Abstract
Opioid analgesics are the standard therapeutic agents for the treatment of pain, but their prolonged use is limited because of the development of tolerance and dependence. Recently, we reported the development of a μ-opioid receptor knock-in (KI) mouse in which the μ-opioid receptor was replaced by a mutant receptor (S196A) using a homologous recombination gene-targeting strategy. In these animals, the opioid antagonist naltrexone elicited antinociceptive effects similar to those of partial agonists acting in wild-type (WT) mice; however, development of tolerance and physical dependence were greatly reduced. In this study, we test the hypothesis that the failure of naltrexone to produce tolerance in these KI mice is attributable to its simultaneous inhibition of δ-opioid receptors and activation of μ-opioid receptors. Simultaneous implantation of a morphine pellet and continuous infusion of the δ-opioid receptor antagonist naltrindole prevented tolerance development to morphine in both WT and KI animals. Moreover, administration of SNC-80 [(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide], a δ agonist, in the naltrexone-pelleted KI animals resulted in a dose-dependent induction in tolerance development to both morphine- and naltrexone-induced analgesia. We conclude that although simultaneous activation of both μ- and δ-opioid receptors results in tolerance development, μ-opioid receptor activation in conjunction with δ-opioid receptor blockade significantly attenuates the development of tolerance.
Keywords: morphine, tolerance, naltrexone, SNC-80, naltrindole, knock-in animals
Introduction
The development of tolerance and physical dependence induced by chronic morphine administration limits its prolonged use in the treatment of pain (Ellison 1993). Both analgesia and tolerance to morphine are abolished in μ-opioid receptor knock-out mice, implicating the μ-opioid receptor as the primary receptor type mediating both of these effects (Fang et al., 1986; Matthes et al., 1996; Sora et al., 1997). However, several lines of evidence suggest the additional involvement of the δ-opioid receptor in morphine tolerance. Initial studies using δ-opioid receptor antagonists (Abdelhamid et al., 1991) and more recent studies using δ-opioid receptor knock-out mice (Nitsche et al., 2002) were shown to disrupt the development of tolerance. Although these studies suggest a role for the δ-opioid receptor in morphine tolerance, they do not provide conclusive evidence for its involvement. For example, in the δ-opioid receptor knock-out mice, adaptive mechanisms, such as the observed novel upregulated “δ-like” receptors (Zhu et al., 1999), might account for the lack of tolerance observed in these animals. In pharmacological studies, the selective δ-receptor antagonist naltrindole (NTI) has been shown to interact with alternate receptors, because NTI binding was still detected in the μ/δ/κ triple knock-out mice (Gaveriaux-Ruff et al., 2001). Furthermore, at high concentrations, NTI has been shown to lose its δ selectivity and act as an agonist in some cell types (Chen et al., 2004).
Recently, we described the development of a μ-opioid receptor knock-in (KI) mouse in which the μ-opioid receptor was replaced by a mutant receptor (S196A) using a homologous recombination gene-targeting strategy (Yang et al., 2003). In these animals, the opioid antagonists naloxone and naltrexone elicited antinociceptive effects similar to those of partial agonists acting in wild-type (WT) mice; however, tolerance development and physical dependence were greatly reduced. The advantages of this animal model over gene deletion and pharmacological antagonist studies reported thus far for studying the role of the δ-opioid receptor in morphine tolerance are as follows: (1) Although the receptor is genetically mutated to recognize naltrexone as an agonist, the functional properties of the μ and δ receptors are still intact in these animals. (2) Naltrexone, unlike NTI, is selective even at high doses in blocking δ-opioid receptors as well as activating μ-opioid receptors in the KI mice. Thus, these KI animals provide us with a unique tool to test the role of δ-opioid receptors in morphine-induced tolerance.
In this study, we specifically test the hypothesis that failure of naltrexone to produce tolerance in the KI mice is attributable to its simultaneous inhibition of δ-opioid receptors and activation of μ-opioid receptors. We first show that simultaneous implantation of a morphine pellet and continuous NTI infusion prevents tolerance development to morphine in both the WT and KI animals. We subsequently show that simultaneous activation of both μ- and δ-opioid receptors results in tolerance development.
Materials and Methods
Generation of KI mice
Homozygous mutant KI mice were generated as described previously (Yang et al., 2003). Only WT and homozygous mice were used in the current study. The handling of animals and experimental procedures were performed as approved by the University of Minnesota Institutional Biosafety Committee.
Tail-flick antinociception test
The tail-flick antinociception test was done as described by Yang et al. (2003). Percentage of maximum possible effect was calculated using the following formula: [(measured latency — baseline latency)/(cutoff time — baseline latency)] × 100. We tested each dose on 8 –10 mice. ED50 values were derived from regression analyses of the linear portion of each dose–response curve or calculated by nonlinear regression. Student’s t tests were used to calculate significance between treatment groups.
Chronic drug treatment
Mice were anesthetized with ketamine/xylazine and implanted subcutaneously with a 75 mg morphine pellet, a 20 mg naltrexone pellet, or a cellulose placebo pellet (National Institute on Drug Abuse, Rockville, MD) encased in a 1 × 1 cm nylon mesh bag to permit easy removal. Based on previous studies (Yang et al., 2003), it was established that mice were tolerant to the analgesic effects of morphine by 72 h after morphine pellet implantation. In all experiments, pellets were left in place for 72 h. Nociceptive tests were done at the same time each day.
Alzet pump delivery of NTI and (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide
Mice were implanted subcutaneously with an Alzet (Cupertino, CA) osmotic minipump infusing either the δ antagonist NTI (10 mg · kg−1 · d−1) or varying doses of the δ agonist (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]- N,N-diethylbenzamide (SNC-80). Two hours after pump placement, animals were implanted with a morphine, naltrexone, or placebo pellet. Pellets and pumps were removed 72 h after pellet implantation. Morphine tolerance was measured 8 h after pellet removal.
Results
We have shown previously (Yang et al., 2003) that the antinociceptive effect of morphine in the KI homozygous mice was similar to its effect in the WT mice, although receptor number [d-Ala2-N-Me-Phe4-glycol5-enkephalin (DAMGO) binding sites] was 85% lower in the KI mice (Bmax, 17.5) compared with the WT mice (Bmax, 113). The affinities (Kd) for DAMGO, however, were similar (2.6 and 3.6 nM for WT and KI, respectively) (Yang et al., 2003). We also showed that naltrexone acted as an agonist in the KI animals and that chronic naltrexone treatment did not result in tolerance development to antinociceptive effects in these animals.
Because naltrexone has antagonist activity at the δ-opioid receptors, we investigated whether this effect contributes to the lack of tolerance. Both KI and WT animals were implanted with a placebo, morphine, or naltrexone pellet for 72 h, and analgesia (tail-flick assay) was tested 8 h after withdrawal.
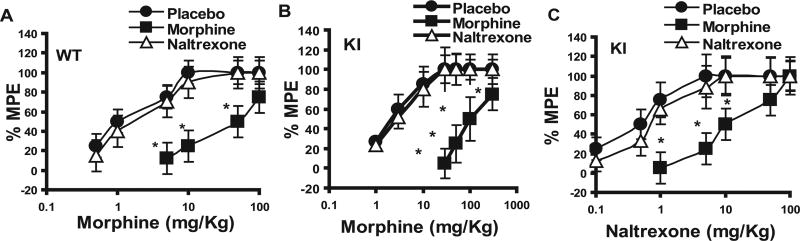
As expected in the WT animals (Fig. 1A), morphine pellet implantation resulted in significant tolerance development, as indicated by a rightward shift in the dose–response curve, with an ED50 of ~12.92 mg/kg. Surprisingly, morphine pellet implantation in the KI animals resulted in a greater degree of tolerance, with a more exaggerated rightward shift and an ED50 of ~65.24 mg/kg (Fig. 1B). As we have observed previously, although significant tolerance developed to morphine in the KI mice after chronic morphine treatment, no tolerance to morphine was observed in the KI mice chronically treated with naltrexone, an agonist in the KI mice (ED50, ~2.05 mg/kg) (Fig. 1B). As expected, chronic naltrexone treatment of WT mice did not produce any significant change in morphine analgesia, with ED50 values being very similar to those of the placebo-treated group (ED50 values, 1.27 and 2.05 mg/kg) (Fig. 1A).
Figure 1.
Dose–response effect of morphine- and naltrexone-induced analgesia in WT and KI animals after chronic placebo, morphine, or naltrexone treatment. WT (A) or KI (B, C) animals were implanted with a placebo, morphine,or naltrexone pellet for 72 h, and morphine-induced (A, B) or naltrexone-induced (C) analgesia was measured by the tail-withdrawal test 8 h after pellet withdrawal. There is a significant difference between the placebo-treated and the morphine-treated group in all three experiments. *p < 0.05, significant difference from the placebo-treated group (Student’s t test). Each dose was tested on at least 8–10 animals. % MPE, Percentage of maximum possible effect. Error bars represent SEM.
We subsequently investigated whether tolerance to naltrexone develops after chronic naltrexone or placebo treatment in the WT and KI animals. Our results show that although morphine-pelleted KI animals displayed significant cross tolerance to naltrexone-induced analgesia, the ED50 for naltrexone-induced analgesia (ED50, ~15.7 mg/kg) (Fig. 1C) was significantly lower than that for morphine-induced analgesia (ED50, 65.24 mg/kg). In naltrexone-pelleted animals, no significant tolerance to naltrexone-induced analgesia was seen (ED50, 0.81 mg/kg), and ED50 values were similar to the placebo group (ED50, 0.69 mg/kg). As expected, naltrexone did not induce any analgesic effect in the WT animals implanted with morphine, placebo, or naltrexone pellets (data not shown).
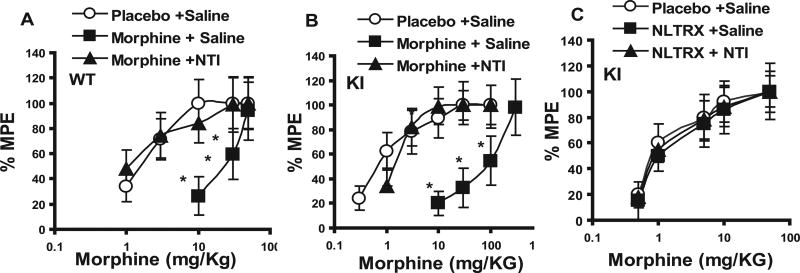
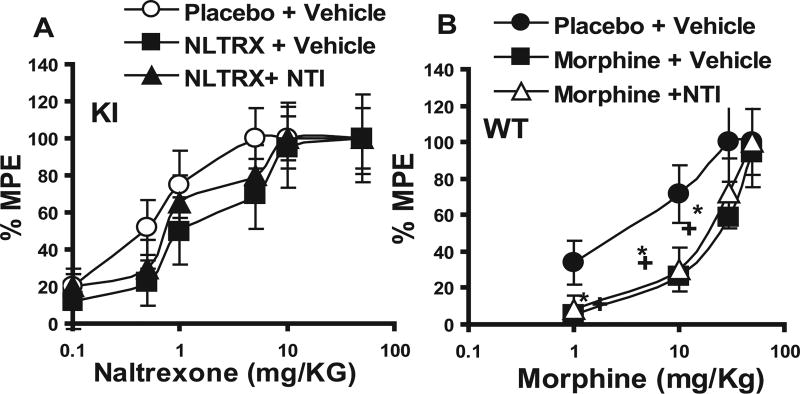
To test the hypothesis that the lack of tolerance development in the KI animals was attributable to blockade of the δ-opioid receptor, both WT and KI animals were first implanted with an Alzet pump delivering NTI (a δ antagonist) at a steady-state rate of 10 mg · kg−1 · 24 h−1. Animals were implanted with a placebo, morphine, or naltrexone pellet 2 h after pump placement. Results show that implantation of a morphine pellet together with continuous NTI infusion prevented tolerance development in the WT animals (Fig. 2A), with a significant leftward shift in the dose–response curve. A similar leftward shift in the morphine dose–response curve was also seen in the KI animals implanted with a morphine pellet and continuously infused with NTI (Fig. 2B). As expected, because both NTI and naltrexone act as antagonists at the δ receptor, no morphine tolerance was observed in KI animals treated with either naltrexone alone or naltrexone plus NTI (Fig. 2C). Similarly, no tolerance to naltrexone was observed in KI animals treated with either naltrexone or naltrexone plus NTI (Fig. 3A)
Figure 2.
Dose–response effect of morphine-induced analgesia in WT (A) and KI (B, C) animals after chronic naltrindole treatment in WT and KI animals. WT(A) or KI (B, C) animals were implanted with placebo, morphine (A, B), or naltrexone (NLTRX) (C) pellets and infused with either saline or naltrindole (Alzet pump; 10 mg · kg−1 · 24 h−1) for 72 h. Morphine-induced analgesia was measured as described above. There was a significant difference between the placebo- plus saline-treated and the morphine- plus saline-treated groups (p < 0.05). There was no significant difference between the placebo plus saline group and the morphine plus NTI group (p > 0.1). *p < 0.05, significant difference from the placebo-treated group (Student’s t test). No significant difference was observed among the three treatment groups in the naltrexone-pelleted animals (C); p > 0.1 (Student’s t test). Each dose was tested on at least 8–10 animals. % MPE, Percentage of maximum possible effect. Error bars represent SEM.
Figure 3.
A, Effect of naltrindole treatment on naltrexone (NLTRX)-induced analgesia in KI animals (as in Fig. 2C, except that naltrexone-induced analgesia was measured). No significant difference was observed among the three treatment groups; p > 0.06 (Student’s t test). Each dose was tested on at least 8–10 animals. B, Effect of naltrindole treatment on morphine-induced analgesia after tolerance development. WT animals were implanted with a placebo or morphine pellet for 72 h. At 24 h before pellet removal, animals were implanted with an Alzet pump delivering 10 mg · kg−1 · 24 h−1 NTI. Morphine-induced analgesia was measured as described above. *p < 0.05, significant difference between the morphine-plus vehicletreated group and the placebo-treated group; + indicates a significant difference between the morphine plus NTI group and the placebo-treated group (Student’s t test). Each dose was tested on at least 8–10 animals. % MPE, Percentage of maximum possible effect. Error bars represent SEM.
We then investigated whether NTI infusion to WT and KI animals after tolerance has developed could reverse the effects of tolerance. Animals were implanted with either morphine and placebo pellets for 72 h and then implanted with an Alzet pump delivering NTI (10 mg · kg−1 · 24 h−1). Once tolerance developed, infusion of NTI failed to reverse tolerance to morphine both in WT (Fig. 3B) and in KI (data not shown) animals. The ED50 for morphine-induced analgesia in the morphine- plus NTI-treated WT animals (ED50, 19.7 ± 9.5 mg/kg) was not significantly different from the morphine- plus vehicle-treated group (ED50, 18.6 + 8.6 mg/kg).
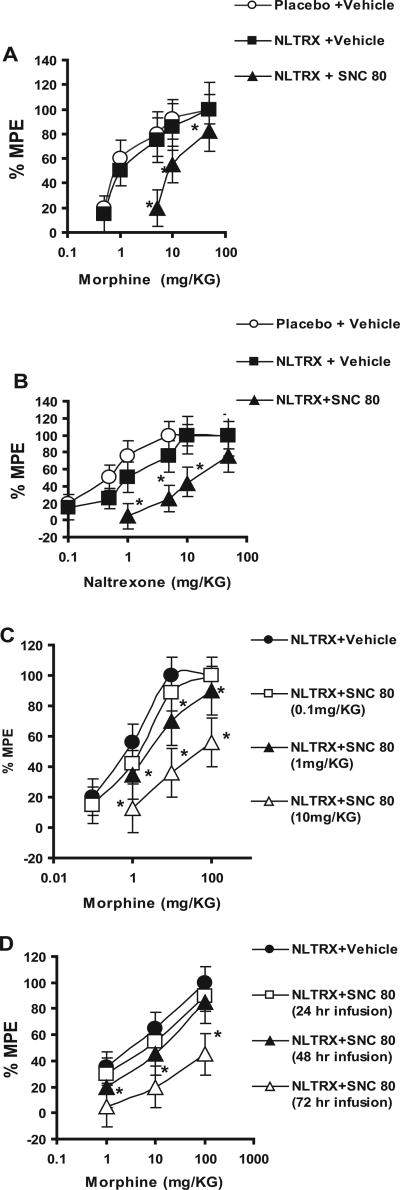
We next tested the hypothesis that activation of δ-opioid receptor with simultaneous activation of μ-opioid receptor in the KI mice would induce tolerance. In these experiments, KI mice were implanted with a naltrexone pellet and also with an Alzet pump delivering either the δ agonist SNC-80 (10 mg · kg−1 · 24 h−1) or vehicle control. Tolerance was measured 8 h after pellet and pump removal. SNC-80 administration in the naltrexone-pelleted KI animals resulted in significant tolerance development to both morphine-induced (ED50, 9.37 ± 1.39 mg/kg) (Fig. 4A) and naltrexone-induced (ED50, 11.45 ± 2.69 mg/kg) (Fig. 4B) analgesia compared with vehicle-infused, naltrexone-pelleted KI animals.
Figure 4.
Effect of SNC-80 treatment on morphine-induced (A) and naltrexone-induced (B) analgesia in KI animals. KI animals were implanted with a placebo or a naltrexone (NLTRX) pellet and infused with either vehicle or SNC-80 (Alzet pump; 10 mg · kg−1 · 24 h−1) for 72 h; morphine-induced (A) or naltrexone-induced (B) analgesia was then measured. There was a significant difference between the placebo- plus vehicle-treated group and the naltrexone- plus SNC-80-treated group; p < 0.05 (Student’s t test). Each asterisk indicates a significant difference from the placebo-treated group. Each dose was tested on at least 8–10 animals. C, Dose–response effect of SNC-80 infusion on morphine-induced analgesia in KI animals. KI animals were implanted with a naltrexone pellet and infused with either vehicle or varying concentrations of SNC-80 (Alzet pump; 0.1, 1, and 10 mg · kg−1 · 24 h−1) for 72 h; morphine-induced analgesia was then measured. There was a significant difference between the naltrexone- plus vehicle-treated group and the naltrexone- plus 1 mg/kg SNC-80-treated and naltrexone- plus 10 mg/kg SNC-80-treated groups; p < 0.05 (Student’s t test). Each dose was tested on at least 8–10 animals. D, Different durations of SNC-80 exposure on morphine-induced analgesia in KI animals. KI animals were implanted with a naltrexone pellet and infused with either vehicle or SNC-80 (Alzet pump; 10 mg · kg−1 · 24 h−1) for 24, 48, and 72 h; morphine-induced analgesia was then measured. There was a significant difference between the naltrexone- plus vehicle-treated group and the naltrexone plus 72 h SNC-80 group; p < 0.05 (Student’s t test). Each asterisk indicates a significant difference from the naltrexone- plus vehicle-treated group. Each dose was tested on at least 8–10 animals.% MPE, Percentage of maximum possible effect. Error bars represent SEM.
To determine the minimum dose of SNC-80 required to induce tolerance, KI mice were implanted with a naltrexone pellet and an Alzet pump delivering varying doses of SNC-80 (0.1, 1, or 10 mg/kg) or vehicle control. Tolerance was measured 8 h after pellet and pump removal. Our data show (Fig.4C) that SNC-80 administration in the naltrexone-pelleted KI animals resulted in a dose-dependent rightward shift in the dose–response curve; significant tolerance was observed with a dose as low as 1 mg · kg−1 · 24 h−1 SNC-80; the ED50 for SNC-80 was 6.48 ± 1.2 mg/kg compared with the ED50 for vehicle, which was 1.02 + 0.8 mg/kg. ED50 values for 0.1 and 10 mg of SNC-80 were 2.089 ± 0.93 and 10.15 ± 2.1 mg · kg−1 · 24 h−1, respectively.
To determine the minimum exposure required to induce tolerance, KI mice were implanted with a naltrexone pellet and also with an Alzet pump delivering either vehicle or SNC-80 for 24, 48, or 72 h. Pumps were filled with 24, 48, or 72 µl of SNC-80 with a delivery rate of 1 µl/h (final SNC-80 dose was 10 mg · kg−1 · 24 h−1). No significant tolerance was observed when SNC-80 was continuously infused for 24 or 48 h. Significant tolerance was observed only when SNC-80 was continuously infused for 72 h (Fig. 4D). These studies indicate that continual administration for the duration that the animal is exposed to morphine is necessary for the development of tolerance.
Discussion
The δ-opioid receptor has been implicated but not conclusively proven to be involved in morphine tolerance (Larson et al., 1980; Lee et al., 1980; Russell et al., 1986; Heyman et al., 1989;Malmberg and Yaksh, 1992; Hepburn et al., 1997; Zhu et al., 1999; Riba et al., 2002). In this paper, we report more compelling evidence for an involvement of δ-opioid receptors in the development of morphine-induced tolerance, using KI mice in which the μ-opioid receptor was replaced by a mutant receptor (S196A). In these animals, an analgesic response was observed with acute administration of naltrexone; however, chronic administration of naltrexone did not result in tolerance to naltrexone itself or to morphine. We hypothesized that the lack of tolerance in these animals was attributable to activation of μ-opioid receptor and blockade of δ-opioid receptor concurrently. To test our hypothesis, both WT and KI mice were infused with naltrindole, a selective δ antagonist, 2 h before morphine pellet implantation. Both the pellet and the Alzet pump were removed after 3 d, and the animals were tested for their response to morphine. Our results show that both WT and KI mice failed to develop tolerance to morphine in the presence of the δ antagonist naltrindole. These studies are in agreement with other reports (Vaught and Takemori, 1979; Fundytus et al., 1995; Schiller et al., 1999; Wells et al., 2001) that show that pharmacological blockade of δ-opioid receptors attenuates morphine tolerance. We then investigated whether δ blockade after tolerance has developed could reverse the effects of tolerance. In these experiments, WT and KI animals were infused with NTI 72 h after morphine pellet implantation. Both WT and KI animals developed a high degree of tolerance to morphine. These results suggest that inhibition of δ-opioid receptor must occur at the time of μ activation to prevent tolerance development. Tolerance development can be prevented by δ blockade but cannot reversed once it has occurred.
If activation of μ-opioid receptor and blockade of δ-opioid receptor are the mechanism for the lack of tolerance development in the KI mice, then we speculate that activation of δ receptor with simultaneous activation of μ-opioid receptor will induce tolerance in the KI animals. To test this hypothesis, experiments were performed to activate δ-opioid receptors with SNC-80, a δ agonist, in the presence of μ activation by naltrexone in the KI animals. Our results support our hypothesis that μ-receptor activation in the presence of a δ-opioid receptor agonist results in tolerance development, because infusion of SNC-80 resulted in a dose-dependent rightward shift in the morphine dose–response curve. A minimal dose of 1 mg · kg−1 · 24 h−1 was sufficient in inducing morphine tolerance. Furthermore, our studies also show that continual administration for the duration that the animal is exposed to morphine is necessary for tolerance development. These results suggest that analgesic agents that have both μ- and δ- (even minimal) agonistic properties would have limitations for long-term analgesic use, because of their potential to induce tolerance.
Although these results provide strong evidence that μ-opioid receptor activation in the presence of δ-opioid receptor blockade reduced the induction of tolerance, it is quite conceivable that the mutant receptor, although having μ-opioid receptor characteristics, may result in a conformational change with naltrexone binding that is distinct from that induced by morphine. This could result in the recruitment or activation of adapter proteins or second messenger systems that require δ-opioid receptor cooperativity. However, because blockade of the δ-opioid receptors in the WT animals also attenuated tolerance, it is unlikely that this phenomenon is an exclusive characteristic of the mutant receptor. Furthermore, in vitro binding and functional (adenylyl cyclase activity) studies using cells transfected with the mutant receptor show that the mutant receptor still retains WT affinity and potency (Claude et al., 1996), implying that ligand–receptor interactions were not altered by the mutation. We speculate that the mutation of the serine in the WT mice to a non-hydrogen-bonding leucine residue as in the KI mice leads to a more permissive receptor activation state, allowing sterically hindered but structurally similar antagonists greater access to the activation domains. This possibility is supported by studies demonstrating that antagonists with high structural homology to agonists (i.e., naloxone, naltrexone, naltriben, and H-Tyr-Tic[CH(2)NH]-Phe-Phe-OH-ψ) are capable of agonist activity if the receptor allows it.
In summary, our study provides additional, compelling evidence that δ-opioid receptors are involved in the development of tolerance to morphine. An important implication of this conclusion is that analgesic agents that have both μ- and δ- (even minimal) agonistic properties would have limitations for long-term analgesic use, because of their potential to induce tolerance. Future studies using this unique model may help to further elucidate the cellular and molecular mechanism underlying morphine tolerance.
Acknowledgments
This research was supported by National Institutes of Health (NIH) Grants DA00564, DA01583, DA11806, and KO5-DA70554, by the F. A. Stark Fund of the Minnesota Medical Foundation (H.H.L.), by NIH Grants RO1 DA12104, KO2 DA015349, and P50 DA 11806 (S.R.), and by NIH Grant T32DA07097 (J.K.).
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of δ opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- Chen YL, Law PY, Loh HH. Inhibition of akt/protein kinase B signaling by naltrindole in small cell lung cancer cells. Cancer Res. 2004;64:8723–8730. doi: 10.1158/0008-5472.CAN-03-3091. [DOI] [PubMed] [Google Scholar]
- Claude PA, Wotta DR, Zhang XH, Prather PL, McGinn TM, Erickson LJ, Loh HH, Law PY. Mutation of a conserved serine in TM4 of opioid receptors confers full agonistic properties to classical antagonists. Proc Natl Acad Sci USA. 1996;93:5715–5719. doi: 10.1073/pnas.93.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison NM. Opioid analgesics for cancer pain: toxicities and their treatment. In: Patt RB, editor. Cancer pain. Philadelphia: JB Lippincott; 1993. pp. 185–194. [Google Scholar]
- Fang FG, Fields HL, Lee NM. Action at the μ receptor is sufficient to explain the supraspinal analgesic effect of opiates. J Pharmacol Exp Ther. 1986;238:1039–1044. [PubMed] [Google Scholar]
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective δ-opioid receptor antagonist TIPP[ψ] Eur J Pharmacol. 1995;286:105–108. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Filliol D, Simonin F, Matthes HW, Kieffer BL. Immunosuppression by δ-opioid antagonist NTI: δ- and triple μ/δ/κ-opioid receptor knockout mice reveal a nonopioid activity. J Pharmacol Exp Ther 2001. 2001;298:1193–1198. [PubMed] [Google Scholar]
- Hepburn MJ, Little PJ, Gingras J, Kuhn CM. Differential effects of NTI on morphine-induced tolerance and physical dependence in rats. J Pharmacol Exp Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- Heyman JS, Jiang Q, Rothman RB, Mosberg HI, Porreca F. Modulation of μ-mediated antinociception by δ agonists: characterization with antagonists. Eur J Pharmacol. 1989;169:43–52. doi: 10.1016/0014-2999(89)90815-7. [DOI] [PubMed] [Google Scholar]
- Larson AA, Vaught JL, Takemori AE. The potentiation of spinal analgesia by leucine enkephalin. Eur J Pharmacol. 1980;61:381–383. doi: 10.1016/0014-2999(80)90077-1. [DOI] [PubMed] [Google Scholar]
- Lee NM, Leybin L, Chang JK, Loh HH. Opiate and peptide interaction: effect of enkephalins on morphine analgesia. Eur J Pharmacol. 1980;68:181–185. doi: 10.1016/0014-2999(80)90319-2. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–146. [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in δ-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba P, Ben Y, Smith AP, Furst S, Lee NM. Morphine tolerance in spinal cord is due to interaction between μ- and δ-receptors. J Pharmacol Exp Ther. 2002;300:265–272. doi: 10.1124/jpet.300.1.265. [DOI] [PubMed] [Google Scholar]
- Russell RD, Leslie JB, Su YF, Watkins WD, Chang KJ. Interaction between highly selective μ and δ opioids in vivo at the rat spinal cord. NIDA Res Monogr. 1986;75:97–100. [PubMed] [Google Scholar]
- Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM, Lemieux C, Chung NN, Coderre TJ. The opioid μ agonist/δ antagonist DIPP-NH(2)[ψ] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J Med Chem. 1999;42:3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- Sora I, Funada M, Uhl GR. The μ-opioid receptor is necessary for [d-Pen2,d-Pen5]enkephalin-induced analgesia. Eur J Pharmacol. 1997;324:R1–R2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- Vaught JL, Takemori AE. Differential effects of leucine and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J Pharmacol Exp Ther. 1979;208:86–90. [PubMed] [Google Scholar]
- Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid μ-agonist/δ-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J Pharmacol Exp Ther. 2001;297:597–605. [PubMed] [Google Scholar]
- Yang W, Law PY, Guo X, Loh HH. In vivo activation of a mutant μ-opioid receptor by antagonist: future direction for opiate pain treatment paradigm that lacks undesirable side effects. Proc Natl Acad Sci USA. 2003;100:2117–2121. doi: 10.1073/pnas.0334906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]