Abstract
In this study, we tested the effect of intratumoral administration of dendritic cells (DCs) with inducible expression of different cytokines, using the novel Rheoswitch Therapeutic System on the experimental models of renal cell cancer (RENCA) and MethA sarcoma. Intratumoral injection of DCs, engineered to express IL-12, IL-21, or IFN-α, showed potent therapeutic effect against established tumor. This effect was associated with the induction of potent tumor antigen-specific CD8+ T-cell responses, as well as the infiltration of tumors with CD4+ and CD8+ T cells but not with the cytotoxic activity of DCs. Combination of i.t. administration of DCs, producing different cytokines, did not enhance the antitumor effect of therapy with single cytokine. These results indicate that RTS can be a potent tool for conditional topical cytokine delivery, in combination with DC administration. However, combination of different cytokines may not necessarily improve the outcome of treatment.
Keywords: Dendritic cells, Cancer immunotherapy, IL-12, IFNα, IL-21
Introduction
Combination of dendritic cell (DC) based vaccines, with cytokine support, was always considered a promising approach to cancer immunotherapy. However, direct administration of different cytokines to patients is associated with toxicity, which limited their clinical utility. Therefore, genetic modification of DCs, engineered to produce different cytokines, was tested in different pre-clinical models and clinical trials [1, 2]. The development of new methods of cytokine delivery, based upon conditional regulation of their release, opened a new opportunity for therapeutic intervention. Recently, the Rheoswitch Therapeutic System (RTS) has been developed by Intrexon [3]. This system consists of two fusion proteins. The first, Gal4-EcR, contains a modified ecdysone receptor (EcR) that is fused with the DNA binding domain of the yeast Gal4 transcription factor. The second, VP16-RXR, consists of a chimeric RXR fused with the transcription activation domain of the VP16 protein of HSV1. The chimeric RXR heterodimerizes efficiently with the Gal-4EcR. Activator ligand (AL) binds specifically to EcR and stabilizes heterodimerization between the two fusion proteins, forming an active transcription factor, which induces the transcription of specific cytokines placed under the control of an inducible promoter containing Gal4-binding sites. Thus, RTS provides a universal gene regulation system for gene and cell therapies that require precise control of gene expression to improve safety and efficacy. In the absence of the AL, cytokine production is not detectable from the engineered DCs.
Various strategies are used to deliver tumor-associated antigens with dendritic cells [4–6]. Tumor cells are the best source of tumor-associated antigens. However, the mere presence of DCs in the vicinity of tumor is insufficient to induce antitumor responses. Immune responses were generated only after combination of intratumoral (i.t.) administration of DCs with radiation or chemotherapy in pre-clinical settings [7–10] and in phase I clinical trials [11–13]. Cytokine support appears to be able to bypass the requirements for tumor radiation, since i.t. injection of DCs transduced with IL-12 or IL-32 resulted in potent antitumor effect [14–16].
Induction of potent antitumor immune responses involves multiple cytokines; therefore, it is possible that administration of several cytokines may provide more superior clinical results than the single one. New RTS technology opened the possibility for testing of this hypothesis using DC platform.
Materials and methods
Mice
Female 6–8 week-old Balb/c mice were purchased from NCI and maintained in microisolator cages. Recognized principles of laboratory animal care were followed (Guide for the Care and Use of Laboratory Animals, National Research Council, 1996), and animal protocols were approved by the University of South Florida Animal Care and Use Committee.
Cell lines and tumor models
The renal adenocarcinoma cell line, RENCA, was maintained in RPMI containing 10% FBS, 0.5% penicillin–streptomycin, 1% non-essential amino acids, 1% sodium pyruvate, and 0.1 M β-mercaptoethanol. The murine fibro-sarcoma cell line, MethA, was propagated in culture in DMEM containing 0.5% penicillin–streptomycin, 11% sodium pyruvate, and 0.1 M β-mercaptoethanol with 10% FBS. The cells were grown as an ascitic tumor in a Balb/c mouse and, after being withdrawn from the mouse, were washed and injected into the experimental mice subcutaneously. Balb/c mice were subcutaneously inoculated with 1 × 106 RENCA or Meth A cells on day 0. Mice were split into various treatment groups around day 10, when the tumors reached an approximate size of 5 mm in diameter. The mice were treated with single intratumoral (i.t.) injections of 5 × 106 transduced DCs. Mice also received i.p. injections of 50 mg/kg activator ligand (AL) in DMSO that were initiated a day before DC administration. In other groups, AL was administered orally with mouse diet. AL was administered for 2 weeks, starting a day prior to DC treatment. Tumor size was determined 3 days a week and recorded as mm2 by determining the product of the largest diameters measured by calipers.
Viral vectors
The VQAd-Rheo-sp1 adenoviral vectors encoding mIL-12, IL-21, or IFN-α that are conditionally activated by a small molecule diacylhydrazine ligand were produced and provided by Intrexon. The RheoSwitch Therapeutic System (RTS) was developed as previously described [14].
Generation of DCs and transduction with adenovirus
DCs were generated from murine bone marrow (BM), using 7-day culture in complete RPMI medium supplemented with 20 ng/ml GM-CSF and 10 ng/ml IL-4 (R&D Systems). DCs were washed in serum-free medium and infected with different adenoviruses (Ad) provided by Intrexon. Infection was performed at DC concentration, 5 × 106/ml, for 2 h in serum-free medium, followed by reconstitution of the medium to 10% FBS and DC concentration to 106/ml. Cells were cultured for an additional 24 h before intratumoral (i.t.) injection. The viral dose required to produce a high level of cytokines was determined in previous and preliminary experiments. Based on the results of those experiments, we used an adenovirus concentration of 5,000 vp/cell.
IFN-γ elispot assay
To measure immune response, MethA sarcoma-bearing mice were used. ELISPOT plates (Millipore) were coated with a 2 μg/ml coating antibody (IFN-γ) (BD, Invitrogen). The following day, spleen cells from the various treatment groups were added at a 4 × 105 cells/well. The MethA sarcoma-associated mutant p53-derived specific peptide (KYICNSSCM) or control peptide (SIYRYYGL) was added at a concentration of 10 μg/ml. The mutant p53-derived peptide binds the MHC class I molecule and therefore is recognized by CD8+ T cells [17]. Spleen cells were incubated overnight at 37°C. Subsequently, plates were washed and incubated with biotinylated detection IFN-γ-specific antibody (BD, Invitrogen) for 2 h at room temperature, followed by Streptavidin-HRP. TMB substrate (Sigma) was used to visualize the results, and counting was performed using an automatic ELISPOT reader (Cellular Technology).
Immunohistochemistry
RENCA tumor-bearing mice were sacrificed 2 days after completion of the treatment; the tumors were harvested and kept frozen in liquid nitrogen. Frozen sections were generated and stained, using antibodies against CD4 or CD8 (BD Biosciences) and the ABC vectastain kit (Vector labs). The cells were counterstained, using hematoxylin, and mounted. The number of cells was counted per mm2.
Statistical analysis
Analysis of tumor growth curves was performed, using a two-way ANOVA test with a Bonferroni posttest. Descriptive statistics, like the number of INF-γ producing T cells and immunohistochemistry, were analyzed using Student t test. P value <0.05 were considered significant.
Results and discussion
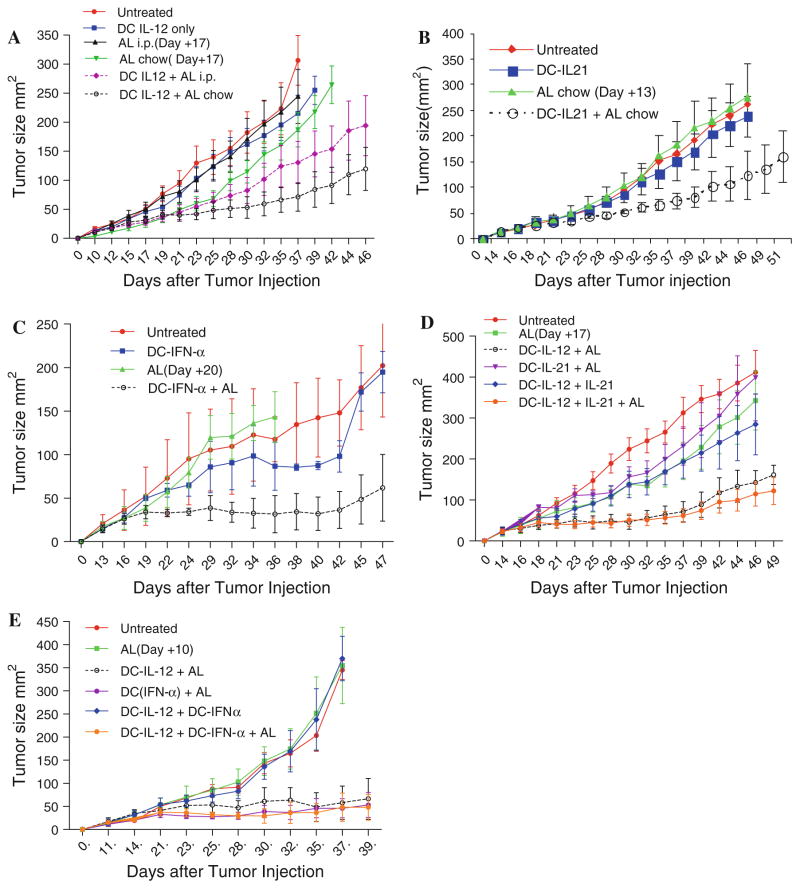
To determine the best route for administrated AL, to induce cytokine production, we compared the daily i.p. injections of AL and the oral administration with chow. The RENCA mouse tumor model of renal cell cancer was used in our study. Tumors were established s.c. and DCs, transduced with IL-12, were injected i.t. with activator delivered for 15 days. Treatment of mice in control groups, with AL alone or with DC-IL-12 without AL, did not significantly affect tumor growth (Fig. 1a). In contrast, treatment of mice with DC-IL-12 and AL resulted in a substantial slow down of the tumor growth (Fig. 1a). However, the effect of i.p. administration of activator was rather modest. The statistical significance was seen only on day 42, after tumor inoculation. A two-way ANOVA test of the all groups did not reach statistical significance. The effect of AL delivered as chow provided a more potent effect with a statistically significant (P = 0.03) difference from all control groups (Fig. 1a). Our data were consistent with previous observation with i.t. injection of DC-IL-12 made in different tumor models [14]. Because AL in chow demonstrated a higher potency than i.p. in all subsequent experiments, the chow form of AL was used.
Fig. 1.
Effect of intratumoral administration of DCs with conditional expression of combination of different cytokines on tumor growth. a Mice bearing RENCA tumors, with an average size of about 50 mm2, were separated into different treatment groups on day 10 after tumor inoculation. DC-IL-12 were injected on day 10 and the administration of AL started on day 9 and continued for 15 days. Each group included 7–10 mice. Differences between mice, treated with DC-IL-12+ AL chow and all control groups, were statistically significant (P < 0.03). b, c Experiments were performed essentially the same way as in Fig. 1a, with the exception of the use of DC-IL-21 (b) or DC-INF-α (c) instead of DC-IL-12. Each group included 6–8 mice. The differences between the DC-IL-21 + AL or DC-IFN-α + AL groups and the controls group were statistically significant (P = 0.01 and P < 0.001, respectively). d, e Mice with established RENCA tumor were treated with i.t. injection of DC-IL-12 on day 14, followed by administration of DC-IL-21 (d) or DC-IFN-α (e) 4 days later. AL was administered from day 13 until day 31. Each group included 6–8 mice. The differences between the combination cytokines + AL groups and all control groups were significant (P < 0.04). However, no differences were seen with DC-IL-12 + AL group (P > 0.1)
IL-21 and IFN-α are two cytokines that are important for the generation of immune responses but have not been previously tested in combination with i.t. DC therapy. In our experiments, DC-IL-21 showed strong antitumor activity, with statistically significant differences between DC-IL-21 + AL group and all control groups (P = 0.01) (Fig. 1b). A similar effect was observed in mice treated with DC-IFN-α (P < 0.001 between AL and DC-IFN-α + AL groups and P < 0.03 between untreated and DC-IFN-α + AL P < 0.03) (Fig. 1c). Thus, DC-IL-12, DC-IL-21, and DC-IFN-α provided significant antitumor activity against RENCA-bearing mice.
Given the observed therapeutic benefits of IL-12, IL-21, and IFN-α as monotherapies, we tested the hypothesis that the combination of IL-12 with sequential administration of other cytokines that may target different types of cells can provide a better therapeutic response than each cytokine alone. In all combination experiments, AL and DC-IL-12 were administered the same way as in the experiments described in Fig. 1a. DC-IL-21 or DC-IFN-α were administered 4–5 days later. AL was delivered for 18 days. The combination of AL and DC-IL-12 had a strong antitumor effect, similar to that observed in previous experiments (Fig. 1d, e). Treatment of mice with DC-IL-21 and AL had no antitumor activity, probably due to the 4-day delay with administration of DC-IL-21 (Fig. 1d). The combination of DC-IL-12 with DC-IL-21 had a strong antitumor effect, but it was not different for the effect of DC-IL-12 + AL. No potentiating effect of two cytokines was seen (Fig. 1d). Similar experiments were performed for the combination of IL-12 and IFN-α. In contrast to the experiments with IL-21, delay with DC-Ad-IFNα administration did not interfere with the antitumor effect of therapy. Treatment with DC-IFNα + AL showed strong antitumor effect (P < 0.01). However, the combination of DC-IL-12 and DC-IFNα did not demonstrate stronger antitumor activity than individual cytokine (Fig. 1e). Thus, the combination of cytokines did not enhance the antitumor effect of the therapy.
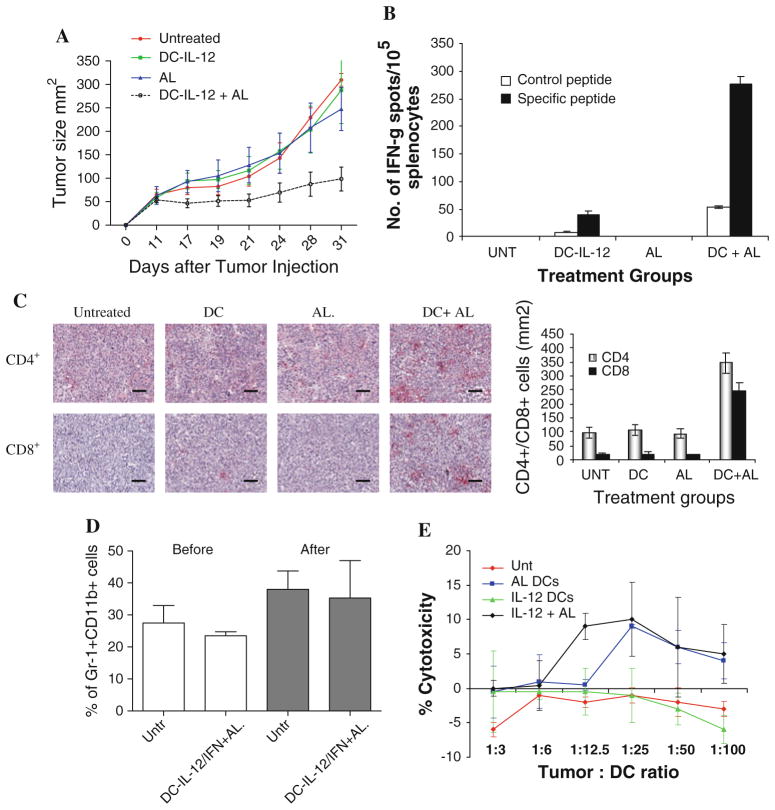
Next, we asked what could be the possible mechanism of the antitumor effect of the DC-cytokine therapy. In order to address this question, we used a MethA sarcoma model with a defined tumor-associated antigen (TAA): p53-derived peptide epitope. We had previously demonstrated that the combination of DC-p53 vaccine with recombinant IL-12 improved the antitumor effect of the therapy in this model [17]. First, we confirmed that treatment of MethA sarcoma with DC-IL-12 and AL resulted in a potent antitumor effect (Fig. 2a). To assess the tumor-specific nature of immune responses in treated mice, we measured T-cell response to MethA using p53-derived or control MHC class I bound peptides in IFNγ ELISPOT assay. A significant p53-specific response was detected only in the group of mice treated with a combination of DC-IL-12 and AL (Fig. 2b). Thus, antitumor activity of DC-IL-12 was associated with the generation of tumor-specific immune responses. To assess tumor infiltration by T cells, we collected tumors 10 days after the start of therapy. Very few T cells were seen in the control groups of mice. In contrast, mice treated with DC-IL-12 and AL demonstrated a strong infiltration of both CD4+ and CD8+ T cells (Fig. 2c).
Fig. 2.
Effect of DC-IL-12 therapy on antitumor immune responses. a Antitumor effect of IL-12 treatment on MethA model. The treatment protocol was the same as in RENCA model. Each group included 7–8 mice. The differences between DC-IL-12 + AL and all control groups were statistically significant (P < 0.05). b Spleens from mice of indicated treatment groups were individually harvested and assayed for IFN-γ secretion, using an ELISPOT assay. Each group included 4 mice. Mean ± SD are shown. The differences between control groups and DC-IL-12 + AL groups were significant (P < 0.05). c Detection of CD4+ and CD8+ T cells in tumor tissues of treated mice. Representative images and statistical analysis of the results are shown. d The proportion of Gr-1+CD11b+ MDSC in spleens of tumor-bearing mice, before and after therapy with DC-IL-12 in combination with DC-IFN-α and AL. Mean ± SD of three mice per group. e Cytotoxic activity of DCs transduced with IL-12 and activated with ligand. Experiments were performed in duplicates in standard 4 h 51Cr-release assay
In recent years, myeloid-derived suppressor cells (MDSC) were demonstrated to play a major role in limiting the effect of cancer immunotherapy [18]. It was suggested that IL-12 could reduced MDSC expansion in tumor-bearing mice and, thus, enhance the effect of the therapy [19]. To test this possibility, we evaluated the MDSC level in spleens of mice treated with an effective combination of DC-IL-12, DC-IFN-α, and AL. Our data showed that, despite the fact that this therapy had very potent antitumor activity, it did not affect the level of MDSC in this model (Fig. 2d).
We also tested the possibility that DC transduced with IL-12 could have direct antitumor activity. We used cyto-toxicity in chromium release assay against RENCA tumor cells. DC-IL-12 cells treated with AL had very little toxicity against tumor not unlike the DCs from any other groups (Fig. 2e).
Thus, our data demonstrated that a novel RTS system, for conditional delivery of several tested cytokines, provides a potent antitumor effect in combination with intratumoral administration of DCs. This effect was associated with the generation of tumor antigen-specific T-cell response and accumulation of CD4+ and CD8+ T cells, but not with the direct cytotoxic effect of DCs.
We selected several cytokines with known activity in antitumor immune responses. IL-12 role in positive regulation of antitumor immune responses is well established [20]. IL-21 can reverse the suppressive effects of regulatory T cells (Tregs) on CD8 + T cells, thereby enhancing the host immunity against tumors [21]. A population of DCs generated by fusion of IL-21 and GM-CSF has been shown to generate tumor antigen-specific response [22]. There have been recent studies demonstrating that type I interferon IFN production, acting on CD8α+ DCs and enhancing their ability to cross-present antigen, is required for effective activation of tumor antigen-specific CD8+ T cells [23, 24]. We expected that these cytokines would support induction of potent antitumor immune responses, which would translate into antitumor activity. Our data have supported this hypothesis. We also suggested that combination of different cytokines may provide additional therapeutic benefits. IL-12 up-regulates critical signaling intermediates like JAK-STAT that sensitizes host immune cells to lower doses of IFN-α, leading to significantly enhanced survival of tumor-bearing mice [25]. Therefore, we hypothesized that using DC-IL-12 as the primary step of treatment would sensitize mice to the antitumor effects of IFN-α leading to increased efficacy and decreased toxicity. We also hypothesized that combination of IL-12, that promotes expansion and activity CTLs, and IL-21, that can neutralizes Tregs, would enhance antitumor effect of DC administration. However, contrary to the initial hypothesis, our data demonstrated that the combination of different cytokines did not necessarily result in an enhanced antitumor effect of therapy. It is possible that there is a certain threshold of T-cell reactivity that can be reached by DCs producing individual cytokines and combination of selected cytokines was not able to enhance it further. In this situation, the limitations of antitumor activity of the therapy could be not the result of inefficient T-cell responses but rather presence of other mechanisms of tumor escape. These factors associated with tumor microenvironment may include immune suppressive cytokines, receptors, and molecules like indolamine 2,3-dioxygenase, peroxynitrite, etc. [26–28].
Acknowledgments
We thank Intrexon for providing us with reagents and support. This work was supported in part by grant 1BT01 from State of Florida Bankhead Coley Cancer Research Program.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Chun Huang, Department of Immunology, H. Lee Moffitt Cancer Center, MRC 2067, 12902 Magnolia Dr. Tampa, Tampa, FL 33647, USA. Department of Immunology, Tianjin Medical University Cancer Institute and Hospital, Huanhuxi Road, Tiyuanbei, Hexi District 300060, Tianjin, People’s Republic of China.
Rupal Ramakrishnan, Department of Immunology, H. Lee Moffitt Cancer Center, MRC 2067, 12902 Magnolia Dr. Tampa, Tampa, FL 33647, USA.
Marko Trkulja, Department of Immunology, H. Lee Moffitt Cancer Center, MRC 2067, 12902 Magnolia Dr. Tampa, Tampa, FL 33647, USA.
Xiubao Ren, Department of Immunology, Tianjin Medical University Cancer Institute and Hospital, Huanhuxi Road, Tiyuanbei, Hexi District 300060, Tianjin, People’s Republic of China.
Dmitry I. Gabrilovich, Email: Dmitry.gabrilovich@moffitt.org, Department of Immunology, H. Lee Moffitt Cancer Center, MRC 2067, 12902 Magnolia Dr. Tampa, Tampa, FL 33647, USA
References
- 1.Chen YZ, Yao XL, Tabata Y, Nakagawa S, Gao JQ. Gene carriers and transfection systems used in the recombination of dendritic cells for effective cancer immunotherapy. Clin Dev Immunol. 2010;2010:565643. doi: 10.1155/2010/565643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterfield LH, Vujanovic L. New approaches to the development of adenoviral dendritic cell vaccines in melanoma. Curr Opin Investig Drugs. 2010;11(12):1399–1408. [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar P, Katakam A. Rheoswitch system: a highly sensitive ecdysone receptro-based gene regulation system induced by synthetic small-molecule ligands. In: Friedman T, Rossi J, editors. Gene transfer: delivery and expression of DNA and rna. Cold Spring Harbor Laboratory Press; NY: 2007. pp. 643–651. [Google Scholar]
- 4.Ishida T, Chada S, Stipanov M, Nadaf S, Ciernik FI, Gabrilovich DI, Carbone DP. Dendritic cells transduced with wild type p53 gene elicit potent antitumor immune responses. Clin Exp Immunol. 1999;117:244–251. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 6.Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer journal (Sudbury, Mass) 2011;17 (5):359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Candido KA, Shimizu K, McLaughlin JC, Kunkel R, Fuller R, Redman BG, Thomas EK, Nickoloff BJ, Mule JJ. Local administration of dendritic cells inhibits established breast tumor growth: implication for apoptosis-inducing agents. Cancer Res. 2001;61:228–236. [PubMed] [Google Scholar]
- 8.Nikitina EY, Gabrilovich DI. Combination of gamma irradiation and dendritic cell administration in treatment of advanced cancer. Intern J Cancer. 2001;94:825–833. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, Chang AE. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 10.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, Gabrilovich D. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]
- 11.Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, Noyes DR, Cheong D, Gonzalez RJ, Heysek RV, Berman C, Lenox BC, Janssen W, Zager JS, Sondak VK, Letson GD, Antonia SJ, Gabrilovich DI. Combination of external beam radiotherapy (ebrt) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2010.12.068. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28(2):129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 13.Mann DL, Celluzzi CM, Hankey KG, Harris KM, Watanabe R, Hasumi K. Combining conventional therapies with intratumoral injection of autologous dendritic cells and activated t cells to treat patients with advanced cancers. Ann N Y Acad Sci. 2009;1174:41–50. doi: 10.1111/j.1749-6632.2009.04934.x. [DOI] [PubMed] [Google Scholar]
- 14.Komita H, Zhao X, Katakam AK, Kumar P, Kawabe M, Okada H, Braughler JM, Storkus WJ. Conditional interleukin-12 gene therapy promotes safe and effective antitumor immunity. Cancer Gene Ther. 2009;16(12):883–891. doi: 10.1038/cgt.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu Y, Taylor JL, Bose A, Storkus WJ. Therapeutic effectiveness of intratumorally delivered dendritic cells engineered to express the pro-inflammatory cytokine, interleukin (il)-32. Cancer Gene Ther. 2011;18(9):663–673. doi: 10.1038/cgt.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayashima H, Toshima T, Okano S, Taketomi A, Harada N, Yamashita Y, Tomita Y, Shirabe K, Maehara Y. Intratumoral neoadjuvant immunotherapy using il-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2010;185(1):698–708. doi: 10.4049/jimmunol.0900187. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Cunningham HT, Carbone DP. Il-12 and mutant p53 peptide-pulsed dendritic cells for the specific immunotherapy of cancer. J Immunother. 1997;19:414–418. doi: 10.1097/00002371-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. 2011;133(2):221–238. doi: 10.1111/j.1365-2567.2011.03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by il-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7(11):1705–1721. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. Il-21 counteracts the regulatory t cell-mediated suppression of human cd4+ t lymphocytes. J Immunol. 2007;178(2):732–739. doi: 10.4049/jimmunol.178.2.732. 178/2/732. [DOI] [PubMed] [Google Scholar]
- 22.Williams P, Bouchentouf M, Rafei M, Romieu-Mourez R, Hsieh J, Boivin MN, Yuan S, Forner KA, Birman E, Galipeau J. A dendritic cell population generated by a fusion of gm-csf and il-21 induces tumor-antigen-specific immunity. J Immunol. 2010;185(12):7358–7366. doi: 10.4049/jimmunol.1002201. [DOI] [PubMed] [Google Scholar]
- 23.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type i interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type i ifn signals are required for antitumor cd8 + t cell responses through cd8{alpha} + dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesinski GB, Badgwell B, Zimmerer J, Crespin T, Hu Y, Abood G, Carson WE., 3rd Il-12 pretreatments enhance ifn-alpha-induced janus kinase-stat signaling and potentiate the antitumor effects of ifn-alpha in a murine model of malignant melanoma. J Immunol. 2004;172(12):7368–7376. doi: 10.4049/jimmunol.172.12.7368. 172/12/7368. [DOI] [PubMed] [Google Scholar]
- 26.Mellor AL, Munn DH. Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 27.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic t cells in mice. J Clin Invest. 2011;121(10):4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes to age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]