Introduction
Chronic pelvic pain is the most common indication for referral to women’s health specialists, encompassing 20% of all secondary care appointments and costing $881.5 million/year in outpatient management in the United States alone [39]. Specifically, vulvodynia affects 4% of women in the United States with a lifetime prevalence of 16% [34,60]. Like other idiopathic pain disorders, vulvodynia is proposed to be of a multifactorial nature and treatment options include non-specific lifestyle interventions, topical or oral medications, injections, physical therapy, and surgical procedures [68]. Clinically, vulvodynia has been associated with other chronic pelvic pain syndromes, most often interstitial cystitis and irritable bowel syndrome, and increased rates of mood disorders such as depression or anxiety [4,9,23,24,29,46,48,52].
Strong evidence has linked early life adverse events with an increased likelihood of vulvodynia in adulthood, which has been attributed to dysfunctional regulation of the hypothalamic-pituitary-adrenal (HPA) axis [4,25,32,50]. The HPA axis regulates the response to stress and influences the perception of pain, largely through release of glucocorticoids initiated by corticotropin-releasing factor (CRF) [6]. Indeed, vulvodynia patients demonstrate blunted serum cortisol cycles [25] and acute stress exposure has been shown to increase symptom severity [31]. Biopsies from vulvodynia patients show increased infiltration and degranulation of mast cells [30,40], which express receptors for CRF [6], and acute stress has been shown to trigger mast cell degranulation and increase CRF peptide content in the surrounding milieu [57,63]. We recently published evidence that early life stress in mice is sufficient to induce increased vaginal sensitivity with a concomitant dysregulation of the HPA axis [49]. Other animal models of neonatal stress or injury in the gastrointestinal [2,5,13,18,59,71] and urinary [20,51,56] systems have demonstrated permanently enhanced sensitivity of the affected organ, increased numbers of dorsal horn neurons responsive to organ distension, a wider expression pattern of the transient receptor potential channels TRPA1 and TRPV1 among dorsal root ganglion (DRG) neurons innervating the affected organ, enhanced peptide content and vascular permeability of the affected organ, and disruption of the descending opioid inhibitory system (reviewed in [14]). Increased colorectal sensitivity has also been observed in adult rats that underwent either neonatal bladder irritation [44] or repeated gastric suctioning during the first two postnatal weeks [59], the latter in an HPA-dependent manner, suggesting that neonatal insult, as well as neonatal stress, is capable of invoking changes within the stress response system that may ultimately affect how noxious input is perceived.
Given the strong clinical evidence linking early life adverse events with an increased likelihood of developing vulvodynia in adulthood [4,25,32,50], we conducted the following study to determine whether neonatal irritation of the vagina leads to permanent vaginal hypersensitivity, as well as comorbid sensitivities, in mice. We investigated the long-term impact of neonatal intravaginal zymosan on primary sensory neurons innervating the vagina and surrounding skin, and peripheral HPA axis output that may contribute towards comorbid visceral hyperalgesia. We also tested the efficacy of antagonizing the CRF receptor, CRF1, prior to neonatal irritation of the vagina.
Materials and Methods
Animals
Experiments were performed on female C57Bl/6 mice (Charles River, Wilmington, MA) born and housed in the Research Support Facility at the University of Kansas Medical Center. Mice received water and food ad libitum. All research performed conformed to NIH guidelines in accordance with the guidelines specified by the University of Kansas Medical Center Institutional Animal Care and Use Committee and the Committee for Research and Ethical Issues of IASP.
Vaginal irritation and CRF1 antagonist (NBI 35965) treatment
To induce neonatal vaginal irritation (NVI), female mice received an intravaginal instillation of 10µl of 5% zymosan (Sigma, St. Louis, MO) in saline via a 26-gauge feeding needle attached to a Hamilton syringe on postnatal days 8 and 10. To determine the effect of the disruption of the vaginal hymen, which occurs during zymosan instillation on postnatal day 8, a separate cohort was treated intravaginally with 10µl saline on postnatal days 8 and 10. A water-based lubricant (KY Jelly, Johnson & Johnson, New Brunswick, NJ) was liberally applied to the perivaginal region prior to instillation of zymosan or saline to avoid sensitization of surrounding somatic tissues. Mice were held briefly (less than 5 minutes) in a secondary container and observed for any adverse effects (e.g. vaginal bleeding) before being returned to their home cages. All female pups from a given litter were assigned to the same treatment group to avoid any cross-exposure between pups. All mice remained undisturbed until weaning on postnatal day 22.
To block activation of the HPA axis during NVI, a separate group of mice was treated with saline or a CRF1 antagonist, NBI 35965 (Tocris, Bristol, UK), prior to zymosan instillation. Female pups received either saline (10µl) or NBI 35965 (20mg/kg in saline; approx. 10µl) i.p. 20 minutes prior to zymosan instillation on both postnatal day 8 and 10. Naïve mice were similarly treated with saline or NBI 35965 and held for 20 minutes without zymosan instillation. Again, all mice remained undisturbed until weaning on postnatal day 22.
To determine the impact of intravaginal zymosan on vaginal sensitivity in adult female mice, 8-week old female mice were anesthetized with inhaled isoflurane (4% induction, 2.5 % maintenance) and secured on a platform that elevated the pelvic region approximately 5cm above the working surface. A water-based lubricant (KY Jelly) was liberally applied to the perivaginal region to avoid sensitization of surrounding somatic tissues. Mice received an intravaginal instillation of 50µl of 5% zymosan (Sigma) in saline via a 26-gauge feeding needle attached to a Hamilton syringe. Mice remained in the elevated position for five minutes to prevent leakage of zymosan. Upon recovery from anesthesia, mice were returned to their home cages. The entire procedure was repeated two days later to replicate the NVI procedure. Following the second zymosan treatment, the mice remained undisturbed outside of routine animal husbandry for an additional eight weeks.
Experimental design
Naïve and NVI mice that did not receive saline or NBI35965 as neonates were assessed for thermal and mechanical hindpaw sensitivity prior to vaginal balloon distension (VBD) or colorectal distension (CRD) and sacrificed three weeks after experimentation was completed (Table 1). Mice that received saline or NBI 35965 as neonates were assessed for either hindpaw or pelvic organ sensitivity; however, only mice that underwent hindpaw assessment were used for further in vitro analysis (Table 1).
Table 1.
Age of mice at experimental time points.
| Thermal Hindpaw |
Mechanical Hindpaw |
Vaginal Balloon Distension |
Colorectal Distension |
Sacrifice | |
|---|---|---|---|---|---|
| Naïve/NVI | |||||
| VBD group | 6 | 7 | 10 | 13 | |
| CRD group | 6 | 7 | 10 | 13 | |
| Saline/NBI 35965-treated | |||||
| Hindpaw | 6 | 7 | 9 | ||
| VBD/CRD | 10 | 12 | |||
Naïve and NVI mice underwent behavioral and/or physiological testing at the above noted ages (in weeks). In the initial study, mice were assessed for thermal and mechanical hindpaw sensitivity prior to vaginal balloon distension (VBD) or colorectal distension (CRD) and sacrificed three weeks after experimentation was completed. In the NBI 35965 study, mice were assessed for either hindpaw or pelvic organ sensitivity; however, only mice that underwent hindpaw assessment were used for further in vitro analysis.
Electromyographic electrode implantation and organ distension
The visceromotor response (VMR) to either vaginal balloon distension (VBD) or colorectal distension (CRD) was evaluated in separate groups of adult (at least 8-week old) mice. Electrode implantation was performed as previously described [15]. Under inhaled isoflurane (4% induction, 2.5% maintenance) and aseptic conditions, the bare ends of two Teflon-coated stainless steel wires (3mm; Grass Technologies, West Warwick, RI) were inserted into the right lateral abdominal musculature, secured via 5-0 prolene sutures, tunneled subcutaneously to a small incision at the nape of the neck, and externalized for access during testing. Skin incisions were closed using 5-0 silk suture. Following recovery from anesthesia, mice were housed singly and allowed to recover for a minimum of 4 days before undergoing testing.
Vaginal balloon distension (VBD): To facilitate balloon insertion and obtain proper restraint during VBD, mice were briefly sedated with inhaled isoflurane and a custom-made latex balloon (1cm in length) was inserted into the vagina and secured to the base of the tail with tape. The mouse was then placed into a Broome rodent holder (Kent Scientific, Torrington, CT), the free ends of the electrode wires were attached to a differential amplifier (Model 1700, A-M Systems, Sequim, WA), and the mice were allowed to recover from anesthesia for 30 minutes. The balloon was inflated with air from a compressed nitrogen tank equipped with a dual-stage low delivery pressure regulator (Matheson-Linweld, Kansas City, MO) and a separate pressure monitor (World Precision Instruments, Sarasota, FL) was used to regulate the intraballoon pressure. Each pressure (40, 60, 80, 100 and 120mmHg) was applied three times for 20 seconds with intervening 4-minute rest periods.
Colorectal distension (CRD): To facilitate balloon insertion and obtain proper restraint during CRD, mice were briefly sedated with inhaled isoflurane and a custom-made polyethylene plastic cylinder (1.5cm in length, 0.7cm diameter) was inserted through the anus until the proximal end of the balloon was 0.5cm from the anal verge (total balloon insertion, 2cm) and secured to the base of the tail with tape. The mouse was then restrained and allowed to recover as described above. The balloon was inflated and monitored as described above and each pressure (15, 30, 45, 60 and 75mmHg) was applied three times for 20 seconds with intervening 4-minute rest periods.
A custom-made distension control device (The University of Iowa Medical Instruments, Iowa City, IA) was used to control the gas flow through the system. Electromyographic (EMG) electrode activity was amplified, filtered, and recorded on a personal computer with Spike 2v7 software (Cambridge Electronic Design, Cambridge, UK) for off-line analysis. The VMR was quantified by measuring the area under the curve for the entire distension period divided by the duration of the distension and expressed as a percent of baseline activity (prior to each distension).
Behavioral analyses
Mice underwent a 30-minute acclimatization period within the testing room on the day before each behavioral test. Mice were allowed to acclimate to each apparatus for 30 minutes prior to testing and the experimenter was blinded to the treatment status of the mice. Mice were tested for thermal sensitivity by measuring paw withdrawal latency to radiant heat [33] and for mechanical sensitivity using calibrated monofilaments. For thermal sensitivity, mice were placed in individual clear plastic chambers (11×5×3.5cm) on the 30°C heated glass surface of a thermal analgesiometer (UARDG; Department of Anesthesiology, University of California San Diego, La Jolla, CA). Hindpaws were tested for a total of three times per side with a minimum of 5 minutes between applications and the latency to hindpaw withdrawal from the stimulus was automatically recorded within 0.01 second. The stimulus terminated automatically at 20 seconds to avoid tissue damage. Individual responses were averaged per mouse and group means were determined as previously described [17]. For mechanical sensitivity, mice were placed into individual clear plastic chambers (11×5×3.5cm) on a wire mesh screen elevated 55 cm above a table. The up-down method was performed to test mechanical sensitivity using a standard set of monofilaments (1.65, 2.36, 2.83, 3.22, 3.61, 4.08, 4.31, 4.74g; Stoelting, Wood Dale, IL) [22]. Beginning with the 3.22g monofilament, mice received a single application of the monofilament to the plantar surface of the right hindpaw. A negative response was followed by the next larger filament and a positive response (considered a brisk withdrawal of the paw) was followed by the next smaller gram filament. The experimenter continued to move up or down the series, depending on the previously elicited response, for an additional four applications after the first positive response was observed for a minimum of five or a maximum of nine total monofilament applications. The value in log10 units of the final monofilament applied in the trial series was used to calculate a 50% g threshold for each mouse and group means were determined as previously described [12].
Retrograde labeling of vaginal and perivaginal neurons
All surgical procedures were performed under aseptic conditions in a designated animal surgery area. Mice were anesthetized by inhaled isoflurane (4% induction, 2.5 % maintenance) and using a Hamilton microsyringe (33 gauge needle), multiple 1.5-2µl injections of Alexa Fluor 488-conjugated cholera toxin-β (CTB, 2mg/ml in saline, 8µl total; Invitrogen) were made circumferentially within the distal region of the vagina, approximately 3–5mm from the external opening. Using a separate Hamilton microsyringe (33 gauge needle), 2–3µl injections of 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; 2% in DMSO, 5µl total; Sigma, St. Louis, MO) were made on the right and left side, subcutaneously, at the junction between the hairy skin and the opening of the vagina. Mice were returned to their home cages and allowed to recover from anesthesia under observation.
Cell culture and calcium imaging
Neurons were retrogradely labeled from the vagina and peri-vaginal skin in naïve and NVI mice as described above. After a recovery period of 7–10 days, mice were overdosed with inhaled isoflurane (>5%) and perfused with ice-cold Ca+2/Mg+2-free Hanks’ Balanced Salt Solution (HBSS; Invitrogen) and lumbosacral (LS; L5-S1) DRG were dissected and prepared for culture as previously described [43]. Dissociated cells were resuspended in F12 media (Invitrogen) containing 10% FBS and penicillin/streptomycin (50U/ml) and plated onto 12mm Poly-D-Lysine/Laminin pre-coated coverslips (BD Biosciences, Franklin Lakes, NJ). Cells were incubated overnight at 37°C and imaged the following day as previously described [13]. Prior to imaging, the cells were incubated in HBSS containing 5mg/ml BSA (Sigma) and 2µM fura-2 acetoxymethyl (AM) ester (Invitrogen) for 30 minutes at 37°C. Coverslips were placed in a QE-1 quick change platform (Warner Instruments, Hamden, CT) and mounted on an inverted Nikon TiE microscope stage (Melville, NY) with HBSS buffer flowing at 5ml/min, controlled by a gravity flow system (Warner Instruments). Perfusate temperature was maintained at 30°C using a dual bipolar temperature controller and a six-line solution heater (Warner Instruments). Chemicals were delivered using a gravity-feed pinch valve control perfusion system (Warner Instruments). Firmly attached, CTB- and DiI-positive neurons were identified and chosen as regions of interest using Nikon Elements Advanced Research Imaging software (Nikon). Unlabeled, adjacent cells were also identified and imaged. Absorbance data at 340nm and 380nm were collected at 1Hz during drug application using a Photometrics HQ2 dual-mode cooled CCD camera (Boyce Scientific, Gray Summitt, MO). Responses were measured as the ratio of 340/380nM excitation and 510nM emission (ΔF340/380) controlled by a high speed Fura/widefield Xenon illuminated filter wheel (Boyce Scientific). All fields were first tested with a brief application (4s) of 30mM K+ (high K+) to ensure that cells were healthy and responsive. Following a five-minute recovery period, 1µM capsaicin (Sigma) or 100µM mustard oil (MO; Sigma) was applied for five or ten seconds, respectively. 10mM capsaicin in 1-methyl-2-pyrrolidinone was used as a stock solution; 1µM capsaicin was made fresh daily in HBSS. 100mM mustard oil in 1-methyl-2-pyrrolidinone was made fresh daily and diluted to 100µM using HBSS. Peak responses >0.1 ΔF340/380 were included in the analysis and were easily distinguishable from optical noise (<0.02 ΔF340/380). The prevalence of capsaicin- or MO-responsive vaginal, perivaginal skin, and unlabeled afferents was determined as a percentage of total healthy (high K+-responsive) CTB-, DiI-positive, and dye-free cells, respectively. Any cell with significantly diminished Fura-2 signal over the duration of the experiment or that did not recover to baseline prior to agonist application was not included in the analysis. Peak Ca2+ influx, total Ca2+ influx, and time to 50% of the peak (T50) were calculated using Microsoft Excel.
Western blot
Total proteins were isolated from approximately 50 mg of snap-frozen DRG, vagina, and colon tissue samples using Cell Extraction Buffer (Invitrogen, Grand Island, NY) containing Halt protease and phosphatase inhibitors (ThermoFisher Scientific, Waltham, MA) and Na3VO4 (Sigma, St. Louis, MO). Protein concentrations were determined using a DC protein assay (ThermoFisher). Samples were reduced by heating to 95°C for 5 minutes in the presence of 2-mercaptoethanol, subjected to SDS-PAGE (Criterion 4% to 12% Bis-Tris gels; Bio-Rad, Hercules, CA), and transferred to Nitrocellulose transfer membrane (Whatman GmbH, Dassel, Germany) by Criterion Blotter wet transfer (Bio-Rad). The membranes were blocked for 1 hour at room temperature in 5% milk in Tris-buffered saline with Tween-20 (TBST) and incubated overnight at 4°C with antisera to CRF1 (1:500; Millipore, Billerica, MA), TRPV1 (1:1000; Alomone Labs, Jerusalem, Israel), or TRPA1 (1:1000; Aviva Systems Biology, San Diego, CA) and GAPDH (1:2000; Cell Signaling Technology, Danvers, MA) diluted in 5% milk in TBST. Membranes were then washed with TBST and incubated for 1 hour with anti-rabbit secondary antibody (1:10,000; Cell Signaling, Danvers, MA). Densitometry was performed using Quantity One 4.6.9 software (Bio-Rad).
Serum corticosterone
Following decapitation, trunk blood was collected in 1.5 ml tubes, allowed to clot on ice for 1 h, and spun at 10,000 RPM at 4°C for 10 min. Serum (clear supernatant) was collected and an enzymatic immunoassay kit, (Enzo Life Sciences, Farmingdale, NY) was used to quantify serum corticosterone content according to the manufacturer’s instructions.
Toluidine blue staining of mast cells
Mice were transcardially perfused with ice cold 4% paraformaldehyde and the entire length of the vagina (minus the cervix) was removed, postfixed, and cryoprotected in 30% sucrose overnight. Tissue was flash frozen in ice-cold heptane, mounted in Tissue-Tek OTC mounting medium (Sakura Finetek, Torrance, CA), and cryosections were cut at 10 µm thickness. Non-serial sections spanning the length of the tissue were stained with acidified toluidine blue and examined with light microscopy (Nikon eclipse 90i), for cells exhibiting metachromatic color change. Digital images were captured using a Photometrics HQ2 dual mode cooled CCD camera (Boyce Scientific) and the total number of non-degranulated mast cells (dense metachromasia with no or faint nuclear outline and/or no granular extrusion around the cell) and degranulated mast cells (less intense metachromasia and obvious clear outline of the nucleus and/or free granules within the cytoplasm), as described in [28], were counted in at least 8 separate sections. The ratio of degranulated/total mast cell infiltrates and total infiltrates per section were calculated for each mouse.
Statistics
Calculations were made using Microsoft Excel and statistical analyses were performed using Student’s t-test or 2-way analysis of variance (ANOVA) followed by Bonferroni’s or Fisher’s Least Significant Difference posttest (GraphPad Prism 6, GraphPad Software, La Jolla, CA) as indicated in the manuscript. All data are expressed as the mean ± SEM. A p value of less than 0.05 was considered significant.
Results
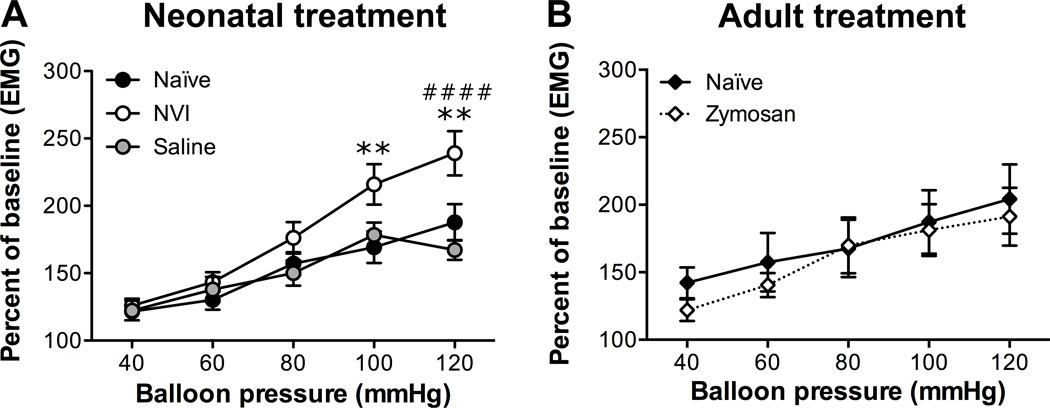
To determine the impact of neonatal vaginal irritation (NVI) on vaginal sensitivity, we measured the electromyographic activity of the abdominal musculature (termed visceromotor response [VMR]) to balloon distension of the vagina. All groups of mice displayed a stepwise and significant increase in VMR as higher intraballoon pressures were administered (p < 0.0001, two-way RM ANOVA; Figure 1). The NVI mice displayed significantly higher VMR than naïve mice over the entire series, particularly at the highest two pressures applied (Figure 1). To control for the impact of rupturing the vaginal hymen during the initial application of zymosan on P8, a separate cohort of female mice was treated intravaginally with saline alone on P8 and 10. Saline-treated mice were not significantly different from naïve mice and had a significantly lower VMR at the highest distension pressure compared to NVI mice (Figure 1A). To determine if intravaginal zymosan administration during adulthood would also produce prolonged vaginal hypersensitivity, we treated 8 week old female mice with intravaginal zymosan on two non-consecutive days and measured the VMR during VBD 8 weeks later. Mice treated with intravaginal zymosan did not have significantly different VMR compared to age-matched control mice (Figure 1B).
Figure 1.
Neonatal vaginal irritation (NVI) significantly increased vaginal sensitivity. A) The visceromotor response (VMR) was measured by recording the electromyographic (EMG) activity of the abdominal musculature during graded balloon distension of the vagina (VBD). NVI mice (n=11) had a significantly higher VMR during VBD than either naïve (n=23) or saline-treated (n=18) mice over the entire distension series (p < 0.05), as well as at the highest intraballoon pressures applied. B) 8 week old female mice received intravaginal zymosan (Zymosan, n=7) or remained undisturbed (naïve, n=8) and the VMR was measured during VBD 8 weeks later. No significant difference in VMR was observed between mice that received zymosan and those that did not. **p < 0.01 vs. naïve, ####p < 0.0001 vs. saline; two-way RM ANOVA and Bonferroni posttest.
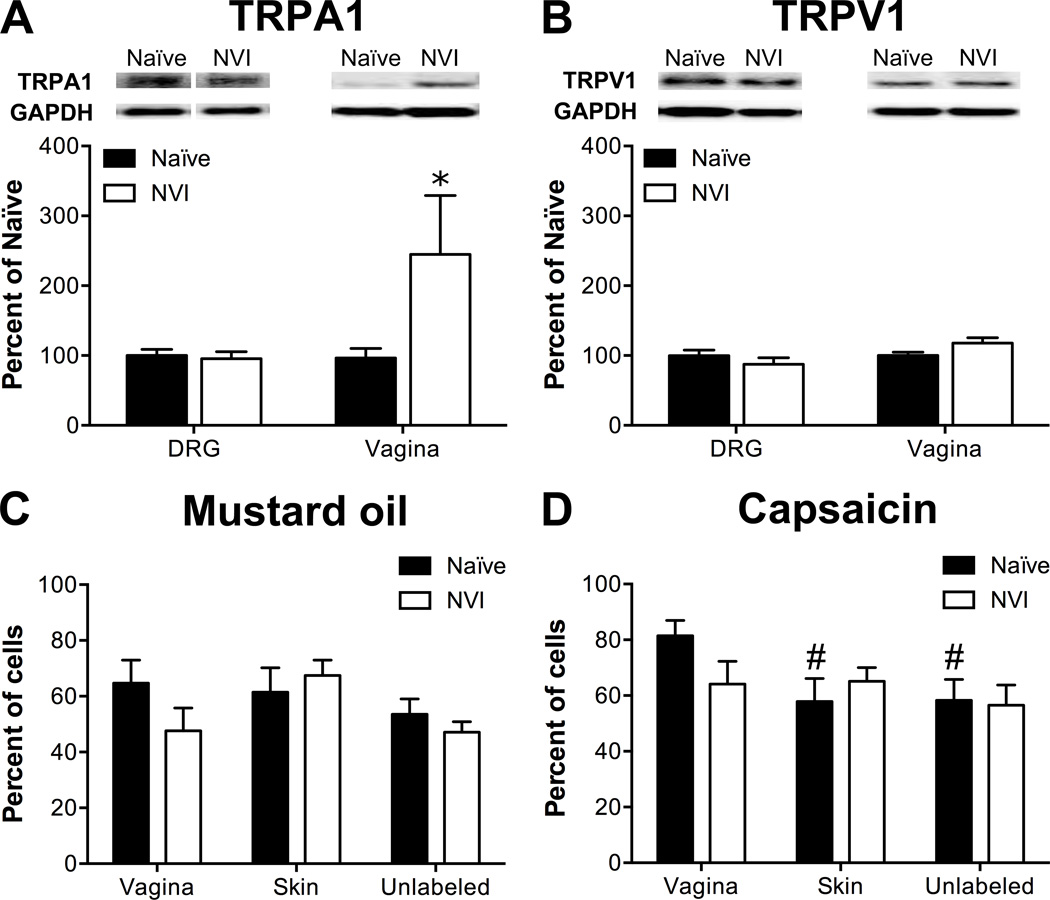
Western blotting and calcium imaging were performed to determine the potential contribution of altered TRP channel protein expression and function, respectively, towards the development of vaginal hypersensitivity in NVI mice. Western blotting revealed a significant increase in TRPA1 protein in the vagina of NVI mice, compared to naïve mice (Figure 2A). No significant change in TRPA1 or TRPV1 protein levels was observed in lumbosacral (LS; L6-S1) DRG from NVI mice compared to naïve mice (Figure 2A–B). Calcium transients in response to 30mM K+ (high K+), 100µM mustard oil (MO; TRPA1-responsive), or 1µM capsaicin (TRPV1-responsive) were measured in dissociated LS DRG retrogradely labeled from the vagina and perivaginal skin, as well as in adjacent unlabeled cells. Functional TRPA1 expression was largely unchanged as no significant effect of NVI or target organ on the percentage of MO-responsive neurons was observed (Figure 2C). Functional TRPV1 expression was also not affected by NVI; however, in naïve mice, the percentage of capsaicin-responsive neurons was significantly higher in vagina-specific DRG neurons compared to either perivaginal skin-specific or unlabeled neurons (Figure 2D). When compared across both MO and capsaicin, NVI significantly reduced the percentage of agonist-responsive vagina-specific neurons (p < 0.05, two-way ANOVA), but NVI had no effect on either perivaginal skin-specific or unlabeled neurons. Analysis of calcium transients revealed a significant impact of both NVI and peripheral target on MO-evoked peak Ca2+ influx and of peripheral target on total Ca2+ influx (Table 2). A significant impact of NVI on peak Ca2+ influx, total Ca2+ influx, and signal decay (T50; time to 50% of peak response) was observed in response to capsaicin, particularly on the population of unlabeled neurons (Table 2). In contrast to agonist-evoked responses, high K+-evoked responses were significantly larger in DRG neurons from NVI mice than from naïve, with high K+ evoking a significantly larger total Ca2+ influx in vagina-specific neurons from NVI mice compared to vagina-specific neurons from naïve mice or unlabeled DRG from NVI mice (Table 2). Time to decay following high K+ application was also significantly longer in vagina-specific DRG from NVI mice compared to vagina-specific DRG from naïve mice and skin-specific or unlabeled DRG from NVI mice (Table 2).
Figure 2.
NVI increased TRPA1 protein expression in the vagina, but not in primary sensory neurons innervating the vagina. A) Representative Western blots are shown for TRPA1 and corresponding GAPDH protein expression with bands at 127 and 35kD, respectively, in both DRG and vagina from naïve and NVI mice. TRPA1 protein expression was significantly increased in vagina, but not DRG, from NVI mice compared to naïve mice. B) Representative Western blots are shown for TRPV1 and corresponding GAPDH protein expression with bands at 85 and 35kD, respectively, in both DRG and vagina from naïve and NVI mice. TRPV1 protein expression was not significantly different in NVI DRG or vagina compared to naïve counterparts. Calcium imaging was performed on lumbosacral (L5-S1) DRG neurons retrogradely labeled by injection of Alexa Fluor-conjugated cholera toxin-β (CTB) into the distal vagina, 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) into the perivaginal skin, and adjacent unlabeled DRG to measure responses to 100µM mustard oil (MO; C) and 1µM capsaicin (D). C) No significant difference in functional TRPA1 expression, measured as the percentage of MO-responsive DRG neurons, was observed between naïve and NVI mice for any population of DRG neurons tested. D) Only in naïve mice, DRG neurons backlabeled from the vagina were significantly more likely to respond to 1capsaicin, suggesting greater functional TRPV1 expression, compared to those backlabeled from the perivaginal skin or unlabeled DRG. When compared across both agonists, vagina-specific DRG neurons from NVI mice had a significantly reduced percentage of responsive neurons compared to naïve (p < 0.05; two-way ANOVA). A–B: naïve, DRG: n=10, vagina: n=5; NVI, DRG: n=8, vagina: n=4; C: naïve, n=8, NVI, n=10; D: naïve, n=8; NVI, n=6. * p < 0.05 vs. naïve, # p < 0.05 vs. vagina; two-way ANOVA and Bonferroni posttest.
Table 2.
Calcium imaging parameters
| Peak Ca2+ influx (ΔF340/380) |
Total Ca2+ influx (ΔF340/380) |
T50 (sec) |
||
|---|---|---|---|---|
| 100mM Mustard oil | ||||
| Vagina | ||||
| Naïve (27) | 0.68 ± 0.067 | 124.5 ± 20.0 | 142.6 ± 22.1 | |
| NVI (41) | 0.54 ± 0.060 | 96.6 ± 15.8 | 151.2 ± 18.4 | |
| Skin | ||||
| Naïve (52) | 0.68 ± 0.047 | 117.1 ± 13.9 | 130.5 ± 16.8 | |
| NVI (67) | 0.62 ± 0.045 | 118.7 ± 13.6 | 153.0 ± 15.6 | |
| Unlabeled | ||||
| Naïve (147) | 0.55 ± 0.029 | 85.8 ± 8.15 | 118.8 ± 12.1 | |
| NVI (174) | 0.50 ± 0.027 | 81.7 ± 7.09 | 130.1 ± 10.6 | |
| *# | # | |||
| 1mM Capsaicin | ||||
| Vagina | ||||
| Naïve (44) | 0.88 ± 0.084 | 142.1 ± 18.9 | 136.0 ± 19.6 | |
| NVI (34) | 0.86 ± 0.095 | 115.9 ± 20.3 | 116.4 ± 20.6 | |
| Skin | ||||
| Naïve (32) | 0.85 ± 0.078 | 149.4 ± 21.7 | 184.3 ± 25.2 | |
| NVI (25) | 0.68 ± 0.103 | 88.9 ± 17.7 | 96.9 ± 12.0 | |
| Unlabeled | ||||
| Naïve (138) | 0.92 ± 0.041 | 172.6 ± 11.9 | 182.6 ± 13.4 | |
| NVI (128) | 0.64 ± 0.044**** | 113.4 ± 10.9** | 150.0 ± 12.6 | |
| * | ** | ** | ||
| 30mm K+ | ||||
| Vagina | ||||
| Naïve (88) | 0.70 ± 0.036 | 38.9 ± 4.29 | 28.0 ± 5.09 | |
| NVI (96) | 0.76 ± 0.048 | 64.1 ± 8.07** | 60.3 ± 9.47**** | |
| Skin | ||||
| Naïve (104) | 0.63 ± 0.30 | 25.6 ± 3.74 | 11.2 ± 2.34 | |
| NVI (85) | 0.78 ± 0.047 | 44.5 ± 7.28 | 23.6 ± 5.07#### | |
| Unlabeled | ||||
| Naïve (490) | 0.59 ± 0.015 | 24.3 ± 1.53 | 14.2 ± 1.21 | |
| NVI (374) | 0.65 ± 0.021 | 33.3 ± 2.63#### | 24.4 ± 2.65*#### | |
| **## | ****#### | ****####+ | ||
Results are expressed as mean ± SEM. The number of cells used for quantification are indicated in parentheses.
Data were analyzed using two-way ANOVA: symbols at the bottom of each column set indicate significant impact of NVI (*,**,****p < 0.05, 0.01, 0.0001), peripheral target (#,##,####p < 0.05, 0.01, 0.0001), or interaction effect (+p < 0.05); and Tukey’s test for multiple comparisons: symbols within the table indicate *,**,****p < 0.05, 0.01, 0.0001 vs. naïve, #### p < 0.05 vs. vagina.
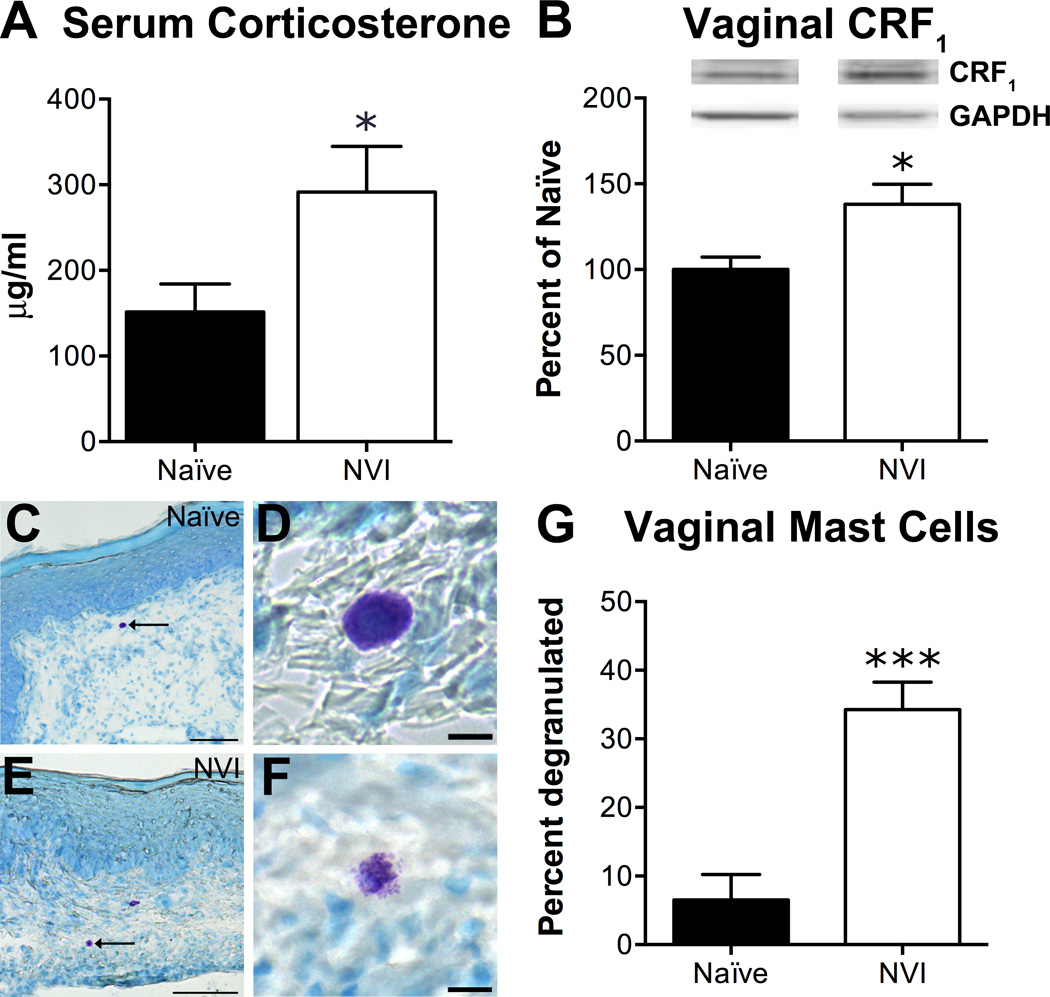
To determine if NVI altered the peripheral output of the HPA axis, we assayed serum corticosterone (CORT) levels and histological evidence of extrusion of mast cell contents and CRF1 protein expression in the vagina. Serum CORT was significantly higher in NVI mice compared to naïve (Figure 3A). Also, CRF1 protein level (Figure 3B) was significantly increased and the percentage of toluidine blue-stained mast cells showing evidence of extruded granules (Figure 3C–G) was significantly higher in NVI vagina compared to naïve mice.
Figure 3.
NVI mice display evidence of increased output from the hypothalamic-pituitary-adrenal (HPA) axis. A) Total corticosterone measured in the serum of NVI mice (n=7) at sacrifice was significantly higher than that of naïve mice (n=9). B) Representative Western blots are shown for CRF1 and corresponding GAPDH protein expression with bands at 55 and 35kD, respectively, in the vagina of naïve and NVI mice. CRF1 protein expression was significantly increased in vagina from NVI mice (n=4) compared to naïve mice (n=4). Mast cells, and evidence of degranulation, were identified by acidified toluidine blue staining in sections of vagina from naïve (n=3; C–D) and NVI mice (n=3; E–F). High magnification photomicrographs show examples of intact mast cells (D) and degranulated mast cells with extruded granules (F) in naïve and NVI vagina, respectively. G) The percentage of mast cells that exhibited evidence of extruded granules was significantly higher in NVI vagina than naïve vagina. *,*** p < 0.05, 0.001 vs. naïve; Student’s t-test. Scale bars equal 100µm (C, E) and 10µm (D, F).
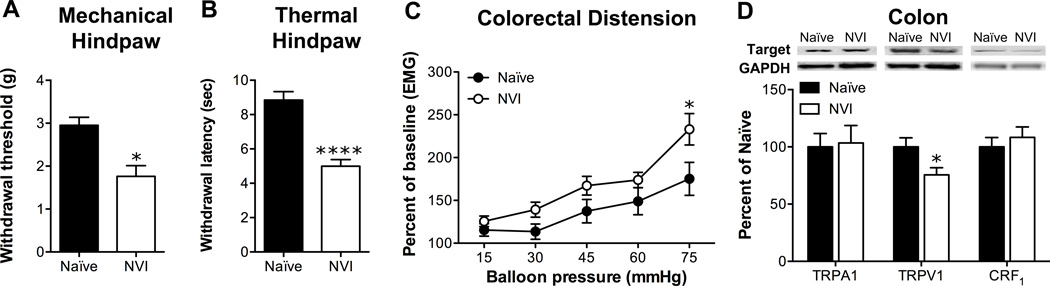
In light of the evidence of increased activity of the HPA axis and previous studies reporting cross-organ sensitization following neonatal organ irritation [13,44,59], we assayed for hindpaw sensitivity and VMR during colorectal distension (CRD). Indeed, NVI mice displayed both a significantly lower withdrawal threshold to mechanical stimulation (Figure 4A) and a significantly shorter withdrawal latency to thermal stimulation of the hindpaw (Figure 4B), compared to naïve. A slight, yet significant, increase in VMR during CRD was observed in NVI mice, compared to naïve mice, most prominently at the highest intraballoon pressure applied (Figure 4C). Western blot analysis revealed that NVI diminished TRPV1 protein in the colon, whereas TRPA1 and CRF1 protein were not affected in NVI mice, compared to naïve (Figure 4D).
Figure 4.
NVI mice display cross-sensitization in both hindpaw and colon. A) The withdrawal threshold of the hindpaw to a mechanical stimulus was significantly lower in NVI mice (n=8) compared to naïve mice (n=7). B) NVI mice (n=8) displayed a significantly shorter withdrawal latency to thermal stimulation of the hindpaw than naïve mice (n=7). C) NVI mice (n=5) also displayed significantly higher VMR during colorectal distension (CRD) than naïve mice (n=5) over the entire dissension series (p < 0.05), as well as at the highest intraballoon pressure applied. D) Representative Western blots are shown for TRPA1, TRPV1, CRF1 and corresponding GAPDH protein expression with bands at 127, 85, 55 and 35kD, respectively, in the colon of naïve and NVI mice. Only TRPV1 protein expression in the colon was affected by NVI (n=4), with a significant decrease compared to naïve (n=6). A-B, D: *, ****p < 0.05, 0.0001 vs. naïve; Student’s t-test. C: *p < 0.05 vs. naïve; two-way RM ANOVA and Bonferroni posttest.
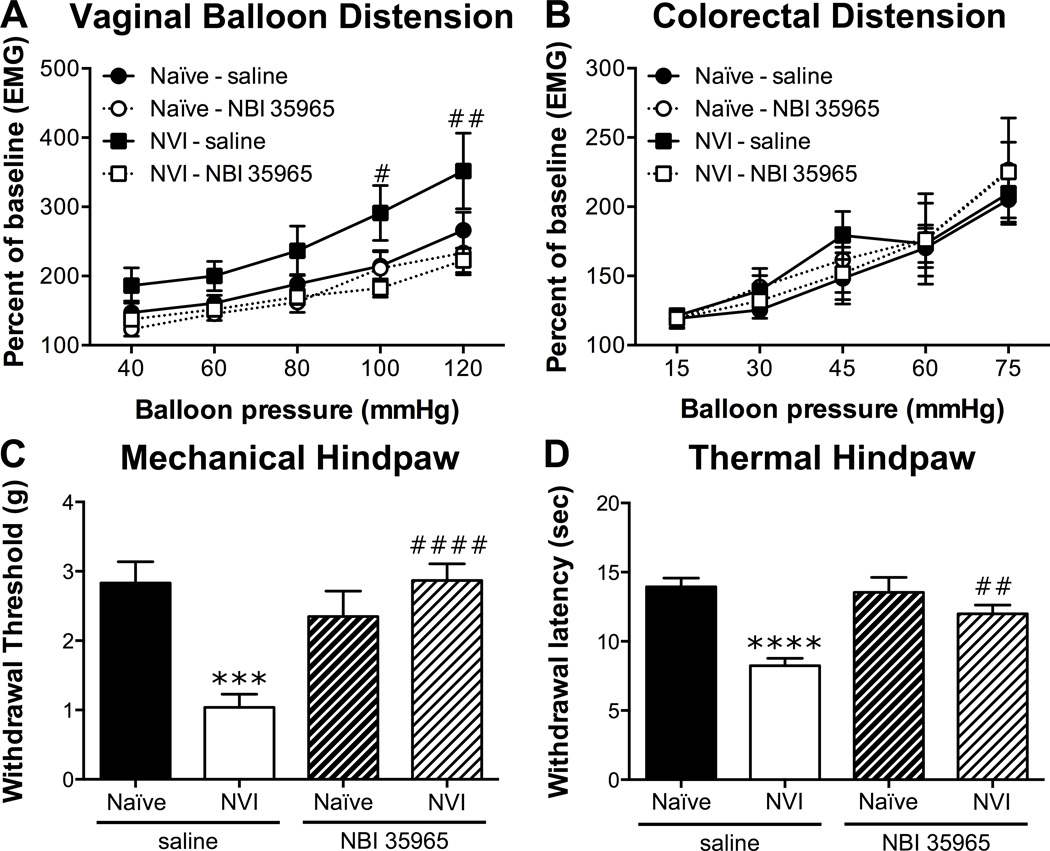
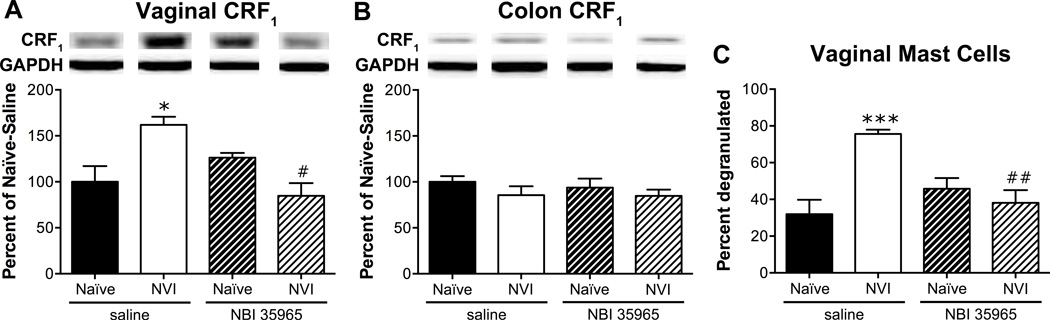
To determine whether activation of the HPA axis is driving the changes following NVI, we treated neonatal mice with a CRF1 antagonist, NBI 35965, 20 minutes prior to each zymosan instillation. NVI mice treated with NBI 35965 displayed a significantly lower VMR during VBD compared to saline-treated NVI mice (Figure 5A). Treatment with NBI 35965 did not affect the VMR of naïve mice during VBD (Figure 5A) or CRD (Figure 5B). The increase in VMR during CRD at 75mmHg that was observed in the initial group of NVI mice was not detected in the saline-treated NVI mice, likewise the VMR of NVI mice treated with NBI 35965 did not differ from any other group during CRD (Figure 5B). Treatment with NBI 35965 completely abolished both mechanical (Figure 5C) and thermal (Figure 5D) hindpaw hypersensitivity in NVI mice and had no effect on naïve mice. Vaginal CRF1 protein level (Figure 6A) and histological evidence of mast cell degranulation (Figure 6C) was significantly lower in NVI mice treated with NBI 35965, compared to saline-treated NVI mice. The level of CRF1 protein in the colon was unaffected by NBI 35965 treatment in any of the groups (Figure 6B).
Figure 5.
Neonatal treatment with NBI 35965 prevented vaginal and hindpaw hypersensitivity in NVI mice. A) The VMR during VBD was significantly lower in NBI 35965-treated NVI mice (n=11) compared to saline-treated NVI mice (n=8) over the entire distension period (p < 0.05), as well as at the two highest pressures applied. Naïve mice treated with saline (n=8) or NBI 35965 (n=8) displayed similar VMR during VBD. B) The VMR during CRD did not differ between any of the groups (n=7–9/group). Neonatal treatment with NBI 35965 significantly attenuated the decrease in mechanical withdrawal threshold (C) and thermal withdrawal latency (D) in NVI mice (n=13) compared to saline-treated NVI mice (n=11). Naïve mice treated with saline (n=10) or NBI 35965 (n=8) had similar mechanical withdrawal thresholds (C) and thermal withdrawal latencies (D). A–B: #,##p < 0.05, 0.01 vs. saline-treated; two-way RM ANOVA and Bonferroni posttest; C–D: ***,****p < 0.001, 0.0001 vs. naïve, ##,####p < 0.01, 0.0001 vs. saline-treated; two-way ANOVA and Bonferroni posttest.
Figure 6.
Neonatal treatment with NBI 35965 prevents increased vaginal CRF1 protein level and mast cell degranulation following NVI. Representative Western blots are shown for CRF1 and corresponding GAPDH protein expression with bands at 55 and 35kD, respectively, in the vagina (A) and colon (B) of naïve and NVI mice treated with saline or NBI 35965. A) Neonatal treatment with NBI 35965 completely abolished the increase in CRF1 protein level in vagina from NVI mice (n=5), compared to saline-treated NVI mice (n=5). Protein levels of CRF1 in vagina were unaffected by neonatal NBI 35965 treatment in naïve mice (n=5), compared to saline treatment (n=4). B) The level of CRF1 protein in colon from naïve and NVI mice treated with neonatal saline (n=4) or NBI 35965 (n=5) did not differ. C) The percentage of mast cells, identified by acidified toluidine blue staining, that demonstrated evidence of degranulation was significantly diminished in the vagina from NVI mice treated with NBI 35965 (n=5) compared to saline (n=5). The extent of mast cell degranulation in the vagina of naïve mice treated with saline (n=4) or NBI 35965 (n=5) did not differ. *,***p < 0.05, 0.001 vs. naïve, #,##p < 0.05, 0.01 vs. saline-treated; two-way ANOVA and Bonferroni posttest.
Discussion
Experiencing adverse events early in life increases the risk of developing chronic pelvic pain disorders, including vulvodynia, during adolescence and adulthood. Here, we have investigated the outcomes of neonatal exposure to intravaginal zymosan, a cell wall component of yeast that has been shown to induce long-lasting hypersensitivity in the bladder of rats following neonatal intravesicular administration [51]. In the current study, we have provided the first evidence that neonatal, but not adult, irritation of the vagina produces long-lasting sensitization of not just the vagina, but also of the hindpaw and possibly colon. Increased downstream output from the HPA axis, which was attenuated by neonatal treatment with a CRF1 antagonist, NBI 35965, and changes in primary neuron signaling were also observed in NVI mice and likely contribute towards the increased visceral and somatic sensitivity.
Permanently heightened visceral sensitivity has been shown to arise following organ irritation or inflammation during the first two weeks of postnatal development. This has been shown extensively to occur in both the gastrointestinal [2,5,13,18,71] and urinary tracts [19,51,56] and the current study provides the first evidence that the reproductive system is also susceptible to neonatal irritation. Mice that received intravaginal zymosan on P8 and 10 displayed an overall increase in VMR during VBD, most prominently at the two highest intravaginal balloon pressures. This observation, as well as that of cross-sensitization of the colon only at the highest intraballoon pressure, is striking in that it more closely resembles changes in colorectal sensitivity following neonatal colon irritation [13] than it does the effect of neonatal maternal separation on vaginal sensitivity, which increased VMR across all pressures [49]. This dichotomy in the qualitative impact of early life insult versus stress on visceral sensitivity is also apparent in rat studies assessing either bladder or colorectal sensitivity following neonatal inflammation or maternal separation, with the former generating increased VMR only at presumed nociceptive pressures and the latter increasing VMR at both non-noxious and noxious pressures [2,18,19,45,51,71]. In the current study, instillation of intravaginal saline, which caused disruption of the vaginal hymen, was not sufficient to produce vaginal hypersensitivity suggesting that physical manipulation of the vagina alone is not sufficient to permanently sensitize the organ. Likewise, recapitulating the neonatal insult in adult female mice was not sufficient to produce a long-lasting vaginal hypersensitivity, indicating that the neonatal period represents a critical time for the induction of chronic pain, as has been illustrated in multiple other studies [2,51,53].
We, and others, have previously shown discrepancies in both immunohistochemical and functional TRP channel expression between viscera-, muscle-, and skin-specific DRG populations, with the former two populations having greater TRP channel expression than the latter population [16,35,41]. Accordingly, DRG neurons back-labeled from the vagina responded more widely to capsaicin than did unlabeled or perivaginal skin-specific DRG neurons. The percentage of perivaginal skin-specific DRG neurons that responded to MO or capsaicin in this study was larger than we have reported previously for cutaneous afferents labeled via injection of retrograde tracer into the saphenous nerve [16,41] and likely reflects differences in the neurochemical phenotype of sensory neurons innervating the ventral hind paw and the perivaginal area. Likewise, functional TRPA1 and TRPV1 expression was higher in unlabeled DRG neurons from both naïve and NVI mice, compared to previous publications of unlabeled primary DRG cultures [11,42]. This is likely due to the relatively high percentage of visceral afferent neurons in our preparations, as only L5-S1 DRG were used in this study and TRPV1 has previously been shown to be enhanced in lumbosacral regions due to the pelvic afferent contribution [16]. Unlike in previous studies of neonatal colon irritation [13,71], functional expression of TRPA1 and TRPV1 was not increased in target-specific DRG neurons from NVI mice. Evidence of increased neuronal excitability, in the form of significantly larger high K+-evoked calcium transients, was observed. Increased depolarization-evoked calcium transients have been reported in adult mouse models of inflammatory-induced interstitial cystitis [21] and chronic pancreatitis [55], the latter of which was shown to be TRP channel-dependent, suggesting that altered calcium regulation may be a common mechanism shared among visceral pain disorders.
Peripheral neuroinflammatory changes likely contribute towards enhanced vaginal sensitivity in NVI mice. Increased TRPA1 protein levels within the vagina were observed, despite no changes in protein or functional expression within the DRG. This observed increase could partially be attributed to enhanced expression on peripheral terminals within the tissue, as TRPA1 is exclusively expressed by TRPV1- and neuropeptide-expressing neurons [26,61], which have been shown to be more prevalent in biopsies from patients with vulvodynia [66,67], IBS [1], and IC/PBS [65]. Pharmacological disruption of TRPA1 signaling has been shown to attenuate visceral hypersensitivity in rodent models of IBS [69,72], IC/PBS [21], and both acute and chronic pancreatitis [54,55], indicating that TRPA1 plays a pivotal role in a wide range of visceral pain syndromes. Increased nerve-mast cell proximity has also been observed in biopsies from vulvodynia [30,40], IC/PBS [38,64], and IBS [62] patients and has been postulated to drive the increased sensitivity in the affected tissues.
A history of early-life adverse events has been routinely shown to increase HPA axis output in both human patients and rodent models [47,70]. In the current study, increased serum corticosterone, histological evidence of increased extruded mast cell contents, and enhanced CRF1 protein expression in the vagina all indicate an increase in HPA axis output resulting from NVI. Indeed, antagonizing CRF1 at the time of neonatal insult abolished both vaginal and hindpaw hypersensitivity, as well as the increase in CRF1 protein level and histological evidence of mast cell degranulation in the vagina. The reversal of hindpaw sensitivity by CRF1 antagonism differs from a similar study that reported a reversal of colorectal sensitivity, but not hindpaw thermal hyperalgesia, in adult rats that were treated with the CRF1 antagonist, antalarmin prior to neonatal gastric suctioning [59]. Considering the consistency with which we have observed comorbid hindpaw hypersensitivity in mouse models of early insult or stress [13,49], it will be important to determine how both potential convergence at the level of the spinal cord and HPA axis dysfunction may contribute towards the development of somatic functional pain disorders, such as fibromyalgia and temporomandibular joint disorder, that are commonly diagnosed alongside chronic pelvic pain disorders [3,4,8]. In order to preserve NVI-induced changes within the HPA axis, the mice used in this study were not subjected to daily vaginal smears to determine estrous cycle. Preliminary work in our laboratory (data not shown) and from others [10,44] has shown that experimental treatment intended to increase visceral sensitivity is sufficient to negate any effect of the estrous cycle.
Finally, many of the observations made in this study, and variations from previous studies of neonatal colon irritation, may be specific to the use of zymosan as the neonatal irritant. Zymosan was chosen for this study not only because of its use in neonatal bladder irritation studies [19,20,51], but because it is a clinically-relevant choice due to the recent rise in Candida infections in young children [36], which has been attributed to both the increasing overuse of antibiotics [7] and infection following hospital-borne Candida exposure in the neonatal intensive care unit [58]. A study by Farmer et al. [27] reported that repeated (<6) weekly intravulvar injections of zymosan produced long-lasting vulvar hypersensitivity in the majority of treated mice, in a similar manner as repeated intravaginal inoculation with Candida. Previous studies of neonatal colon irritation utilized known TRP channel agonists, namely acetic acid and MO, to induce the neonatal irritation, and observed increased expression of the respective TRP channels in adult DRG [13,71]. Intracolonic zymosan has been shown to increase colorectal sensitivity when administered in adult mice and genetic deletion of TRPV1 diminished, but did not completely abolish, the increase in VMR [37], suggesting that zymosan-induced visceral hypersensitivity may partially occur via a non-TRP receptor-mediated mechanism. Previous studies of neonatal bladder irritation using zymosan reported increased bladder weight, plasma extravasation, and neuropeptide content, but no significant change in tissue histology, eosinophil infiltration, or mast cell infiltration or degranulation within either the bladder or colon of adult rats [20,44]. These results, along our observation of increased mast cell degranulation, suggest that intravaginal zymosan likely produced long-lasting neurogenic inflammation in the vaginas of NVI mice.
In conclusion, this study has provided evidence that, similar to the gastrointestinal and urinary systems, the reproductive system is vulnerable to early life insult. Both visceral and somatic secondary hypersensitivity was observed in NVI mice, suggesting that the consequences of NVI were not confined to the primarily affected organ. Depolarization-evoked calcium transients were increased in primary sensory neurons innervating the vagina, and, importantly, antagonizing CRF1 at the time of insult prevented both vaginal and hindpaw hypersensitivity, as well as attenuated peripheral indicators of increased HPA axis output. Considering the extensive diagnostic overlap among chronic pelvic pain disorders, as well as with other somatic pain disorders, such as migraine and fibromyalgia, and mood disorders, taking inventory of a patient’s early life history could provide valuable insight as to how best to treat comorbid symptoms arising from multiple diagnoses.
Acknowledgments
This work was supported by NIH grants R03 DK080182 (JAC), Center of Biomedical Research Excellence (COBRE) grant P20 GM104936 (JAC), start-up funds and core support from the Kansas Institutional Development Award (IDeA) P20 GM103418, core support from the Kansas IDDRC P30 HD002528, and The Madison and Lila Self Fellowship Program (ANP). All authors carried out experiments and analyzed data. A.N.P. and J.A.C. designed experiments. J.A.C. conceived, designed, and supervised the project. A.N.P. and J.A.C. wrote the manuscript. We would like to thank Bilal Hassan for assistance with neonatal vaginal irritation and Drs. Nancy Berman, Brian Davis, Kenneth McCarson, Peter Smith, and Sarah Tague for their insight during manuscript preparation.
Abbreviations
- NVI
Neonatal vaginal irritation
- VMR
visceromotor response
- VBD
vaginal balloon distension
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
- CRF
corticotropin releasing factor
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- IC/PBS
interstitial cystitis/painful bladder syndrome
- DRG
dorsal root ganglion
- CRD
colorectal distension
- EMG
electromyographic
- CTB
cholera toxin-β
- DiI
1, 1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- LS
lumbosacral
- MO
mustard oil
- CORT
corticosterone
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 3.Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49(5A Suppl):52–57. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- 4.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics and gynecology. 2006;107(3):617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53(4):501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain, behavior, and immunity. 2002;16(6):622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 7.Blaser M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature. 2011;476(7361):393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 8.Bullones Rodriguez MA, Afari N, Buchwald DS National Institute of D, Digestive, Kidney Diseases Working Group on Urological Chronic Pelvic P. Evidence for overlap between urological and nonurological unexplained clinical conditions. The Journal of urology. 2013;189(1 Suppl):S66–S74. doi: 10.1016/j.juro.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrico DJ, Sherer KL, Peters KM. The relationship of interstitial cystitis/painful bladder syndrome to vulvodynia. Urologic nursing. 2009;29(4):233–238. [PubMed] [Google Scholar]
- 10.Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44(2):123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 11.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 12.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 13.Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151(2):540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christianson JA, Davis BM. The Role of Visceral Afferents in Disease. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton, FL: 2010. [Google Scholar]
- 15.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc. 2007;2(10):2624–2631. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- 16.Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140(1):247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4(9):493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282(2):G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 19.DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8(12):914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBerry J, Randich A, Shaffer AD, Robbins MT, Ness TJ. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain. 2010;11(3):247–255. doi: 10.1016/j.jpain.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBerry JJ, Schwartz ES, Davis BM. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain. 2014;155(7):1280–1287. doi: 10.1016/j.pain.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 23.Edwards L. New concepts in vulvodynia. American journal of obstetrics and gynecology. 2003;189(3 Suppl):S24–S30. doi: 10.1067/s0002-9378(03)00790-7. [DOI] [PubMed] [Google Scholar]
- 24.Edwards L. Subsets of vulvodynia: overlapping characteristics. The Journal of reproductive medicine. 2004;49(11):883–887. [PubMed] [Google Scholar]
- 25.Ehrstrom S, Kornfeld D, Rylander E, Bohm-Starke N. Chronic stress in women with localised provoked vulvodynia. Journal of psychosomatic obstetrics and gynaecology. 2009;30(1):73–79. doi: 10.1080/01674820802604359. [DOI] [PubMed] [Google Scholar]
- 26.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26(33):8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM, Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Science translational medicine. 2011;3:101ra191. doi: 10.1126/scitranslmed.3002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424(4):651–669. [PubMed] [Google Scholar]
- 29.Gardella B, Porru D, Nappi RE, Dacco MD, Chiesa A, Spinillo A. Interstitial cystitis is associated with vulvodynia and sexual dysfunction--a case-control study. The journal of sexual medicine. 2011;8(6):1726–1734. doi: 10.1111/j.1743-6109.2011.02251.x. [DOI] [PubMed] [Google Scholar]
- 30.Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: a prospective study. American journal of obstetrics and gynecology. 2010;202(6):614, e611–e618. doi: 10.1016/j.ajog.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Gordon AS, Panahian-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: responses to a Web-based survey. Journal of sex & marital therapy. 2003;29(Suppl 1):45–58. doi: 10.1080/713847126. [DOI] [PubMed] [Google Scholar]
- 32.Groysman V. Vulvodynia: new concepts and review of the literature. Dermatologic clinics. 2010;28(4):681–696. doi: 10.1016/j.det.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 34.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88. [PubMed] [Google Scholar]
- 35.Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047(2):261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Jain A, Jain S, Rawat S. Emerging fungal infections among children: A review on its clinical manifestations, diagnosis, and prevention. Journal of pharmacy & bioallied sciences. 2010;2(4):314–320. doi: 10.4103/0975-7406.72131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133(1):184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 38.Larsen MS, Mortensen S, Nordling J, Horn T. Quantifying mast cells in bladder pain syndrome by immunohistochemical analysis. BJU international. 2008;102(2):204–207. doi: 10.1111/j.1464-410X.2008.07576.x. discussion 207. [DOI] [PubMed] [Google Scholar]
- 39.Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC public health. 2006;6:177. doi: 10.1186/1471-2458-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstetrics and gynecology. 2011;117(6):1307–1313. doi: 10.1097/AOG.0b013e31821c33dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 Function and Modulation Are Target Tissue Dependent. J Neurosci. 2011;31(29):10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138(3):484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2(1):152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 44.Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterol Motil. 2011;23(7):683–e281. doi: 10.1111/j.1365-2982.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moloney RD, O'Leary OF, Felice D, Bettler B, Dinan TG, Cryan JF. Early-life stress induces visceral hypersensitivity in mice. Neuroscience letters. 2012;512(2):99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- 46.Nylanderlundqvist E, Bergdahl J. Vulvar vestibulitis: evidence of depression and state anxiety in patients and partners. Acta dermato-venereologica. 2003;83(5):369–373. doi: 10.1080/00015550310003764. [DOI] [PubMed] [Google Scholar]
- 47.O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. 2011;214(1):71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 48.Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. American journal of obstetrics and gynecology. 2002;187(5):1395–1400. doi: 10.1067/mob.2002.127375. [DOI] [PubMed] [Google Scholar]
- 49.Pierce AN, Ryals JM, Wang R, Christianson JA. Vaginal hypersensitivity and hypothalamic-pituitary-adrenal axis dysfunction as a result of neonatal maternal separation in female mice. Neuroscience. 2014;263:216–230. doi: 10.1016/j.neuroscience.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plante AF, Kamm MA. Life events in patients with vulvodynia. Bjog. 2008;115(4):509–514. doi: 10.1111/j.1471-0528.2007.01662.x. [DOI] [PubMed] [Google Scholar]
- 51.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7(7):469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 52.Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner HK. Relationship Between Vulvodynia and Chronic Comorbid Pain Conditions. Obstetrics & Gynecology. 2012;120(1):145–151. doi: 10.1097/AOG.0b013e31825957cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289(5479):628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, Gebhart GF. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140(4):1283–1291. e1281–e1282. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33(13):5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaffer AD, Ball CL, Robbins MT, Ness TJ, Randich A. Effects of acute adult and early-in-life bladder inflammation on bladder neuropeptides in adult female rats. BMC urology. 2011;11:18. doi: 10.1186/1471-2490-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, Theoharides TC. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. The Journal of pharmacology and experimental therapeutics. 1999;288(3):1349–1356. [PubMed] [Google Scholar]
- 58.Singhi S, Rao DS, Chakrabarti A. Candida colonization and candidemia in a pediatric intensive care unit. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2008;9(1):91–95. doi: 10.1097/01.PCC.0000298643.48547.83. [DOI] [PubMed] [Google Scholar]
- 59.Smith C, Nordstrom E, Sengupta JN, Miranda A. Neonatal gastric suctioning results in chronic visceral and somatic hyperalgesia: role of corticotropin releasing factor. Neurogastroenterol Motil. 2007;19(8):692–699. doi: 10.1111/j.1365-2982.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 60.Stockdale CK, Lawson HW. 2013 Vulvodynia Guideline update. Journal of lower genital tract disease. 2014;18(2):93–100. doi: 10.1097/LGT.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 61.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 62.Theoharides TC, Asadi S, Chen J, Huizinga JD. Irritable bowel syndrome and the elusive mast cells. The American journal of gastroenterology. 2012;107(5):727–729. doi: 10.1038/ajg.2012.61. [DOI] [PubMed] [Google Scholar]
- 63.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. Journal of neuroimmunology. 2004;146(1–2):1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 64.Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. The Journal of urology. 1995;153(3 Pt 1):629–636. doi: 10.1097/00005392-199503000-00021. [DOI] [PubMed] [Google Scholar]
- 65.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57(6) Suppl 1:67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 66.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. Eur J Pain. 2004;8(2):129–133. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 67.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. The British journal of dermatology. 2003;148(5):1021–1027. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 68.Ventolini G. Vulvar pain: Anatomic and recent pathophysiologic considerations. Clinical Anatomy. 2013;26(1):130–133. doi: 10.1002/ca.22160. [DOI] [PubMed] [Google Scholar]
- 69.Vermeulen W, De Man JG, De Schepper HU, Bult H, Moreels TG, Pelckmans PA, De Winter BY. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. European journal of pharmacology. 2013;698(1–3):404–412. doi: 10.1016/j.ejphar.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, Mayer EA, Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137(6):1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132(2):615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 72.Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neuroscience letters. 2008;440(3):237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]