Abstract
Innate immune responses to allergens by airway epithelial cells (AECs) help initiate and propagate the adaptive immune response associated with allergic airway inflammation in asthma. Activation of the transcription factor NF-κB in AECs by allergens or secondary mediators via G-protein-coupled receptors (GPCRs) is an important component of this multifaceted inflammatory cascade. Members of the caspase recruitment domain (CARD) family of proteins display tissue specific expression and help mediate NF-κB activity in response to numerous stimuli. We have previously shown that CARMA3 is specifically expressed in AECs and mediates NF-κB activation in these cells in response to stimulation with the GPCR agonist lysophosphatidic acid (LPA). Here we demonstrate that reduced levels of CARMA3 in normal human bronchial epithelial cells decreases the production of pro-asthmatic mediators in response to a panel of asthma-relevant GPCR ligands such as LPA, adenosine tri-phosphate, and allergens that activate GPCRs such as Alternaria alternata and house dust mite. We then show that genetically modified mice with CARMA3-deficient AECs have reduced airway eosinophilia and pro-inflammatory cytokine production in a murine model of allergic airway inflammation. In addition, we demonstrate that these mice have impaired dendritic cell maturation in the lung and that dendritic cells from mice with CARMA3-deficient AECs have impaired antigen processing. In conclusion, we show that AEC CARMA3 helps mediate allergic airway inflammation, and that CARMA3 is a critical signaling molecule bridging the innate and adaptive immune responses in the lung.
Keywords: airway epithelial cells, asthma, cytokines, CARMA3, CARD10, NF-κB
Introduction
Asthma is a syndrome broadly defined by inflammation of the airways associated with airway hyper-responsiveness (AHR) and mucus hypersecretion (1). In most cases, the airway inflammation characteristic of asthma results from an allergic-type reaction to an inhaled substance from the environment. In response to allergen exposure, the airways develop a predominantly eosinophilic inflammation with prominent edema and mucus production. One of the earliest steps in the establishment of allergic sensitization is the generation of an antigen specific T cell response, which results from engagement of T cells by antigen presenting dendritic cells (DCs) (2). A network of DCs reside beneath the epithelium in the airway mucosa where they can survey the airway for invading pathogens and inhaled antigens (3, 4). When appropriately stimulated, these DCs will mature and present antigen with other secondary activating signals to T cells (5).
It is thought that adjuvant signals from airway epithelial cells (AECs), generated in response to inhaled stimuli, influence the migration and maturation state of DCs and T cells, and help determine whether a particular allergen will trigger a Th2-type inflammatory response (3, 6–9). In particular, the production of thymic stromal lymphopoietin (TSLP), granulocyte-macrophage colony-stimulating factor (GM-CSF) and the chemokine CCL20/MIP-3α by epithelial cells is critical for maturation of airway DCs and for the homing of DCs and T cells to the airways (10–19). Consistent with this, both TSLP and GM-CSF are upregulated in the airways of asthmatics and in response to numerous stimuli known to induce allergic airway inflammation (12, 20–24). In addition, the production of chemokines and other inflammatory mediators by AECs in response to these stimuli likely augment both the innate and adaptive immune responses (25–27). These data suggest that AEC production of TSLP, GM-CSF, and CCL20/MIP-3α is likely a critical mechanism for the establishment of allergic airway inflammation, and that understanding the mechanisms that regulate their production in AECs may provide novel insight into the nature of the interaction between innate and adaptive immunity in asthma.
The transcription factor NF-κB regulates TSLP, GM-CSF, and CCL20/MIP-3α expression (23, 28–31) and, therefore, is an ideal therapeutic target for inhibiting the production of these important cytokines. Previous research has also demonstrated that NF-κB is involved in multiple other aspects of asthma pathogenesis including cytokine and mucin production from epithelial cells (32–37), epithelial cell barrier function (38), and airway remodeling (39). Furthermore, NF-κB is activated in airway epithelium in response to numerous asthma relevant stimuli (27, 28, 33–36, 40–44). These data suggest a critical role for the NF-κB pathway in AECs during the development of allergic inflammation.
Many of the molecular scaffolds that organize and facilitate NF-κB activation downstream of plasma membrane receptor signaling contain caspase recruitment domain (CARD) sequences that facilitate protein-protein interactions (45, 46). To investigate the role of CARD proteins in NF-κB signaling in AECs, we performed a functional screen and identified a specific role for caspase recruitment domain-containing membrane-associated guanylate kinase protein-3 (CARMA3) (47). The CARMA proteins are a group of 3 proteins that contain a CARD, a coiled-coil domain, a linker, a PDZ domain, a SH3 domain and a C-terminal membrane-associated guanylate kinase domain (MAGUK) (48). These proteins, known as CARMA1, 2, and 3 (also as CARD11, CARD14, and CARD10, respectively) function as molecular scaffolds for the assembly of multi-protein complexes involved in the activation of NF-κB. CARMA3 is expressed in a wide range of non-hematopoietic cells, including cells in the heart, lung, liver and kidney (49, 50), and has been linked to NF-κB activation through its interactions with Bcl10, MALT1, and NEMO/IKKγ (51, 52). Previous work has demonstrated that CARMA3 mediates pro-inflammatory NF-κB activation in response to G protein-coupled receptor (GPCR) activation in parenchymal cells (47, 53–55). Furthermore, our laboratory has demonstrated that CARMA3 is robustly expressed in AECs and is necessary for production of TSLP and CCL20/MIP-3α in response to lysophosphatidic acid (LPA), a GPCR ligand elevated in the lungs of asthmatics (47, 56). However, the specific role of AEC CARMA3 signaling in inflammatory diseases such as asthma has not been investigated.
Materials and Methods
Reagents
The antibody to CARMA3 was purchased from Abcam (Cambridge, MA). A non-hydrolyzable form of adenosine tri-phosphate (ATP; ATPγS) was purchased from Sigma (Sigma-Aldrich, St. Louis, MO). LPA was purchased from Avanti Polar Lipids (Alabaster, AL) and prepared according to the manufacturer’s instructions. Alternaria alternata and house dust mite (HDM) were purchased from Greer (Greer Laboratories, NC).
Mice
We generated a CARMA3 targeting construct that contained exons 1–3 flanked by loxp sites and a Flippase Recognition Target (FRT) flanked neomycin cassette. The construct was transfected into C57BL/6N x 129SvEv hybrid embryonic stem (ES) cells by inGenious Targeting Laboratory, Inc (Stony Brook, New York). The ES cells were then utilized to generate knock-in mice with germ-line transmission of this altered CARMA3 allele (CARMA3FN/+). These mice were crossed with Actin-Flippase (FLP)-recombinase mice to delete the FRT flanked Neo cassette to generate CARMA3F/+ mice. We then backcrossed the mice to C57BL/6 mice for two generations and crossed these mice to mice that express Cre recombinase driven by the surfactant protein C promoter (SPCCre mice, obtained from Dr. Brigid Hogan, Duke University) to generate SPCCre/CARMA3F/+ mice (57, 58). SPCCre mice have been shown to result in deletion of floxed genes throughout the tracheal epithelium, the bronchiolar epithelium, and in a subset of distal alveolar cells (59, 60). We then crossed these mice to generate SPCCre/CARMA3F/F, SPCCre/CARMA3+/+, and CARMA3F/F mice for experiments. SPCCre/CARMA3F/F mice were born in the predicted Mendelian distribution and were viable and fertile. Transgenic mice that express a T cell receptor specific for chicken ovalbumin 323–339 in the context of I-Ab (OT-II mice) were purchased from Jackson Labs (Bar Harbor, ME). Mice were used at 6–8 weeks of age and were sex matched for all experiments.
Asthma Models
Acute allergic airway inflammation using ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO) was induced in mice as previously described (61). Briefly, mice were immunized with two intraperitoneal (i.p.) injections of 10 μg of OVA bound to 1 mg of alum (Sigma-Aldrich) in 0.5 ml PBS on Days 1 and 7. Starting on Day 14, mice were challenged by aerosol nebulization with 10 mg/ml OVA in PBS or PBS alone (control mice) for 20 minutes daily for 3 days. For DQ™-OVA (Molecular Probes/Invitrogen, Carlsbad, CA) experiments, mice were immunized with either no or one i.p. injection of 10 μg of chicken OVA bound to 1 mg of alum in 0.5 ml PBS on Day 1. On Day 7, 40 μg of DQ-OVA was administered to the mice via intratracheal (i.t.) injection. To assess OT-II cell proliferation, SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice were immunized with two i.p. injections of 10 μg of OVA bound to 1 mg of alum in 0.5 ml PBS on Days 1 and 7. On Days 14 and 15, mice were challenged by aerosol nebulization with 10 mg/ml OVA in PBS for 20 minutes daily. On Day 16, single cell suspensions of thoracic lymph nodes (TLNs) were incubated for 72 hours at 37°C with CFSE-labeled Thy1.1 OT-II CD4 cells. The percentage of divided OT-II cells (Thy1.1+/CFSElow) was measured by flow cytometry. Allergic airway inflammation was induced with HDM as previously described (62). Briefly, 25 μg of HDM in 25 μl of PBS was administered intranasally three times a week for five weeks. For Alexa-488-labeled HDM experiments, mice received 25 μg of HDM three times a week for one week and a fourth dose of 25 μg of Alexa-488-labeled HDM. HDM was labeled with Alexa Fluor 488 Protein Labeling Kit (Invitrogen, Carlsbad, CA). For all in vivo experiments, mice were harvested for analysis 24 hours after the last inhalation.
Mouse Harvest and Analysis
Bronchoalveolar lavage (BAL) and harvest of the lungs and thoracic lymph nodes (TLNs) were performed as previously described (61). Differential cell counts were obtained from BAL fluid after spinning 1.5 × 105 cells onto slides and staining with Hema-3 (Fisher Scientific, Pittsburgh, PA). Differential counts were performed on at least 200 cells per slide. Cells were also analyzed by flow cytometry as described below. Single-cell suspensions of TLNs were prepared. The lungs were flushed free of blood by slowly injecting 10 ml of PBS into the right ventricle before excision. The superior right upper lobe of the lung was collected for RNA analysis with total RNA isolated using Trizol (Invitrogen). The left lung was inflation fixed with 10% buffered formalin for histological analysis and stained with Hematoxylin and eosin (H&E). The remaining lung lobes were removed, minced with scissors, and then digested for 45 min in RPMI 1640 with 0.28 Wunsh U/ml Liberase (Roche Applied Science, Indianapolis, IN) and DNase 30 U/ml (Sigma-Aldrich, St. Louis, MO) at 37°C to extract leukocytes from lung tissue. The digested tissues were then strained through a 70-μm filter before RBC lysis. Samples were blocked with purified CD16/CD32 mAb (BD Biosciences, San Diego, CA) and then stained with fluorescently labeled Abs to CD4, CD8, CD69, CD11c, CD11b, MHCII (I-A), Gr-1/Ly6G, CD80, CD86, OX40L (CD252) and CCR7 (CD197) (R&D Systems, Minneapolis, MN). Flow cytometry was performed on an Accuri C6 or a BD LSR II analytical flow cytometers (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Treestar, Ashland, OR).
Lung tissue homogenization
Snap frozen lung tissue samples were homogenized at 50 mg tissue/ml in HBSS (Invitrogen, Paisley, UK) containing a protease inhibitor cocktail (Roche Diagnostics, Lewes, UK). Samples were them centrifuged (1600 rpm (135 g), 20 minutes) and supernatant collected and stored at −80°C.
Immunohistochemistry
Multiple paraffin-embedded 5 μm sections of the entire mouse lung were prepared. Lung sections were dewaxed in xylene, hydrated, and incubated in 5% normal horse serum to pre-absorb nonspecific immunoglobulin binding sites. The section was flooded with a rabbit polyclonal primary antibody to CARMA3 (1:300; Abcam) and incubated in a humid chamber overnight, followed by a biotinylated goat anti-rabbit secondary antibody (1:200 dilution; Jackson Immunoresearch, West Grove, PA) for 1 hour and then 1:1,000 streptavidin-HRP(Molecular Probes/Invitrogen, Carlsbad, CA) for 1 hour.
Immunofluorescence and Microscopy
Paraffin sections were used and processed as indicated above. Once the paraffin was removed and the tissues were hydrated, antigen retrieval was performed on a pressure cooker for 2 hours using citrate buffer. Tissues were blocked using 1% BSA in PBS-0.1% Triton for 1 hour at room temperature and incubated with primary antibodies diluted in blocking solution overnight at 4°C. After washing, the sections were incubated with secondary antibodies diluted in 1% BSA in PBS-0.1% Triton for 1 hour at room temperature and then washed and counterstained with DAPI. The primary antibodies used were: rabbit anti-CARMA3 (1:100; ab36839, abcam) and rat anti-E-cadherin (ECCD-2) (1:100; 13-1900, Life Technologies). All secondary antibodies were Alexa Fluor conjugates (488 or 594) diluted 1:500 (Invitrogen-Life technologies). Images were obtained using an Olympus IX81 Inverted microscope (Olympus, Center Valley, PA). Representative images are shown.
Epithelial cell isolation
Airway epithelial cells from the lung were dissociated using papain solution and incubated at 37 °C for 2 h. After incubation, dissociated tissues were passed through a cell strainer and centrifuged and pelleted at 500g for 5 min. Cell pellets were dispersed and incubated with Ovo-mucoid protease inhibitor (Worthington Biochemical Corporation) to inactivate residual papain activity by incubating on a rocker at 4 °C for 20 min. Cells were then pelleted and stained with EPCAM–PECy7 (1:50; 25-5791-80, eBioscience) or EPCAM–APC (1:50; 17-5791, eBioscience), GSIβ4 (Griffonia Simplicifolia isolectin beta 4)-Biotin (L2120, Sigma), SSEA-1 eFluor 650NC (1:75, 95-8813-41, eBioscience) and PE anti-mouse CD24 (1:100, 553262, BD Pharmingen) for 30 min in 2.5% FBS in PBS on ice. After washing, cells were sorted on a BD FACS Aria (BD Biosciences) using FACS Diva software.
Epithelial Cell Culture
Mouse tracheal epithelial cells (MTECs) were cultured using a published protocol (63). Briefly, tracheas were removed and digested overnight with pronase (Roche Applied Science, IN). The released cells were collected and then further selected by removing cells that adhered to a culture dish. The cells were then plated onto collagen-coated Transwells (Fisher Scientific, Pittsburgh, PA) and allowed to grow in media supplemented with epidermal growth factor and retinoic acid as described previously (63). After 5 to 7 days, an air–liquid interface (ALI) was created and the cells were allowed to grow for an additional 7 to 10 days. Purity of the culture was determined by the ability to maintain an ALI, the presence of beating cilia, and expression of the AEC transcription factor (TTF-1).
Epithelial Cell Stimulation and CARMA3 knockdown
We have utilized a technique for knockdown of CARMA3 via lentiviral shRNA infection of normal human bronchial epithelial (NHBE) cells, allowing stable knockdown of CARMA3 in cells cultured on an ALI (64). For these experiments, NHBE cells (Lonza, Basel, Switzerland), were cultured in T-75 flasks to 75% confluence in B-ALI™ Growth Medium (Lonza) and then infected by adding 10 ml of packaged CARMA3 shRNA lentivirus or a scrambled shRNA lentivirus with protamine sulphate (5 μg/ml) at a multiplicity of infection (MOI) of 0.25. DNA lentiviral constructs containing shRNA against human CARMA3 (TRCN107248) or the scrambled shRNA were obtained from the RNAi consortium (TRC, http://www.broadinstitute.org/rnai/public/) supply at the Broad Institute in Cambridge, MA (47). The shRNAs are cloned into the pLKO.1 vector with a puromycin resistance gene. Two days after infection, the cells were cultured for 1 day in puromycin (1, 2, or 5 μg/ml) to select out uninfected cells. The cells were then re-plated onto collagen coated 24-well ALI inserts (Costar) with a seeding density of 5×104 in B-ALI™ Growth Medium (Lonza) according to the manufacturer’s instructions. After the cells were confluent on the insert (about 7 to 10 days), the media above the insert was removed to form an ALI, using B-ALI™ Differentiation Media (Lonza) according to manufacturer’s instructions. After 7 more days the cells were used for experiments. For stimulation, normal culture media was replaced with low serum (1%) media for 24 hours. The cells were then stimulated with LPA (10 μM), Alternaria (100 μg/ml), ATP (100 μM) or HDM (100 μg/ml) for 6 hr for RNA extraction.
Quantitative PCR
RNA from stimulated cells and lung lobes was isolated using Trizol (Invitrogen, Carlsbad, CA) and a commercial kit (RNeasy, Qiagen, Valencia, CA). cDNA was prepared using the iScriptcDNA Synthesis Kit (Bio-Rad, Hercules, CA). GPCR expression was determined via the TaqMan® Array Mouse GPCR Panel from Life Technologies (Grand Island, NY). Gene expression was quantified on an iQ5 RT PCR Detection System (Bio-Rad) using SYBR green (iQ SYBR Green Supermix; Bio-Rad) according to the manufacturer’s suggested protocol. All values are shown normalized to GAPDH values. Samples were assayed in duplicate. Primer sequences used were selected using the MGH PrimerBank (pga.mgh.harvard.edu/primerbank/).
Protein Quantification
Supernatants from BAL and lung homogenates were collected and then used undiluted in commercial ELISA kits for KC/mCXCL1, CCL20/MIP-3α, TSLP, GM-CSF, CCL11/eotaxin-1, IL-4, IL-5 and IL-13 (R&D Systems) according to the manufacturer’s protocol.
Measurement of Lung Function in Mice
Lung function measurements were performed using the Flexivent system (Scireq, Montreal, Quebec, Canada), as described before (65). Briefly, mice were anesthetized with an i.p. injection of xylazine (12 mg/kg) and pentobarbital (70 mg/kg). The trachea of anesthetized mice was cannulated and the mice were ventilated with 6 ml/kg tidal volume at 150 breaths per minute. To suppress spontaneous breathing during measurement of lung function, mice were i.p. injected with pancuronium bromide (2 mg/kg). Incremental doses of nebulized methacholine were used to determine total lung resistance (Rn) and compliance (C) according to the Snapshot-150 perturbation provided by the Flexivent equipment. Thirteen data points were collected for each methacholine dose, and only data with a coefficient of determination greater than 0.95 were included in analyses. The survival of mice during the procedure was simultaneously monitored using electrocardiograms.
Data Analysis
Data are expressed as mean ± SEM. Differences between means were tested for statistical significance using unpaired t tests as appropriate to the experiment. For multiple comparisons, a two-way ANOVA test was used for lung function analysis. From such comparisons, differences yielding P < 0.05 were judged to be significant.
Study Approval
All protocols were approved by the Massachusetts General Hospital (MGH) Subcommittee on Research and Animal Care.
Results
AECs express asthma relevant GPCRs
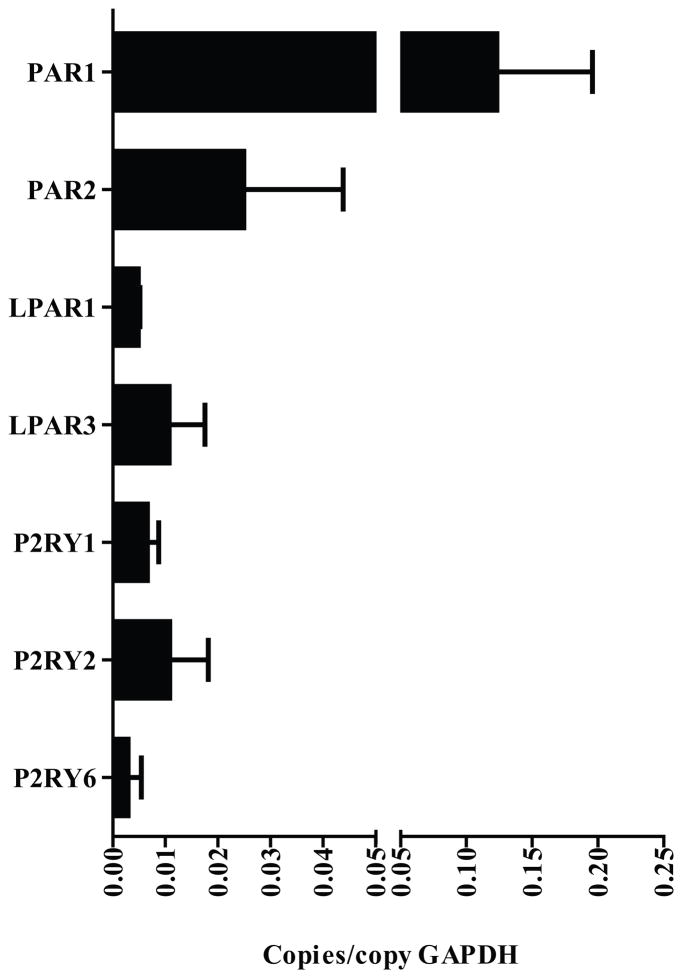
Previous work has demonstrated that CARMA3 is highly expressed in AECs and mediates activation of NF-κB in response to GPCR engagement (47). In order to characterize the profile of GPCRs expressed in AECs, we used a qPCR array to measure the baseline RNA expression of 380 GPCRs in MTECs cultured on an ALI. Multiple GPCRs were identified that are expressed at moderate (0.002 – 0.01 copies/copy GAPDH) or high (>0.01 copies/copy GAPDH) levels at baseline (Supplementary Figure 1A and B). In this list, there were several groups of potentially asthma-relevant pro-inflammatory receptors that can activate NF-κB, including the protease activated receptors (PAR1 and 2), LPA receptors (LPAR1 and 3) and purinergic receptors (P2Y1, 2 and 6) that were expressed at both moderate and high levels (Figure 1).
Figure 1. Asthma-relevant GPCR profile of mouse tracheal epithelial cells.
RNA was isolated from naïve, unstimulated mouse tracheal epithelial cells and the expression profile of a panel of 380 GPCRs was measured using a real-time qPCR mini-array. Shown are an asthma-relevant subset from 67 medium and high abundance GPCRs detected. GAPDH was used to normalize the values of GPCR genes tested. PAR1 and 2 - protease activated receptors 1 and 2; LPAR1 and 3 - LPA receptors 1 and 3 and P2Y1, 2 and 6 - purinergic receptors 1, 2 and 6.
Knockdown of CARMA3 in AECs decreases cytokine production in response to GPCR ligands
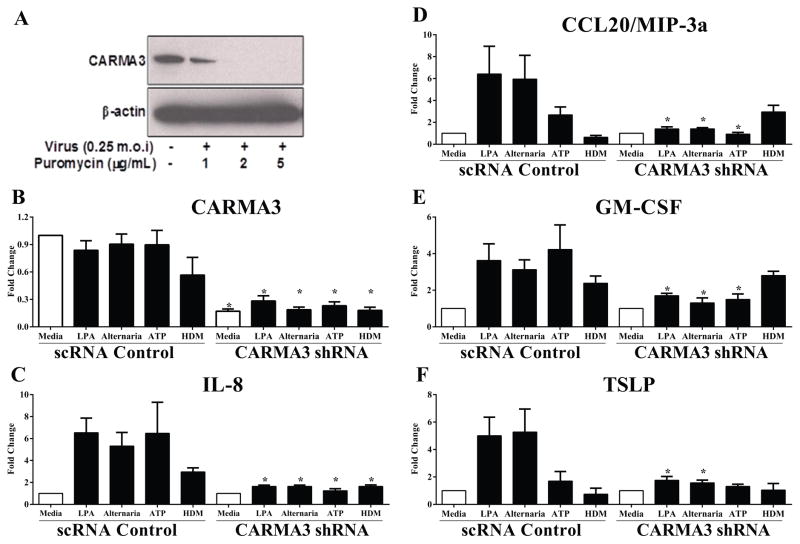
In previous work, it was demonstrated that LPA stimulation of NHBE cells in culture induced expression of TSLP and CCL20/MIP-3α and that the expression was dependent on CARMA3 (47). For those studies, NHBE cells were transfected with plasmids expressing shRNA against CARMA3 to knockdown its expression, but the technique we used only resulted in transient knockdown and was not effective for epithelial cells cultured on an ALI. In order to induce stable knockdown of CARMA3 expression in AECs cultured on an ALI, we utilized lentiviral shRNA infection of NHBE cells for knockdown of CARMA3. Analysis of CARMA3 levels demonstrated effective knockdown of CARMA3 protein (Figure 2A) and RNA (Figure 2B). NHBEs were then stimulated with either LPA (10 μM), Alternaria (100 μg/ml), ATP (100 μM) or HDM (100 μg/ml). LPA- and Alternaria-induced expression of IL-8, CCL20/MIP-3α, GM-CSF and TSLP was attenuated in NHBE cells with CARMA3 knockdown compared to cells infected with a lentivirus containing a non-targeting scrambled shRNA sequence (scRNA) (Figure 2C – F). In addition, knockdown of CARMA3 signaling abolished both ATP- and HDM-induced changes in gene expression in NHBE cells (Figure 2C – F). Together, these results suggest that CARMA3 mediates pro-inflammatory cytokine and chemokine production downstream of multiple different GPCRs.
Figure 2. CARMA3 mediates pro-inflammatory cytokine release from normal human bronchial epithelial cells in response to GPCR stimulation.
(A) A lentivirus containing a shRNA against CARMA3 and a puromycin resistance cassette was used to infect NHBE cells (0.25 m.o.i). The infected cells were selected for puromycin resistance for 5 days and then Western immunoblotting was used to detect CARMA3. (B – F) NHBEs were grown on an ALI after infection with lentivirus containing a non-targeting shRNA (scRNA) or a CARMA3-targetting shRNA. These cells were then stimulated with either 10 μM LPA, 100 μg/ml Alternaria, 100 μM ATP or 100 μg/ml HDM for 6 hr. RNA was then isolated from the cells and the levels of (B) CARMA3, (C) CXCL8/IL-8, (D) GM-CSF, (E) CCL20/MIP-3α and (F) TSLP were determined. GAPDH levels were used to normalize the values of genes tested. Data is expressed as fold change from media. Values are the mean of 3–6 samples ± SEM. *P<0.05 versus scRNA cells by unpaired t test. This experiment was repeated two times. ND - not detected.
Generation of mice deficient in AEC-CARMA3 signaling
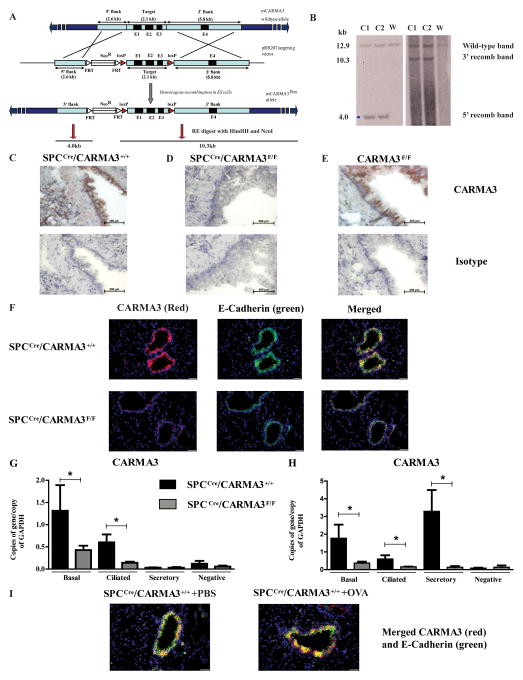
In order to study the role of CARMA3 in AECs in vivo, we generated mice capable of cell-specific deletion of CARMA3. A targeting construct with loxp sites flanking exons 1–3 was generated (Figure 3A and B) and utilized to make CARMA3F/F mice. These mice were then crossed to mice that express Cre recombinase under control of the surfactant protein C promoter (SPCCre) (60) to generate SPCCre/CARMA3F/F mice. Although SPC is predominantly expressed by alveolar type II epithelial cells in adult mice, it is expressed in early endoderm during embryogenesis, leading to Cre-mediated deletion of floxed genes in all respiratory epithelium (59). SPCCre/CARMA3+/+ and CARMA3F/F mice had prominent staining for CARMA3 in the airway epithelium (Figure 3C and E). However, lungs from SPCCre/CARMA3F/F mice had minimal staining in AECs (Figure 3D). These findings were verified by immunofluorescence with co-staining for E-cadherin for airway epithelium. In these experiments, lung sections demonstrated intense CARMA3 staining in the airway epithelium of SPCCre/CARMA3+/+ mice compared to SPCCre/CARMA3F/F mice (Figure 3F). In order to isolate individual AECs, we have developed a protocol to dissociate, isolate and sort epithelial cell subsets from mouse lungs (66). We isolated pure populations of EpCAM+/GSIβ4+ basal cells, EpCAM+/GSIβ4−/SSEA1+ secretory cells and EpCAM+/GSIβ4−/CD24+ ciliated cells from dissociated lung. Basal cells displayed the cell markers p63 and CK5 when stained on a glass slide, secretory cells are CC10 (Scgb1a1)+ and Scgb3a2+, and ciliated cells were verified by expression of acetylated tubulin and FoxJ1 (data not shown). Using exclusion strategies, we sorted each AEC subtype to 98% purity. In AECs that were sorted from the lungs of naive SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice, qPCR showed that in SPCCre/CARMA3+/+ mice, CARMA3 is expressed in all subtypes but expression is higher in the basal and ciliated cells compared to secretory cells (Fig. 3G). As expected, there was a decrease in CARMA3 RNA expression in basal and ciliated cells in the floxed mice (Figure 3G) consistent with deletion of the CARMA3 gene. We also measured CARMA3 RNA levels in epithelial cell subtypes isolated from SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice after OVA/Alum i.p. injection and a single aerosol administration of OVA. Following OVA inhalation, the CARMA3 levels were comparable in basal and ciliated cells; however, there was upregulation of CARMA3 levels in the secretory cells from SPCCre/CARMA3+/+ mice but not SPCCre/CARMA3F/F mice (Figure 3H). Consistent with this, immunofluorescent staining of lung slices from SPCCre/CARMA3+/+ mice following OVA immunization and challenge demonstrated increased staining for CARMA3 in the airway epithelium (Figure 3I).
Figure 3. Generation of SPCCre/CARMA3F/F mice.
(A) Generation of CARMA3 targeting construct. (B) Southern blot analysis to identify correctly targeted ES cell clones obtained from ES cells electroporated with the targeting construct. Two ES cell clones (C1 and C2) were identified in which there was the expected 5′ and 3′ recombinations. ‘W’ indicates wild-type mouse. Immunohistochemistry of lungs from naive (C) SPCCre/CARMA3+/+, (D) SPCCre/CARMA3F/F and (E) CARMA3F/F mice stained with an antibody against CARMA3 (top panels) or an isotype control antibody (bottom panels). Scale bar = 200 μm. (F) Immunofluorescence of lungs from naive SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice stained with an antibodies against CARMA3 and E-Cadherin. All images were taken using the same exposure, scale bar = 20 μm. Basal, ciliated and secretory cells sorted from the lungs of SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice that were either (G) naïve or (H) received one OVA/Alum immunization and one OVA challenge. RNA levels of CARMA3 were determined by qPCR. Data are means ± SEMs of 6 mice per group. *P<0.05 (SPCCre/CARMA3+/+ compared to SPCCre/CARMA3F/F). (I) Immunofluorescence of lungs from PBS and OVA immunized and challenged SPCCre/CARMA3+/+ mice stained for CARMA3. Scale bar = 20 μm.
Deletion of CARMA3 in AECs attenuates allergic airway inflammation in a murine model of asthma
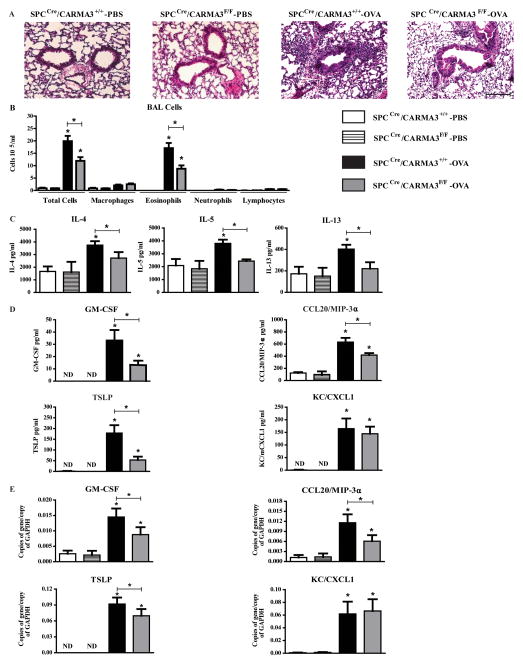
SPCCre/CARMA3F/F, SPCCre/CARMA3+/+ and CARMA3F/F littermate control mice were immunized and challenged with OVA and then analyzed for airway inflammation. H&E stained lung sections showed less airway inflammation in the SPCCre/CARMA3F/F mice compared to the CARMA3-sufficient mice (Figure 4A). SPCCre/CARMA3+/+ and CARMA3F/F showed identical immune responses to OVA and were therefore grouped together as SPCCre/CARMA3+/+ throughout the rest of this manuscript for simplicity. BAL total cell counts and eosinophil counts were reduced in SPCCre/CARMA3F/F mice compared to control mice (Figure 4B). In addition, protein levels of IL-4, IL-5, and IL-13 were all found to be reduced in the lung tissue of SPCCre/CARMA3F/F mice compared to control mice (Figure 4B). Consistent with the data from NHBE cells, the protein levels of GM-CSF, CCL20/MIP-3α and TSLP in BAL were reduced in SPCCre/CARMA3F/F mice (Figure 4D). However, there was no change in the levels of CXCL1/KC or CCL11/eotaxin-1 between the OVA immunized and challenged mice (Figure 4D and data not shown). There was also decreased RNA expression of TSLP and GM-CSF, and CCL20/MIP-3α in the lungs of OVA immunized and challenged SPCCre/CARMA3F/F mice compared to control mice (Figure 4E). Lung RNA levels of a panel of chemokines did not differ (data not shown). These data demonstrate that CARMA3 expression in AECs has an important role in the development of allergic airway inflammation in vivo.
Figure 4. Attenuation of airway inflammation in OVA immunized and challenged SPCCre/CARMA3F/F mice.
(A) Histopathologic analysis of lung sections stained with hematoxylin and eosin (H&E) from SPCCre/CARMA3+/+-PBS, SPCCre/CARMA3F/F-PBS, SPCCre/CARMA3+/+-OVA and SPCCre/CARMA3F/F-OVA treated mice. Scale bar = 50 μm. (B) Total cells, macrophages, eosinophils, neutrophils and lymphocytes were enumerated in BAL fluid. (C)Protein levels of IL-4, IL-5, and IL-13 in the lung quantified by ELISA. (D) Protein levels of GM-CSF, CCL20/MIP-3α, TSLP and CXCL1/KC in the BAL quantified by ELISA. (E) RNA levels of GM-CSF, CCL20/MIP-3α, TSLP and CXCL1/KC in the lung quantified by qPCR. Data are means ± SEMs of 8 mice per group from 2 experiments. *P<0.05 (OVA treatment compared to same genotype that was treated with PBS or SPCCre/CARMA3+/+ OVA compared to SPCCre/CARMA3F/F OVA). ND - not detected.
Deletion of CARMA3 does not attenuate AHR
We assessed whether deletion of CARMA3 expression in AECs would also attenuate AHR. Using mechanically ventilated mice, airway resistance (Rn) and airway compliance (C) were calculated from data obtained with a forced oscillation technique as described previously (65). OVA challenge of both SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice resulted in significant AHR to inhaled methacholine when compared with PBS-challenged mice, however, there was no difference in AHR between the genotypes (Supplementary Figure 2A–D).
Deletion of CARMA3 in AECs leads to impaired lung DC recruitment, maturation and antigen processing
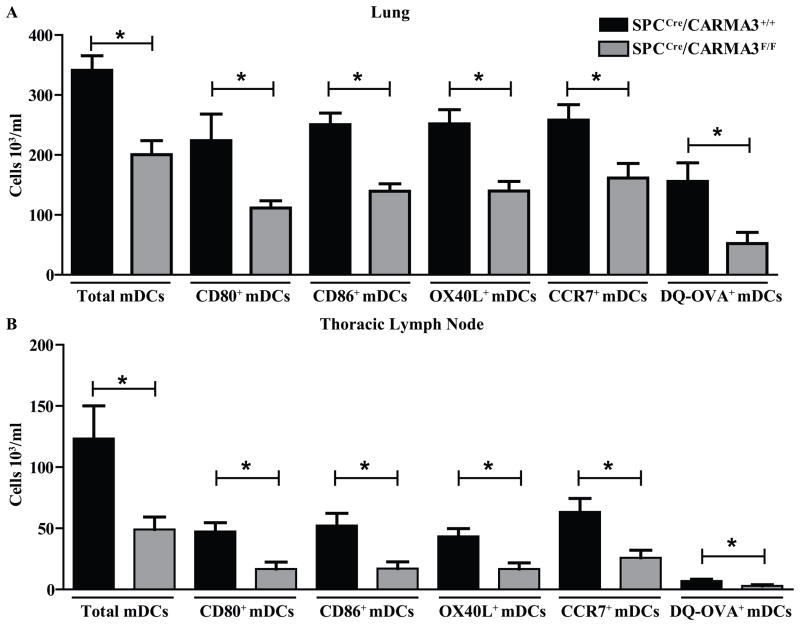
The reduced expression of TSLP and GM-CSF by SPCCre/CARMA3F/F mice suggests that these mice may have impaired lung DC maturation in response to allergens. In addition, reduced CCL20/MIP-3α levels may affect DC recruitment to the lung. To assess this, SPCCre/CARMA3F/F mice and SPCCre/CARMA3+/+ littermate control mice were sensitized to OVA and then challenged i.t. with a fluorescently conjugated form of OVA (DQ™-OVA) that emits green fluorescence when the protein is cleaved after cellular uptake. We then assessed DC migration and antigen processing in the lung and lung draining TLNs. Following OVA sensitization and DQ-OVA challenge, SPCCre/CARMA3F/F mice had lower numbers of myeloid DCs (mDCs) isolated from the lung and TLNs (Figure 5A and B). Myeloid DCs were identified as CD11c+CD11b+MHCII+Gr-1− (representative flow plots are shown in Supplementary Figure 3). In addition, lung and TLN from SPCCre/CARMA3F/F mice had fewer myeloid DCs expressing the maturation markers CD80, CD86, OX40L, the chemokine receptor CCR7 and fluorescent DQ-OVA compared to control mice (Figure 5A and B), suggesting that DC maturation, migration, and antigen processing ability of DCs is hampered in mice lacking CARMA3 in AECs. However, there was no difference in baseline myeloid DC numbers or maturation marker expression in naïve mice (Supplementary Figure 4).
Figure 5. DCs isolated from SPCCre/CARMA3F/F mice show impaired maturation, migration and antigen processing.
SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received one OVA/Alum immunization on day 0, followed by 40 μg of DQ-OVA i.t. on day 7. Twenty four hrs post DQ-OVA administration the (A) lungs and (B) thoracic lymph nodes were isolated and the number of myeloid DCs, CD80+ myeloid DCs, CD86+ myeloid DCs, OX40L+ myeloid DCs, CCR7+ myeloid DCs and DQ-OVA+ myeloid DCs were determined by flow cytometry. Data are means ± SEMs of 8 mice per group from 2 experiments. *P<0.05.
Deletion of CARMA3 in AECs impairs antigen-specific T cell proliferation
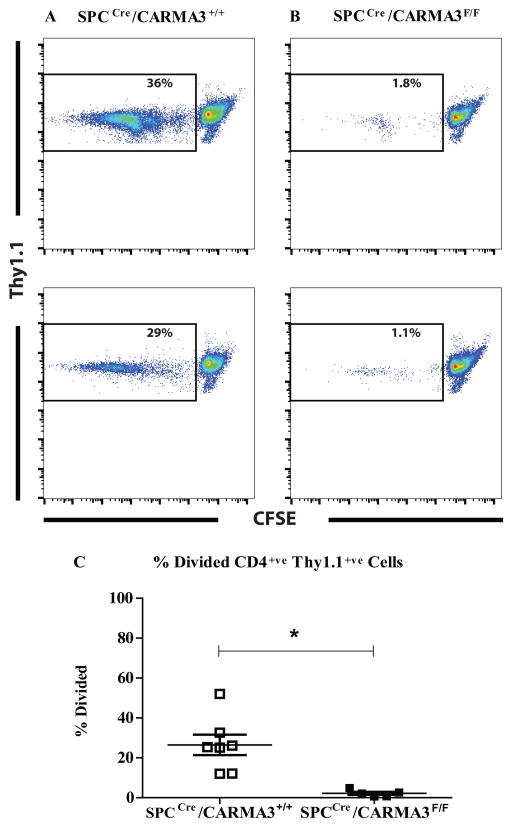
The impairment in DC maturation and antigen processing seen in SPCCre/CARMA3F/F mice should lead to impairment in antigen-specific T cell activation. In order to test this, we used a standard model of antigen-specific T cell activation (67). We sensitized SPCCre/CARMA3F/F mice and SPCCre/CARMA3+/+ littermate control mice to OVA and then challenged them with OVA on 2 consecutive days. One day after the second OVA challenge, single cell suspensions of the TLN from these mice were incubated with CFSE-labelled naïve OVA-specific CD4+ T cells isolated from Thy1.1+ OT-II mice. Prior work has demonstrated that T cell proliferation in this assay is primarily induced by migratory mDCs from the lung (67). After 72 hours, OT-II T cells stimulated with TLN cells from SPCCre/CARMA3F/F mice proliferated less than OT-II T cells stimulated with TLN cells from SPCCre/CARMA3+/+ mice (Figure 6A – C). These data demonstrate that cells from the TLN of SPCCre/CARMA3F/F mice have reduced ability to stimulate antigen-specific T cell proliferation.
Figure 6. Impaired T cell proliferation in SPCCre/CARMA3F/F mice.
SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received two OVA/Alum immunizations on days 0 and 7 followed by two aerosolized OVA challenges on days 14 and 15. On day 16 the thoracic lymph nodes were isolated and single cells suspensions were incubated with CFSE-labeled Thy1.1+ OT-II CD4+ T cells for 3 days. Representative flow cytometry plots from (A) SPCCre/CARMA3+/+ and (B) SPCCre/CARMA3F/F cultures are shown. (C) The percentage of divided OT-II cells (Thy1.1+/CFSElow) was measured. Data are means ± SEMs of 5–7 mice per group from 2 experiments. *P<0.05.
Deletion of CARMA3 in AECs attenuates allergic airway inflammation in response to house dust mite
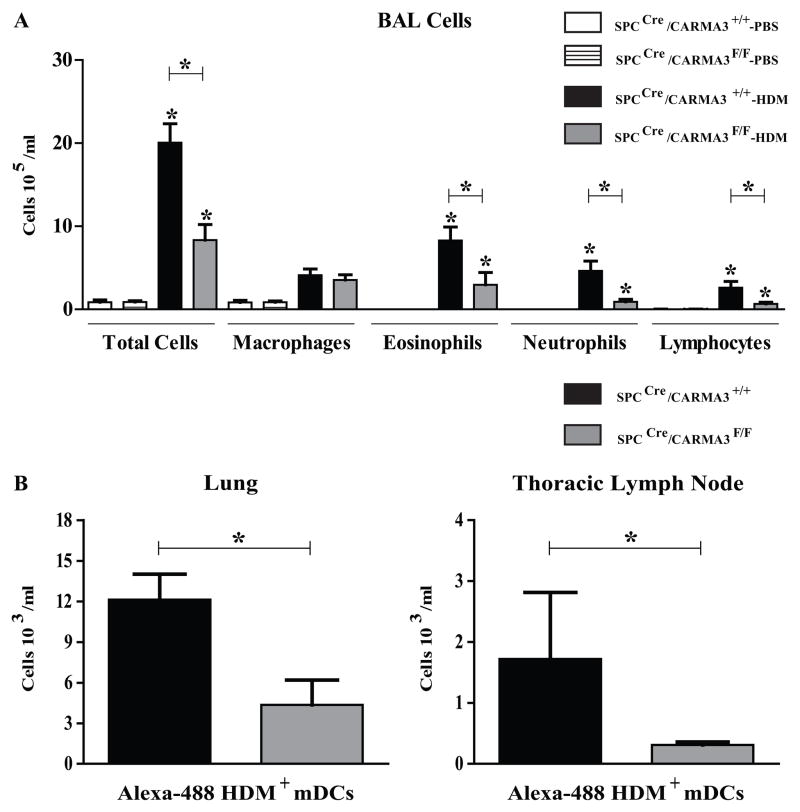
We also tested the role of AEC CARMA3 in the development of allergic airway inflammation in the HDM model of asthma. SPCCre/CARMA3F/F and SPCCre/CARMA3+/+ mice were given HDM intranasally as previously reported. As in the OVA model of asthma, there was a decrease in eosinophilic airway inflammation in SPCCre/CARMA3F/F compared to control animals (Figure 7A). In addition, when mice were given FITC-labelled HDM there were reduced numbers of FITC-labelled mDCs in the draining lymph nodes of SPCCre/CARMA3F/F compared to control animals (Figure 7B). These data confirm the results found with the OVA-model of allergic airway inflammation.
Figure 7. Attenuation of airway inflammation in HDM-treated SPCCre/CARMA3F/F mice.
(A) Total cells, macrophages, eosinophils, neutrophils and lymphocytes were enumerated in BAL fluid. Data are means ± SEMs of 6–8 mice per group from 2 experiments. *P<0.05 (HDM treatment compared to same genotype that was treated with PBS or SPCCre/CARMA3+/+ HDM compared to SPCCre/CARMA3F/F HDM). (B) SPCCre/CARMA3+/+ and SPCCre/CARMA3F/F mice received three doses of HDM followed by 25 μg of Alexa-488-labeled HDM intranasally. Twenty four hrs post Alexa-488-labeled HDM administration the lungs and thoracic lymph nodes were isolated and the number of Alexa-488-labeled HDM+ myeloid DCs were determined by flow cytometry. Data are means ± SEMs of 6–7 mice per group from 1 experiment. *P<0.05.
Discussion
CARMA3 functions as a molecular scaffold for the assembly of multi-protein complexes involved in the activation of NF-κB, a transcription factor involved in regulation of inflammation and immunity. Its role in the pathogenesis of asthma has been suggested on the basis of evidence of its activation in the bronchiolar epithelium from asthmatics (68) and from studies in mouse models of allergic airways disease (36). Indeed, a crucial role for lung epithelial NF-κB in both OVA (40) and HDM models of allergic airways disease has been described (69). Previous work has demonstrated that CARMA3 mediates pro-inflammatory NF-κB activation in response to GPCR activation in parenchymal cells (47, 53–55), and our laboratory has demonstrated that CARMA3 is robustly expressed in AECs and is necessary for production of TSLP and CCL20/MIP-3α in response to LPA, a GPCR ligand elevated in the lungs of asthmatics (47, 56). Despite these prior observations, the importance of airway epithelial CARMA3 signaling and its role in asthma has yet to be determined. Results presented here describe a critical role for AEC CARMA3 in linking the innate and adaptive immune response and promoting airway inflammation in a murine model of allergic asthma.
Results of the current study demonstrate an important relationship between GPCR activation, CARMA3 signaling and the development of airway inflammation. We show that asthma-relevant GPCRs (PAR1, PAR2, LPAR1 LPAR3, P2Y1, P2Y2 and P2Y6) are elevated in AECs and that shRNA-mediated knockdown of CARMA3 in NHBE cells greatly diminishes IL-8, CCL20/MIP-3α, TSLP and GM-CSF production in response to the respective asthma-relevant GPCR ligands (HDM, Alternaria, LPA, and ATP). In addition, we demonstrate that OVA- and HDM-driven eosinophilic airway inflammation is reduced in mice lacking CARMA3 specifically in AECs in vivo. Furthermore, pro-inflammatory cytokine production, DC maturation and migration, antigen processing and resultant T cell proliferation are impaired in mice deficient in CARMA3; however, the development of AHR is not altered. Together, these results suggest a vital role for CARMA3 in the initiation and development of airway inflammation associated with allergic asthma.
The bronchial epithelium is the first line of defense against the abundant array of particles, antigens, and infectious pathogens that are inhaled into the airways. AECs express a diverse array of GPCRs including PARS, P2Y receptors and LPA receptors (70–72), and we found that these specific receptors were elevated without stimulation in AECs, suggesting that the airway epithelium is primed to detect inflammatory mediators and inhaled pathogens. Many of the GPCRs expressed on AECs participate in the initiation and modulation of allergic lung responses via NF-κB activation (73–76) and are linked to common allergens. The expression of PAR2 by AECs allows the recognition of protease active allergens such as HDM and Alternaria, and the release of pro-inflammatory mediators from the airway epithelium has been shown to require PAR2 (74, 77, 78). Upon exposure to protease active HDM allergens, AECs release a vast array of pro-inflammatory mediators, including IL-8, GM-CSF (74, 77, 78) and TSLP (73), that attract neutrophils and DCs to the airways and induce DC maturation. The development of Alternaria-induced lung inflammation has also been shown to rely on PAR2, with IL-8, GM-CSF and TSLP being released from AECs upon Alternaria exposure (73, 79–81). Thus, proteases can activate PAR2 in the airways to generate leukocyte infiltration and to amplify the response to allergens (82–84). The P2Y receptors and the primary ligand ATP have also been linked to the innate and subsequent adaptive response in asthma (85–87). ATP is released in the airways of allergen-challenged patients and contributes to disease pathogenesis via signaling at P2Y receptors expressed at the epithelial surface (85). In addition, the bioactive phospholipid LPA is upregulated in the airways of asthmatics and can stimulate AECs to produce additional pro-inflammatory mediators (88–90). In this manuscript, we show that HDM, ATP, LPA and Alternaria stimulation of NHBEs leads to pro-inflammatory cytokine production. Importantly, we show that knockdown of CARMA3 in vitro attenuates these responses, suggesting that the CARMA3-NF-κB axis acts downstream of multiple GPCR pathways and that CARMA3 contributes to innate cytokine and chemokine production from AECs.
Thus far, in vivo models studying CARMA3 have been severely limited because permanent genetic deletion of CARMA3 in mice results in neural tube defects leading to high mortality (91). Thus, we used conditional deletion of CARMA3 in AECs to study its cell-specific role in asthma pathogenesis. Using this resource, we demonstrate here that deletion of CARMA3 from AECs in mice is sufficient to blunt the eosinophilic inflammatory response observed in the OVA- and HDM-induced models of allergic airways disease. Concomitant with the reduction in airway eosinophils was reduced levels of the Th2-cytokines IL-4, IL-5 and IL-13 in the lung in response to OVA. In addition, the reduced inflammatory response to OVA in mice with CARMA3-deficient AECs was accompanied by reduced expression of the chemokine CCL20/MIP-3α, as well as TSLP and GM-CSF, which have all been shown to be released by AECs (92). CCL20/MIP-3α is central to early DC recruitment acting via CCR6 (93, 94), and TSLP and GM-CSF can activate DCs and induce maturation, thereby promoting T cell activation and Th2 inflammation (95–99). Consistent with these data, there were lower numbers of CD80+, CD86+, OX40L+ and DQ-OVA+ DCs recovered from both the lungs and TLN of CARMA3-deficient mice. The reduced DC numbers in the TLN as well as the lower numbers of DCs expressing co-stimulatory proteins and containing processed antigen likely explains the reduced antigen-specific T cell activation (as measured by proliferation) induced by lymph node cells from CARMA3-deficient mice compared to cells from control mice. Overall, these data suggest that the reduced production of TSLP and GM-CSF in CARMA3-deficient mice likely impairs DC maturation and antigen processing which leads to a defect in T cell activation and Th2 cell development.
The presentation of processed antigens on MHC II complexes by DCs is a crucial step in T cell activation in asthma (100), and thus, our results support the notion that CARMA3 provides an essential link between the innate and adaptive immune response in airway inflammation. These observations, therefore, suggest a mechanism where epithelial GPCR activation and subsequent CARMA3 signaling and NF-κB activation is a crucial step between contact with an allergen and downstream manifestations of airway inflammation. Although our findings support our notion of the central role of airway epithelial CARMA3 signaling in allergic airway inflammation, there are likely additional signaling pathways, additional cell types, and complex interactions between them that contribute to the pathophysiology of allergic airway disease. Consistent with this, despite the significant attenuation of parameters believed to be clinically relevant to the pathophysiology of asthma, the airflow alterations that characterize AHR were not affected in SPCCre/CARMA3F/F mice. We suspect that alternative signaling pathways activated in airways or the residual inflammatory cells present in the CARMA3-deficient mouse lungs may be responsible for the observed AHR (101). Although the inflammatory state is believed to be integral to the development of AHR, it is also possible that residual inflammation and accompanying secretion of mediators observed in the SPCCre/CARMA3F/F mice are sufficient to fully drive the AHR. These results are also in agreement with others, where both OVA- and HDM-induced AHR is unaffected with AEC disruption of NF-κB signaling (40, 69). Indeed, our results are also consistent with other data demonstrating that airway inflammation in mice is at least in part uncoupled from AHR, which has been reported in murine models and human subjects (102), and that changes in allergen-induced airways physiology can occur in the absence of airway inflammation (103, 104).
While our results indicate CARMA3 contributes to allergic inflammation, the precise GPCR-agonist interaction that triggers CARMA3 and NF-κB signaling remains to be identified. One candidate is ATP acting via the P2Y family of receptors. Extracellular ATP serves as a danger signal to alert the immune system of tissue damage and allergen challenge causes accumulation of ATP in the airways of asthmatic subjects and mice with OVA-induced asthma. Indeed, all the cardinal features of asthma, including eosinophilic airway inflammation, Th2 cytokine production and AHR, were abrogated when lung ATP levels were neutralized (85). Thus ATP may act in an autocrine manner at the airway epithelium upon being released. In addition to ATP, PAR2 ligation could also be responsible for the CARMA3-NF-κB cascade. PAR2 has been shown to mediate OVA-induced inflammation and AHR (105) and this again my result from the release of as yet unidentified serine protease in response to OVA acting in an autocrine manner. The specific PAR2 agonist could be mast cell tryptase, which is elevated in the lungs of asthmatics (106–108). Proteases other than tryptase may also activate PAR2 in the airway, such as trypsin-like enzymes which have been detected in AECs (82, 109) and in airway secretions (82, 110). Finally, LPA and uric acid are released into the airway during allergen challenge and could also mediate GPCR activation of AECs in vivo (88, 111, 112).
Global targeting of NF-κB is not a viable therapeutic option as total inhibition of NF-κB activity would interrupt vital physiological processes important for tissue and immune homeostasis. However, these studies suggest that targeting the NF-κB in a cell- and pathway-specific manner could be beneficial in treating asthma. Consistent with this, selectively inhibiting NF-κB in endothelial cells in a murine models of peritonitis and rheumatoid arthritis has been shown to ameliorate both disease courses (113). These data together with the data in this manuscript, suggest that the NF-κB pathway can be selectively inhibited in a cell type- and activation stage-dependent manner (113).
It is important to note that the model used for these experiments tests the importance of CARMA3 in AECs for allergen sensitization and challenge. In fact, our findings suggest that sensitization to allergens is profoundly impaired with CARMA3 deletion in AECs. Thus, it is not clear whether CARMA3 inhibition in AECs following sensitization would be as effective in reducing allergic airway inflammation. However, TSLP, GM-CSF and CCL20/MIP-3α are likely important for exacerbations of allergic inflammation after sensitization given their roles in DC maturation, antigen presentation, and recruitment, so we think it is also likely that CARMA3 will have a role in asthma pathogenesis after initial sensitization. Recent data demonstrating that antibody neutralization of TSLP has a therapeutic effect in human asthma support this premise (114). In future experiments, we can use inducible CARMA3 deletion after the initial sensitization step to better determine its role in exacerbations.
In summary, this study demonstrates that CARMA3 signaling in AECs drives allergic airway inflammation and could be a significant contributor to the generation of the adaptive immune responses associated with asthma. These data suggest that CARMA3 could therefore be a promising therapeutic target to reduce airway inflammation in asthma.
Supplementary Material
Acknowledgments
We would like to thank Dr. Dan Schumperlin and Dr. Jin-Ah Park for the MTEC RNA samples and Dr. Marciela DeGrace for technical help.
This work was supported by grants from the National Institutes of Health R01 HL088297 (to B.D. Medoff and R.J. Xavier), T32 HL07874 (to B.D. Medoff), and an Massachusetts General Hospital Executive Committee on Research Interim Support Award to BDM.
Abbreviations used in this article
- AEC
airway epithelial cell
- AHR
airway hyperreactivity
- ALI
air-liquid interface
- BAL
bronchoalveolar lavage
- CARD
caspase recruitment domain
- CARMA
caspase recruitment domain-containing membrane-associated guanylate kinase protein
- DC
dendritic cell
- GPCR
g-protein coupled receptor
- HDM
house dust mite
- i.t
intra-tracheal
- LPA
lysophosphatidic acid
- MTEC
murine tracheal epithelial cell
- NHBE
normal human bronchial epithelial cell
- qPCR
quantitative PCR
- SPC
surfactant protein C
- SPCCre
cre recombinase driven by SPC promoter
- TLN
thoracic lymph node
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 3.Huh JC, Strickland DH, Jahnsen FL, Turner DJ, Thomas JA, Napoli S, Tobagus I, Stumbles PA, Sly PD, Holt PG. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med. 2003;198:19–30. doi: 10.1084/jem.20021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt PG, Schon-Hegrad MA, Oliver J, Holt BJ, McMenamin PG. A contiguous network of dendritic antigen-presenting cells within the respiratory epithelium. Int Arch Allergy Appl Immunol. 1990;91:155–159. doi: 10.1159/000235107. [DOI] [PubMed] [Google Scholar]
- 5.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 6.Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr. 2004;16:659–666. doi: 10.1097/01.mop.0000145920.00101.e4. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol. 2003;15:620–626. doi: 10.1016/j.coi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol. 2003;3:405–412. doi: 10.1038/nri1084. [DOI] [PubMed] [Google Scholar]
- 9.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, Bottomly K. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz SA, Cundall MJ, Gajewska BU, Alvarez D, Gutierrez-Ramos JC, Coyle AJ, McKenzie AN, Stampfli MR, Jordana M. Granulocyte macrophage colony-stimulating factor-driven respiratory mucosal sensitization induces Th2 differentiation and function independently of interleukin-4. Am J Respir Cell Mol Biol. 2002;27:428–435. doi: 10.1165/rcmb.4824. [DOI] [PubMed] [Google Scholar]
- 11.Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest. 1998;102:1704–1714. doi: 10.1172/JCI4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol. 2006;176:7431–7437. doi: 10.4049/jimmunol.176.12.7431. [DOI] [PubMed] [Google Scholar]
- 14.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 16.Weckmann M, Collison A, Simpson JL, Kopp MV, Wark PA, Smyth MJ, Yagita H, Matthaei KI, Hansbro N, Whitehead B, Gibson PG, Foster PS, Mattes J. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat Med. 2007;13:1308–1315. doi: 10.1038/nm1660. [DOI] [PubMed] [Google Scholar]
- 17.Farrell E, O’Connor TM, Duong M, Watson RM, Strinich T, Gauvreau GM, O’Byrne PM. Circulating myeloid and plasmacytoid dendritic cells after allergen inhalation in asthmatic subjects. Allergy. 2007;62:1139–1145. doi: 10.1111/j.1398-9995.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol. 2007;179:1901–1912. doi: 10.4049/jimmunol.179.3.1901. [DOI] [PubMed] [Google Scholar]
- 19.Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J Exp Med. 2001;194:551–555. doi: 10.1084/jem.194.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan LN, Kon OM, Macfarlane AJ, Meng Q, Ying S, Barnes NC, Kay AB. Attenuation of the allergen-induced late asthmatic reaction by cyclosporin A is associated with inhibition of bronchial eosinophils, interleukin-5, granulocyte macrophage colony-stimulating factor, and eotaxin. Am J Respir Crit Care Med. 2000;162:1377–1382. doi: 10.1164/ajrccm.162.4.9911117. [DOI] [PubMed] [Google Scholar]
- 21.Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 22.Robinson DS, Ying S, Bentley AM, Meng Q, North J, Durham SR, Kay AB, Hamid Q. Relationships among numbers of bronchoalveolar lavage cells expressing messenger ribonucleic acid for cytokines, asthma symptoms, and airway methacholine responsiveness in atopic asthma. J Allergy Clin Immunol. 1993;92:397–403. doi: 10.1016/0091-6749(93)90118-y. [DOI] [PubMed] [Google Scholar]
- 23.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 25.Christman JW, Sadikot RT, Blackwell TS. The role of nuclear factor-kappa B in pulmonary diseases. Chest. 2000;117:1482–1487. doi: 10.1378/chest.117.5.1482. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 27.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 28.Newton R, Holden NS, Catley MC, Oyelusi W, Leigh R, Proud D, Barnes PJ. Repression of Inflammatory Gene Expression in Human Pulmonary Epithelial Cells by Small-Molecule I{kappa}B Kinase Inhibitors. J Pharmacol Exp Ther. 2007;321:734–742. doi: 10.1124/jpet.106.118125. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia F, Delfino S, Merello E, Puppo M, Piva R, Varesio L, Bosco MC. Hypoxia transcriptionally induces macrophage-inflammatory protein-3alpha/CCL-20 in primary human mononuclear phagocytes through nuclear factor (NF)-kappaB. J Leukoc Biol. 2008;83:648–662. doi: 10.1189/jlb.0607349. [DOI] [PubMed] [Google Scholar]
- 30.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 31.Schreck R, Baeuerle PA. NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol. 1990;10:1281–1286. doi: 10.1128/mcb.10.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-kappaB activation through interference with IkappaB alpha function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun. 1997;236:522–526. doi: 10.1006/bbrc.1997.6997. [DOI] [PubMed] [Google Scholar]
- 34.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 35.Wong CK, Li MLY, Wang CB, Ip WK, Tian YP, Lam CWK. House dust mite allergen Der p 1 elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. Int Immunol. 2006;18:1327–1335. doi: 10.1093/intimm/dxl065. [DOI] [PubMed] [Google Scholar]
- 36.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heijink IH, van Oosterhout A, Kapus A. Epidermal growth factor receptor signalling contributes to house dust mite-induced epithelial barrier dysfunction. Eur Respir J. 2010;36:1016–1026. doi: 10.1183/09031936.00125809. [DOI] [PubMed] [Google Scholar]
- 39.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, Holtzman MJ, Whitsett JA, Hershey GK. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414–421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol. 2004;173:7003–7009. doi: 10.4049/jimmunol.173.11.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jedrzkiewicz S, Nakamura H, Silverman ES, Luster AD, Mansharamani N, In KH, Tamura G, Lilly CM. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1058–1065. doi: 10.1152/ajplung.2000.279.6.L1058. [DOI] [PubMed] [Google Scholar]
- 42.Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000;23:182–187. doi: 10.1165/ajrcmb.23.2.4035. [DOI] [PubMed] [Google Scholar]
- 43.Klemm S, Zimmermann S, Peschel C, Mak TW, Ruland J. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-kappaB activation and cytokine production. Proc Natl Acad Sci U S A. 2007;104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, You Y, Lin PC, Xue L, Morris SW, Zeng H, Wen R, Lin X. Bcl10 plays a critical role in NF-kappaB activation induced by G protein-coupled receptors. Proc Natl Acad Sci U S A. 2007;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palsson-McDermott EM, O’Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007;35:1437–1444. doi: 10.1042/BST0351437. [DOI] [PubMed] [Google Scholar]
- 46.Bouchier-Hayes L, Martin SJ. CARD games in apoptosis and immunity. EMBO reports. 2002;3:616–621. doi: 10.1093/embo-reports/kvf139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medoff BD, Landry AL, Wittbold KA, Sandall BP, Derby MC, Cao Z, Adams JC, Xavier RJ. CARMA3 mediates lysophosphatidic acid-stimulated cytokine secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:286–294. doi: 10.1165/rcmb.2008-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 49.Wegener E, Krappmann D. CARD-Bcl10-Malt1 signalosomes: missing link to NF-kappaB. Sci STKE. 2007;2007:pe21. doi: 10.1126/stke.3842007pe21. [DOI] [PubMed] [Google Scholar]
- 50.Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, Ryan C, Duan S, Helms CA, Liu Y, Chen Y, McBride AA, Hwu WL, Wu JY, Chen YT, Menter A, Goldbach-Mansky R, Lowes MA, Bowcock AM. PSORS2 is Due to Mutations in CARD14. Am J Hum Genet. 2012;90:784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stilo R, Liguoro D, Di Jeso B, Formisano S, Consiglio E, Leonardi A, Vito P. Physical and functional interaction of CARMA1 and CARMA3 with Ikappa kinase gamma-NFkappaB essential modulator. J Biol Chem. 2004;279:34323–34331. doi: 10.1074/jbc.M402244200. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Guo Y, Huang WJ, Ke X, Poyet JL, Manji GA, Merriam S, Glucksmann MA, DiStefano PS, Alnemri ES, Bertin J. Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-kappa B. J Biol Chem. 2001;276:21405–21409. doi: 10.1074/jbc.M102488200. [DOI] [PubMed] [Google Scholar]
- 53.Grabiner BC, Blonska M, Lin PC, You Y, Wang D, Sun J, Darnay BG, Dong C, Lin X. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-{kappa}B activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAllister-Lucas LM, Jin X, Gu S, Siu K, McDonnell S, Ruland J, Delekta PC, Van Beek M, Lucas PC. The CARMA3/Bcl10/MALT1 signalosome promotes Angiotensin II-dependent vascular inflammation and atherogenesis. J Biol Chem. 2010;285:25880–25884. doi: 10.1074/jbc.C110.109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang T, Grabiner B, Zhu Y, Jiang C, Li H, You Y, Lang J, Hung MC, Lin X. CARMA3 is crucial for EGFR-Induced activation of NF-kappaB and tumor progression. Cancer Res. 2011;71:2183–2192. doi: 10.1158/0008-5472.CAN-10-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, Natarajan V. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 57.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okubo T, Knoepfler PS, Eisenman RN, Hogan BLM. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 59.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 60.Yang MY, Hilton MB, Seaman S, Haines DC, Nagashima K, Burks CM, Tessarollo L, Ivanova PT, Brown HA, Umstead TM, Floros J, Chroneos ZC, St Croix B. Essential regulation of lung surfactant homeostasis by the orphan G protein-coupled receptor GPR116. Cell reports. 2013;3:1457–1464. doi: 10.1016/j.celrep.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. CARMA1 is critical for the development of allergic airway inflammation in a murine model of asthma. J Immunol. 2006;176:7272–7277. doi: 10.4049/jimmunol.176.12.7272. [DOI] [PubMed] [Google Scholar]
- 62.Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF-β promote TH9 cell–mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012;129:1000–1010.e1003. doi: 10.1016/j.jaci.2011.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 64.Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, Forteza RM. Reactive Oxygen Species and Hyaluronidase 2 Regulate Airway Epithelial Hyaluronan Fragmentation. J Biol Chem. 2010;285:26126–26134. doi: 10.1074/jbc.M110.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramadas RA, Ewart SL, Medoff BD, LeVine AM. Interleukin-1 Family Member 9 Stimulates Chemokine Production and Neutrophil Influx in Mouse Lungs. Am J Respir Cell Mol Biol. 2011;44:134–145. doi: 10.1165/rcmb.2009-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 68.Zhao S, Qi Y, Liu X, Jiang Q, Liu S, Jiang Y, Jiang Z. Activation of NF-κB in bronchial epithelial cells from children with asthma. Chin Med J. 2001;114:909–911. [PubMed] [Google Scholar]
- 69.Tully JE, Hoffman SM, Lahue KG, Nolin JD, Anathy V, Lundblad LK, Daphtary N, Aliyeva M, Black KE, Dixon AE, Poynter ME, Irvin CG, Janssen-Heininger YM. Epithelial NF-kappaB Orchestrates House Dust Mite-Induced Airway Inflammation, Hyperresponsiveness, and Fibrotic Remodeling. J Immunol. 2013;191:5811–5821. doi: 10.4049/jimmunol.1301329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y, Natarajan V. Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling. Cell Signal. 2009;21:367–377. doi: 10.1016/j.cellsig.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Communi D, Paindavoine P, Place GA, Parmentier M, Boeynaems J-M. Expression of P2Y receptors in cell lines derived from the human lung. Br J Pharmacol. 1999;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong JH, Hong JY, Park B, Lee S-I, Seo JT, Kim K-E, Sohn MH, Shin DM. Chitinase Activates Protease-Activated Receptor-2 in Human Airway Epithelial Cells. Am J Respir Cell Mol Biol. 2008;39:530–535. doi: 10.1165/rcmb.2007-0410OC. [DOI] [PubMed] [Google Scholar]
- 73.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 75.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 76.Ye RD. Regulation of nuclear factor kappaB activation by G-protein-coupled receptors. J Leukoc Biol. 2001;70:839–848. [PubMed] [Google Scholar]
- 77.Kauffman HF, Tamm M, Timmerman JABP. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 2006;4:5. doi: 10.1186/1476-7961-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 79.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L605–614. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int Arch Allergy Immunol. 2012;158(Suppl 1):19–29. doi: 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592 e586. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cocks TM, Moffatt JD. Protease-activated Receptor-2 (PAR2) in the Airways. Pulm Pharmacol Ther. 2001;14:183–191. doi: 10.1006/pupt.2001.0285. [DOI] [PubMed] [Google Scholar]
- 83.Lan RS, Stewart GA, Henry PJ. Role of protease-activated receptors in airway function: a target for therapeutic intervention? Pharmacol Ther. 2002;95:239–257. doi: 10.1016/s0163-7258(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 84.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immuno. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 85.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 86.Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, Idzko M. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 87.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, Natarajan V. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 89.Zhao J, He D, Su Y, Berdyshev E, Chun J, Natarajan V, Zhao Y. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am J Physiol Lung Cell Mol Physiol. 2011;301:L547–556. doi: 10.1152/ajplung.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao J, Chen Q, Li H, Myerburg M, Spannhake EW, Natarajan V, Zhao Y. Lysophosphatidic acid increases soluble ST2 expression in mouse lung and human bronchial epithelial cells. Cell Signal. 2012;24:77–85. doi: 10.1016/j.cellsig.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grabiner BC, Blonska M, Lin P-C, You Y, Wang D, Sun J, Darnay BG, Dong C, Lin X. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-κB activation. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 93.Greaves DR, Wang W, Dairaghi DJ, Dieu MC, Saint-Vis Bd, Franz-Bacon K, Rossi D, Caux C, McClanahan T, Gordon S, Zlotnik A, Schall TJ. CCR6, a CC Chemokine Receptor that Interacts with Macrophage Inflammatory Protein 3α and Is Highly Expressed in Human Dendritic Cells. J Exp Med. 1997;186:837–844. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dieu-Nosjean M-C, Massacrier C, Homey B, Vanbervliet B, Pin J-J, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, Caux C. Macrophage Inflammatory Protein 3α Is Expressed at Inflamed Epithelial Surfaces and Is the Most Potent Chemokine Known in Attracting Langerhans Cell Precursors. J Exp Med. 2000;192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y-J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell–mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritz SA, Cundall MJ, Gajewska BU, Alvarez D, Gutierrez-Ramos J-C, Coyle AJ, McKenzie ANJ, Stämpfli MR, Jordana M. Granulocyte Macrophage Colony–Stimulating Factor–Driven Respiratory Mucosal Sensitization Induces Th2 Differentiation and Function Independently of Interleukin-4. Am J Respir Cell Mol Biol. 2002;27:428–435. doi: 10.1165/rcmb.4824. [DOI] [PubMed] [Google Scholar]
- 98.Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest. 1998;102:1704–1714. doi: 10.1172/JCI4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stumbles PA, Thomas JA, Pimm CL, Lee PT, Venaille TJ, Proksch S, Holt PG. Resting Respiratory Tract Dendritic Cells Preferentially Stimulate T Helper Cell Type 2 (Th2) Responses and Require Obligatory Cytokine Signals for Induction of Th1 Immunity. J Exp Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells—not basophils—are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crimi E, Spanevello A, Neri M, Ind PW, Rossi GA, Brusasco V. Dissociation between airway inflammation and airway hyperresponsiveness in allergic asthma. Am J Respir Crit Care Med. 1998;157:4–9. doi: 10.1164/ajrccm.157.1.9703002. [DOI] [PubMed] [Google Scholar]
- 103.Brewer JP, Kisselgof AB, Martin TR. Genetic Variability in Pulmonary Physiological, Cellular, and Antibody Responses to Antigen in Mice. Am J Respir Crit Care Med. 1999;160:1150–1156. doi: 10.1164/ajrccm.160.4.9806034. [DOI] [PubMed] [Google Scholar]
- 104.Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, Peltz G, Wills-Karp M. Quantitative Trait Loci Controlling Allergen-Induced Airway Hyperresponsiveness in Inbred Mice. Am J Respir Cell Mol Biol. 2000;23:537–545. doi: 10.1165/ajrcmb.23.4.4199. [DOI] [PubMed] [Google Scholar]
- 105.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-Activated Receptor 2 Mediates Eosinophil Infiltration and Hyperreactivity in Allergic Inflammation of the Airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 106.Broide DH, Gleich GJ, Cuomo AJ, Coburn DA, Federman EC, Schwarte LB, Wasserman SI. Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol. 1991;88:637–648. doi: 10.1016/0091-6749(91)90158-k. [DOI] [PubMed] [Google Scholar]
- 107.Hart PH. Regulation of the inflammatory response in asthma by mast cell products. Immunol Cell Biol. 2001;79:149–153. doi: 10.1046/j.1440-1711.2001.00983.x. [DOI] [PubMed] [Google Scholar]
- 108.Wenzel SE, Fowler AA, Schwartz LB. Activation of Pulmonary Mast Cells by Bronchoalveolar Allergen Challenge: In Vivo Release of Histamine and Tryptase in Atopic Subjects with and without Asthma. Am Rev Respir Dis. 1988;137:1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- 109.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 110.Yasuoka S, Ohnishi T, Kawano S, Tsuchihashi S, Ogawara M, Masuda K, Yamaoka K, Takahashi M, Sano T. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am J Respir Cell Mol Biol. 1997;16:300–308. doi: 10.1165/ajrcmb.16.3.9070615. [DOI] [PubMed] [Google Scholar]
- 111.Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, Deng J, Ranjan R, Xiao L, Jaffe HA, Corbridge SJ, Kelly EA, Jarjour NN, Chun J, Prestwich GD, Kaffe E, Ninou I, Aidinis V, Morris AJ, Smyth SS, Ackerman SJ, Natarajan V, Christman JW. Autotaxin production of lysophosphatidic Acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med. 2013;188:928–940. doi: 10.1164/rccm.201306-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, Lambrecht BN. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 113.Sehnert B, Burkhardt H, Wessels JT, Schröder A, May MJ, Vestweber D, Zwerina J, Warnatz K, Nimmerjahn F, Schett G, Dübel S, Voll RE. NF-κB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-κB in immune-mediated diseases. Proc Natl Acad Sci U S A. 2013;110:16556–16561. doi: 10.1073/pnas.1218219110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, FitzGerald JM, Boedigheimer M, Davis BE, Dias C, Gorski KS, Smith L, Bautista E, Comeau MR, Leigh R, Parnes JR. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.