Abstract
Cholangiocytes are the epithelial cells that line the bile ducts. Along the biliary tree, two different kinds of cholangiocytes exist; small and large cholangiocytes. Each type has important differences in their biological role in physiological and pathological conditions. In response to injury, cholangiocytes become reactive and acquire a neuroendocrine-like phenotype with the secretion of a number of peptides. These molecules act in an autocrine/paracrine fashion to modulate cholangiocyte biology and determine the evolution of biliary damage. The failure of such mechanisms is believed to influence the progression of cholangiopathies, a group of diseases that selectively target biliary cells. Therefore, the understanding of mechanisms regulating cholangiocyte response to injury is expected to foster the development of new therapeutic options to treat biliary diseases. In the present review, we will discuss the most recent findings in the mechanisms driving cholangiocyte adaptation to damage, with particular emphasis on molecular pathways that are susceptible of therapeutic intervention. Morphogenic pathways (Hippo, Notch, Hedgehog), which have been recently shown to regulate biliary ontogenesis and response to injury, will also be reviewed. In addition, the results of ongoing clinical trials evaluating new drugs for the treatment of cholangiopathies will be discussed.
Keywords: Biliary Epithelium, Primary Biliary Cirrhosis, Primary Sclerosing Cholangitis
INTRODUCTION
The liver is the largest gland in the body and is endowed with critical metabolic functions that involve digestion of food and clearance of toxic substances. At the level of the bile canaliculus, hepatocytes secrete bile, which is then carried to the duodenum through a complex network of bile ducts lined by cholangiocytes 1–3.
Under physiological conditions, cholangiocytes actively contribute to the final composition and volume of bile secretion by basal and hormone regulated events 4. In normal conditions, one of the most important and well-studied functions of cholangiocytes is secretin-induced release of bicarbonate into bile. The binding of secretin to the secretin receptor (SR) on the basolateral membrane of cholangiocytes leads to the formation of cAMP, PKA-dependent phosphorylation of CFTR, and the subsequent extrusion of Cl− in the lumen of bile ducts. Driven by the Cl− gradient across the plasma membrane, the activation of the apical Cl−/HCO3− anion exchanger 2 (AE2) culminates in the net excretion of bicarbonate in bile 5, with passive influx of water (Figure 1). As a result, cholangiocytes participate to up to 40% of the so-called bile salt-independent bile flow 6.
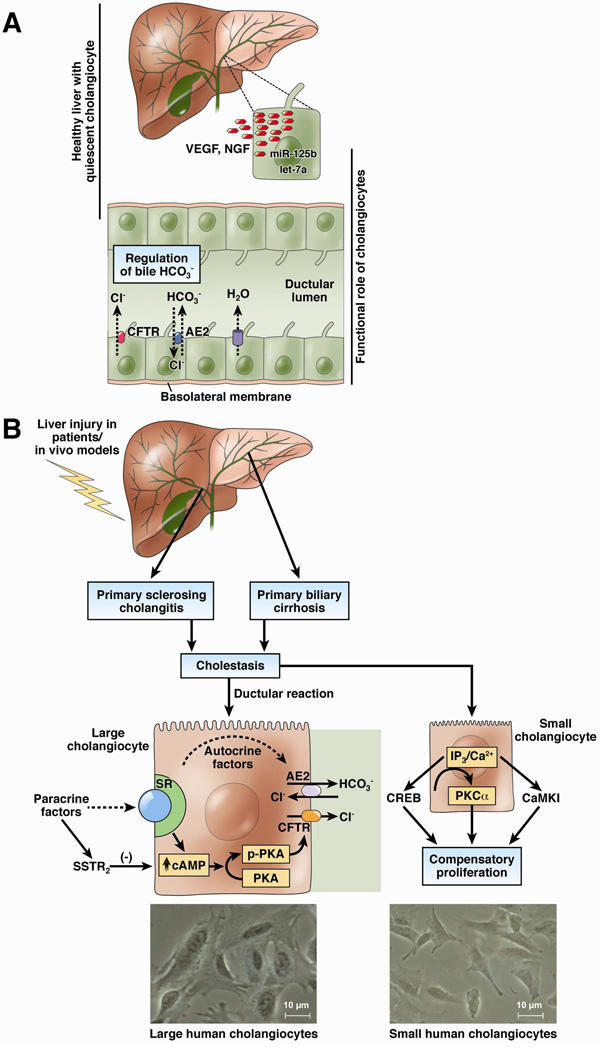
Figure 1.
Overview of cholangiocyte role in biliary functions. [A] Intrahepatic bile ducts are lined by both large and small cholangiocytes. Under physiological conditions, cholangiocytes (large cholangiocytes preferentially) modify ductal bile by a sequence of secretory and absorptive processes mediated by membrane exchangers. This modification mainly leads to the formation of bicarbonate rich bile. Cholangiocytes also secrete VEGF and NGF that are regulated by microRNA 125b and let-7a respectively. [B] The formation of bicarbonate rich bile is enhanced by stimulation with secretin and cAMP, which increase in response to liver insult. Liver behaves as a neuroendocrine compartment in response to injury and starts to respond to hormones and peptides in an autocrine as well as paracrine manner. Liver injury is subsequently followed by large cholangiocyte proliferation under the influence of these factors (neurotransmitters, gastrointestinal peptides, steroids). Large but not small bile ducts express SR and somatostatin receptor 2 (SSTR2) and respond to secretin and somatostatin. Biliary hyperplasia results in cholestasis, which further results in human biliary disorders such as PSC and PBC. Occasionally, in response to specific events injury or drug administration, small cholangiocytes proliferate by an IP3 mediated signaling pathway, often to compensate for the lack of large cholangiocyte proliferation and thus maintain the biliary mass. [Bottom] Isolation of small (right), approximately 9 µm diameter and large (left), approximately 13 µm diameter cholangiocytes from human SV-40 transformed cholangiocytes (H69 cells). Small and large human cholangiocytes were purified by counterflow elutriation followed by immunoaffinity purification. Original magn., × 800.
Cholangiocytes are the specific target of a heterogeneous group of human diseases, termed cholangiopathies, which have deep consequences on the biology of these cells 7. In the present review, we will discuss the differences in the structure and function of cholangiocytes and underline the main findings in biliary pathophysiology of the last 10 years. The clinical implications of ongoing research will also be specifically addressed.
MORPHOLOGY, HEPATIC VASCULATURE AND FUNCTION OF CHOLANGIOCYTES
The biliary epithelium is composed of intra and extra-hepatic bile ducts lined by cholangiocytes 8. The human intrahepatic biliary epithelium is classified by size: hepatic ducts (>800 µm), segmental ducts (400–800 µm), area ducts (300–400 µm), septal bile ducts (100 µm), interlobular ducts (15–100 µm), and bile ductules (<15 µm) 9–11. The intrahepatic biliary epithelium of rodents is formed by ducts of different sizes, small (<15 µm in diameter) and large (>15 µm) 12, 13. Cholangiocytes lining small and large bile ducts have been morphologically and functionally categorized into small and large cholangiocytes, respectively 12–15. With regards to cellular structure, while small cholangiocytes are cuboidal, larger cholangiocytes in larger bile ducts are more columnar in shape 9–11. Moreover, small cholangiocytes are poorly specialized and have a high nucleus/cytoplasm ratio, whereas large cholangiocytes are supplied with plenty of organelles and a small nucleus/cytoplasm ratio 16. Large, but nor small cholangiocytes have cilia, which act as chemo and mechanosensors within bile duct lumen 17. Expression of molecules involved in secretin-stimulated biliary secretion also differs along the biliary epithelium. SR, CFTR and AE2 are only expressed by large cholangiocytes and are responsible for the majority of biliary fluid secretion, through the activation of a cAMP-dependent pathway 12, 13. In small cholangiocytes, on the other hand, Ca2+-activated signaling pathways seem predominant. Indeed, the activation of purinergic receptors in small and large cholangiocytes induces Ca2+-dependent Cl− secretion via TMEM16A, providing an alternative route to the secretin-stimulated cAMP-dependent ductal fluid secretion 18, 19. Functionally, large cAMP-dependent cholangiocytes are more susceptible to damage, whereas small cholangiocytes are more resistant to liver injury 12, 20–22. During damage of large cholangiocytes, however, small cholangiocytes replenish the biliary epithelium. Again, the amplification of Ca2+-dependent signaling pathways in small cholangiocytes are essential in driving the de novo acquisition of large cholangiocyte phenotypes (Figure 1) 20, 21.
Cholangiocytes are normally quiescent in liver but respond to injury or stress by enhanced proliferation 3, 23, 24. Compensatory responses to liver injury include biliary hyperplasia, ductular reaction and ductopenia. Biliary hyperplasia (characterized by proliferation/loss of cholangiocytes as observed in cholestatic liver diseases such as primary sclerosing cholangitis) is associated with enhanced biliary secretion of HCO3− in bile, which may be a compensatory protective mechanism for the injured biliary epithelium 25. On the other hand, ductopenia is evidenced by the damage of bile ducts in response to toxins or in certain diseases such as biliary atresia 15, 20, 21, 26, 27. The hepatic artery is the main blood supplier of the biliary epithelium within the peribiliary vascular plexus (PBP). The PBP secretes a number of angiogenic factors such as VEGF that have been shown to regulate biliary proliferation in experimental models of cholestasis 28–31.
CHANGES OF THE BILIARY EPITHELIUM IN PATHOLOGIC CONDITIONS
Pathophysiology of biliary response to injury
Cholestatic liver diseases represent a heterogeneous group of diseases characterized by an impairment of bile formation or bile flow that can arise at the hepatocellular or cholangiocellular level 32. Emblematic diseases in this group are primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) 33. The current various animal models allow a better insight into the signaling pathways involved in the development of cholestasis. Such studies may provide potential treatment strategies to restore impaired secretory functions of hepatocytes and cholangiocytes, or to modulate the response of these cells to liver injury. Specific types of liver injury activate the proliferation of particular cholangiocyte subpopulations (i.e., large/small) 15, 20, 21. In most instances, biliary proliferation contributes to the major part of the ductular reaction. However, new ductules may also originate from activated progenitor cells or from cells that have entered from the circulation and differentiate into liver cells 23, 34, 35.
Cholangiocyte response to injury is an articulated event, which retains a “double face” in pathophysiologic terms. After an initial insult, cholangiocytes become activated and start to proliferate. This modification is functional to compensate for the anatomical loss of biliary cells and also to sustain their secretory activities 36. In most instances, however, biliary proliferation eventually subsides and apoptotic mechanisms become prevalent with the development of ductopenia 7. Along with proliferation, cholangiocyte response to injury is characterized by the so-called neuroendocrine-like trans-differentiation, which plays an essential role not only in sustaining biliary proliferation itself, but also in immune responses, hepatic inflammation and development of liver fibrosis 4, 23. To this extent, a number of neuroendocrine factors are synthetized de novo by reactive cholangiocyte and have been shown to modulate biliary damage by autocrine/paracrine mechanisms (Table 1) 23. An elegant morphological study has provided strong evidence for the autocrine/paracrine role of VEGF in the regulation of biliary damage. This study has shown that following BDL-induced cholestasis, the PBP undergoes extensive proliferation supporting the increased nutritional and functional needs of the proliferating biliary epithelium. However, the proliferation of the PBP only occurs after cholangiocytes, supporting the autocrine role of the biliary system (secreting angiogenic factors) in the regulation of biliary function 31. To support the important role of angiogenic factors in sustaining biliary growth, we have shown that secretin stimulates biliary proliferation by microRNA 125b and let7a–dependent upregulation of VEGF-A and NGF, respectively 37. Knockout of the secretin receptor (that is only expressed by large cholangiocytes) decreases biliary hyperplasia in cholestatic mice by downregulation of cAMP signaling 38. Other studies have demonstrated the pro-proliferative effect of VEGF-A and VEGF-C, which increased biliary growth of normal rats by interaction with VEGFR2 and VEGFR3, respectively. The same study also showed that the in vivo administration of neutralizing antibodies for VEGF-A/C decreased BDL-induced biliary hyperplasia 29. The paracrine effect of VEGFs on biliary functions was also demonstrated in experiments where the ligation of the hepatic artery resulted in disappearance of the PBP (source of angiogenic factors such as VEGFs), significant reduction of biliary growth (accompanied by enhanced apoptosis), and reduced secretion of VEGF and bicarbonate by cholangiocytes 28. Another study has shown that inhibition of VEGF expression in cholangiocytes by overexpression of miR-125b and knockdown of histidine decarboxylase (HDC, the enzyme regulating histamine synthesis) decreased BDL-induced biliary hyperplasia 39. Consistent with the role of VEGF on biliary functions, a recent study has shown that VEGF plays an important role on infection-induced increase of biliary cystogenesis in cholangiocytes of the polycystic kidney (PCK) rat model 40. Prolonged feeding of taurocholic acid to BDL rats prevents biliary damage induced by hepatic artery ligation or caffeic acid by overexpression of VEGF-A 41, 42. Also, cholangiocyte neuroendocrine-like trans-differentiation, driven by the de novo expression of pancreatic duodenal homeobox-1, has been associated with enhanced biliary VEGF expression 43. Another study has shown that cholangiocytes generate a VEGF gradient that is key during arterial vasculogenesis, whereas angiopoietin-1 signaling from hepatoblasts participates in the remodeling of the hepatic artery to sustain the nutritional demands of the proliferating biliary epithelium 44.
Table 1.
Summarizing the main neuroendocrine factors involved in cholangiocyte response to injury
| Molecule | Functions | Ref. |
|---|---|---|
| Secretin | • Stimulates the proliferation of both normal and BDL large cholangiocytes | 38 |
| • Produced by cholangiocytes and S cell, induces the upregulation of VEGF and NGF via downregulation of microRNA 125b and let7a | 37, 39 | |
| VEGF | • As a component of PDX-1-induced neuroendocrine-like trans-differentiation of cholangiocytes, stimulates biliary proliferation via an autocrine mechanism | 29, 43 |
| • Sustains cholangiocyte proliferation and PBP reactive expansion after BDL | 28 | |
| • Stimulates biliary cystogenesis in cholangiocytes of the polycystic kidney rat model | 40 | |
| • Participates to arterial vasculogenesis during human liver embryogenesis | 44 | |
| FSH | • Stimulates cholangiocyte proliferation | 45 |
| Histamine | • Stimulates small cholangiocyte proliferation via the activation of the histamine receptor H1 | 22, 47 |
| • Stimulates large cholangiocyte proliferation via the activation of the histamine receptor H2 | 47 | |
| • Reduces cholangiocyte proliferation via the activation of the histamine receptor H3 | 57 | |
| • Increases the growth of cholangiocarcinoma cells and the synthesis of VEGF | 59 | |
| Estrogens | • Stimulate cholangiocyte proliferation and prevent apoptosis in BDL rats | 49, 50 |
| NGF | • Stimulates cholangiocyte proliferation (additive effect in combination with estrogens) | 48 |
| Serotonin | • Inhibits cholangiocyte proliferation and secretory activities | 52, 53 |
| Melatonin | • Produced by both pineal gland and cholangiocytes, inhibits biliary proliferation and secretory functions of BDL rats | 24, 54, 55 |
| • Down regulates VEGF synthesis by cholangiocytes | 56 | |
Biliary hyperplasia is also promoted by a number of growth factors such as nerve growth factor (NGF), follicle stimulating hormone (FSH), gonadotropin-releasing hormone (GnRH), estrogens, and the biogenic amine histamine, by the interaction with their specific receptors 22, 45–47. For example, we have shown that: (i) intrahepatic bile ducts secrete NGF and express NGF receptors; and (ii) NGF stimulates (in combination with estrogens) biliary proliferation by activating the ERK pathway as well as the phosphatidylinositol 3-kinase pathway 48. The decrease in intrahepatic bile duct mass (concomitant with reduced expression of estrogen receptor-alpha and beta and enhanced biliary apoptosis) support the role of endogenous estrogens in sustaining the enhanced proliferative and secretory activities of cholangiocytes during cholestasis, which may be important during ductopenic states 49. Supporting this concept, another study has shown that estrogens maintain biliary mass and reduce apoptosis after biliodigestive anastomosis in cholestatic BDL rats 50. A recent study has also shown that cholangiocytes express FSH and its receptor (FSHR) and also secrete FSH. In vivo, FSH increases biliary mass, whereas administration of antide (a gonadotropin-releasing hormone antagonist blocking FSH secretion) and anti-FSH antibody to BDL rats decreased cAMP-dependent cholangiocyte proliferation and biliary mass. Modulation of biliary FSH expression may be a target for the management of cholestatic liver diseases 45. FSH as well as other growth factors including estrogens either directly or by synergizing NGF, IGF1, FSH and VEGF have been shown to regulate the proliferative and secretive activities of cystic epithelium of polycystic liver diseases (PCLDs) in rodent models and human cell lines. Also, GnRH (secreted by the hypothalamus as well as cholangiocytes) has been shown to stimulate biliary proliferation by both paracrine/autocrine pathways 51. Disruption of GnRH/GnRHR cascade may be an important target for the management of cholangiopathies.
Conversely, a number of other molecules have been shown to reduce cholangiocyte proliferation. For example, the activation of serotonin 1A and 1B receptors inhibits biliary hyperplasia in cholestatic rats by enhanced IP3/Ca2+/protein kinase C signaling and subsequent inhibition of the cAMP/protein kinase A/Src/extracellular signal-regulated kinase 1/2 pathway. Cholangiocytes also secrete serotonin that reduces biliary proliferation during the course of cholestasis in an autocrine fashion 52. In addition, they express the neuronal isoform of neuronal tryptophan hydroxylase (TPH) and synthesize serotonin and use serotonin as an autocrine/paracrine signal to regulate biliary remodeling 53. Other studies provide evidence for the growth limiting function of the hormone melatonin. In cholestatic BDL rats, melatonin both in vivo and in vitro decreased biliary hyperplasia by cAMP-dependent downregulation of clock gene expression through the interaction with MT1 receptor subtype 24. Furthermore, when BDL rats were housed in prolonged darkness there was reduced biliary hyperplasia and fibrosis, which was accompanied by a significant increase in the serum levels of melatonin likely originating from the pineal gland 54. Hepatic inhibition of arylalkylamine N-acetyltransferase (AANAT, the rate limiting enzyme regulating melatonin synthesis) by Vivo-Morpholino sequence of AANAT (that decreases melatonin hepatic secretion) increases biliary growth and the expression of angiogenic factors in cholestatic rats 55, 56. A number of elegant studies have also been performed to evaluate the role of histamine on cholangiocyte proliferation. It has been found in rodent models of cholestasis that histamine increases or inhibits biliary proliferation by interacting with either H1-H2HR histamine receptors (stimulatory) or H3-H4HR histamine receptors (inhibitory) 22, 47, 57, 58. Stimulation of H3HR by H3HR agonist decreases BDL-induced cholangiocyte hyperplasia via inhibition of cAMP signaling, thus suggesting a possible beneficial effect of histamine in cholangiopathies. Histamine also interacts with the H1HR receptor and increases the proliferation of small cholangiocytes by activation of IP3/Ca2+/CaMKI/CREB-dependent signaling 58, 59. This differential response induced by histamine may be employed in variable conditions of liver diseases where either reduction in biliary hyperplasia or regeneration of liver would be desirable depending on the injury. It is evident from these studies that modulation of the different receptors could be of prime importance while managing the balance between biliary growth/loss in cholangiopathies or post transplantation. In vivo, GABA induces the damage of cAMP-dependent large cholangiocytes concomitant with de novo proliferation of small cholangiocytes which amplify their Ca2+-dependent signaling and acquire phenotypes of large cholangiocytes to repair the damaged biliary epithelium 20, 21.
Emerging morphogenic pathways regulating biliary pathophysiology
Hippo signaling pathway
The Hippo signaling pathway is an evolutionarily conserved pathway that regulates bile duct differentiation and homeostasis in the liver 60. The core Hippo signaling pathway is a kinase cascade 61. The apical membrane-associated FERM domain protein neurofibromin 2 (NF2) directly binds and recruits the Nuclear Dbf2-related family kinase, large tumor suppressor homolog 1/2 (LATS1/2), to the plasma membrane. Membrane recruitment, in turn, promotes LATS1/2 phosphorylation by the Ste-20 family protein kinase, Mammalian STE20-Like Protein Kinase 1/2 (MST1/2), together with the adaptor protein salvador homolog 1 (SAV1). In turn, LATS1/2, in a complex with small regulator protein Mps One Binder Homolog A (MOB1), phosphorylates Yes-associated protein (YAP), a transcription coactivator. Phosphorylation of YAP deactivates its transcription coactivator activity through sequestering YAP in cytoplasm. YAP is highly expressed in cholangiocytes of both mouse and human liver, suggesting a role of YAP in cholangiocyte biology 62, 63. Using genetically modified mouse models, transcriptional regulation activity of YAP was found to be required for bile duct development 64. Liver specific Yap deletion leads to postnatal bile duct paucity due to failure formation of primitive ductal structures around E18.5. In agreement, increasing YAP activity through ablating upstream negative regulator, Nf2, significantly increases the numbers of primitive ductal structures and results in bile duct hyperplasia 64. However, the YAP downstream targets involved in regulating bile duct development remain to be elucidated. YAP is also important for determining biliary cell fate 62, 65. Comparing to cholangiocytes, hepatocytes keep a lower YAP activity. Increasing hepatocyte YAP activity through ectopic YAP expression or ablating upstream negative regulator Mst1/2 dedifferentiate periportal hepatocytes into ductal cells. Furthermore, Carmago et al. demonstrated that YAP regulates hepatic cell fate determination directly through the Notch signaling, another critical signaling pathway for bile duct development62.
Notch signaling pathway
The Notch signaling pathway contains four transmembrane Notch receptors (Notch-1, −2, −3, −4), and two types of cell surface ligands, Serrate/Jagged (Jag-1, −2) or Delta-like (Dll-1, −3, −4)66. The activation of the Notch signaling requires a cell-cell interaction between the “transmitting” cell expressing Notch ligands and the “receiving” cell expressing Notch receptors. Upon ligand engagement, the Notch receptor is cleaved by the γ-secretase complex, leading to the cytoplasmic release of the Notch intracellular domain (NICD). NICD will then translocate into the nucleus where it binds to the recombination signal binding protein immunoglobulin kappa J (RBP-Jκ) to displace the RBP-Jκ associated co-repressors, thereby allowing the transcription of the Notch-target genes. Among them, the Hairy enhancer of split homologs transcription factors (Hes and Hey), the family of the hepatocyte nuclear factors (HNF) and the Sex determining region Y-box 9 (Sox-9) are involved in biliary cell differentiation. Mouse models deficient in Notch receptor Notch-2 67–69, Notch ligands Jag-1 70,71, Notch nuclear effector RBP-Jκ 72,73, Notch transcription target Hes-1 74, Sox-9 and HNF1β (Ref 13) 75,76, all show defects in intrahepatic bile duct tubulogenesis during fetal development and early post-natal life. Consistently, constitutive activation of the Notch-2 intracellular domain (NICD) in hepatoblasts during development leads to ectopic formation of tubular and cystic structures, resembling early malignant biliary lesions 77,78. In agreement with their physiological role in the commitment of the biliary lineage, Notch2, Jagged1, Hes1, Sox-9 and HNF1β are highly expressed in biliary cells 73, 74.
Hedgehog Signaling
Both immature and mature cholangiocytes produce and respond to the Hedgehog (Hh) signaling ligands, Sonic hedgehog (Shh) and Indian hedgehog (Ihh) 79–81. Shh and Ihh ligands then bind to their transmembrane Hedgehog receptor Patched (Ptc), which relieve the suppression of Smoothened (Smo), leading to activation of the Glioblastoma (Gli) family of transcription factors (Gli1, Gli2, Gli3) 79. The important role of the Hedgehog pathway in cholangiocyte pathogenesis was demonstrated with a cholestatic injury model 80, 82. Dramatic increases in hepatic expression of Hh ligands and up-regulation of Hh pathway activity occur post bile duct ligation (BDL) in rodents. Moreover, mice with a genetic ablation of Ptc exhibited exacerbated ductular and fibrogenic responses. However, the physiological role and the molecular mechanism of the Hedgehog signaling during maintenance of bile duct homeostasis are not fully understood and remains to be further investigated.
Role of cholangiocytes in the development of human chronic cholestatic conditions
Primary Sclerosing Cholangitis
Signaling mechanisms fueling PSC development are being studied in several different animal models. Among the different rodent models, the MDR2−/− mice have been particularly helpful in studying the development of fibrosis in PSC 83. MDR2−/− mice have decreased concentration of phosphatidylcholine in bile, which is known to potentiate the toxicity of bile acids. Additionally, leakage of bile into portal tracts, caused by disrupted tight junctions of the biliary epithelium, has been demonstrated in MDR2−/− mice and is responsible for causing inflammation and fibrosis 84. Fibrosis is regulated by the expression of several pro/anti fibrotic genes. For example, TIMP-1 mRNA expression is increased, whereas MMP-13 is suppressed in this model. Additionally, a number of pro-inflammatory molecules such as TNF-α, IL-1β, IL-6, TGF-β1 and interferon-γ are overexpressed in MDR2−/− mice compared to controls. Mainly based on experiments on MDR2−/− mice 85, new possible treatment options for PSC are currently being evaluated in clinical trials (as discussed below).
In a recent study, cholangiocytes where isolated from livers of PSC patients 86. The authors were able to culture intrahepatic cholangiocytes and further confirm their purity by immunofluorescence studies for cholangiocyte specific markers such as CK-19. The authors also showed that PSC cholangiocytes expressed less tight junction proteins, ZO1 (indicating impaired epithelial junctions) and were enlarged in size with robust filamentous structures throughout the cell body. Further, these cholangiocytes exhibited characteristics of cellular senescence when compared with normal human cholangiocytes and H69. Next Generation Sequencing confirmed the elevated expression of pro-inflammatory cytokines and chemokines compared to controls. Thus, this study has provided targets that could potentially be used for devising treatment protocols for the management of PSC 86.
As for many other diseases, genome-wide association studies (GWASs) represent a promising approach not only for dissecting the pathophysiology of PSC but also for the identification of possible therapeutic targets. To date, a total of 16 genes have been associated with increased risk of PSC 87. Among others, the single nucleotide polymorphism (SNP) located at chromosomal region 2q35 has attracted the interest of researchers. This SNP is in close proximity to the TGR5 gene and has been associated both with PSC and ulcerative colitis 88. TGR5 is the first G-protein coupled receptor for bile acids with important roles in energy expenditure and basal metabolism 89. Interestingly, five mutations in the TGR5 gene have been shown to reduce or abolish the function of the protein 90. The activation of TGR5 in cholangiocytes is thought to stimulate bicarbonate secretion 91, possibly contributing to the protection of the biliary epithelium via the biliary bicarbonate umbrella 25.
Primary Biliary Cirrhosis
Primary Biliary Cirrhosis (PBC) is an immune-mediated pathology of the biliary tree characterized by the generation of anti-mitochondrial antibodies (AMAs) directed against the pyruvate dehydrogenase complex (PDC-E2) 92. Recent studies have shown that TLR9 and CD86 expression is enhanced in B cells of PBC patients 93, 94. Profiling studies for cytokines and chemokines have shown that these molecules are important in the pathogenesis of PBC 95. Further, there is often involvement of autoreactive CD4+ and CD8+ T cells in PBC. A number of animal models for PBC have been proposed. Despite the fact that none of them is able to perfectly recapitulate the complex interactions of the human disease, they have proved to be valuable tools in order to study PBC alterations and explore possible therapeutic targets. Briefly, the NOD.c3c4 mouse was the first animal model to develop PBC like characteristics 96. The second mouse model, which is most frequently used for studying PBC, owing to the similarity of human PBC, is the one expressing the dominant negative form of TGFβ receptor II (dnTGF βRII). This particular mouse model is characterized by higher serum level of TNF-α, IFNY and IL-6 when compared to control animals 97. Similarly, elevated serum cytokines, lymphocyte infiltration around portal tracts and cholangiocyte injury are noted in a third rodent model of PBC. This is called the IL-2Ra knockout mice model98. In genetically susceptible individuals, environmental factors may trigger an immune-mediated injury of cholangiocytes. The immunological events then occur in a step-by-step manner, starting from antigen presentation, T-cell differentiation, proliferation and recruitment, finally resulting in an effector cell response and production of autoantibodies99 . In this context, a number of different signaling pathways have been implicated in this disease development or progression, and as such, any of these steps could theoretically be targeted for treatment of PBC. Since many pathophysiological events of the human disease remain obscure and may differ from animal models, caution should be implemented while evaluating the experimental effects of the manipulation of signaling pathways. Nonetheless, antibody mediated therapy, targeted inhibition of cellular pathways relevant to immune regulation and cell therapy methods directed towards reprogramming the immunomodulatory axis remain an intriguing opportunity to treat PBC patients 100–102.
Biliary Atresia
Biliary atresia (BA) is a disease caused by obstructive cholangiopathy resulting from inflammation and fibrosis of extra-hepatic bile ducts. Inflammatory reactions triggered by viral infection have been proposed as the possible cause of BA by several population studies as well as in murine models 103. Population studies have proved the presence of human papillomavirus, cytomegalovirus and reovirus in livers of BA patients 104–106. Evidences from studies in rhesus-rotavirus infected murine model of BA as well as from fixed liver tissues from BA patients have shown that there are structural as well as pathological changes in the extrahepatic cholangiocytes only. It was observed that primary cilia were selectively lost from the extrahepatic and not intrahepatic cholangiocytes post rotavirus infection in experimental mice 107, 108. Chemokine expression levels were also increased in cholangiocytes isolated from rotavirus-infected mice as well as in virus-infected cholangiocytes in culture 109. In this study, quantitative and qualitative assessments of several chemokines were performed. MIP-2 and monocyte chemotactic protein 1 were up-regulated after rotavirus infection when compared to normal both in vivo and in vitro.
Cholangiocyte proliferation and subsequent enlargement of extrahepatic bile ducts in BA has been linked to over expression of IL-33 and activation of Th-2 cells. Serum levels of IL-33 are elevated in biliary atresia patients, and in livers and bile ducts of experimental mice.. Moreover, treatment of normal wild-type mice with IL-33 promoted cholangiocyte proliferation and cell growth culminating in significantly enlarged extra-hepatic bile ducts. In the same study it was shown that bile ducts genetically primed to cholangiocarcinoma (by constitutive activation of Akt-Yap pathway) responded to administration of IL-33 via development of advanced tumors with intrahepatic metastases compared to controls. Such data suggests that activation of IL-33 pathway may help biliary repair, and disruption of the same may halt carcinogenesis 110. Other studies indicate the involvement of factors such as granzymes, which are secreted by hepatic NK and CD8 T cells, and injure cholangiocytes in short-term culture. Consistent with in vitro data, it has been noted that in infants with BA there are increased hepatic mRNA expression of granzymes A and B 111. Thus these studies offer multiple targets that could be manipulated to manage cholangiocyte proliferation accompanying liver conditions such as BA. Recently, the role of microRNAs in liver diseases is being increasingly recognized 112. For example, miR-21 was found up regulated during early stages of liver regeneration by targeting pellino 1 antibody 113. Let-7 family members, miR-127, miR-26a, miR-34a and miR-23b were all found dysregulated during liver regeneration. Similarly, microRNA expression profiles have been found altered during treatment of mice with rhesus rotavirus, in a time dependent fashion, in the extrahepatic bile ducts from the experimental animals. For instance, changes in expression pattern of miR-30b/c, −133a/b, −195, −200a, −365 have been proposed in the development of BA 114. Hence, these reports could provide alternative treatment protocols for life threatening conditions such as biliary atresia in small children.
CLINICAL IMPLICATIONS OF RECENT ADVANCES IN BILIARY RESEARCH
Despite the enormous progresses of recent years, the pathophysiology of cholangiopathies is far from being completely understood, with severe consequences on the development of effective new treatments.
Important signals come from the clinical practice. To date, orthotopic liver transplantation (OLT) remains the only curative treatment of cholestatic liver diseases (these represent as much as 20% of OLT indications in adults) 115. Moreover, symptoms such as fatigue and pruritus are often scarcely alleviated by standard medical approaches 116,117.
Ursodeoxycholic acid (UDCA) remains the only approved drug for the treatment of fibrosing cholangiopathies. UDCA exert its effects on multiple levels, from the protection of cholangiocytes against toxic bile acid, to the stimulation of choleresis through post-transcriptional effects on hepatocellular and cholangiocyte transporters 118, 119.
The administration of UDCA in a dose of 13–15 mg/kg/day has well-established favorable effects on long-term survival of PBC patients 120, 121. Transplant-free survival in early-stage PBC patients treated with UDCA has been shown to be similar to healthy controls, matched for age and gender 122, 123. However, not all PBC patients respond to UDCA administration. A good biochemical response was achieved only in 61% of PBC patients treated with UDCA, as defined by the “Paris criteria” that strongly correlates with transplant-free survival at 10 years 124.
If the administration of UDCA is universally recognized as the standard treatment for PBC, definitive evidence to recommend its use in PSC is still lacking. Moreover, high doses of 28–30 mg/kg/day of UDCA in PSC patients have been associated with increased risk of liver transplantation and development of esophageal varices 125. As a matter of fact, the latest available European guidelines do not propose any specific recommendation for UDCA use in PSC 126.
In this scenario, the development of alternative therapies for cholestatic liver diseases is utterly needed and intense research is ongoing. Promising results have recently emerged from the study of two bile acids derivatives, obeticholic acid and norursodeoxycholic acid.
Obeticholic acid (OCA), also known as INT-747, is a semisynthetic analogue of chenodeoxycholic acid (CDCA) that possesses a strong farnesoid X receptor (FXR) affinity 127. The role of FXR in bile acid homeostasis has clearly emerged in recent years. Endogenous bile acids bind to FXR which, in turn, is able to repress or induce the expression of various genes involved in their synthesis and secretion, such as CYP7A1, BSEP and NTCP 115. CDCA is the most potent endogenous FXR ligand (with a 100-fold less affinity than OCA), while UDCA has no affinity. Interestingly, Fxr−/− mice have elevated serum bile acids levels and the infusion of OCA in rats is able to stimulate bile flow and protect against lithocholic acid-induced liver damage 127, 128. Given these premises, the efficacy and safety of OCA has been recently tested in 165 PBC patients who failed to achieve a good biochemical response to UDCA alone. The results of the study demonstrated that the administration of 10, 25 or 50 mg of OCA was able to significantly reduced levels of ALP, gamma-GT, and alanine aminotransferase, compared to placebo. However, a significant increase in pruritus was also registered. The severity of itch was worse than placebo with all 3 OCA dosages used, while the incidence of pruritus was higher only in the 2 higher-dosing groups 129. Phase 2 and phase 3 studies involving OCA are currently underway, with extremely promising preliminary results. Indeed, the administration of 5 or 10 mg of OCA has been shown to be superior to placebo in determining the improvement of biochemical parameters correlated with clinical outcome in patients with inadequate response to UDCA 130.
Norursodeoxycholic acid (norUDCA) is a C23 homologue of UDCA, with one fewer methylene group in the side chain of the molecule. The biology of norUDCA has peculiar characteristics; in fact, this bile acid derivative is usually not conjugated with taurine or glycine. It is secreted into the bile canaliculi and reabsorbed by cholangiocytes, from where it returns to the liver. The resulting cholehepatic shunting leads to a bicarbonate rich-choleresis, which is thought to protect cholangiocytes against the toxicity of bile acids 131, 132. Fickert et al. have tested the possible therapeutic effect of norUDCA in Mdr2−/− mice, a model for sclerosing cholangitis 84. The authors demonstrated that the administration of norUDCA was able to ameliorate liver tests and liver histology in Mdr2−/− mice, in contrast to UDCA that had detrimental effects 85. A recent study of the same group confirmed that norUDCA was able to improve liver injury in the selective bile duct ligation model in mice, where UDCA administration resulted again significantly more toxic than norUDCA 133. Based on these results, a phase 2 trial is currently recruiting patients to test the safety and efficacy of norUDCA in PSC patients.
Monoclonal antibodies have also attracted the interest of researches as a possible therapeutic tool to treat cholangiopathies. Given the encouraging results obtained with anti-CD20 antibodies in the dnTGF βRII mice 134, the monoclonal antibody rituximab has been tested in a phase 1 trial in 6 PBC patients with incomplete response to UDCA. Rituximab treatment proved to be safe in PBC patient and was able to transiently reduce serum levels of total IgG, IgM, and IgA and AMAs 135. Based on the results of recent GWAS showing a genetic association between variants of the IL-2 and IL-23 pathways and PBC 136, 137, a phase 2 clinical trial is currently underway evaluating the safety and efficacy of ustekinumab, an anti-p40 monoclonal antibody.
The safety and efficacy of two different monoclonal antibodies (BTT1023 and simtuzumab) are being investigated in PSC patients. This study is currently in phase 2 clinical trials. BTT1023 is a human monoclonal antibody targeting the vascular adhesion protein-1 (VAP-1), a molecule that has been shown to stimulate the recruitment of effector lymphocytes to the liver through the upregulation of the endothelial cell adhesion molecule MadCAM-1 138, 139. Simtuzumab is directed against the lysyl oxidase-like protein 2 (LOXL2), an enzyme that favors the cross-linking of collagen and elastin fibers 140. The results of these studies will hopefully lay the basis for possible new and effective treatments for biliary diseases.
CONCLUSION/FUTURE PERSPECTIVE
The knowledge of the mechanisms regulating biliary cell response to injury has enormously grown in the last few decades 141. The studies of recent years have clarified that cholangiocytes are not the passive targets of biliary diseases. Indeed, reactive cholangiocytes undergo a series of profound modifications and acquire a neuroendocrine-like phenotype that allows cells to regulate the complex molecular interactions that occur in the diseased liver 4, 23. As discussed in the review, a number of molecular pathways have been shown to deeply influence cholangiocyte response to injury. Moreover, animal models have proved an invaluable tool in order to dissect the pathophysiological changes occurring in the biliary tree in response to injury, providing important clues on the complex interactions occurring in vivo. As a result of these continuous efforts, new potential treatments for PBC and PSC have been developed and are currently investigated in clinical trial with promising results. However, the etiology of many cholangiopathies is still obscure, and much work remains to be done to translate the large amount of data collected on disease pathogenesis into effective medical treatments capable of influencing the natural history of biliary diseases.
Acknowledgments
We acknowledge with gratitude the editing assistance of Tami Annable.
This work was supported, in whole or in part, by National Institutes of Health Grants DK054811 and DK076898 (to G. A. and F. M.). This work was also supported by Veterans Affairs Merit Review Grant 1I01BX001724 (to F. M. and G. A.), Veterans Affairs Research Career Award (to G. A.), the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White (to G. A.), and Scott & White Research Grants Program Project 90190 (to F. M.).
Abbreviations
- AE2
anion exchanger 2
- BA
biliary atresia
- BDL
bile duct ligation
- SR
secretin receptor
- cAMP
3',5'-cyclic monophosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- FSH
follicle stimulating hormone
- GnRH
gonadotropin-releasing hormone
- IP3
d-myo-inositol 1,4,5-triphosphate
- MT1
melatonin Receptor 1
- PBC
primary biliary cirrhosis
- PBP
peribiliary plexus
- PKA
protein kinase A
- PSC
primary sclerosing cholangitis
- TMEM16A
transmembrane member 16A
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- UDCA
ursodeoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 2.Cardinale V, Wang Y, Carpino G, et al. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol. 2012;9:231–240. doi: 10.1038/nrgastro.2012.23. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franchitto A, Onori P, Renzi A, et al. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med. 2013;1:27. doi: 10.3978/j.issn.2305-5839.2012.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afroze S, Meng F, Jensen K, et al. The physiological roles of secretin and its receptor. Ann Transl Med. 2013;1:29. doi: 10.3978/j.issn.2305-5839.2012.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 7.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Alpini G, Prall RT, LaRusso NF. In: The pathobiology of biliary epithelia. The Liver: Biology and Pathobiology. 4th edition. Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, editors. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 9.Schaffner F, Popper H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig J. New concepts in biliary cirrhosis. Semin Liver Dis. 1987;7:293–301. doi: 10.1055/s-2008-1040584. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki H, Schaffner F, Popper H. Bile ductules in cholestasis: morphologic evidence for secretion and absorption in man. Lab Invest. 1967;16:84–95. [PubMed] [Google Scholar]
- 12.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 13.Glaser S, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 15.LeSage G, Glaser S, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 16.Benedetti A, Bassotti C, Rapino K, et al. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol. 1996;24:335–342. doi: 10.1016/s0168-8278(96)80014-6. [DOI] [PubMed] [Google Scholar]
- 17.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237:2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo K, Sathe M, Kresge C, et al. Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology. 2010;52:1819–1828. doi: 10.1002/hep.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta AK, Khimji AK, Kresge C, et al. Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium. J Biol Chem. 2011;286:766–776. doi: 10.1074/jbc.M110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancinelli R, Franchitto A, Glaser S, et al. GABA induces the differentiation of small into large cholangiocytes by activation of Ca(2+) /CaMK I-dependent adenylyl cyclase 8. Hepatology. 2013;58:251–263. doi: 10.1002/hep.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis H, Glaser S, DeMorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Renzi A, Glaser S, DeMorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301:G634–G643. doi: 10.1152/ajpgi.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuers U, Hohenester S, de Buy Wenniger LJ, et al. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguti DC, Patricio FR. Morphometrical and immunohistochemical study of intrahepatic bile ducts in biliary atresia. Eur J Gastroenterol Hepatol. 2011;23:759–765. doi: 10.1097/MEG.0b013e32832e9df0. [DOI] [PubMed] [Google Scholar]
- 27.Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186–2194. doi: 10.1038/ajg.2010.216. [DOI] [PubMed] [Google Scholar]
- 28.Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–G317. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 29.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Glaser S, Gaudio E, Alpini G. Vascular factors, angiogenesis and biliary tract disease. Curr Opin Gastroenterol. 2010;26:246–250. doi: 10.1097/MOG.0b013e3283369d19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudio E, Onori P, Pannarale L, et al. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–1124. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 32.Poupon R. Liver alkaline phosphatase: A missing link between choleresis and biliary inflammation. Hepatology. 2015 doi: 10.1002/hep.27715. [DOI] [PubMed] [Google Scholar]
- 33.Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377–1387. doi: 10.1002/hep.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azmaiparashvili E, Berishvili E, Kakabadze Z, et al. Ductular reaction at the early terms of common bile duct ligation in the rats. Acta Biol Hung. 2012;63:321–332. doi: 10.1556/ABiol.63.2012.3.2. [DOI] [PubMed] [Google Scholar]
- 35.Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 36.Lesage G, Glaser SS, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 37.Glaser S, Meng F, Han Y, et al. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaser S, Lam IP, Franchitto A, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng F, Onori P, Hargrove L, et al. Regulation of the histamine/VEGF axis by miR-125b during cholestatic liver injury in mice. Am J Pathol. 2014;184:662–673. doi: 10.1016/j.ajpath.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Ren XS, Sato Y, Harada K, et al. Biliary infection may exacerbate biliary cystogenesis through the induction of VEGF in cholangiocytes of the polycystic kidney (PCK) rat. Am J Pathol. 2011;179:2845–2854. doi: 10.1016/j.ajpath.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glaser S, Onori P, Gaudio E, et al. Taurocholic acid prevents biliary damage induced by hepatic artery ligation in cholestatic rats. Dig Liver Dis. 2010;42:709–717. doi: 10.1016/j.dld.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancinelli R, Onori P, Gaudio E, et al. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med (Maywood) 2009;234:462–474. doi: 10.3181/0808-RM-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marzioni M, Saccomanno S, Candelaresi C, et al. Pancreatic Duodenal Homeobox-1 de novo expression drives cholangiocyte neuroendocrine-like transdifferentiation. J Hepatol. 2010;53:663–670. doi: 10.1016/j.jhep.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Fabris L, Cadamuro M, Libbrecht L, et al. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719–728. doi: 10.1002/hep.22015. [DOI] [PubMed] [Google Scholar]
- 45.Mancinelli R, Onori P, Gaudio E, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–G26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen K, Marzioni M, Munshi K, et al. Autocrine regulation of biliary pathology by activated cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2012;302:G473–G483. doi: 10.1152/ajpgi.00482.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francis HL, DeMorrow S, Franchitto A, et al. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest. 2012;92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gigliozzi A, Alpini G, Baroni GS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–1209. doi: 10.1053/j.gastro.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Alvaro D, Alpini G, Onori P, et al. Effect of ovariectomy on the proliferative capacity of intrahepatic rat cholangiocytes. Gastroenterology. 2002;123:336–344. doi: 10.1053/gast.2002.34169. [DOI] [PubMed] [Google Scholar]
- 50.Svegliati-Baroni G, Ghiselli R, Marzioni M, et al. Estrogens maintain bile duct mass and reduce apoptosis after biliodigestive anastomosis in bile duct ligated rats. J Hepatol. 2006;44:1158–1166. doi: 10.1016/j.jhep.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Ray D, Han Y, Franchitto A, et al. Gonadotropin-releasing hormone stimulates biliary proliferation by paracrine/autocrine mechanisms. Am J Pathol. 2015;185:1061–1072. doi: 10.1016/j.ajpath.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Omenetti A, Yang L, Gainetdinov RR, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol. 2011;300:G303–G315. doi: 10.1152/ajpgi.00368.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han Y, Onori P, Meng F, et al. Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G894–G904. doi: 10.1152/ajpgi.00288.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renzi A, DeMorrow S, Onori P, et al. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology. 2013;57:1130–1141. doi: 10.1002/hep.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renzi A, Mancinelli R, Onori P, et al. Inhibition of the liver expression of arylalkylamine N-acetyltransferase increases the expression of angiogenic factors in cholangiocytes. Hepatobiliary Surg Nutr. 2014;3:4–10. doi: 10.3978/j.issn.2304-3881.2014.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francis H, Meng F, Gaudio E, et al. Histamine regulation of biliary proliferation. J Hepatol. 2012;56:1204–1206. doi: 10.1016/j.jhep.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francis H, DeMorrow S, Venter J, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu FX, Meng Z, Plouffe SW, et al. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201–227. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- 61.Yin F, Yu J, Zheng Y, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai H, Gayyed MF, Lam-Himlin DM, et al. Expression of Yes-associated protein modulates Survivin expression in primary liver malignancies. Hum Pathol. 2012;43:1376–1385. doi: 10.1016/j.humpath.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitamant J, Kottakis F, Benhamouche S, et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morell CM, Fiorotto R, Fabris L, et al. Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis. Clin Res Hepatol Gastroenterol. 2013;37:447–454. doi: 10.1016/j.clinre.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Geisler F, Nagl F, Mazur PK, et al. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 68.Fiorotto R, Raizner A, Morell CM, et al. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol. 2013;59:124–130. doi: 10.1016/j.jhep.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofmann JJ, Zovein AC, Koh H, et al. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loomes KM, Russo P, Ryan M, et al. Bile duct proliferation in liver-specific Jag1 conditional knockout mice: effects of gene dosage. Hepatology. 2007;45:323–330. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- 72.Sparks EE, Huppert KA, Brown MA, et al. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–1400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kodama Y, Hijikata M, Kageyama R, et al. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Antoniou A, Raynaud P, Cordi S, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coffinier C, Gresh L, Fiette L, et al. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 77.Jeliazkova P, Jors S, Lee M, et al. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013;57:2469–2479. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 78.Tchorz JS, Kinter J, Muller M, et al. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology. 2009;50:871–879. doi: 10.1002/hep.23048. [DOI] [PubMed] [Google Scholar]
- 79.Omenetti A, Diehl AM. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol. 2011;27:268–275. doi: 10.1097/MOG.0b013e32834550b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 81.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omenetti A, Popov Y, Jung Y, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 83.Popov Y, Patsenker E, Fickert P, et al. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 84.Fickert P, Fuchsbichler A, Wagner M, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Fickert P, Wagner M, Marschall HU, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 86.Tabibian JH, Trussoni CE, O'Hara SP, et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 2014;94:1126–1133. doi: 10.1038/labinvest.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Folseraas T, Liaskou E, Anderson CA, et al. Genetics in PSC: What Do the "Risk Genes" Teach Us? Clin Rev Allergy Immunol. 2015;48:154–164. doi: 10.1007/s12016-014-8417-z. [DOI] [PubMed] [Google Scholar]
- 88.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 89.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hov JR, Keitel V, Laerdahl JK, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keitel V, Haussinger D. TGR5 in the biliary tree. Dig Dis. 2011;29:45–47. doi: 10.1159/000324127. [DOI] [PubMed] [Google Scholar]
- 92.Dyson JK, Hirschfield GM, Adams DH, et al. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2015;12:147–158. doi: 10.1038/nrgastro.2015.12. [DOI] [PubMed] [Google Scholar]
- 93.Kikuchi K, Lian ZX, Yang GX, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Moritoki Y, Lian ZX, Wulff H, et al. AMA production in primary biliary cirrhosis is promoted by the TLR9 ligand CpG and suppressed by potassium channel blockers. Hepatology. 2007;45:314–322. doi: 10.1002/hep.21522. [DOI] [PubMed] [Google Scholar]
- 95.Manousou P, Kolios G, Drygiannakis I, et al. CXCR3 axis in patients with primary biliary cirrhosis: a possible novel mechanism of the effect of ursodeoxycholic acid. Clin Exp Immunol. 2013;172:9–15. doi: 10.1111/cei.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Irie J, Wu Y, Wicker LS, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oertelt S, Lian ZX, Cheng CM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 98.Wakabayashi K, Lian ZX, Moritoki Y, et al. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 99.Jones DE. Pathogenesis of primary biliary cirrhosis. Gut. 2007;56:1615–1624. doi: 10.1136/gut.2007.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dhirapong A, Yang GX, Nadler S, et al. Therapeutic effect of cytotoxic T lymphocyte antigen 4/immunoglobulin on a murine model of primary biliary cirrhosis. Hepatology. 2013;57:708–715. doi: 10.1002/hep.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanaka H, Zhang W, Yang GX, et al. Successful immunotherapy of autoimmune cholangitis by adoptive transfer of forkhead box protein 3(+) regulatory T cells. Clin Exp Immunol. 2014;178:253–261. doi: 10.1111/cei.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers RP, Swain MG, Lee SS, et al. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933–941. doi: 10.1038/ajg.2013.51. [DOI] [PubMed] [Google Scholar]
- 103.Coots A, Donnelly B, Mohanty SK, et al. Rotavirus infection of human cholangiocytes parallels the murine model of biliary atresia. J Surg Res. 2012;177:275–281. doi: 10.1016/j.jss.2012.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tyler KL, Sokol RJ, Oberhaus SM, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27:1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 105.Drut R, Drut RM, Gomez MA, et al. Presence of human papillomavirus in extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr. 1998;27:530–535. doi: 10.1097/00005176-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 106.Fischler B, Woxenius S, Nemeth A, et al. Immunoglobulin deposits in liver tissue from infants with biliary atresia and the correlation to cytomegalovirus infection. J Pediatr Surg. 2005;40:541–546. doi: 10.1016/j.jpedsurg.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 107.Karjoo S, Hand NJ, Loarca L, et al. Extrahepatic cholangiocyte cilia are abnormal in biliary atresia. J Pediatr Gastroenterol Nutr. 2013;57:96–101. doi: 10.1097/MPG.0b013e318296e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chu AS, Russo PA, Wells RG. Cholangiocyte cilia are abnormal in syndromic and non-syndromic biliary atresia. Mod Pathol. 2012;25:751–757. doi: 10.1038/modpathol.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jafri M, Donnelly B, Bondoc A, et al. Cholangiocyte secretion of chemokines in experimental biliary atresia. J Pediatr Surg. 2009;44:500–507. doi: 10.1016/j.jpedsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Razumilava N, Gores GJ, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–3251. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shivakumar P, Mourya R, Bezerra JA. Perforin and granzymes work in synergy to mediate cholangiocyte injury in experimental biliary atresia. J Hepatol. 2014;60:370–376. doi: 10.1016/j.jhep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Finch ML, Marquardt JU, Yeoh GC, et al. Regulation of microRNAs and their role in liver development, regeneration and disease. Int J Biochem Cell Biol. 2014;54:288–303. doi: 10.1016/j.biocel.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Marquez RT, Wendlandt E, Galle CS, et al. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am J Physiol Gastrointest Liver Physiol. 2010;298:G535–G541. doi: 10.1152/ajpgi.00338.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bessho K, Shanmukhappa K, Sheridan R, et al. Integrative genomics identifies candidate microRNAs for pathogenesis of experimental biliary atresia. BMC Syst Biol. 2013;7:104. doi: 10.1186/1752-0509-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carrion AF, Bhamidimarri KR. Liver transplant for cholestatic liver diseases. Clin Liver Dis. 2013;17:345–359. doi: 10.1016/j.cld.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 116.Griffiths L, Jones DE. Pathogenesis of primary biliary cirrhosis and its fatigue. Dig Dis. 2014;32:615–625. doi: 10.1159/000360515. [DOI] [PubMed] [Google Scholar]
- 117.Beuers U, Kremer AE, Bolier R, et al. Pruritus in cholestasis: facts and fiction. Hepatology. 2014;60:399–407. doi: 10.1002/hep.26909. [DOI] [PubMed] [Google Scholar]
- 118.Roma MG, Toledo FD, Boaglio AC, et al. Ursodeoxycholic acid in cholestasis: linking action mechanisms to therapeutic applications. Clin Sci (Lond) 2011;121:523–544. doi: 10.1042/CS20110184. [DOI] [PubMed] [Google Scholar]
- 119.Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 120.Poupon RE, Lindor KD, Cauch-Dudek K, et al. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–890. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 121.Lindor KD, Therneau TM, Jorgensen RA, et al. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110:1515–1518. doi: 10.1053/gast.1996.v110.pm8613058. [DOI] [PubMed] [Google Scholar]
- 122.Corpechot C, Carrat F, Bahr A, et al. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297–303. doi: 10.1053/j.gastro.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 123.ter Borg PC, Schalm SW, Hansen BE, et al. Prognosis of ursodeoxycholic Acid-treated patients with primary biliary cirrhosis. Results of a 10-yr cohort study involving 297 patients. Am J Gastroenterol. 2006;101:2044–2050. doi: 10.1111/j.1572-0241.2006.00699.x. [DOI] [PubMed] [Google Scholar]
- 124.Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871–877. doi: 10.1002/hep.22428. [DOI] [PubMed] [Google Scholar]
- 125.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.European Association for the Study of the L. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 127.Pellicciari R, Fiorucci S, Camaioni E, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 128.Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 129.Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic Acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic Acid. Gastroenterology. 2015;148:751–761. doi: 10.1053/j.gastro.2014.12.005. e8. [DOI] [PubMed] [Google Scholar]
- 130.Nevens F, Andreone P, Mazzella G, et al. O168 THE FIRST PRIMARY BILIARY CIRRHOSIS (PBC) PHASE 3 TRIAL IN TWO DECADES – AN INTERNATIONAL STUDY OF THE FXR AGONIST OBETICHOLIC ACID IN PBC PATIENTS. Journal of Hepatology. 60:S525–S526. [Google Scholar]
- 131.Cohen BI, Hofmann AF, Mosbach EH, et al. Differing effects of nor-ursodeoxycholic or ursodeoxycholic acid on hepatic histology and bile acid metabolism in the rabbit. Gastroenterology. 1986;91:189–197. doi: 10.1016/0016-5085(86)90457-9. [DOI] [PubMed] [Google Scholar]
- 132.Hohenester S, Wenniger LM, Paulusma CC, et al. A biliary HCO3 umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 133.Fickert P, Pollheimer MJ, Silbert D, et al. Differential effects of norUDCA and UDCA in obstructive cholestasis in mice. J Hepatol. 2013;58:1201–1208. doi: 10.1016/j.jhep.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moritoki Y, Lian ZX, Lindor K, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893–1903. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsuda M, Moritoki Y, Lian ZX, et al. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512–521. doi: 10.1002/hep.24748. [DOI] [PubMed] [Google Scholar]
- 136.Lleo A, Gershwin ME, Mantovani A, et al. Towards common denominators in primary biliary cirrhosis: the role of IL-12. J Hepatol. 2012;56:731–733. doi: 10.1016/j.jhep.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirschfield GM, Liu X, Xu C, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liaskou E, Karikoski M, Reynolds GM, et al. Regulation of mucosal addressin cell adhesion molecule 1 expression in human and mice by vascular adhesion protein 1 amine oxidase activity. Hepatology. 2011;53:661–672. doi: 10.1002/hep.24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moon HJ, Finney J, Ronnebaum T, et al. Human lysyl oxidase-like 2. Bioorg Chem. 2014;57:231–241. doi: 10.1016/j.bioorg.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O'Hara SP, Tabibian JH, Splinter PL, et al. The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol. 2013;58:575–582. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]