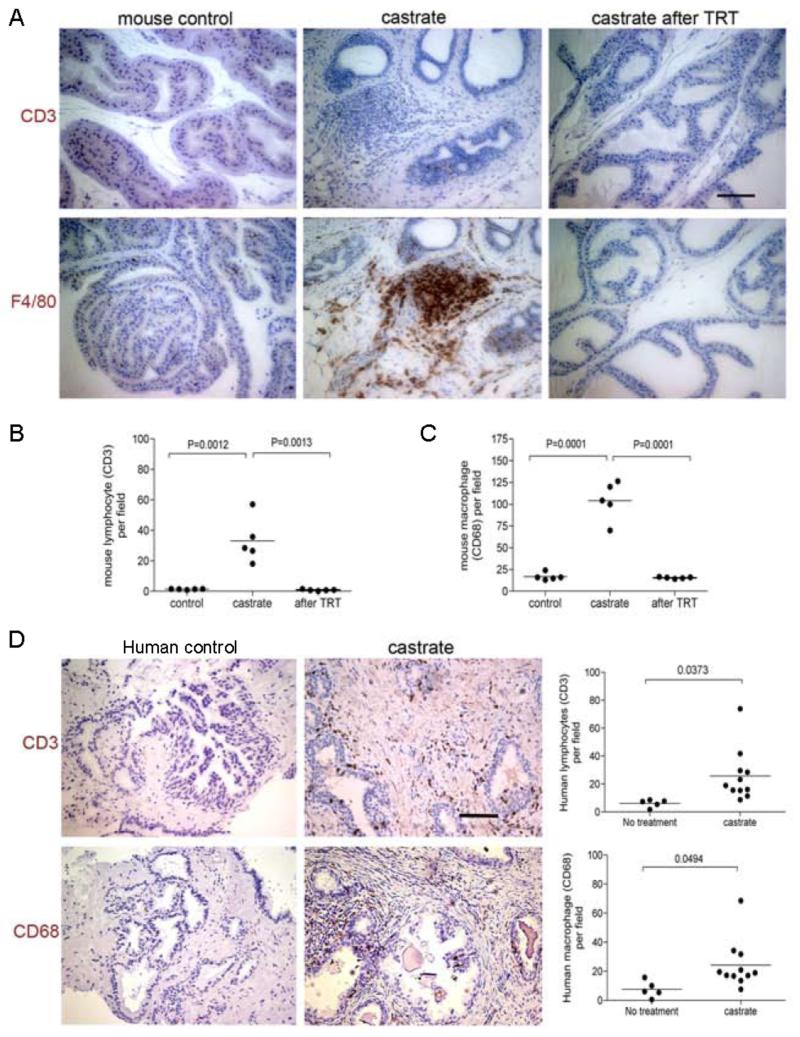
The loss of tight junctions in organs such as the testis has been shown to compromise the blood-testis barrier (22), and loss of this barrier can lead to loss of immune privilege and auto-immune responses (29). To evaluate the effects of extreme androgen deficiency on prostatic inflammation, we stained sections of mouse prostate adjacent to those we used for evaluating tight junction proteins with antibodies directed toward the lymphocyte antigen CD3 and macrophage antigen F4/80. The location of these cell types in stroma and within the glands and acinar structures was evaluated. Overall, the prostates obtained from intact control animals contained very few lymphocytes (1.340 ± 0.1122/field, n=5) or macrophages (16.74 ± 1.950/field, n=5) (Figure 4A). Following castration, significant increases in both lymphocytes (33.16 ± 6.599/field, n=5, p= 0.0013) and macrophages (104.1 ± 9.811/field, n=5, P<0.0001) were quantified in the stroma, along the basal membrane of the epithelium, and within the prostate epithelial layer (Figure 4B &C), consistent with previous observation in human studies
Figure 4. Testosterone suppression is associated with enhanced prostate inflammation.
A substantial CD3 positive lymphocyte infiltrate (brown immunoreactivity) was detected in the prostate glands of castrated mice compared to controls (A). Two weeks after testosterone supplementation, the T cell numbers were substantially reduced relative to castrated mice before testosterone treatment: (10 random fields under 20× lens were examined for each animal, 5 animals in each treatment group; student t-test p=0.0005) (B). Significantly more F4/80 positive macrophages (brown immunoreactivity) were detected in the prostates of castrated mice relative to normal controls. After testosterone replacement treatment, the number of macrophages was significantly reduced in the castrated mice (10 random fields under 20× lens were examined for each animal, 5 animals in each treatment group; student t-test: P=0.0068) (C).
A CD3 positive lymphocyte cell infiltrate and CD68 positive macrophage infiltrate (D) was detectable in the prostates of castrated men compared to eugonadal controls. Ten random fields under the 20× lens were examined for each patient. Lymphocyte numbers increased from 6.158 ± 1.226/field, n=5 in non-treated patients to 25.72 ± 5.600/field, n=11 in men with suppressed testosterone (p=0.0373), and macrophage numbers increased from 7.614 ± 2.545/field, n=5 in non-treated patients to 24.22 ± 4.990/field, n=11 in men with suppressed testosterone (p= 0.0494) Scale bar =50 μm, applied to all images.
To determine if testosterone supplementation could reverse the inflammation associated with androgen deprivation, we embedded testosterone and placebo pellets subcutaneously in castrated mice, and sacrificed cohorts after 2 and 3 weeks. Sera from these mice were obtained weekly and used for quantitating testosterone levels by RIA to confirm adequate replacement (Supplemental Figure 2). After 2 weeks of testosterone treatment to castrated animals, the number of intraprostatic inflammatory cells was dramatically decreased compared to measurements prior to testosterone replacement (Figure 4A): lymphocytes decreased from 2 to 0.8400 ± 0.2249/field (n=5, p= 0.0012); and macrophages decreased from 20 to 15.42 ± 0.4224/field (n=5, p<0.0001) (Figure 4B &C). No difference in lymphocyte or macrophage numbers were observed between normal control mice and castrated mice that also received testosterone supplements (P=0.6 and P=0.17, respectively). These data suggest that androgen treatment can reverse the inflammation associated with low serum androgens.
Consistent with previously published human data (30, 31), as well as observations in the murine prostate, we similarly observed moderate to severe inflammatory cell infiltrates in the prostate of men with suppressed serum testosterone (Figure 4D). In eugonadal patients, prostatic lymphocytes numbered 6.158 ± 1.226 per field (n=5) whereas following androgen suppression the lymphoctyes numbered 25.72 ± 5.600 per field (n=11) (p=0.0373). Similarly, macrophages numbered 7.614 ± 2.545 per field in eugonadal patients and 24.22 ± 4.99 per field in the setting of castrate testosterone levels (p= 0.049) (Figure 4D).
As testosterone suppression resulted in overall down-regulation of prostatic tight junction proteins and increased numbers of inflammatory cells, we sought to determine how these observations were correlated within individual patients. We determined that CLDN4 levels are negatively correlated with the number of intraprostatic lymphocytes (r=−0.5178, p=0.048), and macrophages (r=−0.5899, p=0.0206). CLDN8 levels are also negatively correlated with the number of lymphocytes (r=−0.5702, p=0.0333), and macrophages (r=−0.588, p=0.027).
Testosterone suppression results in an autoimmune response to prostate antigens
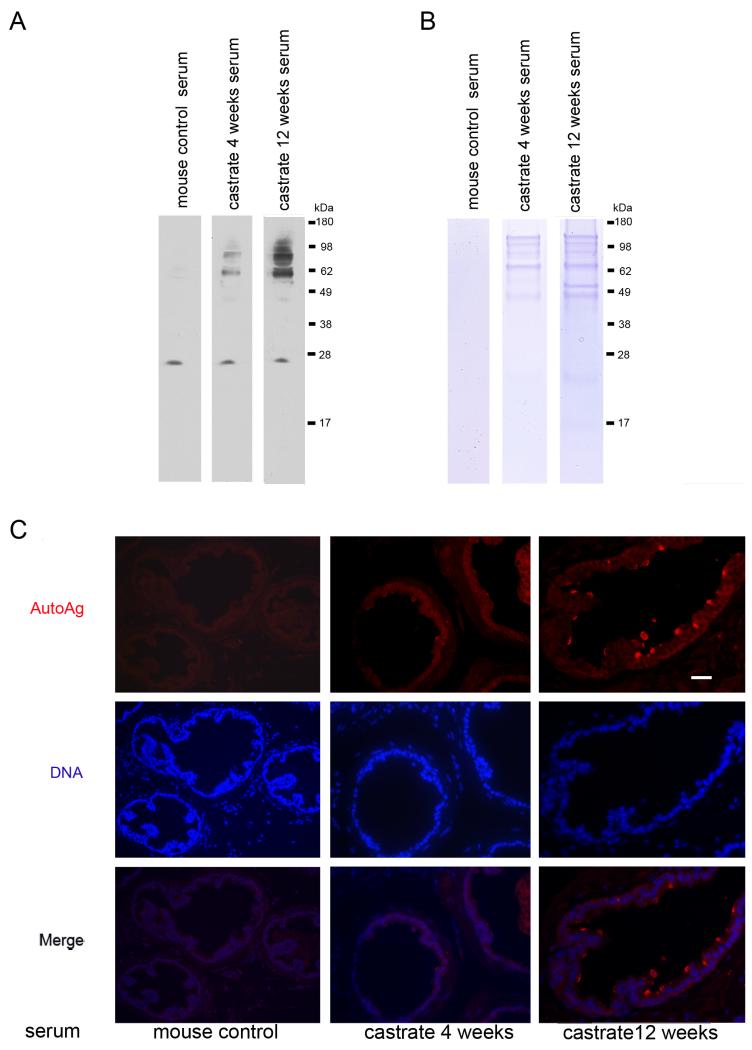
To test the hypothesis that a defective blood-prostate barrier in those individuals with low androgen levels might compromise prostate immune privilege and induce an autoimmune response, we sought to determine if the serum of castrate mice contains auto-antibodies against prostatic proteins. Western blots of prostatic protein extracts from 3-month-old wild-type mice were probed with sera obtained from castrate and normal control mice. Compared to sera from eugonadal mice, distinct protein bands representing polypeptides of different molecular weights were detected using anti-sera from animals castrated for 4 weeks and 12 weeks.
To determine the localization of the auto-antigens, we performed immuno-fluorescence microscopy on prostate glands from eugonadal mice (Figure 5). Using normal mouse serum as the primary antibody, no prostatic reactivity was detected. In contrast, we observed staining of luminal prostate epithelial cells when using sera from 4 week-castrated mice, and the staining intensity increased when sera from 12 week-castrated mice was used (Figure 5).
Figure 5. Testosterone suppression induces autoimmune responses in the prostate.
(A) Detection of prostatic proteins with antisera from intact or castrate mice. Auto-antigens are evident as distinct bands using sera from 4 week and 12 week castrated mice. (B) Coomassie blue stained gel of prostatic proteins immunoprecipitated from mouse prostate using sera from intact or castrate mice. (C) Immunofluorescence staining shows the autoantigens were distributed at the luminal epithelium of the prostate. Scale bar =20 μm, applied to all images.
To identify the prostatic antigens recognized by the anti-sera from castrate mice, we used this sera to immunoprecipitate proteins from lysates of prostate glands from untreated mice. Bound proteins were separated through SDS gels. Those bands visualized by Coomassie blue staining which were unique to castrated mice were excised and subjected to mass spectrometry (Figure 5). Proteins identified with high confidence included Svs2/Semg1, seminogelin 1 (IPI no. 00331164, with 31.7% sequence coverage of the protein), a prostatic secretary protein that has been shown to contribute to autoimmune prostatitis in mice and chronic prostatitis in humans (32), Eapa1, Experimental autoimmune prostatitis antigen1 (IPI no. 00322595, with 34.8% sequence coverage of the protein), and Eapa2, Experimental autoimmune prostatitis antigen2 (IPI no. 00408706, with 29.4% sequence coverage of the protein). These prostatic secretary proteins were previously defined as autoantigens in the context of prostate autoimmunity (33, 34).
DISCUSSION
Cross-sectional and longitudinal studies have consistently determined that normal male aging is associated with gradual and progressive declines in the concentration of testosterone in the blood (35-37). Paralleling the age-related decreases in androgens are substantial increases in the incidence of prostate cancer and BPH, diseases with pathogenesis clearly linked to androgenic hormones (38, 39). The treatments of both prostate cancer and BPH often involve pharmacological agents designed to block the influence of androgens in the prostate epithelium and stroma (6, 40, 41). In addition to effects on resident cells of the prostate, androgen depletion has been shown to increase T cell infiltrates (30, 31), though the mechanism(s) underlying this event is not known. The results in the present study support a hypothesis linking low testosterone with the deterioration of cellular tight junctions that reduce barriers to immune recognition of prostatic proteins, and the consequent generation of an inflammatory response that may contribute to the development and/or progression of neoplasia and other prostate pathology.
Inflammation has been linked to the incidence of a wide range of human cancers that include carcinoma of the liver, stomach, large intestine, and bladder (reviewed in (42). While specific infectious organisms or environmental exposures have been shown to directly induce several types of neoplasms, chronic inflammation also serves to cooperate with environmental exposures, such as dietary carcinogens, to further enhance mechanisms contributing to tumorigenesis. Recent evidence from analyses of model organisms and epidemiological, molecular, and histological studies indicate that inflammation also contributes to the etiology of prostate cancer (43). For example, a lesion termed Proliferative Inflammatory Atrophy (PIA) has been suggested to reflect a ‘field effect’ of epithelium disposed to neoplastic transformation (44). Transitions between PIA and Prostate Intraepithelial Neoplasia (PIN) and adenocarcinoma have been documented to occur frequently (44, 45). Many hypotheses have been put forth to define the inciting events leading to prostatic inflammation that include sexually transmitted infections, urine reflux, physical trauma, dietary agents such as heterocyclic amines, and estrogens (42). However, none of these mechanisms appear to occur at a frequency sufficient to account for rates at which inflammation and neoplasia occur in the prostate. One concept that has been proposed to account for the high incidence of inflammation involves the generation of a self-perpetuating auto-immune reaction directed toward prostatic autoantigens (46). Several proteins exclusive to the prostate are not expressed until androgen-stimulated development of the prostate at puberty, and thus may not be recognized as a self-antigen for the acquisition of immune tolerance. Prostate injury induced by any of the previously described inciting events could expose prostatic antigens to immune recognition leading to sustained immune activity.
One mechanism by which organs such as the testis and brain protect constituent cells from immune attack involves the development of tight cellular junctions that physically restrict the movement and access of immune cells and macromolecules such as antibodies (47). In the brain, tight junctions are formed between endothelial cells and astrocytes, and in the testis tight connections are formed between sertoli cells of the seminiferous tubules. Maintenance of tight junctions in the testis is regulated by testosterone and the androgen receptor though the expression of members of the claudin protein family (22). In the present study, we determined that testosterone regulates two proteins, CLDN4 and CLDN8, which comprise the tight junctions of epithelial cells in the prostate. Further, we found that very low serum testosterone concentrations in men were associated with loss of CLDN4 and CLDN8 protein expression and a marked prostatic inflammatory infiltrate comprised of T cells and macrophages. Restoration of CLDN expression following androgen supplementation was associated with a subsidence of inflammatory cell infiltrates in the mouse prostate. Studies using model systems capable of selectively and temporally altering the expression or function of claudin proteins will clarify the precise relationships between androgens, tight junctions and inflammatory responses.
Though our data do not yet prove causal mechanisms directly linking testosterone, tight junction regulation, inflammation, and prostate pathology, the results do provide a plausible explanation for the juxtaposition of rising rates of BPH, LUTS, and cancer in aging men, with falling testosterone concentrations. Despite a dramatic rise in the use of testosterone replacement therapy in the last decade (48), the incidence of prostate cancer in the US has not risen substantially (49). It is possible that potential detrimental effects of testosterone acting directly on prostate epithelium that involve proliferative and anti-apoptotic signals, are tempered by reductions in deleterious inflammatory responses. Of interest, two small recent clinical studies demonstrated that the administration of testosterone to men with both age-related testosterone deficiency and LUTS produced significant improvements in lower urinary tract symptoms as measured by International Prostate Symptoms Score (IPSS) (50, 51). Planned randomized efficacy studies of testosterone supplementation in men with age-associated hypogonadism may provide further information concerning the beneficial and detrimental effects of androgens on the prostate (ClinicalTrials.gov identifier NCT00799617).
Supplementary Material
Supplemental Figure 1. Claudin expression in normal mouse prostate epithelium
The relative abundance of Cldn1, 3, 4, 7, 8,10, 11, 12 and 23 in the prostate epithelium of normal mice was normalized to Hprt, (Delta CT=CT of Hprt – CT of Claudins).
Supplemental Figure 2. Testosterone replacement induces Claudin expression in prostate epithelium of castrate mice.
(A) Cldn4 protein expression was restored in the prostates of castrated mice following 2 weeks of testosterone supplementation (Lower Panel) compared to the castrated mice before testosterone treatment (Upper Panel). Scale bar =20 μm, applied to all images. (B) Cldn8 protein expression was restored in the prostates of castrated mice (Lower Panel) compared to the cas-trated mice before testosterone treatment (Upper Panel). Scale bar =20 μm, applied to all images.
Supplemental Figure 3. Serum Testosterone levels of castrated and testosterone-treated mice
The average serum T level of intact control mice is 13.1 ± 3.4 ng/ml, n= 4. The serum T level became undetectable in the sera of castrated mice. At 14th day post castration, a group of cas-trated mice were embedded with T pellets, their serum T levels became 30.± 4.2 ng/ml (n=2) at day 2 post T treatment, 28.77 ±9.4 ng/ml (n=3) 1 week post T treatment; and 14.5 ± 0.69 ng/ml (n=3) 3 weeks post T supplementation treatment. Another group of castrated mice were embedded with placebo pellets at day 14 post castration, their serum T levels remained undetectable at 1 week and 3 week post implantation, n=3.
ACKNOWLEDGEMENTS
We thank Phil Gafken and the FHCRC Proteomics Core facility for assistance with mass spectrometry. This work was supported in part by a grant from the AUA Foundation Research Scholars Program (Solvay Pharmaceuticals) (JM), the Prostate Cancer Foundation (EM, PSN), U54 HD042454 (PSN), R01 DK069690, and the NIH/NCI Pacific Northwest Prostate SPORE Grant, P50CA97186 (JM, EM, RBM, LT and PSN).
REFERENCES
- 1.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–62. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard CC, Nelson PS. Gene expression profiling in the developing prostate. Differentiation. 2008;76:624–40. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayward SW, Cunha GR. The prostate: development and physiology. Radiol Clin North Am. 2000;38:1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- 4.Coffey DS, Pienta KJ. New concepts in studying the control of normal and cancer growth of the prostate. Progress in Clinical and Biological Research. 1987;239:1–73. [PubMed] [Google Scholar]
- 5.Huggins C, Hodges CV. Studies on prostate cancer 1: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–7. [Google Scholar]
- 6.Andriole G, Bruchovsky N, Chung LW, et al. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004;172:1399–403. doi: 10.1097/01.ju.0000139539.94828.29. [DOI] [PubMed] [Google Scholar]
- 7.Rittmaster RS. 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22:389–402. doi: 10.1016/j.beem.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 9.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 10.Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol. 2005;174:827–34. doi: 10.1097/01.ju.0000169490.78443.59. [DOI] [PubMed] [Google Scholar]
- 11.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 12.Saad F, Gooren L. The role of testosterone in the metabolic syndrome: a review. J Steroid Biochem Mol Biol. 2009;114:40–3. doi: 10.1016/j.jsbmb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- 14.Schatteman PH, Hoekx L, Wyndaele JJ, Jeuris W, Van Marck E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA density. Eur Urol. 2000;37:404–12. doi: 10.1159/000020161. [DOI] [PubMed] [Google Scholar]
- 15.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171:S36–40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 16.Okada K, Kojima M, Naya Y, et al. Correlation of histological inflammation in needle biopsy specimens with serum prostate-specific antigen levels in men with negative biopsy for prostate cancer. Urology. 2000;55:892–8. doi: 10.1016/s0090-4295(00)00519-7. [DOI] [PubMed] [Google Scholar]
- 17.Palapattu GS, Sutcliffe S, Bastian PJ, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–81. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 18.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The Relationship between Prostate Inflammation and Lower Urinary Tract Symptoms: Examination of Baseline Data from the REDUCE Trial. Eur Urol. 2008;54:1379–84. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page ST, Plymate SR, Bremner WJ, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–63. doi: 10.1152/ajpendo.00484.2005. Epub 2005 Dec 13. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Parker CR., Jr Adrenal androgens and the immune system. Semin Reprod Med. 2004;22:369–77. doi: 10.1055/s-2004-861553. [DOI] [PubMed] [Google Scholar]
- 21.Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:131–42. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 22.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachar B, Reese TS. Formation of misplaced and reflexive tight junction strands in prostate epithelial cells. J Ultrastruct Res. 1983;82:90–5. doi: 10.1016/s0022-5320(83)90099-0. [DOI] [PubMed] [Google Scholar]
- 24.El-Alfy M, Pelletier G, Hermo LS, Labrie F. Unique features of the basal cells of human prostate epithelium. Microsc Res Tech. 2000;51:436–46. doi: 10.1002/1097-0029(20001201)51:5<436::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Itoh M, Chen XH, Takeuchi Y, Miki T. Morphological demonstration of the immune privilege in the testis using adjuvants: tissue responses of male reproductive organs in mice injected with Bordetella pertussigens. Arch Histol Cytol. 1995;58:575–9. doi: 10.1679/aohc.58.575. [DOI] [PubMed] [Google Scholar]
- 26.Fulmer BR, Turner TT. A blood-prostate barrier restricts cell and molecular movement across the rat ventral prostate epithelium. J Urol. 2000;163:1591–4. [PubMed] [Google Scholar]
- 27.Sakai N, Chiba H, Fujita H, et al. Expression patterns of claudin family of tight-junction proteins in the mouse prostate. Histochem Cell Biol. 2007;127:457–62. doi: 10.1007/s00418-007-0269-7. [DOI] [PubMed] [Google Scholar]
- 28.Braun RE, Peschon JJ, Behringer RR, Brinster RL, Palmiter RD. Protamine 3′-untranslated sequences regulate temporal translational control and subcellular localization of growth hormone in spermatids of transgenic mice. Genes Dev. 1989;3:793–802. doi: 10.1101/gad.3.6.793. [DOI] [PubMed] [Google Scholar]
- 29.Morrow CM, Hostetler CE, Griswold MD, et al. ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood testes barrier function and testicular immune privilege. Ann N Y Acad Sci. 2007;1120:144–51. doi: 10.1196/annals.1411.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Hou Y, DeVoss J, Dao V, et al. An aberrant prostate antigen-specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans. J Clin Invest. 2009;119:2031–41. doi: 10.1172/JCI38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimoto N, Akimoto Y, Suzuki T, Kitamura S, Ohta S. Identification of prostatic-secreted proteins in mice by mass spectrometric analysis and evaluation of lobe-specific and androgen-dependent mRNA expression. J Endocrinol. 2006;190:793–803. doi: 10.1677/joe.1.06733. [DOI] [PubMed] [Google Scholar]
- 34.Setiady YY, Ohno K, Samy ET, et al. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+ CD25+ regulatory T cells. Blood. 2006;107:1056–62. doi: 10.1182/blood-2005-08-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab. 1987;65:1118–26. doi: 10.1210/jcem-65-6-1118. [DOI] [PubMed] [Google Scholar]
- 36.Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ. Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol. 1988;43:M163–9. doi: 10.1093/geronj/43.6.m163. [DOI] [PubMed] [Google Scholar]
- 37.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 38.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–7. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6(Suppl 9):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 40.Kawakami J, Cowan JE, Elkin EP, Latini DM, DuChane J, Carroll PR. Androgen-deprivation therapy as primary treatment for localized prostate cancer: data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006;106:1708–14. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]
- 41.Tindall DJ, Rittmaster RS. The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer. J Urol. 2008;179:1235–42. doi: 10.1016/j.juro.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palapattu GS, Sutcliffe S, Bastian PJ, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2004;21:21. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 44.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama M, Bennett CJ, Hicks JL, et al. Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–33. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponniah S, Arah I, Alexander RB. PSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndrome. Prostate. 2000;44:49–54. doi: 10.1002/1097-0045(20000615)44:1<49::aid-pros7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–61. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 48.Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl. 2001;22:718–31. [PubMed] [Google Scholar]
- 49.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 50.Karazindiyanoglu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male. 2008;11:146–9. doi: 10.1080/13685530802290438. [DOI] [PubMed] [Google Scholar]
- 51.Kalinchenko S, Vishnevskiy EL, Koval AN, Mskhalaya GJ, Saad F. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008;11:57–61. doi: 10.1080/13685530801953994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Claudin expression in normal mouse prostate epithelium
The relative abundance of Cldn1, 3, 4, 7, 8,10, 11, 12 and 23 in the prostate epithelium of normal mice was normalized to Hprt, (Delta CT=CT of Hprt – CT of Claudins).
Supplemental Figure 2. Testosterone replacement induces Claudin expression in prostate epithelium of castrate mice.
(A) Cldn4 protein expression was restored in the prostates of castrated mice following 2 weeks of testosterone supplementation (Lower Panel) compared to the castrated mice before testosterone treatment (Upper Panel). Scale bar =20 μm, applied to all images. (B) Cldn8 protein expression was restored in the prostates of castrated mice (Lower Panel) compared to the cas-trated mice before testosterone treatment (Upper Panel). Scale bar =20 μm, applied to all images.
Supplemental Figure 3. Serum Testosterone levels of castrated and testosterone-treated mice
The average serum T level of intact control mice is 13.1 ± 3.4 ng/ml, n= 4. The serum T level became undetectable in the sera of castrated mice. At 14th day post castration, a group of cas-trated mice were embedded with T pellets, their serum T levels became 30.± 4.2 ng/ml (n=2) at day 2 post T treatment, 28.77 ±9.4 ng/ml (n=3) 1 week post T treatment; and 14.5 ± 0.69 ng/ml (n=3) 3 weeks post T supplementation treatment. Another group of castrated mice were embedded with placebo pellets at day 14 post castration, their serum T levels remained undetectable at 1 week and 3 week post implantation, n=3.