Abstract
Background and Purpose
We sought to validate a previously described model combining clinical and MRI thresholds to predict outcome in acute ischemic stroke in a larger cohort, and evaluate effects of reperfusion therapy and stroke side.
Materials and Methods
123 consecutive anterior circulation AIS patients underwent MRI within 6 hours of stroke onset. DWI and PWI volumes were measured. Lesion volume and NIH Stroke Scale Score thresholds were used in models predicting good three-month clinical outcome (mRS 0-2). Patients were stratified by treatment and stroke side.
Results
ROC analysis demonstrated 95.6% and 100% specificity for DWI > 70mL and NIH Stroke Scale Score > 20 to predict poor outcome, and 92.7% and 91.3% specificity for PWI (mean transit time) < 50mL and NIH Stroke Scale Score < 8 to predict good outcome. Combining clinical and imaging thresholds led to 88.8% (71/80) positive predictive value with 65.0% (80/123) prognostic yield. 100 percent specific thresholds for DWI (103 versus 31 mL) and NIHSSS (20 versus 17) to predict poor outcome were significantly higher in treated (intravenous and/or intraarterial) versus untreated patients. Prognostic yield was lower in right versus left-sided strokes for all thresholds (10.4-20.7 vs. 16.9-40.0%). Patients with right-sided strokes had higher 100 percent specific DWI (103.1 vs. 74.8 mL) thresholds for poor outcome, and positive predictive value was lower.
Conclusion
Our predictive model is validated in a much larger patient cohort. Outcome may be predicted in up to two-thirds of patients, and thresholds are affected by stroke side and reperfusion therapy.
Introduction
Reperfusion therapy improves outcomes of patients with acute ischemic stroke (AIS).1, 2 The decision to treat is primarily determined by time from symptom onset. Use of IV-rtPA is restricted to the 31 or 4.5 hour2 time window, resulting in only 1-7% of patients receiving IV-rtPA.3 Advanced neuroimaging provides information about a patient's physiology that may be useful to guide treatment decisions, especially in an extended time window. Selecting patients for reperfusion therapy based on the mismatch between lesions in diffusion-weighted and perfusion images has been proposed,4 but this approach has not uniformly proven to predict a beneficial treatment response.5-8 Based on findings that patients with DWI infarct volumes greater than 70 mL have poor outcome regardless of treatment,9, 10 and that NIH stroke scale score (NIHSSS) is a strong predictor of outcome,11 we recently published a predictive model that combined DWI and mean transit time (MTT) lesion volumes with NIHSSS thresholds to predict outcome in anterior circulation AIS patients.12 DWI lesion volume >72 mL or NIHSSS >20 predicted poor outcome (mRS 3-6), while MTT lesion volume <47mL or NIHSSS <8 predicted good outcome (mRS 0-2) with high positive predictive value (PPV), when used in combination, in two-thirds of patients.
In this study we sought to validate our predictive model in a larger, independent cohort of patients, and investigate the effects of reperfusion therapy and side of involvement on these thresholds.
Methods
Patient Selection and Assessment
We retrospectively examined the clinical and imaging data of consecutive AIS patients admitted to our comprehensive stroke center. Inclusion criteria were (1) DWI and PWI obtained within 6 hours of when the patient was last seen without symptoms, (2) acute MCA stroke, (3) NIHSSS >2, and (4) sufficient clinical follow-up to determine mRS score at 90 days. This study was approved by our institutional review board. Records were maintained in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
We identified 198 patients with AIS who underwent DWI and PWI within 6 hours. Seventy-five were excluded because of the lack of a follow-up mRS score (n=28), posterior circulation infarction (n=29), calculation of perfusion maps with a delay-sensitive deconvolution technique (n=9), or image quality insufficient for analysis (n=9). The remaining 123 patients were included for further analysis. This cohort was independent of the previously published cohort,12 without overlap. In patients who received IV-tPA, treatment was started within 4.5 hours. In patients who received intraarterial (IA) therapy, treatment was initiated within 8 hours after symptom onset.
Admission NIHSSS was determined by a stroke neurologist. Clinical outcome was evaluated using the mRS score 90 days after stroke onset, which was determined by a stroke neurologist in 59 instances (48%), and retrieved from a neurological exam in the patient's medical record for the remaining patients. Patient outcomes were dichotomized into good (mRS 0-2) versus poor (mRS 3-6).
Image Acquisition, Post-Processing, and Analysis
MR examinations were performed on a 1.5-Tesla Signa scanner (GE Medical Systems, Milwaukee, WI). DWI was performed with a single-shot echo-planar spin-echo sequence with two 180-degree radiofrequency pulses. Three images/slice were acquired at b=0 s/mm2, followed by 25 at b=1000 s/mm2. Imaging parameters were TR/TE 5000/80-110 ms, FOV 22-cm, matrix 128×128 zero-filled to 256×256, 5-mm thickness, 1-mm gap.
PWI was performed using a dynamic susceptibility technique. Serial echoplanar gradient-echo images acquired with TR/TE 1500/40ms, FOV 22cm, matrix 128×128, slice thickness 5mm with 1mm gap. 20 mL of gadopentetate dimeglumine 0.5 mmol/mL (Magnevist, Bayer Healthcare, Pittsburgh, PA) was injected IV at 5 mL/s, beginning 10 seconds after scanning started, followed by 20 mL of normal saline.
PWI data were processed using locally written, automated software. Signal-versus-time curves for each pixel were converted to delta-R2-versus-time curves. CBV was calculated by integrating the area under these curves, CBF as the amplitude of the scaled residue function yielded by delay-insensitive singular value deconvolution13, Tmax as the time at which the scaled residue function reached its maximum, and MTT as CBV/CBF.
For quantitative measurement, visually detected DWI and MTT abnormalities were segmented in randomized order by two experienced neuroradiologists blinded to clinical information using a semiautomated commercial analysis program (Analyze 7.0/AnalyzeDirect. Overland Park, KS). Tmax maps were thresholded to 6 seconds. Absolute mismatch was defined as MTT–DWI, percent mismatch as (MTT–DWI)/DWI*100%.
Statistical Analysis
Statistical analysis was performed using MedCalc 11.2.1 (MedCalc Software, Mariakerke, Belgium). P-values <0.05 were considered significant. Clinical and imaging data were compared using the Student's t test or Mann-Whitney U test and categorical data using the Chi-Square test. Receiver operating characteristics (ROC) curves were calculated for DWI, MTT, Tmax, and NIHSSS relative to clinical outcome. Areas under the curve (AUC) were compared and stratified by reperfusion therapy and stroke side. Prognostic yield was defined as the proportion of patients fulfilling at least one threshold criteria.
Results
Baseline Clinical and Imaging Characteristics, Treatment, Outcomes and Predictors of 3-Month mRS (Table 1)
Table 1. Baseline Clinical and Imaging Characteristics, Treatment, and predictors of 3-month mRS.
| All patients (N=123) | mRS 0-2 (N=68) | mRS 3-6 (N=55) | P-value | |
|---|---|---|---|---|
| Age | 69.8±15.9 | 68.9±16.9 | 70.8±14.7 | 0.727* |
| Baseline NIHSSS | 11(6–16) | 7(5–11.5) | 16(14–20.75) | <0.0001* |
| Female gender | 55(44.7%) | 31(45.6%) | 24(43.6%) | 0.973‡ |
| Right hemisphere | 74(47.2%) | 34(50.0%) | 24(43.6%) | 0.602‡ |
| Time to MRI | 3:57±1:26 | 3:45±1:29 | 4:12±1:20 | 0.085† |
| DWI volume, mL | 37.4±47.7 | 16.3±20.1 | 63.4±58.2 | <0.0001* |
| MTT volume, mL | 129.5±101.3 | 80.6±78.3 | 189.9±94.1 | <0.0001* |
| Tmax volume, mL | 148.1±114.9 | 89.5±86.5 | 217.3±106.0 | <0.0001* |
| Absolute mismatch, mL | 92.7±81.4 | 64.9±69.1 | 127.1±82.9 | <0.0001* |
| Relative mismatch | 8.7±21.6 | 10.9±27.8 | 6.0±8.8 | 0.541* |
| Reperfusion therapy | None: 37(30.1%) | 22(32.4%) | 15(25.4%) | |
| IV-TPA: 61(49.6%) | 36(52.9%) | 25(45.5%) | 0.20‡ | |
| IAT +/- IV-TPA: 25(20.3%) | 10(14.7%) | 15(27.3%) | ||
| Occlusion level | ICA: 24(19.5%) | 6(8.8%) | 18(32.7%) | |
| MCA M1: 39(31.7%) | 15(22.1%) | 24(43.6%) | <0.0001‡ | |
| MCA M2/3: 36(29.3%) | 24(35.3%) | 12(21.8%) | ||
| None: 24(19.5%) | 23(33.8%) | 1(1.8%) |
Mann-Whitney U test.
Student's t-test.
χ2-test.
68 patients (55.3%) had good clinical outcome 90 days after stroke onset. There were no significant differences in age, gender, right hemisphere involvement, relative mismatch volume, or time from symptom onset to MRI, between patients with good versus poor outcome. Median admission NIHSSS was significantly higher (16 vs. 7, P<0.0001), and mean DWI, MTT, Tmax and absolute mismatch volumes were significantly larger (63.4 vs. 16.3, 189.9 vs. 80.6, 217.3 vs. 89.5, and 127.1 vs. 64.9 mL, respectively, all P<0.0001) in patients with poor outcome. More patients with poor outcome than with good outcome had proximal occlusions (ICA or MCA M1) versus MCA M2/M3 or no identifiable occlusion (P<0.0001). 61 patients (49.6%) received IV-tPA, and 25 (20.3%) received IAT. 37 (30.1%) patients did not receive IV- or IA-therapy.
Validation of the Previously Described Combined Thresholds to Predict Outcome in AIS (Table 2)
Table 2.
Validation of the previously described model combining clinical and imaging thresholds to predict outcome in acute ischemic stroke.
| Threshold | Positive predictive value (%) | Prognostic yield (%) | ||
|---|---|---|---|---|
| DWI >70mL |
|
for poor outcome | 86.4* | 17.9 |
| NIHSSS >20 | 100.0* | 11.4 | ||
| MTT <50mL |
|
for good outcome | 89.5* | 30.9 |
| Tmax <50mL | 88.9* | 29.3 | ||
| NIHSSS <8 | 92.3* | 31.7 | ||
|
| ||||
| Combined Clinical thresholds (NIHSSS) | 94.3* | 43.1 | ||
| Combined Imaging thresholds (DWI + MTT) | 88.3* | 48.8 | ||
| Combined imaging thresholds (DWI + Tmax>6s) | 87.9* | 47.2 | ||
|
| ||||
| Combined Clinical and Imaging thresholds | 88.8* | 65.01) | ||
No significant difference in PPV between any of the applied thresholds.
Significantly increased over use of single parameters (p <0.0001) and combined clinical or imaging thresholds (P=0.01).
When applying the previously reported thresholds of DWI >70mL or NIHSSS >20 to predict poor outcome and MTT <50mL or NIHSSS <8 to predict good outcome to our patient cohort, we found the following: Combining DWI with MTT (or Tmax) led to 88.3% (or 87.9%) PPV, with 48.8% (or 47.2%) prognostic yield. For NIHSSS <8 or >20, PPV was 94.3% with 43.1% yield. Combining NIHSSS with imaging thresholds improved prognostic yield to 65.0%, higher than for NIHSS or DWI and MTT alone (P<0.01), with high PPV (88.8%, 71/80 patients). Figure 1 shows scatter plots of all patients dichotomized into good versus poor outcome for the different thresholds. ROC analysis revealed 95.6% (87.6 – 99.1%) specificity and 36.4% (23.8–50.4%) sensitivity to predict poor outcome for DWI volume >70mL. For MTT volume <50mL, 92.7% (82.4–98.0%) specificity and 50.0% (37.6–62.4%) sensitivity to predict good outcome were found. For Tmax volume <50mL, 92.7% (82.4–98.0%) specificity and 46.2% (33.7–59.0%) sensitivity to predict good outcome were found. AUCs were 0.802±0.04 for DWI, 0.816±0.039 for MTT, and 0.819±0.039 for Tmax (all P<0.0001).
Figure 1. Baseline imaging volumes and NIHSSS of patients with good (mRS 0-2) and poor (mRS 3-6) clinical outcome at 90 days after stroke.
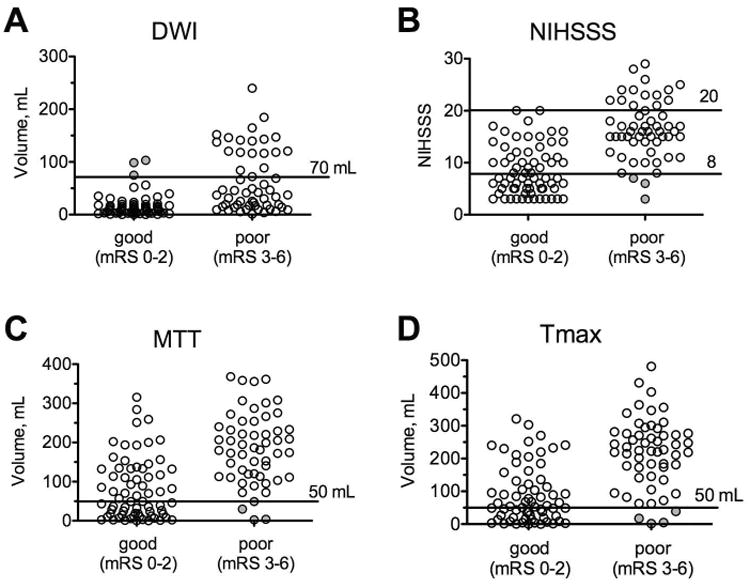
Horizontal lines indicate the thresholds used to predict outcome with (A) DWI (>70mL predicts poor outcome), (B) NIHSSS (<8 or >20 predicts good or poor outcome respectively), (C) MTT (<50mL predicts good outcome), and (D) Tmax (<50mL predicts good outcome). The filled circles mark patients in whom prediction was incorrect.
Intravenous or Intraarterial Reperfusion Therapy Affects Clinical and Imaging Thresholds
Stratification by reperfusion therapy (no IV/IA therapy versus IV-tPA alone or IA recanalization +/- preceding IV-TPA) resulted in similar NIHSSS (11.0±7.9 vs. 12.5±5.8, P=0.23), as well as DWI (44.5±55.0 vs. 35.1±44.4 mL, P=0.70), MTT (110.3±101.8 vs. 137.8±100.6 mL, P=0.17), and Tmax mean lesion volumes (125.0±108.2 vs. 157.6±116.9 mL, P=0.16). However, significant differences in ROC curves and DWI and NIHSSS thresholds for poor outcome were found. Setting specificity to 95% resulted in optimal thresholds of >74.8 mL for DWI and >19 for NIHSSS. For untreated patients, optimal thresholds were lower: >20.5 mL for DWI and >15 for NIHSSS. The AUC was greater for DWI in untreated versus treated patients (0.935 vs. 0.724, P=0.011). Prognostic yield utilizing DWI was higher for untreated patients (42.3 to 35.1 vs. 19.5 to 10.4%, Table 3). The mean DWI volume was lower in untreated versus treated patients with good outcome (9.2±1.7 vs. 19.8±3.4 mL, P=0.033), and the median NIHSSS (5 vs. 9.5, P=0.004, Figure 2A) was also lower. Probability plots for poor outcome based on logistic regression show a flatter curve for treated patients, which plateaus at approximately 100 mL DWI infarct volume. In contrast, the curve plateaus at approximately 50 mL for untreated patients (Figure 2B).
Table 3.
Differences of clinical and imaging thresholds in patients receiving thrombolysis versus untreated patients.
| No reperfusion therapy | IV- and/or IA-therapy | P-value | |
|---|---|---|---|
| DWI | |||
|
| |||
| Area under the curve (AUC) ±SE for predicting poor outcome | 0.935±0.06 | 0.724±0.05 | 0.011 |
| 90% specificity for poor outcome (prognostic yield), mL | >19.7(42.3%) | >51.8(19.5%) | 0.016* |
| 95% specificity for poor outcome (prognostic yield), mL | >20.5(40.5%) | > 74.8(16.1%) | 0.007* |
| 100% specificity for poor outcome (prognostic yield), mL | >30.6(35.1%) | > 103.1(10.4%) | 0.003* |
|
| |||
| NIHSSS | |||
|
| |||
| Area under the curve (AUC) ±SE for predicting poor outcome | 0.903±0.06 | 0.827±0.04 | 0.314 |
| 90% specificity for poor outcome (prognostic yield) | >12(37.8%) | > 16(23.0%) | 0.142* |
| 95% specificity for poor outcome (prognostic yield) | >15(27.0%) | >19(14.9%) | 0.182* |
| 100% specificity for poor outcome (prognostic yield) | >17(16.2%) | >20(9.2%) | 0.414* |
P-value for comparison between prognostic yields is stated.
Figure 2. Baseline infarct volumes, clinical status, and probability for poor outcome in treated versus untreated patients.
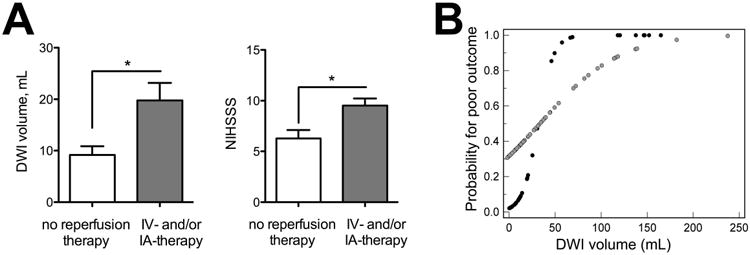
(A) Mean DWI volume (left) and NIHSSS (right) in patients with good outcome stratified by treatment; asterisks indicate significant difference. (B) Probability (determined by logistic regression) for poor outcome versus DWI plots between untreated and treated patients (black circles represent untreated, grey circles treated).
Side of Involvement Affects Clinical and Imaging Thresholds
The DWI threshold set at 95% specificity for poor outcome was lower with left-sided (>51.8 mL) than right-sided strokes (>98.5 mL), and NIHSSS thresholds were higher (<9 or >20 vs. <5 or >16) when the left hemisphere was affected. Prognostic yield was higher with strokes involving the left side: For DWI, yield was 40.0 to 16.9 versus 20.7 to 10.4%, and for NIHSSS yield was 66.2 to 38.5 versus 29.3 to 1.7% (Table 4). Furthermore, the AUC was higher for DWI with left-sided strokes (0.875 vs. 0.706, P=0.043), and there was a trend towards a higher AUC with NIHSSS (0.911 vs. 0.808, P=0.077). The mean DWI volume in patients with good outcome was smaller in left versus right-sided strokes (Figure 3A). A probability plot for poor outcome demonstrates better predictability for left-sided strokes (Figure 3B). Scatter plots for good versus poor outcome show that with left-sided strokes, no patient with a NIHSSS >20 (or <8) had good (or poor) outcome, while for right-sided strokes no patients with NIHSSS >17 had good outcome, and some patients with NIHSSS <8 had poor outcome (Figure 3B). PPV of our model was 97.4% for left-sided strokes, and 82.1% for right-sided strokes (P=0.028).
Table 4.
Differences of clinical and imaging thresholds in patients stratified by affected cerebral hemisphere.
| Right Side | Left Side | P-value | |
|---|---|---|---|
| DWI | |||
|
| |||
| Area under the curve (AUC) ±SE | 0.706±0.07 | 0.875±0.04 | 0.043 |
| 90% specificity for poor outcome (prognostic yield), mL | >39.6(20.7%) | >23.4(40.0%) | 0.034* |
| 95% specificity for poor outcome (prognostic yield), mL | >98.5(12.1%) | > 51.8(26.2%) | 0.082* |
| 100% specificity for poor outcome (prognostic yield), mL | >103.1(10.4%) | > 74.8(16.9%) | 0.435* |
|
| |||
| NIHSSS | |||
|
| |||
| Area under the curve (AUC) ±SE | 0.808±0.06 | 0.911±0.03 | 0.077 |
| 90% specificity for poor or good outcome (prognostic yield) | >15 or <6(29.3%) | >17 or <10(66.2%) | <0.001* |
| 95% specificity for poor or good outcome (prognostic yield) | >16 or <5(17.2%) | >20 or <9(49.2%) | <0.001* |
| 100% specificity for poor or good outcome (prognostic yield) | >16 or <3(1.7%) | >20 or <7(38.5%) | <0.001* |
P-value for comparison between prognostic yields is stated.
Figure 3. Baseline infarct volumes, probability for poor outcome, and NIHSSS thresholds in left-versus right-sided strokes.
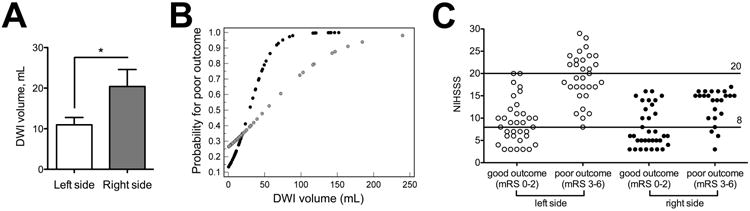
(A) Mean DWI volume in patients with good outcome stratified by side of involvement. Asterisks indicate significant difference. (B) Probability (determined by logistic regression) for poor outcome versus DWI plots between left and right-sided strokes (left=black circles, right=grey circles). (C) NIHSSS scatter plots and thresholds stratified by side of involvement.
Patients in whom Imaging or Clinical Thresholds Predicted Clinical Outcome Incorrectly (Table S1)
Three patients had poor outcomes despite an admission NIHSSS <8. All three had right-sided MCA strokes, and two had critical ICA stenoses with substantial infarct growth after initial evaluation (Figure S1, patient 3). Three patients had MTT abnormalities < 50 mL, but poor outcome. All three had significant comorbidities and/or complicated recoveries. Also, patient 5 (Figure S1) had an infarct in the precentral gyrus, a highly eloquent region strongly represented on the mRS score. Three patients had DWI abnormalities >70 mL but good outcome. These were relatively young patients (26-45 years) who all underwent reperfusion therapy (2/3 had received IAT) and had uncomplicated recoveries without significant infarct extension. Two also had right-sided strokes.
Discussion
We have validated our previously described predictive thresholds of DWI >70 mL or NIHSS >20 for poor outcome of acute ischemic stroke patients, and of MTT <50 mL or NIHSSS <8 for good outcome, with high PPV in this much larger, entirely independent cohort. In the 65% of patients who met at least one of these criteria, outcome was predictable with 89% PPV. Indeed, we identified only 9 of 123 patients in whom an imaging or clinical threshold predicted clinical outcome incorrectly. Furthermore, we extended our previous results by comparing treated (with IV or IA therapy) versus untreated patients, and by comparing right versus left-sided strokes.
Factors involved in patient selection for reperfusion therapy are nuanced, and currently based on time from onset and neurological deficit. Advanced imaging may help further refine these parameters, and more appropriately select patients for intervention. Currently, the most frequently proposed advanced MRI method to select patients for intravenous reperfusion therapy is the mismatch between the DWI and certain PWI lesion sizes.4 Although some evidence supports this strategy,14, 15 it has not conclusively predicted a favorable treatment response.5-8 In addition, relative mismatch measurements fail to account for the absolute sizes of the infarct core and abnormally perfused areas.7 In the EPITHET trial, absolute Tmax and DWI lesion volumes influenced response to reperfusion, but relative mismatch did not.16 Finally, DWI/PWI mismatch does not reflect the patient's clinical status. Clinical status, as measured by NIHSSS, is an important independent predictor of outcome,11 and neurological worsening.17 In general, in our predictive model, patients with DWI lesions >70 mL or NIHSSS >20 have high likelihood of poor outcome, and reperfusion therapy in these patients may offer minimal or no clinical benefit. Patients with MTT lesions <50 mL or NIHSSS <8 have high likelihood of good outcome. Aggressive treatments may unnecessarily expose this group to the risks of invasive reperfusion therapy. The outcomes of the remaining 35% of patients, those with DWI volume <70 mL, MTT volume >50 mL and NIHSSS >8 and <20, could not be predicted accurately by initial clinical and imaging assessments, suggesting that these patients may represent a group to direct early invasive therapy towards.
We hypothesized that patients who received IV thrombolytic and/or intra-arterial recanalization therapy would have higher DWI and NIHSSS thresholds for poor outcome than untreated patients. Indeed, the 100% specific DWI threshold for poor outcome was 103 mL in treated compared to 31 mL in untreated patients, and the 100% specific NIHSSS thresholds were similarly 20 versus 17 respectively, suggesting that treated patients may tolerate larger baseline infarcts. This is also supported by significantly larger DWI lesions and higher NIHSS scores in treated versus untreated patients with good outcome. An explanation for this finding might be that in patients treated with reperfusion therapy infarcts grow less than in untreated patients. When predicting good outcome with NIHSSS, MTT, or Tmax we did not find conclusive evidence for differences by treatment. We did not further stratify patients by different treatment strategies and recanalization status (e.g., IV- versus IAT), because recanalization status was unknown in the 61 patients treated with IV therapy only and our sample size was too small.
Since the dominant (usually left) hemisphere harbors important functions represented in the mRS, we hypothesized that patients with right-sided strokes would tolerate larger DWI lesions, and that our model would be less accurate. Indeed, the AUCs were significantly higher for DWI (0.88 vs. 0.71, P=0.04) with left-sided strokes. The difference in AUC for NIHSSS in left versus right-sided strokes also approached significance (0.91 versus 0.81, P=0.08) Moreover, patients with right-sided strokes could tolerate larger DWI lesions (100% specific DWI volume for poor outcome = 103 versus 75 mL), and on average patients with right-sided strokes and good outcome had significantly larger DWI lesions (20 vs. 10 mL, P<0.05). No patient with an infarct affecting the right hemisphere had a NIHSSS greater 17, making outcome prediction in these patients less reliable.
In the 9 patients for whom the above clinical or imaging thresholds predicted outcome incorrectly, we identified four scenarios: (1) Young patients undergoing reperfusion may do well with DWI lesion volumes >70 mL, in accordance with studies suggesting better outcome in patients <40 years.18 (2) Factors delaying rehabilitation or causing infarct growth, such as comorbidities, complicated recovery, or a stuttering clinical course, might lead to poor outcome despite MTT lesion volumes <50 mL; (3) Infarcts in areas affecting functions highly represented in the mRS score (e.g., motor function in precentral gyrus) may result in poor outcome despite small MTT lesion volumes. This is underscored by a recent study, in which infarct location on MRI correlated highly with outcome.19 4) NIHSSS and mRS are strongly biased towards dominant (usually left) hemisphere functions. All patients with false prediction based on NIHSSS <8 had right-sided infarcts and two-thirds of patients with infarct volumes >70 mL and good outcome had right-sided strokes. The challenges to prediction represented by these variables are important to keep in mind when evaluating patients for therapy.
Limitations of our investigation are consequences of the retrospective design, and have been described elsewhere.12 Briefly, we could not obtain data such as reperfusion status, final infarct volumes, and clinical status at day 30 for most patients. There were variable treatments, variable times from stroke onset to imaging and from imaging to follow-up clinical assessment. There was potential selection bias related to using MRI, the decision to offer thrombolysis, and our technical approach to performing intra-arterial therapy. While assessment of Tmax maps was based on a 6-second threshold, DWI and MTT lesions were outlined by visual estimation. Thresholds have been proposed to increase reliability, but most studies demonstrating a clinical benefit from perfusion imaging-based patient selection have used visual estimation.14, 15 No thresholding software is universally accepted and our data suggest that both methods can be used in our model with similar yield and PPV. Finally, our method for measuring DWI and PWI lesion volumes is too time-consuming for routine clinical use. The faster ABC/2 volume estimation method, based on three orthogonal measurements easily obtained on an MR scanner console, could be a feasible substitute.20
Conclusion
The previously proposed predictive model combining DWI, MTT and NIHSSS thresholds is validated in a much larger patient cohort. For AIS, thresholds applied to acute DWI and MTT lesion volumes and NIHSS can be used to predict good and poor clinical outcomes with high PPV. Our prediction model shows higher PPV in left-sided strokes. Younger patients, those treated with IV and/or IA therapy and those with right-sided strokes may have good outcomes despite larger DWI infarcts. Those treated with reperfusion therapy may also tolerate a higher NIHSSS. These variables, alone and in combination, may help guide decisions related to patient selection for therapy. Patients who do not meet these thresholds have variable outcomes and may represent a target population for more aggressive revascularization approaches.
Supplementary Material
Figure S1. Patients in whom outcome predication was incorrect. (A-D) Patient 3, a 73-year-old male, with poor outcome (mRS 3) after balloon angioplasty despite an initial NIHSSS of 7. CTA (A) shows severe right MCA M1 stenosis (arrows). Initial DWI (B, left column) demonstrates only subtle asymmetry in signal intensity in the right corona radiata and frontal subcortical white matter, but there is prolonged MTT involving much of the right MCA territory (B, right column). DWI 24 hours after symptom onset (C) shows multiple acute infarcts in the right MCA territory (total volume, 53.9 mL). CT after 4 months shows infarct extension into the initial MTT lesion (final infarct volume 173.5 mL). (E-F) Patient 6, a 60-year-old male, who underwent IV-tPA and angioplasty, with poor outcome (mRS 3) despite an initial MTT volume <50ml. Initial DWI (E, top row) shows acute ACA and MCA infarcts involving the left superior frontal gyrus, motor cortex, and premotor cortex (volume 42.1mL). Elevated MTT is seen in the motor and premotor cortex and in part of the superior frontal gyrus (E, bottom row, volume 29.8mL). No infarct extension is seen on follow-up NCCT 5 days after MRI (F, volume 40.6mL).
Table S1. Patients in whom imaging or clinical thresholds falsely predicted clinical outcome.
Acknowledgments
Disclosures: Joshua Hirsch—UNRELATED: Consultancy: CareFusion, Comments: In >24 months, < 36 months I received compensation related to vertebral augmentation; Royalties: CareFusion (as above); Stock/Stock Options: Intratech, Comments: Development-stage company for interventional stroke products. Lee Schwamm—UNRELATED: Consultancy: Lundbeck, Comments: Member DSMB for DIAS 3/4 trials of desmoteplase; Grants/Grants Pending: NINDS/Genentech,* Comments: Genentech provides drug free of charge and some supplemental site payments for the MR WITNESS trial of extended window thrombolysis (Schwamm PI). Ona Wu—RELATED: Grant: NIH,* Comments: R01NS059775, R01NS063925, P50NS051343, P41EB015896; UNRELATED: Consultancy: Penumbra, Comments: Provided consultancy regarding DICOM servers and clients; Grants/Grants Pending: Genentech,* Comments: Genentech has provided funds to MR WITNESS to help per subject reimbursement for each site; OTHER RELATIONSHIPS: I have a patent on “Delay-compensated calculation of tissue blood flow,” US Patent 7,512,435. March 31, 2009, and the patent has been licensed to General E, Siemens, Imaging Biometrics and Olea Medical. Albert Yoo—UNRELATED: Grants/Grants Pending: Penumbra,* Comments: Core imaging lab.
*money paid to institution
Abbreviations
- AIS
acute ischemic stroke
- AUC
area under the curve
- IA
intraarterial
- MTT
mean transit time
- NIHSSS
NIH stroke scale score
- PPV
positive predictive value
- ROC
receiver operating characteristics
- Tmax
time at which the scaled residue function reached its maximum
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Cocho D, Belvis R, Marti-Fabregas J, et al. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology. 2005;64:719–20. doi: 10.1212/01.WNL.0000152041.20486.2F. [DOI] [PubMed] [Google Scholar]
- 4.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–37. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 5.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 6.Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke. 2010;41:e25–33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–50. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanak D, Nosal V, Horak D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–9. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 10.Yoo AJ, Verduzco LA, Schaefer PW, et al. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–54. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, Toyoda K, Uehara T, et al. Baseline NIH Stroke Scale Score predicting outcome in anterior and posterior circulation strokes. Neurology. 2008;70:2371–7. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 12.Yoo AJ, Barak ER, Copen WA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41:1728–35. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]
- 13.Wu O, Ostergaard L, Weisskoff RM, et al. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50:164–74. doi: 10.1002/mrm.10522. [DOI] [PubMed] [Google Scholar]
- 14.Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–31. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 16.Parsons MW, Christensen S, McElduff P, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–25. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGraba TJ, Hallenbeck JM, Pettigrew KD, et al. Progression in acute stroke: value of the initial NIH stroke scale score on patient stratification in future trials. Stroke. 1999;30:1208–12. doi: 10.1161/01.str.30.6.1208. [DOI] [PubMed] [Google Scholar]
- 18.Galldiks N, Zaro-Weber O, Dohmen C, et al. Systemic thrombolysis with rt-PA in patients under 40 years of age: a subgroup analysis of the Cologne Stroke Experience. Cerebrovasc Dis. 2010;30:514–8. doi: 10.1159/000319776. [DOI] [PubMed] [Google Scholar]
- 19.Phan TG, Chen J, Donnan G, et al. Development of a new tool to correlate stroke outcome with infarct topography: a proof-of-concept study. Neuroimage. 2010;49:127–33. doi: 10.1016/j.neuroimage.2009.07.067. [DOI] [PubMed] [Google Scholar]
- 20.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patients in whom outcome predication was incorrect. (A-D) Patient 3, a 73-year-old male, with poor outcome (mRS 3) after balloon angioplasty despite an initial NIHSSS of 7. CTA (A) shows severe right MCA M1 stenosis (arrows). Initial DWI (B, left column) demonstrates only subtle asymmetry in signal intensity in the right corona radiata and frontal subcortical white matter, but there is prolonged MTT involving much of the right MCA territory (B, right column). DWI 24 hours after symptom onset (C) shows multiple acute infarcts in the right MCA territory (total volume, 53.9 mL). CT after 4 months shows infarct extension into the initial MTT lesion (final infarct volume 173.5 mL). (E-F) Patient 6, a 60-year-old male, who underwent IV-tPA and angioplasty, with poor outcome (mRS 3) despite an initial MTT volume <50ml. Initial DWI (E, top row) shows acute ACA and MCA infarcts involving the left superior frontal gyrus, motor cortex, and premotor cortex (volume 42.1mL). Elevated MTT is seen in the motor and premotor cortex and in part of the superior frontal gyrus (E, bottom row, volume 29.8mL). No infarct extension is seen on follow-up NCCT 5 days after MRI (F, volume 40.6mL).
Table S1. Patients in whom imaging or clinical thresholds falsely predicted clinical outcome.


