Abstract
Objective
Over the past decade, clinical data has accumulated showing that inflammation might contribute to the pathophysiology of suicide. To evaluate associations and identify support for pathways linking inflammatory processes with suicidal behavior, a comprehensive review of the literature was undertaken.
Method
The search terms “cytokine”, “risk factors”, “kynurenine”, “asthma”, “allergy”, “autoimmunity”, “traumatic brain injury”, “infection” along with the terms “inflammation” and “suicide” were entered into PubMed and a thorough analysis of the publications and their reference lists was performed.
Results
The effects of inflammation on mood and behavior could partially be mediated by kynurenine pathway metabolites, modulating neuroinflammation and glutamate neurotransmission. At the same time, the triggers of the inflammatory changes documented in suicidal patients may be attributed to diverse mechanisms such as autoimmunity, neurotropic pathogens, stress or traumatic brain injury.
Conclusion
Targeting the inflammatory system might provide novel therapeutic approaches as well as potential biomarkers to identify patients at increased risk. For the goal of improved detection and treatment of suicidal individuals to be achieved, we need to develop a detailed understanding of the origin, mechanisms and outcomes of inflammation in suicidal behavior.
Introduction
Suicide is defined as the intentional termination of one's own life, and constitutes the 14th leading cause of global years of life lost (1). According to a recent report by the World Health Organization over 800,000 deaths by suicide occur around the world each year, but the actual number may be higher (2). Cultural taboos and the fact that suicide is considered a criminal act in some countries may affect the statistical reporting of such events (3). Suicide attempts are estimated to be 10 to 20 times more frequent than the number of completed suicides. Both deaths by suicide and attempts are signs of severe psychological suffering, and a completed suicide carries with it an emotional burden that can impact family for years to come.
The pharmacological treatment of depressed and suicidal individuals has increased over the past decades, but the incidence rates of suicide and suicide attempts are still increasing. 1.5 million people will die from suicide in 2020, if the current trends remain unaltered (4). Accurate suicide risk determination is a very difficult task for clinicians, considering that a patient at high risk for suicide is likely to minimize this symptom. The health care system is in many cases unable to accurately detect suicidality, in spite of the fact that almost half of the suicidal patients actually contact health care providers in the months prior to their attempt (5). Consequently, there is a great need for both improved methods for the detection of suicide risk and for effective, novel pharmacological treatment options for suicidal patients.
The pharmacological treatment options for suicidal patients today are likely to depend on the patient's primary psychiatric diagnosis, and often include antidepressants and anxiolytic medications (6). The positive effects of pharmacotherapy on symptoms of depression take weeks to months to develop, and moreover there may be an increased risk for suicidal behavior during this initial period of treatment, especially in children, adolescents and adults up to age 25 (7). Besides the conventional anti-depressant drugs, clozapine (in schizophrenia) and lithium (in patients with mood disorders) are sometimes indicated specifically for reduction of suicide risk. So-called treatment-resistant depression, which often includes symptoms of suicidality, can be treated with electroconvulsive therapy in many countries, in addition to pharmacological treatment. Accumulating studies suggest that subanesthetic doses of intravenous ketamine exerts rapid anti-depressant and anti-suicidal effects (8, 9). The underlying biological mechanisms by which these treatment options modulate suicidal symptoms are not fully understood.
Aims of the study
The aim of this literature review is to evaluate associations and identify support for pathways linking inflammatory processes with suicidal behavior, and on the basis of the findings, provide guidance on how to relate to this emerging scientific evidence in clinical practise.
Material and methods
The search terms “risk factors”, “cytokine”, “kynurenine”, “asthma”, “allergy”, “autoimmunity”, “traumatic brain injury”, “infection” along with the terms “inflammation” and “suicide” were entered into PubMed and a thorough analysis of the publications and their reference lists was performed.
Results
Risk factors for suicide
Suicidal behavior is thought to be driven by complex interactions between genetic predisposition and environmental factors (10). Up to 90% of individuals who complete suicide have an underlying psychiatric disorder, frequently Major Depressive Disorder (MDD) and bipolar disorder (11). Men and women with major depression have a 20.9 and 27.0, standardized mortality ratio for suicide, respectively, and the suicide mortality is approximately 20 fold higher than in the general population (12). However, for the determination of suicide risk in the clinic, the psychiatric history is still of limited value. Currently, a previous suicide attempt is the best predictor of a future completed suicide (13). Certain personality traits, such as impulsivity, aggression (14, 15) and hopelessness (16), are also coupled to an increased risk of suicide. The genetic component of suicidal behavior has been estimated to be as high as 43% (17). Still, the neurobiological mechanisms involved in suicidal behavior are poorly characterized. To date, some of the most frequently reported neurobiological changes in suicidal individuals and suicide victims are abnormalities in serotonergic neurotransmission (18) and hypothalamic–pituitary–adrenal (HPA) axis activity (19). As the topic of this review suggests, accumulating evidence indicate that inflammation contributes to the pathophysiology of suicidality.
Evidence of inflammation in suicidality
Treatment with interferon (IFN) for patients with certain forms of cancer and infections is known to induce depression around one month after the beginning of medication (20). Such immunotherapy trials indicate a causal relationship between the induced inflammation and subsequent onset of depression. They also provide neurobiological proof of principle that peripheral inflammatory factors can transmit to the central nervous system, and their levels correlate with depressive symptoms (21, 22). Several case reports also describe the development of suicidal ideation and suicide attempts in this patient population (23, 24). Similarly, suicidal ideation and attempts have been documented in previously psychiatrically healthy patients with multiple sclerosis during and after treatment with interferon-β (IFN-β) (25). In a blinded study that directly confirmed the causality, Reichenberg, et al. administered a low dose of lipopolysaccharide (LPS, bacterial endotoxin) or placebo to healthy subjects, and found significantly increased depressive symptoms in the LPS-injected subjects (26). Over the past years, converging lines of evidence point to inflammation as a possible causal factor, underlying the pathophysiology of suicidality in primary psychiatric patients.
In 1992, an early study discovered increased levels of the soluble interleukin-2 receptor (IL-2R) in blood samples from suicide attempters (27). It took more than fifteen years for new reports on the topic to follow. Steiner et al. examined the brains of suicide victims for inflammatory changes and found significantly increased microgliosis in the brains of suicide victims with depression and schizophrenia compared to subjects with the same diagnoses who died from other causes (28). The same year, another group demonstrated increased levels of mRNA transcription for the cytokines interleukin-4 (IL-4) and IL-13 in the orbitofrontal cortical area of suicide victims (29). Supporting of these key observations, Lindqvist et al. found elevated cerebrospinal fluid (CSF) levels of IL-6 in patients who attempted suicide (5.3 ± 3.2; mean ± SEM) compared to healthy controls (0.6 ± .1; mean ± SEM). Higher levels of CSF IL-6 were associated with increasing severity of depression, as evaluated using the Montgomery-Asberg Depression Rating Scale (MADRS) (Pearson's r = .3; p = .016) (30). Recently, increased levels of IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) at both the mRNA and protein levels were shown in the anterior prefrontal cortex (Brodmann area 10) of teenage suicide victims (31).
Further studies have confirmed that suicidal behavior is also accompanied by changes in peripheral cytokine levels. The levels of plasma IL-6 and TNF-α are increased, and IL-2 levels are decreased, in suicide attempters compared to both non-suicidal depressed patients and healthy controls (32). In vitro stimulated whole blood from suicidal patients with MDD had a decreased production of both IL-2 and IL-6 compared to non-suicidal MDD patients (33). In agreement with the original post-mortem data from Steiner et al. (28), these studies suggest that it might be possible to distinguish suicidal- from non-suicidal depressed patients based on peripheral inflammatory markers. However, cytokine levels are known to display a large biological spread and increased levels of inflammatory markers are also seen in other psychiatric and non-psychiatric disorders, including PTSD, non-suicidal depression and neurodegenerative disorders. To circumvent these problems it is likely that a combination of biomarkers will be required to increase sensitivity and specificity rates for clinical prediction of suicide risk. Additional studies on peripheral cytokines in suicidal patients are clearly needed to establish which biomarkers are most useful. The proteins S100B and C-reactive protein (CRP) are other peripheral markers of inflammation that increase during injury and inflammation in the central nervous system (CNS). Falcone et al. analyzed serum S100B in teenagers with depression and psychosis, and found that the levels were related to the intensity of suicidal ideation irrespective of diagnosis (34). O'Donovan et al examined the relation of suicidal ideation to a composite inflammatory index, consisting of CRP, IL-6, IL-10 and TNF-α, in patients with depression. They found that suicidal ideation was significantly associated with an elevated inflammatory index, and this was independent of both the severity of depression and whether the patients had recently attempted suicide (35).
Importantly, a novel meta-analysis on inflammation in suicidal patients concluded that there are aberrant cytokine levels in blood, CSF, and postmortem brain samples from suicidal patients (36). The levels of IL-1β and IL-6 were most robustly associated with suicidality, and these cytokines may help distinguish suicidal from nonsuicidal patients. In another review of the inflammatory changes in suicidal subjects, Serafini et al also concluded that most suicide attempters, or subjects with suicidal ideation, show an imbalance in the immune system, although they stress that cross-sectional studies are not able to demonstrate causal links between inflammation and suicidality (37). In support of these meta-analyses, a recent gene-expression study showed that biological mechanisms related to stress, inflammation and apoptosis may underlie suicidality, at least in part (38).
Biological mechanisms underlying suicidal ideation and behavior
As clearly learned from the interferon-treatment studies mentioned above, activation of the immune system can exert profound effects on mood and behavior. The clinical syndrome “sickness behavior” is well defined, and consists of behavioral and emotional changes that occur in patients and animals in conjunction with a known infectious/inflammatory trigger (39). A number of models have demonstrated how peripherally produced cytokines reach and convey signals to the CNS, including active or passive transportation over the blood-brain barrier (BBB) and by vagus-nerve mediated signaling (40). Cytokines are also secreted locally, by microglia, astrocytes and endothelial cells in the brain, and play an important role in the development and maintenance of brain function (for review, see (41)). Experimental data show that there are several biological mechanisms by which cytokines could contribute to behavioral and emotional symptoms relevant for suicidality. First, cytokine receptors are present on neurons in specific brain regions in both animals and man. For example, IL-6 receptors are expressed on serotonergic neurons in the medulla oblongata, as well as in the hypothalamus, hippocampus, cerebellum and selective cortical areas (42, 43). IL-1β receptors are located on hippocampal, amygdaloid and thalamic neurons, on 5-HT2 receptor-expressing neurons in the hypothalamus, and on cerebellar Purkinje cells (44). The presence of cytokine receptors on neurons might indicate that they have specific and direct effects on neuronal function. In fact, IL-6, IL-1β and TNF-α have all been implicated in the regulation of synaptic transmission and plasticity (45, 46). Cytokines can also modulate the concentration of monoaminergic neurotransmitters and their metabolites in various regions of the CNS (47).
There are no animal models that directly mimic human suicidal behavior. However, suicidal behavior is thought to depend on several critical personality traits and symptoms, for example aggression and helplessness (48). Animal studies have demonstrated that cytokines might actually influence these key behaviors. IL-1β injected into the medial hypothalamus or periaqueductal gray (PAG), acts on 5-HT2 receptors to potentiate aggressive behaviors in cats (49, 50). IL-2 injected into the PAG promoted aggression through neurokinin NK(1) receptors (51). TNF-α is also involved in the regulation of aggression, as TNF-α receptor deficient mice did not exhibit aggressive behavior in the resident-intruder test (52). Clinical studies have confirmed that peripheral cytokines are associated with aggression and hopelessness. IL-6 correlates positively with anger (53) and IL-1β is associated with hostility in patients with self-harm (54). Correspondingly, increased anger and hostility were observed in patients with hepatitis C that underwent IFN-α treatment (55). Higher levels of anger in these patients were found linked to a genetic variability in the TNF-α gene (56).
A second biological mechanism, that could have profound impact on emotion and behavior, is the activation of the kynurenine pathway of tryptophan catabolism (Fig 1). Aberrations in this pathway could constitute a particular pathogenic mechanism linking inflammation and suicidal/depressive symptoms (57). Tryptophan degradation along the enzymatic kynurenine pathway produces several neuroactive compounds, including quinolinic acid (QUIN), and kynurenic acid (KYNA) (58). The pro-inflammatory cytokines, especially IFN-γ but also IL-1β and IL-6 are potent inducers of indoleamine 2,3 dioxygenase (IDO-1) and tryptophan 2,3-dioxygenase (TDO2), two enzymes regulating the first step of the kynurenine pathway (59, 60). As tryptophan is also the precursor of serotonin, inflammation might cause a decrease in serotonin levels by shifting the catabolism of tryptophan to breakdown via the kynurenine pathway (61). This mechanism could potentially contribute to the lowered levels of monoamine metabolites found in CSF of suicide attempters (62). However, it has not yet been firmly established if inflammation does indeed cause depletion of brain tryptophan and serotonin levels in patients with primary depression or suicidality.
Figure 1.
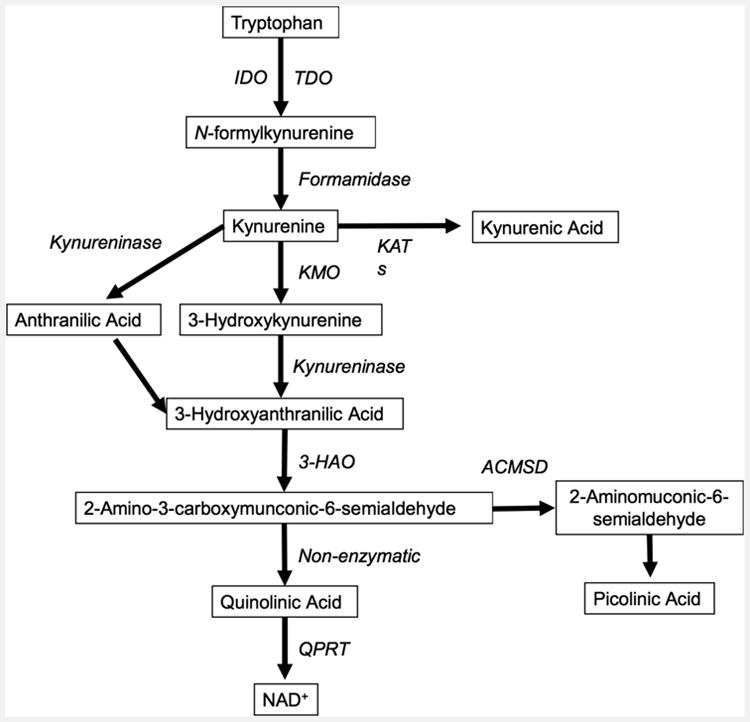
Simplified diagram of the kynurenine pathway. Enzymes in italics and metabolites boxed. Abbreviations: Indoleamine-2,3-dioxygenase, IDO; tryptophan-2,3-dioxygenase, TDO; Kynurenine aminotransferase, KAT; Kynurenine-3-monooxygenase, KMO 3-Hydroxyanthranilate-3,4-dioxygenase, 3-HAO; aminocarboxymuconate-semialdehyde-decarboxylase, ACMSD; quinolinate phosphoribosyltransferase, QPRT; Nicotinamide adenine dinucleotide, NAD.
Interestingly, the kynurenine pathway accounts for over 90% of tryptophan degradation in the periphery (63) and enzymes of this pathway are found in many organs/cell types, including liver, kidney, brain- and immune cells. In the brain, the two main branches of the kynurenine pathway are segregated into astrocytes and microglia, with a differential production of metabolites (64). QUIN is an N-methyl-D-aspartic acid (NMDA) receptor agonist, acting through NMDA receptors containing the NR1 + NR2A and the NR1 + NR2B subunits (65, 66). It is formed to a large extent in microglial cells in the brain. KYNA, on then other hand, antagonizes the glycine-site of the NMDA-receptor, blocks the cholinergic α7 nicotinic receptor (α7nAChR) and is produced by astrocytes (65, 67). QUIN is a potent excitotoxin with pro-inflammatory and immunoregulatory properties. In addition to being a direct NMDA receptor agonist, QUIN increases neuronal glutamate release and decreases glutamate uptake and recycling by astrocytes (68). KYNA has neuroprotective and anti-convulsive properties, although elevated levels may be associated with the development of psychotic symptoms and are observed in patients with schizophrenia (69, 70).
Evidence of kynurenine pathway alterations in suicidality
In 2011, Sublette et al reported that the blood level of kynurenine, the first metabolite produced along the kynurenine pathway, is significantly elevated in suicide attempters with depression compared to non-suicidal patients with depression (t(58) = 2.1, p = .04) (71). This suggests that the activation of the kynurenine pathway in the periphery may serve as a marker, potentially distinguishing suicidal from non-suicidal depressed patients. Kynurenine can freely pass through the BBB, and while in the brain, can initiate inflammation and lead to further production of the secondary kynurenine metabolites (58). It has not been firmly established whether peripheral QUIN, KYNA and several other metabolites cross the BBB, and, therefore, central measures of these markers in psychiatric patients are currently highly relevant to increase our understanding of their contribution to psychiatric illness. In a study where we measured KYNA and QUIN in the CSF of suicide attempters, QUIN levels were found to be more than twice as high as those in healthy controls, while KYNA levels were unaltered (72). This gave rise to an increased QUIN/KYNA quotient, indicative of a potential net positive effect on NMDA-receptor transmission in suicidality. Moreover, CSF QUIN correlated with CSF IL-6 levels, suggesting that the kynurenine pathway and the generation of QUIN had been induced by an active inflammatory process (72). A further example suggesting a role for QUIN in suicidality was the finding of a positive association between high CSF QUIN levels and high suicidal intent (Spearman ρ = .3, p = .03). In a follow-up study we measured CSF levels of the metabolites repeatedly over the first years following a suicide-attempt (73). We found that the QUIN levels were continuously elevated in the patients who had performed a suicide attempt, while the levels were highest in close proximity to the attempt. The magnitude of their depressive and suicidal symptoms fluctuated with increasing cytokine levels and decreasing KYNA-levels. In line with this, decreased plasma KYNA levels in depressed patients have previously been observed (74), although recent studies indicate that patients with depression without suicidality may not have any significant changes in the peripheral kynurenine pathway (75).
Interestingly, this accumulating evidence suggest that subsets of patients, prone to suicidal behavior, could be sensitive to inflammatory changes, and may develop symptoms upon such challenges; possibly as a result of an enzymatic imbalance of the kynurenine pathway in the CNS. The increased levels of QUIN could also contribute to the cell loss and structural changes in cortical and subcortical regions that has been reported in patients with suicidal behavior, due to the neurotoxic effects of the metabolite (76). Recent post-mortem data from suicide completers supports the data gathered in attempters, by showing increased counts of QUIN-reactive microglia cells in the subgenual anterior cingulate cortex (sACC) and anterior midcingulate cortex (aMCC) of those with depression who completed suicide (77). The findings that an NMDA receptor agonist (QUIN) is increased, and an NMDA receptor antagonist (KYNA) is found to be decreased in patients support a role for a glutamatergic mechanism in the generation of suicidality. Noteworthy, the findings of exceptionally high levels of the NMDA-receptor agonist QUIN in the CNS of suicidal patients could provide a neurobiological rationale for the rapid remedial effects of ketamine, an NMDA-receptor antagonist, on suicidal ideation (78, 79). It remains to be described how enzymes in the kynurenine pathway are regulated. It is currently not known whether specific cytokines activate some of the enzymes in the kynurenine pathway in preference over others, or why some metabolites would accumulate in favor of another. In this respect, it has been suggested that genetic variants could pay a role (80).
Suicidal ideation and behavior in somatic conditions with increased inflammation
The studies described above shows that inflammation is present in some cohorts of suicidal patients, and that inflammation also is specifically associated with clinically important symptoms of suicidal behavior. In addition, studies in both animals and human subjects confirm the causal relation between cytokines and symptoms of hopelessness, aggression and hostility, intermediate phenotypes for suicidal behavior (14-16). Therefore, it is possible that inflammation observed in patients with so called “primary psychiatric conditions” is actually part of a pathogenic mechanism that contributes to suicidal symptoms. Many somatic conditions are associated with increased peripheral and central inflammation. If our hypothesis is true, then suicidal ideation and behavior should also be increased in some of these somatic conditions.
Suicide, asthma and allergies
Asthma and allergies consist of an array of innate and adaptive immune responses, locally as well as systemically in the peripheral blood (81). To our knowledge, it is currently not known if there are any central nervous system inflammatory changes in these conditions. This topic is of interest, as several epidemiological studies have shown a link between suicide, allergies and asthma. There is an increased risk for both suicidal ideation, completed suicides and suicide attempts in asthmatic patients (82). A large epidemiological study from Taiwan investigated suicide rates in more than 160,000 high-school students, with and without asthma, over 12 years (83). The suicide rate in students with asthma was more than double that of the control group, with no difference in the number of natural deaths. In another epidemiological study from the Unites States (US) both suicidal ideation and suicide attempts were found to be associated with a current diagnosis of asthma (84). The risk increase for suicidal ideation and attempts seems to be approximately 2-3 fold, with numbers varying between different studies.
A spring peak of suicides is observed each year around the world but there is no current explanation for this phenomenon (85). Hypothetically, the increased amount of seasonal aeroallergens known to peak in spring, such as airborne pollen, could lead to increased upper airway inflammation and an increased risk of suicidal behavior, in particular in sensitized individuals. A study from the US over four years did indeed report an increased rate of suicides during the peaks in tree-pollen in females (86). While a subsequent study in the US failed to replicate the original finding (87), this was later replicated by a large population study in Denmark (88). It is important to note that the seasonal variation may be related to several factors, such as the exposure to visible and UV-light, and seasonal variations of vitamin D levels, as discussed below. In addition, intranasal corticosteroids, first line treatment for allergic rhinitis, are known to reduce cytokine production in the nasal airways and have been pharmaco-ecologically shown to be negatively associated with suicide rates. (89).
Vitamin D, inflammation and suicidality
Vitamin D is the common term for a group of related substances, particularly cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). The effects of vitamin D on the immune system include a shift in the T helper (Th)-balance towards a Th2-phenotype, which in simplified terms can be said to lead to a less pro-inflammatory state. As there is an over-representation of vitamin D deficiency in patients with psychiatric illness (90), the lack of vitamin D has been proposed to contribute to the underlying disease mechanisms (91). Two studies to date have addressed the association between vitamin D and suicidality (92, 93). Umhau et al investigated the vitamin D levels in blood samples from around 1000 active duty US military service members, of which half completed suicide over the year following the blood sample. They found that low vitamin D status was common in active military members, and the lowest levels were associated with an increased risk for suicide (92). In the study by Grudet et al, it was found that as many as 90% of patients with a recent suicide attempt had suboptimal levels of vitamin D, and 60% had a clear clinical deficiency. As expected, vitamin D in the blood correlated inversely with the levels of pro-inflammatory cytokines (93).
Autoimmunity and suicidality
Increased rates of suicidality are also observed in autoiummune disorders such as Systemic Lupus Erythematosus (SLE), Multiple Sclerosis (MS) and coeliac disease. Approximately 40% of SLE and MS patients develop depression at some point during their illness (94, 95). The neurobiological changes in SLE frequently includes antibodies against the NMDA receptor, as well as microglial activation and BBB dysfunction (96). The prevalence of suicidal ideation was as high as 34% in a sample of around 300 Chinese patients with SLE (97). In a cohort of over 12,000 hospitalized MS patients, Fredrikson et al. found an increased suicide risk (odds-risk ratio = 2.3) when compared to the general population (98). A smaller scale study of around 100 MS patients in Denmark confirmed the two-fold increased risk of dying by suicide (99). A large epidemiological study on a total of over 29.000 individuals with coeliac disease in Sweden established that the patients with inflammatory disease also have a two-fold increased risk for suicide (100).
Traumatic brain injury and suicidality
Traumatic brain injury (TBI) is a well-known cause of neuroinflammation and glial activation (101). The effects on the brain parenchyma can be long lasting, and has been proposed to underlie the increased suicidal behavior observed in veterans as well as to constitute a risk factor in sports associated with frequent head trauma (102). Interestingly, in a recent large epidemiological study, the death records were compared between around 200.000 individuals who had been hospitalized due to TBI in Sweden over a 40-year period and over 2 million controls, never hospitalized for TBI, as well as 150.000 unaffected siblings of the TBI victims (103). It was found that the TBI victims were more than 3 times as likely than the general population and unaffected siblings to die from suicide. Juengst et al measured serum and CSF levels of TNF-α in patients with moderate and severe TBI as well as in controls (104). They found that the TBI patients had significantly higher TNF-α levels than healthy controls, and that the levels were associated both with disinhibition and suicidal ideation up to 12 months after the injury. Among 559 patients with mild to severe TBI, 25% reported suicidal ideation at one or more time points over the first year after the injury (105).
Infections and suicidality
Infectious agents in the central nervous system are likely to greatly increase the CSF levels of cytokines and kynurenine metabolites (106). Therefore, such infections can give important insights into the role of neuroinflammation in the development of suicidal symptoms. It is known that neuroborreliosis and neurosyphilis, as well as late-stage HIV and AIDS all frequently lead to neuropsychiatric symptoms. However, the prevalence and incidence rates of suicidality in these conditions still remain to be established. Suicide rates in patients that suffered from bacterial and viral meningitis is also unknown, but would be of relevance, as such patients may be at heightened risk and could benefit from increased surveillance. There are also neurotrophic pathogens of low virulence, known to reside relatively quietly within the central nervous system of immuno-competent hosts after infection. Such pathogens include the parasite Toxoplasma Gondii (T. Gondii). Interestingly, accumulating research now shows that such chronic, low-grade infections may exert effects on the host brain to a much larger extent than previously thought. Repeated studies now confirm that there is a significantly increased risk for suicidal behavior in individuals that are positive for T. Gondii infection (107-110). About 30% of the world's population is infected with the parasite in developing, as well as industrial, parts of the world. The initial peripheral infection is associated with none- or very limited flu-like symptoms in immunocompetent hosts, whereafter the parasite enters the brain, to form intracellular cysts in neurons and glial cells. A chronic infection with T. Gondii can lead to increased neuroinflammation and increased production of kynurenine metabolites within the brain, potentially at the localized sites of the parasitic cysts (111). Moreover, it is possible that the parasite and the surrounding inflammatory changes could show varying degrees of activity, depending on the immunocompetence of the host. Finally, it has been shown that the T. Gondii parasite contains two genes encoding tyrosine hydroxylase, the enzyme responsible for producing L-dopa (112). Changes in impulsivity and the regulation of fear might increase the risk for suicidal behavior, perhaps particularly in combination with inflammatory changes in the CNS. We analyzed the antibody titers to T. Gondii in a small study of recent suicide attempters and controls, and found that seropositivity was associated with a greatly increased odds-risk ratio for suicide attempt (adjusted odds-risk ratio = 7.1; p = .01) (109). In a large epidemiological study of 45.000 Danish mothers, seropositive subjects had an increased relative risk of 1.8 for violent suicide attempts and 2.1 for suicides compared to non-infected mothers (110). These results are consistent with previously reported small samples of patients with mood disorders and younger patients with schizophrenia, both studies eliminating the potential confound of mental illness by including both healthy controls and also psychiatric controls as comparison(107, 108). Recently, T. gondii infection has been linked, independent of mental illness, with intermediate phenotypes of suicidal behavior such as trait aggression (in women) and impulsivity (in younger men) (113).
Discussion
In summary, there is a growing body of evidence that inflammation, as manifested by increased levels of pro-inflammatory cytokines and inflammatory metabolites, is present in patients with suicidal behavior and ideation. The inflammatory changes can be detected in the periphery, cerebrospinal fluid and brain parenchyma of affected patients. Inflammation activates the kynurenine pathway; with a down-stream production of metabolites with effects on glutamate neurotransmission, which could be an important biological mechanism responsible for symptom generation. The rapid anti-suicidal effect of the NMDA-receptor antagonist, ketamine, may be due to its effect on the same biological pathway. In addition, cytokines induce site-specific effects on behavior and emotion in different brain areas. Our knowledge in this field is just beginning to evolve. In order to find the optimal treatment regimen for suicidal symptoms, it is of critical importance to identify both the upstream triggers of inflammation, the downstream neurobiological effectors, as well as moderators that convey vulnerability or resilience. Importantly, there are several anti-inflammatory treatments that are clinically approved for other indications. The effects of these common anti-inflammatory agents could be tested in patients with suicidal behavior and ideation in randomized controlled trials (RCTs), after it has been concluded which ones of them have the optimal effects in reducing CNS inflammation. Future clinical trials should also, for example, evaluate the effects of anti-parasitic treatment on T. Gondii infection in patients with suicidal behavior. Other novel therapeutic strategies could include inhibition of the first enzyme of the kynurenine pathway, IDO-1, or target microglial activation by means of drugs such as minocycline. As knowledge increases about the role of specific cytokines for symptom generation, specific cytokine blockers, potentially with access to the CNS, could also be developed and tested. Finally, it makes sense to screen patients prior to clinical trials to identify subgroups of patients with increased inflammation, or vulnerability to inflammation, in order to minimize heterogeneity of treatment response (114). Identification of such subgroups of patients with an inflammatory profile is expected to increase the precision of anti-inflammatory treatments in reducing suicide risk, at the same time preventing unnecessary exposure to anti-inflammatory treatments in those unlikely to benefit.
Clinical Recommendations
Clinicians should be aware that medical conditions associated with inflammation and infections can be linked to symptoms of depression and suicidality.
Clinicians should attempt to establish whether any underlying potentially treatable condition is present in patients presenting with suicidal ideation or history of suicidal behavior.
Treatable somatic conditions linked to depressive and suicidal symptoms include chronic infections, autoimmune disease and certain vitamin deficiencies.
Additional Comments
While there is evidence from both clinical and experimental settings that inflammation can lead to depressive and suicidal symptoms in both clinical and experimental settings, there is still not sufficient data from placebo-controlled trials indicating that eradicating inflammation will actually reduce risk of suicide.
The paucity of trials, utilizing available anti-inflammatory, anti-cytokine and antibiotic drugs (approved for other indications) may require non-profit agency sponsorship.
Repurposing of drugs already approved for other conditions might be an important path forward, with the potential of aiding psychiatric patients suffering from treatment-resistant depressive- and suicidal symptoms.
Acknowledgments
Lena Brundin and Eric Achtyes were supported by the National Institute of Mental Health, Bethesda, MD, R01MH104622-01, Michigan State University and Van Andel Research Institute. Elena Bryleva was supported by Van Andel Research Institute. Sophie Erhardt was supported by Swedish Research Council Grants 2009-7052, 2013-2838 and an independent research grant from Astra Zeneca. Teodor Postolache was supported by an American Foundation for Suicide Prevention Distinguished Investigator Award and by Veterans Integrated Service Network 19, Rocky Mountain Mental Illness Research Education and Clinical Center, (MIRECC) Denver, CO, USA and the Veterans Integrated Service Network 5, MIRECC, Baltimore, MD, USA. Partial funding for Teodor Postolache was also provided by the Mid-Atlantic Nutrition and Obesity Research Center (NORC) (P30DK072488) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
The views, opinions and findings contained in this article belong to the authors and should not be construed as an official position of the NIH, American Foundation for Suicide Prevention, Department of Veterans Affairs or any other funding organization.
Declaration of interests: Eric Achtyes has received research funding to support work related to depression from AssurEx, Janssen, Novartis, Pine Rest Foundation, Priority Health, and the University of Chicago. He has also served as a consultant to Roche for work on schizophrenia.
References
- 1.Mortality Gbd, Causes Of Death C. Global, Regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 Jan 10;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Preventing suicide: A global imperative. [cited April 30, 2015];Geneva. 2014 updated 2014. Available from: http://apps.who.int/iris/bitstream/10665/131056/1/9789241564779_eng.pdf.
- 3.Varnik P. Suicide in the world. International journal of environmental research and public health. 2012 Mar;9:760–71. doi: 10.3390/ijerph9030760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertolote JM, Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry. 2002 Oct;1:181–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Da Cruz D, Pearson A, Saini P, et al. Emergency department contact prior to suicide in mental health patients. Emergency medicine journal: EMJ. 2011 Jun;28:467–71. doi: 10.1136/emj.2009.081869. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman D, Rihmer Z, Rujescu D, et al. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. European psychiatry: the journal of the Association of European Psychiatrists. 2012 Feb;27:129–41. doi: 10.1016/j.eurpsy.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Archives of general psychiatry. 2006 Mar;63:332–9. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 8.Zarate C, Duman RS, Liu G, Sartori S, Quiroz J, Murck H. New paradigms for treatment-resistant depression. Annals of the New York Academy of Sciences. 2013 Jul;1292:21–31. doi: 10.1111/nyas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology. 2014 Sep;231:3663–76. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- 10.Roy A, Sarchiopone M, Carli V. Gene-Environment interaction and suicidal behavior. Journal of psychiatric practice. 2009 Jul;15:282–8. doi: 10.1097/01.pra.0000358314.88931.b5. [DOI] [PubMed] [Google Scholar]
- 11.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997 Mar;170:205–28. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Osby U, Brandt L, Correia N, Ekbom A, Sparen P. Excess mortality in bipolar and unipolar disorder in Sweden. Archives of general psychiatry. 2001 Sep;58:844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 13.Hawton K, Van Heeringen K. Suicide. Lancet. 2009 Apr 18;373:1372–81. doi: 10.1016/S0140-6736(09)60372-X. [DOI] [PubMed] [Google Scholar]
- 14.Dumais A, Lesage AD, Alda M, et al. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. The American journal of psychiatry. 2005 Nov;162:2116–24. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- 15.Perroud N, Baud P, Mouthon D, Courtet P, Malafosse A. Impulsivity, Aggression and suicidal behavior in unipolar and bipolar disorders. Journal of affective disorders. 2011 Nov;134:112–8. doi: 10.1016/j.jad.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 16.David Klonsky E, Kotov R, Bakst S, Rabinowitz J, Bromet EJ. Hopelessness as a predictor of attempted suicide among first admission patients with psychosis: a 10-year cohort study. Suicide Life Threat Behav. 2012 Feb;42:1–10. doi: 10.1111/j.1943-278X.2011.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcguffin P, Perroud N, Uher R, et al. The genetics of affective disorder and suicide. European psychiatry: the journal of the Association of European Psychiatrists. 2010 Jun;25:275–7. doi: 10.1016/j.eurpsy.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Bach H, Arango V. Neuroanatomy of Serotonergic Abnormalities in Suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- 19.Mann JJ. Neurobiology of suicidal behaviour. Nature reviews Neuroscience. 2003 Oct;4:819–28. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 20.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. The Journal of clinical psychiatry. 2005 Jan;66:41–8. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biological psychiatry. 2009 Feb 15;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Molecular psychiatry. 2010 Apr;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. General hospital psychiatry. 2004 May-Jun;26:237–40. doi: 10.1016/j.genhosppsych.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Janssen HL, Brouwer JT, Van Der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. Journal of hepatology. 1994 Aug;21:241–3. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 25.Fragoso YD, Frota ER, Lopes JS, et al. Severe depression, Suicide attempts, And ideation during the use of interferon beta by patients with multiple sclerosis. Clinical neuropharmacology. 2010 Nov-Dec;33:312–6. doi: 10.1097/WNF.0b013e3181f8d513. [DOI] [PubMed] [Google Scholar]
- 26.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-Associated emotional and cognitive disturbances in humans. Archives of general psychiatry. 2001 May;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 27.Nassberger L, Traskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta psychiatrica Scandinavica. 1993 Jul;88:48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- 28.Steiner J, Bielau H, Brisch R, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of psychiatric research. 2008 Jan;42:151–7. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Tonelli LH, Stiller J, Rujescu D, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta psychiatrica Scandinavica. 2008 Mar;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biological psychiatry. 2009 Aug 1;66:287–92. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Pandey GN, Rizavi HS, Ren X, et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. Journal of psychiatric research. 2012 Jan;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain, Behavior, and immunity. 2011 Feb;25:335–9. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim YK, Lee SW, Kim SH, et al. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Progress in neuro-psychopharmacology & biological psychiatry. 2008 Feb 15;32:356–61. doi: 10.1016/j.pnpbp.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 34.Falcone T, Fazio V, Lee C, et al. Serum S100B: a potential biomarker for suicidality in adolescents? PloS one. 2010;5:E11089. doi: 10.1371/journal.pone.0011089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'donovan A, Rush G, Hoatam G, et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depression and anxiety. 2013 Apr;30:307–14. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- 36.Black C, Miller BJ. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biological psychiatry. 2014 Oct 30; doi: 10.1016/j.biopsych.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Serafini G, Pompili M, Elena Seretti M, et al. The role of inflammatory cytokines in suicidal behavior: a systematic review. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2013 Dec;23:1672–86. doi: 10.1016/j.euroneuro.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Le-Niculescu H, Levey DF, Ayalew M, et al. Discovery and validation of blood biomarkers for suicidality. Molecular psychiatry. 2013 Dec;18:1249–64. doi: 10.1038/mp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008 Jan;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009 May 1;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends in neurosciences. 2015 Mar;38:145–57. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Schobitz B, De Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. The European journal of neuroscience. 1993 Nov 1;5:1426–35. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 43.Schele E, Fekete C, Egri P, et al. Interleukin-6 receptor alpha is co-localised with melanin-concentrating hormone in human and mouse hypothalamus. Journal of neuroendocrinology. 2012 Jun;24:930–43. doi: 10.1111/j.1365-2826.2012.02286.x. [DOI] [PubMed] [Google Scholar]
- 44.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, Regulation, and relationship to sites of IL-1-induced cellular activation. The Journal of comparative neurology. 1995 Oct 30;361:681–98. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 45.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, Interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008 May 14;28:5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Oscos F, Salgado H, Hall S, et al. The stress-induced cytokine interleukin-6 decreases the inhibition/excitation ratio in the rat temporal cortex via trans-signaling. Biological psychiatry. 2012 Apr 1;71:574–82. doi: 10.1016/j.biopsych.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clinical neuroscience research. 2006 Aug;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malkesman O, Pine DS, Tragon T, et al. Animal models of suicide-trait-related behaviors. Trends in pharmacological sciences. 2009 Apr;30:165–73. doi: 10.1016/j.tips.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassanain M, Zalcman S, Bhatt S, Siegel A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: role of serotonin-2 receptors. Neuroscience. 2003;120:227–33. doi: 10.1016/s0306-4522(03)00264-1. [DOI] [PubMed] [Google Scholar]
- 50.Bhatt S, Bhatt R, Zalcman SS, Siegel A. Role of IL-1 beta and 5-HT2 receptors in midbrain periaqueductal gray (PAG) in potentiating defensive rage behavior in cat. Brain, Behavior, and immunity. 2008 Feb;22:224–33. doi: 10.1016/j.bbi.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatt S, Siegel A. Potentiating role of interleukin 2 (IL-2) receptors in the midbrain periaqueductal gray (PAG) upon defensive rage behavior in the cat: role of neurokinin NK(1) receptors. Behavioural brain research. 2006 Feb 28;167:251–60. doi: 10.1016/j.bbr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Patel A, Siegel A, Zalcman SS. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain, Behavior, and immunity. 2010 Nov;24:1276–80. doi: 10.1016/j.bbi.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carroll JE, Low CA, Prather AA, et al. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and immunity. 2011 Feb;25:232–8. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westling S, Ahren B, Traskman-Bendz L, Brundin L. Increased IL-1beta reactivity upon a glucose challenge in patients with deliberate self-harm. Acta psychiatrica Scandinavica. 2011 Oct;124:301–6. doi: 10.1111/j.1600-0447.2011.01734.x. [DOI] [PubMed] [Google Scholar]
- 55.Bezemer G, Van Gool AR, Fekkes D, et al. Psychiatric side effects and fluctuations in serotonergic parameters in the treatment of chronic hepatitis C infection. Neuropsychobiology. 2012;65:126–32. doi: 10.1159/000330585. [DOI] [PubMed] [Google Scholar]
- 56.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clinical neuropharmacology. 2010 Jul;33:191–7. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller AH. Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013 Aug;38:1607–8. doi: 10.1038/npp.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews Neuroscience. 2012 Jul;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwieler L, Larsson MK, Skogh E, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. Journal of psychiatry & neuroscience: JPN. 2015 Mar;40:126–33. doi: 10.1503/jpn.140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urata Y, Koga K, Hirota Y, et al. Il-1Beta increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. American journal of reproductive immunology. 2014 Nov;72:496–503. doi: 10.1111/aji.12282. [DOI] [PubMed] [Google Scholar]
- 61.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, Which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), Both of which contribute to the onset of depression. Progress in neuro-psychopharmacology & biological psychiatry. 2011 Apr 29;35:702–21. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 62.Asberg M, Traskman L, Thoren P. 5-Hiaa in the cerebrospinal fluid. A biochemical suicide predictor? Archives of general psychiatry. 1976 Oct;33:1193–7. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 63.Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. The American journal of clinical nutrition. 1971 Jun;24:659–72. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- 64.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001 Aug;78:842–53. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 65.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993 Sep;45:309–79. [PubMed] [Google Scholar]
- 66.De Carvalho LP, Bochet P, Rossier J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochemistry international. 1996 Apr;28:445–52. doi: 10.1016/0197-0186(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 67.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001 Oct 1;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guillemin GJ. Quinolinic acid, The inescapable neurotoxin. The FEBS journal. 2012 Apr;279:1356–65. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 69.Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS drugs. 2009;23:91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- 70.Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophrenia bulletin. 2012 May;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sublette ME, Galfalvy HC, Fuchs D, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain, Behavior, and immunity. 2011 Aug;25:1272–8. doi: 10.1016/j.bbi.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erhardt S, Lim CK, Linderholm KR, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013 Apr;38:743–52. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain, Behavior, and immunity. 2015 Jan;43:110–7. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 74.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. Journal of affective disorders. 2007 Feb;98:143–51. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Dahl J, Andreassen OA, Verkerk R, et al. Ongoing episode of major depressive disorder is not associated with elevated plasma levels of kynurenine pathway markers. Psychoneuroendocrinology. 2015 Jun;56:12–22. doi: 10.1016/j.psyneuen.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Van Heeringen K, Bijttebier S, Desmyter S, Vervaet M, Baeken C. Is there a neuroanatomical basis of the vulnerability to suicidal behavior? A coordinate-based meta-analysis of structural and functional MRI studies. Frontiers in human neuroscience. 2014;8:824. doi: 10.3389/fnhum.2014.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steiner J, Walter M, Gos T, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological psychiatry. 2009 Sep 1;66:522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diazgranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010 Dec;71:1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claes S, Myint AM, Domschke K, et al. The kynurenine pathway in major depression: haplotype analysis of three related functional candidate genes. Psychiatry research. 2011 Aug 15;188:355–60. doi: 10.1016/j.psychres.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nature medicine. 2012 May;18:726–35. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 82.Goodwin RD. Asthma and suicide: current knowledge and future directions. Current psychiatry reports. 2012 Feb;14:30–5. doi: 10.1007/s11920-011-0243-x. [DOI] [PubMed] [Google Scholar]
- 83.Kuo CJ, Chen VC, Lee WC, et al. Asthma and suicide mortality in young people: a 12-year follow-up study. The American journal of psychiatry. 2010 Sep;167:1092–9. doi: 10.1176/appi.ajp.2010.09101455. [DOI] [PubMed] [Google Scholar]
- 84.Goodwin RD, Demmer RT, Galea S, Lemeshow AR, Ortega AN, Beautrais A. Asthma and suicide behaviors: results from the Third National Health and Nutrition Examination Survey (NHANES III) Journal of psychiatric research. 2012 Aug;46:1002–7. doi: 10.1016/j.jpsychires.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 85.Christodoulou C, Douzenis A, Papadopoulos FC, et al. Suicide and seasonality. Acta psychiatrica Scandinavica. 2012 Feb;125:127–46. doi: 10.1111/j.1600-0447.2011.01750.x. [DOI] [PubMed] [Google Scholar]
- 86.Postolache TT, Stiller JW, Herrell R, et al. Tree pollen peaks are associated with increased nonviolent suicide in women. Molecular psychiatry. 2005 Mar;10:232–5. doi: 10.1038/sj.mp.4001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woo JM, Gibbons RD, Rogers CA, et al. Pollen counts and suicide rates. Association not replicated. Acta psychiatrica Scandinavica. 2012 Feb;125:168–75. doi: 10.1111/j.1600-0447.2011.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qin P, Waltoft BL, Mortensen PB, Postolache TT. Suicide risk in relation to air pollen counts: a study based on data from Danish registers. BMJ open. 2013;3 doi: 10.1136/bmjopen-2012-002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woo JM, Gibbons RD, Qin P, et al. Suicide and prescription rates of intranasal corticosteroids and nonsedating antihistamines for allergic rhinitis: an ecological study. The Journal of clinical psychiatry. 2011 Oct;72:1423–8. doi: 10.4088/JCP.10m06765. [DOI] [PubMed] [Google Scholar]
- 90.Mccue RE, Charles RA, Orendain GC, Joseph MD, Abanishe JO. Vitamin d deficiency among psychiatric inpatients. The primary care companion for CNS disorders. 2012;14 doi: 10.4088/PCC.11m01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kjaergaard M, Joakimsen R, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with depression in an adult Norwegian population. Psychiatry research. 2011 Dec 30;190:221–5. doi: 10.1016/j.psychres.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 92.Umhau JC, George DT, Heaney RP, et al. Low vitamin D status and suicide: a case-control study of active duty military service members. Plos one. 2013;8:E51543. doi: 10.1371/journal.pone.0051543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grudet C, Malm J, Westrin A, Brundin L. Suicidal patients are deficient in vitamin D, Associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology. 2014 Dec;50:210–9. doi: 10.1016/j.psyneuen.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 94.Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. The Journal of clinical psychiatry. 2012 Jul;73:993–1001. doi: 10.4088/JCP.11r07425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. The American journal of psychiatry. 2002 Nov;159:1862–8. doi: 10.1176/appi.ajp.159.11.1862. [DOI] [PubMed] [Google Scholar]
- 96.Lauvsnes MB, Omdal R. Systemic lupus erythematosus, The brain, And anti-NR2 antibodies. Journal of neurology. 2012 Apr;259:622–9. doi: 10.1007/s00415-011-6232-5. [DOI] [PubMed] [Google Scholar]
- 97.Xie LF, Chen PL, Pan HF, et al. Prevalence and correlates of suicidal ideation in SLE inpatients: Chinese experience. Rheumatology international. 2012 Sep;32:2707–14. doi: 10.1007/s00296-011-2043-3. [DOI] [PubMed] [Google Scholar]
- 98.Fredrikson S, Cheng Q, Jiang GX, Wasserman D. Elevated suicide risk among patients with multiple sclerosis in Sweden. Neuroepidemiology. 2003 Mar-Apr;22:146–52. doi: 10.1159/000068746. [DOI] [PubMed] [Google Scholar]
- 99.Bronnum-Hansen H, Stenager E, Nylev Stenager E, Koch-Henriksen N. Suicide among Danes with multiple sclerosis. Journal of neurology, Neurosurgery, and psychiatry. 2005 Oct;76:1457–9. doi: 10.1136/jnnp.2004.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ludvigsson JF, Sellgren C, Runeson B, Langstrom N, Lichtenstein P. Increased suicide risk in coeliac disease--a Swedish nationwide cohort study. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2011 Aug;43:616–22. doi: 10.1016/j.dld.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 101.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain, Behavior, and immunity. 2012 Nov;26:1191–201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Coughlin JM, Wang Y, Munro CA, et al. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiology of disease. 2015 Feb;74:58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fazel S, Wolf A, Pillas D, Lichtenstein P, Langstrom N. Suicide, Fatal injuries, And other causes of premature mortality in patients with traumatic brain injury: a 41-year Swedish population study. JAMA psychiatry. 2014 Mar;71:326–33. doi: 10.1001/jamapsychiatry.2013.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Juengst SB, Kumar RG, Arenth PM, Wagner AK. Exploratory associations with Tumor Necrosis Factor-alpha, Disinhibition and suicidal endorsement after traumatic brain injury. Brain, Behavior, and immunity. 2014 Oct;41:134–43. doi: 10.1016/j.bbi.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 105.Mackelprang JL, Bombardier CH, Fann JR, Temkin NR, Barber JK, Dikmen SS. Rates and predictors of suicidal ideation during the first year after traumatic brain injury. American journal of public health. 2014 Jul;104:e100–7. doi: 10.2105/AJPH.2013.301794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heyes MP, Saito K, Crowley JS, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain: a journal of neurology. 1992 Oct;115(Pt 5):1249–73. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 107.Arling TA, Yolken RH, Lapidus M, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. The Journal of nervous and mental disease. 2009 Dec;197:905–8. doi: 10.1097/NMD.0b013e3181c29a23. [DOI] [PubMed] [Google Scholar]
- 108.Okusaga O, Langenberg P, Sleemi A, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophrenia research. 2011 Dec;133:150–5. doi: 10.1016/j.schres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Traskman-Bendz L, Janelidze S, et al. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. J Clin Psychiatry. 2012 Aug;73:1069–76. doi: 10.4088/JCP.11m07532. [DOI] [PubMed] [Google Scholar]
- 110.Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Archives of general psychiatry. 2012 Nov;69:1123–30. doi: 10.1001/archgenpsychiatry.2012.668. [DOI] [PubMed] [Google Scholar]
- 111.Notarangelo FM, Wilson EH, Horning KJ, et al. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophrenia research. 2014 Jan;152:261–7. doi: 10.1016/j.schres.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaskell EA, Smith JE, Pinney JW, Westhead DR, Mcconkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. Plos one. 2009;4:E4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cook TB, Brenner LA, Cloninger CR, et al. “Latent” infection with Toxoplasma gondii: association with trait aggression and impulsivity in healthy adults. Journal of psychiatric research. 2015 Jan;60:87–94. doi: 10.1016/j.jpsychires.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 114.Miller AH, Raison CL. Are Anti-inflammatory Therapies Viable Treatments for Psychiatric Disorders?: Where the Rubber Meets the Road. JAMA psychiatry. 2015 Apr 8; doi: 10.1001/jamapsychiatry.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]


