Abstract
Background
Gestational weight gain (GWG) is potentially modifiable and is associated with infant size and body composition; however, long-term effects on childhood obesity have not been reported among multi-ethnic urban populations.
Methods
We examined the association between GWG and child anthropometric measures and body composition at 7 years [waist circumference (WC), body-mass-index Z-score (BMIZ), obesity (BMIZ≥95%ile), and bioelectrical impedance analysis estimates of percentage body fat (%fat)] in African American and Dominican dyads (n=323) in the Columbia Center for Children’s Environmental Health prospective birth cohort study from 1998–2013. Linear and logistic regression evaluated associations between excessive GWG (>Institute of Medicine (IOM) 2009 guidelines) and outcomes, adjusting for prepregnancy BMI and covariates.
Results
Pre-pregnancy BMI (mean±SD, all such values) and total GWG were 25.8±6.2 kg/m2 (45% overweight/obese) and 16.4±7.9 kg (64%>IOM guidelines), respectively. Excessive GWG was associated with higher BMIZ [0.44 (95% CI: 0.2, 0.7), p<0.001], waist circumference [β: 2.8 cm (95% CI: 1.1, 4.6), p=0.002], %fat at 7 years [β: 2.2 % (95% CI: 1.0, 3.5), p=0.001)] and obesity [OR: 2.92 (95% CI: 1.5, 5.7), p=0.002]. Prepregnancy BMI was positively associated with child size, adiposity and obesity (all p<0.05).
Conclusions
Excessive GWG was highly prevalent and was associated with child obesity, greater percentage body fat and abdominal adiposity. Strategies to support healthy GWG are warranted to promote healthy growth and prevent childhood obesity.
Keywords: Pregnancy, Weight gain, life course, childhood adiposity, childhood obesity, body composition
Introduction
Childhood overweight and obesity remains pervasive in the United States and globally,(Ogden et al., 2014, Skinner and Skelton, 2014, Black et al., 2013) and stems from multiple individual, proximal and distal causes. The intrauterine environment contributes to metabolic programming (Dahly et al., 2009, Gluckman et al., 2008) and therefore health (and obesity risk) across the life course. Maternal nutritional status in pregnancy is an indicator of the intrauterine environment (Dahly et al., 2009, Kuzawa and Adair, 2004, Bollen et al., 2013). Gestational weight gain (GWG), in particular, is a measurable and potentially modifiable marker of nutritional availability to the growing fetus (Kuzawa and Adair, 2004, Dello Russo et al., 2013) and is associated with both short and potentially longer-term health of mother and child. Therefore the Institute of Medicine (IOM) recommends that pregnant women gain weight within ranges according to prepregnancy body mass index (BMI) in order to optimize prenatal, birth and possibly longer-term health outcomes (Institute of Medicine, 2009).
Recent studies suggest that both total (continuous, kg) and excessive GWG (defined as exceeding the IOM recommendations) are positively associated with adiposity in the neonatal period and infancy (Deierlein et al., 2012, Hull et al., 2011, Crozier et al., 2010) and these effects may persist into childhood (Dello Russo et al., 2013, Hinkle et al., 2012, Margerison-Zilko et al., 2012, Lawlor et al., 2011, Fraser et al., 2010, Schack-Nielsen et al., 2010, Ensenauer et al., 2013, Stamnes Kopp et al., 2012, Ludwig and Currie, 2010, Crozier et al., 2010). However, most studies examining longer-term effects of GWG on childhood size typically only have measures of height and weight rather than estimates of body composition (Margerison-Zilko et al., 2012, Hinkle et al., 2012, Crozier et al., 2010). Further, evidence of long-term effects of GWG is limited in vulnerable populations, such as low-income multi-ethnic cohorts, who may be at increased risk of obesity and associated sequelae.
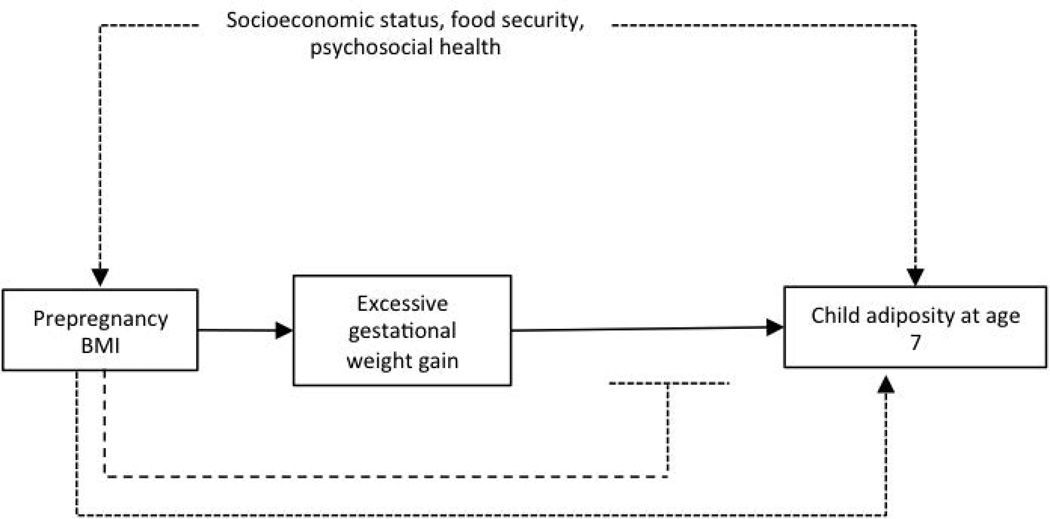
In the Columbia Center for Children’s Environmental Health prospective birth cohort, we investigated the association between GWG and childhood size, body composition and obesity in African-American and Dominican youth at 7 years. We hypothesized that excessive GWG (>IOM guidelines) and total GWG (continuous, kg) are associated with offspring obesity and increased adiposity, and further that prepregnancy BMI modifies these associations (Figure 1).
Figure 1.
Conceptual framework
Methods
Data are from the Columbia Center for Children’s Environmental Health (CCCEH) Mothers and Newborns Study in Northern Manhattan and the South Bronx, New York, a prospective birth cohort that has been previously described (Perera et al., 2003, Whyatt et al., 2003). From 1998–2006, 727 mothers self-identifying as African American or Dominican were enrolled during the third trimester of pregnancy if they had resided in the study area for at least 1 year. Women were excluded if the first prenatal visit was after 20 weeks gestation or if the mother self-reported diabetes, hypertension, known HIV status, or use of illicit drugs or cigarettes during pregnancy.
Self-report of prepregnancy weight, maternal education, receipt of public assistance, ability to afford food in pregnancy and previous pregnancies was obtained at the baseline interview during the second or third trimester. At delivery, maternal and infant medical records were abstracted by research staff to ascertain prenatal medical history, birth outcomes, and the last prenatal visit weight. Maternal report of breastfeeding was obtained at follow-up visits at 3, 6, 12 and 24 months. Maternal height was obtained by self-report at the baseline prenatal interview, measured in the medical record, and at subsequent study visits measured with a stadiometer (Before January 2010: Cardinal Scale Manufacturing Company, Webb City, MO; After January 2010: Holtain Limited, Crymych, UK). Prenatal self-report, medical record and postpartum study visit measured heights were compared to each other. If height values were discrepant a data-cleaning algorithm along with verification of original study documents was used to re-code maternal height. If the algorithm identified a systematic conversion error from data entry, study documents were reviewed to confirm and if identified, the value was recoded (i.e. 5’6” self-report & 56” data entered: recoded to 66”). If medical record and self-reported heights were within ±2.54 cm, the self-reported heights were retained. If medical record and self-reported heights were within ±2.55–5.08 cm, the two height measurements were 1) averaged if no subsequent measured height measurements were available and 2) if other values were available, the height values within ±5.08 cm were averaged. Finally, if height values were greater than ±5.09 cm different, study charts were reviewed for accuracy and values within ±5.08 cm were averaged.
Total GWG was determined by subtracting the last prenatal visit weight from the prepregnancy weight. GWG percent adequacy was determined by dividing the observed total GWG by the expected GWG [GWG percent adequacy= (observed total GWG/ expected GWG)× 100], where expected GWG = IOM recommended first trimester gain + (gestational age – 13 wk ) × recommended rate of GWG for the second and third trimesters (Institute of Medicine, 2009). IOM recommended first trimester gains used to calculate expected GWG were 2 kg for underweight and normal weight women, 1 kg for overweight women and 0.5 kg for obese women (Bodnar et al., 2011). Ratios exceeding the 2009 Institute of Medicine (IOM) recommended ranges for each prepregnancy BMI group were coded as excessive GWG, as previously described (Mehta et al., 2011, Bodnar et al., 2011, Deierlein et al., 2012). This approach for estimating adherence to the IOM recommendations reflects that longer gestations may have greater weight gain.
Child measurements were conducted at 7.1±0.2 y from 2005 to 2013. Children wore light clothing and no shoes. Weight and height were obtained by a digital scale/stadiometer (Cardinal Scale Manufacturing Company, Webb City, MO) until January 2010. After January 2010, height was obtained with the Holtain-Harpenden Wall Mounted Stadiometer Counter 602VR (Holtain Limited, Crymych, UK). Waist circumference was measured halfway between the iliac crest and the lowest rib to the nearest 0.5 cm using non-stretchable tape. Bioelectrical impedance estimates of percentage body fat (%fat) were obtained with the Tanita Digital Body Mass Indicator Scale BC-418 (Tanita Corporation of America, Arlington Heights, IL). Child BMI-Z-scores were determined using the SAS programs provided by the Centers for Disease Control and Prevention (CDC (Centers for Disease Control and Prevention 2014)); BMI-Z scores reflect age and sex specific standard deviations from the mean values in the CDC 2000 growth charts. For example, a BMI-Z score of +1.0 indicates one standard deviation above the mean.
Study procedures at enrollment and follow-up were approved by the Columbia University Institutional Review Board. Written informed consent was obtained by all enrolled women, and written assent was obtained from children at 7 years.
Statistical analysis
Statistical analyses were conducted with Stata 12.0 (College Station, TX) with an alpha of 0.05 for statistical tests. Baseline characteristics were compared between included and excluded dyads using parametric tests for continuously, normally distributed variables and non-parametric tests, as appropriate.
Multivariable linear and logistic regression models were used to assess the association between gestational weight gain (GWG) and the following childhood outcomes at 7 years: offspring size, childhood body composition and obesity (BMIZ>95%ile). GWG was modeled as both total gain (continuous variable, kg) and adequacy of GWG (dichotomous variable: excessive/not-excessive (referent)) (Institute of Medicine, 2009). Effect modification was evaluated using interaction terms for GWG exposures and 1) prepregnancy BMI, modeled as a continuous variable, and 2) infant sex with an alpha of 0.1 (Marshall, 2007). No effect modification was observed, thus both infant sex and prepregnancy BMI were included as covariates in all models. Other covariates included maternal education (>12 years, yes/no), maternal race/ethnicity (categorical), receipt of public assistance (yes/no), ability to afford food in pregnancy (yes/no), parity (continuous), prenatal demoralization (i.e. psychological distress, previously described (Reyes et al., 2011, Wallace et al., 2003)) (score >1.55 coded as high, yes/no), and child age at measurement (months, continuous). Gestational age at the last measured weight was also included in the total GWG models (weeks, continuous). Models initially controlled for marital status, which did not influence observed associations, thus it was not included in the final adjustment set. For all models, only effect estimates for primary exposures (GWG) and prepregnancy BMI are presented in order to limit potential misunderstandings of secondary effects (defined as a ‘table 2 fallacy’) (Westreich and Greenland, 2013). The models are presented as follows: Model 1 (unadjusted model with GWG, prepregnancy BMI and gestational age), and Model 2 (GWG, prepregnancy BMI, gestational age and all covariates).
Table 2.
Child anthropometry and body composition at 7 years
| Characteristic | All n=323 |
Male n=146 |
Female n=177 |
|---|---|---|---|
| Weight, kg | 28.3 ± 7.0 | 28.7 ± 6.9 | 27.9 ± 7.2 |
| Height, cm | 125.2 ± 5.7 | 125.9 ± 5.2 | 124.5 ± 5.9 |
| BMIZ | 0.80 ± 1.1 | 0.86 ± 1.1 | 0.74 ± 1.1 |
| Waist circumference, cm | 58.6 ± 8.0 | 59.0 ± 8.44 | 58.1 ± 7.6 |
| Fat mass, kg | 7.2 ± 3.8 | 7.0 ± 3.9 | 7.3 ± 3.7 |
| Fat-free mass, kg | 21.1 ± 3.7 | 21.7 ± 3.4 | 20.6 ± 3.8 |
| Percentage fat, % | 24.1 ± 5.8 | 23.0 ± 6.0 | 25.1 ± 5.5 |
Mean ± SD, all such values. BMIZ, Body mass index Z-score.
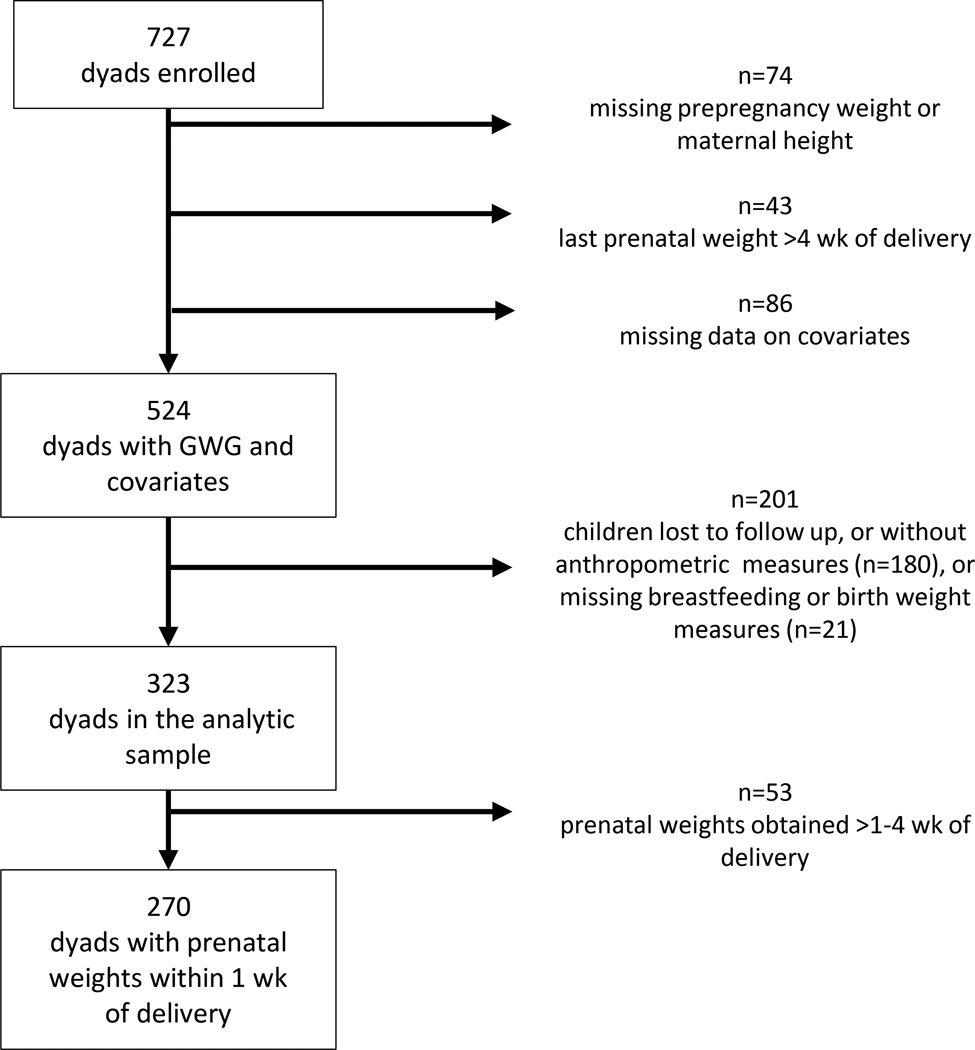
It is important to note, the last measured pregnancy weight abstracted from the medical record was missing in some women and was not always obtained in close proximity to delivery. Of women with prepregnancy weight, covariates and childhood data, 323 women had prenatal weights obtained within 4 wk of delivery, which were obtained on average 4 d prior to delivery (Median: 3 (Interquartile range: 1–6 d). Sensitivity analyses were conducted assessing whether exclusion of women with weights obtained 7–28 d (n=53) before delivery influenced observed associations in multivariable models. In addition, analyses were conducted using inverse probability weights for successful follow-up at 7 years to assess the effects of sample attrition and incomplete follow up on effect estimates, as previously described in this cohort (Mueller et al., 2014, Rundle et al., 2012). Briefly, analyses using IPW allow for estimation and correction for bias due to incomplete follow up and missing data, which can bias results if attrition or missing data was differential by primary exposure or important covariates (Hernan et al., 2004). A logistic regression model was used to estimate the covariate adjusted probability of successful follow up using baseline data from this cohort; the model used for estimating the predicted probability of follow up included the following variables: prepregnancy obesity, parity, total pregnancy weight gain (GWG), race/ethnicity, maternal age, education, parity, linguistic isolation, neighborhood poverty rate, birth weight, child sex, receiving public assistance and an indicator variable for missing income data. The inverse of the predicted probability of follow up (IPW) was used for sample weighting in subsequent analyses. Logistic and linear regression models assessing effects between GWG and child outcomes were re-analyzed with weighting by IPW for follow-up using the survey command in Stata 12.0.
Results
Table 1 shows baseline characteristics and risk-factors for the overall cohort with GWG data and for children included in this analysis at age 7. Data on body composition and key covariates were available for 323 dyads. The primary missing data element was childhood measurements (Figure 2). Most characteristics were similar between those included (n=323) and excluded from this analysis (n=201); however, compared to those excluded from the analytic sample, a smaller proportion of included mothers were Dominican and a larger proportion were African American (Table 1, p=0.005).
Table 1.
Sample characteristicsa
| Characteristic | Enrolled cohort with pregnancy weight gain data (n=524) |
Excluded due to missing childhood or covariate data (n=201) |
Analytic sample (n=323) |
|---|---|---|---|
| Prenatal maternal | |||
| Prepregnancy BMI, kg/m2 | 25.6 ± 5.9 | 25.3 ± 5.3 | 25.8 ± 6.2 |
| Prepregnancy BMI, n (%) | |||
| Underweight | 27 (5.2) | 11 (5.5) | 16 (5.0) |
| Normal | 266 (50.8) | 101 (50.3) | 165 (51.1) |
| Overweight | 122 (23.3) | 52 (25.9) | 70 (21.7) |
| Obese | 109 (20.8) | 37 (18.4) | 72 (22.3) |
| Total GWG, kg | 16.7 ± 7.7 | 17.1 ± 7.4 | 16.4 ± 7.9 |
| Excessive total GWGb, n (%) | 341 (65.1) | 134 (66.7) | 207 (64.1) |
| Primiparous, n (%) | 131 (25.0) | 47 (23.4) | 84 (26.0) |
| Ethnicity | |||
| Dominican, n (%) | 334 (63.7) | 143 (71.1) | 191 (59.1) |
| African-American, n (%) | 190 (36.3) | 58 (28.9) | 132 (40.9) |
| Maternal education >12 y, n (%) | 152 (29.0) | 55 (27.4) | 97 (30.0) |
| Received public assistance, n (%) | 213 (40.7) | 85 (42.3) | 128 (39.6) |
| Unable to afford food, n (%) | 91 (17.4) | 37 (18.4) | 54 (16.7) |
| Demoralization, score | 1.1 ± 0.63 | 1.2 ± 0.63 | 1.1 ± 0.63 |
| Demoralization >1.55, n (%) | 133 (25.4) | 50 (24.9) | 83 (25.7) |
| Post-natal child | |||
| Birth weight, kg | 3.3 ± 0.45 (514) | 3.4 ± 0.44 (191) | 3.4 ± 0.46 (323) |
| Breastfeeding duration, wk | 10.2 ± 13.5 (470) | 7.7 ± 10.6 (147) | 11.3 ± 14.5 (323) |
| Male, n (%) | 246 (47.0) | 100 (49.8) | 146 (45.2) |
Abbreviations: BMI, body mass index; GWG, gestational weight gain.
Mean±SD (n) or Mean±SD, all such values.
GWG > Institute of Medicine 2009 Guidelines.
Figure 2.
Participant flow diagram. GWG, gestational weight gain.
At 7 years, 71 (22% of 323) of children were obese; mean percentage body fat was 24.1±5.8 % (7.2±3.8 kg of fat mass) (Table 2). Table 3 shows the multivariable associations between excessive GWG, covariates and childhood outcomes. Excessive GWG was associated with higher child BMI z-score, percentage body fat and larger waist circumference. Prepregnancy BMI was positively associated with childhood size outcomes (waist circumference, percentage body fat and BMI z-score); these estimates are the effect of a 5-kg/m2 increase in prepregnancy BMI on child outcomes, controlling for the average effect of GWG (Westreich and Greenland, 2013). Associations were similar in models with continuous total GWG, where higher total GWG was associated with larger childhood size outcomes. For example, every 5-kg increase in total GWG was associated with a 0.11 higher child BMIZ [β=0.11, 95% CI: 0.03, 0.18), p<0.01], 0.71% higher body fat [β=0.71, (95% CI: 0.32, 1.10), p<0.001], and waist circumference is 0.95 cm greater [B=0.95, (95% CI: 0.41, 0.1.50), p=0.001].
Table 3.
Associations of gestational weight gain and prenatal factors with childhood size and body composition at 7 years (n=323)1
| BMIZ | % Body Fat | Waist circumference, cm | ||||
|---|---|---|---|---|---|---|
| I | II | I | II | I | II | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | ||
| Excessive GWG model | ||||||
| Excessive GWG2 | 0.43 (0.18, 0.67) | 0.44 (0.20,0.68) | 2.09 (0.81,3.38) | 2.23 (0.97, 3.49) | 2.81 (1.02, 4.60) | 2.85 (1.07, 4.62) |
| Prepregnancy BMI3, 5 kg/m2 | 0.26 (0.16, 0.35) | 0.29 (0.19, 0.39) | 1.15 (0.65, 1.64) | 1.20 (0.70, 1.70) | 1.31 (0.62,2.00) | 1.60 (0.89, 2.30) |
| Total GWG model | ||||||
| Total GWG4, kg | 0.02 (0.01, 0.04) | 0.02 (0.01, 0.04) | 0.13 (0.05, 0.21) | 0.14 (0.06, 0.22) | 0.18 (0.07, 0.29) | 0.19 (0.08, 0.30) |
| Prepregnancy BMI3, 5 kg/m2 | 0.31 (0.22, 0.41) | 0.30 (0.21, 0.40) | 1.37 (0.87, 1.88) | 1.50 (0.99, 2.00) | 1.69 (0.98, 2.39) | 1.98 (1.27, 2.70) |
Model I includes GWG, prepregnancy BMI and gestational age; Model II (Fully adjusted), includes Model I and covariates [parity (continuous), child age (months), maternal race/ethnicity (categorical), maternal education >12 y (yes/no, categorical), child sex (categorical), receipt of public assistance (yes/no), prenatal demoralization score >1.55 (yes/no), and ability to afford food in pregnancy (yes/no)]. Abbreviations: BMI, body mass index; CI, confidence interval; GWG, gestational weight gain
Adequate/inadequate GWG was the reference group
Prepregnancy BMI was modeled as a continuous variable; effects shown are per 5 kg/m2increase in prepregnancy BMI
Total GWG modeled as a continuous variable; effects shown are per 1-kg increase in GWG
Excessive GWG was associated with an almost 300% increased risk of childhood obesity (BMIZ>95%ile), while every 1-kg increase in total GWG (continuous, kg) was associated with a 4% higher risk of child obesity (Table 4). Controlling for the average effect of GWG, every 5-kg/m2 increase in prepregnancy BMI was positively associated with a 50% increase in child obesity risk at 7 years.
Table 4.
Risk of child obesity at age 7 years according to gestational weight gain and prenatal factors (n=323)1
| Obesity Risk (BMIZ>95%ile) RR (95% CI) |
|||
|---|---|---|---|
| I | II | ||
| Excessive GWG model | |||
| Excessive GWG2 | 2.75 (1.43, 5.29) | 2.93 (1.49,5.75) | |
| Prepregnancy BMI3, 5-kg/m2 | 1.31 (1.07, 1.61) | 1.40 (1.12,1.75) | |
| Total GWG model | |||
| Total GWG4, kg | 1.07 (1.03, 1.12) | 1.04 (1.01,1.08) | |
| Prepregnancy BMI3, 5-kg/m2 | 1.41(1.14, 1.74) | 1.53 (1.21,1.92) | |
Model I includes GWG (total or excessive), prepregnancy BMI (continuous) and gestational age (weeks) for total GWG model; Model II (Fully adjusted), includes Model I and covariates [parity (continuous), child age (months), maternal race/ethnicity (categorical), maternal education >12 y (yes/no), child sex, receipt of public assistance (yes/no); ability to afford food in pregnancy (yes/no) and high prenatal demoralization (yes/no)]
Adequate/inadequate GWG was the reference group.
Prepregnancy BMI was modeled as a continuous variable; effects shown are per 5 kg/m2increase in prepregnancy BMI
Total GWG modeled as a continuous variable; effects shown are per 1-kg increase in GWG
Abbreviations: BMI, body mass index; CI, confidence interval; GWG, gestational weight gain
We conducted further analyses to assess whether the results were sensitive to the timing of GWG measurement relative to delivery. In total, 53 women in the analytic sample (16%) had last prenatal weights obtained within 1–4 wk prior to delivery (mean = 1.8±0.69 wk prior to delivery; range: 1.1–3.7 wk). After excluding women with weight measurements obtained within 1–4 wk of delivery, associations between GWG and childhood weight and body composition outcomes were essentially the same or were negligibly different from observed associations in the larger cohort (data not shown).
Analyses were also conducted using inverse probability weights for successful follow-up at 7 years to assess for effects of sample attrition and incomplete follow up on effect estimates. Calculation of IPW for follow up at age 7 showed that parity, birth weight, black race and maternal age were associated with successful follow up, while prepregnancy obesity, GWG, maternal education, linguistic isolation, receiving public assistance, poverty rate, and missing income data were not associated follow up (all p>0.15). Weighting the data for successful follow up did not appreciably alter associations between GWG and childhood outcomes (data not shown).
Discussion
To our knowledge, this is the first study to characterize long-term effects of GWG in a contemporary low-income multi-ethnic urban cohort; a population characterized by a high risk of obesity and associated consequences. Our findings suggest that weight gain in pregnancy greater than the IOM’s recommendations is strongly associated with obesity in childhood; children of mothers with excessive GWG had almost a 300% increased odds of obesity [OR: 2.93; 95% CI: 1.49, 5.75]. In our study, as expected, higher prepregnancy BMI was positively associated with higher BMIZ and obesity in childhood.
Our results are generally consistent with previous studies reporting associations between total/excessive GWG and childhood obesity and body composition (Margerison-Zilko et al., 2012, Hinkle et al., 2012, Fraser et al., 2010, Schack-Nielsen et al., 2010, Ensenauer et al., 2013, Lawlor et al., 2011, Crozier et al., 2010). However, in our cohort, the effects of excessive GWG on childhood obesity (300% increased risk) were stronger than other reports, where obesity risk was increased by ~30–60%. This likely reflects sample differences and highlights the importance of supporting healthy GWG in populations similar to ours. Previously, in a nationally representative sample of predominately normal weight mothers (n=3600, 64% normal weight, 34% overweight/obese) in the United States, excessive GWG (>2009 IOM guidelines) was associated with higher BMIZ at 5 years, but only in offspring of mothers with normal and overweight prepregnancy BMI (Hinkle et al., 2012). In another diverse United States based cohort (n=3,015; 24% overweight/obese), total GWG was positively associated with childhood overweight (Margerison-Zilko et al., 2012). Excessive GWG was also associated with increased odds of childhood overweight (BMIZ>85%ile) [OR: 1.27, (95% CI: 1.10–1.48)] (Margerison-Zilko et al., 2012). Similarly, in a subset of the ALSPAC Study, a United Kingdom based cohort (n=5114; 69% normal weight, 17% overweight, 7% obese prepregnancy), excessive GWG (>2009 IOM guidelines) was associated with 1075 g (95% Confidence interval (CI): 773, 1378) greater fat mass in children (measured by Dual-energy X-ray Absorptiometry), compared to women gaining within the recommendations (Fraser et al., 2010). Early GWG (<14 weeks) was incrementally associated with offspring fat mass among all women, but only later GWG (between 14–36 wk) was associated with greater offspring fat mass among women gaining >500 g/week (Fraser et al., 2010). Prevalence of prepregnancy obesity in this cohort was only 7% (Fraser et al., 2010); therefore, these findings may not be fully generalizable to our findings, as prepregnancy weight status is associated with the magnitude and composition of gestational weight gain (Lederman et al., 1997, Butte et al., 2003). In a European cohort (n=12,775) high total GWG was associated with 0.34 (95% CI: 0.28–0.40, p<0.01) higher BMIZ in childhood (ages 2–9 years) and risk of child obesity [OR: 1.33 (95% CI: 1.09–1.62] (Dello Russo et al., 2013). In this cohort, reliable estimates of prepregnancy BMI were not available (Dello Russo et al., 2013). In predominately normal-weight German mothers (n=6,837) (26% overweight/obese), excessive GWG (>2009 IOM guidelines) was associated with child overweight [OR 1.57 (95% CI: 1.30–1.91)] and abdominal adiposity (WC>90%ile) [OR: 1.39, (95% CI: 1.19, 1.63)] (Ensenauer et al., 2013). In the University of Southampton Women’s Study (n=948) (43% overweight/obese), excessive GWG (>2009 IOM) was associated with childhood fat mass at 6 years (β: 0.30, (95% CI: 0.11, 0.49) (Crozier et al., 2010).
Consistent with previous reports a majority of women in our analytic sample gained above the current IOM recommendations (Institute of Medicine, 2009, Centers for Disease Control and Prevention, 2012). However, the proportion of women in our sample who gained above the guidelines (64%) was higher than most other reports (Centers for Disease Control and Prevention, 2012, Sangi-Haghpeykar et al., 2014). Based on data from the CDC Pregnancy Nutrition Surveillance System, which collects data from low-income women in federally funded maternal and child health programs, prevalence of excessive GWG among black, non-Hispanic women was approximately 48% (Range: 47.7% to 48.7%), and was approximately 43% for Hispanic women (Range: 42.4% to 44.5%) for the years 2002–2011 (Centers for Disease Control and Prevention, 2012). Mean total GWG in our cohort (16.4 ± 7.9 kg) was similar to another cohort of African American women (n=47) in New York City (17.4±5.6 kg), where about 2/3 of all women and all overweight/obese women gained above the 1990 IOM recommendations (Lederman et al., 2002).
This study has limitations that should be noted. Prepregnancy weight was obtained by self-report. Previously, in a multi-ethnic cohort of New York women, self-report of prepregnancy weight was highly correlated with measured weights across BMI categories (r=0.92–0.99) and was only significantly different in underweight women (Lederman and Paxton, 1998); but, in other non-pregnant populations there is evidence that weight under-reporting weight-varies by ethnicity (Richmond et al., 2014, Wen and Kowaleski-Jones, 2012). If weight was underreported, our estimates of prepregnancy BMI would be underestimated and GWG would be overestimated (Deierlein et al., 2011). Due to insufficient sample size, we were unable to evaluate for heterogeneity by prepregnancy BMI, which has been observed in some previous reports (Margerison-Zilko et al., 2012, Hinkle et al., 2012) but not others (Deierlein et al., 2012, Fraser et al., 2010). Moreover, the last prenatal weight measurement was not available for some women within 1 wk of delivery, thus we conducted a sensitivity analysis excluding women with last prenatal weights obtained 1–4 wk before delivery. In this analysis, we found that the results were essentially unchanged. As with any longitudinal study, we had sample attrition and missing data. Reanalysis of the data using inverse probability weighting for successful follow-up suggests that incomplete follow up did not bias the results. Lastly, childhood body composition was assessed with bioelectrical impedance analysis, which has been validated in some populations but has not been compared to gold-standard reference methods in a population of African American and Dominican children similar to ours (Haroun et al., 2009, Jebb et al., 2000, Pietrobelli et al., 2004, Xie et al., 1999); yet, similar associations were observed for all outcomes (BMIZ, waist circumference and %fat from bioelectrical impedance analysis).
In conclusion, GWG above the IOM recommendations was associated with a markedly increased risk of child obesity. Total GWG was positively associated with childhood size and adiposity, but only marginally associated with obesity. These results suggest that effects of excessive GWG persist into childhood; thus supporting controlled and healthy GWG is essential to promote healthy growth in childhood and thereafter. The strengths of our study include a prospective cohort design, medical record report of GWG and use of body composition measurements in childhood. As previously mentioned, this is one of the first studies to report long-term effects of GWG on childhood fatness and obesity in a multi-ethnic urban and predominately low-income cohort. We were able to adjust for potential confounders including socioeconomic status and psychosocial health in pregnancy. Future studies with measurement of maternal body composition changes in pregnancy and childhood adiposity measures may provide further insight to mechanisms underlying these associations.
Acknowledgements
We would like to thank the CCCEH Participants and research team, especially: Virginia Rauh, Greg Freyer, Howard Andrews, Deliang Tang, Diurka Diaz, Fred Hua, and Darrell Holmes. This publication was made possible by U.S. Environmental Protection Agency (US EPA) grant RD83214101 and National Institute for Environmental Health Sciences (NIEHS) grant 1RC2ES018784 and P01 ES09600. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
This study was also supported by the Irving General Clinical Research Center (grant RR00645), the Educational Foundation of America, the John and Wendy Neu Family Foundation, the New York Community Trust, and the Trustees of the Blanchette Hooker Rockefeller Fund. Finally, NTM and EMW received fellowships from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK T32DK091227-03).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Hutcheon JA, Platt RW, Himes KP, Simhan HN, Abrams B. Should gestational weight gain recommendations be tailored by maternal characteristics? Am J Epidemiol. 2011;174:136–146. doi: 10.1093/aje/kwr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA, Noble MD, Adair LS. Are gestational age, birth weight, and birth length indicators of favorable fetal growth conditions? A structural equation analysis of Filipino infants. Stat Med. 2013;32:2950–2961. doi: 10.1002/sim.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–1432. doi: 10.1067/s0002-9378(03)00596-9. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2011 Pregnancy Nutrition Surveillance: Summary of Trends in Maternal Health Indicators by Race/Ethnicity. 2012 [Google Scholar]
- Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr. 2010;91:1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahly DL, Adair LS, Bollen KA. A structural equation model of the developmental origins of blood pressure. Int J Epidemiol. 2009;38:538–548. doi: 10.1093/ije/dyn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr. 2011;158:221–226. doi: 10.1016/j.jpeds.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierlein AL, Siega-Riz AM, Herring AH, Adair LS, Daniels JL. Gestational weight gain and predicted changes in offspring anthropometrics between early infancy and 3 years. Pediatr Obes. 2012;7:134–142. doi: 10.1111/j.2047-6310.2011.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Russo M, Ahrens W, De Vriendt T, Marild S, Molnar D, Moreno LA, et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: the IDEFICS project. Int J Obes (Lond) 2013;37:914–919. doi: 10.1038/ijo.2013.35. [DOI] [PubMed] [Google Scholar]
- Ensenauer R, Chmitorz A, Riedel C, Fenske N, Hauner H, Nennstiel-Ratzel U, et al. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: results from a retrospective cohort study. Int J Obes (Lond) 2013;37:505–512. doi: 10.1038/ijo.2012.226. [DOI] [PubMed] [Google Scholar]
- Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121:2557–2564. doi: 10.1161/CIRCULATIONAHA.109.906081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun D, Croker H, Viner RM, Williams JE, Darch TS, Fewtrell MS, et al. Validation of BIA in obese children and adolescents and re-evaluation in a longitudinal study. Obesity (Silver Spring) 2009;17:2245–2250. doi: 10.1038/oby.2009.98. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142:1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211, e211–e217. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- Jebb SA, Cole TJ, Doman D, Murgatroyd PR, Prentice AM. Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model. Br J Nutr. 2000;83:115–122. doi: 10.1017/s0007114500000155. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS. A supply-demand model of fetal energy sufficiency predicts lipid profiles in male but not female Filipino adolescents. Eur J Clin Nutr. 2004;58:438–448. doi: 10.1038/sj.ejcn.1601826. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Lichtenstein P, Fraser A, Langstrom N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr. 2011;94:142–148. doi: 10.3945/ajcn.110.009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman SA, Alfasi G, Deckelbaum RJ. Pregnancy-associated obesity in black women in New York City. Matern Child Health J. 2002;6:37–42. doi: 10.1023/a:1014364116513. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern Child Health J. 1998;2:123–126. doi: 10.1023/a:1022996924094. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–488. doi: 10.1016/s0029-7844(97)00355-4. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J. 2012;16:1215–1223. doi: 10.1007/s10995-011-0846-1. [DOI] [PubMed] [Google Scholar]
- Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4. doi: 10.1186/1742-5573-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta UJ, Siega-Riz AM, Herring AH. Effect of body image on pregnancy weight gain. Matern Child Health J. 2011;15:324–332. doi: 10.1007/s10995-010-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobelli A, Rubiano F, St-Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr. 2004;58:1479–1484. doi: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- Reyes M, Perzanowski MS, Whyatt RM, Kelvin EA, Rundle AG, Diaz DM, et al. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allergy Asthma Immunol. 2011;107:42–49. e41. doi: 10.1016/j.anai.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TK, Thurston I, Sonneville K, Milliren CE, Walls CE, Austin SB. Racial/ethnic differences in accuracy of body mass index reporting in a diverse cohort of young adults. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175:1163–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangi-Haghpeykar H, Lam K, Raine SP. Gestational weight gain among Hispanic women. Matern Child Health J. 2014;18:153–160. doi: 10.1007/s10995-013-1248-3. [DOI] [PubMed] [Google Scholar]
- Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sorensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 2010;34:67–74. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999–2012. JAMA Pediatr. 2014 doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- Stamnes Kopp UM, Dahl-Jorgensen K, Stigum H, Frost Andersen L, Naess O, Nystad W. The associations between maternal pre-pregnancy body mass index or gestational weight change during pregnancy and body mass index of the child at 3 years of age. Int J Obes (Lond) 2012;36:1325–1331. doi: 10.1038/ijo.2012.140. [DOI] [PubMed] [Google Scholar]
- Wallace D, Wallace R, Rauh V. Community stress, demoralization, and body mass index: evidence for social signal transduction. Soc Sci Med. 2003;56:2467–2478. doi: 10.1016/s0277-9536(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Wen M, Kowaleski-Jones L. Sex and ethnic differences in validity of self-reported adult height, weight and body mass index. Ethn Dis. 2012;22:72–78. [PMC free article] [PubMed] [Google Scholar]
- Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Kolthoff N, Barenholt O, Nielsen SP. Validation of a leg-to-leg bioimpedance analysis system in assessing body composition in postmenopausal women. Int J Obes Relat Metab Disord. 1999;23:1079–1084. doi: 10.1038/sj.ijo.0801034. [DOI] [PubMed] [Google Scholar]