Abstract
Impaired γ-aminobutyric acid (GABA) signaling may contribute to the emergence of cognitive deficits and subcortical dopaminergic hyperactivity in patients with schizophrenia and related psychotic disorders. Against this background, it has been proposed that pharmacological interventions targeting GABAergic dysfunctions may prove useful in correcting such cognitive impairments and dopaminergic imbalances. Here, we explored possible beneficial effects of the benzodiazepine positive allosteric modulator SH-053-2′F-S-CH3 with partial selectivity at the α2, α3, and α5 subunits of the GABAA receptor in an immune-mediated neurodevelopmental disruption model. The model is based on prenatal administration of the viral mimetic poly(I:C) in mice, which is known to capture various GABAergic, dopamine-related, and cognitive abnormalities implicated in schizophrenia and related disorders. Real-time PCR analyses confirmed the expected alterations in GABAA receptor α subunit gene expression in the medial prefrontal cortex and ventral hippocampus of adult poly(I:C) offspring relative to control offspring. Systemic administration of SH-053-2′F-S-CH3 failed to normalize the poly(I:C)-induced deficits in working memory and social interaction, but instead impaired performance in these cognitive and behavioral domains both in control and poly(I:C) offspring. In contrast, SH-053-2′F-S-CH3 was highly effective in mitigating the poly(I:C)-induced amphetamine hypersensitivity phenotype without causing side effects in control offspring. Our preclinical data suggest that benzodiazepine-like positive allosteric modulators with activity at the α2, α3, and α5 subunits of the GABAA receptor may be particularly useful in correcting pathological overactivity of the dopaminergic system, but they may be ineffective in targeting multiple pathological domains that involve the coexistence of psychotic, social, and cognitive dysfunctions.
Keywords: GABA, Infection, Poly(I:C), Schizophrenia, Working Memory
INTRODUCTION
The central γ-aminobutyric acid (GABA) system is strongly implicated in cognitive processes. Accumulating evidence suggests that GABAergic interneurons critically regulate neuronal oscillatory activity (Gonzalez-Burgos and Lewis, 2012), which in turn is believed to serve various complex functions, including perception, cognition, and memory (Gonzalez-Burgos et al, 2011; Lewis et al, 2012). On these bases, various cognitive deficits found in psychiatric disorders with neurodevelopmental components may, at least in part, stem from a dysregulated inhibitory GABAergic interneuron network (Gonzalez-Burgos et al, 2011; Lewis et al, 2012; Volk and Lewis, 2013). Altered pre- and post-synaptic markers of cortical and hippocampal GABAergic neurotransmission are, in fact, among the most consistently observed abnormalities in developmental psychiatric disorders, most notably schizophrenia (Benes and Berretta, 2001; Volk and Lewis, 2013). Post-mortem studies conducted in schizophrenic patients report reduced expression levels of specific GABAergic interneuron markers, including parvalbumin and somatostatin (Fung et al, 2010; Hashimoto et al, 2008a; Konradi et al, 2011; Morris et al, 2008), along with deficient expression of various presynaptic regulators of GABA neurotransmission such as the 67 kDA isoform of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD67) and the GABA transporter 1 (Hashimoto et al, 2008b). These changes are further accompanied by altered levels of GABAA receptor subunits, including increased α2 subunits and decreased α1, α4 and α5 subunits in cortical layers of patients with schizophrenia (Beneyto et al, 2011; Duncan et al, 2010; Hashimoto et al., 2008a,b).
In addition to their involvement in cognitive processes, GABA-mediated inhibitory networks are also believed to critically regulate subcortical dopaminergic functions. According to a recent hypothesis (Grace, 2012; Lodge and Grace, 2011), disinhibition of the (ventral) hippocampus resulting from intrahippocampal impairments in GABAergic signaling could lead to a pathological hyperactivity of the (ventral) hippocampus and subsequent increase in mesoaccumbal dopamine system function. Such hippocampal abnormalities and states of subcortical hyperdopaminergia are prominent features of schizophrenia and related psychotic disorders (Lodge and Grace, 2011; Zierhut et al, 2010).
Against these backgrounds, it has been proposed that pharmacological interventions targeting GABA abnormalities may prove useful in correcting both cognitive impairments and dopaminergic dysfunctions present in patients with schizophrenia (Guidotti et al, 2005; Stan and Lewis, 2012). A first line of evidence supporting this hypothesis was derived from a small placebo-controlled clinical trial suggesting that treatment with a benzodiazepine-like agent with preferential activity at the α2/α3 subunit of GABAA receptors can improve cognitive and electrophysiological measures of prefrontal functions in individuals with chronic schizophrenia (Lewis et al, 2008). Such pro-cognitive effects associated with positive allosteric modulation of α2/α3 subunit, however, could not be replicated in a larger randomized clinical trial (Buchanan et al, 2011). In contrast, a novel α5 GABAA receptor positive allosteric modulator has been shown to reverse hyperactivation of the dopamine system in the methylazoxymethanol acetate (MAM)-based neurodevelopmental disruption model of schizophrenia (Gill et al, 2011), indicating that such GABAergic modulation may be useful in targeting positive symptoms of schizophrenic disease.
In the present study, we used an established immune-mediated neurodevelopmental disruption model to test the behavioral effects of SH-053-2′F-S-CH3, a novel benzodiazepine positive allosteric modulator (PAM) with activity/partial selectivity at the α2, α3, and α5 subunits (Fischer et al, 2010; Savić et al, 2010). The chosen model is based on prenatal administration of the viral mimetic poly(I:C) (= polyriboinosinic-polyribocytidilic acid) in mice, which is known to capture a wide spectrum of behavioral and cognitive abnormalities relevant to neurodevelopmental psychiatric disorders (reviewed in Meyer, 2013; Meyer and Feldon, 2010). The prenatal poly(I:C) model has been established in relation to epidemiological studies showing increased risk of schizophrenia and related disorders following prenatal maternal exposure to infection or inflammation (Brown and Derkits, 2010). Importantly, prenatal poly(I:C) treatment in mice is capable of inducing a wide range of schizophrenia-relevant prefrontal and hippocampal GABAergic abnormalities in adult offspring, including reduced mRNA and/protein expression of GAD65, GAD67, and parvalbumin (Meyer et al, 2008; Piontkewitz et al, 2012; Richetto et al, 2013a,b). Prenatally poly(I:C)-exposed mice also show diminished prefrontal expression of the α4 and α5 subunits of GABAA receptors (Richetto et al, 2013a,b) and increased α2-GABAA receptor immunoreactivity at axon initial segments (Meyer et al, 2008; Nyffeler et al, 2006) akin to post-mortem findings in schizophrenia (Beneyto et al, 2011; Hashimoto et al, 2008a; Volk et al, 2002). These GABAergic changes are further paralleled by schizophrenia-relevant behavioral and cognitive dysfunctions, including impaired working memory and cognitive flexibility, reduced social approach behavior, and increased amphetamine (AMPH) sensitivity (Bitanihirwe et al, 2010a,b; Connor et al, 2012; Meyer et al, 2008; Richetto et al, 2013a,b; Zuckerman et al, 2003). Based on these findings, we tested whether positive allosteric modulation of the α2, α3, and α5 GABAA receptor subunits by systemic SH-053-2′F-S-CH3 treatment may mitigate working memory deficiency, social interaction deficits, and AMPH hypersensitivity in adult offspring prenatally exposed to poly(I:C).
MATERIALS AND METHODS
Animals
C57BL6N mice were used throughout the study. Female and male breeders were obtained from our in-house specific pathogen free colony at the age of 12–14 weeks. Breeding began after 2 weeks of acclimatization to the new animal holding room, which was a temperature and humidity controlled (21±11C, 55±5%) holding facility under a reversed 12-h light and 12-h dark cycle (lights off at 0800 h). All animals had ad libitum access to standard rodent chow (Kliba 3430, Klibamühlen, Kaiseraugst, Switzerland) and water throughout the study. All procedures described in the present study had been previously approved by the Cantonal Veterinarian’s Office of Zurich and are in agreement with the principles of laboratory animal care in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86-23, revised 1985).
Maternal Immune Activation during Pregnancy
Female mice were subjected to a timed mating procedure as described previously (Meyer et al, 2005). Pregnant dams on gestation day 17 (GD 17) received either a single injection of poly(I:C) (potassium salt; Sigma-Aldrich) at a dose of 5 mg/kg or vehicle (sterile pyrogen-free 0.9% NaCl) according to protocols established before (Bitanihirwe et al, 2010a,b,: Meyer et al, 2008; Richetto et al, 2013a,b). The late gestational stage (i.e., GD 17) was selected because of our previous findings showing that GD17 poly(I:C) treatment is capable of inducing working memory deficiency and various pre- and postsynaptic GABAergic abnormalities in adulthood (Meyer et al, 2008; Richetto et al, 2013a,b). Poly(I:C) was dissolved in sterile pyrogen-free 0.9% NaCl (= vehicle) solution to yield a final concentration of 1.0 mg/ml and was administered via the intravenous (i.v) route at the tail vein under mild physical constraint as fully described elsewhere (Meyer et al, 2005). All solutions were freshly prepared at the day of administration and injected with a volume of 5 ml/kg.
Allocation and Testing of Offspring
All offspring were weaned and sexed at postnatal day (PND) 21. Littermates of the same sex were caged separately and maintained in groups of 2–4 animals per cage as described above. Only male mice were included in all experiments to circumvent bias arising from sexual dimorphism. A first cohort of behaviorally naïve male animals comprising of 11 control (CON) and 9 poly(I:C) (POL) offspring was allocated to gene expression analyses of GABAA receptor subunits in prefrontal and hippocampal tissues (see below). A second cohort of male animals comprising of 34 control (CON) and 34 poly(I:C) (POL) offspring was assigned to the behavioral and cognitive testing, which included (1) spatial working memory in the Y-maze, (2) social approach and cognition, and (3) AMPH sensitivity tests (see below). To minimize the number of animals required to complete all tests of interest, mice were repeatedly tested starting with the spatial working memory test and completing with the AMPH sensitivity test. A resting period of 1 week was inserted between each of these tests so as to allow sufficient drug wash-out from one test to another. Previous drug histories (i.e., SH-053-2′F-S-CH3 or vehicle treatment, see below) were counterbalanced across the prenatal treatment conditions from one test to another in order to further minimize possible confounds from drug carryover effects. CON and POL offspring in both cohorts used in this study stemmed from multiple independent litters (16 CON litters and 14 POL litters) in order to avoid litter effects (Zorrilla et al, 1997). Hence, 1–2 offspring per litter were used either for the gene expression analyses or the behavioral and cognitive testing.
The behavioral testing was always conducted during the animals’ active phase (i.e. during the dark phase of the reversed light-dark cycle between 0900 and 1800 h). The time of daily testing was counterbalanced across the different experimental groups. To facilitate the visualization of spatial cues and to enable adequate video recordings, behavioral testing required that the animals were briefly exposed to a dimly lit room. All experiments were conducted once the offspring reached the adult stage of development (i.e., from 14 weeks of age onwards).
Gene Expression Analysis by Quantitative Real-Time PCR
Fresh brain tissue was collected and prepared for gene expression analysis by quantitative real-time PCR as fully described in the Supplementary Materials and Methods. Following total RNA extraction, the samples were processed for real-time PCR to assess the five different α subunits (α1-α5) of GABAA receptors using protocols established previously (Guidotti et al, 2012; Richetto et al, 2013a,b; see also Supplementary Materials and Methods). Probe and primer sequences were purchased from Eurofins MWG-Operon and are summarized in Table 1. Relative target gene expression was calculated according to the 2(−Delta Delta C(T)) method (Livak and Schmittgen, 2001), and the data was expressed and analyzed as percentage of mRNA levels in control offspring. Gene expression was examined in medial prefrontal cortex (mPFC; including anterior cingulate, prelimbic, and infralimbic subregions, bregma:+2.3 to +1.3 mm), the dorsal hippocampus (dHPC; bregma −1.5 to −2.5 mm), and the ventral hippocampus (vHPC; bregma −2.5 to −3.5 mm).
Table 1.
Primer Sequences Used in the Real-Time PCR Analyses.
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Alpha1 | 5′-TGAGAGCTGAATGCCCAATG-3′ | 5′-TCTGCTACAACCACTGAACG-3′ | 5′-CCTGCCCACTAAAATTCGGAAGCTATGC-3′ |
| Alpha2 | 5′-CCATGAGGCTTACAGTCCAAG-3′ | 5′-ACGGAGTCAGAAGCATTGTAAG-3′ | 5′-CGTAGCTTCCAAATTTCAGTGGGCA-3′ |
| Alpha3 | 5′-ACAATATGACCACACCCAACA-3′ | 5′-AGCTTCCAAACTTCAGTGGG-3′ | 5′-CAATACACGCTGAATGCCCCATGC-3′ |
| Alpha4 | 5′-GCCTGCCCTTTGAAATTTGG-3′ | 5′-GATACAGTCTGCCCAATGAGG-3′ | 5′-ATCTACACCTGGACCAAAGGCCC-3′ |
| Alpha5 | 5′-GGGAATGGACAATGGAATGC-3′ | 5′-TGTCATTGGTCTCGTCTTGTAC-3′ | 5′-CATTTGCGAAAAGCCAAAGTGACTGGA-3′ |
| 36B4 | 5′-AGATGCAGCAGATCCGCAT-3′ | 5′-GTTCTTGCCCATCAGCACC-3′ | 5′-CGCTCCGAGGGAAGGCCG-3′ |
Working Memory in a Spatial Novelty Preference Y-maze Paradigm
Working memory was tested using a spatial novelty preference task in the Y-maze as established before (Bitanihirwe et al, 2010b). The spatial novelty preference test in the Y-maze assesses spatial working memory and uses the natural tendency of rodents to explore novel over familiar spatial environments (Dellu et al, 1992). The apparatus and test protocol are fully described in the Supplementary Materials and Methods.
Social Interaction Test
The test of social interaction was performed in a modified Y-maze as fully described in the Supplementary Materials and Methods. The test consisted of two phases, namely the ‘dummy phase’ and the ‘novelty phase’, which measure social approach behavior and social recognition, respectively. During the first phase (‘dummy phase’), the test mouse could freely explore an unfamiliar C57BL6 mouse (‘live mouse’) and an inanimate object (‘dummy mouse’). The percent time spent with the live mouse was calculated by the formula (time spent with the live mouse/(time spent with the live mouse + time spent with the dummy object)) × 100 and used to assess relative exploration time between a congenic mouse and an inanimate dummy object. During the second phase (‘dummy phase’), the test mouse could freely explore another unfamiliar C57BL6 mouse (‘novel mouse’) and the ‘familiar mouse’ previously used in the ‘dummy phase’. This phase served as a measure of social recognition. The percent time spent with the novel mouse was calculated by the formula (time spent with the novel mouse/(time spent with the novel mouse + time spent with the familiar mouse)) × 100 and used to assess relative exploration time between the familiar and unfamiliar congenic mouse.
Amphetamine Sensitivity Test
An amphetamine (AMPH)-induced locomotor hyperactivity test in the open field was used to assess the animals’ behavioral response to acute dopaminergic stimulation. The test apparatus and protocol are fully described in the Supplementary Materials and Methods. D-amphetamine sulfate (= AMPH; Sigma–Aldrich) was dissolved in isotonic 0.9% NaCl solution to achieve the desired concentration for injection. AMPH was administered via the intraperitoneal (i.p.) route at a dose of 2.5 mg/kg. The dose was selected based on our previous studies showing that poly(I:C) offspring display enhanced locomotor responses to AMPH at this dose (e.g., Meyer et al, 2005, 2008).
SH-053-2′F-S-CH3 Preparation and Administration
A detailed description of SH-053-2′F-S-CH3 synthesis has been described previously (Cook et al, 2009). Synthesis of the S stereoisomer of 8-ethynyl-6-(2-fluorophenyl)-4-methyl-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester was performed at the Department of Chemistry and Biochemistry, University of Wisconsin, Milwaukee, USA. SH-053-2′F-S-CH3 has been demonstrated to have a greater relative affinity for α5 (Ki = 19.2), α2 (Ki = 33.3), and α3 (Ki = 291.5) compared with the relative affinity for α1 (Ki = 468.2) (Fisher et al, 2010; Savić et al, 2010). Moreover, SH-053-2′F-S-CH3 has been shown to have a greater efficacy at α5 (218/389), α2 (170/348), and α3 (138/301) compared with the relative efficacy at α1 (116/164) (efficacy expressed as %of control current at 100nM and 1μM) (Fisher et al, 2010; Savić et al, 2010). SH-053-2′F-S-CH3 was dissolved with the aid of sonication in a solvent containing 85% deionized water, 14% propylene glycol, and 1% Tween 80. Corresponding vehicle (VEH) solution consisted of the solvent only. SH-053-2′F-S-CH3 was administered at a dose of 15 or 30 mg/kg (i.p.) according to dose ranges reported previously (e.g., Savić et al, 2010). All solutions were freshly prepared on the day of administration and were injected using an injection volume of 5ml/kg (i.p.).
Statistical Analysis
All gene expression (real-time PCR) data were analyzed using independent Student’s t tests (two-tailed). In the Y-maze working memory test, the relative time spent in the novel arm and distance moved during the choice phase were analyzed using a 2 × 3 (prenatal treatment × drug treatment) ANOVA. The data obtained in the social interaction test were separately analyzed for the two successive phases. In the first phase, a 2 × 3 (prenatal treatment × drug treatment) ANOVA of the percent time spent with the live mouse was used to assess relative exploration time between a congenic mouse and an inanimate dummy object. In the second phase, a 2 × 3 (prenatal treatment × drug treatment) ANOVA of the percent time spent with the unfamiliar mouse was used to assess the relative exploration time between a novel congenic mouse and a familiar congenic mouse. In the AMPH sensitivity test, the distance moved was expressed as a function of 5-min bins and analyzed using a 2 × 3 × 6 (prenatal treatment × drug treatment × bins) repeated-measure ANOVA for the initial pre-AMPH phase, and using a 2 × 3 × 12 (prenatal treatment × drug treatment × bins) repeated-measure ANOVA for the subsequent AMPH phase. All ANOVAs were followed by Fisher’s least significant difference (LSD) post-hoc comparisons or restricted ANOVAs whenever appropriate. Statistical significance was set at P < 0.05. All statistical analyses were performed using the statistical software StatView (version 5.0) implemented on a PC running the Windows XP operating system.
RESULTS
Effects of Prenatal Immune Activation on the Gene Expression Profile of the GABAA Receptor α(1-5) Subunits
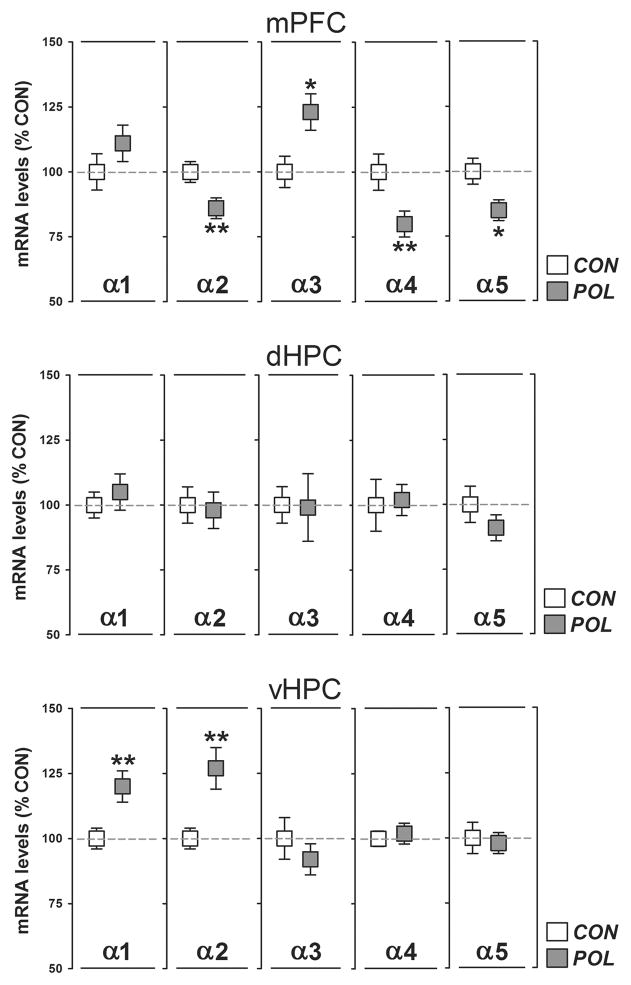
We first determined the gene expression levels of the α1-5 subunits of the GABAA receptor in the mPFC, dHPC, and vHPC of prenatally poly(I:C)-exposed and control offspring. As summarized in Figure 1, poly(I:C) offspring displayed a significant reduction in the expression levels of the α2 (−15%; P < 0.01, t18 = 3.76), α4 (−20%; P < 0.01, t18 = 2.91) and α5 (−15%; P < 0.05, t18 = 2.73) subunits in the mPFC compared to controls. Moreover, prenatal poly(I:C) treatment significantly increased the expression of α3 in the mPFC by 23% (P < 0.05, t18 = 2.70; Figure 1). Prenatal poly(I:C) exposure did not significantly affect the expression levels of the α1-5 subunits in the dHPC (Figure 1). Poly(I:C) offspring displayed, however, a significant increase in the expression levels of the α1 (+20%; P < 0.01, t18 = 2.93) and α2 (+25%; P < 0.01, t18 = 2.96) subunits in the vHPC relative to controls (Figure 1).
Figure 1.
Prenatal immune activation alters the gene expression profile of the GABAA receptor α(1-5) subunits in the medial prefrontal cortex and ventral hippocampus. The graphs depict the levels of normalized mRNA expression in the medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC), and ventral hippocampus (vHPC) of control (CON) and poly(I:C)-exposed (POL) offspring. N(CON) = 11 and N(POL) = 9 for each region and subunit. *P < 0.05 and **P < 0.01. All values are means ± SEM.
Effects of SH-053-2′F-S-CH3 on Working Memory Deficits Induced by Prenatal Immune Activation
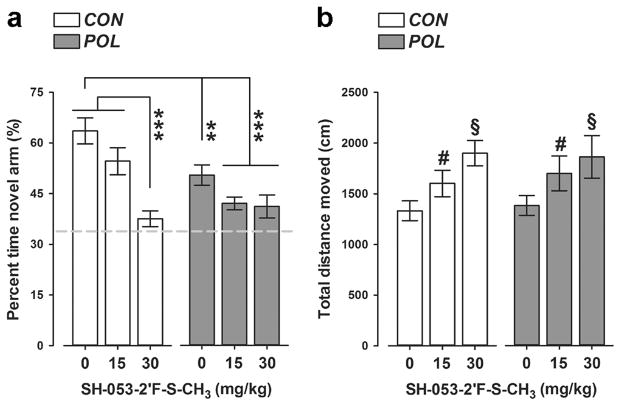
We evaluated possible pro-cognitive effects of SH-053-2′F-S-CH3 using a spatial recognition working memory test in the Y-maze. The critical measure of spatial recognition memory is the relative time spent in the novel (previously unexplored) arm during the choice phase of this test. As expected, VEH-treated control offspring displayed a noticeable preference towards the novel arm, indicating intact working memory in these animals (Figure 2a). Poly(I:C) offspring exhibited a significant reduction in this measure regardless of whether they were treated with VEH or SH-053-2′F-S-CH3 (Figure 2a), suggesting that the PAM failed to restore working memory deficits in poly(I:C) offspring. Moreover, administration of the higher dose of SH-053-2′F-S-CH3 (30 mg/kg) in prenatal control offspring also impaired working memory (Figure 2a), with both groups regressing to chance level of performance after treatment with the PAM. ANOVA of relative time spent in the novel arm revealed a significant main effect of prenatal treatment (F(1,62) = 7.51, P < 0.01), drug treatment (F(2,62) = 14.45, P < 0.001), and their interaction (F(2,62) = 4.16, P < 0.05). Subsequent post-hoc analysis confirmed the significant difference between VEH-treated control and poly(I:C) offspring (P < 0.01); between VEH-treated control offspring and poly(I:C) offspring treated with SH-053-2′F-S-CH3 (P’s < 0.001); and between VEH-treated control offspring and control offspring treated with SH-053-2′F-S-CH3 at 30 mg/kg (P < 0.001) (see Figure 2a).
Figure 2.
SH-053-2′F-S-CH3 administration produces working memory deficits in the Y-maze spatial recognition paradigm in both control and poly(I:C) offspring. (a) The bar plot depicts the percent time spent in the novel (previously unexplored) arm during the choice phases of the test following vehicle (= 0 mg/kg SH-053-2′F-S-CH3) or SH-053-2′F-S-CH3 (at 15 or 30 mg/kg) treatment in control (CON) and poly(I:C) (POL) offspring. The dashed line represents the chance level. **P < 0.01 and ***P < 0.001, based on post-hoc tests (b) The graph shows the total distance moved during the choice phases of the test. #P < 0.05 and §P < 0.01, reflecting the increase in distance moved displayed by animals treated with 15 mg/kg and 30 mg/kg SH-053-2′F-S-CH3, respectively, relative to vehicle treatment (= 0 mg/kg SH-053-2′F-S-CH3). N(CON, 0 mg/kg) = 12, N(CON, 15 mg/kg) = 11, N(CON, 30 mg/kg) = 11, N(POL, 0 mg/kg) = 12, N(POL, 15 mg/kg) = 11, and N(POL, 30 mg/kg) = 11. All values are means ± SEM.
Prenatal poly(I:C) exposure did not significantly affect the distance moved during the Y-maze test (Figure 2b). Administration of SH-053-2′F-S-CH3, however, led to an increase in the total distance moved independent of the prenatal treatment histories (Figure 2b), as supported by the significant main effect of drug treatment in the ANOVA of total distance moved (F(2,62) = 5.67, P < 0.01). Subsequent post-hoc tests verified the significant difference between animals treated with VEH and SH-053-2′F-S-CH3 at 15 mg/kg (P < 0.05), as well as between animals treated with VEH and SH-053-2′F-S-CH3 at 30 mg/kg (P < 0.01) (see Figure 2b).
Effects of SH-053-2′F-S-CH3 on Social Interaction and Recognition Deficits Induced by Prenatal Immune Activation
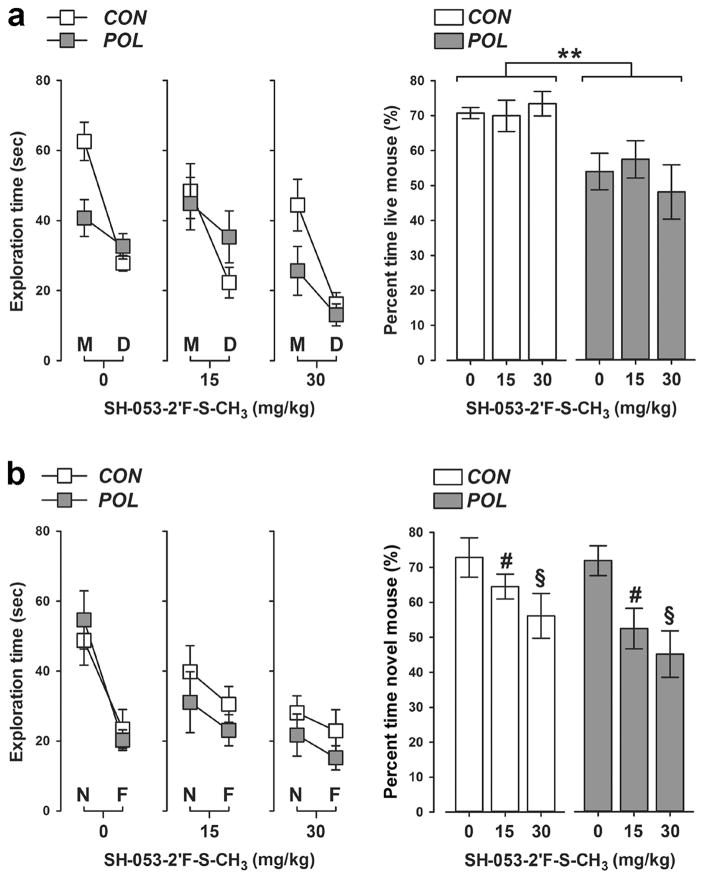
In a next step, we investigated whether SH-053-2′F-S-CH3 would be effective in normalizing social interaction deficits that are typically seen following prenatal immune challenge. The relative exploration time between an unfamiliar congenic mouse and an inanimate dummy object was used to assess social approach behavior in the first phase of the social interaction test. As shown in Figure 3a, prenatal control offspring displayed a clear preference towards the unfamiliar live mouse regardless of whether they were treated with VEH or SH-053-2′F-S-CH3. Such social approach behavior was significantly disrupted in poly(I:C) offspring independent of whether they received VEH or SH-053-2′F-S-CH3 treatment. Indeed, poly(I:C) offspring did not display a clear preference towards the unfamiliar live mouse such that the percent time spent with the live mouse was approximately at the 50%-chance level in these animals (see Figure 3a). ANOVA of the percent time spent with the live mouse revealed only a significant main effect of prenatal treatment (F(1,62) = 7.05, P < 0.01).
Figure 3.
SH-053-2′F-S-CH3 does not impair social approach behavior but interferes with social recognition performance in both control and poly(I:C) offspring. (a) Behavioral outcomes in the social approach test (“dummy phase”). The line plot shows absolute exploration time of an unfamiliar live mouse (M) and inanimate dummy object (D) separately for control (CON) and poly(I:C) (POL) offspring treated with vehicle (= 0 mg/kg SH-053-2′F-S-CH3) or SH-053-2′F-S-CH3 (at 15 or 30 mg/kg). The bar plot depicts the percent time spent with the live mouse in the social approach test. **P < 0.01, reflecting the significant main effect of prenatal poly(I:C) exposure. (b) Behavioral outcomes in the social recognition test (“novelty phase”). The line plot shows absolute exploration time of a novel live mouse (N) and the familiar live mouse (F) separately for CON and POL offspring treated with vehicle (= 0 mg/kg SH-053-2′F-S-CH3) or SH-053-2′F-S-CH3 (at 15 or 30 mg/kg). The bar plot depicts the percent time spent with the novel mouse in the social recognition test. #P < 0.05 and §P < 0.01, reflecting the significant decrease displayed by animals treated with 15 mg/kg and 30 mg/kg SH-053-2′F-S-CH3, respectively, relative to vehicle-treated animals. N(CON, 0 mg/kg) = 11, N(CON, 15 mg/kg) = 12, N(CON, 30 mg/kg) = 11, N(POL, 0 mg/kg) = 11, N(POL, 15 mg/kg) = 11, and N(POL, 30 mg/kg) = 12. All values are means ± SEM.
In the second phase of the test, the relative exploration time between the previously explored live mouse and a novel unfamiliar mouse was then used to assess social recognition memory. Both control and poly(I:C) offspring treated with VEH displayed a clear preference towards the novel unfamiliar mouse relative to the previously explored mouse, suggesting that prenatal poly(I:C) exposure did not affect social recognition memory (see Figure 3b). Administration of SH-053-2′F-S-CH3, however, significantly impaired social recognition memory regardless of the prenatal treatment histories (Figure 3b). Indeed, the percent time spent with the novel unfamiliar mouse decreased with increasing doses of SH-053-2′F-S-CH3, leading to a significant main effect of drug treatment in the ANOVA of this measure (F(2,62) = 5.42, P < 0.01). Subsequent post-hoc analyses confirmed the significant difference between animals treated with VEH and SH-053-2′F-S-CH3 at 15 mg/kg (P < 0.05), as well as between animals treated with VEH and SH-053-2′F-S-CH3 at 30 mg/kg (P < 0.01) (Figure 3b).
Effects of SH-053-2 ′F-S-CH3 on AMPH Hypersensitivity Induced by Prenatal Immune Activation
Finally, we also explored whether SH-053-2′F-S-CH3 may be effective in normalizing potentiated AMPH sensitivity typically emerging following prenatal immune activation. For this purpose, we assessed the effects of the PAM on AMPH-induced hyperactivity in the open field test.
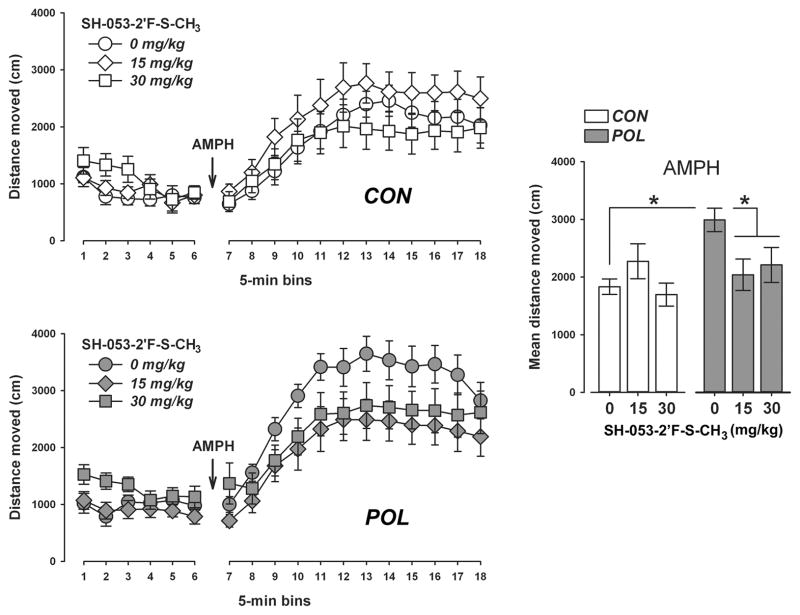
Neither prenatal poly(I:C) exposure nor SH-053-2′F-S-CH3 pretreatment significantly affected the total distance moved during the initial 30-min period of the open field test (Figure 4). Poly(I:C) and control offspring treated with the higher dose of SH-053-2′F-S-CH3 (30 mg/kg) tended to show higher locomotor activity scores during this initial testing period, but this effect did not attain statistical significance (Figure 4). ANOVA of the total distance moved only revealed a significant main effect of bins (F(5,310) = 9.04, P < 0.001), reflecting the overall changes in locomotor activity resulting from habituation to the open field.
Figure 4.
SH-053-2 ′F-S-CH3 selectively reverses the potentiated amphetamine locomotor response in poly (I:C) offspring. The line plots show the distance moved in the open field as a function of 5-min bins during the initial habituation phase and the subsequent amphetamine (AMPH; 2.5 mg/kg, i.p.) exposure phase separately for control (CON) and poly(I:C) (POL) offspring treated with vehicle (= 0 mg/kg SH-053-2′F-S-CH3) or SH-053-2′F-S-CH3 (at 15 or 30 mg/kg). The bar plot depicts the mean distance moved during the AMPH exposure phase for all groups. *P < 0.05, based on post-hoc analyses. N(CON, 0 mg/kg) = 11, N(CON, 15 mg/kg) = 11, N(CON, 30 mg/kg) = 12, N(POL, 0 mg/kg) = 11, N(POL, 15 mg/kg) = 12, and N(POL, 30 mg/kg) = 11. All values are means ± SEM.
Subsequent administration of AMPH led to a general increase in the total distance moved, as supported by the main effect of bins (F(11,682) = 77.78, P < 0.001) in the AMPH phase of the test. VEH-treated poly(I:C) offspring showed a marked potentiation of the AMPH-induced hyperactivity response compared with the locomotor-enhancing effects of AMPH in VEH-treated control offspring (Figure 4). Most interestingly, SH-053-2′F-S-CH3 pretreatment fully normalized the potentiation of the AMPH-induced hyperactivity response in poly(I:C) offspring without significantly affecting the locomotor-enhancing effects of AMPH exposure in control offspring (Figure 4). These impressions were supported by ANOVA revealing a significant interaction between prenatal treatment and drug treatment (F(2,62) = 3.56, P < 0.05), and a significant three-way interaction between prenatal treatment, drug treatment and bins (F(22,682) = 1.60, P < 0.05). Subsequent post-hoc analyses of the mean distance moved across the 60-min AMPH test period confirmed a significant difference between VEH-treated control and poly(I:C) offspring (P < 0.05), as well as between VEH-treated poly(I:C) offspring and poly(I:C) offspring treated with SH-053-2′F-S-CH3 (P’s < 0.05).
DISCUSSION
The present study confirms that prenatal immune activation by the viral mimic poly(I:C) alters GABAergic gene expression in the adult CNS (Richetto et al, 2013a,b). Here, we replicated our initial findings of impaired α2, α4, and α5 gene expression in the mPFC of adult poly(I:C) offspring (Richetto et al, 2013b). Similar reductions in cortical α4 and α5 gene expression have been found in schizophrenia and other neurodevelopmental disorders with prenatal infectious etiologies, including autism (Beneyto et al, 2011; Blatt and Fatemi, 2011; Duncan et al, 2010). On the other hand, our cortical findings do not parallel the reports of decreased and increased α1 and α2 mRNA levels, respectively, in cortical areas of schizophrenia patients (Beneyto et al, 2011; Hashimoto et al, 2008a). Adult poly(I:C) offspring, however, displayed a significant increase in α1 and α2 gene expression in the vHPC, the latter being consistent with previous immunohistochemical studies showing increased α2 protein expression in the vHPC of poly(I:C)-exposed offspring (Meyer et al, 2008). Interestingly, the dHPC was largely spared by the prenatal manipulation with respect to GABAA receptor alterations, and similar region-specific effects have been reported for other GABAergic markers such as parvalbumin and reelin (Meyer et al, 2008). It thus appears that the vHPC may be more susceptible to the disrupting effects of prenatal immune challenge compared to the dHPC. This notion fits well with accumulating evidence supporting a pivotal role of ventral hippocampal (GABAergic) abnormalities in developmental psychiatric disorders such as schizophrenia (Grace, 2010, 2012; Lodge and Grace, 2011; Steullet et al, 2010).
Based on the GABAergic effects reported here and previously (Meyer et al, 2008; Richetto et al, 2013a,b), we speculated that administration of SH-053-2′F-S-CH3, a positive allosteric modulator with high affinity for the α2 and α5 (and to a lesser extent for α3) subunits of the GABAA receptor, may exert beneficial effects against prenatal infection-induced behavioral abnormalities. Consistent with previous studies (Bitanihirwe et al, 2010a,b; Connor et al, 2012; Richetto et al, 2013a,b), poly(I:C) offspring were found to display impaired working memory in the Y-maze spatial recognition test and reduced social approach behavior in the social interaction test. Furthermore, they exhibited increased sensitivity to the locomotor-enhancing effects of the indirect dopamine receptor agonist AMPH compared to control offspring, as reported before (Meyer et al, 2008; Piontkewitz et al, 2011; Zuckerman et al, 2003). In contrast to our hypothesis, the PAM SH-053-2′F-S-CH3 did not mitigate the poly(I:C)-induced working memory and social interaction deficits, two behavioral abnormalities commonly found in people with schizophrenia and related disorders (Foussias and Remington, 2010; Lewis and Moghaddam, 2006). In fact, SH-053-2′F-S-CH3 administration to prenatal control offspring impaired performance in the Y-maze working memory and social interaction tests. In the latter, SH-053-2′F-S-CH3 pretreatment led to a selective impairment in social recognition but not social approach behavior, suggesting that the drug negatively affected short-term retention of social cues rather than social approach behavior per se. Several previous studies have shown that prenatal poly(I:C)-induced cognitive deficits in rats and mice can be restored or even prevented by atypical antipsychotic drugs such as clozapine and risperidone (Meyer et al, 2010; Ozawa et al, 2006; Piontkewitz et al, 2009; Zuckerman et al, 2003). Therefore, one implication is that a normalization of prenatal poly(I:C)-induced spatial and social recognition deficits would require a modulation of neurotransmitter systems that go beyond those primarily mediated by GABAA receptors.
The detrimental effects of SH-053-2′F-S-CH3 on cognitive functions revealed here are in contrast to previous findings in rats suggesting that this PAM does not negatively affect spatial reference memory as assessed in the Morris water maze test (Savic et al, 2010). We have no parsimonious explanation for this discrepancy, but it could be related to differential cognitive processes involved (short-term working memory versus long-term reference memory) and/or potentially important species differences (mice versus rats). The amnesic effects of SH-053-2′F-S-CH3 on short-term spatial and social recognition memory may also seem surprising in view of previous findings indicating that GABAergic agonists acting at the at the α2/α3 subunits induce pro-cognitive effects (Buchanan et al, 2010; Castner et al, 2010; Lewis et al, 2008). It needs to be pointed out, however, that the pro-cognitive effects of α2/α3 agonists remain controversial (Buchanan et al, 2011). Furthermore, accumulating evidence suggests that reduced and increased activity of the α5 subunit of the GABAA receptor facilitates and impairs cognitive functions, respectively (Collinson et al, 2002; Crestani et al, 2002; Dawson et al, 2006; Milić et al, 2013; Redrobe et al, 2012; Tan et al, 2011). Hence, the negative consequences of SH-053-2′F-S-CH3 on short-term memory revealed here may also be explained by positive modulation of the α5 subunit, given that the drug is characterized by strong α5 activity (Fisher et al, 2010; Savić et al, 2010).
Despite the inability of SH-053-2′F-S-CH3 to correct prenatal infection-induced cognitive impairments, it was highly effective in mitigating AMPH hypersensitivity in adult poly(I:C) offspring without concomitant effects in prenatal control offspring. AMPH exposure can produce psychosis-like states in healthy human subjects and exacerbate existing psychoses in patients with schizophrenia (Laruelle, 2000). Moreover, potentiation of AMPH-induced dopamine release in schizophrenia patients tends to correlate with the severity of positive symptoms (Laruelle and Abi-Dargham, 1999). The efficacy of SH-053-2′F-S-CH3 to correct the poly(I:C)-induced potentiation of AMPH sensitivity may thus be relevant especially for attempts to establish and validate GABA-based treatments targeting positive symptoms.
Our data are also highly congruent with previous findings showing that acute treatment with a selective α5 GABAA-receptor PAM can fully reverse AMPH hypersensitivity and hyperactivity of ventral midbrain dopamine neurons in the MAM-based neurodevelopmental model of schizophrenia (Gill et al, 2011). The fact that prenatal MAM-induced AMPH hypersensitivity can be normalized by a selective α5 GABAA-receptor PAM indicates that the beneficial effects of SH-053-2′F-S-CH3 revealed here may also be largely attributable to the drug’s activity at the α5 subunit. Whatever precise molecular mechanisms may be involved, the findings obtained in the prenatal poly(I:C) and MAM administration models emphasize the possibility that alterations in the adult central GABA system may represent a critical pathological convergence point for various prenatal adversities implicated in the etiology of neurodevelopmental brain abnormalities (Volk and Lewis, 2013). These models also support the hypothesis that there is a causal link between altered signaling at α subunits of the GABAA receptor and the emergence of schizophrenia-relevant dysfunctions in neurodevelopmentally comprised offspring (Stan and Lewis, 2011; Volk and Lewis, 2013), at least with respect to AMPH hypersensitivity and related hyperdopaminergic functions. Consistent with this notion, reduced GABAergic signaling at the α5 subunit has previously been implicated in other dopamine-dependent behaviors: Genetically induced deficits in α5 expression impair prepulse inhibition (PPI) of the acoustic startle reflex (Hauser et al, 2005) and selective associative learning as assessed by the latent inhibition (LI) paradigm (Gerdjikov et al, 2007).
In conclusion, our study provides preclinical support for the use of benzodiazepine-positive allosteric modulators in the symptomatic treatment of AMPH hypersensitivity that emerges following (immune-mediated) neurodevelopmental disruption. Together with the recent findings obtained in the prenatal MAM administration model (Gill et al, 2011), our data suggest that positive allosteric modulation of the GABAA receptor α5 subunit may be particularly useful in mitigating pathological overactivity of the dopaminergic system and, consequently, in alleviating dopamine-mediated psychosis. At the same time, however, our study falls short in detecting possible pro-cognitive effects of PAM treatment with preferential affinity for the α5 and α2 (and to a lesser extent the α3) subunits. Evaluating whether the preferentially α5 selective compounds tested previously (Gill et al., 2011) shares this cognitive disruptive effect could lend further support to the disruptive influence on working memory of α5 subunit potentiation. The lack of such pro-cognitive effects may raise concerns regarding the effective use of some types of GABA subunit selective compounds with the aim to target multiple pathological domains that involve the co-existence of psychotic, social, and cognitive dysfunctions.
Supplementary Material
Acknowledgments
We remain indebted to Ulrike Stadlbauer and Sandra Giovanoli for animal husbandry and care. The present study was supported by The European Union’s (EU) Seventh Framework Programme (FP7/2007–2011) under grant agreement No. 259679 and by the Swiss National Science Foundation (grant 310030_146217/1) awarded to U.M.; and by Italian Ministry of University and Research (PRIN 20107MSMA4) and Fondazione CARIPLO (grant n. 2012-0503) to M.A.R.
Footnotes
FUNDING AND DISCLOSURE
All authors declare that they have no conflicts of interest to disclose. The present work is purely academic.
References
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010a;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010;13:981–996. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec. 2011;294:1646–1652. doi: 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RS, Lieberman JA, Barch DM, Csernansky JG, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry. 2011;69:442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RS, Lieberman JA, Barch DM, Csernansky JG, Goff DC, Gold JM, Green MF, Jarskog LF, Javitt DC, Kimhy D, Kraus MS, McEvoy JP, Mesholam-Gately RI, Seidman LJ, Ball MP, McMahon RP, Kern RS, Robinson J, Marder SR. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry. 2011;69:442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Arriza JL, Roberts JC, Mrzljak L, Christian EP, Williams GV. Reversal of ketamine-induced working memory impairments by the GABAAalpha2/3 agonist TPA023. Biol Psychiatry. 2010;67:998–1001. doi: 10.1016/j.biopsych.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012;140:175–184. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Zhou H, Huang S, Sarma PV, et al. Stereospecific and anticonvulsant agents with reduced muscle-relaxant, sedative-hypnotic and ataxic effects. United States of America. 7,618,458 US Patent. 2009
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace Fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–5. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr, Cook JM, Furtmüller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167(12):1479–88. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal alpha 5 subunit-containing GABA A receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem. 2008;89:87–94. doi: 10.1016/j.nlm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18:367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–1348. doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Guidotti G, Calabrese F, Auletta F, Olivier J, Racagni G, Homberg J, Riva MA. Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: modulation by antidepressant treatment. Neuropsychopharmacology. 2012;37:746–758. doi: 10.1038/npp.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psychiatry. 2005;10:201–207. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–73. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.011. in press (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Knuesel I, Nyffeler M, Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology. 2010;208:531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Milić M, Timić T, Joksimović S, Biawat P, Rallapalli S, Divljaković J, Radulović T, Cook JM, Savić MM. PWZ-029, an inverse agonist selective for α5 GABAA receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats. Behav Brain Res. 2013;241:206–213. doi: 10.1016/j.bbr.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex (2008) 2008;18:1575–87. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry. 2011;70:842–851. doi: 10.1016/j.biopsych.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Bernstein HG, Dobrowolny H, Bogerts B, Weiner I, Keilhoff G. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun. 2012;26:353–363. doi: 10.1016/j.bbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Elster L, Frederiksen K, Bundgaard C, de Jong IE, Smith GP, Bruun AT, Larsen PH, Didriksen M. Negative modulation of GABAA α5 receptors by RO4938581 attenuates discrete sub-chronic and early postnatal phencyclidine (PCP)-induced cognitive deficits in rats. Psychopharmacology. 2012;221:451–468. doi: 10.1007/s00213-011-2593-9. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Meyer U, Riva MA. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain Behav Immun. 2013a;33:190–200. doi: 10.1016/j.bbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull. 2013b doi: 10.1093/schbul/sbs195. in press (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Majumder S, Huang S, Edwankar RV, Furtmüller R, Joksimović S, Clayton T, Sr, Ramerstorfer J, Milinković MM, Roth BL, Sieghart W, Cook JM. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan AD, Lewis DA. Altered cortical GABA neurotransmission in schizophrenia: insights into novel therapeutic strategies. Curr Pharm Biotechnol. 2012;13:1557–1562. doi: 10.2174/138920112800784925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Rudd JA, Yew DT. Gene expression changes in GABA(A) receptors and cognition following chronic ketamine administration in mice. PLoS One. 2011;6:e21328. doi: 10.1371/journal.pone.0021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248C:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Zierhut K, Bogerts B, Schott B, Fenker D, Walter M, Albrecht D, Steiner J, Schütze H, Northoff G, Düzel E, Schiltz K. The role of hippocampus dysfunction in deficient memory encoding and positive symptoms in schizophrenia. Psychiatry Res. 2010;183:187–194. doi: 10.1016/j.pscychresns.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.